Abstract
STUDY QUESTION
Does supplementation with vaginal tablets of progesterone after frozen-thawed embryo transfer in natural cycles improve the live birth rate?
SUMMARY ANSWER
Supplementation with vaginal tablets of progesterone after frozen-thawed embryo transfer in natural cycles significantly improves the number of live births.
WHAT IS KNOWN ALREADY
Progesterone supplementation during luteal phase and early pregnancy may improve the number of live births after frozen-thawed embryo transfer. However, due to the limited number of previous studies, being mainly retrospective, evidence is still limited.
STUDY DESIGN, SIZE, DURATION
This is a prospective randomized controlled trial, performed at two university clinics. In total, 500 subjects were randomized with a 1:1 allocation into two groups, during the period February 2013 to March 2018. Randomization was performed after a frozen embryo transfer in a natural cycle by use of opaque sealed envelopes. The primary outcome was live birth rate; secondary outcomes were pregnancy, biochemical pregnancy, clinical pregnancy and miscarriage rate, and if there was a possible association between the serum progesterone concentration on the day of embryo transfer and live birth rate.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Women, receiving embryo transfer in natural cycles participated in the study. The embryos were frozen on Day 2, 3, 5 or 6. In total, 672 women having regular menstrual cycles were invited to participate in the study; of those, 500 agreed to participate and 488 were finally included in the study. Half of the study subjects received progesterone supplementation with progesterone vaginal tablets, 100 mg twice daily, starting from the day of embryo transfer. The other half of the subjects were not given any treatment. Blood samples for serum progesterone measurements were collected from all subjects on the day of embryo transfer.
MAIN RESULTS AND THE ROLE OF CHANCE
There were no differences in background characteristics between the study groups. In the progesterone supplemented group, 83 of 243 patients (34.2%) had a live birth, compared to 59 of 245 patients (24.1%) in the control group (odds ratio 1.635, 95% CI 1.102–2.428, P = 0.017*). The number of pregnancies was 104 of 243 (42.8%) and 83 of 245 (33.9%), respectively (odds ratio 1.465, 95% CI 1.012–2.108, P = 0.049*) and the number of clinical pregnancies was 91 of 243 (37.4%) and 70 of 245 (28.6%), respectively (odds ratio 1.497, 95% CI 1.024–2.188, P = 0.043*). There were no significant differences in biochemical pregnancy rate or miscarriage rate. There was no correlation between outcome and serum progesterone concentration.
LIMITATIONS, REASONS FOR CAUTION
The study was not blinded because placebo tablets were not available. Supplementation started on embryo transfer day, regardless of the age of the embryos, which resulted in a shorter supplementation time for Day 5/6 embryos compared to Day 2/3 embryos.
WIDER IMPLICATIONS OF THE FINDINGS
Supplementation with progesterone in natural cycles improved the number of live births after frozen-thawed embryo transfer and should therefore be considered for introduction in clinical routine.
STUDY FUNDING/COMPETING INTEREST(S)
The study was funded by Uppsala University, the Uppsala-Family Planning Foundation, and Ferring Pharmaceuticals AB, Malmö, Sweden. The authors have no personal conflicting interests to declare.
TRIAL REGISTRATION NUMBER
NL4152.
TRIAL REGISTRATION DATE
5 December 2013.
DATE OF FIRST PATIENT’S ENROLMENT
18 February 2013.
Keywords: frozen embryo transfer, natural cycles, SET, progesterone, supplementation, RCT, live birth, clinical pregnancy, miscarriage, blastocyst
Introduction
Progesterone production from the corpus luteum is crucial in natural menstrual cycles for a number of reasons such as normal development of the endometrium to a receptive phase, optimal embryo implantation and continuation of pregnancy (Csapo et al., 1972, 1973a,b; Gellersen and Brosens, 2014). However, normal levels of serum progesterone levels vary considerably between women (Guerrero et al., 1976), and may only to some extent predict live birth among infertile women (Murto et al., 2013).
Despite regular menstrual cycles and normal serum progesterone levels, some women undergoing fertility treatment might have insufficient progesterone production during their luteal phase (Gaggiotti-Marre et al., 2020). In studies performed in artificial and natural cycles, it has been noticed that low levels of serum progesterone on the day before, on the day of frozen-thawed embryo transfer (FET) or on the day of pregnancy test are associated with decreased number of pregnancies and reduced live birth rates (LBRs; Labarta et al., 2017; Alsbjerg et al., 2018; Gaggiotti-Marre et al., 2019; Gaggiotti-Marre et al., 2020). Consequently, supplementation with vaginal progesterone during the luteal phase and early pregnancy could improve the outcome after FET, which has been suggested by one earlier prospective randomized controlled study (Bjuresten et al., 2011) and two retrospective studies (Veleva et al., 2013; Kim et al., 2014). However, contradictory results have been reported, in one small randomized controlled trial (RCT; Eftekhar et al., 2013) and one large retrospective study (Montagut et al., 2016). In two recent meta-analyses, it was indicated that LBR is improved by progesterone supplementation after frozen embryo transfer in natural cycles (NC-FET), but then again, it was stated that additional large RCTs are needed to confirm the results (Seol et al., 2020; Mizrachi et al., 2021).
There is still no consensus on which regimen is most optimal for endometrial preparation in FET cycles (van der Linden et al., 2015; Ghobara et al., 2017; Groenewoud et al., 2017; Mackens et al., 2017). Therefore, the main objective of this trial was to study if supplementation with vaginal progesterone improves the number of live births after FET in natural cycles. Secondary endpoints were pregnancy rate, biochemical pregnancy rate, clinical pregnancy rate (CPR), miscarriage rate and serum progesterone levels during embryo transfer in relation to live birth.
Materials and methods
Study material
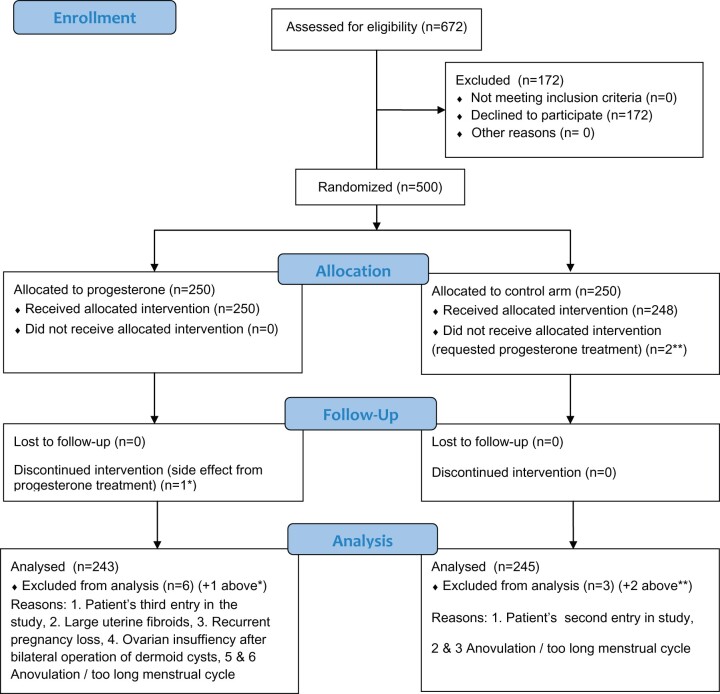
In the present study, 672 infertile women were invited to participate in this RCT. Of these, 500 study entries (74.2%) were included in the study and after additional exclusions 488 women were finally included, all staying in their assigned group throughout the study (Fig. 1). The first 201 women were included at Uppsala University Hospital between 18 February 2013 to 31 October 2014 and the following 299 at Karolinska University Hospital between 17 September 2015 and 14 May 2018. All women were healthy, had regular menstrual cycles and were scheduled for embryo transfer in natural cycles.
Figure 1.
CONSORT 2010 flow diagram.
Exclusion criteria were women who did not want to participate in the study or those who had adverse effects from previous progesterone supplementation. Women undergoing donor egg cycles, preimplantation testing cycles or testicular sperm cycles were also excluded from the study. Twelve subjects were excluded for the following reasons: Two women requested progesterone supplementation, despite being randomized to the control group. One patient discontinued intervention because she experienced side effects (‘felt ill’) from the progesterone treatment. One woman was included in the study three times, which was corrected by only including her first entry in the study sample and excluding the following two entries. Five women were not eligible for inclusion for medical reasons, four because of anovulation or too long menstrual cycles (more than 35 days) and one due to recurrent pregnancy loss. Furthermore, one woman with ovarian insufficiency after bilateral operations of ovarian dermoid cysts and one woman with large uterine fibroids were excluded from the study.
Progesterone supplementation
The included women were randomized into two groups, by use of numbered opaque sealed envelopes stratified in blocks of 10. The allocation sequence was generated by K.W. and the envelopes were distributed to the two study centres before the subjects were included in the study. Opening of envelopes and assignment to study group was performed by a doctor or midwife, after having completed the FET. The women were only allowed to participate in the study once. Half of the women were allocated to progesterone vaginal tablets (Lutinus®, Ferring Pharmaceuticals, Malmö, Sweden), 100 mg twice daily, which is the dose routinely used at the study centres. The other half of the women had no treatment. No additional follicle stimulation or ovulation induction medication was given to any of the patients. Progesterone supplementation was initiated on the day of FET and continued for six full weeks corresponding to 8 weeks of pregnancy. In cases of negative pregnancy test, the treatment ended immediately. In cases of positive pregnancy test, the pregnancies were followed up until the end of pregnancy, regardless of pregnancy outcome. To obtain pregnancy outcome, the patients were instructed to send a report to the fertility clinic after the pregnancy had ended by regular mail, e-mail or telephone. If no report was received, information about the pregnancy outcome was obtained from the patient record at the delivery ward, or by contacting the patient by phone.
Definition of infertility diagnoses
Infertility diagnoses were endometriosis, male factor, tubal factor, unexplained infertility, social indication and other diagnoses. Endometriosis was diagnosed by medical history and/or vaginal ultrasonography or laparoscopy. Male factor infertility was defined as abnormal semen analysis according to the WHO criteria (World Health Organization, 2010). Tubal factor was diagnosed by hystero-salpingo-sonography when uni- or bilateral absence or partial filling of the fallopian tube with contrast was observed. Patients with unexplained infertility had regular menstruations, normal endometrial and ovarian development as determined by use of vaginal ultrasonography, normal tubal patency tested by hystero-salpingo-sonography and a partner with normal semen analysis. The social indication group consisted of healthy same-sex female couples, or in one case a single woman, all with normal menstrual cycles and no signs of gynaecological disorders.
Other diagnoses included 15 women who did not fit into any of the previous diagnoses, i.e. other causes. In detail, four women had a history of fertility preservation prior to cancer treatment (one woman with tongue cancer, one treated for arm osteosarcoma and two for breast cancer), two women were treated because of infectious disease in the male partner (one with Hepatitis B and one with HIV infection) and five women were diagnosed with polycystic ovaries, but still with regular ovulatory cycles. One patient had a double uterus, and one a unicorn uterus. One patient was successfully operated for uterine septum and one had undergone kidney transplantation.
Embryo transfer
FET was performed after positive morning urinary LH test (Clearblue, SPD, Swiss Precision Diagnostics, Geneva, Switzerland), depending on the day the embryo was frozen. Embryos frozen on Day 2 were transferred 3 days after a positive LH test and Day 3 embryos were transferred 4 days after a positive LH test. Day 5 and 6 embryos were transferred 6 days after a positive LH test. All embryo transfers were ultrasonography-guided. At Uppsala University Hospital, all transfers were performed by specialists in obstetrics and gynaecology with special training in reproductive medicine, while at Karolinska University Hospital, the transfers were either performed by one ESHRE certified midwife trained in reproductive medicine or by a specialist in obstetrics and gynaecology with special training in reproductive medicine. A great majority of the transfers, 476 (97.5%), were single embryo transfers (SET) and only 12 (2.5%) were double embryo transfers (DET). In the progesterone treatment group, 97.9% were SET compared to 97.1% in the control group (Table I).
Table I.
Characteristics of study subjects included in the study (n = 488).
| Descriptive data | Treated (n = 243) | Controls (n = 245) |
|---|---|---|
| Age at embryo transfer (year) | 34.1 (23–42) | 34.1 (22–44) |
| Age at embryo freeze (year) | 32.8 (21–39) | 32.8 (21–42) |
| BMI (kg/m2) | 23.9 ± 3.8 | 23.5 ± 4.1 |
| Serum progesterone (nmol/l) | 37.0 ± 15.6 | 37.4 ± 16.0 |
| Cycle day of positive urinary LH test | 13.4 ± 2.4 | 13.7 ± 2.6 |
| Parity | 1.1 ± 0.8 | 1.0 ± 0.8 |
| Embryo thaw number | 1.9 ± 1.2 | 2.0 ± 1.5 |
| Antral follicle count | 17.0 ± 7.1 | 16.7 ± 7.3 |
| Blastocyst transfer | 154 of 236 (65.2%) | 154 of 242 (63.6%) |
| ICSI | 31 of 237 (13.1%) | 31 of 242 (12.8%) |
| Vitrification | 153 of 234 (65.4%) | 163 of 242 (67.4%) |
| SET | 229 of 234 (97.9%) | 235 of 242 (97.1%) |
Age was not normally distributed, median and range are shown. For normally distributed data, mean and standard deviation are shown. For number of blastocyst transfers, number of ICSI, number of vitrified embryos and single embryo transfers (SET), numbers and percentages are shown. There were no significant differences between the groups.
Embryo scores
All cleavage stage embryos were slow frozen and all blastocysts were vitrified. The embryo quality was assessed before embryo freezing and after the thawing procedure pre-transfer. In cases of too low-quality embryos after thawing, a new embryo was thawed to secure that the embryo used was of good quality or top quality.
Day 2/3 embryo quality was assessed morphologically, by a trained embryologist, based on the number of blastomeres, the fragmentation rate and the multinucleation of blastomeres (Mohr et al., 1985; Ziebe et al., 1997). Each cleavage stage embryo received a grade of 0 (top quality), 1 (good quality), 2 (fair quality) and 3 (poor quality). Embryos with a score of 0, top quality, and 1, good quality, were used in the present study.
Blastocysts were scored according to Gardner et al. (2000), where embryos graded as A were considered top quality, B as median quality and C as low quality. Blastocysts with score A or B were used in the present study.
Blood samples
To evaluate the potential association between serum progesterone and pregnancy outcome, a blood sample was collected on the day of embryo transfer. Progesterone analysis was performed at the laboratory of clinical chemistry and pharmacology at Uppsala University Hospital, by use of competitive immunometrics and electro-chemical luminescence detection (Roche Cobas e602). Detection limit of the method was 0.636 nmol/l, the within assay coefficient was 2.4% and the between assay coefficient was 3.2%. Normal level of progesterone during the luteal phase is 5.8–76 nmol/l according to the clinical chemistry laboratories at the study centres. Serum progesterone level in fertile women is considered to be at least 32 nmol/l on Day 21 in a normal ovulatory cycle (Landgren et al., 1980). In the present study, the limit was set to 29 nmol/l because the embryo transfer in several cases was performed on cycle Day 18 (Gaggiotti-Marre et al., 2019) and to 10 nmol/l for sub-analysis of extremely low serum progesterone concentration.
Outcomes
The primary outcome was LBR. Secondary outcome measures were pregnancy, biochemical pregnancy, clinical pregnancy and miscarriage rate, and if there was a possible association between the serum progesterone concentration on day of embryo transfer and LBR. Pregnancy was defined as a positive urinary pregnancy test (Unistep hCG, Hangzhou AllTest Biotech Co., Ltd., Hangzhou, China) conducted 18 days after the FET. Clinical pregnancy was defined as a visible gestational sac determined by use of vaginal ultrasonography performed at 7–8 weeks of pregnancy. Biochemical pregnancy was defined as a positive hCG urine measurement and absence of clinical pregnancy. Miscarriage was defined as loss of pregnancy up to 21 + 6 weeks of pregnancy, following an initially positive pregnancy test. Live birth was defined as delivery of a living offspring from 22 + 0 weeks of pregnancy.
Statistics
Statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) software (SPSS 15.0 for Windows; SPSS Inc. Chicago, IL, USA). Sample size calculation was conducted for LBR and was based on the previous randomized controlled study (Bjuresten et al., 2011), where 30% of the progesterone supplemented patients and 20% of the untreated patients had an LBR after NC-FET. With an alpha = 0.05 and power = 0.80, the recommended sample size was 588 study participants.
An interim analysis was performed in December 2014, after the recruitment of 200 subjects. Ongoing pregnancy and LBRs were calculated and compared between the two study groups. The analysis showed a non-significant rise in ongoing pregnancy rate/LBR among progesterone supplemented compared to untreated women (30% compared to 19%, respectively, P = 0.100). A second analysis after 500 individuals had been included revealed that significance was reached. To account for multiple looks (n = 2), the corrected significance level regarding the primary outcome (LBR) was set to P < 0.025. A power calculation of 500 subjects showed a predicted power of 73.4%.
Differences in descriptive data between study groups were analysed by use of Mann–Whitney Rank-Sum test or Student’s T-test. Comparison of study outcomes between the two groups, in the total population as well as after stratification for Day 2/3 and 5/6 embryos, was performed by use of Fisher’s exact test. A P-value <0.05 was considered as statistically significant.
Ethics
The study was approved by the Regional Ethics Review Board in Stockholm (Dnr: 2012/1845-32). All participants received detailed information and gave written consent to participate specifically in this study prior to enrolment. The trial registration number was NL4152.
Results
Study material
There were no differences in background characteristics between controls and treated women (Table I). The distribution of study subjects into different diagnoses is shown in Table II.
Table II.
Diagnoses of the subjects included in the study (n = 488).
| Infertility diagnosis | Treated (n = 243) | Controls (n = 245) |
|---|---|---|
| Endometriosis | 12 (4.9%) | 19 (7.8%) |
| Tubal factor | 12 (4.9%) | 17 (6.9%) |
| Unexplained infertility | 105 (43.2%) | 93 (38.0%) |
| Social indication | 36 (14.8%) | 29 (11.8%) |
| Male factor | 69 (28.4%) | 80 (32.7%) |
| Other diagnoses | 9 (3.7%) | 7 (2.9%) |
Total numbers and percentages are shown. There were no significant differences between the groups.
Pregnancy outcomes
LBR was significantly improved by progesterone supplementation. In the progesterone supplemented group, 83 of 243 patients (34.2%) and in the control group, 59 of 245 patients (24.1%) had a live birth (odds ratio 1.635, 95% CI 1.102–2.428, P = 0.017; Table III). Four patients in the progesterone treatment group and two patients in the control group had twin births following three SET and one DET in the treatment group and two SET in the control group. Both pregnancy rate and CPR were higher in the treatment group compared to the control group but there were no significant differences in biochemical pregnancy rate or miscarriage rate (Table III).
Table III.
Pregnancy outcomes in treated and control groups (n = 488).
| Outcome | Treated | Controls | P-value | OR | 95 % CI |
|---|---|---|---|---|---|
| Pregnancy rate | 104 of 243 (42.8%) | 83 of 245 (33.9%) | 0.049* | 1.465 | 1.012–2.108 |
| Biochemical pregnancy rate | 13 of 104 (12.5 %) | 13 of 83 (15.7%) | 0.671 | 0.769 | 0.336–1.763 |
| Clinical pregnancy rate | 91 of 243 (37.4%) | 70 of 245 (28.6%) | 0.043* | 1.497 | 1.024–2.188 |
| Miscarriage rate | 8 of 91 (8.8%) | 11 of 70 (15.7%) | 0.22 | 0.517 | 0.196–1.364 |
| Live birth rate | 83 of 243 (34.2%) | 59 of 245 (24.1%) | 0.017* | 1.635 | 1.102–2.428 |
Numbers and percentages are shown. Fisher’s exact test, odds ratio (OR) and 95% CI were calculated. P-value <0.05 was considered as statistically significant (*); regarding the primary outcome (LBR), results were significant even after correction for multiple looks.
LBR, live birth rate.
In order to be certain that the excluded subjects did not influence the primary outcome, we performed an intention-to-treat analysis. Data including all 500 subjects showed that 84 of 250 (33.6%) had a live birth in the treatment group compared to 59 of 250 (23.6%) in the control group (P = 0.017). The numbers needed-to-treat was calculated to 10.
There was no significant difference in the number of live births between the two centres, 48 of 193 (24.9%) at the Uppsala University Hospital centre compared to 94 of 295 (31.9%) at the Karolinska University Hospital centre (P = 0.104). However, the Uppsala University Hospital centre had 16.1% blastocyst transfers while the Karolinska University Hospital centre had 93% blastocyst transfers, which did not influence the LBR after Day 2/3 transfer or blastocyst transfer (Supplementary Table SI). There were no significant differences in the LBR after embryo transfer between physicians and the midwife 58 of 241 (24.1%) versus 83 of 259 (32%), P = 0.059.
Embryo data
We did not find any significant differences in pregnancy, clinical pregnancy or LBRs between cleavage stage embryos and blastocysts (Table IV), although there was a tendency towards better results in the blastocyst transfer group (Table IV). However, after dividing the embryos into groups for each single day, higher numbers of live births were seen in the progesterone-supplemented groups for Day 3 and 5 embryos (Table IV). Other characteristics did not vary depending on the age of the embryo (Supplementary Table SII).
Table IV.
Pregnancy outcomes across embryos among controls and treated study subjects.
| Day 2/3 embryos | Treated | Controls | P-value | OR | 95% CI |
|---|---|---|---|---|---|
| Top-quality embryos | 54 of 82 (66%) | 63 of 88 (72%) | 0.508 | 0.765 | 0.399–1.467 |
| Pregnancy | 29 of 82 (35%) | 23 of 88 (26%) | 0.244 | 1.546 | 0.802–2.982 |
| Clinical pregnancy | 28 of 82 (34%) | 20 of 88 (23%) | 0.125 | 1.763 | 0.897–3.466 |
| Live birth | 26 of 82 (32%) | 18 of 88 (21%) | 0.115 | 1.806 | 0.900–3.622 |
| Serum progesterone, no live birth | 17.7 ± 11.5, n = 51 | 24.8 ± 9.5, n = 67 | |||
| Serum progesterone, live birth | 13.0 ± 3.6, n = 26 | 17.3 ± 11.5, n = 19 | |||
| Day 5/6 embryos | |||||
| Top-quality embryos | 33 of 153 (22%) | 33 of 154 (21%) | 1.000 | 1.008 | 0.585–1.738 |
| Pregnancy | 72 of 153 (47%) | 60 of 154 (39%) | 0.167 | 1.393 | 0.885–2.192 |
| Clinical pregnancy | 60 of 153 (39%) | 50 of 154 (32%) | 0.235 | 1.342 | 0.840–2.143 |
| Live birth | 54 of 153 (35%) | 41 of 154 (27%) | 0.110 | 1.503 | 0.923–2.448 |
| Serum progesterone, no live birth | 38.7 ± 12.9, n = 101 | 37.0 ± 15.7, n = 113 | – | ||
| Serum progesterone, live birth | 39.11 ± 17.6, n = 54 | 42.2 ± 15.6, n = 41 | – | ||
| Day 2 embryos, Live birth | 11 of 46 (24%) | 12 of 51 (24%) | 1.000 | 1.021 | 0.400–2.606 |
| Day 3 embryos Live birth | 15 of 38 (40%) | 6 of 37 (16%) | 0.039* | 3.37 | 1.133–10.018 |
| Day 5 embryos Live birth | 43 of 105 (41%) | 27 of 115 (24%) | 0.006* | 2.26 | 1.265–4.040 |
| Day 6 embryos Live birth | 11 of 49 (22%) | 14 of 39 (36%) | 0.234 | 0.517 | 0.202–1.320 |
The embryos used in this study were either good- or top-quality embryos. The number of top-quality embryos as percentage of the total number of embryos is shown. Pregnancy outcomes are presented as numbers and percentages while serum progesterone values are presented as mean values (nmol/l), standard deviation and number of subjects. Fisher’s exact test, odds ratio (OR) and 95% CI were calculated. P-value <0.05 was considered as statistically significant (*).
Progesterone levels
There were no significant differences in the mean values of serum progesterone between controls without (36.2 nmol/l) and with a live birth (40.5 nmol/l, P = 0.409) and progesterone supplemented women without (36.7 nmol/l) and with a live birth (37.6 nmol/l, P = 0.218).
Women in the progesterone supplemented group without a live birth receiving Day 2/3 embryos had a tendency of lower serum progesterone (17.7 nmol/l) compared to control group women without a live birth (24.8 nmol/l; Table IV). As expected, women receiving Day 5/6 embryos had higher serum progesterone levels as the serum samples were taken later in the cycle.
There was no difference in LBR between women in the control group with serum progesterone ≤29 nmol/l, 22 of 90 (24.4%) compared to those who had serum progesterone >29 nmol/l, 36 of 149 (24.2%; P = 1). The same pattern was found in the progesterone supplemented group, with 29 of 86 (33.7%) LBR if serum progesterone was ≤29 nmol/l compared to 52 of 149 (34.9%) when serum progesterone was >29 nmol/l (P = 0.888).
There was no statistical difference in LBR between controls who had a serum progesterone ≤10 nmol/l, two of 18 (11.1%) compared to controls with a serum progesterone >10 nmol/l, 56 of 221 (25.3%, P = 0.255). Progesterone supplemented women with a serum progesterone ≤10 nmol/l and a live birth were 5 of 12 (41.7%) compared to those with serum progesterone >10 nmol/l, 76 of 223 (34.1%, P = 0.756).
Discussion
The main finding of the present study is the beneficial role of progesterone supplementation after NC-FET, resulting in a significant, 10% increase in live births. These data confirm the results from the previous study published by Bjuresten et al. (2011) and strengthens the evidence for luteal phase supplementation with vaginal progesterone in NC-FET shown in two recent meta-analyses (Seol et al., 2020; Mizrachi et al., 2021). Nevertheless, one large retrospective study by Montagut et al. (2016; n = 2353) reported contradictory results for CPR when NC-FET was compared to modified NC-FET with luteal phase support (LPS). However, when CPR after NC-FET was compared to CPR after NC-FET and LPS, no significant difference could be shown. One reason for the contradictory results could be the early initiation of LPS already on the day after LH rise which may have caused early closure of the implantation window (Montagut et al., 2016). A second reason might be that clinical pregnancy does not correspond to the live birth, the latter being the purpose of fertility treatment. In an RCT including 102 subjects, there was no significant improvement in CPR after LPS, probably due to the small sample size (Eftekhar et al., 2013). However, there was a trend for higher CPR in the progesterone supplemented group compared to the control group, which is in line with the results of the present study and also the previous RCT by Bjuresten et al. (2011). This is also in agreement with data regarding embryo transfer in artificial cycles, where high doses of progesterone administration after embryo transfer have been shown to improve LBRs (Alsbjerg et al., 2013).
The start of LPS is likely to be of importance. In this study, LPS started 3–6 days after a positive LH test, at the time of embryo transfer, which is at a similar time point as the rise of serum progesterone in a normal menstrual cycle. One previous study reported lower CPRs in fresh IVF cycles, when progesterone supplementation started before ovum pick up than after ovum pick up (Sohn et al., 1999). The CPR was lower in cases when LPS started 6 days after ovum pick up compared to 3 days after ovum pick up (Williams et al., 2001). Therefore, as the time for initiation of LPS affects outcomes, it cannot be ruled out that start of progesterone supplementation 3 days after a positive LH test also for the blastocyst transfer could have resulted in even better LBRs, which calls for further studies.
The LPS continued over the first 8 weeks of pregnancy, in cases where the pregnancy test was positive. Previous studies in fresh embryo transfer cycles found that LBR was similar when LPS was terminated at pregnancy test or at pregnancy Week 6–7 (Liu et al., 2012). However, the optimal length of LPS has not been thoroughly studied for embryo transfer in natural cycles and further studies are needed to determine this aspect.
The embryos used for transfer in the present study were either cleavage stage embryos ranked as of good or top quality or blastocysts scored to be of median or top quality. There was no difference in the proportion of transferred blastocysts compared to cleavage stage embryos between the treatment and control groups. LBR was higher in the treatment group regardless of the stage of the embryo. When dividing the embryos further, significantly higher LBR was seen on Day 3 and 5 embryos, but the same tendency was present also on Day 2 and 6 embryos. However, the number of embryos in these groups was small which makes it difficult to draw any conclusions regarding the age of the embryo.
LBR after FET has improved over the years, probably due to initiation of vitrification rather than slow freezing and a higher number of good-quality blastocysts available for transfer (Rienzi et al., 2017; Saket et al., 2021). This was also the case in the present study where there was a shift towards the transfer of vitrified blastocysts during the study resulting in a tendency towards higher LBR during the second part of the study performed at Karolinska University Hospital, compared to the first part of the study undertaken at Uppsala University Hospital. This can also explain the tendency towards higher LBR after transfers performed by the specially trained fertility midwife who performed a majority of transfers at Karolinska University Hospital, compared to the transfers made by fertility doctors at both centres. It has previously been shown that the basic university education to medical doctor or midwife does not determine the success rate of embryo transfer performed by trained personnel (Bjuresten et al., 2003).
There was no difference in the initial serum progesterone levels between the groups regarding study outcomes. A single progesterone measurement may not be the perfect predictive marker for successful implantation in natural cycles, due to substantial variation in serum progesterone concentration between women (Murto et al., 2013). However, low levels of serum progesterone in artificial and natural cycles have previously been shown to correlate with lower ongoing pregnancy and LBRs and higher miscarriage rates (Labarta et al., 2017; Alsbjerg et al., 2018; Ku et al., 2018; Gaggiotti-Marre et al., 2019; Gaggiotti-Marre et al., 2020). In the present study, these findings could not be reproduced for the 29 nmol/l threshold. There was a lower percentage of live births in the few controls who had a serum progesterone ≤10 nmol/l compared to those with a serum progesterone >10 nmol/l on the day of embryo transfer, but this was not a statistical difference, and the present study was not designed to evaluate this issue.
Sponsorship by the pharmaceutical company manufacturing the progesterone tablets can be considered as a possible bias. However, the company did not have any influence on the study whatsoever, and was not involved in the study design, data analysis or interpretation of study results.
The two-centre setting could be considered as a study strength, providing a large sample size with enough statistical power to detect differences between groups. Although there was a significant difference in the proportion of blastocysts transferred there was no statistically significant difference in LBR between the two centres and the clinical quality can be considered comparable. Therefore, data from the two centres can be analysed together. The time difference between inclusion in the two centres could be a disadvantage as the time gap to continue the study with new personnel was the reason for the delay. However, besides the higher proportion of blastocysts, the clinical procedures were the same at the two centres.
The vast majority of SET can also be considered as a strength of the study. The groups of control and treated women were balanced as we included a variety of diagnosis. Furthermore, transfer of embryos frozen on either Day 2–3 and 5–6 were included, and we can therefore assume that the result is valid for most patients with infertility diagnoses included in the present study, undergoing NC-FER.
In our study, 500 out of 672 invited women (74.4%) initially agreed to participate in the study. One limitation due to ethical reasons is that the causes for opting out from the study were not registered. The per-protocol analysis in the primary analysis of the results can also be considered as a limitation, since the intention-to-treat analysis is usually recommended for an RCT. However, our intention-to-treat analysis did not substantially alter the main outcome. A power calculation showed that a total of 588 women should have been included in the study. However, as the primary aim to determine LBR was significant after 500 subjects, it was decided not to include additional women. Yet another limitation is that because of the study protocol the start of progesterone treatment differed depending on the stage of the embryo, which theoretically could affect the results negatively for the patients having blastocysts transferred.
Moreover, the study was not double-blinded since it was not possible to manufacture a placebo tablet. The placebo effect is well known in all types of medical treatments and is likely to affect the result and interpretation of the study in favour of progesterone supplementation. However, the performance of embryo transfer should be unaffected since randomization was performed after the transfer. The lack of psychosomatic effect from the missing placebo may have negatively affected the outcome in the untreated group. However, it is well known that progesterone is the most important hormone for successful implantation and pregnancy outcome. Therefore, women not given progesterone supplementation will not get the positive medical effect of the progesterone administration. Although a double-blinded study is preferred, we still believe that progesterone supplementation favours the number of live birth outcomes after FET in natural cycles.
In conclusion, the present study shows that progesterone supplementation during the luteal phase improves LBR after FET in natural cycle, which also confirms previous data. Therefore, we suggest that patients undergoing FET in natural cycles should be offered LPS with progesterone.
Supplementary Material
Acknowledgements
We would like to sincerely thank all the women who participated in the study, and the staff at the two reproductive medicine centres. We also thank Dr Lena Wånggren, Department of English Literature, University of Edinburgh, for linguistic revision of the manuscript.
Authors’ roles
K.W. and A.S.-E. designed the study. K.W., M.D.G. and J.G. executed the study. S.I. has contributed to data retrieval and management. All authors have been involved in data analysis, manuscript drafting and critical discussion. All authors have approved the final version of the manuscript submitted for publication.
Funding
The study was funded by grants from Uppsala University, Uppsala Family Planning fund and Ferring pharmaceuticals AB, Malmö, Sweden.
Conflict of interest
The authors have no personal conflicting interests to declare. Some funding of the study was obtained from Ferring pharmaceuticals AB, Malmö, Sweden. The sponsor was not involved in the design, performance or interpretation of the findings of the study.
Contributor Information
K Wånggren, Division of Obstetrics and Gynaecology, Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Stockholm, Sweden; Department of Women’s and Children’s Health, Uppsala University, Uppsala, Sweden.
M Dahlgren Granbom, Division of Obstetrics and Gynaecology, Department of Clinical Science, Intervention and Technology, Karolinska Institutet, Stockholm, Sweden.
S I Iliadis, Department of Women’s and Children’s Health, Uppsala University, Uppsala, Sweden.
J Gudmundsson, Department of Women’s and Children’s Health, Uppsala University, Uppsala, Sweden.
A Stavreus-Evers, Department of Women’s and Children’s Health, Uppsala University, Uppsala, Sweden; The Centre for Reproductive Biology in Uppsala, CRU, Uppsala, Sweden.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- Alsbjerg B, Polyzos NP, Elbaek HO, Povlsen BB, Andersen CY, Humaidan P.. Increasing vaginal progesterone gel supplementation after frozen-thawed embryo transfer significantly increases the delivery rate. Reprod Biomed Online 2013;26:133–137. [DOI] [PubMed] [Google Scholar]
- Alsbjerg B, Thomsen L, Elbaek HO, Laursen R, Povlsen BB, Haahr T, Humaidan P.. Progesterone levels on pregnancy test day after hormone replacement therapy-cryopreserved embryo transfer cycles and related reproductive outcomes. Reprod Biomed Online 2018;37:641–647. [DOI] [PubMed] [Google Scholar]
- Bjuresten K, Hreinsson J, Fridström M, Rosenlund B, Ek I, Hovatta O.. Embryo transfer by midwife or gynecologist: a prospective randomized study. Acta Obstet Gynecol Scand 2003;82:462–466. [DOI] [PubMed] [Google Scholar]
- Bjuresten K, Landgren BM, Hovatta O, Stavreus-Evers A.. Luteal phase progesterone increases live birth rate after frozen embryo transfer. Fertil Steril 2011;95:534–537. [DOI] [PubMed] [Google Scholar]
- Csapo AI, Pulkkinen MO, Kaihola HL.. The effect of estradiol replacement therapy on early pregnant luteectomized patients. Am J Obstet Gynecol 1973a;117:987–990. [DOI] [PubMed] [Google Scholar]
- Csapo AI, Pulkkinen MO, Kaihola HL.. The effect of luteectomy-induced progesterone-withdrawal on the oxytocin and prostaglandin response of the first trimester pregnant human uterus. Prostaglandins 1973b;4:421–429. [DOI] [PubMed] [Google Scholar]
- Csapo AI, Pulkkinen MO, Ruttner B, Sauvage JP, Wiest WG.. The significance of the human corpus luteum in pregnancy maintenance. I. Preliminary studies. Am J Obstet Gynecol 1972;112:1061–1067. [DOI] [PubMed] [Google Scholar]
- Eftekhar M, Rahsepar M, Rahmani E.. Effect of progesterone supplementation on natural frozen-thawed embryo transfer cycles: a randomized controlled trial. Int J Fertil Steril 2013;7:13–20. [PMC free article] [PubMed] [Google Scholar]
- Gaggiotti-Marre S, Alvarez M, Gonzalez-Foruria I, Parriego M, Garcia S, Martinez F, Barri PN, Polyzos NP, Coroleu B.. Low progesterone levels on the day before natural cycle frozen embryo transfer are negatively associated with live birth rates. Hum Reprod 2020;35:1623–1629. [DOI] [PubMed] [Google Scholar]
- Gaggiotti-Marre S, Martinez F, Coll L, Garcia S, Alvarez M, Parriego M, Barri PN, Polyzos N, Coroleu B.. Low serum progesterone the day prior to frozen embryo transfer of euploid embryos is associated with significant reduction in live birth rates. Gynecol Endocrinol 2019;35:439–442. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB.. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril 2000;73:1155–1158. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens JJ.. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 2014;35:851–905. [DOI] [PubMed] [Google Scholar]
- Ghobara T, Gelbaya TA, Ayeleke RO.. Cycle regimens for frozen-thawed embryo transfer. Cochrane Database Syst Rev 2017;7:CD003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewoud ER, Macklon NS, Cohlen BJ; ANTARCTICA Study Group. The effect of elevated progesterone levels before HCG triggering in modified natural cycle frozen-thawed embryo transfer cycles. Reprod Biomed Online 2017;34:546–554. [DOI] [PubMed] [Google Scholar]
- Guerrero R, Aso T, Brenner PF, Cekan Z, Landgren BM, Hagenfeldt K, Diczfalusy E.. Studies on the pattern of circulating steroids in the normal menstrual cycle. I. Simultaneous assays of progesterone, pregnenolone, dehydroepiandrosterone, testosterone, dihydrotestosterone, androstenedione, oestradiol and oestrone. Acta Endocrinol (Copenh) 1976;81:133–149. [PubMed] [Google Scholar]
- Kim CH, Lee YJ, Lee KH, Kwon SK, Kim SH, Chae HD, Kang BM.. The effect of luteal phase progesterone supplementation on natural frozen-thawed embryo transfer cycles. Obstet Gynecol Sci 2014;57:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku CW, Allen JC Jr, Lek SM, Chia ML, Tan NS, Tan TC.. Serum progesterone distribution in normal pregnancies compared to pregnancies complicated by threatened miscarriage from 5 to 13 weeks gestation: a prospective cohort study. BMC Pregnancy Childbirth 2018;18:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarta E, Mariani G, Holtmann N, Celada P, Remohi J, Bosch E.. Low serum progesterone on the day of embryo transfer is associated with a diminished ongoing pregnancy rate in oocyte donation cycles after artificial endometrial preparation: a prospective study. Hum Reprod 2017;32:2437–2442. [DOI] [PubMed] [Google Scholar]
- Landgren BM, Unden AL, Diczfalusy E.. Hormonal profile of the cycle in 68 normally menstruating women. Acta Endocrinol (Copenh) 1980;94:89–98. [DOI] [PubMed] [Google Scholar]
- Liu XR, Mu HQ, Shi Q, Xiao XQ, Qi HB.. The optimal duration of progesterone supplementation in pregnant women after IVF/ICSI: a meta-analysis. Reprod Biol Endocrinol 2012;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackens S, Santos-Ribeiro S, van de Vijver A, Racca A, Van Landuyt L, Tournaye H, Blockeel C.. Frozen embryo transfer: a review on the optimal endometrial preparation and timing. Hum Reprod 2017;32:2234–2242. [DOI] [PubMed] [Google Scholar]
- Mizrachi Y, Horowitz E, Ganer Herman H, Farhi J, Raziel A, Weissman A.. Should women receive luteal support following natural cycle frozen embryo transfer? A systematic review and meta-analysis. Hum Reprod Update 2021;27:643–650. [DOI] [PubMed] [Google Scholar]
- Mohr LR, Trounson A, Freemann L.. Deep-freezing and transfer of human embryos. J In Vitro Fert Embryo Transf 1985;2:1–10. [DOI] [PubMed] [Google Scholar]
- Montagut M, Santos-Ribeiro S, De Vos M, Polyzos NP, Drakopoulos P, Mackens S, van de Vijver A, van Landuyt L, Verheyen G, Tournaye H. et al. Frozen-thawed embryo transfers in natural cycles with spontaneous or induced ovulation: the search for the best protocol continues. Hum Reprod 2016;31:2803–2810. [DOI] [PubMed] [Google Scholar]
- Murto T, Bjuresten K, Landgren BM, Stavreus-Evers A.. Predictive value of hormonal parameters for live birth in women with unexplained infertility and male infertility. Reprod Biol Endocrinol 2013;11:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, Vanderpoel S, Oocyte RC.. Embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update 2017;23:139–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saket Z, Källén K, Lundin K, Magnusson Å, Bergh C.. Cumulative live birth rate after IVF: trend over time and the impact of blastocyst culture and vitrification. Hum Reprod Open 2021;3:hoab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol A, Shim YJ, Kim SW, Kim SK, Lee J, Jee C, Chang Suk S, Kim S.. Effect of luteal phase support with vaginal progesterone on pregnancy outcomes in natural frozen embryo transfer cycles: a meta-analysis. Clin Exp Reprod Med 2020;47:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn SH, Penzias AS, Emmi AM, Dubey AK, Layman LC, Reindollar RH, DeCherney AH.. Administration of progesterone before oocyte retrieval negatively affects the implantation rate. Fertil Steril 1999;71:11–14. [DOI] [PubMed] [Google Scholar]
- van der Linden M, Buckingham K, Farquhar C, Kremer JA, Metwally M.. Luteal phase support for assisted reproduction cycles. Cochrane Database Syst Rev 2015;7:CD009154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veleva Z, Orava M, Nuojua-Huttunen S, Tapanainen JS, Martikainen H.. Factors affecting the outcome of frozen-thawed embryo transfer. Hum Reprod 2013;28:2425–2431. [DOI] [PubMed] [Google Scholar]
- Williams SC, Oehninger S, Gibbons WE, Van Cleave WC, Muasher SJ.. Delaying the initiation of progesterone supplementation results in decreased pregnancy rates after in vitro fertilization: a randomized, prospective study. Fertil Steril 2001;76:1140–1143. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing Human Semen,5th edn. Geneva: WHO Press, 2010. [Google Scholar]
- Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN.. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod 1997;12:1545–1549. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.