Abstract
Background:
It is unclear whether the results of osteochondral transplant using autografts or allografts for talar osteochondral defect are equivalent.
Purpose:
A systematic review of the literature was conducted to compare allografts and autografts in terms of patient-reported outcome measures (PROMs), MRI findings, and complications.
Study Design:
Systematic review; Level of evidence, 4.
Methods:
This study was conducted according to the PRISMA guidelines. The literature search was conducted in February 2021. All studies investigating the outcomes of allograft and/or autograft osteochondral transplant as management for osteochondral defects of the talus were accessed. The outcomes of interest were visual analog scale (VAS) score for pain, American Orthopaedic Foot and Ankle Society (AOFAS) score, and Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART) score. Data concerning the rates of failure and revision surgery were also collected. Continuous data were analyzed using the mean difference (MD), whereas binary data were evaluated with the odds ratio (OR) effect measure.
Results:
Data from 40 studies (1174 procedures) with a mean follow-up of 46.5 ± 25 months were retrieved. There was comparability concerning the length of follow-up, male to female ratio, mean age, body mass index, defect size, VAS score, and AOFAS score (P > .1) between the groups at baseline. At the last follow-up, the MOCART (MD, 10.5; P = .04) and AOFAS (MD, 4.8; P = .04) scores were better in the autograft group. The VAS score was similar between the 2 groups (P = .4). At the last follow-up, autografts demonstrated lower rate of revision surgery (OR, 7.2; P < .0001) and failure (OR, 5.1; P < .0001).
Conclusion:
Based on the main findings of the present systematic review, talar osteochondral transplant using allografts was associated with higher rates of failure and revision compared with autografts at midterm follow-up.
Keywords: talus, osteochondral defect, osteochondral transplant, OAT, allograft, autograft
Osteochondral lesions of the talus are common. 50 Given the avascular and hypocellular nature of articular cartilage, the management of talar osteochondral lesions is challenging.15,49 Several surgical procedures have been described.57,66 Smaller defects up to 1.5 cm 2 can be treated arthroscopically using the microfractures technique.4,52,74 For larger defects, osteochondral transplant has been commonly used.16,69 Osteochondral transplant can be performed using single or multiple plugs (mosaicplasty).13,68 Indications for osteochondral transplant are full-thickness symptomatic defects of 1 cm 2 up to approximately 4 cm 2 .23,24 Both osteochondral allografts and autografts can be used.39,47 Common donor sites for autologous osteochondral plugs are the anterosuperior condyle region and the lateral aspect of the intercondylar notch of the ipsilateral femur. 68 The use of allografts avoids donor site morbidity.9,76 Several clinical studies have evaluated the outcomes of autografts and allografts.** Whether osteochondral transplant using autografts performs better than that using allografts has not been fully clarified. The present study updates current evidence concerning the use of allografts and autografts for osteochondral transplant in talar osteochondral defects at midterm follow-up. A systematic review of the literature was conducted to compare allografts versus autografts in terms of patient-reported outcome measures (PROMs), MRI findings, and complications.
Methods
Search Strategy
This study was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. 51 The PICO algorithm was as follows:
P (Problem): talus osteochondral defects
I (Intervention): osteochondral transplant
C (Comparison): autograft versus allograft
O (Outcomes): clinical scores, MRI findings, and complications
Data Source
The literature search was conducted in February 2021. Two independent authors (F.M. and H.S.) accessed the main online databases: PubMed, Google Scholar, Embase, and Scopus. The following keywords were used in combination: talus, ankle, chondral, cartilage, articular, osteochondral, damage, defect, injury, chondropathy, pain, autologous, allograft, autograft, transplantation, therapy, management, surgery, outcomes, failure, revision, reoperation, recurrence. The same reviewers selected the articles of interest and accessed the full-text. The bibliographies were also checked. Disagreements were solved by a third author (N.M.).
Eligibility Criteria
All studies investigating the surgical outcomes of allograft and/or autograft osteochondral transplant as management for osteochondral defects of the talus were accessed. Given the authors’ language capabilities, articles in English, German, Italian, French, and Spanish were considered. Studies with level of evidence 1 to 4, according to the Oxford Centre for Evidence-Based Medicine, 37 were eligible. Registries, letters, abstracts, reviews, editorials, and opinions were not considered. Animal, computational, biomechanics, and in vitro studies were excluded. Studies augmenting autologous matrix-induced chondrogenesis (AMIC) or matrix-induced autologous chondrocyte implantation (mACI) with less committed cells (eg, mesenchymal stem cells) were not considered. Studies reporting data on patients with end-stage joint osteoarthritis were not considered. Missing data under the outcomes of interest warranted exclusion from this study.
Data Extraction
Two authors (F.M. and H.S.) separately performed data extraction. Study generalities (author, year, journal, study design) and length of follow-up were retrieved. Baseline patient data were collected: length of symptoms before intervention, number of procedures, mean body mass index (BMI), mean age, sex, and mean defect size. Further, data concerning the following scores were retrieved: visual analog scale (VAS) for pain, American Orthopaedic Foot and Ankle Society (AOFAS), 75 and Magnetic Resonance Observation of Cartilage Repair Tissue (MOCART). 48 Rate of failures and revision surgeries were also collected.
Methodological Quality Assessment
The methodological quality assessment was performed by 2 independent authors (F.M. and H.S.). The risk of bias graph tool of the Review Manager Software (The Nordic Cochrane Collaboration) was used. Selection, detection, attrition, reporting, and other sources of bias were considered for evaluation.
Statistical Analysis
The statistical analyses were performed by the main author (F.M.) using the IBM SPSS Version 25 program. Continuous data were reported as mean difference (MD), whereas binary variables were reported using the odds ratio (OR) effect measure. The confidence interval (CI) was set at 95%. We performed t tests and χ2 tests for continuous and binary data, respectively, with P < .05 considered statistically significant.
The meta-analyses were performed using Editorial Manager Software Version 5.3 (The Nordic Cochrane Collaboration). Dichotomous data were analyzed through the Mantel-Haenszel method and OR effect measure. The CI was set at 95% in all comparisons. A fixed model effect was set as default. If moderate or high heterogeneity was detected, a random model effect was adopted. Heterogeneity was evaluated through Higgins I2 and χ2 tests. Values of Higgins I2 were interpreted as low (<30%), moderate (30%-60%), and high (>60%). Values of P > .05 were considered statistically significant.
Results
Search Result
The literature search resulted in 357 articles. Of them, 104 were duplicates. A further 213 articles were excluded because of incompatibility with the inclusion criteria: not matching the topic (n = 139), not focusing on the talus (n = 17), study design (n = 21), not reporting quantitative data under the outcomes of interest (n = 11), augmented with other cells (n = 7), reporting data on patients with end-stage joint degeneration (n = 5), language limitations (n = 1), and other (n = 12). This left 40 articles for the present study: 35 retrospective and 4 prospective studies and 1 randomized clinical trial. The literature search results are shown in Figure 1.
Figure 1.
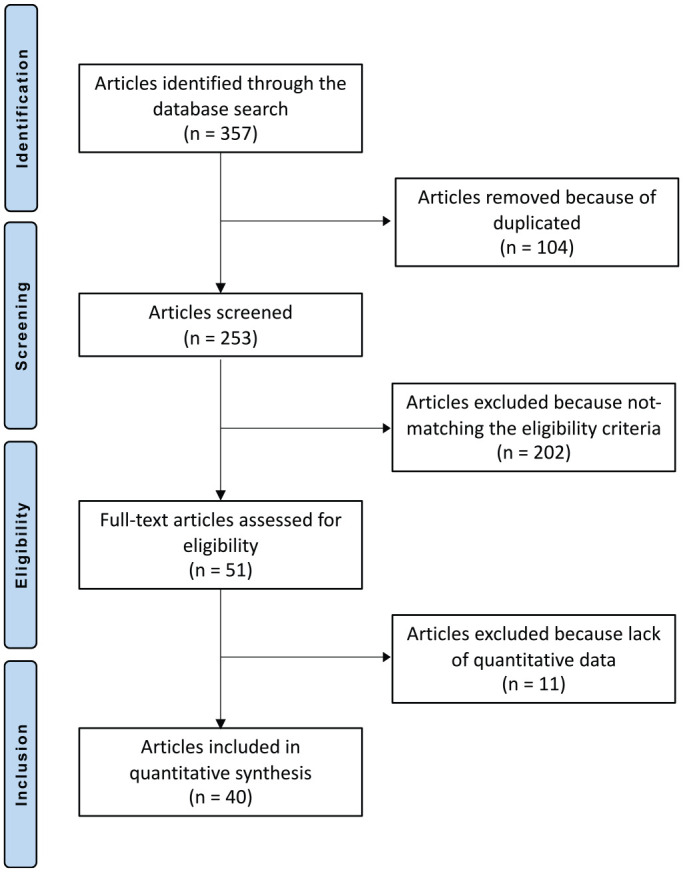
Flow chart of the literature search.
Methodological Quality Assessment
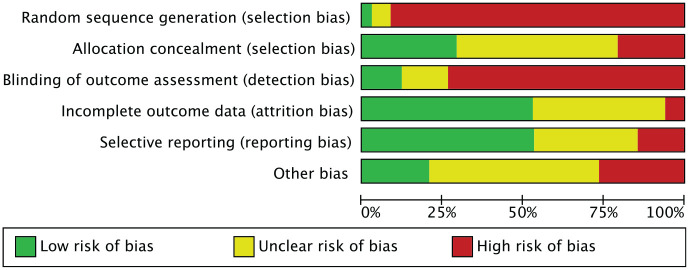
Given the retrospective design of 85% (35/40) of the included studies, the risk of selection bias was moderate-high. Given the overall lack of blinding, detection bias was high. The risk of attrition and reporting bias among all included studies was moderate, as was the risk of other bias. The overall quality of the methodological assessment was fair. The risk of bias graph is shown in Figure 2.
Figure 2.
Methodological quality assessment.
Patient Characteristics
Data from 1174 procedures, with a mean follow-up of 46.5 ± 25 months, were retrieved. Study generalities and patient characteristics are shown in Table 1. There was no difference at baseline between the allograft and autograft groups (Table 2) concerning the duration of symptoms before surgery (P = .05), length of follow-up (P = .6), male to female ratio (P = .5), mean age (P = .4), mean BMI (P = .1), defect size (P = .6), VAS score (P = .8), and AOFAS score (P = .1).
Table 1.
Generalities and Descriptions of the Included Studies a
| Lead Author (Year) | Journal | Design | Follow-up, mo | Treatment | Procedures | Female, % | Mean Age, y |
|---|---|---|---|---|---|---|---|
| Adams 2 (2011) | J Bone Joint Surg Am | Retrospective | 48 | Osteochondral allograft transplant | 8 | 62.5 | 31.4 |
| Adams 1 (2018) | Foot Ankle Int | Prospective | 55.0 | Osteochondral allograft transplant | 14 | 42.9 | 40.0 |
| Ahmad 3 (2016) | Foot Ankle Int | Randomized | 40.5 | Osteochondral allograft transplant | 16 | 37.5 | 39.7 |
| 35.2 | Osteochondral autograft transplant | 20 | 45.0 | 41.3 | |||
| El-Rashidy 12 (2011) | J Bone Joint Surg Am | Retrospective | 37.7 | Osteochondral allograft transplant | 38 | 42.1 | 44.2 |
| Emre 13 (2012) | J Foot Ankle Surg | Retrospective | 16.8 | Mosaicplasty | 32 | 9.4 | 27.5 |
| de l’Escalopier 10 (2015) | Orthop Traumatol Surg Res | Retrospective | 76.0 | Mosaicplasty | 37 | 33.0 | 21.6 |
| Fraser 16 (2016) | Knee Surg Sports Traumatol Arthrosc | Retrospective | 70.8 | Osteochondral autograft transplant | 36 | 33.3 | 31.0 |
| Gaul 19 (2019) | Foot Ankle Int | Retrospective | 116.4 | Osteochondral allograft transplant | 20 | 47.0 | 34.7 |
| Gaul 18 (2018) | Foot Ankle Int | Retrospective | 123.6 | Osteochondral allograft transplant | 20 | 55.0 | 43.6 |
| Gautier 20 (2002) | J Bone Joint Surg Br | Retrospective | 24.0 | Osteochondral autograft transplant | 11 | 66.5 | 32.0 |
| Georgiannos 21 (2016) | Knee Surg Sports Traumatol Arthrosc | Retrospective | 66 | Osteochondral autograft transplant | 48 | 19.5 | 36 |
| Gobbi 22 (2006) | Arthroscopy | Prospective | 53.0 | Control group | 10 | 40.0 | 24.0 |
| Control group | 11 | 45.5 | 32.0 | ||||
| Osteochondral autograft transplant | 12 | 33.3 | 27.8 | ||||
| Gül 28 (2016) | J Foot Ankle Surg | Retrospective | 30.5 | Osteochondral autograft transplant | 15 | 33.3 | 32.6 |
| 28.9 | Osteochondral autograft transplant | 13 | 8.3 | 36.7 | |||
| Guney 29 (2016) | Knee Surg Sports Traumatol Arthrosc | Prospective | 47.3 | Control group | 19 | 37.4 | 47, 4 |
| 40.4 | Control group | 22 | 43.9 | 50.0 | |||
| 30.1 | Mosaicplasty | 13 | 37.6 | 15.4 | |||
| Haleem 33 (2014) | Am J Sports Med | Retrospective | 93.0 | Osteochondral autograft transplant | 14 | 50.0 | 42.8 |
| 85.3 | Osteochondral autograft transplant | 28 | 39.3 | 44.1 | |||
| Haasper 30 (2008) | Arch Orthop Trauma Surg | Retrospective | 24.0 | Mosaicplasty | 14 | 57.1 | 24.8 |
| Hahn 32 (2010) | Foot Ankle Int | Retrospective | 47.9 | Osteochondral allograft transplant | 13 | 61.5 | 30.4 |
| Hangody 34 (1997) | Foot Ankle Int | Retrospective | 19.0 | Mosaicplasty | 11 | NR | 25.1 |
| Hangody 35 (2001) | Foot Ankle Int | Retrospective | 50.4 | Mosaicplasty | 36 | NR | 27.0 |
| Imhoff 38 (2011) | Am J Sports Med | Retrospective | 84.0 | Osteochondral autograft transplant | 26 | 46.2 | 33.0 |
| Jackson 39 (2019) | J Foot Ankle Surg | Retrospective | 21.0 | Osteochondral allograft transplant | 31 | 9.7 | 33.6 |
| Kreuz 42 (2006) | Am J Sports Med | Retrospective | 48.9 | Mosaicplasty | 35 | 48.6 | 30.9 |
| Lee 44 (2003) | Foot Ankle Int | Retrospective | 36.0 | Mosaicplasty | 18 | 5.6 | 22.7 |
| Li 45 (2017) | BMC Musculoskelet Disord | Retrospective | 21.2 | Osteochondral autograft transplant | 11 | 63.6 | 55.4 |
| Liu 46 (2011) | Foot Ankle Int | Prospective | 36.3 | Osteochondral autograft transplant | 16 | 37.5 | 33.9 |
| Liu 47 (2020) | Foot Ankle Int | Retrospective | 18.0 | Osteochondral autograft transplant | 14 | 21.4 | 29.6 |
| Nguyen 55 (2019) | Am J Sports Med | Retrospective | 44.7 | Osteochondral autograft transplant | 38 | 0.0 | 26.0 |
| Orr 56 (2017) | Foot Ankle Spec | Retrospective | 28.5 | Osteochondral allograft transplant | 8 | 0.0 | 34.4 |
| Park 58 (2018) | Am J Sports Med | Retrospective | 71.4 | Osteochondral autograft transplant | 18 | 41.6 | NR |
| Osteochondral autograft transplant | 28 | 41.6 | NR | ||||
| Park 57 (2020) | Bone Joint J | Retrospective | 22.0 | Osteochondral allograft transplant | 25 | 40.0 | 19.6 |
| Paul 59 (2012) | Am J Sports Med | Retrospective | 60.0 | Osteochondral autograft transplant | 131 | 38.2 | 31.0 |
| Ross 64 (2016) | Arthroscopy | Retrospective | 51.0 | Osteochondral autograft transplant | 76 | 34.2 | 35.8 |
| Sabaghzadeh 65 (2020) | Chin J Traumatol | Retrospective | Mosaicplasty | 19 | 42.1 | 43.0 | |
| Sadlik 66 (2017) | Foot Ankle Surg | Retrospective | 46.4 | Osteochondral autologous transposition | 10 | 40.0 | 37.0 |
| Shimozono 69 (2018) | Am J Sports Med | Retrospective | 52.0 | Osteochondral autograft transplant | 63 | 42.9 | 36.0 |
| 45.0 | Osteochondral autograft transplant | 31 | 32.3 | 34.0 | |||
| Shimozono 70 (2018) | J Bone Joint Surg Am | Retrospective | 26.3 | Osteochondral autograft transplant | 25 | 64.0 | 38.4 |
| 22.3 | Osteochondral allograft transplant | 16 | 37.5 | 43.6 | |||
| Woelfle 77 (2013) | Knee Surg Sports Traumatol Arthrosc | Retrospective | 29.0 | Osteochondral autograft transplant | 32 | 24.5 | 46.9 |
| Yoon 78 (2014) | Am J Sports Med | Retrospective | 45.0 | Osteochondral autograft transplant | 22 | 31.8 | 37.1 |
| Retrospective | 50.0 | Control group | 22 | 18.2 | 41.6 | ||
| Zhu 79 (2016) | Foot Ankle Int | Retrospective | 25.4 | Osteochondral autograft and cancellous allograft transfer | 12 | 38.5 | 40.5 |
NR, not reported.
Table 2.
Characteristics of the 2 Cohorts at Baseline a
| Allograft | Autograft | MD | P | |
|---|---|---|---|---|
| Procedures, n | 219 | 955 | ||
| Follow-up, mo | 50.8 ± 34.3 | 46.0 ± 21.6 | 4.7 | .6 |
| Duration of symptoms, mo | 54.3 ± 39.5 | 21.9 ± 16.8 | 32.4 | .05 |
| Female, % | 39.6 ± 18.5 | 36.1 ± 16.3 | 3.5 | .5 |
| Mean age, y | 36.0 ± 7.0 | 33.5 ± 8.3 | 2.5 | .4 |
| Body mass index | 27.9 ± 2.4 | 25.0 ± 1.4 | 1.9 | .1 |
| Defect size, cm2 | 1.8 ± 0.8 | 2.6 ± 4.3 | −0.9 | .6 |
| VAS score | 6.9 ± 0.9 | 6.7 ± 0.9 | 0.1 | .8 |
| AOFAS score | 58.8 ± 13.8 | 51.5 ± 9.1 | 7.3 | .1 |
| Lesion site, % (n/N) | ||||
| Medial | 72.9 (102/140) | 72.4 (417/576) | ||
| Central | 1.4 (2/140) | 0.5 (3/576) | ||
| Lateral | 25.7 (36/140) | 27.1 (156/576) | ||
Values for allograft and autograft are expressed as mean ± SD unless otherwise noted. AOFAS, American Orthopaedic Foot and Ankle Society; MD, mean difference; VAS, visual analog scale.
Outcomes of Interest
At last follow-up, the autograft group had higher MOCART scores (MD, 10.5; P = .04). Similarly, the AOFAS score was higher in the autograft group (MD, 4.8; P = .04). The VAS score was similar between the 2 groups (P = .4) (Table 3).
Table 3.
Results of VAS, MOCART, and AOFAS Scores a
| Score | Allograft | Autograft | MD | P |
|---|---|---|---|---|
| VAS | 2.3 ± 1.3 | 2.5 ± 1.3 | 0.2 | .4 |
| MOCART | 72.5 ± 4.2 | 83.0 ± 8.7 | 10.5 | .04 |
| AOFAS | 81.6 ± 6.0 | 86.4 ± 5.6 | 4.8 | .04 |
AOFAS, American Orthopaedic Foot and Ankle Society; MD, mean difference; MOCART, Magnetic Resonance Observation of Cartilage Repair Tissue; VAS, visual analog scale.
Complications
The autograft group demonstrated lower rates of revision surgery (OR, 7.2; P < .0001) and failure (OR, 5.1; P < .0001) (Table 4).
Table 4.
Results of Complications
| Endpoint | Allograft, % (n/N) | Autograft, % (n/N) | OR | 95% CI | P |
|---|---|---|---|---|---|
| Revision | 44.9 (58/129) | 10.2 (50/490) | 7.19 | 4.5665-11.3167 | <.0001 |
| Failure | 14.7 (24/163) | 3.3 (16/490) | 5.08 | 2.6263-9.8367 | <.0001 |
Meta-analysis
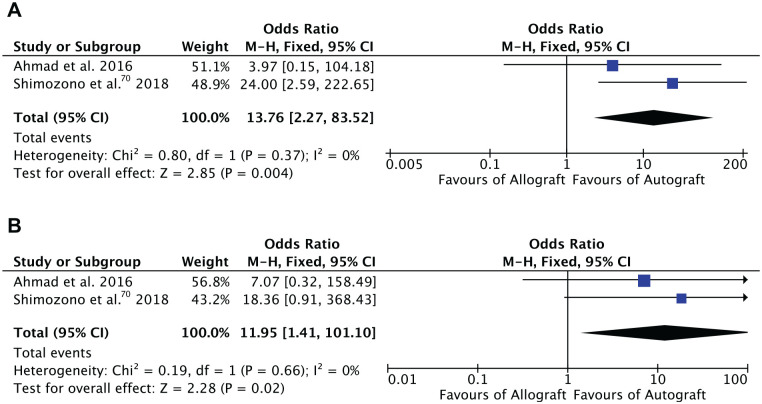
Two comparative studies including 77 patients were included in the meta-analysis.3,70 We noted statistically significant lower rates of revision surgery (OR, 12.0; P = .02) and failure (OR, 13.8; P = .004) in the autograft group. The forest plots are shown in Figure 3.
Figure 3.
Forest plots of the comparison: (A) failures and (B) revision surgeries.
Discussion
According to the main findings of the present study, osteochondral transplant for osteochondral defects of the talus using autografts or allograft evidenced similar VAS and AOFAS scores at midterm follow-up. However, given the lower rates of failure and revision, autografts should be preferred during primary surgeries for osteochondral defects of the talus. The primary use of allograft should probably be reserved for patients who do not have a suitable donor site.
Osteochondral autograft transplant has been used for small to medium-sized defects of the talus, with a rate of satisfaction up to 95% for primary procedures.41,67 Osteochondral autograft transplant has been used in revision settings as well, with considerable improvement of AOFAS and VAS scores.42,78 Allografts show a higher rate of failure and higher costs, greater risk of disease transmission, and limited availability, whereas the autologous techniques can entail donor site morbidity. 14 The effect of donor site morbidity on outcomes has not been fully clarified. Considering low-weightbearing, dimensional properties and surface curvature, only 3 harvest sites are available within the knee: the lateral side of the intercondylar notch, the superomedial trochlea, and the superolateral trochlea.27,36,63 In a systematic review by Bexkens et al 7 that included 190 patients, the rate of donor site morbidity was 7.8% (10/128 patients). Of those patients, 7% (9/128 patients) reported knee pain during activity and 0.8% (1/128 patients) reported locking episodes. Shimozono et al, 71 in a meta-analysis of 24 studies (915 procedures), estimated the rate of donor site morbidity to be from 6.7% to 10.8%. Nakagawa et al 53 investigated the clinical outcomes of donor sites in the knee after osteochondral autograft transplant. Those investigators found that 34 of 40 patients (85%) were asymptomatic at a mean follow-up of 43.1 months.
Despite the encouraging results with autografts, the use of allografts has become more prevalent to avoid donor site morbidity. Studies have shown that allografts achieve similar clinical outcomes as autografts and avoid donor site morbidity but have a greater rate of revision and failure.12,26 Two studies compared allografts versus autografts for osteochondral defects of the talus. Ahmad et al 3 conducted a prospective study comparing 16 autograft procedures with grafts from the ipsilateral femoral condyle versus 20 fresh size-matched allografts; the mean VAS scores improved similarly in both groups. Shimozono et al 70 retrospectively evaluated 25 patients treated with autografts versus 16 patients treated with allografts for talar defects and found that AOFAS and MOCART scores were better in the autograft group. Both of these studies found higher rates of failure and revision surgery in the allograft group.
The present study found a rate of failure of 14.7% (24/163 patients). This finding agrees with previous evidence of failure rates between 12% and 30%. Ahmad et al 3 found that 18.8% of allografts did not heal properly as shown on computed tomography scans or plain radiographs after 6 months. Gross et al 26 reported fragmentation and resorption of the allograft in one-third of patients (3/9). Haene et al 31 evaluated the failure rate of allografts in 16 patients at 48 months of follow-up. Those investigators evidenced failure in graft incorporation in 13% (2/16), osteolysis in 31% (5/16), and cysts in 50% (8/16) of patients at 4 years.
The higher rate of failure in allografts may derive from immune-mediated mechanisms. 54 Immune responses similar to those implicated in allogenic organ transplant rejections have been documented in human and animal osteochondral allograft transplant.17,60,72,73 Sirlin et al 72 demonstrated that 31% (11/36) of patients who underwent allograft implantation in the knee presented anti-human antibody. These patients had a statistically significant greater rate of edema, thicker interface, abnormal graft marrow, and surface collapse. 72 Pomajzl et al 61 demonstrated a substantial loss of sulfated glycosaminoglycans and osteocalcin in the graft-host interface, along with a high osteoclast activity. They further demonstrated the presence of T-helper, T-cytotoxic, and NK cells. Moreover, Pomajzl et al demonstrated the presence of tumor necrosis factor α throughout the allograft. These features discourage the use of osteochondral allograft transplant. If a suitable harvest site is unavailable, other techniques can be used, such as AMIC, mACI, and matrix-induced stem cell transplant (mAST); these are feasible and reliable options that exploit the regenerative potential of autologous tissue.5,6,8,25,62 Particulated juvenile articular cartilage (PJAC) transplant has been also used to manage osteochondral defects of the talus. However, given its limited evidence and controversial results, PJAC was not included in this analysis.11,40,43
This study has some limitations. The analyses were performed regardless of the surgical approach (arthroscopy, mini-arthrotomy, arthrotomy). Furthermore, the limited number of included articles and procedures may have negatively affected our results. The retrospective design of most of the studies represents an important limitation; however, the current literature lacks high-quality studies, and further investigations are required. Furthermore, given the lack of studies directly comparing the 2 types of graft, the meta-analysis collected data from only 2 trials.3,70 Thus, even if no heterogeneity was detected, the reliability of the results is questionable. Most authors did not clarify the type of allograft used and did not specify its provenance or any additional procedures (eg, sterilization) before transplant. Given the lack of data, primary and revision surgeries were often mixed, and some authors combined the surgeries with other procedures. This may have influenced the results, increasing the risk of selection bias. Given the lack of data, the analyses were performed regardless of the cause of the defect or the talar location of the defect (eg, medial vs lateral). Thus, our results must be interpreted within the limitations of the present investigation. Future studies are required to overcome these limitations.
Conclusion
Based on the main findings of the present systematic review, talar osteochondral transplant using allografts was associated with higher rates of failure and revision compared with autografts at midterm follow-up. However, given the limited published data, the strength of the conclusions is weak and further high-quality comparative studies are required.
Acknowledgments
The authors thank Mr Michael Berner for his support and motivation during the study conceptualization. They also thank Dr Andreas Bell and Dr Jens Schneider for their perpetual clinical support and education.
Submitted January 1, 2021; accepted April 9, 2021.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
An online CME course associated with this article is available for 1 AMA PRA Category 1 Credit™ at http://www.sportsmed.org/aossmimis/Members/Education/AJSM_Current_Concepts_Store.aspx. In accordance with the standards of the Accreditation Council for Continuing Medical Education (ACCME), it is the policy of The American Orthopaedic Society for Sports Medicine that authors, editors, and planners disclose to the learners all financial relationships during the past 12 months with any commercial interest (A ‘commercial interest’ is any entity producing, marketing, re-selling, or distributing health care goods or services consumed by, or used on, patients). Any and all disclosures are provided in the online journal CME area which is provided to all participants before they actually take the CME activity. In accordance with AOSSM policy, authors, editors, and planners’ participation in this educational activity will be predicated upon timely submission and review of AOSSM disclosure. Noncompliance will result in an author/editor or planner to be stricken from participating in this CME activity.
ORCID iDs: Filippo Migliorini  https://orcid.org/0000-0001-7220-1221
https://orcid.org/0000-0001-7220-1221
Nicola Maffulli  https://orcid.org/0000-0002-5327-3702
https://orcid.org/0000-0002-5327-3702
References
- 1. Adams SB, Dekker TJ, Schiff AP, et al. Prospective evaluation of structural allograft transplantation for osteochondral lesions of the talar shoulder. Foot Ankle Int. 2018;39(1):28-34. [DOI] [PubMed] [Google Scholar]
- 2. Adams SB, Jr, Viens NA, Easley ME, Stinnett SS, Nunley JA, II. Midterm results of osteochondral lesions of the talar shoulder treated with fresh osteochondral allograft transplantation. J Bone Joint Surg Am. 2011;93(7):648-654. [DOI] [PubMed] [Google Scholar]
- 3. Ahmad J, Jones K. Comparison of osteochondral autografts and allografts for treatment of recurrent or large talar osteochondral lesions. Foot Ankle Int. 2016;37(1):40-50. [DOI] [PubMed] [Google Scholar]
- 4. Anders S, Volz M, Frick H, Gellissen J. A randomized, controlled trial comparing autologous matrix-induced chondrogenesis (AMIC®) to microfracture: analysis of 1- and 2-year follow-up data of 2 centers. Open Orthop J. 2013;7:133-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basad E, Wissing FR, Fehrenbach P, et al. Matrix-induced autologous chondrocyte implantation (MACI) in the knee: clinical outcomes and challenges. Knee Surg Sports Traumatol Arthrosc. 2015;23(12):3729-3735. [DOI] [PubMed] [Google Scholar]
- 6. Baumfeld T, Baumfeld D, Prado M, Nery C. All-arthroscopic AMIC(®) (AT-AMIC) for the treatment of talar osteochondral defects: a short follow-up case series. Foot (Edinb). 2018;37:23-27. [DOI] [PubMed] [Google Scholar]
- 7. Bexkens R, Ogink PT, Doornberg JN, et al. Donor-site morbidity after osteochondral autologous transplantation for osteochondritis dissecans of the capitellum: a systematic review and meta-analysis. Knee Surg Sports Traumatol Arthrosc. 2017;25(7):2237-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carey JL, Remmers AE, Flanigan DC. Use of MACI (autologous cultured chondrocytes on porcine collagen membrane) in the United States: preliminary experience. Orthop J Sports Med. 2020;8(8):2325967120941816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Czitrom AA, Keating S, Gross AE. The viability of articular cartilage in fresh osteochondral allografts after clinical transplantation. J Bone Joint Surg Am. 1990;72(4):574-581. [PubMed] [Google Scholar]
- 10. de l’Escalopier N, Barbier O, Mainard D, et al. Outcomes of talar dome osteochondral defect repair using osteocartilaginous autografts: 37 cases of mosaicplasty®. Orthop Traumatol Surg Res. 2015;101(1):97-102. [DOI] [PubMed] [Google Scholar]
- 11. DeSandis BA, Haleem AM, Sofka CM, O’Malley MJ, Drakos MC. Arthroscopic treatment of osteochondral lesions of the talus using juvenile articular cartilage allograft and autologous bone marrow aspirate concentration. J Foot Ankle Surg. 2018;57(2):273-280. [DOI] [PubMed] [Google Scholar]
- 12. El-Rashidy H, Villacis D, Omar I, Kelikian AS. Fresh osteochondral allograft for the treatment of cartilage defects of the talus: a retrospective review. J Bone Joint Surg Am. 2011;93(17):1634-1640. [DOI] [PubMed] [Google Scholar]
- 13. Emre TY, Ege T, Cift HT, et al. Open mosaicplasty in osteochondral lesions of the talus: a prospective study. J Foot Ankle Surg. 2012;51(5):556-560. [DOI] [PubMed] [Google Scholar]
- 14. Familiari F, Cinque ME, Chahla J, et al. Clinical outcomes and failure rates of osteochondral allograft transplantation in the knee: a systematic review. Am J Sports Med. 2018;46(14):3541-3549. [DOI] [PubMed] [Google Scholar]
- 15. Filardo G, Perdisa F, Roffi A, Marcacci M, Kon E. Stem cells in articular cartilage regeneration. J Orthop Surg Res. 2016;11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fraser EJ, Harris MC, Prado MP, Kennedy JG. Autologous osteochondral transplantation for osteochondral lesions of the talus in an athletic population. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1272-1279. [DOI] [PubMed] [Google Scholar]
- 17. Friedlaender GE, Horowitz MC. Immune responses to osteochondral allografts: nature and significance. Orthopedics. 1992;15(10):1171-1175. [DOI] [PubMed] [Google Scholar]
- 18. Gaul F, Tirico LEP, McCauley JC, Bugbee WD. Long-term follow-up of revision osteochondral allograft transplantation of the ankle. Foot Ankle Int. 2018;39(5):522-529. [DOI] [PubMed] [Google Scholar]
- 19. Gaul F, Tirico LEP, McCauley JC, Pulido PA, Bugbee WD. Osteochondral allograft transplantation for osteochondral lesions of the talus: midterm follow-up. Foot Ankle Int. 2019;40(2):202-209. [DOI] [PubMed] [Google Scholar]
- 20. Gautier E, Kolker D, Jakob RP. Treatment of cartilage defects of the talus by autologous osteochondral grafts. J Bone Joint Surg Br. 2002;84(2):237-244. [DOI] [PubMed] [Google Scholar]
- 21. Georgiannos D, Bisbinas I, Badekas A. Osteochondral transplantation of autologous graft for the treatment of osteochondral lesions of talus: 5- to 7-year follow-up. Knee Surg Sports Traumatol Arthrosc. 2016;24(12):3722-3729. [DOI] [PubMed] [Google Scholar]
- 22. Gobbi A, Francisco RA, Lubowitz JH, Allegra F, Canata G. Osteochondral lesions of the talus: randomized controlled trial comparing chondroplasty, microfracture, and osteochondral autograft transplantation. Arthroscopy. 2006;22(10):1085-1092. [DOI] [PubMed] [Google Scholar]
- 23. Godin JA, Sanchez G, Cinque ME, et al. Osteochondral allograft transplantation for treatment of medial femoral condyle defect. Arthrosc Tech. 2017;6(4):e1239-e1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gomoll AH, Madry H, Knutsen G, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434-447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gotze C, Nieder C, Felder H, Migliorini F. AMIC for focal osteochondral defect of the talar shoulder. Life (Basel). 2020;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gross AE, Agnidis Z, Hutchison CR. Osteochondral defects of the talus treated with fresh osteochondral allograft transplantation. Foot Ankle Int. 2001;22(5):385-391. [DOI] [PubMed] [Google Scholar]
- 27. Gudas R, Gudaite A, Mickevicius T, et al. Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: a prospective study with a 3-year follow-up. Arthroscopy. 2013;29(1):89-97. [DOI] [PubMed] [Google Scholar]
- 28. Gul M, Cetinkaya E, Aykut US, et al. Effect of the presence of subchondral cysts on treatment results of autologous osteochondral graft transfer in osteochondral lesions of the talus. J Foot Ankle Surg. 2016;55(5):1003-1006. [DOI] [PubMed] [Google Scholar]
- 29. Guney A, Yurdakul E, Karaman I, et al. Medium-term outcomes of mosaicplasty versus arthroscopic microfracture with or without platelet-rich plasma in the treatment of osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1293-1298. [DOI] [PubMed] [Google Scholar]
- 30. Haasper C, Zelle BA, Knobloch K, et al. No mid-term difference in mosaicplasty in previously treated versus previously untreated patients with osteochondral lesions of the talus. Arch Orthop Trauma Surg. 2008;128(5):499-504. [DOI] [PubMed] [Google Scholar]
- 31. Haene R, Qamirani E, Story RA, Pinsker E, Daniels TR. Intermediate outcomes of fresh talar osteochondral allografts for treatment of large osteochondral lesions of the talus. J Bone Joint Surg Am. 2012;94(12):1105-1110. [DOI] [PubMed] [Google Scholar]
- 32. Hahn DB, Aanstoos ME, Wilkins RM. Osteochondral lesions of the talus treated with fresh talar allografts. Foot Ankle Int. 2010;31(4):277-282. [DOI] [PubMed] [Google Scholar]
- 33. Haleem AM, Ross KA, Smyth NA, et al. Double-plug autologous osteochondral transplantation shows equal functional outcomes compared with single-plug procedures in lesions of the talar dome: a minimum 5-year clinical follow-up. Am J Sports Med. 2014;42(8):1888-1895. [DOI] [PubMed] [Google Scholar]
- 34. Hangody L, Kish G, Karpati Z, Szerb I, Eberhardt R. Treatment of osteochondritis dissecans of the talus: use of the mosaicplasty technique—a preliminary report. Foot Ankle Int. 1997;18(10):628-634. [DOI] [PubMed] [Google Scholar]
- 35. Hangody L, Kish G, Modis L, et al. Mosaicplasty for the treatment of osteochondritis dissecans of the talus: two to seven year results in 36 patients. Foot Ankle Int. 2001;22(7):552-558. [DOI] [PubMed] [Google Scholar]
- 36. Hangody L, Vasarhelyi G, Hangody LR, et al. Autologous osteochondral grafting—technique and long-term results. Injury. 2008;39(suppl 1):S32-S39. [DOI] [PubMed] [Google Scholar]
- 37. Howick J, Chambers I, Glasziou P, et al. The 2011 Oxford CEBM Levels of Evidence. Oxford Centre for Evidence-Based Medicine; 2011. Accessed February 2021. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/levels-of-evidence-introductory-document
- 38. Imhoff AB, Paul J, Ottinger B, et al. Osteochondral transplantation of the talus: long-term clinical and magnetic resonance imaging evaluation. Am J Sports Med. 2011;39(7):1487-1493. [DOI] [PubMed] [Google Scholar]
- 39. Jackson AT, Drayer NJ, Samona J, et al. Osteochondral allograft transplantation surgery for osteochondral lesions of the talus in athletes. J Foot Ankle Surg. 2019;58(4):623-627. [DOI] [PubMed] [Google Scholar]
- 40. Karnovsky SC, DeSandis B, Haleem AM, et al. Comparison of juvenile allogenous articular cartilage and bone marrow aspirate concentrate versus microfracture with and without bone marrow aspirate concentrate in arthroscopic treatment of talar osteochondral lesions. Foot Ankle Int. 2018;39(4):393-405. [DOI] [PubMed] [Google Scholar]
- 41. Kim YS, Park EH, Kim YC, Koh YG, Lee JW. Factors associated with the clinical outcomes of the osteochondral autograft transfer system in osteochondral lesions of the talus: second-look arthroscopic evaluation. Am J Sports Med. 2012;40(12):2709-2719. [DOI] [PubMed] [Google Scholar]
- 42. Kreuz PC, Steinwachs M, Erggelet C, et al. Mosaicplasty with autogenous talar autograft for osteochondral lesions of the talus after failed primary arthroscopic management: a prospective study with a 4-year follow-up. Am J Sports Med. 2006;34(1):55-63. [DOI] [PubMed] [Google Scholar]
- 43. Lanham NS, Carroll JJ, Cooper MT, Perumal V, Park JS. A comparison of outcomes of particulated juvenile articular cartilage and bone marrow aspirate concentrate for articular cartilage lesions of the talus. Foot Ankle Spec. 2017;10(4):315-321. [DOI] [PubMed] [Google Scholar]
- 44. Lee CH, Chao KH, Huang GS, Wu SS. Osteochondral autografts for osteochondritis dissecans of the talus. Foot Ankle Int. 2003; 24(11):815-822. [DOI] [PubMed] [Google Scholar]
- 45. Li X, Zhu Y, Xu Y, et al. Osteochondral autograft transplantation with biplanar distal tibial osteotomy for patients with concomitant large osteochondral lesion of the talus and varus ankle malalignment. BMC Musculoskelet Disord. 2017;18(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu W, Liu F, Zhao W, et al. Osteochondral autograft transplantation for acute osteochondral fractures associated with an ankle fracture. Foot Ankle Int. 2011;32(4):437-442. [DOI] [PubMed] [Google Scholar]
- 47. Liu X, An J, Zhang H, et al. Autologous osteochondral graft for early posttraumatic arthritis of tibiotalar joints after comminuted pilon fractures in young patients. Foot Ankle Int. 2020;41(1):69-78. [DOI] [PubMed] [Google Scholar]
- 48. Marlovits S, Singer P, Zeller P, et al. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16-23. [DOI] [PubMed] [Google Scholar]
- 49. Mei-Dan O, Carmont MR, Laver L, et al. Platelet-rich plasma or hyaluronate in the management of osteochondral lesions of the talus. Am J Sports Med. 2012;40(3):534-541. [DOI] [PubMed] [Google Scholar]
- 50. Migliorini F, Berton A, Salvatore G, et al. Autologous chondrocyte implantation and mesenchymal stem cells for the treatments of chondral defects of the knee—a systematic review. Curr Stem Cell Res Ther. 2020;15(6):547-556. [DOI] [PubMed] [Google Scholar]
- 51. Moher D, Liberati A, Tetzlaff J, Altman DG; the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mundi R, Bedi A, Chow L, et al. Cartilage restoration of the knee: a systematic review and meta-analysis of level 1 studies. Am J Sports Med. 2016;44(7):1888-1895. [DOI] [PubMed] [Google Scholar]
- 53. Nakagawa Y, Mukai S, Setoguchi Y, et al. Clinical outcomes of donor sites after osteochondral graft harvest from healthy knees. Orthop J Sports Med. 2017;5(10):2325967117732525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Neri S, Vannini F, Desando G, et al. Ankle bipolar fresh osteochondral allograft survivorship and integration: transplanted tissue genetic typing and phenotypic characteristics. J Bone Joint Surg Am. 2013;95(20):1852-1860. [DOI] [PubMed] [Google Scholar]
- 55. Nguyen A, Ramasamy A, Walsh M, McMenemy L, Calder JDF. Autologous osteochondral transplantation for large osteochondral lesions of the talus is a viable option in an athletic population. Am J Sports Med. 2019;47(14):3429-3435. [DOI] [PubMed] [Google Scholar]
- 56. Orr JD, Dunn JC, Heida KA, Jr, et al. Results and functional outcomes of structural fresh osteochondral allograft transfer for treatment of osteochondral lesions of the talus in a highly active population. Foot Ankle Spec. 2017;10(2):125-132. [DOI] [PubMed] [Google Scholar]
- 57. Park CH, Song KS, Kim JR, Lee SW. Retrospective evaluation of outcomes of bone peg fixation for osteochondral lesion of the talus. Bone Joint J. 2020;102(10):1349-1353. [DOI] [PubMed] [Google Scholar]
- 58. Park KH, Hwang Y, Han SH, et al. Primary versus secondary osteochondral autograft transplantation for the treatment of large osteochondral lesions of the talus. Am J Sports Med. 2018;46(6):1389-1396. [DOI] [PubMed] [Google Scholar]
- 59. Paul J, Sagstetter M, Lammle L, et al. Sports activity after osteochondral transplantation of the talus. Am J Sports Med. 2012; 40(4):870-874. [DOI] [PubMed] [Google Scholar]
- 60. Phipatanakul WP, VandeVord PJ, Teitge RA, Wooley PH. Immune response in patients receiving fresh osteochondral allografts. Am J Orthop (Belle Mead NJ). 2004;33(7):345-348. [PubMed] [Google Scholar]
- 61. Pomajzl RJ, Baker EA, Baker KC, et al. Case series with histopathologic and radiographic analyses following failure of fresh osteochondral allografts of the talus. Foot Ankle Int. 2016;37(9):958-967. [DOI] [PubMed] [Google Scholar]
- 62. Richter M, Zech S, Meissner S, Naef I. Comparison matrix-associated stem cell transplantation (MAST) with autologous matrix induced chondrogenesis plus peripheral blood concentrate (AMIC+PBC) in chondral lesions at the ankle—a clinical matched-patient analysis. Foot Ankle Surg. 2020;26(6):669-675. [DOI] [PubMed] [Google Scholar]
- 63. Robert H. Chondral repair of the knee joint using mosaicplasty. Orthop Traumatol Surg Res. 2011;97(4):418-429. [DOI] [PubMed] [Google Scholar]
- 64. Ross AW, Murawski CD, Fraser EJ, et al. Autologous osteochondral transplantation for osteochondral lesions of the talus: does previous bone marrow stimulation negatively affect clinical outcome? Arthroscopy. 2016;32(7):1377-1383. [DOI] [PubMed] [Google Scholar]
- 65. Sabaghzadeh A, Mirzaee F, Shahriari Rad H, et al. Osteochondral autograft transfer (mosaicplasty) for treatment of patients with osteochondral lesions of talus. Chin J Traumatol. 2020;23(1): 60-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sadlik B, Kolodziej L, Blasiak A, Szymczak M, Warchal B. Biological reconstruction of large osteochondral lesions of the talar dome with a modified “sandwich” technique—midterm results. Foot Ankle Surg. 2017;23(4):290-295. [DOI] [PubMed] [Google Scholar]
- 67. Scranton PE, Jr, Frey CC, Feder KS. Outcome of osteochondral autograft transplantation for type-V cystic osteochondral lesions of the talus. J Bone Joint Surg Br. 2006;88(5):614-619. [DOI] [PubMed] [Google Scholar]
- 68. Sherman SL, Thyssen E, Nuelle CW. Osteochondral autologous transplantation. Clin Sports Med. 2017;36(3):489-500. [DOI] [PubMed] [Google Scholar]
- 69. Shimozono Y, Donders JCE, Yasui Y, et al. Effect of the containment type on clinical outcomes in osteochondral lesions of the talus treated with autologous osteochondral transplantation. Am J Sports Med. 2018;46(9):2096-2102. [DOI] [PubMed] [Google Scholar]
- 70. Shimozono Y, Hurley ET, Nguyen JT, Deyer TW, Kennedy JG. Allograft compared with autograft in osteochondral transplantation for the treatment of osteochondral lesions of the talus. J Bone Joint Surg Am. 2018;100(21):1838-1844. [DOI] [PubMed] [Google Scholar]
- 71. Shimozono Y, Seow D, Yasui Y, Fields K, Kennedy JG. Knee-to-talus donor-site morbidity following autologous osteochondral transplantation: a meta-analysis with best-case and worst-case analysis. Clin Orthop Relat Res. 2019;477(8):1915-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sirlin CB, Brossmann J, Boutin RD, et al. Shell osteochondral allografts of the knee: comparison of MR imaging findings and immunologic responses. Radiology. 2001;219(1):35-43. [DOI] [PubMed] [Google Scholar]
- 73. Stevenson S. The immune response to osteochondral allografts in dogs. J Bone Joint Surg Am. 1987;69(4):573-582. [PubMed] [Google Scholar]
- 74. Valtanen RS, Arshi A, Kelley BV, Fabricant PD, Jones KJ. Articular cartilage repair of the pediatric and adolescent knee with regard to minimal clinically important difference: a systematic review. Cartilage. 2020;11(1):9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Van Lieshout EM, De Boer AS, Meuffels DE, et al. American Orthopaedic Foot and Ankle Society (AOFAS) Ankle-Hindfoot Score: a study protocol for the translation and validation of the Dutch language version. BMJ Open. 2017;7(2):e012884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Williams SK, Amiel D, Ball ST, et al. Analysis of cartilage tissue on a cellular level in fresh osteochondral allograft retrievals. Am J Sports Med. 2007;35(12):2022-2032. [DOI] [PubMed] [Google Scholar]
- 77. Woelfle JV, Reichel H, Nelitz M. Indications and limitations of osteochondral autologous transplantation in osteochondritis dissecans of the talus. Knee Surg Sports Traumatol Arthrosc. 2013;21(8):1925-1930. [DOI] [PubMed] [Google Scholar]
- 78. Yoon HS, Park YJ, Lee M, Choi WJ, Lee JW. Osteochondral autologous transplantation is superior to repeat arthroscopy for the treatment of osteochondral lesions of the talus after failed primary arthroscopic treatment. Am J Sports Med. 2014;42(8):1896-1903. [DOI] [PubMed] [Google Scholar]
- 79. Zhu Y, Xu X. Osteochondral autograft transfer combined with cancellous allografts for large cystic osteochondral defect of the talus. Foot Ankle Int. 2016;37(10):1113-1118. [DOI] [PubMed] [Google Scholar]