Abstract
Conjugal transfer of Agrobacterium tumefaciens Ti plasmids is regulated by quorum sensing via TraR and its cognate autoinducer, N-(3-oxo-octanoyl)-l-homoserine lactone. We isolated four Tn5-induced mutants of A. tumefaciens C58 deficient in TraR-mediated activation of tra genes on pTiC58ΔaccR. These mutations also affected the growth of the bacterium but had no detectable influence on the expression of two tester gene systems that are not regulated by quorum sensing. In all four mutants Tn5 was inserted in a chromosomal open reading frame (ORF) coding for a product showing high similarity to RNase D, coded for by rnd of Escherichia coli, an RNase known to be involved in tRNA processing. The wild-type allele of the rnd homolog cloned from C58 restored the two phenotypes to each mutant. Several ORFs, including a homolog of cya2, surround A. tumefaciens rnd, but none of these genes exerted a detectable effect on the expression of the tra reporter. In the mutant, traR was expressed from the Ti plasmid at a level about twofold lower than that in NT1. The expression of tra, but not the growth rate, was partially restored by increasing the copy number of traR or by disrupting traM, a Ti plasmid gene coding for an antiactivator specific for TraR. The mutation in rnd also slightly reduced expression of two tested vir genes but had no detectable effect on tumor induction by this mutant. Our data suggest that the defect in tra gene induction in the mutants results from lowered levels of TraR. In turn, production of sufficient amounts of TraR apparently is sensitive to a cellular function requiring RNase D.
Quorum-dependent conjugation of Agrobacterium Ti plasmids is controlled by a hierarchical cascade designed to sense environmental conditions conducive to interbacterial transfer of these virulence elements (22). Activation of expression of the three operons of the Ti plasmid tra regulon requires the LuxR homolog TraR and its acyl-homoserine lactone (acyl-HSL) ligand, N-(3-oxo-octanoyl)-l-HSL (3-oxo-C8-HSL) (24, 52, 64). However, induction of transfer also requires a plasmid-specific subset of opines (37), nutritional factors produced by the crown gall tumors induced by pathogenic agrobacteria (17). Opines are required for induction of transfer because on the Ti plasmids traR itself is invariably a member of an operon regulated by these substrates. For example, conjugal transfer of the nopaline-type Ti plasmid pTiC58 is induced by the sugar phosphodiester opines agrocinopines A and B (21). The traR gene of this Ti plasmid is a member of the five-gene arc operon, expression of which is controlled by AccR, a transcriptional repressor that responds to the agrocinopines (5, 53). Thus, in the absence of the opines, AccR represses expression of the arc operon and TraR is not produced at levels sufficient to activate the tra regulon.
Synthesis of functional components of quorum-sensing systems can be dependent upon specialized host functions. For example, expression of signal-activatable LuxR requires GroESL, suggesting that proper folding during translation is critical for the activity of this transcription factor (1, 18). To date, only TraR has been purified in an active form and this has occurred only with cells grown with the acyl-HSL signal (54, 65). The inability to directly purify other members of the LuxR family in their native, biologically active form emphasizes the importance of correct folding in the activities of these proteins. In addition, specialized transcription factors are required for expression of some quorum-sensing systems. For example, in Pseudomonas aeruginosa, a mutation in rpoS diminishes expression of rhlI, suggesting that production of the Rhl-associated quorum-sensing signal, N-butyryl-HSL, is controlled by this transition-phase sigma factor (60). Since RhlR, the cognate LuxR homolog (40, 47) requires N-butyryl-HSL for activation (50), quorum-dependent expression of the rhl regulon by activated RhlR is influenced by RpoS.
Several lines of evidence suggest that the TraR-mediated Ti plasmid quorum-sensing system also is subject to host factors. First, TraR in conjunction with its acyl-HSL signal does not activate expression of a tra promoter in heterologous bacteria such as Escherichia coli (43), suggesting that host-specific factors play some mechanistic role in the Ti plasmid quorum-sensing system. Second, TraR expressed in A. tumefaciens in the absence of its acyl-HSL signal is extremely unstable and apparently is rapidly degraded by a host proteolysis system (65, 66). Finally, addition of the acyl-HSL signal to saturating levels at the time of opine induction does not result in immediate expression of the tra regulon (51). Instead, expression is delayed between 6 and 8 h after simultaneous addition of the two signals. On the other hand, addition of the acyl-HSL to a reporter system in which traR is constitutively expressed results in virtually immediate activation of a tra::lacZ reporter fusion (52). This observation suggests that expression of traR from the arc promoter, or accumulation of TraR protein following expression, is influenced by one or more additional factors.
To approach the question of host factors important to quorum sensing, we developed a genetic screen to search for functions of Agrobacterium tumefaciens that are required for the TraR-mediated expression of Ti plasmid conjugal transfer genes. We report here that, of the four mutants defective in TraR-mediated gene activation that we isolated from this screen, all mapped to the same chromosomal gene, which by sequence analysis is a homolog of rnd from E. coli. Genetic and physiological analyses indicate that the product of the rnd gene of A. tumefaciens (rndA.t.), the homolog of which in E. coli probably participates in tRNA processing, is required for accumulation of TraR to levels necessary to induce the tra regulon.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Plasmids and strains of E. coli and A. tumefaciens used in this study are listed in Table 1. E. coli strains were grown at 37°C in L broth or on L agar plates. Agrobacterium strains were grown at 28°C in L broth, nutrient agar (NA) (Difco Laboratories, Detroit, Mich.), or MG/L medium (11). AB medium (13) supplemented with 0.2% mannitol (ABM medium) as the sole carbon source was used as the defined minimal medium for Agrobacterium strains. To select transconjugants containing pTiC58 and its derivatives, a mixture of nopaline and arginine at final concentrations of 1 and 10 mM respectively, was included in AB agar as the sole carbon source (6). The following antibiotics were used at the indicated concentrations (in micrograms per milliliter); for E. coli kanamycin, 50; tetracycline, 10; and ampicillin, 100; and for A. tumefaciens, kanamycin, 50; carbenicillin, 50 or 100; and tetracycline, 2. X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Sigma, St. Louis, Mo.) was included in the medium at 40 μg per ml to monitor the production of β-galactosidase. When necessary, cell growth was monitored by measuring culture turbidity by Klett colorimetry (red filter) or by optical density at 600 nm (OD600) using a Spectronic 20 spectrophotometer.
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strains or plasmid | Characteristicsa | Reference(s) or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | F− φ80dlacZΔM15 endA1 recA1 hsdR17(rK−mK+) supE44 thi-1 gyrA96 Δ(lacZYA-argF)U169 | 55 |
| S17-1 | Pro− r− m+recA, integrated RP4-Tet::Mu-Kan::Tn7, Mob+ | 57 |
| 1830(pJB4JI) | Met-63 Pro-22 Kmr Nmr, Tn5 donor | 8 |
| A. tumefaciens | ||
| NT1 | Derivative of strain C58 cured of pTiC58 | 59 |
| NTM4 | Tn5-induced mutant of NT1 deficient in TraR/AAI-mediated activation of tra genes | This study |
| NTM5 | Tn5-induced mutant of NT1 deficient in TraR/AAI-mediated activation of tra genes | This study |
| NTM6 | Tn5-induced mutant of NT1 deficient in TraR/AAI-mediated activation of tra genes | This study |
| NTM7 | Tn5-induced mutant of NT1 deficient in TraR/AAI-mediated activation of tra genes | This study |
| A136 | Derivative of NT1 resistant to rifampin and nalixic acid, Rifr Nalr | 59 |
| A136(miaA) | miaA::Tn5 mutant of A136, deficient in VirA/VirG-mediated activation of vir genes | 28 |
| C58C1RS | Ti plasmid-cured C58, recipient strain for Ti plasmid conjugation, Rifr Strr | 21 |
| Plasmids | ||
| pBluescript SK(+) | Cloning vector, Apr | Stratagene |
| pRK415 | Broad-host-range cloning vector, InP1α Tcr | 36 |
| pMGm | Source of gentamicin resistance cassette, Apr Gmr | 46 |
| pBBR1MCS-5 | Broad-host-range cloning vector, unknown Inc group, Gmr | 39 |
| pTiC58ΔaccR | Derivative of pTiC58 with a deletion in the accR gene, Trac | 5, 6 |
| pDCKI41 | Derivative of pTiC58ΔaccR with a lacZ fusion to traG, TraR reporter strain, Tra− Cbr | 32 |
| pKPK12 | Derivative of pTiC58ΔaccR with a lacZ fusion to traR, Tra− Kmr | 53 |
| pKMI41 | Derivative of pDCKI41 with an nptII cassette inserted into the traM gene, Kmr Cbr | 32 |
| pYPQ | lac promoter region of pZLQ (43) cloned by PCR into pBBR1MCS-5 as an SstI-KpnI fragment | Our collection |
| pPLE2-25 | pDSK519 carrying a fragment from pTiC58 with a regulated trbE::lacZ fusion, IncQ Kmr Cbr | 41 |
| pRK415GIII | pRK415 with a gentamicin cassette cloned into the unique XmnI site as a 1.7-kb end-blunted EcoRI-SphI fragment, IncP1α Tcr Gmr | This study |
| pRK415GIIIE33 | EcoRI fragment 33 of pTiC58 containing traR cloned into pRK415GIII | This study |
| pRKL17 | Ca. 8-kb BglII fragment containing a trbE::lacZ fusion from pPLE2-25 cloned into pRK415GIIIE33, IncP1α Tcr Gmr | This study |
| pYDH208::lacZ#6 | mocE::lacZ fusion in pYDH208, IncP1α Tcr Cbr | 31, 38 |
| pSOM303::lacZ#39 | recA::lacZ fusion cloned in pRK290, IncP1α Tcr Cbr | 23 |
| pZL5E33 | EcoRI fragment 33 of pTiC58 containing traR cloned into pBBR1MCS-5, Gmr | This study |
| pTiA6pinF::lacZ | pinF::lacZ (virH::lacZ) fusion in pTiA6 | 28, 35 |
| pSM358 | virE::lacZ fusion from pTiA6 cloned into pVK101, IncP1α Apr Kmr | 58 |
| pZLQ9 | Cosmid clone of the NT1 genome in pCP13/B that complements the mutation in NTM7, Tcr | This study |
| pZLQ10 | Cosmid clone of the NT1 genome in pCP13/B that complements the mutation in NTM7, Tcr | This study |
| pZLB11 | 11-kb SalI fragment from pZLQ10 cloned into pBluescript SK(+) | This study |
| pZLR11 | 11-kb SalI fragment from pZLB11 cloned into pRK415GIII | This study |
| pZLB7 | 7-kb SalI-BglII fragment from pZLB11 cloned into pBluescript SK(+) | This study |
| pZLG7 | 7-kb XbaI-BglII fragment from pZLB7 cloned into pRK415GIII | This study |
| pZLE2.7 | 2.7-kb EcoRI fragment from pZLB7 cloned into pBluescript SK(+) | This study |
| pZLE252 | 2.7-kb EcoRI fragment from pZLB7 cloned into pRK415GIII | This study |
| pZLE253 | Same as pZLE252, but the fragment is cloned in the opposite orientation | This study |
| pZLP161 | 1.6-kb PstI fragment containing the rnd gene cloned into pRK415GIII and oriented correctly with respect to the lac promoter of the vector | This study |
| pZLP162 | Same as pZLP162, but the insert is oriented in the opposite direction | This study |
| pZLmeP1 | 2-kb NdeI-BamHI fragment from pZLQ10 containing the ycaD homolog and 1 kb of downstream DNA cloned into pYPQ | This study |
| pZlmeP1.9 | Same as pZlmeP1, but the insert is oriented in the opposite direction | This study |
| pZL41 | Tn5-tagged EcoRI fragment from NTM4 cloned into pBluescript SK(+) | This study |
| pZL51 | Tn5-tagged EcoRI fragment from NTM5 cloned into pBluescript SK(+) | This study |
| pBSM62 | Tn5-tagged EcoRI fragment from NTM6 cloned into pBluescript SK(+) | This study |
| pBSM72 | Tn5-tagged EcoRI fragment from NTM7 cloned into pBluescript SK(+) | This study |
| pZLK6 | 3.8-kb SalI-EcoRI fragment from pBSM62 cloned into pBluescript SK(+) | This study |
| pZLA6 | 3.2-kb SalI-EcoRI fragment from pBSM62 cloned into pBluescript SK(+) | This study |
| pZLK7 | 4.8-kb SalI-EcoRI fragment from pBSM62 cloned into pBluescript SK(+) | This study |
| pZLA7 | 3.6-kb SalI-EcoRI fragment from pBSM62 cloned into pBluescript SK(+) | This study |
| pDB14 | E. coli rnd gene cloned in pUC18 | 62 |
| pZLD14 | E. coli rnd gene from pDB14 cloned as a 1.4-kb EcoRI-BamHI fragment into pRK415GIII | This study |
Abbreviations: Apr, ampicillin resistance; Cbr, carbenicillin resistance; Cmr, chloramphenicol resistance; Gmr, gentamicin resistance; Kmr, kanamycin resistance; Nalr, naladixic acid resistance; Rifr, rifampin resistance; Spcr, spectinomycin resistance; Strr, streptomycin resistance; Tcr, tetracycline resistance; Trac, transfer constitutive.
General DNA manipulations.
Plasmids with sizes less that 50 kb were isolated from E. coli or A. tumefaciens strains by alkaline lysis methods (29, 55). To isolate Ti plasmid DNA for restriction analysis or for electroporation, 5-ml cultures of A. tumefaciens strains were grown in MG/L medium to late exponential phase (OD600, ∼0.8). The cells were harvested by centrifugation and washed with a 1-ml volume of Agrowash (0.5 M NaCl, 50 mM Tris · HCl, 20 mM Na2EDTA [pH 8.0], 0.1% Sarkosyl), and the Ti plasmids were extracted by a modified alkaline lysis method as described previously (30). Following lysis, all extractions, mixings, and other manipulations were performed as gently as possible to minimize shearing of the plasmid DNA.
Cloning was conducted using standard recombinant-DNA techniques (55). Restriction digestions were carried out according to the instructions of the manufacturers (Gibco BRL and New England Biolabs). Digestion products were separated by electrophoresis in 0.8 or 1.5% agarose gels, depending upon the size of the fragments to be separated, using Tris-borate-Na2EDTA buffer. DNA fragments were recovered from agarose gels using GenElute spin columns (Supelco, Inc., Bellefonte, Pa.). Plasmids were introduced into E. coli by CaCl2-mediated transformation (55) and into A. tumefaciens by S17-1-mediated biparental matings (57) or by electroporation (11).
Preparation of genomic DNA.
Genomic DNAs were prepared from Agrobacterium strains by a modification of the method of Glickmann et al. (27) as follows. Bacterial cells grown in 2 ml of MG/L medium to late exponential phase were collected and washed with 1.5 ml of Agrowash. After incubation at room temperature for 5 min, cells were collected and were subjected to two washes with 1.5 ml of 5 M NaCl. In the second wash, a volume of 50 μl of 5% Sarkosyl was included in the NaCl solution. Following incubation at room temperature for another 5 min, cells were collected and resuspended in 900 μl of LTE (55) and volumes of 300 μl of 5% Sarkosyl and 50 μl of proteinase K (5 mg per ml) were added to the cell suspension. The mixture was gently vortexed and was incubated at 37°C for 3 h or until the cells were completely lysed, as judged by the loss of turbidity of the cell suspension. The DNA was gently sheared by pipetting the mixture for 5 min, and the lysate was extracted three times with equal volumes of phenol saturated with 3% NaCl. Following one extraction with 400 μl of chloroform-isoamyl alcohol (24:1, vol/vol), the supernatant was extracted once with diethyl ether. DNA present in the lower, aqueous phase was precipitated with 2 volumes of ethanol and washed twice with cold 70% ethanol. After drying in air for 2 h, the DNA was dissolved in 200 to 300 μl of LTE buffer containing RNase (40 μg per ml) (Ambion, Austin, Tex.).
Reporter strain construction and mutant screening.
A derivative of A. tumefaciens NT1 containing two independent reporters was constructed to reduce the possibility of obtaining trivial mutants by simply inactivating one of the known components of the tra quorum-sensing system. The first reporter plasmid, pDCKI41, is a derivative of pTiC58ΔaccR (Table 1), which contains a traG::lacZ fusion marker-exchanged into the tra region of the Ti plasmid (32). This reporter expresses the fusion constitutively, since both TraR and the acyl-HSL are produced at high levels (32, 51, 53). For the second reporter, a gentamicin resistance cassette was isolated from pMGm (46) as a ca.-1.7-kb EcoRI-SphI fragment and, after being blunted with mung bean nuclease, cloned into the unique XmnI site of the IncP1α plasmid pRK415 (36) to generate the new vector pRK415GIII. The activator gene traR and a trbE::lacZ fusion from pPLE2-25 (34, 41) were cloned, respectively, as a 1.8-kb EcoRI fragment and a ca. 8-kb BgII fragment into pRK415GIII to give pRKL17. Similar to what occurs with pDCKI41, in A. tumefaciens NT1 this plasmid constitutively expresses the lacZ reporter fusion. pRKL17 was transferred into NT1(pDCKI41) to give NT1(pDCKI41, pRKL17), a strain that contains two plasmids, each with a copy of traR and traI and each harboring a lacZ fusion that reports TraR activity.
NT1(pDCKI41, pRKL17) was mutagenized with Tn5 by mating it on filters with E. coli 1830(pJB4JI) (Table 1) as follows. Saturated cultures of both strains were diluted 1:10 in L broth, and after a period of regrowth (4 h for E. coli and 6 h for A. tumefaciens), 1-ml volumes of the donor culture were mixed with 1.5-ml volumes of recipient cells. The cells in the mixtures were collected onto 11 membrane filters (0.2-μm pore size; Millipore, Bedford, Mass.). and the filters were incubated for 10 h at 28°C on NA plates, after which the cells were washed off the filters with 0.9% NaCl solution. A portion of cells from each filter were individually plated onto ABM medium containing X-Gal, 3-oxo-C8-HSL, and appropriate antibiotics. Pale blue or white colonies growing on this medium constitute candidate mutants that no longer support expression of the traG::lacZ and trbE::lacZ fusions.
Conjugal transfer assay.
The transfer-constitutive Ti plasmid pTiC58ΔaccR was introduced into cured derivatives of the Tn5-induced mutants using the spot plate mating method as previously described (6). Cells of the mutants were spread as a confluent lawn onto AB medium containing 1 mM nopaline, 10 mM arginine, and kanamycin. Five-microliter volumes of serial 10-fold dilutions of the donor strain NT1(pTiC58ΔaccR), grown in ABM medium (OD600 = 0.5 to ∼0.6), were then spotted onto the medium on which the recipients had been spread. After 4 to 5 days, transconjugants appeared within the areas in which the drops were applied. Transconjugant colonies were purified, and the presence and integrity of the Ti plasmid were confirmed by restriction endonuclease analysis. A similar method was used to assess the ability of the mutants to transfer pTiC58ΔaccR. The recipient strain C58C1RS was spread as a confluent lawn over the surface of the selection medium containing rifampin and streptomycin. Ten-microliter volumes of donor cells at decreasing cell concentrations were spotted onto the surface of the recipient lawn, and the cultures were incubated at 28°C for 72 to 96 h. Transconjugant colonies appearing within the donor inoculum spots were enumerated with the aid of a dissecting microscope. Each set of matings was repeated twice, and frequencies are expressed as transconjugants arising per input donor.
Southern hybridization.
After digestion with the appropriate restriction endonucleases, DNA fragments were separated on 0.7% agarose gels and transferred by diffusion to a nitrocellulose membrane. DNA probes were randomly labeled using a Genius digoxigenin kit (Roche Biochemicals, Indianapolis, Ind.) by following the manufacturer's instructions. Protocols for hybridizations, washings, and detection were those provided by the manufacturer. Hybridization and washing were performed under conditions of high stringency.
Cloning the Tn5-disrupted locus.
Samples of total genomic DNA of the mutants were digested with EcoRI, an enzyme that does not cleave Tn5 (7), thus generating fragments that contain the entire transposon and the flanking chromosomal DNA. The digested DNA was ligated to EcoRI-digested pBluescript SK(+), and the ligation mixtures were transformed into E. coli strain DH5α with selection for resistance to kanamycin.
Complementation using a genomic clone bank.
A small portion of a genomic bank of NT1 represented by cosmid clones harbored in E. coli strain DH1 (23) was grown overnight on L agar. Cells were washed off with a 0.9% NaCl solution, total plasmid DNA was isolated, and the pooled cosmid bank was introduced by electroporation into one of the mutants, NTM7, harboring pDCKI41 as the reporter. Cells were spread onto ABM medium plates containing X-Gal, and the cultures were incubated at 28°C for 3 days. Blue colonies growing on the medium were retained as harboring candidate cosmids carrying the locus complementing the Tn5-disrupted gene.
Acyl-HSL detection.
ABM agar-based acyl-HSL detection plates were prepared as follows. A 20-ml volume of a saturated culture of the acyl-HSL reporter strain NT1(pDCKE33141) (56) was mixed with 100 ml of soft ABM medium (0.7% agar) containing X-Gal, and a 6-ml volume of the culture suspension was laid over a 25-ml base of ABM agar medium. Culture supernatants, extracts of culture supernatants, or cells to be tested were spotted onto the solidified indicator medium, and the plates were incubated at 28°C for 14 to 18 h. The presence of the acyl-HSL was indicated by the appearance of a diffusing blue zone around the samples.
Virulence assays.
Tumorigenesis was assessed on tomato plants using a stem inoculation method (49). Bacterial strains were grown in ABM medium to saturation. A 10-fold dilution series of each culture was prepared by diluting the cell suspensions in a 0.9% solution of NaCl. For each strain, volumes of 10 μl of several of the dilutions were inoculated into the stems of 3-week-old tomato plants. Tumors appearing at the inoculation sites were scored 20 days after inoculation.
Induction media and assays for β-galactosidase activity.
For expression of tra genes, all assays were carried out using cells grown in liquid ABM medium. Unless otherwise specified, when necessary, the acyl-HSL was added at a final concentration of 25 nM. To assay for the induction of mannopine utilization genes, bacterial strains were grown in AT medium supplemented with 0.2% mannitol and 0.15% (NH4)2SO4 (16). Cultures at an OD600 of about 0.8 were diluted 1:10 into fresh medium and were allowed to grow for 4 h. Mannopine (Sigma Chemical Co.) was added to a final concentration of 10 mM, the cultures were incubated for another 6 h, and cells were harvested and assayed for the expression of the reporter gene. Strains used to assay for the expression of the recA::lacZ fusion were grown in ABM medium. To measure the expression of vir::lacZ reporters, we employed a method described by Gray et al. (28). Briefly, A. tumefaciens strains were grown to mid-exponential phase in YEP medium (28) and diluted to an OD600 of 0.1 in filter-sterilized AB medium-based vir induction medium. This medium, with the final pH adjusted to 5.6, contains the salts for AB medium, 0.1% glucose, 2 mM phosphate, and 30 mM morpholine ethanesulfonic acid (MES). In all cases, the vir inducer acetosyringone was added to a final concentration of 200 μM. Cultures were incubated with shaking at 28°C for 12 to 14 h, and cells were harvested for determining culture titers and assayed for β-galactosidase activity. In all cases, enzyme activity is expressed as units of β-galactosidase per 109 CFU (48).
Nucleotide sequencing and sequence analysis.
Subcloned DNA fragments were sequenced on both strands using automated methods by the Genetic Engineering Facility at the University of Illinois at Urbana-Champaign. To ensure that no short fragments were present between the restriction sites used for subcloning, we determined the sequence across each site using pZLB7 (Table 1) as the template with primers designed from the adjacent regions. Nucleotide sequences were assembled and analyzed using DNA Strider (45). The BLAST (2) protocols were used for DNA and protein database searches and analyses. The GAP subroutine of the GCG program (Genetics Computer Group, Madison, Wis.) was used to compare sequences for similarity.
Determination of the Tn5 insertion sites.
The sites of the Tn5 insertions in the mutants were determined using a primer designed from the end of the transposon as follows. To avoid interference by the repeated DNA sequence at the ends of Tn5, plasmids harboring the transposon-tagged genomic DNAs from mutants NTM4, NTM5, and NTM7 were digested with EcoRI and SalI. EcoRI does not cut within Tn5, and SalI, which cleaves the transposon at one site, does not cut within the flanking chromosomal DNA (determined by digesting each clone with appropriate enzymes). The resulting two EcoRI-SalI fragments from each mutant were individually cloned into pBluescript SK(+) (Table 1). A primer homologous to the end of Tn5 (5′-AAGGTTCCGTTCAGGACGCTAC-3′) was used to sequence through the junction site between the transposon and the chromosomal DNA. For mutant NTM6, in which the transposon inserted into a relatively short (∼1-kb) EcoRI fragment, the site of insertion was determined by sequencing through the junction sites with the universal primers from the cloning vector, pBluescript SK(+).
Nucleotide sequence accession number.
The sequence of the 5.3-kb region containing rndA.t. from wild-type C58 was deposited in the GenBank database under accession no. AY026066.
RESULTS
Tn5 mutagenesis of A. tumefaciens and screening of mutants defective in tra gene induction.
To identify functions required for quorum sensing, we mutagenized NT1(pDCKI41, pRKL17) with Tn5 and screened for mutants unable to activate the tra::lacZ reporters. From approximately 15,000 kanamycin-resistant mutants recovered, 11 pale blue or white colonies were chosen for further study. In four of the candidates, NTM4, NTM5, NTM6, and NTM7, the insertion did not directly inactivate traR or the lacZ reporter. The other seven candidates all contained Tn5 inserted into the traR gene of pDCKI41 (data not shown).
Based on the level of tra gene induction from the two reporters, the four mutants were divided into two groups. The first, consisting of only NTM6, is partially deficient in tra gene induction, expressing the two fusions at a level about half of that in the parent strain (Table 2). The second group, consisting of NTM4, NTM5, and NTM7, produced barely detectable levels of β-galactosidase activity (Table 2).
TABLE 2.
The mutants deficient in tra gene induction are altered in their ability to transfer pTiC58ΔaccR
| Strain | Relative level of expression of reportersa | Conjugal transfer frequencyb |
|---|---|---|
| NT1 | ++++ | 2.7 × 10−2 |
| NTM4 | +/− | <10−8 |
| NTM5 | +/− | <10−8 |
| NTM6 | ++ | 3.5 × 10−6 |
| NTM7 | +/− | <10−8 |
Assessed on ABM medium containing X-Gal after 2 days of incubation. Each strain harbored the two reporter plasmids pDCKI41 and pRKL17. ++++, intensely blue colonies; ++, medium-blue colonies; +/−, very pale blue colonies.
Conjugal transfer of pTiC58ΔaccR from the parent and the four mutants to C58C1RS was tested as described in Materials and Methods. The frequency of transfer is expressed as the number of transconjugants obtained per input donor.
The mutants are defective in Ti plasmid conjugation.
To assess the effect of the mutation on conjugal transfer, we introduced pTiC58ΔaccR (Table 1) into each of the mutants which had been cured of their two reporters. NTM6 transferred pTiC58ΔaccR at a frequency about 4 orders of magnitude lower than that of the parent, while each of the other three mutants failed to transfer the Ti plasmid at a detectable level (Table 2).
The mutants grow more slowly than the wild-type parent.
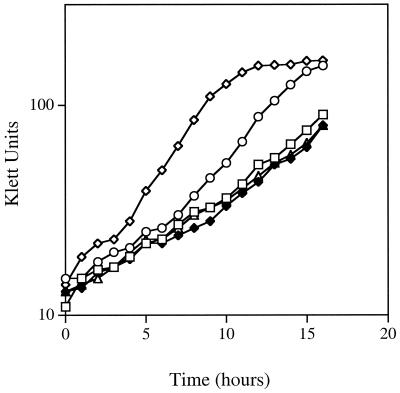
The growth rate of NTM6 was slightly lower than that of the wild-type strain in minimal and rich media (Fig. 1 and data not shown). However, NTM4, NTM5, and NTM7, while all growing at similar rates, grew considerably more slowly than NT1 and also NTM6 in both media (Fig. 1 and data not shown).
FIG. 1.
Growth properties of the Tn5-induced mutants of A. tumefaciens NT1. Saturated cultures of the bacterial strains were diluted into 20 ml of ABM minimal medium to the same population density (ca. 15 Klett units) in 150-ml sidearm flasks. The cultures were incubated with shaking at 28°C, and culture growth was monitored by Klett colorimetry (red filter) at 30-min intervals. ◊, NT1; □, NTM4; ⧫, NTM5; ○, NTM6; ▵, NTM7.
Cloning and characterization of the mutated gene.
As assessed by genomic Southern analysis, the Tn5 probe hybridized with a single fragment in each mutant, indicating that each is derived from a single transposition event (data not shown). In three mutants, the probe hybridized with a ca. 8.5-kb fragment, while in NTM6 the probe hybridized with a ca. 7-kb fragment (data not shown). We cloned the regions of the chromosomal DNA tagged by Tn5 from each mutant and determined the sizes of the chromosomal EcoRI fragments associated with the transposon. Consistent with the results of the Southern analysis, in NTM6, the transposon had inserted into a ca. 1.0-kb EcoRI fragment while in NTM4, NTM5, and NTM7, the transposon had inserted into a ca. 2.7-kb EcoRI fragment. Mapping experiments (Fig. 2) and nucleotide sequence analysis (see below) showed that the two EcoRI fragments are contiguous and that all four Tn5 elements are inserted in the same gene. Thus, we focused our study on one class II mutant, NTM7.
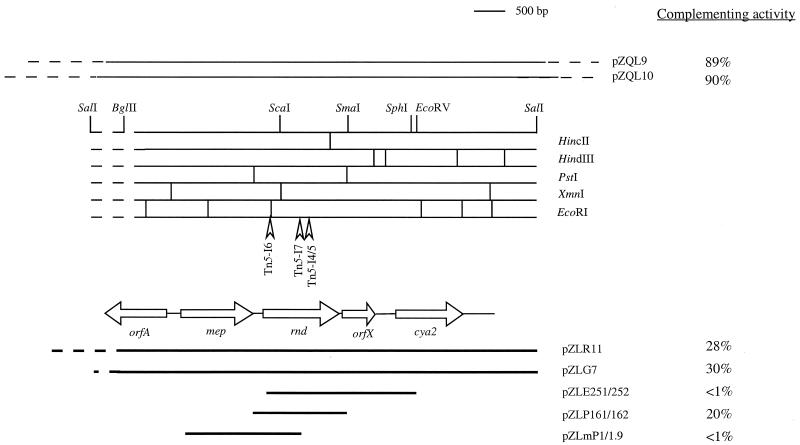
FIG. 2.
Genetic organization of the rnd region from the genome of A. tumefaciens C58. The restriction maps and the locations of the identified ORFs, shown as open horizontal arrows below the maps, are based on nucleotide sequence analysis as described in Materials and Methods and the text. The arrowheads show the sites of the Tn5 insertions in the mutants. Tn5-I6 corresponds to NTM6, Tn5-I7 corresponds to NTM7, and Tn5-I4/5 corresponds to NTM4 and NTM5. The two lines above the map depict the two cosmid genomic clones, with the solid lines denoting those portions of the inserts that overlap one another in the rnd region. The bold lines below the map show the extents of the various subclones derived from pZQL10. The percentages to the right indicate the ability of each clone to restore TraR-mediated activation of the traG::lacZ reporter fusion on pDCKI41 in NTM7 relative to levels of expression in NT1 harboring the same plasmids.
Identification and characterization of the gene affected in NTM7.
To isolate the wild-type version of the Tn5-disrupted gene, we introduced a cosmid bank of NT1 (23) into NTM7(pDCKI41) and screened for clones in which expression of the traG::lacZ reporter on the Ti plasmid was restored. Of about 50 blue colonies obtained, the cosmid clones from 10 were purified and analyzed. Restriction analysis revealed that the 10 cosmids are representatives of only two different clones, which we designated pZQL9 and pZQL10 (Table 1). pZQL9 contains an insert of about 28 kb, and pZQL10 contains an insert of about 23 kb (data not shown).
By reciprocal Southern blot analysis, several fragments common to both clones were identified, including one 1.6-kb PstI fragment, two HindIII fragments with sizes of ca. 0.9 and 1.4 kb, and at least three EcoRI fragments with sizes of ca. 2.7, 1.2, and 0.9 kb (Fig. 2 and data not shown). In a series of subclonings from this common region, we obtained a ca. 7-kb BglII-SalI fragment that, when cloned into pRK415GIII to give pZLG7, complemented the defect in expression of the traG::lacZ fusion in NTM7 and in each of the other three mutants (Fig. 2, Table 3, and data not shown).
TABLE 3.
Complementation analysis of traG::lacZ induction in mutant NTM7 with two genomic cosmids and their subclones
| Strain | Complementing cosmid or clone | β-Galactosidase activity from traG::lacZa | % of complementationb |
|---|---|---|---|
| NT1 | pRK415GIII | 94 | NAc |
| NTM7 | pRK415GIII | <1 | NA |
| NTM7 | pZQL9 | 83 | 88 |
| NTM7 | pZQL10 | 85 | 90 |
| NTM7 | pZLR11 | 28 | 30 |
| NTM7 | pZLG7 | 30 | 32 |
| NTM7 | pZLP162 | 20 | 21 |
| NTM7 | pZLP163 | 23 | 24 |
| NTM7 | pZLD14 | 4 | 4 |
β-Galactosidase activity expressed from pDCKI41 present in each mutant was analyzed as described in Materials and Methods (expressed as units per 109 CFU). No significant difference was observed when exogenous 3-oxo-C8-HSL was provided at a final concentration of 25 nM. Similar results were obtained in three independent experiments.
Calculated relative to the level of β-galactosidase activity present in NT1(pDCKI41, pRK415GIII).
NA, not applicable.
We determined the restriction map of this 7-kb fragment (Fig. 2) and subcloned several segments from this region into pBluescript SK(+) for DNA sequence analysis. Several fragments common to pZQL9 and pZQL10 also were cloned in both orientations into pRK415GIII to test for their ability to complement the mutation in NTM7 (Table 3, Fig. 2, and data not shown). The 1.6-kb PstI fragment present in clones pZLP161 and pZLP162, but not the 2.7-kb EcoRI fragment in pZLE251 and pZLE252, partially restored expression of the traG::lacZ reporter in NTM7 (Table 3 and Fig. 2). The two clones containing this PstI fragment also completely restored the growth defect of the mutant (Fig. 3). That such complementation is independent of the orientation of the insert with respect to the lac promoter on pRK415GIII suggested that this DNA fragment harbors at least some of the cis-acting promoter elements necessary for expression of the complementing gene.
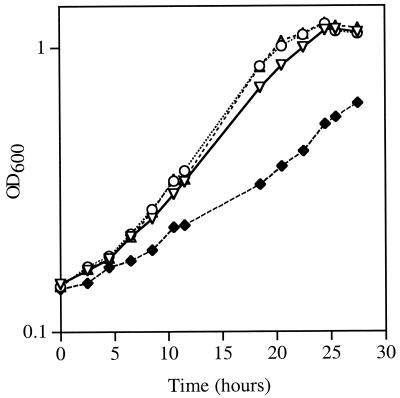
FIG. 3.
Subclones of rndA.t. and E. coli rnd restore wild-type growth properties to NTM7. Cultures of NTM7 harboring various clones were initiated in ABM medium and incubated as described in the legend to Fig. 1. Growth was monitored at 30-min intervals by spectrophotometry at 600 nm (OD600). ▿, NT1(pDCKI41, pRK415GIII); ⧫, NTM7(pDCKI41, pRK415GIII); ○, NTM7(pDCKI41, pZPL162); ▵, NTM7(pDCKI41, pZLD14).
DNA sequence analysis of a 5.3-kb region containing the 1.6-kb PstI fragment and the 2.7-kb EcoRI fragment revealed four complete open reading frames (ORFs), all transcribed in the same orientation, and one partial ORF oriented in the opposite direction (Fig. 2). The incomplete ORF, called orfA, codes for the first 153 amino acids of a protein that is related to YabK of E. coli (Table 4) (9). This 536-residue putative translation product is itself related to permease components of several transport systems. The first complete ORF, which we call mep (for membrane protein), is divergently oriented to orfA (Table 4). The product of this ORF is similar to the predicted product of the ycaD gene (previously orfY) of E. coli (Table 4) (9) and also to several putative multidrug efflux transporter homologs, including the YfkF protein of Bacillus subtilis (39% similarity and 22% identity; GeneBank accession no. D83967). Sequences resembling canonical −35 and −10 promoter elements were not present in the 660 bp of sequence upstream of this ORF (data not shown). The second ORF, which is preceded by a good ribosomal binding site sequence, may code for a protein with an Mr of 43,209 that is related to the product of the rnd gene of E. coli (Table 4 and Fig. 4). This gene, which we designated rndA.t., is separated from mep by an 82-bp intergenic region that contains the canonical −35 element TTGACA and a weak −10 sequence, the two being separated by an optimal 17-bp interval (data not shown). Downstream of rndA.t. is a small ORF, which we call orfX, the 185-residue translation product of which has no significant homologs in the databases (Table 4). Within the 55-bp intergenic region between rndA.t. and orfX, there are no DNA elements significantly similar to standard bacterial promoter components and there is no recognizable ribosome binding site candidate sequence adjacent to the putative translation initiation codon of this ORF (data not shown). The fourth ORF codes for a polypeptide exhibiting strong homology with several adenyl cyclases from both prokaryotes and eukaryotes, with highest similarity to the cya2 gene product from Sinorhizobium meliloti strain F34 (4) (Table 4). orfX and the cya2 homolog are separated by a 253-bp intergenic region containing sequences similar to −35 and −10 elements (data not shown).
TABLE 4.
Characteristics of the genes and their products coded for by the rndA.t. locus of C58
| A. tumefaciens gene | Coordinates (bp)a | Size (aa)d | Mass (kDa) | Related protein | Relatednessb | GenBank accession no. | Possible function |
|---|---|---|---|---|---|---|---|
| orfA | 459–1 | >153 | NAc | YabK | 46/61 | AE000117 | Putative transport protein |
| mep | 665–1955 | 394 | 46.2 | YcaD | 22/35 | AE000192 | Putative transport protein |
| rnd | 2041–3205 | 388 | 43.2 | RNase D | 30/44 | X07055 | RNase D |
| orfX | 3262–3816 | 185 | 20.1 | None | NA | NA | Unknown |
| cya2 | 4074–5102 | 343 | 37.6 | Cya2 | 53/62 | X80991 | Adenylate cyclase |
The first number is the first nucleotide of the initiation codon, and the second number is the last nucleotide of the final codon.
Numbers represent the percentages of identical residues over the percentages of residues exhibiting conserved substitutions between the two proteins.
NA, not applicable.
aa, amino acids.
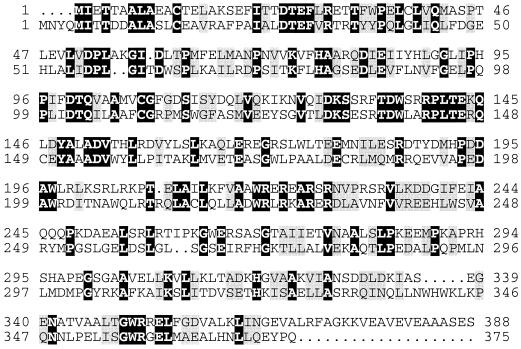
FIG. 4.
Relatedness of the translation products of the rnd genes of A. tumefaciens C58 and E. coli K-12. Alignments of amino acid sequences were performed using the GAP algorithm from the GCG package as described in Materials and Methods. Identical amino acids are shown as white letters on a black background, and conserved amino acid substitutions are shown as black letters on a shaded background.
By sequence analysis we located the insertion in mutant NTM6 at bp 41 of the rndA.t. gene, whereas in mutant NTM7, the transposon is inserted at bp 519 of this gene (Fig. 2 and data not shown). In mutants NTM4 and NTM5 Tn5 is inserted at the same location at bp 572 of the rndA.t. gene (Fig. 2 and data not shown). We do not know whether these two mutants are siblings or if each represents an independent insertion event.
The rnd mutation in NTM7 abolishes the expression of genes regulated by TraR on the Ti plasmid.
NTM7 containing the two reporter constructs expressed the traG::lacZ and the trbE::lacZ fusions at levels about sevenfold lower than those expressed by the wild-type strain (Table 5). However, when tested alone in NTM7, expression of traG::lacZ on pDCKI41, a Ti plasmid that represents the natural, transfer-induced conditions, was completely abolished (Table 5). Addition of excess exogenous 3-oxo-C8-HSL did not restore the expression of the fusion. We also examined the production of this acyl-HSL by NTM7 harboring pTiC58ΔaccR. As indicated by the sizes of the blue zones formed around the tested colonies, NTM7 (pDCKI41) produced about 10-fold less acyl-HSL than NT harboring the same plasmid (data not shown). Similar results were obtained when culture supernatants of these strains were spotted onto the detection plates (data not shown).
TABLE 5.
Mutant NTM7 fails to express tra genes
| Strain | Reporter fusion(s) | β-Galactosidase activitya
|
|
|---|---|---|---|
| Without acyl-HSL | With acyl-HSLb | ||
| NT1(pDCKI41, pRKL17) | traG + trbE | 332 | 327 |
| NTM7(pDCKI41, pRKL17) | traG + trbE | 48 | 53 |
| NT1(pDCKI41, pRK415GIII) | traG | 89 | 90 |
| NTM7(pDCKI41, pRK415GIII) | traG | <1 | <1 |
β-Galactosidase activity from traG::lacZ on pDCKI41 and trbE::lacZ on pRKL17 was assayed as described in Materials and Methods and is expressed as units per 109 CFU. The experiment was repeated two times with a similar pattern of results.
Provided at a final concentration of 100 nM.
The rnd mutation does not affect expression of recA or a mannopine utilization gene.
The four rnd mutants grow considerably more slowly than the wild-type strain (Fig. 1), suggesting that the mutations affect expression of many other genes or the activities of their products. We tested NTM7 for expression of two gene systems not regulated by quorum sensing. Plasmids pSOM303, containing a recA::lacZ fusion (23), and pYDH208-6, a clone containing lacZ fused to the mocE gene from the mannopine catabolism region of pTi15955 (31), each were introduced into NTM7. Both reporters were expressed in the mutant at levels indistinguishable from those observed in the wild-type strain (data not shown).
Expression of traR in the mutant is slightly lowered.
The transcriptional activator TraR is indispensable for expression of tra genes (52). We examined the expression of a translational traR::lacZ fusion carried on pKPK12, a derivative of pTiC58ΔaccR (53), in NTM7 and in NT1. As judged by levels of β-galactosidase, the TraR::LacZ fusion protein was expressed in the mutant at a level about twofold lower than that in strain NT1 (47 U per 109 CFU in the mutant versus 101 U per 109 CFU in the parent).
A mutation in traM or overexpression of traR partially restores the expression of tra genes in NTM7.
The observation that traR expresses at a lower level in NTM7 raised the possibility that the deficiency in tra gene induction in the mutant is due to insufficient amounts of the activator (51). Such a possibility is likely since pDCKI41, the plasmid we used to analyze the expression of the traG::lacZ reporter fusion, also codes for the antiactivator TraM, a protein that specifically inhibits TraR activity (25, 32, 33, 44). The presence of TraM in a strain expressing traR at a level lower than that of normal cells may lead to amounts of activator insufficient to initiate transcription. To examine this hypothesis, pKMI41, a derivative of pDCKI41 with a null mutation in traM (32) (Table 1), was introduced into NTM7. Strain NTM7(pKMI41) expressed the traG::lacZ reporter at a significantly higher level than that expressed by NTM7(pDCKI41), which is traM+ (Table 6). These results suggest that in NTM7, TraM completely inhibits TraR activity expressed from pDCKI41.
TABLE 6.
A mutation in traM partially restores the expression of the traG::lacZ reporter fusion in NTM7
| Test strain | rnd genotype | Reporter Ti plasmid | traM genotype | β-Galactosidase activitya |
|---|---|---|---|---|
| NT1 | + | pDCKI41 | + | 92 |
| pKMI41b | − | 114 | ||
| NTM7 | − | pDCKI4 | + | <1 |
| pKMI41b | − | 46 |
β-Galactosidase activity, assayed as described in Materials and Methods, is expressed as units per 109 CFU.
pKMI41 is a derivative of pDCKI41 with an nptII insertion in traM (32).
The results also suggest that overexpression of the activator gene in the mutant should at least partially restore the induction of the traG::lacZ fusion. We tested this hypothesis by introducing clones of traR into strain NTM7(pDCKI41). When pRK415GIIIE33, a low-copy-number clone (5 to 10 copies [36]), was introduced into this strain, no significant induction of the traG::lacZ reporter was observed (Table 7). In wild-type strain NT1, TraR expressed from this plasmid is sufficient to activate the tra and trb genes to high levels (Table 7 and data not shown). However, the mutant harboring pZL5E33, a higher-copy-number (25 to 30 copies [3]) clone of traR, expressed the reporter at a level significantly higher than the level in NTM7(pKCKJI41) (Table 7). Thus, the mutation in NTM7 apparently affects tra gene induction by negatively influencing the amount of TraR present in the cells.
TABLE 7.
Induction of the traG::lacZ reporter in NTM7 can be restored by overexpressing traR
| TraR plasmid | traR | No. of copies | β-Galactosidase activity from traG::lacZ of pDCKI41 ina:
|
|
|---|---|---|---|---|
| NT1 | NTM7 | |||
| pRK415GIII | − | 5–10 | 89 | <1 |
| pRK415GIIIE33 | + | 5–10 | 97 | 3 |
| pBBR1MCS-5 | − | 25–30 | 91 | <1 |
| pZL5E33 | + | 25–30 | 105 | 33 |
β-Galactosidase activity was assayed as described in Materials and Methods and is expressed as units per 109 CFU. The experiment was repeated once with a similar pattern of values.
The rnd mutation slightly affects vir gene induction but does not detectably affect virulence.
Disruption of miaA, a gene coding for a tRNA processing function (62, 63), affects induction of vir genes (28). Given their similar functions, we determined if the rnd mutation affects expression of two vir reporters, virE2::lacZ and pinF::lacZ (now called virH [35]) (58). Under conditions necessary for vir induction, the two reporters expressed at levels about threefold lower in NTM7 than in the wild-type strain (data not shown). However, when tested on tomato seedlings, each of the four mutants containing pTiC58ΔaccR induced tumors at infective-dose levels indistinguishable from those of the wild-type parent strain (data now shown). We also tested whether the miaA mutation affects TraR-mediated induction of tra genes by introducing pDCKI41 into A136 (miaA) (Table 1) and its parent, A136 (Table 1). The traG::lacZ fusion in A136 (miaA) expressed at a level about twofold lower than that in the miaA+ parent (data not shown).
The rnd gene of E. coli complements the growth defect of NTM7 but not the defect in induction of tra gene expression.
Given the similarity between the rnd genes from E. coli and A. tumefaciens, we examined whether the E. coli homolog could complement the defects exhibited by NTM7. A 1.4-kb EcoRI-BamHI fragment carrying the E. coli rnd gene with its promoter was cloned from pDB14 (Table 1) into pRK415GIII to generate pZLD14 (Table 1). This plasmid was introduced into NTM7(pDCKI41), and the construct was assayed for growth and for tra gene induction. The E. coli rnd gene restored the growth rate of the mutant to that of the wild-type parent (Fig. 3). However, expression of the traG::lacZ reporter in NTM7(pDCKI41, pZLD14) was only slightly higher than that in NTM7(pDCKI41, pRK415GIII), the vector control (Table 3).
DISCUSSION
Of the three clearly independent mutants that are affected in TraR-mediated activation of Ti plasmid tra genes, all contain inserts in a gene homologous to rnd. In E. coli, this gene codes for RNase D, a 3′-exoribonuclease thought to be involved in processing the 3′ ends of select tRNA precursors (14, 15, 26). While we have no direct proof that rndA.t. codes for such an activity, our observation that the rnd gene of E. coli almost completely complemented the growth rate defect of NTM7 strongly suggests that the products of the genes of the two organisms have common activities. In turn, these results suggest that functions involved in RNA metabolism and in tRNA processing in particular are important for quorum-dependent gene expression controlled by TraR. Ours are not the first observations concerning the importance of tRNA processing in regulating gene expression. Also in A. tumefaciens, the product of the miaA gene, coding for a tRNA:isopentenyltransferase, is required for efficient expression of the vir regulon necessary for the processing and transfer of T-strand DNA from the bacterium to its plant host (28). Similarly, miaA is required for expression of several virulence genes in Shigella flexneri (19). Mutations in genes coding for other tRNA-modifying enzymes, including RNase R and a tRNA-guanine transglycosylase, also negatively affect expression of virulence determinants in S. flexneri and in E. coli (12, 20).
How tRNA modification functions influence the regulation of gene expression remains unknown. However, three lines of evidence indicate that the rnd mutation in NTM7 affects the amount of TraR present in the cells. First, expression of traR is reduced severalfold in the rnd mutant. Second, expression of the traG::lacZ reporter on the Ti plasmid can be restored by overexpressing traR (Table 7). Third, the mutant phenotype can also be restored by a mutation in traM in pDCKI41 (Table 6). This gene codes for an antiactivator that strongly inhibits the activity of activated TraR (32, 33, 44). In the rnd+ parent strain harboring pDCKI41, traR is expressed at a level such that the activator is present in excess over the available TraM. The facts that the rnd mutation results in the loss of TraR-mediated gene expression and that this expression can be restored by mutating traM suggest that, in the rnd mutant, TraR is produced but at a level that is no longer sufficient to overcome the effect of the antiactivator.
The traR reporter on pKPK12 is a translational fusion between the activator gene and lacZ (53) and, as such, does not allow us to differentiate between effects on transcription and on translation. Furthermore, TraR autoregulates its own expression at the level of transcription (53), complicating any differentiation between transcriptional and posttranscriptional effects of the rnd mutation. Although we cannot rule out an inhibition of transcription, we favor an effect on translation since mutations in other tRNA-modifying genes, including miaA and tgt of S. flexneri, clearly affect virF expression at the posttranscriptional level (19).
It is not clear what role RNAse D plays in E. coli; strains with null mutations in rnd do not show any obvious defect in growth or in the biosynthesis of mature tRNA species (10, 61). In contrast to these observations, the growth rates of all of the rndA.t. mutants are significantly lower than that of the parent strain (Fig. 1). The association of a phenotype with mutations in rndA.t. may serve as a model for investigating the substrates of this RNase and the role of the enzyme in the physiology of the bacterium.
Although the mutation in rnd exerts pleiotropic effects, it does not affect expression of all genes. The levels of expression of recA do not differ detectably between NT1 and NTM7 (data not shown). Moreover, the induction of expression of mocE, a Ti plasmid gene required for catabolism of the opine mannopine (38), is not affected detectably by the rnd mutation (data not shown). However, the reduced growth rates exhibited by the four mutants suggest that mutations in rnd affect the expression of other genes in addition to traR. We know of two such examples. First, the cryptic chromosomal tetAR gene unit of C58, when mutationally derepressed, confers resistance to high levels of tetracycline to strain C58 and its derivatives, including NT1, the immediate parent of NTM7 (42). However, such derepressed mutants of NTM7 express the tetracycline resistance phenotype at a considerably lower level than that of their rnd+ parent (42). In the second case, expression of the Ti plasmid vir regulon, as assessed by lacZ fusions to virE2 and virH, was reduced some two- to threefold in NTM7 compared to the level expressed by the parent strain (data not shown).
With respect to mechanism, the effect of the rnd mutation on expression of the Ti plasmid vir regulon may be significant; a mutation in miaA also decreases the levels of induction of expression of several of the vir operons (28). Interestingly, the mutation in miaA negatively affects the expression of virG, which codes for the response regulator of the two-component signal transduction system that controls the vir regulon. This observation raises the possibility that the decrease in expression of the vir operons in the miaA mutant is due to effects of the mutation on the production of VirG (28). Thus, like TraR of the Ti plasmid conjugal transfer system, a mutation in a tRNA processing function apparently inhibits expression of the vir system by negatively affecting the expression of a specific transcription factor. This hypothesis is supported by our observation that, like its effect on vir, the miaA mutation lowers the expression of the Ti plasmid traG::lacZ reporter some two- to threefold (data not shown). Thus, mutations in two genes associated with tRNA processing negatively affect expression of two sets of Ti plasmid transfer genes. Moreover, the phenotypes are mediated through effects of the mutations on production of cognate transcriptional activators: TraR on the one hand and VirG on the other. However, compared to the effect of the miaA mutation on vir, the effect of the rnd mutation on expression of the tra regulon is much stronger. While a two- to threefold reduction in production of VirG is not sufficient to affect tumorigenesis, a similar reduction in the production of TraR results in a complete loss of conjugal transfer. We suspect that this pronounced effect on expression of the tra regulon results from the TraM-mediated inactivation of TraR. Thus, because of the inhibitory effect of the antiactivator, it is not necessary to completely block production of TraR to inhibit expression of the tra regulon. This conclusion is consistent with our observation that a mutation in traM restores the TraR-mediated induction of tra genes in the rnd mutant (Table 6).
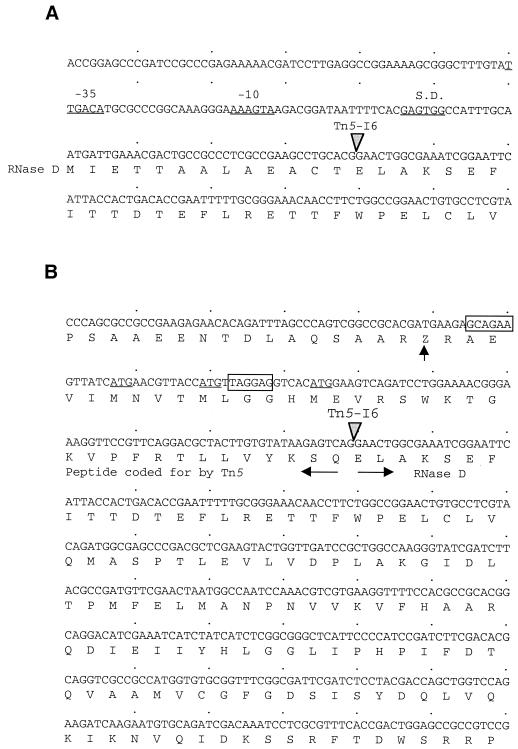
Insertions in the middle of rnd lead to significantly lower growth rates and the complete loss of tra gene expression on the Ti plasmid. However, mutant NTM6, in which Tn5 is inserted at the far 5′ end of the gene, grows faster than the other mutants (Fig. 1) and still expresses the traG::lacZ reporter, albeit at a level considerably lower than that of the wild-type parent (Table 2). It is conceivable that the insertion in NTM6 created a configuration that allows the cell to produce a partially active hybrid RNase composed of a peptide coded for by the transposon fused to the majority of the rnd gene product. Examination of the insertion site in NTM6 indicates that this hypothesis indeed is possible. There are three ATG codons in the sequence of Tn5 upstream of the junction site with rnd, each of which is in frame to and may serve as the translational start site for the downstream Agrobacterium gene (Fig. 5). Among these potential initiation codons, two are preceded by a good ribosome binding site spaced at the optimal distance. If transcribed from an upstream promoter within Tn5, this hybrid ORF may be translated as a fusion protein in which the first 13 amino acids of RNase D are replaced by a peptide of 22 to 31 residues coded for by the transposon (Fig. 5). Expression of genes driven by a promoter located in an upstream Tn5 are not without precedent (7). Of particular relevance, Beck von Bodman et al. (6) isolated a transfer-constitutive Ti plasmid in which the normally repressed traR gene is expressed from a promoter associated with a Tn5 inserted just upstream of the gene (52).
FIG. 5.
The Tn5 insertion in NTM6 may generate a fusion composed of the majority of the rndA.t. translation product and an N-terminal oligopeptide coded for by the transposon. (A) Nucleotide sequence of the 5′ region of the rnd gene. The sequence shows the locations of the putative rnd−10 and −35 promoter elements and a putative ribosome binding (Shine-Dalgarno [S.D]) site, as well as the ATG initiation codon. The inverted shaded arrowhead indicates the location of the Tn5 insertion in NTM6. (B) Nucleotide and predicted protein sequences resulting from the fusion formed between Tn5 and the rnd gene in NTM6. The fusion site is indicated by the inverted shaded arrowhead, while the two horizontal arrows indicate polypeptides of Tn5 origin (pointing left) and of rnd origin (pointing right). The three in-frame ATG codons from Tn5 are underlined, while the two potential ribosomal binding sites are boxed. The translation stop codon from the transposon closest to the first likely ATG initiation codon is indicated by the vertical filled arrow.
Our analyses indicate that the insertion mutations in rnd are solely responsible for the defects in growth and tra gene activation exhibited by the mutants. Each mutant contains a single copy of Tn5, and only clones overlapping the sites of the insertions complement the phenotypes. Moreover, all clones containing the rnd gene tested restored the growth rate of NTM7 to wild-type levels (Fig. 3). However, none of the clones fully complemented the defect in tra gene induction (Fig. 2 and Table 3). Moreover, it is not clear why the two cosmids containing the rnd gene restored TraR-meditated gene activation to a level somewhat higher than that in the mutant harboring smaller subclones of rnd. It is possible that the expression of rnd on the two cosmids differs from that on the shorter clones or that overexpression of rnd exerts some deleterious effect on the expression of traR. For example, it is conceivable that RNase D in some way regulates the amount of traR messenger RNA. No matter the reason, our results mirror those of Gray et al. (28); in their study, the cloned miaA gene did not fully complement the miaA mutant of A. tumefaciens for induction of the vir regulon. These results suggest that subtle differences in the amounts or activities of RNA-processing enzymes can have significant effects on normal cell processes. Consistent with this interpretation, in E. coli elevated levels of rnd are subtly deleterious to the cells (62).
The fact that all of our mutants contain insertions in the same gene points to the importance of rnd in the production of TraR and therefore in quorum sensing. Given that it is the only gene we identified in a screen of better than 15,000 random insertion events, it is tempting to conclude that rnd is the only host factor required for proper expression of traR. However, by using Tn5 as the mutagen, we would not have identified mutations in essential genes such as groES or groEL, which are known to be required for production of functional LuxR (1, 18). In addition, our screen may have other biases of which we are not aware. However, our results do indicate that tRNA processing is important for production of TraR, and they reinforce the notion that production of the LuxR-like activators is very sensitive to perturbations in functions required for messenger translation and for proper folding of newly translated proteins.
ACKNOWLEDGMENT
This work was supported in part by grant R01-GM52465 from the NIH to S.K.F.
REFERENCES
- 1.Adar Y Y, Simaan M, Ulitzur S. Formation of the LuxR protein in Vibrio fischeri lux system is controlled by HtpR through GroESL proteins. J Bacteriol. 1992;174:7138–7143. doi: 10.1128/jb.174.22.7138-7143.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Antoine R, Locht C. Isolation and molecular characterization of a novel broad-host-range plasmid of Bordetella brochiseptica with sequence similarities to plasmids from Gram-positive organisms. Mol Microbiol. 1992;6:1785–1799. doi: 10.1111/j.1365-2958.1992.tb01351.x. [DOI] [PubMed] [Google Scholar]
- 4.Archdeacon J, Talty J, Boesten B, Danchin A, O'Gara F. Cloning of the second adenylate cyclase gene (cya2) from Rhizobium meliloti F34: sequence similarity to eukaryotic cyclases. FEMS Microbiol Lett. 1995;128:177–184. doi: 10.1111/j.1574-6968.1995.tb07519.x. [DOI] [PubMed] [Google Scholar]
- 5.Beck von Bodman S, Hayman G T, Farrand S K. Opine catabolism and conjugal transfer of the nopaline Ti plasmid pTiC58 are coordinately regulated by a single repressor. Proc Natl Acad Sci USA. 1992;89:643–647. doi: 10.1073/pnas.89.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck von Bodman S, McCutchan J E, Farrand S K. Characterization of conjugal transfer functions of Agrobacterium tumefaciens Ti plasmid pTiC58. J Bacteriol. 1989;171:5281–5289. doi: 10.1128/jb.171.10.5281-5289.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berg C M, Berg D E. Uses of transposable elements and maps of known insertions. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington, D.C.: American Society for Microbiology; 1987. pp. 1071–1109. [Google Scholar]
- 8.Beringer J E, Beynon J L, Buchanon-Wollaston A V, Johnston A W B. Transfer of the drug resistance transposon Tn5 to Rhizobium. Nature. 1978;276:633–634. [Google Scholar]
- 9.Blattner F R, Plunkett G R, Bloch C A, Perna N T, Burland V, Riley M, Collado-Vides J, Glasner J D, Rode C K, Mayhew G F, Gregor J, Davis N W, Kirkpatrick H A, Goeden M A, Rose D J, Mau B, Shao Y. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 10.Blouin R T, Zaniewski R, Deutscher M P. Ribonuclease D is not essential for the normal growth of Escherichia coli or bacteriophage T4 or for the biosynthesis of a T4 suppressor tRNA. J Biol Chem. 1983;258:1423–1426. [PubMed] [Google Scholar]
- 11.Cangelosi G A, Best E A, Marinetti G, Nester E W. Genetic analysis of Agrobacterium. Methods Enzymol. 1991;204:384–397. doi: 10.1016/0076-6879(91)04020-o. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Z F, Zuo Y, Li Z, Rudd K E, Deutscher M P. The vacB gene required for virulence in Shigella flexneri and Escherichia coli encodes the exoribonuclease RNase R. J Biol Chem. 1998;273:14077–14080. doi: 10.1074/jbc.273.23.14077. [DOI] [PubMed] [Google Scholar]
- 13.Chilton M-D, Currier T C, Farrand S K, Bendich A J, Gordon M P, Nester E W. Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA not detected in crown gall tumors. Proc Natl Acad Sci USA. 1974;71:3672–3676. doi: 10.1073/pnas.71.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cudny H, Deutscher M P. Apparent involvement of ribonuclease D in the 3′ processing of tRNA precursors. Proc Natl Acad Sci USA. 1980;77:837–841. doi: 10.1073/pnas.77.2.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cudny H, Zaniewski R, Deutscher M P. Escherichia coli RNase D. Catalytic properties and substrate specificity. J Biol Chem. 1981;256:5633–5637. [PubMed] [Google Scholar]
- 16.Dessaux Y, Tempé J, Farrand S K. Genetic analysis of mannityl opine catabolism in octopine-type Agrobacterium tumefaciens strain 15955. Mol Gen Genet. 1987;208:301–308. doi: 10.1007/BF00330457. [DOI] [PubMed] [Google Scholar]
- 17.Dessaux Y, Petit A, Farrand S K, Murphy P J. Opines and opine-like molecules involved in plant-Rhizobiaceae interactions. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae, molecular biology of model plant-associated bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 173–197. [Google Scholar]
- 18.Dolan K M, Greenberg E P. Evidence that GroEL, not sigma 32, is involved in transcriptional regulation of the Vibrio fischeri luminescence genes in Escherichia coli. J Bacteriol. 1992;174:5132–5135. doi: 10.1128/jb.174.15.5132-5135.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durand J M, Bjork G R, Kuwae A, Yoshikawa M, Sasakawa C. The modified nucleoside 2-methylthio-N6-isopentenyladenosine in tRNA of Shigella flexneri is required for expression of virulence genes. J Bacteriol. 1997;179:5777–5782. doi: 10.1128/jb.179.18.5777-5782.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durand J M, Okada N, Tobe T, Watarai M, Fukuda I, Suzuki T, Nakata N, Komatus K, Yoshikawa M, Sasakawa C. vacC, a virulence-associated chromosomal locus of Shigella flexneri, is homologous to tgt, a gene encoding tRNA-guanine transglycosylase (Tgt) of Escherichia coli K-12. J Bacteriol. 1994;176:4627–4634. doi: 10.1128/jb.176.15.4627-4634.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ellis J G, Kerr A, Petit A, Tempé J. Conjugal transfer of the nopaline and agropine Ti-plasmid—the role of agrociopines. Mol Gen Genet. 1982;186:269–273. [Google Scholar]
- 22.Farrand S K. Conjugation in the Rhizobiaceae. In: Spaink H P, Kondorosi A, Hooykaas P J J, editors. The Rhizobiaceae, molecular biology of model plant-associated bacteria. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1998. pp. 199–233. [Google Scholar]
- 23.Farrand S K, O'Morchoe S P, McCutchan J. Construction of an Agrobacterium tumefaciens C58 recA mutant. J Bacteriol. 1989;171:5314–5321. doi: 10.1128/jb.171.10.5314-5321.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuqua C, Winans S C. A LuxR-LuxI type regulatory system activates Agrobacterium Ti plasmid conjugal transfer in the presence of a plant tumor metabolite. J Bacteriol. 1994;176:2796–2806. doi: 10.1128/jb.176.10.2796-2806.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fuqua C, Burbea M, Winans S C. Activity of the Agrobacterium Ti plasmid conjugal transfer regulator TraR is inhibited by the product of the traM gene. J Bacteriol. 1995;177:1367–1373. doi: 10.1128/jb.177.5.1367-1373.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghosh R K, Deutscher M P. Purification of potential 3′ processing nucleases using synthetic tRNA precursors. Nucleic Acids Res. 1978;5:3831–3842. doi: 10.1093/nar/5.10.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glickmann E, Gardan L, Jacquet S, Hussain S, Elasri M, Petit A, Dessaux Y. Auxin production is a common feature of most pathovars of Pseudomonas syringae. Mol Plant-Microbe Interact. 1998;11:156–162. doi: 10.1094/MPMI.1998.11.2.156. [DOI] [PubMed] [Google Scholar]
- 28.Gray J, Wang J, Gelvin S B. Mutation of the miaA gene of Agrobacterium tumefaciens results in reduced vir gene expression. J Bacteriol. 1992;174:1086–1098. doi: 10.1128/jb.174.4.1086-1098.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayman G T, Farrand S K. Characterization and mapping of the agrocinopine-agrocin 84 locus on the nopaline Ti plasmid pTiC58. J Bacteriol. 1988;170:1759–1767. doi: 10.1128/jb.170.4.1759-1767.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hayman G T, Farrand S K. Agrobacterium plasmids encode structurally and functionally different loci for catabolism of agrocinopine-type opines. Mol Gen Genet. 1990;223:465–473. doi: 10.1007/BF00264455. [DOI] [PubMed] [Google Scholar]
- 31.Hong S B, Dessaux Y, Chilton W S, Farrand S K. Organization and regulation of the mannopine cyclase-associated opine catabolism genes in Agrobacterium tumefaciens 15955. J Bacteriol. 1993;175:401–410. doi: 10.1128/jb.175.2.401-410.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hwang I, Cook D M, Farrand S K. A new regulatory element modulates homoserine lactone-mediated autoinduction of Ti plasmid conjugal transfer. J Bacteriol. 1995;177:449–458. doi: 10.1128/jb.177.2.449-458.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang I, Smyth A J, Luo Z-Q, Farrand S K. Modulating quorum sensing by antiactivation: TraM interacts with TraR to inhibit activation of Ti plasmid conjugal transfer genes. Mol Microbiol. 1999;34:282–294. doi: 10.1046/j.1365-2958.1999.01595.x. [DOI] [PubMed] [Google Scholar]
- 34.Hwang I, Li P-L, Zhang L, Piper K R, Cook D M, Tate M E, Farrand S K. TraI, a LuxI homologue, is responsible for production of conjugation factor, the Ti plasmid N-acylhomoserine lactone autoinducer. Proc Natl Acad Sci USA. 1994;91:4639–4643. doi: 10.1073/pnas.91.11.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalogeraki V S, Zhu J, Eberhard A, Madsen E L, Winans S C. The phenolic vir gene inducer ferulic acid is O-demethylated by the VirH2 protein of an Agrobacterium tumefaciens Ti plasmid. Mol Microbiol. 1999;34:512–522. doi: 10.1046/j.1365-2958.1999.01617.x. [DOI] [PubMed] [Google Scholar]
- 36.Keen N T, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene. 1988;70:191–197. doi: 10.1016/0378-1119(88)90117-5. [DOI] [PubMed] [Google Scholar]
- 37.Kerr A, Manigault P, Tempé J. Transfer of virulence in vivo and in vitro in Agrobacterium. Nature. 1977;265:560–561. doi: 10.1038/265560a0. [DOI] [PubMed] [Google Scholar]
- 38.Kim K-S, Farrand S K. Ti plasmid-encoded genes responsible for catabolism of the crown gall opine mannopine by Agrobacterium tumefaciens are homologs of the T-region genes responsible for synthesis of this opine by the plant tumor. J Bacteriol. 1996;178:3275–3284. doi: 10.1128/jb.178.11.3275-3284.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kovach M E, Elzer P H, Hill D S, Robertson G T, Farris M A, Roop R M I, Peterson K M. Four new derivatives of the broad-host-range cloning vector pBBR1MCS carrying different antibiotic resistance cassettes. Gene. 1995;166:175–176. doi: 10.1016/0378-1119(95)00584-1. [DOI] [PubMed] [Google Scholar]
- 40.Latifi A, Winson K M, Foglino M, Wycroft B W, Stewart G S A B, Lazdunski A, Williams P. Multiple homologs of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol Microbiol. 1995;17:333–344. doi: 10.1111/j.1365-2958.1995.mmi_17020333.x. [DOI] [PubMed] [Google Scholar]
- 41.Li P-L, Everhart D M, Farrand S K. Genetic and sequence analysis of the pTiC58 trb locus, encoding a mating-pair formation system related to members of the type IV secretion family. J Bacteriol. 1998;180:6164–6172. doi: 10.1128/jb.180.23.6164-6172.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo Z-Q, Farrand S K. Cloning and characterization of a tetracycline resistance determinant present in Agrobacterium tumefaciens C58. J Bacteriol. 1999;181:618–626. doi: 10.1128/jb.181.2.618-626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo Z-Q, Farrand S K. Signal-dependent DNA binding and functional domains of the quorum-sensing activator TraR as identified by repressor activity. Proc Natl Acad Sci USA. 1999;96:9009–9014. doi: 10.1073/pnas.96.16.9009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo Z Q, Qin Y, Farrand S K. The antiactivator TraM interferes with the autoinducer-dependent binding of TraR to DNA by interacting with the C-terminal region of the quorum-sensing activator. J Biol Chem. 2000;275:7713–7722. doi: 10.1074/jbc.275.11.7713. [DOI] [PubMed] [Google Scholar]
- 45.Mark C. “DNA Strider”: a “C” program for fast analysis of DNA and protein sequences on the Apple Macintosh family of computers. Nucleic Acids Res. 1988;16:1829–1836. doi: 10.1093/nar/16.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murillo J, Shen H, Grehold D, Sharma A, Cooksey D A, Keen N T. Characterization of pPT23B, the plasmid involved in syringolide production by Pseudomonas syringae pv. tomato PT23. Plasmid. 1994;31:275–287. doi: 10.1006/plas.1994.1029. [DOI] [PubMed] [Google Scholar]
- 47.Ochsner U A, Reiser J. Autoinducer-mediated regulation of rhamnolipid bisurfactant synthesis in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:6424–6428. doi: 10.1073/pnas.92.14.6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oger P, Kim K-S, Sackett R L, Piper K R, Farrand S K. Octopine-type Ti plasmids code for a mannopine-inducible dominant-negative allele of traR, the quorum-sensing activator that regulates Ti plasmid conjugal transfer. Mol Microbiol. 1998;27:277–288. doi: 10.1046/j.1365-2958.1998.00671.x. [DOI] [PubMed] [Google Scholar]
- 49.Palanichelvam K, Oger P, Clough S J, Cha C, Bent A F, Farrand S K. A second T-region of the soybean-supervirulent chrysopine-type Ti plasmid pTiChry5, and construction of a fully disarmed vir helper plasmid. Mol Plant-Microbe Interact. 2000;13:1081–1091. doi: 10.1094/MPMI.2000.13.10.1081. [DOI] [PubMed] [Google Scholar]
- 50.Pearson J P, Passadore L, Iglewski B H, Greenberg E P. A second N-acylhomoserine lactone signal produced by Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 1995;92:1490–1494. doi: 10.1073/pnas.92.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piper K R, Farrand S K. Quorum-sensing but not autoinduction of Ti plasmid conjugal transfer requires control by the opine regulon and the antiactivator TraM. J Bacteriol. 2000;182:1080–1088. doi: 10.1128/jb.182.4.1080-1088.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piper K R, Beck von Bodman S, Farrand S K. Conjugation factor of Agrobacterium tumefaciens regulates Ti plasmid transfer by autoinduction. Nature. 1993;362:448–450. doi: 10.1038/362448a0. [DOI] [PubMed] [Google Scholar]
- 53.Piper K R, Beck von Bodman S, Hwang I, Farrand S K. Hierarchical gene regulatory systems arising from fortuitous gene associations: controlling quorum sensing by the opine regulon in Agrobactrium. Mol Microbiol. 1999;32:1077–1089. doi: 10.1046/j.1365-2958.1999.01422.x. [DOI] [PubMed] [Google Scholar]
- 54.Qin Y, Luo Z-Q, Smyth A J, Gao P, Beck von Bodman S, Farrand S K. Quorum-sensing signal binding results in dimerization of TraR and its release from membranes into the cytoplasm. EMBO J. 2000;19:5212–5221. doi: 10.1093/emboj/19.19.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 56.Shaw P D, Ping G, Daly S L, Cha C, Cronan J E, Jr, Rinehart K L, Farrand S K. Detecting and characterizing N-acyl-homoserine lactone signal molecules by thin-layer chromatography. Proc Natl Acad Sci USA. 1997;94:6036–6041. doi: 10.1073/pnas.94.12.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:37–45. [Google Scholar]
- 58.Stachel S E, An G, Flores C, Nester E W. A Tn3lacZ transposon for the random generation of β-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985;4:891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Watson B, Currier T C, Gordon M P, Chilton M-D, Nester E W. Plasmid required for virulence of Agrobacterium tumefaciens. J Bacteriol. 1975;123:255–264. doi: 10.1128/jb.123.1.255-264.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whiteley M, Parsek M R, Greenberg E P. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J Bacteriol. 2000;182:4356–4360. doi: 10.1128/jb.182.15.4356-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zaniewski R, Petkaitis E, Deutscher M P. A multiple mutant of Escherichia coli lacking the exoribonucleases RNase II, RNase D, and RNase BN. J Biol Chem. 1984;259:11651–11653. [PubMed] [Google Scholar]
- 62.Zhang J R, Deutscher M P. Cloning, characterization, and effects of overexpression of the Escherichia coli rnd gene encoding RNase D. J Bacteriol. 1988;170:522–527. doi: 10.1128/jb.170.2.522-527.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang J R, Deutscher M P. Transfer RNA is a substrate for RNase D in vivo. J Biol Chem. 1988;263:17909–17912. [PubMed] [Google Scholar]
- 64.Zhang L, Murphy P J, Kerr A, Tate M E. Agrobacterium conjugation and gene regulation by N-acyl-l-homoserine lactones. Nature. 1993;362:446–448. doi: 10.1038/362446a0. [DOI] [PubMed] [Google Scholar]
- 65.Zhu J, Winans S C. Autoinducer binding by the quorum-sensing regulator TraR increases affinity for target promoters in vitro and decreases TraR turnover rates in whole cells. Proc Natl Acad Sci USA. 1999;96:4832–4837. doi: 10.1073/pnas.96.9.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu J, Winans S C. The quorum-sensing transcriptional regulator TraR requires its cognate signaling ligand for protein folding, protease resistance, and dimerization. Proc Natl Acad Sci USA. 2001;98:1507–1512. doi: 10.1073/pnas.98.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]