Abstract
STUDY QUESTION
Is a mechanical hand-held device for removing a single-rod subdermal contraceptive implant safe for implant users?
SUMMARY ANSWER
In terms of safety, the device is non-inferior to the standard technique for implant removal.
WHAT IS KNOWN ALREADY
An easy-to-use device for removing a subdermal contraceptive implant may be helpful in settings where skilled providers are in short supply. Prior to this study, the only report on the world’s first hand-held, mechanical device with build-in incisor was a Swedish study using earlier versions of the product.
STUDY DESIGN, SIZE, DURATION
From December 2019 to November 2020, we conducted a three-arm, open-label non-inferiority randomized trial involving 225 Ugandan women to assess safety (primary outcome) and measure implant removal efficacy (secondary outcomes) of a newly developed, hand-held device, compared to the standard removal technique.
PARTICIPANTS/MATERIALS, SETTING, METHODS
We randomized participants desiring removal of their one-rod contraceptive implant in a 1:1:1 ratio: standard technique/lidocaine injection, new device/lidocaine patch or new device/lidocaine injection. For primary safety endpoints, we examined removal complications and grouped them according to severity. For secondary endpoints on efficacy, we defined three device outcomes: intact implant removed without additional tools (primary), implant removed allowing implant breakage, but without tools (secondary) and implant removed allowing implant breakage and non-scalpel tools (tertiary). We assessed provider feedback on the device and used chi-square tests for all comparisons.
MAIN RESULTS AND THE ROLE OF CHANCE
We recruited 225 participants and randomly assigned (n = 75) to each group. For safety, no primary complications occurred in any treatment group, while only one secondary complication occurred in each treatment group (1%). Primary efficacy was 100% (standard technique), 85% (new device/lidocaine patch) and 73% (new device/lidocaine injection) (P < 0.0001). Secondary efficacy was 100% (standard technique), 92% (new device/lidocaine patch) and 79% (new device/lidocaine injection) (P < 0.0001). Tertiary efficacy was 100% (standard technique), 96% (new device/lidocaine patch) and 91% (new device/lidocaine injection) (P = 0.017). Unsuccessful removals with the new device did not hinder subsequent implant extractions with standard back-up tools. In over 90% of the 150 device procedures, providers agreed or strongly agreed that the product is an acceptable alternative to standard removal technique.
LIMITATIONS, REASONS FOR CAUTION
We tested a new removal device in the hands of Ugandan nurses who were adept at standard removal techniques; our estimates of removal efficacy may not apply to lower-level providers who arguably may be the prime beneficiaries of this technology.
WIDER IMPLICATIONS OF THE FINDINGS
The study was conducted in a region of the world where the new device could be used to expand access to implant removal services. Intended beneficiaries of the new product are implant users who cannot easily find skilled providers for traditional scalpel-dependent removals and/or users who are intimidated by scalpel procedures, and lower-level providers who can be trained to help deliver services to meet a growing demand. The new device is a safe, acceptable alternative; efficacy was high, but not on par with standard technique.
STUDY FUNDING/COMPETING INTEREST(S)
Funding for this study was provided by the RemovAid AS of Norway with grants from Research Council of Norway (GLOBVAC number 228319), Bill & Melinda Gates Foundation (grant INV-007571) and SkatteFUNN. M.B. is founder and former CEO of RemovAid AS, Norway. M.B. holds contraceptive rod remover patents (2012 1307156.8 and 2015), pre-removal test (filed) and shares in RemovAid AS. All of the remaining authors’ institutions received payments in the form of contracts to help conduct the study; the funds for these contracts emanated from RemovAid AS.
TRIAL REGISTRATION NUMBER
TRIAL REGISTRATION DATE
9 October 2019
DATE OF FIRST PATIENT’S ENROLMENT
23 December 2019
Keywords: female contraception, randomized controlled trials, subdermal implant, device, implant removal
Introduction
In sub-Saharan Africa, the prevalence of subdermal contraceptive implant use among females is ∼6% (Population Reference Bureau, 2019); given the population of 250 million, women this calculates to ∼15 million current users. In this region of the world, implants have become the second most popular form of contraception (injectable prevalence is 12%). Just 20 years ago, the prevalence of implant use in sub-Saharan Africa was about 1% (United Nations, 2013).
The current high level of implant use in the region is no accident. Many factors came together: women’s preferences for this form of contraception, country-level commitments to expand access to wide varieties of products, collaborations and actions among international donor agencies and implant manufacturers to increase purchases through guaranteed low commodity costs and procurement guarantees (Jacobstein, 2018). The 2012 London Summit on Family Planning, involving philanthropic foundations, international donor agencies, non-governmental organizations and contraceptive manufacturers, led to formation of the Implant Access Program and subsequent international commitments to increase availability of one- and two-rod implants (Jacobstein, 2018; Braun and Grever, 2020). Subsequent impact was clear; for example, in Burkina Faso (where modern method use rose from 15.7% in 2014 to 26.4% in 2017), implant use rose quickly to constitute 50% of the contraceptive method mix (Ahmed et al., 2019).
Subdermal contraceptive implants are placed on the inner side of the non-dominant upper arm and provide 3+ years of highly effective protection from unintended pregnancy (Merck Sharp & Dohme Ltd., 2020). For the one-rod product, the insertion procedure does not require highly technical skills, thanks to the product’s delivery device that has a built-in trocar and easy instructions on proper use. The advent of an easy-to-insert mechanism for implants has led to provider task-sharing initiatives to expand access to this important contraceptive. Ethiopia’s Integrated Family Health Program, for example, recorded 1.2 million one-rod insertion procedures performed by Health Extension Workers (Tilahun et al., 2017). Nigeria’s task-shifting pilot study showed that health extension workers can correctly perform insertion tasks 90% of the time (Charyeva et al., 2015). Task-shifting and task-sharing activities in family planning are encouraged by the World Health Organization (2017) and both Nigeria and Ethiopia have demonstrated that these service-delivery approaches can be accomplished safely for insertion of one-rod contraceptive implants.
Traditionally, an implant removal procedure is generally considered to be minor surgery (Fraser, 2006). It should be performed with local anaesthesia in aseptic conditions, using scalpel and forceps (Levine et al., 2008; Stoddard et al., 2011; Merck Sharp & Dohme Ltd., 2020). The key steps in a standard removal are injection of lidocaine beneath the implant, incision with scalpel at the distal end of the subdermal implant, removal with forceps and placement of sterile adhesive wound closure with pressure bandage; no sutures are typically required post-removal. Removals currently require adequate facilities, sterile instruments and trained healthcare providers to safely use scalpels (Bahamondes and Peloggia, 2019). However, lack of experienced operators or geographical isolation or other reasons can prevent timely removal (Fraser, 2006; Callahan et al., 2020; Costenbader et al., 2020; Hernandez et al., 2020).
The disconnect between rapid uptake of implants and commensurate access to timely removal services has created some documented backlogs. In Ethiopia, 61% of current implant users who received insertion services from a Health Extension Worker (HEW) reported they had experienced a barrier to removal (Costenbader et al., 2020). Sixteen percent (16%) of former implant users who received insertion services from a Health Extension Worker reported barriers; this level was significantly higher than the prevalence of barriers (10%) reported by women who received services from a clinic. In the Democratic Republic of Congo, a survey of implant users who did not fulfil their desire for removal found that about 7% cited barriers as the reasons for continued use (Hernandez et al., 2020). Among Ghanaian implant users who sought removal, the prevalence of unsuccessful results varied by location of services. For example, in public facilities, 8% of users did not succeed and an additional 14% had to wait over 1 week for services (Callahan et al., 2020); among users who received insertions from outreach mobile services, 23% did not succeed in having their implant removed and an additional 5% waited over 1 week.
As expanded uptake of implants continues, more effort must be made to ensure that all women can easily have the product removed when desired. New approaches to implant removal and task sharing/shifting may be needed, particularly in regions where access to skilled providers is limited. Our main aim in this study was to measure and compare safety and efficacy of an innovative, easy-to-use removal device versus the standard technique, in a population of Ugandan women seeking removal of a one-rod subdermal implant. The RemovAid™ device used in Uganda was an improved version of the earlier models used in pilot studies in Sweden (Iwarsson et al., 2020).
Materials and methods
Study design
We conducted a three-arm parallel group, open-label, non-inferiority randomized trial in Kampala, Uganda to compare subdermal contraceptive implant removal techniques: RemovAid™ device/lidocaine injection, standard technique/lidocaine injection or RemovAid™ device/lidocaine patch. The research was conducted at Kawempe National Referral Hospital from 2019 to 2021, and in accordance with the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice and local regulations. The Institutional Review Boards of the School of Medicine Research and Ethics Committee at Makerere University (REC REF No 2018-104) and the Karolinska Institutet approved the research (reference protocol 2018-103). In addition, the Uganda National Council for Science and Technology (HS 2526) also approved the use of the device for investigational purposes as described in the approved protocol. Participants received oral and written information and were provided the opportunity to ask questions before they signed informed consent forms to voluntarily join the study. We used English or Luganda language forms, as preferred by the participant.
We designed a three-arm trial to accommodate two different approaches to anaesthetizing the skin when using RemovAid™. We included lidocaine injection because it is currently the predominant approach used with the gold standard technique of removing an implant and we included lidocaine patch because of potential advantages.
Participants
Women presenting for contraceptive implant removal services at Kawempe National Referral Hospital were screened for eligibility. We applied the following eligibility criteria: 18+ years of age, seeking voluntary removal of a one-rod subdermal implant, completely and easily palpable subdermal implant that could be pinched and lifted with the fingers, and willing to provide follow-up information. We excluded women who had a previous failed removal of the current implant and women who had any known allergies to skin preparation products or local anaesthetics.
Randomization
The study statistician prepared opaque and sealed envelopes with an externally printed sequential number and used a random number generator with random block sizes of 3, 6 or 9 for the allocation sequence. We randomly assigned participants in a 1:1:1 ratio to one of the three removal techniques. The study nurse or research assistant opened the envelopes in sequential order for each participant after eligibility was confirmed and then proceeded to perform the implant removal procedure. No masking of assignment was used.
Interventions
Prior to study initiation, the Ugandan nurses participated in RemovAid’s Experienced Provider Training Program. They learned proper device usage and safety during the didactic portion of the training. Then they practiced removals using placebo implants and model arms. When the nurses were proficient at removing placebo implants from model arms, correctly operating RemovAid™, they were certified for competency.
To anaesthetize the skin before implant removal, the study nurse used either lidocaine injection (1–2 ml of 1% lidocaine) or a lidocaine patch (Emla® lidocaine/prilocaine 5% patch, containing 25 mg of lidocaine and 25 mg of prilocaine). Per manufacturer’s instructions (Astra USA, Inc, 2000), the lidocaine patch required at least 1 h of continuous contact with skin before the procedure, whereas the lidocaine injections required <5 min before procedure.
For the implant removal procedure, the study nurse used either the standard implant removal tools (scalpel and forceps/tweezers) or RemovAid™, the hand-held mechanical device with built-in incisor (Fig. 1) developed by RemovAid AS of Norway. The study nurse positioned the device over the midpoint of the subdermal implant and perpendicular to the skin, clamped the skin around the implant and temporarily locked the clamp with the slider. Then the study nurse pressed down on the thumb knob to incise the skin and deployed the pincher knob to grasp the implant through the incision. Finally, the study nurse unlocked the clamp on the skin, and with the pincher still gripping the midpoint of the implant, rotated the angle of the device to be more plane with the skin and pulled the laterally positioned device up and away from the incision point. If successful, the implant folded in half while exiting the skin.
Figure 1.
Picture of RemovAid™ device and description of components.
Prior to starting the removal procedure, study nurses collected sociodemographic information, recorded height/weight of the participant for BMI calculations and measured mid-arm circumference and characterized its muscularity and fattiness. Also, the nurse recorded the reason for seeking removal and the duration of use of the implant.
To address the primary outcome of safety, nurses recorded any complications that occurred during the removal procedure. For efficacy, the nurses recorded the success/failure to remove the implant, the additional tools used for the RemovAid™ procedures and mechanical failures of the device. We gave participants an appointment card for a 2-week follow-up visit; when participants returned to the clinic, the study nurse examined the wound and assessed how well it had healed.
Participants were asked to mark the level of pain they experienced at two separate times: at the time of lidocaine application and at the time of implant removal. Participants used a 10-point visual analogue scale, from 0 (no pain) to 10 (worst pain imaginable) to mark the spot on the line that characterized their level of pain (Sriwatanakul et al., 1983).
We measured the duration of the procedures with a timer. The starting time for measuring duration was from the moment the scalpel or RemovAid™ were ‘in hand’ and the skin was being touched to prepare for incision. Duration was measured to four different endpoints: time to incision, time to full implant extraction, time to when a final bandage was placed on the wound after the removal, and in the case of a RemovAid™ failure event, time when the device was abandoned, and finger manipulation or traditional instruments were used.
After each procedure, providers recorded whether they thought RemovAid™ was an acceptable alternative to the standard removal technique (strongly disagree, disagree, neutral, agree, strongly agree). We asked participants to respond to these three statements/questions: I would recommend this procedure to a friend/relative (strongly disagree, disagree, neutral, agree, strongly agree); If needed in future, clinicians should use this method (strongly disagree, disagree, neutral, agree, strongly agree); and How satisfied were you with the procedure (very dissatisfied, dissatisfied, neutral, satisfied, very satisfied)?
Study nurses recorded all data on paper case report forms, then the data manager used EpiData to enter the data with double-entry for verification. The data manager and statisticians helped generate data queries for resolution by the study nurses.
Outcomes
We created two safety outcomes: primary complications and secondary complications. Primary complications included any of the following: some or all of implant remains in arm (after reasonable removal attempts with any available equipment), probable or confirmed nerve damage, excessive bleeding during removal procedure, use of sutures to repair skin after removal, excessive post-removal bleeding or subsequent infection that results in sepsis. Secondary complications included unusual level of trauma to the skin (bruising/hematoma) or unhealed wound at the 2-week visit that required additional treatment (including localized infection requiring treatment). In addition, we defined a separate category of non-safety complications: implant breakage from incision, implant breakage during extraction or wound still healing at 2-week visit but does not need any additional intervention. Implant breakage included complete severing of the product at the time of incision and/or breakage into two pieces during the extraction step when the implant folds to exit the skin.
For the secondary outcome of efficacy, we defined three measures to fully characterize RemovAid™.
Primary efficacy: Implants successfully removed without breaking the implant, and in the case of RemovAid™, without using scalpel, tweezers, or forceps.
Secondary efficacy: Implants successfully removed (implant breakage allowed), and in the case of RemovAid™, without the use of scalpel, tweezers, or forceps.
Tertiary efficacy: Implants successfully removed (implant breakage allowed), and in the case of RemovAid™, without the use of a scalpel (tweezers and/or forceps allowed).
We also defined these additional outcomes: mean pain levels experienced from lidocaine application and removal procedure, mean duration of procedures, provider and participant feedback on device.
Statistical analysis
We hypothesized that RemovAid™ would be non-inferior to standard removal technique in terms of safety. To claim non-inferiority, we stipulated that the upper one-sided 95% confidence bound for the difference in complication rates (RemovAid™ versus standard procedure) should be no more than 10%. We assumed that the true complication rate would be no more than 10% in each group and desired 80% power. In our design, the study size of n = 225 (randomly allocated in a 2:1 ratio, RemovAid™ vs standard technique) was deemed sufficient if RemovAid™ has a lower complication rate than the standard technique, or if the common complication rate is less than 10%. The study size justification assumed that the lidocaine patch and injection subgroups would have similar complication rates among the RemovAid™ device users; thus, the planned approach was to combine the patch and lidocaine results to achieve a 2:1 ratio for comparisons to the standard technique. Previous research has shown that complications rates for removal of a one-rod subdermal implant are <10% (Levine et al., 2008; Creinin et al., 2017).
We used the intent-to-treat population to compare baseline characteristics across the three randomized groups. We used the treated population for analysis of outcomes; participants who received the incorrect treatment were analysed according to treatment received. We did a sensitivity analysis of baseline characteristics and outcomes, with the per-protocol population, to examine any threats to validity based on the treated population.
As stipulated in the protocol, an interim analysis was conducted when 50% of participants were successfully enrolled. The Haybittle–Peto stopping boundary was used to consider ending the trial early for ethical reasons if the treatment group clearly showed evidence of benefit or harm (with a probability 0.0005). The results of the interim analysis were shared with the Safety Review Committee (SRC). The SRC recommended that the trial continue without changes, taking into consideration the pattern of complications, efficacy and other secondary outcomes. No adjustments were made to the final P-values for declaring harm or non-inferiority based on the interim analysis.
All significant tests were two-sided and at 0.05 significance level. All analyses were done in SAS®. This trial is registered on www.clinicaltrials.gov (NCT04120337).
Role of the funding source
The funder of the study contributed to the overall study design and the interpretation of data. The funder had no role in data collection, data analysis or writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
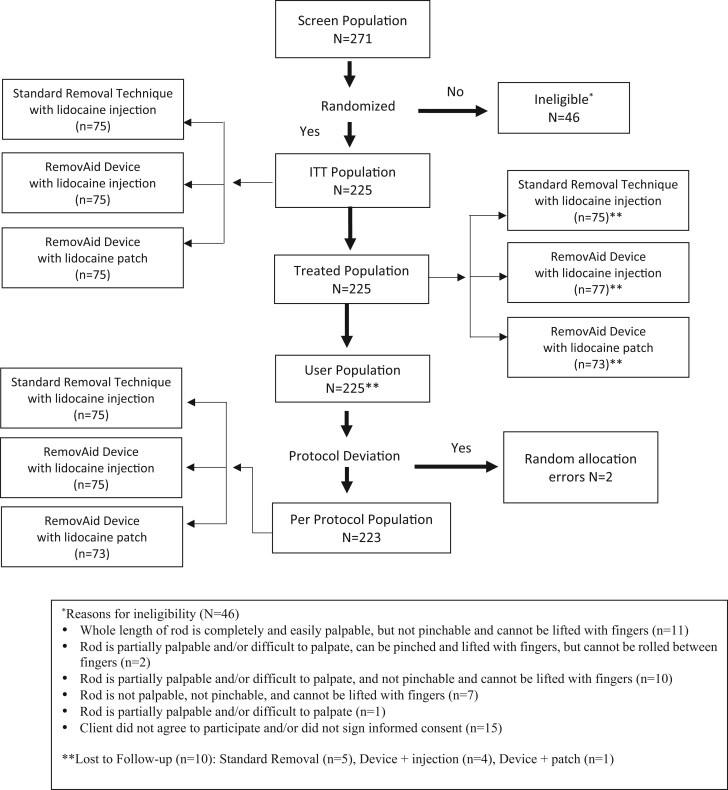
Between 23 December 2019 and 9 November 2020, 271 women seeking removal of their subdermal contraceptive implant were screened and 256 consented to participate. Thirty-one participants (12.1%: 31/256) were ineligible due to the deep location of the subdermal implant, leaving a total of 225 enrolled. All enrolled participants were randomly assigned to one of three groups (n = 75 in each group): RemovAid™ device/lidocaine injection, standard technique/lidocaine injection or RemovAid™ device/lidocaine patch (Fig. 2).
Figure 2.
Trial profile.
None of the baseline characteristics varied by randomized group: mean age (28.0), mean BMI (25.9), mean years using implant (2.2), reasons for seeking implant removal (product expiration: 47%, side effects: 27%, desire for pregnancy: 24%), arm circumference/fat/muscularity (Table I).
Table I.
Baseline characteristics, reasons for seeking implant removal, arm/implant characteristics.*
| Characteristics | Standard procedure (N = 75) | RemovAid™ with injection (N = 75) | RemovAid™ with patch (N = 75) | Total (N = 225) |
|---|---|---|---|---|
| Age, years: mean (SD) | 28.5 (6.59) | 27.1 (5.39) | 28.5 (5.88) | 28.0 (5.98) |
| Number of children, mean (SD) | 2.5 (1.59) | 2.1 (1.24) | 2.3 (1.39) | 2.3 (1.42) |
| Want more children (%) | 58 (77) | 55 (73) | 53 (71) | 166 (74) |
| Weight, mean (SD) | 65.7 (13.43) | 64.4 (13.19) | 63.5 (11.24) | 64.5 (12.63) |
| BMI | ||||
| Mean (SD) | 26.6 (5.54) | 25.8 (5.55) | 25.2 (4.63) | 25.9 (5.26) |
| Underweight (<18.5) | 4 (5) | 4 (5) | 4 (5) | 12 (5) |
| Normal (18.6–24.9) | 26 (35) | 38 (51) | 35 (47) | 99 (44) |
| Overweight (25–29) | 27 (36) | 16 (21) | 25 (33) | 68 (30) |
| Obese (30+) | 18 (24) | 17 (23) | 11 (15) | 46 (20) |
| Years using current implant, mean (SD) | 2.1 (1.05) | 2.2 (0.99) | 2.2 (1.02) | 2.2 (1.02) |
| Reasons for seeking implant removal† | ||||
| Desire pregnancy | 19 (25) | 17 (23) | 18 (24) | 54 (24) |
| Product expiration | 34 (45) | 34 (45) | 37 (49) | 105 (47) |
| Side effects | 21 (28) | 21 (28) | 18 (24) | 60 (27) |
| In natural state, are contours of implant visible under skin? | ||||
| Yes | 73 (97) | 75 (100) | 74 (99) | 222 (99) |
| Subcutaneous fat in arm | ||||
| Below normal | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Normal | 69 (92) | 69 (92) | 69 (92) | 207 (92) |
| Above normal | 6 (8) | 6 (8) | 6 (8) | 18 (8) |
| Total | 75 | 75 | 75 | 225 |
| Muscularity of arm | ||||
| Below normal | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Normal | 70 (93) | 69 (92) | 69 (92) | 208 (92) |
| Above normal | 5 (7) | 6 (8) | 6 (8) | 17 (8) |
| Total | 75 | 75 | 75 | 225 |
| Arm circumference, mean (SD) | 30.3 (4.18) | 29.6 (3.66) | 29.7 (3.33) | 29.9 (3.74) |
| Evidence of past trauma at insertion site | ||||
| None | 41 (55) | 39 (52) | 45 (60) | 125 (56) |
| Visible healed trauma normal scar | 34 (45) | 36 (48) | 29 (39) | 99 (44) |
| Visible healed trauma larger than usual scar | 0 (0) | 0 (0) | 1 (1) | 1 (0) |
Data are n (%) unless stated otherwise.
Participants were analysed according to the treatment they were initially randomized into (Intent to Treat Population).
Multiple reasons allowed.
No primary complications occurred, and only three (3) secondary complications occurred, distributed evenly in the three groups (1%) (Table II). RemovAid™ was found to be non-inferior to the standard procedure in terms of safety; the exact upper bound of the 95% CI for the difference in secondary complications was 3.9%, which was below the protocol’s pre-specified margin of 10% to claim non-inferiority. Non-safety-related complications did not vary by group either, however, the components of this outcome did. For example, of the 150 RemovAid™ participants, 14 (9%) experienced implant breakage during the removal procedure, compared to zero in the standard technique. At the follow-up visit, 7% of standard technique participants’ wounds did not heal completely, compared to <1% of RemovAid™ participants (P = 0.010). No serious adverse events occurred during the trial.
Table II.
Implant removal complications.*
| Characteristics | Standard procedure (N = 75) | RemovAid™ with injection (N = 77) | RemovAid™ with patch (N = 73) | Total (N = 225) | P-value |
|---|---|---|---|---|---|
| Any primary complications?† | |||||
| No | 70 (100) | 73 (100) | 72 (100) | 215 (100) | — |
| Yes | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Any secondary complications?‡ | |||||
| No | 69 (99) | 72 (99) | 71 (99) | 212 (99) | 1.0 |
| Yes | 1 (1) | 1 (1) | 1 (1) | 3 (1) | |
| Total§ | 70 | 73 | 72 | 215 | |
| Unusual level of trauma to the skin bruising/hematoma | |||||
| No | 74 (99) | 76 (99) | 73 (100) | 223 (99) | 1.0 |
| Yes | 1 (1) | 1 (1) | 0 (0) | 2 (1) | |
| Total | 75 | 77 | 73 | 225 | |
| Wound needed additional treatment (at follow-up visit) | |||||
| No | 70 (100) | 73 (100) | 71 (99) | 214 (99) | 0.66 |
| Yes | 0 (0) | 0 (0) | 1 (1) | 1 (1) | |
| Total§ | 70 | 73 | 72 | 215 | |
| Wound needed additional treatment (a subset of follow-up visits that occurred 14 days (±4d) after implant removal) | |||||
| No | 38 (100) | 48 (100) | 50 (98) | 136 (99) | 0.43 |
| Yes | 0 (0) | 0 (0) | 1 (2) | 1 (1) | |
| Total§ | 38 | 48 | 51 | 137 | |
| Any non-safety complications?¶ | |||||
| No | 65 (93) | 66 (89) | 66 (92) | 197 (91) | 0.79 |
| Yes | 5 (7) | 8 (11) | 6 (8) | 19 (9) | |
| Total** | 70 | 74 | 72 | 216 | |
| Implant breakage from incision | |||||
| No | 75 (100) | 75 (97) | 70 (96) | 220 (98) | 0.25 |
| Yes | 0 (0) | 2 (3) | 3 (4) | 5 (2) | |
| Total | 75 | 77 | 73 | 225 | |
| Implant newly broken during extraction | |||||
| No | 75 (100) | 69 (92) | 67 (96) | 211 (96) | 0.036 |
| Yes | 0 (0) | 6 (8) | 3 (4) | 9 (4) | |
| Total | 75 | 75 | 70 | 220 | |
| Wound still healing, no treatment needed (at follow-up visit) | |||||
| No | 65 (93) | 73 (100) | 71 (99) | 209 (97) | 0.010 |
| Yes | 5 (7) | 0 (0) | 1 (1) | 6 (3) | |
| Total§ | 70 | 73 | 72 | 215 | |
| Wound still healing, no treatment needed (a subset of follow-up visits that occurred 14 days (±4d) after implant removal) | |||||
| No | 34 (89) | 48 (100) | 50 (98) | 132 (96) | 0.03 |
| Yes | 4 (11) | 0 (0) | 1 (2) | 5 (4) | |
| Total§ | 38 | 48 | 51 | 137 |
Data are n (%).
Two participants who received the incorrect implant removal procedure are included and analysed according to the treatment they actually received (Treated Population).
Primary complications included any of the following: inability to remove the implant (after reasonable attempts with any available equipment), probable or confirmed nerve damage, excessive bleeding during removal procedure, use of sutures to repair skin after removal, excessive post-removal bleeding and subsequent infection (sepsis).
Secondary complications included any of the following: unusual level of trauma to the skin (bruising/hematoma), wound still healing at time of 2-week visit and needing additional treatment, implant breakage or severing at time of removal.
These totals show everyone in the analyses who had a follow-up visit (n = 215). Ten subjects did not have a follow-up visit: standard procedure (5), RemovAid™ + injection (4) and RemovAid™ + patch (1).
Non-safety complications included any of the following: Implant breakage from incision, implant newly broken during extraction step, wound still healing at 2 weeks, but not requiring additional treatment.
One participant with implant breakage during extraction did not return for the 2-week follow-up visit, but is included in these totals.
Note: Fisher’s exact test for all P values.
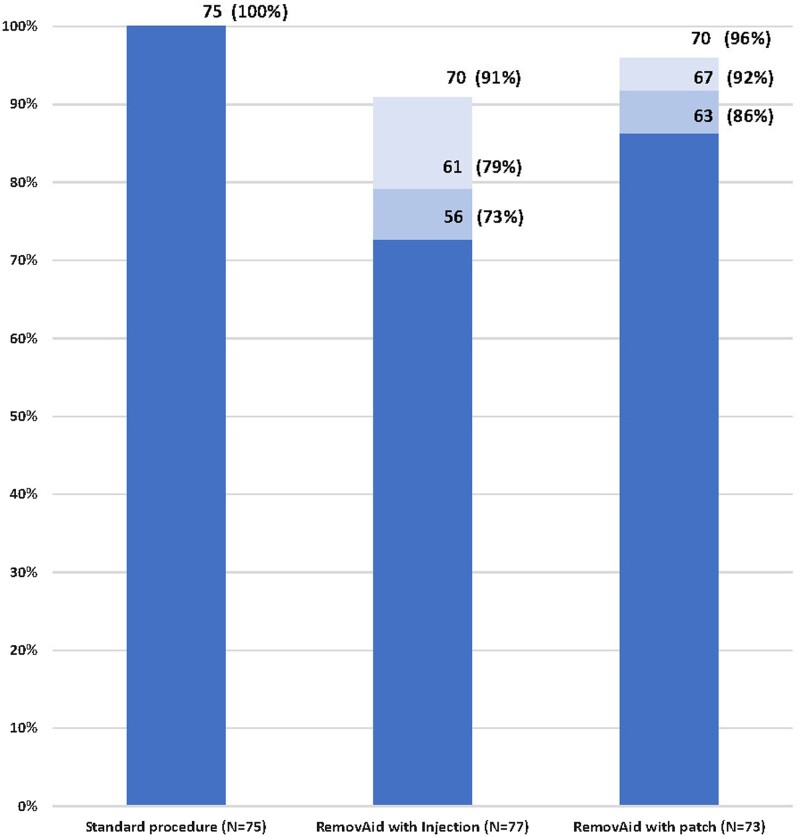
For primary efficacy, implant removal success was 100% (standard technique), 73% (RemovAid™ with lidocaine injection) and 86% (RemovAid™ with lidocaine patch) (P < 0.0001) (Fig. 3 and Table III). For secondary efficacy, implant removal success was 100% (standard technique), 79% (RemovAid™ with lidocaine injection) and 92% (RemovAid™ with lidocaine patch) (P < 0.0001). Secondary efficacy of RemovAid™ was higher than the primary measure due to allowing implant breakage. In the tertiary measure of efficacy, RemovAid™ improved to 91% with lidocaine injection and 96% with lidocaine patch; still, overall differences across the three techniques were statistically significant (P = 0.017). Within both primary and tertiary efficacy measures, RemovAid™ with lidocaine injection was statistically equivalent to RemovAid™ with lidocaine patch; combining results for all 150 RemovAid™ procedures yielded a primary efficacy of 79% (95% CI: 71.2–84.9%) and a tertiary efficacy of 93% (95% CI: 88.1–96.8%).
Figure 3.
Efficacy of implant removal procedures.  Primary efficacy (most stringent definition): Implant successfully removed without implant breakage, and additionally for device procedures, without scalpel or forceps, P < 0.0001
Primary efficacy (most stringent definition): Implant successfully removed without implant breakage, and additionally for device procedures, without scalpel or forceps, P < 0.0001  Secondary efficacy: Implant successfully removed yet allowing implant breakage, and additionally for device procedures, without scalpel or forceps, P < 0.0001
Secondary efficacy: Implant successfully removed yet allowing implant breakage, and additionally for device procedures, without scalpel or forceps, P < 0.0001  Tertiary efficacy: Implant successfully removed yet allowing implant breakage, and additionally for device procedures, without the use of a scalpel, P = 0.017. Note: the standard procedure was 100% effective on all three efficacy measures.
Tertiary efficacy: Implant successfully removed yet allowing implant breakage, and additionally for device procedures, without the use of a scalpel, P = 0.017. Note: the standard procedure was 100% effective on all three efficacy measures.
Table III.
Efficacy of implant removal procedure.*
| Characteristics | Standard procedure (N = 75) | RemovAid™ with injection (N = 77) | RemovAid™ with patch (N = 73) | Total (N = 225) | P-value† |
|---|---|---|---|---|---|
| Primary efficacy | |||||
| Failure | 0 (0) | 21 (27) | 10 (14) | 31 (14) | <0.0001 |
| Success | 75 (100) | 56 (73) | 63 (86) | 194 (86) | |
| Total | 75 | 77 | 73 | 225 | |
|
|||||
| Secondary efficacy | |||||
| Failure | 0 (0) | 16 (21) | 6 (8) | 22 (10) | <0.0001 |
| Success | 75 (100) | 61 (79) | 67 (92) | 203 (90) | |
| Total | 75 | 77 | 73 | 225 | |
| Tertiary efficacy | |||||
| Failure | 0 (0) | 7 (9) | 3 (4) | 10 (4) | 0.017 |
| Success | 75 (100) | 70 (91) | 70 (96) | 215 (96) | |
| Total | 75 | 77 | 73 | 225 | |
|
|||||
Data are n (%).
Primary efficacy: Implant removed without breakage for all procedures, and additionally for RemovAid™ procedures, without additional tools (besides fingers).
Secondary efficacy: Implant removed for all procedures, and additionally for RemovAid™ procedures, with no additional tools (besides fingers).
Tertiary efficacy: Implant removed for all procedures, and additionally for RemovAid™ procedures, without the use of a scalpel.
Two participants who received the incorrect implant removal procedure are included and analysed according to the treatment they actually received (Treated Population).
Fisher’s exact test for categorical variables.
Combined efficacy due to statistical equivalence of components.
The combined 31 failures on primary efficacy among 150 RemovAid™ attempts (Table IV) were due to implant breakage from incision (5) and/or breakage of implants into two pieces during the extraction step (9). In addition, the following reasons caused failure (some failures had more than one reason): inability to clamp around the implant (2, data not shown), unsuccessful incision (6, data not shown), pincher failed to lock around implant (2, data not shown), failure to maintain grip on implant (11) and use of any additional tools (22). The breakdown of additional tools used with RemovAid™ included scalpel (10) and/or use of forceps (18). Of note, none of the standard technique removals resulted in implant breakage.
Table IV.
Analysis of 31 RemovAid™ failures on primary efficacy.*
| Characteristics | RemovAid™ with injection (N = 21) | RemovAid™ with patch (N = 10) | Total (N = 31) | P-value† |
|---|---|---|---|---|
| Did the device malfunction? | ||||
| No | 9 (43) | 4 (40) | 13 (42) | 1.0 |
| Yes | 12 (57) | 6 (60) | 18 (58) | |
| Total | 21 | 10 | 31 | |
| Was implant cut into 2+ separate pieces during the incision? | ||||
| No | 19 (90) | 7 (70) | 26 (84) | 0.30 |
| Yes | 2 (10) | 3 (30) | 5 (16) | |
| Total | 21 | 10 | 31 | |
| Did implant newly break into 2+ pieces during the extraction?‡ | ||||
| No | 13 (68) | 4 (57) | 17 (65) | 0.66 |
| Yes | 6 (32) | 3 (43) | 9 (35) | |
| Total | 19 | 7 | 26 | |
| Was implant partially cut, not sliced all the way through? | ||||
| No | 20 (95) | 9 (90) | 29 (94) | 1.0 |
| Yes | 1 (5) | 1 (10) | 2 (6) | |
| Total | 21 | 10 | 31 | |
| Were additional tools required to extract the implant? | ||||
| No | 5 (24) | 4 (40) | 9 (29) | 0.42 |
| Yes | 16 (76) | 6 (60) | 22 (71) | |
| Total | 21 | 10 | 31 | |
| For procedures that needed additional tools: | ||||
| Was a scalpel used? | ||||
| No | 9 (56) | 3 (50) | 12 (55) | 1.0 |
| Yes | 7 (44) | 3 (50) | 10 (45) | |
| Total | 16 | 6 | 22 | |
| Were forceps used? | ||||
| No | 4 (25) | 0 (0) | 4 (18) | 0.54 |
| Yes | 12 (75) | 6 (100) | 18 (82) | |
| Total | 16 | 6 | 22 | |
| Were other tools used? | ||||
| No | 16 (100) | 6 (100) | 22 (100) | — |
| Yes | 0 (0) | 0 (0) | 0 (0) | |
| Total | 16 | 6 | 22 | |
| Did the device remain affixed for the entire procedure? | ||||
| No | 7 (58) | 4 (44) | 11 (52) | 0.67 |
| Yes | 5 (42) | 5 (56) | 10 (48) | |
| Total | 12 | 9 | 21 |
Data are n (%).
Treated Population.
Fisher’s exact test for categorical variables.
Among intact implants after incision.
Mean pain levels were low for lidocaine applications and for the implant removal procedures (Table V). For the lidocaine applications, mean pain levels were 2.1 (standard procedure), 1.9 (RemovAid™ with injection) and 0.1 (RemovAid™ with patch). As expected, pain from application of the lidocaine patch was significantly lower than pain due to lidocaine injection (P < 0.0001).
Table V.
Mean level of pain from removal procedure,* duration of procedures and participant/provider feedback on device.†
| Characteristics | Standard procedure (N = 75) | RemovAid™ with injection (N = 77) | RemovAid™ with patch (N = 73) | Total (N = 225) | P-value‡ |
|---|---|---|---|---|---|
| Pain from procedures | |||||
| Pain from lidocaine in cm: mean (SD) | 2.1 (1.56) | 1.9 (1.37) | 0.1 (0.37) | 1.4 (1.51) | <0.0001 |
| Pain from implant removal in cm: mean (SD) | 0.8 (1.36) | 1.3 (1.86) | 1.6 (2.51) | 1.2 (1.98) | 0.11 |
| Total | 75 | 77 | 73 | 225 | |
| Duration of procedures | |||||
| Time to first incision in minutes: mean (SD) | 0.4 (0.38) | 0.9 (1.27) | 0.7 (0.77) | 0.7 (0.92) | <0.0001 |
| Time to extraction in minutes: mean (SD) | 1.1 (1.01) | 2.4 (2.01) | 1.7 (1.34) | 1.7 (1.59) | <0.0001 |
| Time to bandage in minutes: mean (SD) | 1.7 (1.33) | 3.0 (2.16) | 2.4 (1.52) | 2.3 (1.79) | <0.0001 |
| Total | 75 | 77 | 73 | 225 | |
|
| |||||
| Participant feedback | |||||
|
| |||||
| I would recommend this procedure to a friend/relative | |||||
| Strongly disagree | 0 (0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.84 |
| Disagree | 1 (1) | 1 (1) | 2 (3) | 4 (2) | |
| Neutral | 3 (4) | 1 (1) | 2 (3) | 6 (3) | |
| Agree | 28 (37) | 34 (44) | 25 (34) | 87 (39) | |
| Strongly agree | 43 (57) | 41 (53) | 44 (60) | 128 (57) | |
| Total | 75 | 77 | 73 | 225 | |
| How satisfied were you with the procedure? | |||||
| Very dissatisfied | 1 (1) | 0 (0) | 0 (0) | 1 (0) | 0.88 |
| Dissatisfied | 0 (0) | 1 (1) | 2 (3) | 3 (1) | |
| Neutral | 3 (4) | 2 (3) | 1 (1) | 6 (3) | |
| Satisfied | 30 (40) | 31 (40) | 28 (38) | 89 (40) | |
| Very satisfied | 41 (55) | 43 (56) | 42 (58) | 126 (56) | |
| Total | 75 | 77 | 73 | 225 | |
| If needed in future, clinician should use this method | |||||
| Strongly disagree | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.51 |
| Disagree | 1 (1) | 0 (0) | 2 (3) | 3 (1) | |
| Neutral | 3 (4) | 3 (4) | 1 (1) | 7 (3) | |
| Agree | 29 (39) | 29 (38) | 21 (29) | 79 (35) | |
| Strongly agree | 42 (56) | 45 (58) | 49 (67) | 136 (60) | |
| Total | 75 | 77 | 73 | 225 | |
|
| |||||
| Provider feedback | |||||
|
| |||||
| Provider considers RemovAid an acceptable alternative | |||||
| Strongly disagree | 0 (0) | 0 (0) | 0 (0) | 0.064 | |
| Disagree | 0 (0) | 0 (0) | 0 (0) | ||
| Neutral | 6 (8) | 4 (5) | 10 (7) | ||
| Agree | 21 (28) | 10 (14) | 31 (21) | ||
| Strongly agree | 49 (64) | 59 (81) | 108 (72) | ||
| Total | 76 | 73 | 149 | ||
Data are n (%) unless stated otherwise.
Using visual analog scale (0 = no pain to 10 = worst pain imaginable).
Two participants who received the incorrect implant removal procedure are included and analysed according to the treatment they actually received (Treated Population).
Wilcoxon test for continuous variables.
Pain levels from the implant removal step appeared to vary by procedure; mean pain levels were 0.8 (standard procedure), 1.3 (RemovAid™ with injection) and 1.6 (RemovAid™ with patch). However, these differences were not statistically significant (P = 0.11).
Standard removal procedures took less time than removals with RemovAid™. The mean duration to placement of bandage was 1.7 min for the standard procedure, 3.0 min for RemovAid™ with lidocaine injection and 2.4 min for RemovAid™ with lidocaine patch.
Participants who received the RemovAid™ procedure had levels of satisfaction (about 95%) that were equivalent to those who received the standard procedure; also, about 95% stated they would recommend RemovAid™ to friends/relatives and that clinicians should use it in the future. Finally, in over 90% of the 150 RemovAid™ procedures, providers agreed or strongly agreed that RemovAid™ is an acceptable alternative to standard removal technique.
Discussion
In terms of safety, RemovAid™ was found to be non-inferior compared to standard technique; on efficacy, RemovAid™ performed well, but did not achieve a 100% scalpel-free removal rate. All implants were removed during the initial visit in all groups, yet 7% (10/150) of RemovAid™ procedures required a scalpel.
The overall estimated efficacy of RemovAid™ was confounded by type of lidocaine used. The injection of lidocaine lowered successful implant removal compared to the lidocaine patch. Some removal failures with injection were likely attributable to volume of lidocaine injected, which caused swelling around the implant and made it more difficult for RemovAid™ to fixate the implant and sustain a grip on the implant during the extraction step. Because RemovAid™ is a mechanical device, operator skill certainly played a role in successful use. The positioning and angle of RemovAid™ during extraction (relative to the plane of the arm’s surface), likely affects efficacy. Operator skills are a key factor in successful implant removal (Fraser, 2006). In traditional scalpel removals, it is possible that subdermal injection of lidocaine beneath the implant helps ‘push up’ the implant for easier extraction.
Pain from the complete procedure involves both pain from the lidocaine application (2 ml in the control group, 1 ml in the RemovAid™ with injection group) and pain from the implant extraction. We observed an important trade-off in the use of the lidocaine patch versus the lidocaine injection: the injection caused significantly more pain than the patch but was better though, not significantly, at reducing pain during the implant extraction stage. This observation warrants more investigation, partly because clinicians were inconsistent in the amount of time they kept the patch on the skin before starting the removal procedure. The amount of lidocaine that is systemically absorbed from the patch is directly related to the duration of application (Astra USA, Inc, 2000).
In terms of future scale-up, the combination approach of lidocaine patch with RemovAid™ could avert other problems. For example, mishandling of scalpels can cause sharps injuries to implant users and clinicians (Brewer, 2003; Laumonerie et al., 2018). Also, mishaps from injection needles are quite common worldwide (Liyew et al., 2020). Moreover, safe disposal challenges for sharps materials are a perennial problem in sub-Saharan Africa and increase the risk of transmission of infectious diseases (Mengiste et al., 2021). While disposal requirements for RemovAid™ are not averted, the blade is not exposed like that of a scalpel and is not easily used for other purposes; in addition, a reusable product for sterilization is theoretically feasible, but would need to be investigated in a future study. Implant users may prefer ‘no pain’ with lidocaine patch application, even if it means applying the patch 60+ min before the procedure while waiting for services; in Sweden, women often apply the patch before arriving at the clinic. On the other hand, waiting 60+ min may not be possible for walk-in clinic attendees. In our study, 12% of potential participants could not enroll since their implant was placed too deep; this will limit full potential of the product and implant users would need to be referred to an appropriate clinic as usual.
The version of the RemovAid™ product used in Uganda performed better than the previous versions used in pilot studies in Sweden (Iwarsson et al., 2020) (note: the secondary efficacy definition in Uganda is comparable to the definition used in the Swedish study). In that previous trial, the device successfully removed the implant 58.5% of the time. The 41.5% failure was attributable to clamping problems (14.6%) and incision/extraction problems (26.8%). The comparable figures in Uganda were a 15% failure rate (22/150), which was attributable to clamping problems (1%) and incision/extraction problems (13%). After the first Swedish study, it was determined that the device’s fixation platform needed modifications to better grip the implant. In a subsequent modification, the built-in scalpel was shortened by 0.5 mm to reduce the chances of severing the implant yet still be sufficient to incise the skin correctly.
RemovAid™ is designed to be easy to use, easy to teach and easy to scale. Moreover, it is a one-size-fits-all device (i.e. no need for individual adjustment). This design feature, largely obviates the need for other tools, including sharps. The complex interplay between user (operator), device mechanisms, and client characteristics (including subdermal implant characteristics) might limit the ability of any device to perform flawlessly all the time. Frequency of implant breakage could potentially be reduced with product refinement and/or increased clinical experience. However, even standard removals, as described by the implant’s manufacturer, require alternative approaches in certain circumstances.
The current RemovAid™ product is designed to remove only a one-rod implant. In the 2016–2020 period, international donor agencies collaborated with over 90 recipient countries to procure ∼40 million implants2; ∼49% of implants were one-rod products (Reproductive Health Supplies Coalition, 2021). RemovAid™ may be particularly useful in countries with a high proportion of one-rod products and additionally, where lower-level cadres are trained to insert them (e.g. Ethiopia).
Implant removals in our study were performed by experienced nurses. It is unknown whether properly trained lower-level health workers can match a 92% no-tools success rate of removing implants with lidocaine patch and RemovAid™. Certainly, the lidocaine patch with RemovAid™ would be safer for implant users and lower-level workers, compared to lower-level workers using lidocaine injection and scalpel. In situations where a lower-level provider failed to remove an implant, the arm would need to be bandaged as usual, and referred to experienced clinicians. In our study, we observed that the wound from RemovAid™ was minimal and thus amenable to temporary bandaging; only 1 of 145 RemovAid™ procedures did not heal completely in 2 weeks, compared to 5 of 70 scalpel procedures. Lower-level providers would need proper training in how to use forceps and/or their gloved fingertips to remove severed implants left behind in the extraction step; temporary bandages and referral to a facility might be required in some situations. Additional research is needed to corroborate removal efficacy in the hands of lower-level providers.
The one-rod contraceptive implant with its easy-to-use trocar system was designed to place implants just under the subdermal fascia for simple removals. Eighty-eight percent of our study population indeed had properly placed implants (completely palpable and pinchable) and were thus eligible for participation. This high percentage of properly inserted implants is a testament to good training and perhaps to the latest insertion technology (being used worldwide by 2016 for one-rod implants) that was designed to prevent deep implant insertions. The insertion technology enables lower-level health workers in many regions of the world to provide services; an easy-to-use removal device can do the same. These paired technologies can help expand access to long-acting reversible contraception (Shelton and Burke, 2016) through task sharing (Ouedraogo et al., 2021) to help achieve the ambitious goals of providing family planning information, services and supplies to an additional 120 million women and girls in 69 of the world’s poorest countries (FP2020, 2021).
Acknowledgements
We would like to thank the participants who volunteered to be in the study, the Ugandan research team (Nakazibwe Rehema, Ssentongo Gertrude, Namuli Lillian Muwonge, Ayo Dorcas and Wasswa Damien), Emily Mayaka of FHI Clinical, Kirunda Ramadhan and Alissa Bernholc of FHI360 and Valerie Robertson of RemovAid AS.
Authors’ roles
D.H., J.B., M.B. and K.G.-D. conceived and designed the study. J.B., O.K., H.N., K.G.-D. and D.H. oversaw implementation. D.H., J.B. and K.G.-D. oversaw the analysis, and write-up. K.E.I. led the training and participated in design of study tools and writing. P.-L.C. generated the random allocation and did the statistical analysis. J.B., H.N., D.H. and K.G.-D. all verified the data. Data were accessible to D.H., J.B., K.G.-D. and P.-L.C. after data freezing. All authors read and approved the manuscript.
Funding
Funding for this study was provided by the RemovAid AS of Norway with grants from Research Council of Norway (GLOBVAC number 228319) and Bill & Melinda Gates Foundation (grant INV-007571).
Conflict of interest
M.B. is founder and former CEO of RemovAid AS, Norway. M.B. holds contraceptive rod remover patents (2012 1307156.8 and 2015), pre-removal test (filed) and shares in RemovAid AS. All of the remaining authors’ institutions received payments in the form of contracts to help conduct the study; the funds for these contracts emanated from RemovAid AS.
Contributor Information
David Hubacher, FHI 360, Durham, NC, USA.
Josaphat Byamugisha, Department of Obstetrics and Gynaecology, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
Othman Kakaire, Department of Obstetrics and Gynaecology, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
Hadija Nalubwama, Department of Obstetrics and Gynaecology, School of Medicine, College of Health Sciences, Makerere University, Kampala, Uganda.
Karin Emtell Iwarsson, Department of Women’s and Children’s Health, Karolinska Institutet, Stockholm, Sweden; Division of Gynaecology and Reproductive Medicine, Karolinska University Hospital, Stockholm, Sweden.
Marte Bratlie, RemovAid AS, Norway.
Pai-Lien Chen, FHI 360, Durham, NC, USA.
Kristina Gemzell-Danielsson, Department of Women’s and Children’s Health, Karolinska Institutet, Stockholm, Sweden; Division of Gynaecology and Reproductive Medicine, Karolinska University Hospital, Stockholm, Sweden.
Data Availability
Metadata will be available for public access without application. De-identified individual participant data and a data dictionary defining each raw data variable will be made available free of charge to any research institute and/or university through application to the Norwegian Center for Research Data (www.nsd.no). The datasets in excel format, the study protocol, statistical analysis plan and informed consent form will be made available with publication.
References
- Ahmed S, Choi Y, Rimon JG, Alzouma S, Gichangi P, Guiella G, Kayembe P, Kibira SP, Makumbi F, OlaOlorun F. et al. Trends in contraceptive prevalence rates in sub-Saharan Africa since the 2012 London Summit on Family Planning: results from repeated cross-sectional surveys. Lancet Glob Health 2019;7:e904–e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astra USA, Inc. EMLA Anesthetic Disc US. Silver Spring, MD USA: Food and Drug Administration, 2000. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/19941s11lbl.pdf (6 August 2021, date last accessed). [Google Scholar]
- Bahamondes L, Peloggia A.. Modern contraceptives in sub-Saharan African countries. Lancet Glob Health 2019;7:e819–e820. [DOI] [PubMed] [Google Scholar]
- Braun R, Grever A.. Scaling up access to implants: a summative evaluation of the implants access program. Glob Health Sci Pract 2020;8:205–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer S. Risks and effects of sharps injuries. Nurs Times 2003;99:46. [PubMed] [Google Scholar]
- Callahan R, Lebetkin E, Brennan C, Kuffour E, Boateng A, Tagoe S, Coolen A, Chen M, Aboagye P, Brunie A. et al. What goes in must come out: a mixed-method study of access to contraceptive implant removal services in Ghana. Glob Health Sci Pract 2020;8:220–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charyeva Z, Oguntunde O, Orobaton N, Otolorin E, Inuwa F, Alalade O, Abegunde D, Danladi S.. Task shifting provision of contraceptive implants to community health extension workers: results of operations research in Northern Nigeria. Glob Health Sci Pract 2015;3:382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costenbader E, Cartwright AF, McDowell M, Assefa B, Tejeji MY, Tenaw E.. Factors associated with delayed contraceptive implant removal in Ethiopia. Glob Health Sci Pract 2020;8:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creinin MD, Kaunitz AM, Darney PD, Schwartz L, Hampton T, Gordon K, Rekers H.. The US etonogestrel implant mandatory clinical training and active monitoring programs: 6-year experience. Contraception 2017;95:205–210. [DOI] [PubMed] [Google Scholar]
- FP2020. Building FP2030: A Collective Vision for Family Planning Post-2020. 2021. https://familyplanning2020.org/Building2030 (6 August 2021, date last accessed).
- Fraser IS. The challenges of location and removal of Implanon contraceptive implants. J Fam Plann Reprod Health Care 2006;32:151–152. [DOI] [PubMed] [Google Scholar]
- Hernandez JH, Akilimali P, Glover A, Bertrand JT.. Feasibility and acceptability of using medical and nursing students to provide Implanon NXT at the community level in Kinshasa, Democratic Republic of Congo. BMC Womens Health 2020;20:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwarsson KE, Sakaria A, Bratlie M, Hubacher D, Gemzell-Danielsson K.. Hand-held device to remove a single-rod subdermal contraceptive implant: results of early trials in Sweden. Contraception 2020;102:424–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobstein R. Liftoff: the blossoming of contraceptive implant use in Africa. Glob Health Sci Pract 2018;6:17–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumonerie P, Blasco L, Tibbo ME, Leclair O, Kerezoudis P, Chantalat E, Mansat P.. Peripheral nerve injury associated with a subdermal contraceptive implant: illustrative cases and systematic review of literature. World Neurosurg 2018;111:317–325. [DOI] [PubMed] [Google Scholar]
- Levine JP, Sinofsky FE, Christ MF; Implanon US Study Group. Assessment of Implanon insertion and removal. Contraception 2008;78:409–417. [DOI] [PubMed] [Google Scholar]
- Liyew B, Sultan M, Michael M, Tilahun AD, Kassew T.. Magnitude and determinants of needlestick and sharp injuries among nurses working in Tikur Anbessa Specialized Hospital, Addis Ababa, Ethiopia. Biomed Res Int 2020;2020:6295841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengiste DA, Dirbsa AT, Ayele BH, Hailegiyorgis TT.. Hepatitis B virus infection and its associated factors among medical waste collectors at public health facilities in eastern Ethiopia: a facility-based cross-sectional study. BMC Infect Dis 2021;21:233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck Sharp & Dohme Ltd. Package Leaflet: Information for the User. Nexplanon® 68 mg Implant for Subdermal Use. 2020. https://www.medicines.org.uk/emc/files/pil.5720.pdf (14 February 2020, date last accessed).
- Ouedraogo L, Habonimana D, Nkurunziza T, Chilanga A, Hayfa E, Fatim T, Kidula N, Conombo G, Muriithi A, Onyiah P et al Towards achieving the family planning targets in the African region: a rapid review of task sharing policies. Reprod Health 2021;18:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Population Reference Bureau. Family Planning Worldwide 2019 Data Sheet. 2019. https://www.prb.org/2019-family-planning-data-sheet-highlights-family-planning-method-use-around-the-world/ (2 November 2019, date last accessed).
- Reproductive Health Supplies Coalition. Family Planning Procurement Database. 2021. https://www.rhsupplies.org/activities-resources/tools/rh-viz/tool/#c11039 (23 July 2021, date last accessed).
- Shelton JD, Burke AE.. Effective LARC providers: moving beyond training. Glob Health Sci Pract 2016;4:356–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriwatanakul K, Kelvie W, Lasagna L, Calimlim JF, Weis OF, Mehta G.. Studies with different types of visual analog scales for measurement of pain. Clin Pharmacol Ther 1983;34:234–239. [DOI] [PubMed] [Google Scholar]
- Stoddard A, McNicholas C, Peipert JF.. Efficacy and safety of long-acting reversible contraception. Drugs 2011;71:969–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilahun Y, Lew C, Belayihun B, Lulu Hagos K, Asnake M.. Improving contraceptive access, use, and method mix by task sharing Implanon insertion to frontline health workers: the experience of the Integrated Family Health Program in Ethiopia. Glob Health Sci Pract 2017;5:592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations. World Contraceptive Patterns 2013, Department of Economic and Social Affairs, Population Division. 2013. http://www.un.org/en/development/desa/population/publications/family/contraceptive-wallchart-2013.shtml (10 January 2018, date last accessed).
- World Health Organization. Task Sharing to Improve Access to Family Planning/Contraception. 2017. https://www.who.int/reproductivehealth/publications/task-sharing-access-fp-contraception/en/ (8 June 2020, date last accessed).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Metadata will be available for public access without application. De-identified individual participant data and a data dictionary defining each raw data variable will be made available free of charge to any research institute and/or university through application to the Norwegian Center for Research Data (www.nsd.no). The datasets in excel format, the study protocol, statistical analysis plan and informed consent form will be made available with publication.