Abstract
Oxytocin is hypothesized to promote social interactions by enhancing the salience of social stimuli. While previous neuroimaging studies have reported that oxytocin enhances amygdala activation to face stimuli in autistic men, effects in autistic women remain unclear. In this study, the influence of intranasal oxytocin on activation and functional connectivity of the basolateral amygdala—the brain’s ‘salience detector’—while processing emotional faces vs shapes was tested in 16 autistic and 21 non-autistic women by functional magnetic resonance imaging in a placebo-controlled, within-subject, cross-over design. In the placebo condition, minimal activation differences were observed between autistic and non-autistic women. However, significant drug × group interactions were observed for both basolateral amygdala activation and functional connectivity. Oxytocin increased left basolateral amygdala activation among autistic women (35-voxel cluster, Montreal Neurological Institute (MNI) coordinates of peak voxel = −22 −10 −28; mean change = +0.079%, t = 3.159, PTukey = 0.0166) but not among non-autistic women (mean change = +0.003%, t = 0.153, PTukey = 0.999). Furthermore, oxytocin increased functional connectivity of the right basolateral amygdala with brain regions associated with socio-emotional information processing in autistic women, but not in non-autistic women, attenuating group differences in the placebo condition. Taken together, these findings extend evidence of oxytocin’s effects on the amygdala to specifically include autistic women and specify the subregion of the effect.
Keywords: autism, basolateral amygdala, emotional face processing, oxytocin, salience
Background
For more than a decade, the neuropeptide oxytocin has been considered a candidate treatment for promoting social well-being in people with psychiatric conditions (Meyer-Lindenberg et al., 2011). The effects of oxytocin among autistic people have attracted particular attention, with early studies reporting that intranasal administration of oxytocin enhances emotion recognition (Guastella et al., 2010) and eye contact during social interactions (Auyeung et al., 2015), among various effects. In efforts to clarify the clinical applications of oxytocin, neuroimaging studies have sought to identify the neurological underpinnings of oxytocin’s effects on social behavior and cognition. Such studies have reported that a single dose of intranasal oxytocin influences amygdala activation in neurotypical participants (Kirsch et al., 2005; Gamer et al., 2010; Pincus et al., 2010; Radke et al., 2017; Geng et al., 2018; Xin et al., 2020), in people with affective or anxiety-related disorders (Labuschagne et al., 2010; Dodhia et al., 2014; Frijling et al., 2015; Gorka et al., 2015; Koch et al., 2016; Lorenz et al., 2019) and in animal models (Amico et al., 2004; Sobota et al., 2015). Effects on the amygdala may help to explain two of oxytocin’s broad effects, namely increased attention and orientation to social stimuli (Shamay-Tsoory and Abu-Akel, 2016) and reduced anxiety responses to social stimuli (Bethlehem et al., 2013, 2014).
In light of its central role in social information processing, the amygdala is one of the first brain regions implicated in the neurobiology of autism and continues to be seen as key to understanding autistic behavioral phenotypes (Hennessey et al., 2018). The amygdala theory of autism (Baron-Cohen et al., 1999) proposed that diminished amygdala activation underlies alterations in attention and response to social stimuli. The neuroimaging literature supports this theory to some degree, with evidence of lower amygdala activation to emotional faces among autistic participants relative to controls (e.g. Baron-Cohen et al., 1999; Ashwin et al., 2007; Herrington et al., 2016; for exceptions, see Piggot et al., 2004; Wang et al., 2004; Dalton et al., 2005). Notably, intranasal oxytocin is reported to influence amygdala activation to social stimuli in autistic people. In a study involving 14 autistic men and 14 non-autistic men who performed a face-matching task, a 24 IU dose of oxytocin increased right amygdala activation specifically among autistic participants, attenuating the group difference observed in the placebo condition (Domes et al., 2013). Oxytocin was also reported to increase left amygdala activation during an emotion recognition task involving the same sample of autistic men (Domes et al., 2014). In a study where 20 autistic adults (19 men and 1 woman) played a virtual ball-tossing game, amygdala activation increased in the oxytocin condition (24 IU) when the virtual partner behaved in an unfair manner but decreased when the partner behaved in an equitable manner. By contrast, a study of 19 autistic youth (16 boys and 3 girls, aged 8–16.5 years) found no effect of oxytocin (12–24 IU, depending on the participant age) on amygdala activation during an emotion recognition task (Gordon et al., 2013). However, a positive correlation was observed between amygdala activation and pre- to post-administration salivary oxytocin levels, suggesting that participants who experienced larger oxytocin increases tended to show increased amygdala activation. Taken together, these studies support that oxytocin enhances amygdala activation to socially relevant stimuli in autistic people, with effects varying with context and individual differences.
In oxytocin studies involving neurotypical participants, biological sex has emerged as a likely variable moderating oxytocin’s effects on brain and behavior. Importantly, several studies have found the effect of oxytocin on amygdala activation in female participants to be opposite to that originally reported in all-male samples (Rilling et al., 2014; Luo et al., 2017; Lieberz et al., 2020). While the precise cause of sex differential effects of oxytocin remains unclear, they may arise due to interactions with sex steroid hormones or baseline sex differences in the neural oxytocin system (Borland et al., 2019; Winterton et al., 2021). As the few studies examining the effects of oxytocin on amygdala activation in autistic people have involved predominantly men, the ability of oxytocin to enhance amygdala response to social stimuli should not be assumed to generalize to autistic women.
Aiming to extend knowledge of oxytocin’s effects on the amygdala to include autistic women, the present study used functional magnetic resonance imaging (fMRI) to examine the influence of a single 24 IU dose of intranasal oxytocin on neural activation to emotional face stimuli in autistic and non-autistic women. In light of our specific interest in the effects of oxytocin on the salience of social stimuli, our analysis focused on the basolateral amygdala, which has been described as the brain’s ‘salience detector’ due to its responsivity to both reward and threat (Sander et al., 2003; Terburg et al., 2018). Informed by the above-described studies, we predicted that autistic women would show lower basolateral amygdala activation relative to non-autistic women in the placebo condition and that oxytocin would enhance basolateral amygdala activation in both autistic and non-autistic women. Furthermore, we predicted that oxytocin would enhance functional connectivity of the basolateral amygdala with other brain regions involved in processing socio-emotional information. Lastly, to explore individual differences that may moderate oxytocin’s effects, we explored the relationships of autistic-like traits, social anxiety and salivary oxytocin levels with amygdala activation.
Materials and methods
Study design and drug administration
Adopting a double-blind, placebo-controlled, cross-over design, participants completed two experimental sessions with the drug order randomly determined. Of the non-autistic participants, 10/21 received oxytocin first; of the autistic participants, 9/16 received oxytocin first. To minimize variation in endogenous hormone levels, both sessions were scheduled during the follicular phase of the menstrual cycle; for participants who self-reported use of hormonal contraceptives, sessions were scheduled at least 1 week apart. On each experiment day, participants underwent a health screening by a clinician and were then instructed to self-administer nasal spray containing oxytocin (4 IU per puff; total dose 24 IU; Syntocinon, Novartis, Switzerland) or placebo. Participants rested for ∼20 min before scanning. The emotional face-matching task described here began ∼55 min after administration. A time window of 45–70 min after administration of 24 IU oxytocin is reported to be optimal for assessing effects on the amygdala (Spengler et al., 2017). Saliva samples were collected pre- and post-administration (immediately and 90 min post-administration), and salivary oxytocin was quantified by radioimmunoassay performed by an external lab [full details of salivary hormone analyses in these participants are reported in Procyshyn et al. (2020a)]. In advance of the experimental sessions, participants completed the Autism-Spectrum Quotient (AQ), which assesses autistic-like traits (Baron-Cohen et al., 2001), and the Liebowitz Social Anxiety Scale (LSAS), which assesses social phobia and avoidance across various situations (Heimberg et al., 1999). This work is part of a larger study of the effects of oxytocin in autistic and non-autistic women, and the effects of oxytocin on resting-state connectivity are reported elsewhere (Bethlehem et al., 2017; Procyshyn et al., 2020b).
The a priori power calculation indicated that a sample size of 34 was needed for a repeated measures mixed-effect design with two measures across two groups, with α = 0.05, an estimated effect size of 0.35 and a correlation between measures of 0.4. A recent meta-analysis of common fMRI tasks reported an intraclass correlation coefficient of 0.397, which is consistent with the value used in our a priori power calculation (Elliott et al., 2020).
All participants provided written informed consent prior to participation. This work was approved by the NHS Research Ethics Service (NRES Committee East of England-Cambridge Central, 14/EE/0202) and conducted following the Declaration of Helsinki. The UK Medicines and Healthcare Regulatory Agency exempted this study from clinical trial status.
Participants
A total of 42 women were recruited from Cambridge, UK, and the surrounding area to participate in this study and completed both neuroimaging sessions. Four non-autistic women were excluded due to data acquisition errors and one non-autistic woman was excluded due to excessive motion, as detailed in the ‘fMRI data acquisition and pre-processing’ section. Table 1 presents the demographic characteristics, hormonal contraceptive use, questionnaire scores and salivary oxytocin levels for the final sample of 16 autistic and 21 non-autistic women. As shown, the groups did not differ significantly in age, full-scale intelligence quotient (IQ) or salivary oxytocin levels. Autistic women had significantly higher AQ and LSAS scores relative to non-autistic women.
Table 1.
Characteristics of the autistic and non-autistic women included in the analysis
| Autistic (n = 16) | Non-autistic (n = 21) | P | |
|---|---|---|---|
| Age (years) | 29.9 ± 8.4 | 26.4 ± 7.9 | 0.20 |
| Full-scale IQ | 121 ± 16.4 | 117.9 ± 13.7 | 0.52 |
| Hormonal contraceptive use (n) | 0 | 6 | 0.01* |
| Psychological traits | |||
| AQ | 37.1 ± 5.2 | 13.2 ± 6.6 | <0.001** |
| LSAS | 73.3 ± 24.2 | 31.71 ± 13.6 | <0.001** |
| Salivary oxytocin (pg/ml) | |||
| Placebo condition | |||
| Baseline | 3.3 ± 0.9 | 3.0 ± 0.9 | 0.17 |
| Immediately post-administration | 3.0 ± 0.9 | 2.7 ± 0.8 | 0.32 |
| 90-min post-administration | 2.9 ± 1.0 | 2.6 ± 0.7 | 0.30 |
| Oxytocin condition | |||
| Baseline | 2.8 ± 0.9 | 2.9 ± 0.8 | 0.89 |
| Immediately post-administration | 112.9 ± 15.1 | 113.5 ± 16.0 | 0.91 |
| 90-min post-administration | 46.1 ± 15.6 | 35.3 ± 21.3 | 0.08 |
Full-scale IQ = Wechsler abbreviated scale of intelligence,
P < 0.05,
P < 0.01.
Autism was determined as a clinical diagnosis of an autistic disorder/childhood autism or Asperger’s disorder/syndrome based on Diagnostic and Statistical Manual of Mental Disorders, fourth edition or International Classification of Diseases, tenth revision criteria. Exclusion criteria were pregnancy, cigarette smoking, substance dependence, epilepsy, a genetic syndrome related to autism, intellectual disability and a diagnosis of bipolar, obsessive-compulsive, panic or psychotic disorder.
fMRI task
Participants completed an established emotional face-matching paradigm (Hariri et al., 2002; Terburg et al., 2012). To minimize potential context-related effects of varying emotional expressions, we used a variant involving only negatively valanced faces (angry or fearful) shown to elicit robust bilateral amygdala response in autistic and non-autistic participants (Kleinhans et al., 2010). The task design is shown in Supplementary Figure S1. Participants completed four blocks of face-matching interspersed with five blocks of shape-matching. Each block comprised six trials. Participants were instructed to press a button corresponding to which of the two images at the bottom of the screen matched the image at the top of the screen. Response time was recorded for each trial. For face-matching trials, images were matched for the same emotion; for shape-matching trials, images were matched for the same orientation. As shown, the shape stimuli were scrambled images of the face stimuli.
fMRI data acquisition and pre-processing
Scanning was performed at the Wolfson Brain Imaging Centre in Cambridge, UK, using a 3T Siemens MAGNETOM Tim Trio MRI scanner. Functional images were acquired with a multi-echo echo planar imaging (EPI) sequence (field-of-view (FOV) = 240 mm; flip angle = 80°; 3 echoes at echo time (TE) = 12, 29, and 46 ms; repetition time (TR) = 2300 ms; 33 oblique slices, interleaved slice acquisition, slice thickness = 3.8 mm, 11% slice gap; generalized autocalibrating partial parallel acquisition acceleration factor = 2, bandwidth = 2368 Hz pixel−1). Anatomical images were acquired using a T1-weighted magnetization prepared rapid gradient echo sequence (FOV = 256 mm; flip angle = 9°; TE = 2.98 ms; TI = 900 ms; TR = 2250 ms; voxel size = 1 mm3; acquisition matrix size = 256 × 256 × 256 mm).
Multi-echo functional images were combined and denoized using the AFNI-integrated multi-echo independent component analysis (meica.py v3) pipeline (Kundu et al., 2012). After deleting the first four volumes, which were dummy scans collected before task onset, each TE functional dataset was slice-time corrected and decomposed into independent components (ICs). ICs were categorized as representing blood oxygen level-dependent (BOLD) or non-BOLD signals based on their linear relationship with TE (Kundu et al., 2013). Removal of non-BOLD ICs increases the signal-to-noise ratio by the systematic removal of motion, physiological and scanner artifacts based on the characteristics of T2* decay and has been shown to improve estimates of effect size (Kundu et al., 2013; Lombardo et al., 2016). Additional pre-processing included removal of non-brain areas, co-registration of structural images to the first TE functional image, spatial smoothing with a 6 mm full width at half maximum Gaussian kernel and registration to the MNI152 2 mm template.
Four participants were excluded due to data acquisition issues, and one participant was excluded due to excessive motion (mean framewise displacement (FD) = 0.75 mm, max FD = 6 mm) in the placebo session. Average FD in the included 37 participants did not differ between drug conditions (placebo = 0.157 ± 0.09 mm, oxytocin = 0.155 ± 0.06 mm, P = 0.92).
Subject-level fMRI analysis
FEAT (FMRI Expert Analysis Tool), part of FSL 6.00 (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl), was used to fit a general linear model for each participant for each session. Two regressors of interest were included, representing the onset times of the face-matching and shape-matching blocks. Neural response to emotional face stimuli was obtained as the contrast of activation to faces relative to shapes (Faces > Shapes). The generated contrast images for each participant were then used in the group-level analyses.
Group-level fMRI analyses
Planned analyses included (i) group differences (autistic vs non-autistic) in the baseline (placebo) condition, (ii) drug × group differences and (iii) correlations between oxytocin-associated amygdala activation and autistic-like traits (AQ), social anxiety (LSAS) and salivary oxytocin levels.
Group analyses were conducted as higher-level mixed-effect analyses using the FMRIB Local Analysis of Mixed Effects (FLAME) tool in FSL. For analyses of group differences in the placebo condition, drug order (i.e. whether the placebo session occurred first or second) and hormonal contraceptive use were included as regressors of no interest. Analyses were performed separately for maps of the left and right basolateral amygdala, which were determined using probabilistic maps from the Juelich Histological Atlas implemented in FSLeyes. Voxels were included if ≥50% probability of belonging to the laterobasal nucleus of the amygdala complex (based on the study by Amunts et al., 2005; see Supplementary Figure S2). Significant results were determined as Z > 2.3 and a cluster-corrected threshold of P < 0.05. In the case of significant clusters, the contrast estimates for Faces > Shapes were extracted for the cluster using featquery as percent signal change and imported into R for post hoc analyses. In addition to this region of interest (ROI) analysis focused on the basolateral amygdala, exploratory whole-brain analyses were conducted using an uncorrected statistical threshold of P < 0.001 and a cluster extent threshold k > 10 voxels.
Functional connectivity analysis
Task-based functional connectivity of the basolateral amygdala with other brain regions was examined using the psychophysiological interaction (PPI) approach. The left and right basolateral amygdala masks described above were used as the functional seeds. In FSL, PPI is modeled as the interaction between the task regressor (Faces > Shapes) and the time series of the ROI (extracted for each participant using fslmeants; see Supplementary Materials for further details). The subject-level results were then used in group-level random effects models (FLAME1) to compare connectivity of the amygdala seeds with other brain regions implemented in FEAT as a 2 (drug) × 2 (group) repeated measures analysis of variance (ANOVA). PPI analyses were performed for the whole brain, and results were considered significant at Z > 2.3 and cluster-corrected P < 0.05. Post hoc analyses were performed by extracting the parameter estimates for the contrast Faces > Shapes for the significant clusters using featquery.
Statistical analysis
Statistical analyses were performed using R software (R Core Team, 2020). The effects of oxytocin on task performance and clusters in autistic vs non-autistic women were assessed using the ‘lmerTest’ package (Kuznetsova et al., 2017), with drug and group as fixed effects and participant ID as a random effect, controlling for drug order and hormonal contraceptive use. Fixed effects are reported as type III ANOVA with Satterwaithe’s method. Post hoc tests were performed by comparing pairwise differences in estimated marginal means (‘emmeans’ package), with Tukey adjustment for multiple comparisons. Change in the activation of the basolateral amygdala ROI between drug conditions was computed as activationOXYTOCIN − activationPLACEBO; thus, a positive value indicates increased activation in the oxytocin condition. Relationships between activation and psychological/hormonal variables were then assessed using correlation analyses.
Results
Behavioral results
Participants’ reaction times were recorded for all trials and compared between groups (autistic vs non-autistic), drug conditions (oxytocin vs placebo) and stimulus type (shapes vs faces). As shown in Figure 1, reaction times were significantly faster for matching shapes than matching faces (F(1,112) = 844, P < 0.001). No other effects or their interaction were statistically significant.
Fig. 1.
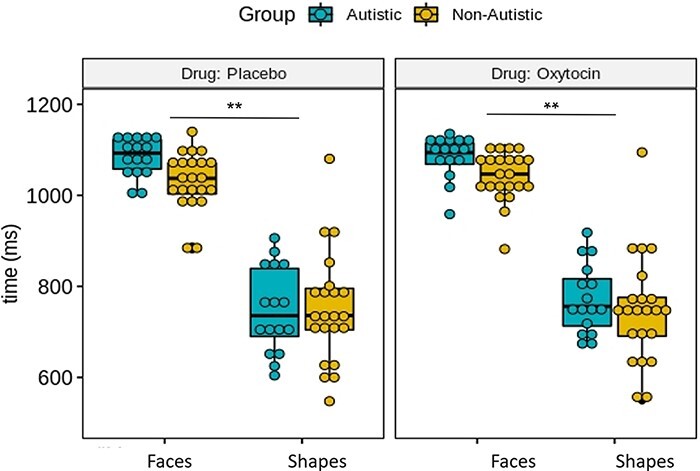
Boxplots showing median response time (in ms) for face-matching and shape-matching trials between groups (autistic vs non-autistic) and drug conditions (placebo vs oxytocin). Only the effect of task (matching faces vs shapes) was significant. **P < 0.01.
Functional activation analyses
Group differences
Among all participants, the contrast Faces > Shapes resulted in significant bilateral amygdala activation as well as activation of the frontal cortex, visual areas, motor areas, temporal occipital fusiform cortex and cerebellum (Supplementary Figure S3). No significant group differences in basolateral amygdala activation were observed between autistic and non-autistic women in the baseline (placebo) condition (Supplementary Figures S4 and S5). Exploratory whole-brain analyses (Supplementary Table S1) indicated greater angular gyrus and cerebellum activation in non-autistic women relative to autistic women, while autistic women relative to non-autistic women showed greater activation of the middle frontal gyrus.
Effect of oxytocin on basolateral amygdala activation
The ROI analysis indicated a significant drug × group interaction for the left basolateral amygdala (Z = 2.96, P = 0.0266, k = 35 voxels, MNI coordinates of peak activation = −22 −10 −28; Figure 2). Similar findings were obtained when the six women taking hormonal contraceptives (all non-autistic) were excluded (see Supplementary Materials). Post hoc tests showed higher activation in the oxytocin relative to placebo condition among autistic women (pairwise difference = 0.079, t = 3.159, PTukey = 0.0166), but no significant changes among non-autistic women (pairwise difference = 0.003, t = 0.153, PTukey = 0.999). As shown in Figure 2, 14 of the 16 (88%) autistic women participants showed an activation increase in the oxytocin condition; by contrast, 13 of the 21 (62%) non-autistic women showed an activation increase in the oxytocin condition.
Fig. 2.
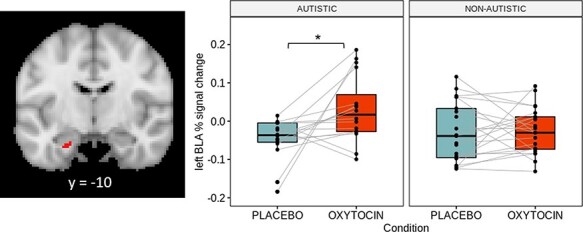
Boxplots showing activation of the left basolateral amygdala cluster identified in the group analysis (35 voxels, peak activation = −22 −10 −28) between drug conditions and groups. Each dot represents one participant and the gray lines connect their activation values for the contrast Faces > Shapes between drug conditions. Image is shown such that the left side is the left hemisphere. *P < 0.05.
Relationship of amygdala activation with psychological and hormonal variables
With the aim of understanding individual variation in the effects of oxytocin on amygdala activation among our participants, we explored the relationships between activation of the left basolateral amygdala ROI and autistic-like traits, social anxiety and salivary oxytocin levels. No relationship was observed between AQ score and left basolateral amygdala activation in non-autistic women (Supplementary Figure S6). A moderate, but not statistically significant, correlation was observed between self-reported social anxiety score and oxytocin-associated change in left basolateral amygdala activation in non-autistic women (r = 0.34, P = 0.15; Supplementary Figure S7), whereas autistic women showed a moderate, but not statistically significant, correlation between pre- to post-administration change in salivary oxytocin and left basolateral amygdala activation (r = 0.48, P = 0.06; Supplementary Figure S8). Additional details and results are provided in the Supplementary Materials.
Functional connectivity analyses
Group differences
For the contrast Faces > Shapes in the baseline (placebo) condition, non-autistic women showed higher connectivity of bilateral basolateral amygdala seeds with large clusters that included the frontal lobe, temporal/occipital fusiform cortex, precuneus, putamen and cerebellum (Z > 2.3, cluster-corrected P < 0.05; Supplementary Table S2, Supplementary Figure S9). No group differences were observed in the oxytocin condition.
Effect of oxytocin on functional connectivity of basolateral amygdala
Using the right basolateral amygdala as the seed, a significant main effect of drug (Table 2A; Figure 3) was observed such that connectivity with two large clusters comprising frontal areas (cluster 2) and cerebellar/occipitotemporal areas associated with visual processing (cluster 1) was higher in the oxytocin relative to placebo condition in the combined participant sample. In addition, significant drug × group interactions were observed for four clusters (Table 2A; Figure 3). Post hoc analyses showed that, for autistic women, functional connectivity of all four clusters was significantly higher in the oxytocin relative to placebo condition (all PTukey < 0.01; see Supplementary Table S3 for the mean value for each cluster); by contrast, non-autistic women showed non-significant decreases in connectivity of the right basolateral amygdala seed with the four clusters. Similar results were obtained when the six women taking hormonal contraceptives (all non-autistic) were excluded (see Supplementary Materials).
Table 2.
Summary of oxytocin effects on functional connectivity of right basolateral amygdala seed with other brain regions in autistic and non-autistic women during the experimental task (Faces > Shapes; Z > 2.3 and cluster-corrected P < 0.05)
| k | P | Z | x | y | z | ||
|---|---|---|---|---|---|---|---|
| (A) Main effect of drug | |||||||
| Cluster 1 | 944 | 0.0012 | Cerebellum | 3.82 | −44 | −60 | −44 |
| Lateral occipital cortex | 3.48 | −48 | −78 | −14 | |||
| Inferior temporal gyrus/temporal occipital fusiform cortex | 3.38 | −48 | −50 | −22 | |||
| Cerebellum | 3.26 | −42 | −56 | −30 | |||
| Cerebellum | 3.21 | −48 | −70 | −38 | |||
| Lateral occipital cortex | 3.21 | −50 | −72 | −16 | |||
| Cluster 2 | 1083 | 0.000423 | Precentral gyrus | 4.05 | −52 | −10 | 38 |
| Superior frontal gyrus | 3.36 | −18 | 8 | 52 | |||
| Precentral gyrus | 3.31 | −32 | −6 | 54 | |||
| Superior frontal gyrus | 3.3 | −24 | 0 | 54 | |||
| Precentral gyrus | 3.15 | −48 | −4 | 42 | |||
| (B) Group × drug interaction | |||||||
| Cluster 1 | 737 | 0.00626 | Cerebellum | 3.58 | −32 | −48 | −46 |
| Cerebellum | 3.32 | −40 | −68 | −50 | |||
| Lateral occipital cortex | 3.22 | −52 | −68 | −22 | |||
| Cerebellum | 3.21 | −40 | −50 | −42 | |||
| Cluster 2 | 1100 | 0.000374 | Lateral occipital cortex | 3.99 | −30 | −64 | 46 |
| Superior parietal lobule | 3.57 | −36 | −56 | 56 | |||
| Lateral occipital cortex | 3.47 | −32 | −76 | 42 | |||
| Lateral occipital cortex | 3.39 | −28 | −74 | 42 | |||
| Supramarginal gyrus/superior parietal lobule | 3.37 | −44 | −50 | 54 | |||
| Angular gyrus | 3.14 | −36 | −52 | 34 | |||
| Cluster 3 | 2364 | P < 0.000001 | Middle frontal gyrus | 3.86 | −42 | 36 | 20 |
| Middle frontal gyrus | 3.74 | −44 | 32 | 28 | |||
| Middle frontal gyrus | 3.57 | −38 | 22 | 30 | |||
| Frontal pole | 3.49 | −50 | 40 | 4 | |||
| Superior frontal gyrus | 3.41 | −14 | 34 | 42 | |||
| Frontal pole | 3.31 | −32 | 48 | 18 | |||
| Cluster 4 | 4275 | P < 0.000001 | Lingual gyrus | 3.98 | −4 | −78 | −16 |
| Cerebellum | 3.94 | 0 | −76 | −26 | |||
| Lingual gyrus/visual cortex | 3.73 | 12 | −62 | −4 | |||
| Occipital fusiform gyrus | 3.71 | 2 | −82 | −24 | |||
| Lingual gyrus/visual cortex | 3.6 | 18 | −58 | −2 | |||
| Precuneus cortex | 3.59 | 22 | −58 | 16 | |||
k = cluster size in voxels, x, y, z = MNI coordinates of peak activation.
Fig. 3.
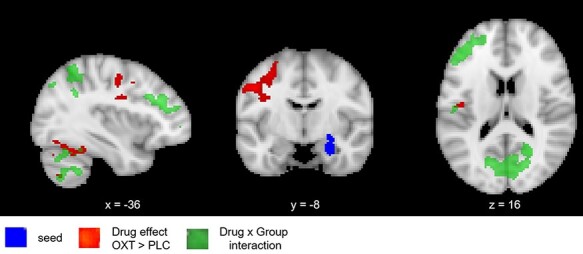
Effects of oxytocin (vs placebo) on functional connectivity. Using the right basolateral amygdala as the seed, oxytocin increased connectivity with temporal lobe and occipital lobe clusters. A significant Drug × Group interaction was also observed such that oxytocin significantly increased connectivity with frontal lobe regions and the cerebellum among autistic women. All tests Z > 2.3, P < 0.05 cluster-corrected. Image is shown such that the left side is the left hemisphere.
Discussion
Despite more than a decade of research efforts, intranasal oxytocin as a treatment for social cognition challenges has not been realized, in part due to remaining uncertainties about its mechanism of action, optimal treatment course and heterogeneity in effects related to sex, age and clinical phenotype (Andari et al., 2018; Erdozain and Peñagarikano, 2020; Winterton et al., 2021). Given the underrepresentation of women in both oxytocin research (Williams and Trainor, 2018) and autism research (Lai et al., 2015), the influence of oxytocin on amygdala activation in autistic women was essentially unknown prior to this study.
The present study used fMRI to test the effects of a single 24 IU dose of intranasal oxytocin on basolateral amygdala response to emotional face stimuli in a sample of autistic and non-autistic women matched for age and IQ. Autistic women, but not non-autistic women, showed a significant increase in left basolateral amygdala activation in the oxytocin condition. This result is broadly consistent with the findings of Domes et al. (2014), who reported increased left amygdala activation (11 voxels, x = −18, y = −1, z = −23) to images of the eye or mouth area of emotional faces among autistic men, but no change among non-autistic men. Moreover, as was the case in the study by Domes et al., the oxytocin-associated increase in activation resulted in, on average, higher left amygdala activation in autistic vs non-autistic participants in the oxytocin condition, rather than attenuation of a group difference. Domes et al. (2014) interpreted increased left amygdala activation in autistic men as indicating greater salience of the emotional face stimuli, as there was a positive correlation between amygdala activation and performance on the emotion recognition task. Although oxytocin had no significant effects on the performance of the emotional face-matching task in our study, we interpret the effect specific to the basolateral amygdala to reflect an alteration in salience processing. Domes et al. did not address the amygdala subregion of their finding, but we note that the peak voxel has a >20% probability of belonging to the basolateral group. Despite early oxytocin administration research reporting amygdala subregion-specific effects (e.g. Kirsch et al., 2005), many oxytocin- and autism-related studies have treated the amygdala as a homologous structure, potentially obscuring the findings. Whereas the ‘amygdala theory of autism’, as detailed above, did not make amygdala subregion-related predictions, a model of amygdala alterations in psychiatric conditions specifically predicts the hypoactivity of the basolateral amygdala (Moul et al., 2012) to be associated with reduced reflexive shifting of gaze to the eye area, manifesting in social and emotional challenges. Thus, we suggest that future studies consider amygdala subregions to clarify the functional significance of their findings and possible clinical implications.
As the oxytocin administration literature grows, it has become increasingly clear that its effects vary across individuals (Bartz et al., 2011). Indeed, not all autistic women in our study showed increased basolateral amygdala activation in the oxytocin condition. However, the additional variables considered (AQ and LSAS) did not significantly explain the observed variation. Although there are no other studies of autistic women with which to compare our findings, several studies have reported that oxytocin decreased amygdala activation to social stimuli in women with borderline personality disorder (Bertsch et al., 2013; Lischke et al., 2017). While this result contrasts with our main finding that oxytocin increased amygdala activation in autistic women, the borderline personality neurophenotype also contrasts with autism, with the borderline personality participants reported to show higher amygdala activation to social stimuli than controls at baseline (and some evidence of lower amygdala activation in autism, as detailed above). Taken together, these findings suggest that the effects of oxytocin on amygdala activation in women vary with the clinical condition and may ‘normalize’ activation only in cases of deviation from optimal amygdala responsivity to social and emotional stimuli.
In the present study, we also performed functional connectivity analyses to examine group differences and effects of oxytocin on the functional coupling of the amygdala with other brain regions during the emotional face-matching task. In contrast to the similarities in basolateral amygdala activation between groups, widespread differences in functional connectivity were observed in the placebo condition, with autistic women showing lower connectivity of bilateral basolateral amygdala seeds with frontal, striatal and occipitotemporal areas associated with reward and processing of socio-emotional information (see Supplementary Table S2). In the combined sample, oxytocin increased functional connectivity of the right basolateral amygdala seed with frontal, cerebellar and occipitotemporal areas. However, significant drug × group effects were also present, with post hoc analyses indicating that oxytocin’s enhancement of functional connectivity was limited to autistic women, with non-autistic women showing non-significant decreases in connectivity. Notably, these opposite directional changes in autistic and non-autistic women attenuated the group differences in functional connectivity present in the placebo condition. Several previous studies have reported lower amygdala functional connectivity during socially relevant tasks in autistic relative to non-autistic participants (Kleinhans et al., 2008; Monk et al., 2010; Swartz et al., 2013) and that oxytocin influences resting-state connectivity of the amygdala in autistic people (Alaerts et al., 2019, 2020). However, to the best of our knowledge, this is the first report of oxytocin’s effects on task-associated amygdala functional connectivity in autistic people. A study using a comparable emotional face-matching task reported that oxytocin enhanced connectivity of the amygdala with the insula and cingulate while processing the images of fearful faces in a sample of men with generalized social anxiety disorder but not in controls (Gorka et al., 2015). Similar to our findings, the oxytocin-associated increases in connectivity in men with anxiety attenuated the group differences observed in the placebo condition. While our functional connectivity findings should be considered preliminary, they suggest that examining connectivity of an amygdala-related network, rather than amygdala activation in isolation, may be useful for understanding the differences in how social–emotional information is processed between autistic and non-autistic people and how oxytocin influences coupling of relevant brain networks.
This study is subject to several limitations. Although this women-focused study has increased the representation of autistic women in oxytocin research, the sample size of 16 is relatively small. As a result, effects of oxytocin of a small effect size are unlikely to have been detected, which is a limitation of many intranasal oxytocin studies (Quintana, 2020). Further studies in larger samples are warranted to confirm these findings. Second, we lack a true baseline condition for the comparison of amygdala activation between groups. Although we found no significant differences, it is possible that placebo administration exerted its own effect on the amygdala, obscuring group differences. Third, measures of salivary oxytocin following intranasal oxytocin administration are greatly elevated due to ‘drip down’ into the throat. In our analysis, changes in oxytocin were scaled relative to participants’ pre-administration oxytocin levels (% change) as an effort to account for inflated post-administration values. Nevertheless, the finding of a relationship between oxytocin-associated changes in the present study and previous work involving autistic people (Parker et al., 2017; Greene et al., 2018; Yamasue et al., 2020) highlights the need for a more reliable marker of individual differences in the oxytocin system than saliva or plasma levels (Martins et al., 2020). Lastly, this was a single administration study and we did not collect data on participants’ subjective emotional responses to the face stimuli or effects of the experiment on mood or arousal levels. As the ultimate aim of oxytocin administration is to improve the quality of life for people interested in a pharmacological intervention, studies examining the effects of long-term oxytocin use on amygdala reactivity to naturalistic social stimuli, as well as subjective social well-being, are needed. While several studies have now reported positive effects of long-term oxytocin administration in autistic people (e.g. Bernaerts et al., 2020; Peled-Avron et al., 2020), autistic women continue to be underrepresented in such work. Future oxytocin administration studies need to include sex-balanced and gender-inclusive samples.
Supplementary Material
Acknowledgements
None.
Contributor Information
Tanya L Procyshyn, Department of Psychiatry, Autism Research Centre, University of Cambridge, Cambridge CB2 8AH, UK.
Michael V Lombardo, Department of Psychiatry, Autism Research Centre, University of Cambridge, Cambridge CB2 8AH, UK; Laboratory for Autism and Neurodevelopmental Disorders, Center for Neuroscience and Cognitive Systems @UniTn, Istituto Italiano di Tecnologia, Rovereto 38068, Italy.
Meng-Chuan Lai, Department of Psychiatry, Autism Research Centre, University of Cambridge, Cambridge CB2 8AH, UK; Department of Psychiatry, Centre for Addiction and Mental Health and The Hospital for Sick Children, University of Toronto, Toronto, ON M5G 1X8, Canada; Department of Psychiatry, National Taiwan University Hospital and College of Medicine, Taipei 10002, Taiwan.
Nazia Jassim, Department of Psychiatry, Autism Research Centre, University of Cambridge, Cambridge CB2 8AH, UK.
Bonnie Auyeung, Department of Psychiatry, Autism Research Centre, University of Cambridge, Cambridge CB2 8AH, UK; Department of Psychology, School of Philosophy, Psychology and Language Sciences, University of Edinburgh, Edinburgh EH8 9AD, UK.
Sarah K Crockford, Department of Psychiatry, Autism Research Centre, University of Cambridge, Cambridge CB2 8AH, UK; Department of Theoretical and Applied Linguistics, University of Cambridge, Cambridge CB3 9DA, UK.
Julia B Deakin, Department of Psychiatry, University of Cambridge, Cambridge CB2 0SZ, UK; Cambridgeshire and Peterborough NHS Foundation Trust, Cambridge CB21 5EF, UK.
Sentil Soubramanian, South West London and St George’s Mental Health NHS Trust, London SW17 7DJ, UK; Liaison Psychiatry Service, St Helier Hospital, Epsom and St Helier University Hospitals NHS Trust, Surrey KT18 7EG, UK.
Akeem Sule, Behavioural and Clinical Neuroscience Institute, University of Cambridge, Cambridge CB2 3EB, UK.
David Terburg, Department of Experimental Psychology, Utrecht University, Utrecht 3584, The Netherlands; Department of Psychiatry and Mental Health, Groote Schuur Hospital, MRC Unit on Anxiety & Stress Disorders, University of Cape Town, Cape Town 7925, South Africa.
Simon Baron-Cohen, Department of Psychiatry, Autism Research Centre, University of Cambridge, Cambridge CB2 8AH, UK.
Richard A I Bethlehem, Department of Psychiatry, Autism Research Centre, University of Cambridge, Cambridge CB2 8AH, UK; Department of Psychiatry, University of Cambridge, Cambridge CB2 0SZ, UK.
Funding
TLP was supported by the Autism Research Trust, Cambridge Trust, and Natural Sciences and Engineering Research Council of Canada. MVL was supported by an European Research Council Starting Grant (ERC-2017-STG; 755816). MCL was supported by a Canadian Institutes of Health Research (CIHR) Sex and Gender Science Chair (GSB 171373), the O’Brien Scholars Program within the Child and Youth Mental Health Collaborative at the Centre for Addiction and Mental Health (CAMH) and The Hospital for Sick Children, Toronto, the Academic Scholars Award from the Department of Psychiatry, University of Toronto, the CAMH Foundation, and the Ontario Brain Institute. SBC received funding from the Wellcome Trust, Autism Centre of Excellence, Simons Foundation Autism Research Initiative, the Templeton World Charitable Fund, the Medical Research Council UK, and the National Institute for Health Research (NIHR). Any views expressed are those of the author(s) and not necessarily those of the funder. RB was supported by the MRC UK, Pinsent Darwin Trust and British Academy post-doctoral fellowship. For the purpose of open access, the authors have applied a CC BY public copyright license for any author-accepted manuscript version arising from this submission.
Conflict of interest
The authors declare that they have no conflict of interest with respect to their authorship or the publication of this article.
Supplementary data
Supplementary data is available at SCAN online.
Author contributions
T.L.P. designed the analysis plan, analyzed and interpreted the data and wrote the manuscript. M.V.L., M.-C.L., N.J., D.T. and B.A. helped design the study, assisted in data analysis and contributed to writing the manuscript. S.K.C., J.B.D., S.S. and A.S. assisted with study design and data collection. S.B.-C. and R.A.I.B. designed the study, assisted in data analysis and interpretation and contributed to writing the manuscript. All authors approved the final manuscript.
References
- Alaerts K., Bernaerts S., Vanaudenaerde B., Daniels N., Wenderoth N. (2019). Amygdala–hippocampal connectivity is associated with endogenous levels of oxytocin and can be altered by exogenously administered oxytocin in adults with autism. Biological Psychiatry Cognitive Neuroscience and Neuroimaging, 4, 655–63. [DOI] [PubMed] [Google Scholar]
- Alaerts K., Bernaerts S., Prinsen J., Dillen C., Steyaert J., Wenderoth N. (2020). Oxytocin induces long-lasting adaptations within amygdala circuitry in autism: a treatment-mechanism study with randomized placebo-controlled design. Neuropsychopharmacology, 45, 1141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amico J.A., Mantella R.C., Vollmer R.R., Li X. (2004). Anxiety and stress responses in female oxytocin deficient mice. Journal of Neuroendocrinology, 16(4), 319–24.doi: 10.1111/j.0953-8194.2004.01161.x. [DOI] [PubMed] [Google Scholar]
- Amunts K., Kedo O., Kindler M., et al. (2005). Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anatomy and Embryology, 210(5–6), 343–52.doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Andari E., Hurlemann R., Young L.J. (2018). A precision medicine approach to oxytocin trials. Current Topics in Behavioral Neurosciences, 35, 559–90.doi: 10.1007/7854_2017_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwin C., Baron-Cohen S., Wheelwright S., O’Riordan M., Bullmore E.T. (2007). Differential activation of the amygdala and the “social brain” during fearful face-processing in Asperger syndrome. Neuropsychologia, 45, 2–14. [DOI] [PubMed] [Google Scholar]
- Auyeung B., Lombardo M.V., Heinrichs M., et al. (2015). Oxytocin increases eye contact during a real-time, naturalistic social interaction in males with and without autism. Translational Psychiatry, 5, e507.doi: 10.1038/tp.2014.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S., Ring H.A., Wheelwright S., et al. (1999). Social intelligence in the normal and autistic brain: an fMRI study. European Journal of Neuroscience, 11(6), 1891–8.doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S., Wheelwright S., Skinner R., Martin J., Clubley E. (2001). The autism spectrum quotient: evidence from Asperger syndrome/high functioning autism, males and females, scientists and mathematicians. Journal of Autism and Developmental Disorders, 31, 5–17. [DOI] [PubMed] [Google Scholar]
- Bartz J., Zaki J., Bolger N., Ochsner K.N. (2011). Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences, 15, 301–9. [DOI] [PubMed] [Google Scholar]
- Bernaerts S., Boets B., Bosmans G., Steyaert J., Alaerts K. (2020). Behavioral effects of multiple-dose oxytocin treatment in autism: a randomized, placebo-controlled trial with long-term follow-up. Molecular Autism, 11(1), 6.doi: 10.1186/s13229-020-0313-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertsch K., Gamer M., Schmidt B., et al. (2013). Oxytocin and reduction of social threat hypersensitivity in women with borderline personality disorder. The American Journal of Psychiatry, 170(10), 1169–77. [DOI] [PubMed] [Google Scholar]
- Bethlehem R.A.I., Van H.J., Auyeung B., Baron-cohen S. (2013). Oxytocin, brain physiology, and functional connectivity: a review of intranasal oxytocin fMRI studies. Psychoneuroendocrinology, 38, 962–74. [DOI] [PubMed] [Google Scholar]
- Bethlehem R.A.I., Lombardo M.V., Lai M.C., et al. (2017). Intranasal oxytocin enhances intrinsic corticostriatal functional connectivity in women. Translational Psychiatry, 7(4), e1099.doi: 10.1038/tp.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethlehem R.A.I.I., Baron-Cohen S., van Honk J., Auyeung B., Bos P.A. (2014). The oxytocin paradox. Frontiers in Behavioral Neuroscience, 8, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borland J.M., Rilling J.K., Frantz K.J., Albers H.E. (2019). Sex-dependent regulation of social reward by oxytocin: an inverted U hypothesis. Neuropsychopharmacology, 44, 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton K.M., Nacewicz B.M., Johnstone T., et al. (2005). Gaze fixation and the neural circuitry of face processing in autism. Nature Neuroscience, 8(4), 519–26.doi: 10.1038/nn1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodhia S., Hosanagar A., Fitzgerald D.A., et al. (2014). Modulation of resting-state amygdala-frontal functional connectivity by oxytocin in generalized social anxiety disorder. Neuropsychopharmacology, 39, 2061–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domes H.M., Kumbier E., Grossmann A., Hauenstein K., Herpertz S.C. (2013). Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biological Psychiatry, 74, 164–71. [DOI] [PubMed] [Google Scholar]
- Domes K.E., Heinrichs M., Herpertz S.C. (2014). Oxytocin promotes facial emotion recognition and amygdala reactivity in adults with Asperger syndrome. Neuropsychopharmacology, 39, 698–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott M.L., Knodt A.R., Ireland D., et al. (2020). What is the test-retest reliability of common task-functional MRI measures? New empirical evidence and a meta-analysis. Psychological Science, 31, 792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdozain A.M., Peñagarikano O. (2020). Oxytocin as treatment for social cognition, not there yet. Frontiers in Psychiatry, 10, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijling J.L., van Zuiden M., Koch S.B.J., Nawijn L., Veltman D.J., Olff M. (2015). Effects of intranasal oxytocin on amygdala reactivity to emotional faces in recently trauma-exposed individuals. Social Cognitive and Affective Neuroscience, 11, 327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamer M., Zurowski B., Büchel C. (2010). Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proceedings of the National Academy of Sciences, 107, 9400–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y.Y., Zhao W., Zhou F., et al. (2018). Oxytocin facilitates empathic- and self-embarrassment ratings by attenuating amygdala and anterior insula responses. Frontiers in Endocrinology, 9, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I., Vander Wyk B.C., Bennett R.H., et al. (2013). Oxytocin enhances brain function in children with autism. Proceedings of the National Academy of Sciences, 110, 20953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorka S.M., Fitzgerald D.A., Labuschagne I., et al. (2015). Oxytocin modulation of amygdala functional connectivity to fearful faces in generalized social anxiety disorder. Neuropsychopharmacology, 40(2), 278–86.doi: 10.1038/npp.2014.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R.K., Spanos M., Alderman C., et al. (2018). The effects of intranasal oxytocin on reward circuitry responses in children with autism spectrum disorder. Journal of Neurodevelopmental Disorders, 10, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guastella A.J., Einfeld S.L., Gray K.M., et al. (2010). Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry, 67, 692–4. [DOI] [PubMed] [Google Scholar]
- Hariri A.R., Tessitore A., Mattay V.S., Fera F., Weinberger D.R. (2002). The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage, 17(1), 317–23.doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- Heimberg R.G., Horner K.J., Juster H.R., et al. (1999). Psychometric properties of the Liebowitz Social Anxiety Scale. Psychological Medicine, 29(1), 199–212.doi: 10.1017/S0033291798007879. [DOI] [PubMed] [Google Scholar]
- Hennessey T., Andari E., Rainnie D.G. (2018). RDoC-based categorization of amygdala functions and its implications in autism. Neuroscience and Biobehavioral Reviews, 90, 115–29.doi: 10.1016/j.neubiorev.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington J.D., Miller J.S., Pandey J., Schultz R.T. (2016). Anxiety and social deficits have distinct relationships with amygdala function in autism spectrum disorder. Social Cognitive and Affective Neuroscience, 11, 907–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P., Esslinger C., Chen Q., et al. (2005). Oxytocin modulates neural circuitry for social cognition and fear in humans. Journal of Neuroscience, 25(49), 11489–93.doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinhans N.M., Richards T., Sterling L., et al. (2008). Abnormal functional connectivity in autism spectrum disorders during face processing. Brain, 131, 1000–12. [DOI] [PubMed] [Google Scholar]
- Kleinhans N.M., Richards T., Weaver K., et al. (2010). Association between amygdala response to emotional faces and social anxiety in autism spectrum disorders. Neuropsychologia, 48, 3665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S.B.J., Van Zuiden M., Nawijn L., Frijling J.L., Veltman D.J., Olff M. (2016). Intranasal oxytocin administration dampens amygdala reactivity towards emotional faces in male and female PTSD patients. Neuropsychopharmacology, 41, 1495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P., Inati S.J., Evans J.W., Luh W.M., Bandettini P.A. (2012). Differentiating BOLD and non-BOLD signals in fMRI time series using multi-echo EPI. Neuroimage, 60, 1759–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundu P., Brenowitz N.D., Voon V., et al. (2013). Integrated strategy for improving functional connectivity mapping using multiecho fMRI. Proceedings of the National Academy of Sciences, 110, 16187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznetsova A., Brockhoff P.B., Christensen R.H.B. (2017). lmerTest package: tests in linear mixed effects models. Journal of Statistical Software, 82(13), 1–26.doi: 10.18637/jss.v082.i13. [DOI] [Google Scholar]
- Labuschagne I., Phan K.L., Wood A., et al. (2010). Oxytocin attenuates amygdala reactivity to fear in generalized social anxiety disorder. Neuropsychopharmacology, 35(12), 2403–13.doi: 10.1038/npp.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M.C., Baron-Cohen S., Buxbaum J.D. (2015). Understanding autism in the light of sex/gender. Molecular Autism, 6, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberz J., Scheele D., Spengler F.B., et al. (2020). Kinetics of oxytocin effects on amygdala and striatal reactivity vary between women and men. Neuropsychopharmacology, 45, 1134–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lischke A., Herpertz S.C., Berger B., Domes G., Gamer M. (2017). Divergent effects of oxytocin on (para-)limbic reactivity to emotional and neutral scenes in females with and without borderline personality disorder. Social Cognitive and Affective Neuroscience, 12(11), 1783–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo M.V., Auyeung B., Holt R.J., et al. (2016). Improving effect size estimation and statistical power with multi-echo fMRI and its impact on understanding the neural systems supporting mentalizing. Neuroimage, 142, 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz T.K., Cheng H., Heiman J.R. (2019). Neural correlates of emotion processing comparing antidepressants and exogenous oxytocin in postpartum depressed women: an exploratory study. PLoS One, 14(5), e0217764.doi: 10.1371/journal.pone.0217764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L., Becker B., Geng Y., et al. (2017). Sex-dependent neural effect of oxytocin during subliminal processing of negative emotion faces. Neuroimage, 162, 127–37. [DOI] [PubMed] [Google Scholar]
- Martins D., Gabay A.S., Mehta M., Paloyelis Y. (2020). Salivary and plasmatic oxytocin are not reliable trait markers of the physiology of the oxytocin system in humans. Elife, 9, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A., Domes G., Kirsch P., Heinrichs M. (2011). Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nature Reviews Neuroscience, 12, 524–38. [DOI] [PubMed] [Google Scholar]
- Monk C.S., Weng S.J., Wiggins J.L., et al. (2010). Neural circuitry of emotional face processing in autism spectrum disorders. Journal of Psychiatry and Neuroscience, 35, 105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moul C., Killcross S., Dadds M.R. (2012). A model of differential amygdala activation in psychopathy. Psychological Review, 119(4), 789–806.doi: 10.1037/a0029342. [DOI] [PubMed] [Google Scholar]
- Parker O.O., Libove R.A., Sumiyoshi R.D., et al. (2017). Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proceedings of the National Academy of Sciences, 114(30), 8119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled-Avron L., Abu-Akel A., Shamay-Tsoory S. (2020). Exogenous effects of oxytocin in five psychiatric disorders: a systematic review, meta-analyses and a personalized approach through the lens of the social salience hypothesis. Neuroscience and Biobehavioral Reviews, 114, 70–95.doi: 10.1016/j.neubiorev.2020.04.023. [DOI] [PubMed] [Google Scholar]
- Piggot J., Kwon H., Mobbs D., et al. (2004). Emotional attribution in high-functioning individuals with autistic spectrum disorder: a functional imaging study. Journal of the American Academy of Child and Adolescent Psychiatry, 43, 473–80. [DOI] [PubMed] [Google Scholar]
- Pincus D., Kose S., Arana A., et al. (2010). Inverse effects of oxytocin on attributing mental activity to others in depressed and healthy subjects: a double-blind placebo controlled fMRI study. Frontiers in Psychiatry / Frontiers Research Foundation, 1, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procyshyn T.L., Lombardo M.V., Lai M.C., et al. (2020a). Effects of oxytocin administration on salivary sex hormone levels in autistic and neurotypical women. Molecular Autism, 11, 20.doi: 10.1186/s13229-020-00326-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procyshyn T.L., Lombardo M., Lai M.C., et al. (2020b). Intranasal oxytocin differentially affects resting-state functional connectivity of social brain regions in autistic and non-autistic women. OSF Prepr.doi: 10.31219/osf.io/2mkd8. [DOI] [Google Scholar]
- Quintana D.S. (2020). Most oxytocin administration studies are statistically underpowered to reliably detect (or reject) a wide range of effect sizes. Comprehensive Psychoneuroendocrinology, 4.doi: 10.1016/j.cpnec.2020.100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team . (2020). R: a language and environment for statistical computing.
- Radke S., Volman I., Kokal I., Roelofs K., ERA D.B., Toni I. (2017). Oxytocin reduces amygdala responses during threat approach. Psychoneuroendocrinology, 79, 160–6.doi: 10.1016/j.psyneuen.2017.02.028. [DOI] [PubMed] [Google Scholar]
- Rilling J.K., DeMarco A.C., Hackett P.D., et al. (2014). Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology, 39, 237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander D., Grafman J., Zalla T. (2003). The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences, 14(4), 303–16.doi: 10.1515/REVNEURO.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Abu-Akel A. (2016). The social salience hypothesis of oxytocin. Biological Psychiatry, 79, 194–202. [DOI] [PubMed] [Google Scholar]
- Sobota R., Mihara T., Forrest A., Featherstone R.E., Siegel S.J. (2015). Oxytocin reduces amygdala activity, increases social interactions, and reduces anxiety-like behavior irrespective of NMDAR antagonism. Behavioral Neuroscience, 129(4), 389–98.doi: 10.1037/bne0000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler F.B., Schultz J., Scheele D., et al. (2017). Kinetics and dose dependency of intranasal oxytocin effects on amygdala reactivity. Biological Psychiatry, 82, 885–94. [DOI] [PubMed] [Google Scholar]
- Swartz J.R., Wiggins J.L., Carrasco M., Lord C., Monk C.S. (2013). Amygdala habituation and prefrontal functional connectivity in youth with autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry, 52(1), 84–93.doi: 10.1016/j.jaac.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terburg D., Morgan B.E., Montoya E.R., et al. (2012). Hypervigilance for fear after basolateral amygdala damage in humans. Translational Psychiatry, 2(5), e115.doi: 10.1038/tp.2012.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terburg D., Scheggia D., Triana R., et al. (2018). The basolateral amygdala is essential for rapid escape: a human and rodent study. Cell, 175, 723–35.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A.T., Dapretto M., Hariri A.R., Sigman M., Bookheimer S.Y. (2004). Neural correlates of facial affect processing in children and adolescents with autism spectrum disorder. Journal of the American Academy of Child and Adolescent Psychiatry, 43, 481–90. [DOI] [PubMed] [Google Scholar]
- Williams A.V., Trainor B.C. (2018). The impact of sex as a biological variable in the search for novel antidepressants. Frontiers in Neuroendocrinology, 50, 107–17.doi: 10.1016/j.yfrne.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterton A., Westlye L.T., Steen N.E., Andreassen O.A., Quintana D.S. (2021). Improving the precision of intranasal oxytocin research. Nature Human Behaviour, 5, 9–18. [DOI] [PubMed] [Google Scholar]
- Xin F., Zhou X., Dong D., et al. (2020). Oxytocin differentially modulates amygdala responses during top-down and bottom-up aversive anticipation. Advanced Science, 7, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasue H., Okada T., Munesue T., et al. (2020). Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: a randomized clinical trial. Molecular Psychiatry, 25(8), 1849–58.doi: 10.1038/s41380-018-0097-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


