Abstract
Background
Malignant gliomas are treated with temozolomide chemoradiotherapy. Because pneumocystis pneumonia (PCP) can occur in patients receiving temozolomide, the product monograph recommends PCP prophylaxis during temozolomide chemoradiotherapy. Not all neuro-oncologists follow these recommendations, though.
Methods
We performed a population-based retrospective cohort study of glioma patients undergoing temozolomide chemoradiotherapy 2005 to 2019 in Ontario, Canada. A propensity score model was used to predict the use of PCP prophylaxis. We compared the risk of PCP within 90 days of starting radiotherapy with versus without PCP prophylaxis using inverse probability of treatment weighting (IPTW). We also examined overall survival, hospitalizations, and myelosuppression.
Results
There were 3,225 patients included in the cohort (648 received antibiotics and 2,434 did not). Only 18 patients developed PCP within 90 days of therapy. The IPTW-adjusted absolute risk reduction in PCP with antibiotics was 0.0035 (95% CI, −0.0013 to 0.0083), number needed to treat: 288. Neither overall survival nor hospitalization count differed between the groups. The number needed to harm by causing grade 3/4 neutropenia was 39.
Conclusions
In regions (like Ontario) where PCP is rare, routine PCP prophylaxis with trimethoprim-sulfamethoxazole should not be offered, since the harms may outweigh the benefits.
Keywords: glioma, pneumocystis pneumonia, temozolomide
Key Points.
In this province-wide cohort of glioma patients receiving chemoradiotherapy, pneumocystis pneumonia (PCP) was rare (18/3,225 patients, 0.6% within 90 days of treatment).
This is the first-ever population-based study examining safety and effectiveness of PCP prophylaxis for temozolomide chemoradiotherapy.
If we treat 288 glioma patients with antibiotic PCP prophylaxis, one case of PCP will be prevented, but there would be seven cases of severe neutropenia and 21 patients will miss doses of chemotherapy due to myelosuppression.
Importance of the Study.
We report the results of a population-based, province-wide retrospective analysis of pneumocystis pneumonia (PCP) in glioma patients receiving temozolomide chemoradiotherapy. We showed that PCP is rare and that many (288) patients need to be prescribed antibiotics to prevent one case of PCP. The number needed to harm with trimethoprim-sulfamethoxazole was 39 for grade 3/4 neutropenia and 14 for potential missed doses of adjuvant chemotherapy. Especially where the incidence of PCP is low the routine prescription of PCP antibiotic prophylaxis with temozolomide for the treatment of glioma should be revisited.
The standard treatment of glioblastoma includes surgery, external beam radiotherapy, temozolomide chemotherapy, and in some places around the world, tumor treating fields. Temozolomide chemotherapy is also routinely used for high-risk or recurrent low-grade gliomas or for newly diagnosed grade 3 astrocytoma or oligodendroglioma.
A 2002 phase II trial of temozolomide in 64 patients with newly diagnosed glioblastoma showed good clinical and imaging responses to 75 mg/m2 daily temozolomide with radiation.1 Two of the first 15 patients in this trial developed pneumocystis pneumonia (PCP) with concomitant chemoradiotherapy. Prophylaxis against PCP was introduced for the remaining 49 patients. In March 2005, the Food and Drug Administration (FDA) approved temozolomide for newly diagnosed glioblastoma.2 The FDA and Health Canada recommended PCP prophylaxis with concomitant chemoradiotherapy. The Temodar product monograph says that antibiotics are “required” during chemoradiotherapy.
Prophylaxis is recommended for patients receiving any chemotherapy regimens associated with >3.5% risk for pneumonia from Pneumocystis jirovecii.3 It is also recommended for patients receiving ≥20 mg prednisone equivalents daily for ≥1 month.4 Antibiotic options for PCP prophylaxis include trimethoprim-sulfamethoxazole (TMP-SMX), dapsone, atovaquone, and pentamidine. In Ontario, TMP-SMX is the most common prophylactic agent, followed by pentamidine. The side effects of TMP-SMX include hyperkalemia, skin reactions, hepatitis, agranulocytosis, and other blood dyscrasias.5
There are a few known risk factors for PCP infection in glioma patients. Corticosteroids are known to increase one’s risk of PCP. In one retrospective review, corticosteroids at a dose of 3 mg dexamethasone equivalent or more for eight or more weeks significantly increased the risk of PCP.6,7 Radiation therapy may increase the risk for PCP, perhaps through its resultant lymphopenia.7 Lymphopenia (especially lymphocyte count < 0.5 × 109 cells/L) is associated with higher risk of PCP.7
There are several arguments why PCP prophylaxis might not be routinely indicated in temozolomide chemoradiotherapy patients. First, PCP is rare in glioma patients receiving temozolomide, likely less than 1% risk among these patients.8,9 According to a 2007 meta-analysis, when the risk of PCP is less than 3.5%, the harms of PCP prophylaxis outweigh the benefits.3 These data informed the Infectious Diseases Society of America (IDSA) guidelines that recommend PCP prophylaxis only when the risk of PCP is 3.5% or greater.4 Second, most cases of PCP respond well to antibiotics. Lastly, antibiotics can cause side effects, including low white blood cell counts with TMP-SMX, which can limit the use of chemotherapy.
The purpose of this study was to (1) determine the risk of PCP in temozolomide chemoradiotherapy patients with antibiotic prophylaxis compared with patients without prophylaxis and (2) to determine (a) the overall survival, (b) the hospitalization rate, and (c) the degree of myelosuppression with antibiotic prophylaxis compared to without prophylaxis.
Methods
Data Sources
We conducted a population-based retrospective cohort study using linked administrative databases held at ICES, an independent, nonprofit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement.10,11 These datasets were linked deterministically using unique encoded identifiers and analyzed at ICES. In accordance with ICES’ commitments in data sharing agreements and to minimize risk of reidentification, ICES prohibits the presence of small cells (counts less than six) in any report. The Research Ethics Board at Sunnybrook Health Sciences Centre confirmed that this project was exempt from review under section 45 of Ontario’s Personal Health Information Protection Act.
To define the cohort, three core databases were used: the Ontario Cancer Registry (OCR), the Ontario Drug Benefit (ODB) database, and the Cancer Activity Level Reporting (ALR) , database. The OCR is a registry of all incident cancer diagnoses in Ontario.12 The ODB provides free drug coverage to patients aged 65 and older, those on social assistance, those living in long-term care homes, and to recipients of home care, and the database contains drug records for these patients. The ALR database contains records of all radiation treatments in Ontario.13 The Canadian Institute for Health Information Discharge Abstract Database (DAD) was used to define the primary outcome.10 After each patient discharge, a medical records coder at the hospital draws up an abstract from the chart, compiling information on that admission. A single most responsible diagnosis is coded for each hospital discharge. In addition, up to 24 other diagnoses, including pre- and post-admission diagnoses, are enumerated and are coded with International Statistical Classification of Diseases and Related Health Problems, 10th Revision, Canada (ICD-10-CA) values. More details on the databases used can be found in the Supplementary Materials.
Cohort Creation
Adult patients with brain cancer were identified using the OCR. Using ICD-10-CA codes, we selected only patients with glioblastoma, grade 2 or 3 astrocytoma, or grade 2 or 3 oligodendroglioma from the OCR (see Supplementary Materials). Only those patients who received central nervous system external beam radiotherapy planning (from ALR database) were included. Only those patients with a temozolomide prescription within 30 days of (before or after) the first day of radiotherapy (from ALR or ODB databases) were included. The Index Date was defined as the first day of radiotherapy (or day of planned radiotherapy if none was delivered). For each patient with more than one course of concomitant chemoradiotherapy, we included only the first course and used that first treatment date as the Index Date. The maximum follow-up date was December 31, 2020. The lookback window was 2 years.
Patients < 18 years or > 105 years were excluded. Patients were excluded if they were missing an ICES patient “IKN” identifier, missing age or sex, or if they were a non-Ontario resident. Patients who died on or before the Index Date were excluded. Patients with a radiotherapy body region codes outside the central nervous system were excluded. Those with a hospital discharge diagnosis of PCP prior to Index Date were excluded. Known HIV diagnosis was an exclusion. Patients on baseline non-dexamethasone prolonged high-dose steroids were excluded. These patients were identified using ODB prescriptions: (1) steroid prescription (ie, prednisone, cortisone, hydrocortisone, methylprednisolone, or prednisolone); (2) duration of prescription is more than 30 days; (3) prescription within 90 days prior to Index Date; (4) prednisone equivalent dose > 15 mg daily.
Exposure Variable
The “prophylaxis” cohort were patients who received a prescription for a PCP-preventing antibiotic up to 30 days before the Index Date. The antibiotics for PCP prophylaxis in the ODB database are TMP-SMX (co-trimoxazole), dapsone, and atovaquone. Information on aerosolized pentamidine prescriptions is not available.
The “no prophylaxis” cohort were those who did not receive a prescription for a PCP-preventing antibiotic between 0 and 30 days before the Index Date. Patients who had ODB temozolomide coverage ought to have ODB coverage for antibiotics, too. Most patients without ODB coverage will be excluded from the cohort (except those with ALR antibiotic and temozolomide records). For the purposes of this analysis, all antibiotic prescriptions must have been for >14 days.
Outcome Variables
The primary outcome was risk of PCP with antibiotic prophylaxis compared with no prophylaxis. Because PCP is a serious infection, we expected that essentially all patients diagnosed with it are hospitalized. As a result, hospital discharge diagnoses ought to be an accurate way of finding incident cases of PCP. In the DAD, we looked for patients with the ICD-10-CA code B24 for a diagnosis of PCP within 90 days of the Index Date.
There were three secondary outcomes: (1) overall survival, (2) rate of hospitalization for any reason, and (3) rate of severe neutropenia—absolute neutrophil count < 1.0 × 109/L. Data sources for secondary outcomes are outlined in the Supplementary Materials.
There were several exploratory outcomes. An exploratory antibiotic efficacy outcome was risk of any pneumonia diagnosis, based on Ontario Health Insurance Plan (OHIP) billing codes. Exploratory antibiotic safety outcomes included risk of hyperkalemia and risk of chemotherapy delays (via anemia, thrombocytopenia, neutropenia).
Covariates
Model covariates were selected a priori based on clinical relevance. Where variable collinearity was hypothesized, the most relevant variable was selected for inclusion in the model. The variables included in the propensity model and additional variables are shown in Table 1.
Table 1.
Patient Demographic and Clinical Variable Definitions
| Variable | Data source(s) | Calculation | Included in propensity score model |
|---|---|---|---|
| Age | RPDB | At the time of Index Date | Yes |
| Sex | RPDB | – | Yes |
| Deprivation index | Ontario Marginalization Index, Statistics Canada census results | Based on patient’s neighborhood; quintile 1 is the least marginalized | Yes |
| Elixhauser comorbidity index | DAD | Two-year lookback | Yes |
| Diagnosis | OCR | Based on ICD-O-3 codes | Yes |
| Time since neurosurgery | OCR, DAD | Time from first recorded brain surgery to Index Date | Yes |
| Extent of surgery | DAD | Based on administrative codes for surgical operations; either biopsy or resection | Yes |
| Dexamethasone dose | ODB | Prescriptions between –30 and 0 days; daily dose calculated from prescribed dose, quantity, and duration | Yes |
| Radiation dose per fraction | ALR | Dose per fraction for the first radiation treatment after the Index Date | Yes |
| Income quintile | RPDB, Statistics Canada census results | Median household income of patient’s neighborhood; quintile 5 has the highest income | No—collinearity with deprivation index |
| Rurality | RPDB | Binary rural versus urban classification based on postal code | No—not thought to correlate with outcomes |
| Baseline leukocyte count | OLIS | The latest leukocyte count between the Index Date and 30 days prior | No—too much missingness |
| Baseline lymphocyte count | OLIS | The latest lymphocyte count between the Index Date and 30 days prior | No—too much missingnes |
| Tumor grade | OCR | Based on ICD-O-3 codes | No—collinearity with diagnosis |
| Tumor location | OCR | Based on ICD-O-3 topography codes | No—not thought to correlate with outcomes |
| Year of diagnosis | OCR | – | No—not thought to correlate with outcomes |
| Temozolomide dose | ODB | Initial daily temozolomide dose, calculated from prescribed dose, quantity, and duration | No—not enough variability in dose per body surface area |
| Concomitant radiotherapy duration | ALR | Number of contiguous (maximum 14-day break) radiation treatment days | No—collinearity with dose per fraction |
| Concomitant radiotherapy cumulative dose | ALR | Sum of dose of contiguous radiation treatments | No—collinearity with dose per fraction |
| Trough lymphocyte count | OLIS | Minimum lymphocyte count between 0 and 90 days after Index Date | No—time-varying covariate |
| Hospital neuro-oncology volume | ALR | Treating hospital glioma radiotherapy volume tertile—high, medium, low volume | No—instrumental variable |
RPDB, Registered persons database; DAD, Discharge abstract database; OLIS, Ontario Laboratories Information System; OCR, Ontario Cancer Registry; ALR, Cancer Activity Level Reporting.
Analytic Methods and Statistics
Given that PCP is a rare infection, we expected very few cases in either the PCP prophylaxis or the no prophylaxis cohorts. A multivariable logistic regression model was thus inappropriate. To overcome this limitation, we used inverse probability of treatment weighting (IPTW) using the propensity score.14 A propensity score model was created that incorporated all available variables that were felt to be potentially prognostic for the binary outcome (ie, 90-day PCP infection). Variables with presumed collinearity were not included in the initial model. Instrumental variables, not thought to be associated with the outcome, were also excluded. We estimated the average treatment effect. The propensity score was defined as the probability of receiving PCP-preventing antibiotics (vs no antibiotics) conditional on the covariates included in the analysis (see Table 1).
A logistic regression model was used to model the propensity score. Large weights were trimmed such that weights that exceeded the 99th percentile of weights were set equal to the 99th percentile, and weights below the first percentile were set to the first percentile. Weights were stabilized to improve variance estimation.14 Balance tables were created. The propensity score model was adjusted in an iterative fashion until a good balance between groups was achieved.
Once the final propensity score model was ready, weighted logistic regression was performed on the binary outcome of 90-day PCP infection. For the weighted logistic regression, the R “svydesign” package was used.15,16 Point estimates were calculated for risk of PCP infection with and without antibiotics. From this, both absolute risk reduction and number needed to treat (NNT) could be calculated. For confidence intervals of absolute risk reduction and NNT, a standard calculation was performed.17
For the overall survival analysis, weighted Kaplan–Meier plots were created based on the exposure group. An IPTW-weighted Cox proportional hazard model was created. For the any hospitalization analysis, a propensity score–weighted Poisson regression model was fit to hospitalization rates. A likelihood ratio test compared a model with the exposure group to one without the exposure group, to determine whether PCP prophylaxis affects overall hospitalization rate. For the myelosuppression analysis, risks of different grades of Common Terminology Criteria for Adverse Events (CTCAE) neutropenia, thrombocytopenia, or anemia were compared between the PCP prophylaxis and no prophylaxis cohorts. Patients with no relevant blood tests were removed from each analysis. Unweighted risks of these were compared between exposure groups using Chi-square tests. Using an IPTW-adjusted logistic regression model, point estimates were calculated for risk of grade 3/4 neutropenia with and without antibiotics. From this, both absolute risk increase and number needed to harm (NNH) were calculated.
Results
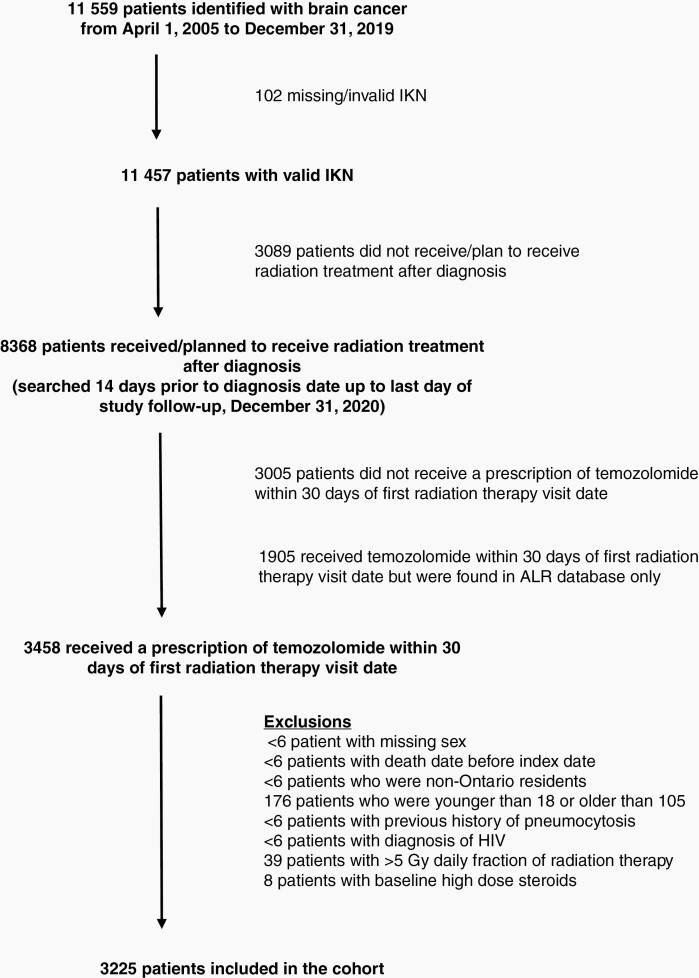
Cohort
There were 3225 patients included in the study. Of all patients with gliomas identified in the study period, 73% received radiotherapy (see Figure 1). Of these patients, 36% were excluded because they did not receive a prescription for concomitant temozolomide. Almost 23% of patients had a prescription for temozolomide noted in the ALR database, but not in the ODB database, so they were also excluded (since no information about antibiotic prescriptions could be inferred). The other exclusions were rare.
Fig. 1.
Patient inclusion and exclusion flow diagram. IKN, ICES number; ALR, Cancer Activity Level Reporting; HIV, human immunodeficiency virus.
There were 2,577 patients in the “no prophylaxis” cohort. Within this cohort, 2,434 patients (80%) had no antibiotic prescriptions at all, 143 had an antibiotic prescription between day 1 and 30 after Index Date, and 44 patients had a TMP-SMX prescription before Index Date but less than 14 days prescription duration. There were 648 patients (20%) in the “prophylaxis” cohort. Within this cohort, almost all prescriptions were for TMP-SMX and <6 were for dapsone; there were eight patients with a TMP-SMX prescription noted in the ALR database, but not in the ODB database.
The baseline characteristics of the cohorts are presented in Table 2, with and without propensity score adjustment by IPTW. The median (unadjusted) age was 63 years in the “no prophylaxis” group and 65 in the “prophylaxis” group. See Supplementary Materials for details about construction of the propensity score model. There was some baseline imbalance in baseline dexamethasone, in radiation dose, in surgery type, and age, but these covariates were balanced after IPTW adjustment. There was also baseline imbalance in the radiation treatment center volume, which was not included in the propensity score volume. In the high-volume centers, only 1% of patients received PCP prophylaxis; in the medium volume centers, 52% received prophylaxis; and in the low volume centers, 19% received prophylaxis (see Table 3).
Table 2.
Baseline Patient Characteristics Before and After Propensity Score Adjustment
| Variable | No prophylaxis (N = 2,577) |
Prophylaxis (N = 648) |
Abs. SMD | No prophylaxis | Prophylaxis | Abs. SMD |
|---|---|---|---|---|---|---|
| Crudea | Crudea | Crude | Adjusted | Adjusted | Adjusted | |
| Age (years) | 59.1 | 61.25 | 0.156 | 59.58 | 60.22 | 0.047 |
| Sex (proportion female) | 1074 (41.7%) | 247 (38.1%) | 0.073 | 41% | 41.9% | 0.017 |
| Deprivation index | 2.84 | 2.84 | 0.004 | 2.84 | 2.79 | 0.038 |
| Elixhauser comorbidity index | 2.15 | 2.49 | 0.088 | 2.23 | 2.24 | 0.003 |
| Histology | ||||||
| Glioblastoma | 1957 (75.9%) | 483 (74.5%) | 0.032 | 75.6% | 75% | 0.014 |
| Diffuse astrocytoma | 126 (4.89%) | 33 (5.09%) | 0.009 | 5% | 5.3% | 0.014 |
| Anaplastic astrocytoma | 230 (8.93%) | 43 (6.64%) | 0.092 | 8.5% | 8.8% | 0.011 |
| Oligodendroglioma | 53 (2.06%) | 19 (2.93%) | 0.052 | 2.2% | 2.3% | 0.003 |
| Anaplastic oligodendroglioma | 115 (4.46%) | 23 (3.55%) | 0.049 | 4.3% | 4.2% | 0.003 |
| Unspecified glioma | 96 (3.73%) | 47 (7.25%) | 0.136 | 4.4% | 4.4% | 0.001 |
| Extent of surgery | ||||||
| Biopsy | 413 (16.0%) | 151 (23.3%) | 0.172 | 17.3% | 16.5% | 0.021 |
| Resection | 2069 (80.3%) | 470 (72.5%) | 0.174 | 78.9% | 79.7% | 0.021 |
| None | 95 (3.69%) | 27 (4.17%) | 0.024 | 3.8% | 3.8% | 0.003 |
| Time from diagnosis to index (months) | 3.56 | 4.56 | 0.002 | 3.74 | 3.61 | 0.0003 |
| Baseline daily dexamethasone dose (mg) | 3.49 | 5.21 | 0.342 | 3.83 | 3.99 | 0.034 |
| Radiation dose per fraction | ||||||
| 0 to 2 Gy | 67.7% | 54.5% | 0.266 | 65% | 64.1% | 0.018 |
| 2.1 to 3 Gy | 29.9% | 43.5% | 0.274 | 32.7% | 33.5% | 0.017 |
| 3.1 to 5 Gy | 1.4% | 1.5% | 0.012 | 1.4% | 1.5% | 0.003 |
| Unknown | 1% | < 6 | 0.075 | 0.9% | 0.9% | 0.005 |
Abs. SMD, absolute standardized mean difference.
aMean or N and proportion.
Table 3.
Additional Unadjusted Patient Characteristics
| Variable | No prophylaxisa (N = 2,577) |
Prophylaxisa (N = 648) |
|---|---|---|
| Treating hospital patient volume | ||
| Lowest tertile | 924 (35.9%) | 213 (32.9%) |
| Medium tertile | 395 (15.3%) | 420 (64.8%) |
| Highest tertile | 1258 (48.8%) | 15 (2.31%) |
| Index year | ||
| 2005–2009 | 468 (18.2%) | 157 (24.2%) |
| 2010–2014 | 894 (34.7%) | 149 (23.0%) |
| 2015–2020 | 1215 (47.1%) | 342 (52.8%) |
| Rurality | ||
| Urban | 2237 (86.8%) | 504 (77.8%) |
| Rural | 340 (13.2%) | 144 (22.2%) |
| Elixhauser comorbidity index | ||
| Low (< 4) | 1808 (70.2%) | 427 (65.9%) |
| High (≥ 4) | 769 (29.8%) | 221 (34.1%) |
| Baseline daily dexamethasone | ||
| None | 1338 (51.9%) | 207 (31.9%) |
| Nonzero but < 3 mg daily | 87 (3.3%) | 48 (7.4%) |
| Dose ≥ 3 mg daily | 1152 (44.7%) | 393 (60.6%) |
| Total radiation dose (Gy) | 52.7 (11.4) | 50.4 (13.7) |
| Total radiation treatments (days) | 25.7 (7.37) | 24.3 (8.14) |
| Temozolomide dose during radiation (mg/day) | 143 (31.4) | 145 (22.9) |
| Tumor location | ||
| Frontal lobe | 917 (35.6%) | 234 (36.1%) |
| Occipital lobe | 128 (4.97%) | 34 (5.25%) |
| Parietal lobe | 418 (16.2%) | 102 (15.7%) |
| Temporal lobe | 741 (28.8%) | 206 (31.8%) |
| Baseline lymphocyte count (×109/L) | 1.45 (1.37) | 1.41 (0.98) |
aNumber and proportion or mean and standard deviation.
PCP
In both the “prophylaxis” and “no prophylaxis” cohorts, PCP was rare. Overall there were 18 patients with PCP within 90 days. Most patients who developed PCP did so within 90 days of the Index Date, with <6 patients developing PCP between 90 days and 365 days after Index Date. Of the 18 patients who were hospitalized for PCP within 90 days of their Index Date, <6 patients received antibiotics for PCP prophylaxis. When looking at all patients who developed PCP within 1 year of the Index Date, the unadjusted baseline lymphocyte count did not differ compared to the remainder of the cohort (who did not develop PCP): 1.39 × 109/L versus 1.44 × 109/L (Student’s t-test P-value .7). The baseline dexamethasone dose also did not differ between these groups: 3.84 mg/day in no PCP group versus 2.85 mg/day in PCP group (Student’s t-test P-value .3).
None of the patients in this province-wide, multi-year cohort died of PCP (ie, none had PCP listed as the cause of death). All patients who had PCP listed as a diagnostic code for an emergency department visit subsequently had PCP listed as a discharge diagnosis for an inpatient hospital admission.
In the IPTW analysis, the adjusted risk of PCP without antibiotics was 0.59% (95% CI, 0.36 to 0.97). The adjusted risk of PCP with antibiotics was 0.24% (95% CI, 0.06 to 0.99). The adjusted relative risk reduction with antibiotics was 2.42. The adjusted absolute risk reduction with antibiotics was 0.0035 (95% CI, −0.0013 to 0.0083). Note that this 95% CI crosses zero. As a result, the adjusted NNT with antibiotics to prevent one hospitalization with PCP within 90 days was 288 (95% CI, NNH 744 to ∞ to number needed to benefit 121). The use of PCP prophylaxis was associated with a nonsignificant reduction in the odds of developing PCP: odds ratio 0.41 (95% CI, 0.09 to 1.81), P = .24.
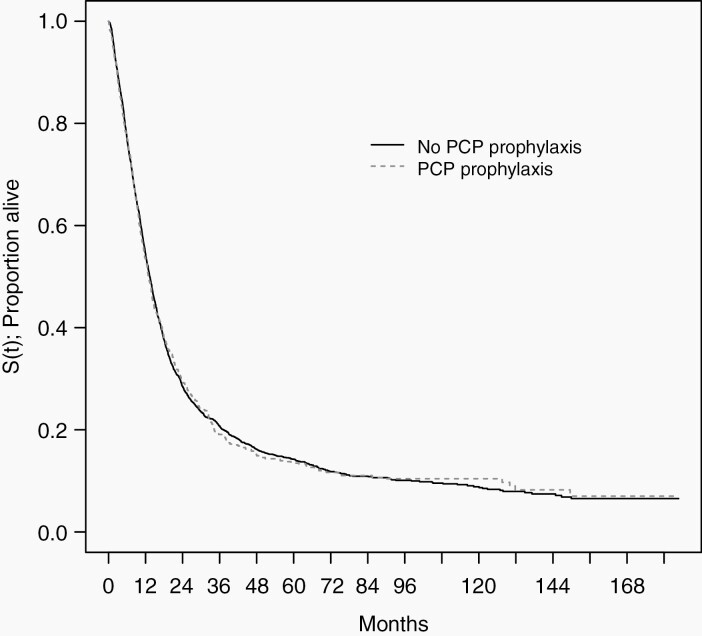
Overall Survival
The unadjusted median survival in the overall cohort was 13.3 months. Among patients with glioblastoma, the median survival was 11.4 months. The unadjusted median survival among patients receiving PCP prophylaxis was 12.0 months compared with 13.7 months in the no prophylaxis group. The IPTW-adjusted median survival among patients receiving PCP prophylaxis was 13.0 months compared with 13.4 months in the no prophylaxis group (see Figure 2). The survival curves are superimposable. In a single-variable Cox proportional hazards model (adjusted using weights from the propensity score model), the hazard ratio for PCP prophylaxis was 0.996 (95% CI, 0.906 to 1.11), Wald P-value = .94. This indicates no association between PCP prophylaxis and overall survival.
Fig. 2.
Inverse probability of treatment weighting–adjusted Kaplan–Meier plot of overall survival by exposure. PCP, pneumocystis pneumonia.
Any Hospitalizations
The propensity score–weighted Poisson regression model had a rate ratio for PCP prophylaxis versus no prophylaxis of 0.92 (95% CI, 0.79 to 1.07). A likelihood ratio test comparing this model to a model without the PCP prophylaxis variable revealed a P-value of .27, indicating low likelihood of PCP prophylaxis affecting overall hospitalizations. Patients had infrequent hospitalizations. In the no PCP prophylaxis group, 178 (7.4%) were hospitalized more than once after Index Date, compared with 44 (7.3%) in the PCP prophylaxis group.
Myelosuppression
Bloodwork data (through Ontario Laboratories Information System) were available for 1,656 (64%) patients without PCP prophylaxis and 412 (64%) patients with PCP prophylaxis. Between the Index Date and 90 days, there was a median of six complete blood counts per patient (range 1 to 120) among those who had bloodwork. Neither anemia nor thrombocytopenia was more common in the PCP prophylaxis group than the no prophylaxis group. Leukopenia was more common in the PCP prophylaxis cohort than the no prophylaxis cohort, and this was driven by neutropenia (see Table 4). The propensity score–weighted risk of CTCAE grade 3 or 4 neutropenia within 90 days was 3.1% without PCP prophylaxis and 5.6% with PCP prophylaxis. The absolute risk increase (with PCP prophylaxis) was 2.5% (95% CI, 0.6 to 4.4) with a corresponding NNH of 39 patients (95% CI, 23 to 155). The use of PCP prophylaxis was associated with a 1.82 increase in the odds of developing grade 3 or 4 neutropenia (95% CI for odds ratio, 1.19 to 2.80).
Table 4.
Unadjusted Severity of Myelosuppression by Antibiotic Prophylaxis Status
| Cytopenia | CTCAEGrade | No prophylaxisa | Prophylaxisa | P-valueb |
|---|---|---|---|---|
| Leukopenia | 1 | 152 (5.90%) | 45 (6.94%) | 0.5 |
| 2 | 137 (5.32%) | 53 (8.18%) | 0.02 | |
| 3 | 65 (2.52%) | 27 (4.17%) | 0.07 | |
| 4 | 43 (1.67%) | 12 (1.85%) | 0.9 | |
| Neutropenia | 1 | 117 (4.54%) | 55 (8.49%) | <0.001 |
| 2 | 97 (3.76%) | 40 (6.17%) | 0.02 | |
| 3 | 67 (2.60%) | 28 (4.32%) | 0.04 | |
| 4 | 49 (1.90%) | 18 (2.78%) | 0.2 | |
| Anemia | 1 | 561 (21.8%) | 142 (21.9%) | 0.95 |
| 2 | 171 (6.64%) | 44 (6.79%) | 0.95 | |
| 3 | 48 (1.86%) | 12 (1.85%) | 0.97 | |
| 4 | 15 (0.58%) | < 6 | > 0.9 | |
| Thrombocytopenia | 1 | 405 (15.7%) | 121 (18.7%) | 0.1 |
| 2 | 132 (5.12%) | 32 (4.94%) | 0.96 | |
| 3 | 102 (3.96%) | 27 (4.17%) | 0.93 | |
| 4 | 69 (2.68%) | 12 (1.85%) | 0.5 |
CTCAE, Common Terminology Criteria for Adverse Events.
aNumber and proportion.
bChi-square test comparing presence, absence, and missing.
Exploratory Outcomes
There did not seem to be a difference in 90-day any pneumonia diagnosis between the groups: 158 (13.8%) without prophylaxis and 49 (12.5%) with prophylaxis, Chi-square P-value .622.
Hyperkalemia was more common in patients receiving PCP prophylaxis than those not receiving PCP prophylaxis in an unadjusted analysis. TMP-SMX seemed to especially cause grade 1 hyperkalemia, and to a lesser degree caused grade 2, 3, or 4 hyperkalemia. In the no prophylaxis group, the rates of grade 1–4 CTCAE hyperkalemia were 4.5%, 3.8%, 2.6%, and 1.9%, compared with 8.5%, 6.2%, 4.3%, and 2.8% (P-values <.001, .02, .04, and .2, respectively).
Based on bloodwork cutoffs for temozolomide continuation (ie, platelets > 100, hemoglobin > 100, and neutrophils > 1.5), potential temozolomide interruptions were estimated. The risk of potential concomitant temozolomide interruption was 7% with or without PCP prophylaxis (P-value 0.6). The risk of any potential adjuvant chemotherapy interruption was 23% without prophylaxis and 30% with prophylaxis (P-value 0.001). The average patient potentially missed 0.32 cycles of adjuvant temozolomide without prophylaxis and 0.54 cycles with prophylaxis (P-value < .001).
Discussion
In this large 15-year study of Ontario glioma patients treated with radiotherapy and concomitant temozolomide, the risk of PCP within 90 days of treatment was very low: 18 out of 3,225 (0.56%) with 80% of patients not receiving prophylaxis. The 0.56% risk of PCP in this Ontario cohort can be compared to other published papers. In the pre-temozolomide era, the risk of PCP in brain tumor patients was approximately 0.88%.18 The 0.56% risk is clearly lower than the 13% risk in a phase II trial that led to the monograph recommendations for PCP prophylaxis for all glioma patients receiving temozolomide chemoradiotherapy.1,19 In more modern series, the rates of PCP are low. At Oregon Health and Sciences University over 13 years, only one patient was diagnosed with PCP out of 252 who did not receive PCP prophylaxis (0.40%).9 At one center in Italy, 103 patients were treated for glioma without PCP prophylaxis and none developed PCP (ie, rate < 0.96%).8 These are similar to the rate we found.
Crucially, the IDSA looked at the risks and benefits of PCP prophylaxis, and decided that prophylaxis among cancer patients is only indicated if the risk of PCP is greater than 3.5%.4 In all but the original phase II trial data, the rate of PCP among temozolomide-treated glioma patients is decidedly less than 3.5%.
Despite the temozolomide monograph recommendation of PCP prophylaxis for all glioma patients receiving temozolomide chemoradiotherapy, we would need to treat 288 patients with PCP prophylaxis to prevent one PCP hospitalization within 90 days. Our findings of a high NNT to prevent PCP infection contrast with the lower numbers found in the Green et al. meta-analysis (which did not look at temozolomide).3 They found that antibiotic PCP prophylaxis was effective at preventing PCP (NNT = 15) among non-HIV immunocompromised patients with either leukemia or organ transplantation. This is well outside the 95% NNT confidence interval found in our study, where the best possible NNT in the interval was 121. The difference in NNTs can be ascribed to (1) different patient populations and (2) publication bias which became incorporated into the meta-analysis.
Some neuro-oncologists use baseline dexamethasone dose or lymphocyte counts to try and stratify patients for risk of PCP and thus need for PCP prophylaxis.20,21 Based on a dexamethasone sensitivity analysis (see Supplementary Materials), it seems that the benefit of PCP prophylaxis is low even in this higher risk group. We showed that the baseline lymphocyte count did not differ (1.43 × 109/L versus 1.44 × 109/L) between patients who developed PCP and those who did not. It thus does not make sense to use baseline dexamethasone dose nor baseline lymphocyte to select patients to receive PCP prophylaxis.
Patients who remain on high-dose steroids for a prolonged period of time (ie, >4 weeks) are at higher risk of developing PCP. Patients who go on to develop severe lymphopenia are at higher risk of PCP, too. We showed that patients who developed PCP had a trough lymphocyte count of 0.39 × 109/L compared with 0.74 × 109/L in the rest of the cohort. Thus, choosing PCP prophylaxis antibiotics just for patients on prolonged dexamethasone or those who develop severe lymphopenia can be supported. This treatment strategy requires ongoing monitoring and vigilance.
There was no change in survival or hospitalizations with PCP prophylaxis. There were important bloodwork abnormalities more common with PCP prophylaxis. The NNH by causing grade 3 or 4 neutropenia with PCP prophylaxis was 39 (with a rate of neutropenia with prophylaxis of 5.6%). This neutropenia likely translated to missed doses of adjuvant temozolomide (unadjusted NNH = 14). Even though more patients in the PCP prophylaxis group missed adjuvant temozolomide cycles, their overall survival did not differ. There was a higher rate of hyperkalemia with TMP-SMX, but this was likely of no clinical consequence.
Few others have published about the toxicities of PCP prophylaxis in this population. Streeter et al. compared 32 chemoradiotherapy patients who received TMP-SMX with 35 who received no prophylaxis.22 The rate of dose-limiting neutropenia was 3.1% versus 2.9% in the two groups, respectively, with wide confidence margins. Our study’s larger sample size gives a more stable estimate of the risk of neutropenia with TMP-SMX.
Our analysis revealed wide discrepancies in patterns of PCP prophylaxis prescribing between cancer centers in Ontario. It is exactly because of this discrepancy that all analyses in this natural experiment were possible. The field of neuro-oncology is small in Ontario. As of 2021, there are nine neurology-trained neuro-oncologists practicing in Ontario; there are also < 20 medical oncologists who treat brain cancer. The antibiotic prescribing patterns of each oncologist have an outsized influence on that center’s prescribing patterns. The antibiotic prescribing patterns in centers where the oncologist trained likely strongly influenced their future prescribing patterns.
In this Ontario-wide study of PCP prophylaxis in glioma patients receiving temozolomide chemoradiotherapy, the risks of PCP prophylaxis seemed to outweigh the benefits. If we treated 288 Ontario glioma patients with antibiotic PCP prophylaxis, one case of PCP would be prevented, but there would be seven cases of severe neutropenia and approximately 21 patients would miss doses of chemotherapy due to myelosuppression. We would not expect a difference in overall survival or hospitalizations, and we would not expect a difference in PCP-attributable death. These data suggest that the risks of prophylaxis outweigh the benefits.
The risk-benefit analysis of PCP prophylaxis for temozolomide chemoradiotherapy patients depends on the baseline incidence of PCP in a given location. We know that there is regional variation in PCP incidence, in part due to the number of susceptible (i.e. immunocompromised) hosts, in part due to a given strain’s transmissibility, and in part due to natural reservoirs of the fungus.23,24 In areas with high population risk of PCP (i.e. incidence much greater than 1.6 per 100,000 per year), Ontario’s data will not apply. Wherever the risk of PCP among patients receiving temozolomide chemoradiotherapy is > 3.5%, following IDSA guidelines and prescribing antibiotics is warranted.
In Ontario, the risk of PCP in patients receiving temozolomide chemoradiotherapy is 0.56%. In Ontario, the annual risk of PCP in the general population is 1.6 per 100,000. A crude calculation estimates that the population annual risk of PCP would have to be 10 per 100,000 per year for the temozolomide-specific risk to be > 3.5%. It stands to reason that the routine use of PCP prophylaxis is not indicated unless the population annual risk of PCP is > 10 per 100,000.
This study has several strengths, but also some limitations. This is by far the largest study of PCP infections among glioma patients receiving temozolomide chemoradiotherapy. Our cohort was 3225 patients; the largest comparable cohort in the literature was just 252 patients.9 This highlights the power of large administrative datasets to answer focused clinical questions. The propensity score analysis in our study accounts, to some degree, for confounding by indication. We believe that the important baseline covariates are captured for our cohort.
The fact that PCP was rarely seen in this cohort is itself a valuable insight, indicating that PCP prophylaxis is not likely warranted according to IDSA guidelines. Still, the small number of patients who developed PCP limits potential statistical conclusions and limits our ability to perform multivariable logistic regression. Even with the IPTW propensity score analysis, there is residual risk of confounding by indication. The cohort is not representative of the entire Ontario glioma cohort. Only patients with ODB-funded temozolomide, and patients < 65 years with ODB access are in this cohort, so 36% (i.e. 1905/5363) of Ontarians who received temozolomide chemoradiotherapy were excluded. Patients < 65 years without ODB access are different from those with access. Most patients without ODB coverage were excluded from the no prophylaxis cohort (except those with ALR antibiotic and temozolomide records). Some patients were likely treated with pentamidine but were misclassified as not having received PCP prophylaxis. Pentamidine is rarely used for PCP prophylaxis among medium- and high-volume centers in Ontario. Still, this misclassification bias could skew the results and make TMP-SMX look less effective than it really is. Only patients hospitalized with PCP will be classified as having PCP, but most PCP diagnoses require inpatient hospitalization anyway for diagnosis and initial management. There is a chance for confounding by cancer center patient volume. The cause of death analysis might be underpowered since most patients with glioma will have the glioma listed as cause of death, rather than a more proximate cause.
These results cannot be extrapolated to other antibiotics (such as dapsone, atovaquone, or pentamidine). Almost all patients who received PCP prophylaxis received TMP-SMX. The efficacy and safety profiles of dapsone, pentamidine, and atovaquone in this patient population are separate and unanswered questions. Given the prescribing patterns of Ontario physicians, these questions cannot be answered using ICES data.
In conclusion, patients with brain cancer receiving temozolomide chemoradiotherapy are at risk for PCP, a serious fungal pneumonia, but this risk is low even without the use of antibiotics. In this large, province-wide analysis of PCP among patients who received temozolomide chemoradiotherapy, the risks of PCP prophylaxis seemed to outweigh the benefits. The use of PCP-preventing antibiotics, especially TMP-SMX, reduced the risk of PCP slightly. Patients treated with or without PCP prophylaxis had no difference in overall survival or in hospitalizations. Patients receiving PCP prophylaxis had a higher risk of grade 3/4 neutropenia, more potential temozolomide dose interruptions, and more hyperkalemia. Antibiotics can likely be reserved for patients thought to be at high risk of developing PCP such as those on prolonged high-dose steroids or those who develop low through lymphocyte counts, but these factors cannot always be determined at the baseline assessment. Otherwise, based on the findings of our study, the routine use of PCP antibiotic prophylaxis cannot be recommended for glioma patients receiving temozolomide and radiotherapy in a setting of low background risk of PCP. The FDA, Health Canada, and other similar entities should update their requirement for PCP prophylaxis with temozolomide chemoradiotherapy.
Supplementary Material
Acknowledgments
Parts of this material are based on data and information compiled and provided by Ontario Ministry of Health. The analyses, conclusions, opinions, and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred. Parts of this material are based on data and/or information compiled and provided by CIHI. However, the analyses, conclusions, opinions and statements expressed in the material are those of the author(s), and not necessarily those of CIHI. We thank IQVIA Solutions Canada Inc. for use of their Drug Information File. Parts of this material are based on data and information provided by Ontario Health. The opinions, results, view, and conclusions reported in this paper are those of the authors and do not necessarily reflect those of Ontario Health. No endorsement by Ontario Health is intended or should be inferred. We thank the Toronto Community Health Profiles Partnership for providing access to the Ontario Marginalization Index.
Contributor Information
Seth A Climans, Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Ontario, Canada; Department of Oncology, Western University, London, Ontario, Canada.
Eva Grunfeld, Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Ontario, Canada.
Warren P Mason, Department of Medicine, Division of Neurology, University of Toronto, Toronto, Ontario, Canada; Department of Medical Oncology and Hematology, University of Toronto, Toronto, Ontario, Canada.
Kelvin K W Chan, Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Ontario, Canada.
Funding
This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health (MOH) and the Ministry of Long-Term Care (MLTC). This study also received funding from the Canadian Centre for Applied Research in Cancer Control and is funded by the Canadian Cancer Society (grant 2015-703549).
Conflict of interest statement. None of the authors has a conflict of interest.
Authorship statement. Manuscript design and conception: S.A.C., K.K.W.C. Statistical analysis: S.A.C. Critical revision of manuscript for intellectual content: S.A.C., E.G., W.P.M., K.K.W.C.
References
- 1. Stupp R, Dietrich PY, Kraljevic SO, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20(5):1375–1382. [DOI] [PubMed] [Google Scholar]
- 2. Cohen MH, Johnson JR, Pazdur R. Food and Drug Administration Drug approval summary: temozolomide plus radiation therapy for the treatment of newly diagnosed glioblastoma multiforme. Clin Cancer Res 2005;11(19 Pt 1):6767–6771. [DOI] [PubMed] [Google Scholar]
- 3. Green H, Paul M, Vidal L, Leibovici L. Prophylaxis of pneumocystis pneumonia in immunocompromised non-HIV-infected patients: systematic review and meta-analysis of randomized controlled trials. Mayo Clin Proc. 2007;82(9):1052–1059. [DOI] [PubMed] [Google Scholar]
- 4. Taplitz RA, Kennedy EB, Flowers CR. Antimicrobial prophylaxis for adult patients with cancer-related immunosuppression: ASCO and IDSA clinical practice guideline update summary. J Oncol Pract. 2018;14(11):692–695. [DOI] [PubMed] [Google Scholar]
- 5. AA Pharma. Sulfatrim Product Monograph; 2019:48. [Google Scholar]
- 6. Yale SH, Limper AH. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illnesses and prior corticosteroid therapy. Mayo Clin Proc. 1996;71(1):5–13. [DOI] [PubMed] [Google Scholar]
- 7. De Vos FY, Gijtenbeek JM, Bleeker-Rovers CP, van Herpen CM. Pneumocystis jirovecii pneumonia prophylaxis during temozolomide treatment for high-grade gliomas. Crit Rev Oncol Hematol. 2013;85(3):373–382. [DOI] [PubMed] [Google Scholar]
- 8. Rifino N, Rigamonti A, Guida FM, et al. Lack of development of Pneumocystis jirovecii pneumonia in a cohort of 103 Italian glioblastoma patients not receiving prophylaxis during post-surgical chemoradiotherapy. J Neurol Sci. 2019;405:116431. [DOI] [PubMed] [Google Scholar]
- 9. Neuwelt AJ, Nguyen TM, Fu R, et al. Incidence of Pneumocystis jirovecii pneumonia after temozolomide for CNS malignancies without prophylaxis. CNS Oncol. 2014;3(4):267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Juurlink D, Preyra C, Croxford R, et al. Canadian Institute for Health Information Discharge Abstract Database: A Validation Study. Toronto, ON: Institute for Clinical Evaluative Sciences; 2006. [Google Scholar]
- 11. Goel V, Williams JI, Anderson GM, et al., eds. The ICES Practice Atlas: Patterns of Health Care in Ontario. Ottawa, ON: Canadian Medical Association; 1996. [Google Scholar]
- 12. Robles SC, Marrett LD, Aileen Clarke E, Risch HA. An application of capture-recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol. 1988;41(5):495–501. [DOI] [PubMed] [Google Scholar]
- 13. Mittmann N, Porter JM, Rangrej J, et al. Health system costs for stage-specific breast cancer: a population-based approach. Curr Oncol 2014;21(6):281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Austin PC, Yu AYX, Vyas MV, Kapral MK. Applying propensity score methods in clinical research in neurology. Neurology 2021;97(18):856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. http://www.R-project.org/ [Google Scholar]
- 16. Lumley T. Package “Survey.”; 2012. https://cran.r-project.org/web/packages/survey/survey.pdf [Google Scholar]
- 17. Schechtman E. Odds ratio, relative risk, absolute risk reduction, and the number needed to treat—which of these should we use? Value Health. 2002;5(5):431–436. [DOI] [PubMed] [Google Scholar]
- 18. Mahindra AK, Grossman SA. Pneumocystis carinii pneumonia in HIV negative patients with primary brain tumors. J Neurooncol. 2003;63(3):263–270. [DOI] [PubMed] [Google Scholar]
- 19. Merck Canada Inc. Temodal® Product Monograph: Consumer Information; 2019:48. [Google Scholar]
- 20. Chen JY, Hovey E, Rosenthal M, Livingstone A, Simes J. Neuro-oncology practices in Australia: a Cooperative Group for Neuro-Oncology patterns of care study. Asia Pac J Clin Oncol 2014;10(2):162–167. [DOI] [PubMed] [Google Scholar]
- 21. Skorupan N, Ranjan S, Mehta S, et al. Pneumocystis jirovecii prophylaxis in patients treated for high-grade gliomas: a survey among neuro-oncologists. Neuro-Oncol Pract. 2019;6(4):321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Streeter JC, Cohen A, Gilreath J, Sageser D, Ye X. Choice of Pneumocystis jiroveci pneumonia prophylaxis does not increase hematologic toxicity of temozolomide and radiation in patients with glioblastoma. Neuro-Oncology. 2012;14:vi65– vi85. [Google Scholar]
- 23. Beck JM. Pneumocystis carinii and Geographic Clustering. Am J Respir Crit Care Med. 2000;162(5):1605–1606. [DOI] [PubMed] [Google Scholar]
- 24. De Armas Rodríguez Y, Wissmann G, Müller AL, et al. Pneumocystis jirovecii pneumonia in developing countries. Parasite J Société Fr Parasitol 2011;18(3):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.