Abstract
Background
Sub-Saharan African (SSA) neuro-oncologists report high workloads and challenges in delivering evidence-based care; however, these reports contrast with modeled estimates of adult neuro-oncology disease burden in the region. This scoping review aimed to better understand the reasons for this discrepancy by mapping out the SSA adult brain tumor landscape based on published literature.
Methods
Systematic searches were conducted in OVID Medline, Global Index Medicus, African Journals Online, Google Scholar, and faculty of medicine libraries from database inception to May 31, 2021. The results were summarized quantitatively and narratively. English and French peer-reviewed articles were included (title, abstract, and full text).
Results
Of the 819 records identified, 119 articles by 24 SSA countries (42.9%) were included in the final review. Odeku published the first article in 1967, and nine of the ten most prolific years were in the 21st century. The greatest contributing region was Western Africa (n = 58, 48.7%) led by Nigeria (n = 37, 31.1%). Central Africa had fewer articles published later than the other SSA regions (P = .61). Most studies were nonrandomized (n = 75, 63.0%) and meningiomas (n = 50, 42.0%) were the most common brain tumors reported. Less than 30 studies reported on adjuvant treatment or patient outcomes.
Conclusions
Most publications were hospital-based, and there was significant heterogeneity in the quality of evidence and reporting. This study highlights the need for rapid and sustainable investments and brain tumor research capacity in SSA.
Keywords: epidemiology, global neurosurgery, neuro-oncology, scoping review, Sub-Saharan Africa
Key Points.
Most adult brain Sub-Saharan African (SSA) research is noninterventional.
There are regional differences in adult neuro-oncology research and service delivery.
Importance of the Study.
This article identifies regional differences in the landscape of adult brain tumors in Sub-Saharan Africa (SSA), highlighting disparities in research output, disease epidemiology, service delivery, and patient outcome. This review of the existing SSA adult neuro-oncology literature highlights a need for more and higher-quality evidence in the region, especially regarding population-level epidemiology, access to adjuvant therapies, and patient survival. The study findings will inform future research questions and capacity-building within the region.
Primary and secondary (metastatic) brain tumors contribute significantly to morbidity and mortality worldwide. Primary brain tumors are responsible for 0.34% of total disability-adjusted life years (DALYs) globally1; however, secondary brain tumors contribute to more disease burden. Secondary brain tumors are ten times as common as primary brain tumors.1,2 Secondary brain tumors are caused by at least three of the five primary organ cancers with the highest disease burden, and these three organ cancers account for 3.5% of the total global disease burden.1 For example, 10% of lung cancer patients,3 4% of colorectal cancer patients,4 and up to one in four breast cancer patients develop secondary brain tumors.5
Recent data suggest that the burden of disease attributable to brain tumors is not evenly distributed worldwide. In a 2018 review, Barnholtz-Sloan et al.6 estimated the age-standardized incidence rate of brain tumors between 6 and 8 per 100 000 people in most of Europe and North America and less than 2 per 100 000 in Sub-Saharan Africa (SSA). These statistics were generated from the World Health Organization’s International Agency for Research on Cancer (IARC) database, curating national mortality data.7 Unfortunately, most SSA countries do not report brain tumor data to the World Health Organization.7 Due to the lack of brain tumor registries, thus an absence of reliable data.6,8 The burden of disease data for cancers is often modeled for these countries, based on reported cases from neighboring countries.9 In 2020, this was the case for Burundi, Central African Republic, Chad, Comoros, the Democratic Republic of Congo, Djibouti, Equatorial Guinea, Eritrea, Lesotho, Madagascar, Sao Tome and Principe, Somalia, South Africa, South Sudan.7 In addition, many SSA countries report to international organizations, although they are often excluded from international publications at higher rates than other regions.7,10
The available modeled national and regional data have drawn attention to brain tumors in SSA. However, there has always been a discrepancy between the modeled statistics and observations on the ground. For example, it was widely accepted that certain brain tumors were rare or absent among black Africans; however, African neurosurgeons have since disproved these misconceptions.11–15 The discrepancies between practice and published data have been attributed to inadequate information management infrastructure, low scholarly output, suboptimally organized referral systems, and challenges to seeking, reaching, and receiving care.16–18
The past two decades have seen a rapid increase in specialist training, research, and organized activity in the region.19,20 These changes are best illustrated by the creation of the Society for Neuro-Oncology Sub-Saharan Africa (SNOSSA) in 2018 “to improve outcomes, sustaining hope for adult and pediatric brain tumor patients in [SSA].21” The first step to improving brain tumor outcomes in SSA is understanding the regional and national landscape through the lens of scholarly output on brain tumors. This will serve as a foundation for organizations such as SNOSSA to understand the different subspecialty human resources needs, infrastructural limitations, and needs in neuro-oncology and thus a springboard for the design and execution of educational opportunities and collaborations that will maximize the available resources on the continent and pave ways for the evolution of the contemporary neuro-oncology practice.
The authors conducted a scoping review to outline the epidemiology, management, and outcomes of adult brain tumors in SSA. The findings should help identify challenges, solutions, and high-, medium-, and low-performing countries within the region. This information will inform targeted interventions to strengthen SSA health systems and improve patient outcomes sustainably.
Materials and Methods
This scoping review is reported per PRISMA-ScR guidelines22 and conducted per the Arksey and O’Malley framework.23 First, the authors identified the research question through consultation of an expert panel of African neurosurgeons with neuro-oncological experience (J.A.B., C.K., and T.L.) and a researcher with formal information management training (U.S.K.) They decided to review the adult neuro-oncology literature in Africa based on previous experience and preliminary literature searches. Next, the authors identified relevant databases. The challenge was identifying relevant databases to render a comprehensive and accurate overview of adult neuro-oncology literature. Many African journals are not indexed in standard databases, and a significant proportion of research can only be found in institutional databases. The authors searched OVID Medline, Global Index Medicus, African Journals Online, Google Scholar, and faculty of medicine databases. These searches were complemented by a backward citation analysis of relevant articles and recommendations by regional focal points. The search strategy used is available in the Supplemental File 1.
A preliminary search was done, and the results were presented to the expert panel for calibration. From inception to May 31, 2021, the databases were searched for French and English articles, abstracts, reviews, case reports, letters to the editor, theses, memoirs, and other academic reports. The language choice was based on the authors’ fluency and a lack of funding to contract professional translation services. The authors included articles on or about neuro-oncology epidemiology, treatment, and practice in Africa irrespective of author affiliations.
The search results were imported into Rayyan,24 a free online screening platform. The citations were deduplicated, and the reviewers participated in a pilot review to ensure consistency and clarify ambiguities. The inclusion and exclusion criteria were refined following the pilot article review. Each title and abstract independently (J.A.B., C.K., L.T., or U.S.K.) All conflicts were resolved among the two reviewers, but persistent disagreements were arbitrated by a third reviewer. Next, the authors reviewed the full articles independently. Articles without full manuscripts were excluded if they were unavailable despite good-faith efforts, that is, searching for an online copy and contacting the corresponding author at least two times.
The data extraction sheet was designed in Microsoft Excel and collected information on the article title, author names, journal, country, publication language, publication year, study design, sample size, descriptive patient data, type of intervention, diagnosis, interventions, and outcomes. Two authors (A.D.N. and A.K.A.) independently extracted data from included studies after pilot data extraction. In comparison, three authors (J.A.B., C.K., and U.S.K.) examined the references to identify relevant articles. The authors requested disaggregated data from the corresponding authors of articles with aggregated pediatric and brain tumors. When the corresponding authors did not respond or could not share the disaggregated data, the articles were excluded.
The review process data were presented as a PRISMA flow diagram, and the article data were summarized in narrative and tabular forms. The results were presented to regional focal points, submitted for presentation at African and international conferences, and written up as a manuscript for publication in a peer-reviewed journal. The published manuscript will be promoted via social media using visual and video abstracts in French and English.
Results
Screening
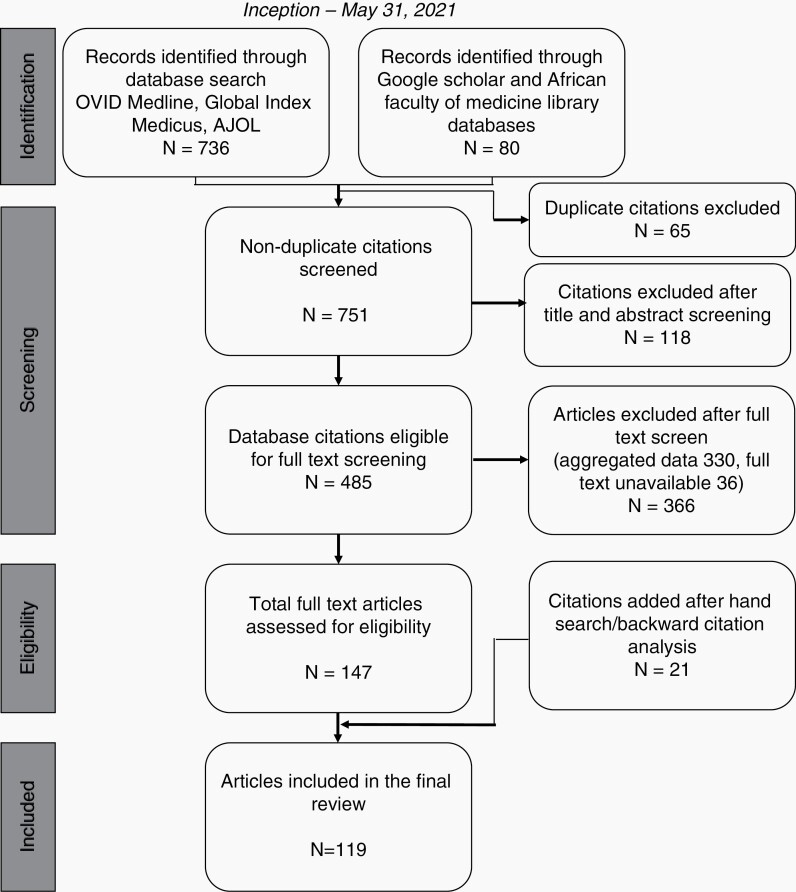
The database search returned 816 articles. After deduplication and title and abstract screening, four hundred and eighty-five articles (59.4%) remained. Most articles were excluded because they aggregated adult and pediatric data without distinctions (n = 330), while the rest were excluded because they lacked full texts (n = 36). Only 119 articles were included after the full-text screening and complementary hand search (Figure 1). The complete article data is available in Supplemental File 2 (DOI 10.17605/OSF.IO/FJCTS).
Fig. 1.
PRISMA flow diagram of the scoping review search, deduplication, and screening.
Scholarly Productivity
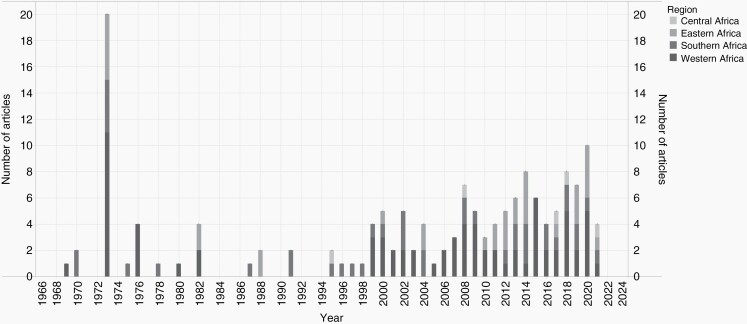
Twenty-four of the 46 SSA countries (52.2%) contributed to the 119 articles. The greatest contributors were Western Africa (n = 58, 48.7%), Southern Africa (n = 33, 27.7%), Eastern Africa (n = 23, 19.3%), and Central Africa (n = 5, 4.2%). Nigeria contributed the most articles (n = 37, 31.1%), followed by South Africa (n = 26, 21.8%), Senegal (n = 10, 8.4%), Sudan (n = 6, 5.0%), Ethiopia (n = 7, 5.9%), Ghana (n = 6, 5.0%), and Zimbabwe (n = 5, 4.2%). The first article was published in 1969 by Adeloye A and Odeku EL in Nature.25 To this date, 1973 remains the most prolific year with 10 articles (8.4%) but nine of the top ten most prolific years were in the 21st century (Figure 2). Central African articles had been published more recently (median publication year [MPY] = 2017, range:1995–2021) than Eastern (MPY = 2012, range:1973–2021), Southern (MPY = 2004, range:1970–2021), and Western (MPY = 2003, range:1970–2021) articles (P = .61).
Fig. 2.
Distribution of adult Sub-Saharan African brain tumor research disaggregated regionally.
All the studies were nonrandomized: 75 (63.0%) were cross-sectional, 39 (32.8%) were case reports or series, and the rest were either case-control or cohort studies (n = 5, 4.2%).
The most studied tumors were meningiomas (n = 50, 42.0%), pituitary adenomas (n = 37, 31.1%), gliomas (n = 36, 30.3%), metastasis (n = 29, 24.4%), and craniopharyngiomas (n = 20, 16.8%). Pineal tumors (n = 11, 9.2%),26–36 lymphomas (n = 7, 5.9%),36–42 acoustic neuromas (n = 3, 2.5%),28,29,43 and chordomas (n = 2, 1.7%)44,45 were less common. Four papers (3.4%) reported access to molecular biology techniques.46–49 Access to radiotherapy was reported in 11 studies (9.2%)26,43,44,48,50–56 and the most commonly available modality was whole-brain radiotherapy.26,48,51–53,55,56 Three studies (2.5%)52,57,58 reported the use of chemotherapy most commonly for metastatic brain lesions.52,57 Only nine studies (7.6%) reported the Karnofsky Performance Score.50,51,59–65 Twenty studies (16.8%) reported mortality rates44,48,50,55,57,60,64–76 and the mortality rates in studies with ten or more patients varied between 8% and 88%.50,60,64,65,70,73,76
In their 2014 publication, Ibebuike and Ouma77 found meningiomas (33.8%) were the most common primary tumor in Johannesburg (South Africa), followed by pituitary adenomas (24.6%), gliomas (22.5%), craniopharyngiomas (2.8%), ependymomas (2.8%), and medulloblastomas (2.8%). They found black South Africans were more likely to have meningiomas than white or Indian South Africans (75% vs. 12.5% vs. 4.2%).77
Discussion
This scoping review summarizes evidence on adult brain tumors in SSA. All the study data were hospital-based and, as such, cannot accurately represent the epidemiology of brain tumors in SSA because patients face numerous barriers to care. Nevertheless, the data from these studies give an insight into the adult neuro-oncology practice in SSA. In addition, this finding highlights the need for emphasis on population-based research.
Mbi Feh et al.78 performed a systematic review of brain tumors in Africa from 1960 to 2017 and only found 26 articles. The low number of articles is surprising considering that they did not discriminate between adult and pediatric brain tumors. However, this discrepancy can be explained because they searched major peer-review databases.78 They found brain tumor prevalence as high as 5902 cases per 100 000 population, with Nigeria, Egypt, and Uganda reporting some of the highest frequencies.78 In addition, they reported a male predominance (54%) and found that the most common tumors were astrocytomas (24.7%), meningiomas (22.2%), pituitary adenomas (8.4%), and medulloblastomas (4.2%).78 Our review revealed a wide spectrum of tumors similar to Mbi Feh et al.78 However, it is interesting that there were fewer studies on acoustic neuromas. It will be interesting to explore the genetic or socioeconomic correlates of acoustic neuromas in the SSA population. These studies can build on past meningioma studies. For example, Ibebuike and Ouma77 found a higher prevalence among black patients. This disparity has not been confirmed by Anzalone et al.,79 who found a higher prevalence among white Americans (9.52 per 100 000 people vs. 3.43 per 100 000 people). These discordances highlight a need for further epidemiological studies in SSA.
Studies on metastatic brain tumors were less common than meningiomas, pituitary adenomas, and gliomas, even though brain metastases are widely regarded as the most common type of brain tumor. The reported adult brain metastasis frequency never exceeded 23.7%.80 The discrepancy may be due to late presentation and poor survivorship of patients with cancers such as breast, thyroid, lung that metastasize to the brain.81 Patients with brain metastases may present terminally with other extracranial metastases. This observation creates an opportunity for organized neuro-oncology groups like SNOSSA that can leverage on relationships with other supporting sister bodies such as the Society of Neuro-Oncology (SNO), European Association of Neuro-Oncology (EANO), Asian Society for Neuro-Oncology (ASNO), and African Organisation for Research & Training in Cancer (AORTIC) to improve expertise in clinical trials and draw industry support to Africa. The lack of clinical trials of brain tumors in SSA raises concerns about the generalization of findings from clinical trials involving other ethnic groups to Africans without consideration to the possible uniqueness of both the tumors and response to the medications by Africans.
All four SSA regions were well represented in the neuro-oncology scholarly output. The increase in the number of published papers on brain tumors seems to coincide with the increase in the number of training centers for neurosurgeons in SSA.19 We found regional disparities in scholarly output, including that Central Africa published almost a decade after Southern and Western Africa on average. This temporal disparity might explain the regional discrepancy in scholarly productivity. In addition, we noted sustained publication since 1995. The temporal and scholarly output differences can be understood because the first brain tumor excision in SSA by an African was in West Africa by E-Latunde Odeku in 1962 in Ibadan, with the first published manuscript on intracranial masses by him in 1967.82 We equally found regional disparities in access to imaging, molecular biology, and adjuvant therapy. These findings reinforce the widely held view that SSA is not a monolith, so interventions aiming to improve neuro-oncology care should be tailored to the needs and resources of each SSA nation.
Five papers reported access to molecular diagnostics reflective of the changing landscape of brain tumor diagnosis and prognosis. The limited information on access to molecular diagnosis hinders comparison to other regions. In addition, it limits the estimation and classification of the burden of brain tumors in this region. Most articles were observational, specifically case reports. This high number of case reports/series and lack of basic and interventional research points to the enormous gap in locally-generated and high-quality evidence research that needs to be filled. The reason for this huge gap is multifactorial, including out-of-pocket payment for the care of brain tumors, the competition of cancer care with treatment of infectious diseases, with brain tumors further down the ladder of cancers considered a priority, and the lack of expertise in neuro-oncology across the multiple subspecialties. This gap highlights a need for neuro-oncology research capacity-building to increase the quality and quantity of publications. Ngulde et al.16 have suggested a multi-pronged strategy to improve brain tumor research in SSA, including raising awareness, research capacity-building, infrastructure development, research funding, and research collaboration.16
The African oncology situation remains worrisome and uncertain due to the lack of research, absence of national or regional tumor registries, and patients facing numerous barriers to care.78 The reported variations in the incidence of adult brain tumors by African countries can be explained by the varying availability of resources and contrasts in health systems infrastructure. Previous studies have shown significant differences in the neurosurgical workforce, infrastructure, service delivery, funding, governance, and information management.83,84 Even though neurosurgeons are gradually increasing on the continent, access to neuro-oncology care remains insufficient; neuro-oncological specialists are still very few.85 This is a concern because successful neuro-oncology practice requires a multidisciplinary team of neurosurgeons, neurologists, nursing providers, medical oncologists, radiation oncologists, neuroradiologists, neuropathologists, and palliative care specialists. The lack of these specialists still makes a dedication to neuro-oncological practice difficult. As a result, medical centers with established neuro-oncology facilities and oncologic centers remain rare, diagnostic modalities and complementary treatment options (chemotherapy and radiation) are equally limited.86,87 As a result, brain tumors are diagnosed at very late stages, leading to high morbidity and mortality rates.88
The diagnosis and treatment of cancers remain in Africa remains a challenge. The IARC predicts the regional cancer burden will increase over the next decades, further complicating the management of cancers.7 Challenges include underdiagnoses, limited access to medical imaging and histopathology, insufficient or inadequate surgical, medical, and radiotherapy treatment, lack of specialized workforce, and unclear referral patterns. These challenges are common to all cancers10,89; however, brain cancers face an added burden because they receive less attention than other cancers.
SSA’s challenges are compounded by rapid advancements in neuro-oncology diagnosis and management. Recent brain tumor classification requires molecular and genetic testing,90 but access to these modalities is limited by availability and affordability.88 Additionally, the high rate of patients lost to follow-up makes the little available data incomplete. There is an undeniable need for alternative solutions that better suit the African health systems without compromising the scientific evidence, which can only be possible through locally-driven research.91
Despite the importance of a scoping review to provide an overview of the available literature, it may explicitly hinder comparing data from various study designs and methods.92 This limitation is accentuated when the included studies use different outcome measures. We found heterogeneity in the reporting of adjuvant therapy, patient performance status, and mortality rates. Hence, our findings are susceptible to publication bias. Also, no randomized studies met our inclusion criteria, and we only included French and English publications. These limitations reduced the quality of evidence and limited the scope of our analysis. Although English is the predominant language for research globally, many countries publish research primarily in official local languages such as Arabic, Portuguese, or Spanish.93 We chose to limit our search to French and English, the two most common official languages in SSA because our authors were fluent in these languages. We equally excluded a significant number of articles due to stringent exclusion criteria. While this helped preserve the quality of our results, it equally led to the loss of valuable information. For example, the exclusion of Odeku EL’s 1967 publication on intracranial masses in Nigeria.82 This article is the earliest recorded publication on brain tumors in SSA; however, it was excluded due to a lack of abstract and full-text access. Another limitation is excluding articles that lacked disaggregated data on adult tumors.
Despite these limitations, this study provides a range of new information on adult brain tumors in SSA that will allow stakeholders to strengthen health systems through research, practice, and advocacy.
Conclusion
Crossdisciplinary and holistic health system investments can only improve brain tumor diagnosis and management. Our findings highlight an urgent need to increase the quantity and quality of brain tumor research in SSA. Also, the study findings give an insight into the stakeholder and priorities of a future international SSA brain tumor research consortium. We have identified at least 22 countries in need of research and a need for interventional, basic science, and population-based research. The African neuro-oncology research community should leverage the experience of higher-performing nations such as Nigeria, South Africa, Senegal, and Sudan to champion this initiative. Moreover, it has become apparent that SSA needs a dedicated neuro-oncology journal that can address her peculiarities. This publication must be indexed on major databases to facilitate international access.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
A spreadsheet of all 119 articles included in this study is available at DOI 10.17605/OSF.IO/FJCTS.
Conflict of interest statement. The authors declare no conflict of interest
Authorship statement. USK: Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing—original draft, and Writing—review and editing; CK: Conceptualization, Data curation, Investigation, Supervision, Validation, Writing—original draft, and Writing—review and editing; ADN: Data curation, Investigation, Writing—original draft, and Writing—review and editing; AKA: Data curation, Investigation, Writing—original draft; TL: Data curation, Supervision, Validation, Writing—review and editing; and JAB: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Supervision, Validation, Writing—original draft, and Writing—review and editing.
Contributor Information
Ulrick Sidney Kanmounye, Research Department, Association of Future African Neurosurgeons, Yaounde, Cameroon.
Claire Karekezi, Neurosurgery Unit, Department of Surgery, Rwanda Military Hospital, Kigali, Rwanda.
Arsene Daniel Nyalundja, Research Department, Association of Future African Neurosurgeons, Yaounde, Cameroon; Center for Tropical Diseases and Global Health (CTDGH), Faculty of Medicine, Université Catholique de Bukavu, Bukavu, Democratic Republic of Congo.
Ahmed K Awad, Research Department, Association of Future African Neurosurgeons, Yaounde, Cameroon; Faculty of Medicine, Ain Shams University, Cairo, Egypt.
Tsegazeab Laeke, Neurosurgery Unit, Surgery Department, Addis Ababa University, College of Health Sciences, Addis Ababa, Ethiopia.
James A Balogun, Division of Neurosurgery, Department of Surgery, College of Medicine, University of Ibadan, Ibadan, Nigeria.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. IHME. GBD Compare. IHME Viz Hub. Accessed July 30, 2019. http://vizhub.healthdata.org/gbd-compare.
- 2. Lu-Emerson C, Eichler AF. Brain metastases. Contin Minneap Minn. 2012; 18(2):295–311. [DOI] [PubMed] [Google Scholar]
- 3. Lim JH, Um SW. The risk factors for brain metastases in patients with non-small cell lung cancer. Ann Transl Med 2018; 6(Suppl 1):S66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan WS, Ho KS, Eu KW. Brain metastases in colorectal cancers. World J Surg. 2009; 33(4):817–821. [DOI] [PubMed] [Google Scholar]
- 5. Rostami R, Mittal S, Rostami P, Tavassoli F, Jabbari B. Brain metastasis in breast cancer: a comprehensive literature review. J Neurooncol. 2016; 127(3):407–414. [DOI] [PubMed] [Google Scholar]
- 6. Barnholtz-Sloan JS, Ostrom QT, Cote D. Epidemiology of brain tumors. Neurol Clin. 2018; 36(3):395–419. [DOI] [PubMed] [Google Scholar]
- 7. WHO. Cancer mortality database (IARC). https://www-dep.iarc.fr/WHOdb/WHOdb.htm. Accessed November 11, 2021.
- 8. Dewan MC, Rattani A, Fieggen G, et al. Global neurosurgery: the current capacity and deficit in the provision of essential neurosurgical care. Executive Summary of the Global Neurosurgery Initiative at the Program in Global Surgery and Social Change. J Neurosurg. 2018;130(4):1–10. [DOI] [PubMed] [Google Scholar]
- 9. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010; 127(12):2893–2917. [DOI] [PubMed] [Google Scholar]
- 10. Population-Based Cancer Registration in Sub-Saharan Africa. Its role in research and cancer control. JCO Glob Oncol. https://ascopubs.org/doi/full/10.1200/GO.20.00294. Accessed January 27, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levy LF. Brain tumours in Malawi, Rhodesia and Zambia. Afr J Med Sci. 1973; 4(4):393–397. [PubMed] [Google Scholar]
- 12. Levy LF, Auchterlonie WC. Primary cerebral neoplasia in Rhodesia. Int Surg. 1975; 60(5):286–292. [PubMed] [Google Scholar]
- 13. Adeloye A. Neoplasms of the brain in the African. Surg Neurol. 1979; 11(4):247–255. [PubMed] [Google Scholar]
- 14. Odeku EL, Osuntokun BO, Adeloye A, Williams AO. Tumors of the brain and its coverings. An African series. Int Surg. 1972; 57(10):798–801. [PubMed] [Google Scholar]
- 15. Collomb H, Dumas M, Girard PL. Expanding intracranial processes among the Negroes of Senegal. J Neurol Sci. 1973; 19(4):437–452. [DOI] [PubMed] [Google Scholar]
- 16. Ngulde SI, Fezeu F, Ramesh A, et al. Improving brain tumor research in resource-limited countries: a review of the literature focusing on West Africa. Cureus. 2015; 7(11):e372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wiafe BA, Karekezi C, Jaklitsch MT, Audisio RA. Perspectives on surgical oncology in Africa. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2020; 46(1):3–5. [DOI] [PubMed] [Google Scholar]
- 18. Haizel-Cobbina J, Chen JW, Belete A, Dewan MC, Karekezi C. The landscape of neuro-oncology in East Africa: a review of published records. Childs Nerv Syst ChNS Off J Int Soc Pediatr Neurosurg. 2021; 37(10):2983–2992. [DOI] [PubMed] [Google Scholar]
- 19. Dada OE, Karekezi C, Mbangtang CB, et al. State of neurosurgical education in Africa: a narrative review. World Neurosurg. 2021; 151(7): 172–181. [DOI] [PubMed] [Google Scholar]
- 20. Kanmounye US, Zolo Y, Nguembu S, et al. Training the next generation of academic global neurosurgeons: experience of the Association of Future African Neurosurgeons. Front Surg. 2021; 8(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balogun J, Haynes C, Lwin Z, et al. SNO 25th anniversary history series: providing a global platform for communication and exchange in neuro-oncology. Neuro-Oncol. 2020; 22(11):1551–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018; 169(7):467–473. [DOI] [PubMed] [Google Scholar]
- 23. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005; 8(1):19–32. [Google Scholar]
- 24. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016; 5(1):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adeloye A, Odeku EL. Metastatic neoplasms of the brain in Nigeria. Br J Cancer. 1969; 23(2):340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elmezughi KK, Pirie FJ, Motala AA. Characteristics and outcome of surgically treated pituitary tumours in South Africa: a single-centre experience. Clin Endocrinol (Oxf). 2017; 86(4):534–540. [DOI] [PubMed] [Google Scholar]
- 27. Salami A, Azeez A, Malomo A, et al. Correlation of intraoperative cytological and final histological diagnoses: a retrospective 10-year study of neurosurgical cases from I badan, Nigeria. Diagn Cytopathol. 2015; 43(3):195–201. [DOI] [PubMed] [Google Scholar]
- 28. Froman C, Lipschitz R. Demography of tumors of the central nervous system among the Bantu (African) population of the Transvaal, South Africa. J Neurosurg. 1970; 32(6):660–664. [DOI] [PubMed] [Google Scholar]
- 29. Motah M, Gams Massi D, Fouda Bekolo F, et al. Epidemiological profile of brain tumors in Cameroon: a retrospective study. Egypt J Neurol Psychiatry Neurosurg. 2021; 57(1):1–5. [Google Scholar]
- 30. Girard PL, Dumas M, Collomb H. “Gliomas” (neuroectodermal tumors) in Senegal. Afr J Med Sci. 1973; 4(2):265–274. [PubMed] [Google Scholar]
- 31. Jibrin P, Ibebuike K, Ado-Wanka AN. Histo-pathological pattern of intracranial tumours in the National Hospital, Abuja. Afr Health Sci. 2018; 18(2):281–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Odeku EL, Adeloye A. Gliomas of the brain among Nigerians. Afr J Med Med Sci. 1976; 5(1):31–33. [PubMed] [Google Scholar]
- 33. Idowu O, Doherty A, Tiamiyu O. Initial experience with endoscopic third ventriculostomy in Nigeria, West Africa. Childs Nerv Syst. 2008; 24(2):253–255; discussion 257. [DOI] [PubMed] [Google Scholar]
- 34. Onakpoya O, Komolafe E, Akintomide F, et al. Ophthalmic manifestations in patients with intracranial tumours. Afr J Neurol Sci. 2009; 28(1):53–60. [Google Scholar]
- 35. Ekpene U, Ametefe M, Akoto H, et al. Pattern of intracranial tumours in a tertiary hospital in Ghana. Ghana Med J. 2018; 52(2):79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Templeton AC. Tumours of the brain. In: Tumours in a Tropical Country. Berlin, Heidelberg: Springer; 1973:200–202. [Google Scholar]
- 37. Eyenga VC, Ngah JE, Atangana R, et al. Central nervous system tumours in Cameroon: histopathology and demography. Cah Détudes Rech Francoph. 2008; 18(1):39–42. [DOI] [PubMed] [Google Scholar]
- 38. Millogo A, Sawadogo AB, Lankoande D, Ouedraogo I. Diagnostic problems of expansive intracranial process in HIV infected patients of the Bobo-Dioulasso Central Hospital (Burkina Faso). Bull Soc Pathol Exot 1990. 2001; 94(4):315–318. [PubMed] [Google Scholar]
- 39. Olasode B, JA. Pathological review of intracranial tumours seen at the University College Hospital, Ibadan between 1980 and 1990. Niger Postgrad Med J. 2002; 9(1):23–28. [PubMed] [Google Scholar]
- 40. Mwanda OW, Whalen C, Scot CR, et al. Anatomical site predilections of non-Hodgkin’s lymphoma in human immunodeficiency virus infection: a report on 54 cases. East Afr Med J. 2004;. 8(8):S90–S96. [DOI] [PubMed] [Google Scholar]
- 41. Ibebuike K, Ouma J, Gopal R. Meningiomas among intracranial neoplasms in Johannesburg, South Africa: prevalence, clinical observations and review of the literature. Afr Health Sci. 2013; 13(1):118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Levy LM, Coutts AM, Abayomi EA, Mandisodza M. Primary cerebral lymphoma in Zimbabwe: a report of three patients. Cent Afr J Med. 1997; 43(11):328–331. [PubMed] [Google Scholar]
- 43. Seedat RY, Claassen AJ, Mol DA. Incidence and management of acoustic neuromas in South Africa. Otol Neurotol. 2002; 23(6):996–998. [DOI] [PubMed] [Google Scholar]
- 44. Chetty R, Levin CV, Kalan MR. Chordoma: a 20-year clinicopathologic review of the experience at Groote Schuur Hospital, Cape Town. J Surg Oncol. 1991; 46(4):261–264. [DOI] [PubMed] [Google Scholar]
- 45. Collomb H, Dumas M. Intracranial space occupying lesions in Snenegal. Afr J Med Med Sci. 1973; 4(2):143. [PubMed] [Google Scholar]
- 46. Raidoo DM, Sawant S, Mahabeer R, Bhoola KD. Kinin receptors are expressed in human astrocytic tumour cells. Immunopharmacology. 1999; 43(2-3):255–263. [DOI] [PubMed] [Google Scholar]
- 47. Fatima Salih S. Brain Tumors among Sudanese Patients (A Histopathological Study) (Doctoral Dissertation, U of K). University of Khartoum. Published online 2011. https://ils.uofk.edu/cgi-bin/koha/opac-detail.pl?biblionumber=20450. [Google Scholar]
- 48. Gadji M, Crous-Tsanaclis AM, Mathieu D, et al. new der (1; 7)(q10; p10) leading to a singular 1p loss in a case of glioblastoma with oligodendroglioma component. Neuropathology. 2014; 34(2):170–178. [DOI] [PubMed] [Google Scholar]
- 49. Abdelmontalab FY, Fadl EI, Abushama H, Kreskowski K, Liehr T. Molecular cytogenetic study of the NF2 gene deletion in meningioma in Sudanese patients. Balk J Med Genet BJMG. 2013; 16(2):29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Doléagbénou KA, Kpélao E, Adani-Ifè AO, et al. Intracranial meningiomas among intracranial tumors in the Neurosurgical Unit of Sylvanus Olympio Teaching Hospital. Open J Mod Neurosurg. 2020; 10(3):345–352. [Google Scholar]
- 51. Ayandipo OO, Adeleye AO, Ulasi IB, Ogundiran TO. Outcome of cerebral metastasectomy in select cases of brain metastases from breast cancer in Ibadan, Nigeria. World Neurosurg. 2019; 127(7):186–193. [DOI] [PubMed] [Google Scholar]
- 52. Semple PL, Denny L, Coughlan M, Soeters R, Van Wijk L. The role of neurosurgery in the treatment of cerebral metastases from choriocarcinoma: a report of two cases. Int J Gynecol Cancer. 2004; 14(1):157–161. [DOI] [PubMed] [Google Scholar]
- 53. Olasinde TA, Adamu A, Jimeta JD, Chukwuocha IC. Palliative care in patients who receive whole brain radiotherapy for brain metastases in Ahmadu Bello University Teaching Hospital, Zaria. Niger J Med. 2016; 25(3):215–219. [PubMed] [Google Scholar]
- 54. Tagoe NN, Essuman VA, Bankah P, et al. Visual outcome of patients with pituitary adenomas following surgery and its contributory factors at a tertiary hospital in Ghana. Ethiop J Health Sci. 2019; 29(1):895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mwang’Ombe NJ, Ombachi RB. Brain tumours at the Kenyatta National Hospital, Nairobi. East Afr Med J. 2000; 77(8):444–447. [PubMed] [Google Scholar]
- 56. Ibrahim H, Yaroko AA. Palliative external beam radiotherapy for advanced breast cancer patients with brain metastasis in the University College Hospital Ibadan. Ann Afr Med. 2019; 18(3):127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Komolafe MA, Sunmonu TA, Oke O. Stroke-like syndrome in a middle aged Nigerian woman with metastatic brain cancer. West Afr J Med. 2009; 28(4):266–269. [PubMed] [Google Scholar]
- 58. Domingo Z, Fisher-Jeffes ND, de Villiers JC. Malignant occipital astrocytoma in a patient with Lhermitte-Duclos disease (cerebellar dysplastic gangliocytoma). Br J Neurosurg. 1996; 10(1):99–102. [DOI] [PubMed] [Google Scholar]
- 59. Ndubuisi. Some characteristics of gliomas managed at a Neurosurgery centre in Nigeriahttps://www.npmj.org/article.asp?issn=1117-1936;year=2017;volume=24;issue=1;spage=44;epage=47;aulast=Ndubuisi. Accessed November 11, 2021. [DOI] [PubMed]
- 60. Andrews NB, Ramesh R, Odjidja TA. Preliminary survey of central nervous system tumors in Tema, Ghana. West Afr J Med. 2003; 22(2):167–172. [DOI] [PubMed] [Google Scholar]
- 61. Arbab M, Deaf S, Ahmed L, et al. Cranial meningioma in Sudanese patients: clinical and pathological characteristics. Int J Res IJR. 2014; 1(7):452–461. [Google Scholar]
- 62. Adeleye AO. Low rates of post-craniotomy surgical site infections in a developing country: surgical technique and results. Br J Neurosurg. 2018; 32(2):136–140. [DOI] [PubMed] [Google Scholar]
- 63. Jokonya L, Musara A, Esene I, et al. Landscape, presentation, and characteristics of brain gliomas in Zimbabwe. Asian J Neurosurg 2021; 16(2):294–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kakusa BW, Xu LW, Vaca SD, et al. Central nervous system tumors in Uganda: outcomes of surgical treatment and complications assessed through telephone survey. World Neurosurg. 2019; 129((Kakusa B.W.; Xu L.W.; Vaca S.D.; Grant G.A., ggrant2@stanford.edu) Department of Neurosurgery, Stanford University School of Medicine, Stanford, CA, United States):e866–e880. [DOI] [PubMed] [Google Scholar]
- 65. Laeke T, Biluts H, Sahlu A. Clinical outcome of operated intracranial meningiomas: an Ethiopian experience. World Neurosurg 2019; 128(8): e81–e86. [DOI] [PubMed] [Google Scholar]
- 66. Goncalves N, Lubbe DE. Transorbital endoscopic surgery for sphenoid wing meningioma: long-term outcomes and surgical technique. J Neurol Surg Part B Skull Base. 2020; 81(4):357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Egbohou P, Mouzou T, Beketi K, et al. Anesthesiological aspects and complications of intracranial meningiomas operated at the Sylvanus Olympio University Hospital Center, Lomé: about 21 cases. Pan Afr Med J. 2017; 28(42):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Loembe PM, Epaly GO, Ramarojoana R, Mwanyombet-Ompounga L. Tumor-related epilepsy in adults in Gabon: diagnostic problems and therapeutic management. Med Trop (Mars). 1995; 55(1):68–72. [PubMed] [Google Scholar]
- 69. Ejiro BA, Edomwonyi NP. Audit of intensive care unit (ICU) admissions from the operation room: experience at the University of Benin Teaching Hospital, Benin City, Nigeria. J Med Biomed Res. 2012; 11(2):9–17. [Google Scholar]
- 70. Thiam AB, Kessely YC, Thioub M, et al. Our experience of intracranial meningioma in Dakar: about 50 cases. Pan Afr Med J. 2015; 20 (379):379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Badiane SB, Sakho Y, Ba MC, et al. Intracranial meningiomas. The Dakar experience apropos of 79 cases. Neurochirurgie. 1999; 45(2):134–138. [PubMed] [Google Scholar]
- 72. Keita AD, Kane M, Guinto CO, et al. Using CT to diagnose brain tumors at the Point G Hospital in Mali. Mali Med. 2007; 22(2):14–18. [PubMed] [Google Scholar]
- 73. Igun GO. Diagnosis and management of brain tumours at Jos University Teaching Hospital, Nigeria. East Afr Med J. 2001; 78(3):148–151. [DOI] [PubMed] [Google Scholar]
- 74. Andrews NB. Initial experience with neuroendoscopic surgery in West Africa. Afr J Neurol Sci. 2005; 24(2):55–61. [Google Scholar]
- 75. Biluts H, Laeke T. Microscopic transphneoidal surgery experience from Christian Medical Center Addis Abeba Ethiopia. Ethiop Med J. 2014; 52(2):67–76. [PubMed] [Google Scholar]
- 76. N’dri Oka D, Broalet EMY, Kakou M, et al. Intracranial meningiomas in Ivory Coast. A surgical study. Afr J Neurol Sci. 2008; 27(1):57–66. [Google Scholar]
- 77. Ibebuike K, Ouma J. Demographic profile of patients diagnosed with intracranial meningiomas in two academic hospitals in Johannesburg, South Africa: a 12-month prospective study. Afr Health Sci. 2014; 14(4):939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Mbi Feh MKN, Lyon KA, Brahmaroutu AV, Tadipatri R, Fonkem E. The need for a central brain tumor registry in Africa: a review of central nervous system tumors in Africa from 1960 to 2017. Neuro-Oncol Pract. 2021; 8(3):337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Anzalone CL, Glasgow AE, Gompel JJV, Carlson ML. Racial differences in disease presentation and management of intracranial meningioma. J Neurol Surg Part B Skull Base. 2019; 80(6):555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Olasode BJ, Shokunbi MT, Aghadiuno PU. Intracranial neoplasmin Ibadan, Nigeria. East Afr Med J. 2000; 77(1): 4–8. [PubMed] [Google Scholar]
- 81. Nieder C, Spanne O, Mehta MP, Grosu AL, Geinitz H. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer. 2011; 117(11):2505–2512. [DOI] [PubMed] [Google Scholar]
- 82. Odeku EL, Janota I. Intracranial masses--Ibadan. West Afr Med J Niger Pract. 1967; 16(1):31–42. [PubMed] [Google Scholar]
- 83. Kanmounye US, Lartigue JW, Sadler S, et al. Emerging trends in the neurosurgical workforce of low- and middle-income countries: a cross-sectional study. World Neurosurg. 2020; 142(4):420–433. [DOI] [PubMed] [Google Scholar]
- 84. Kanmounye US, Ghomsi NC, Djiofack D, et al. The implications of global neurosurgery for low- and middle-income countries. The case of Cameroon. Iran J Neurosurg. 2020; 6(2):2–2. [Google Scholar]
- 85. Kanmounye US, Robertson FC, Thango NS, et al. Needs of young African neurosurgeons and residents: a cross-sectional study. Front Surg. 2021; 8(5):20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Elmore SNC, Polo A, Bourque JM, et al. Radiotherapy resources in Africa: an International Atomic Energy Agency update and analysis of projected needs. Lancet Oncol. 2021; 22(9):e391–e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ndom P. Challenges of anticancer chemotherapy in Africa. Can J Urol. 2008; 15(1):3909–3911. [PubMed] [Google Scholar]
- 88. Nguembu S, Kanmounye US, Tétinou F, Djiofack D, Takoukam R. Barriers to the management of non-traumatic neurosurgical diseases at two Cameroonian neurosurgical centers: a cross-sectional study. World Neurosurg. 2020; 139(7):774–783. [DOI] [PubMed] [Google Scholar]
- 89. Vanderpuye V, Dadzie MA, Huo D, Olopade OI. Assessment of breast cancer management in Sub-Saharan Africa. JCO Glob Oncol. 2021; 7(12): 1593–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro-Oncol. 2021; 23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Adegboyega G, Ozair A, Kanmounye US, Bandyopadhyay S, Vaqas B; on behalf of InciSion UK Collaborative. Letter: Is the Stupp protocol an expensive and unsustainable standard of care for glioblastoma in low- and middle-income country settings? A call to action! Neurosurgery. 2021; 89(4):E249–E251. [DOI] [PubMed] [Google Scholar]
- 92. Sucharew H, Sucharew H. Methods for research evidence synthesis: the scoping review approach. J Hosp Med. 2019; 14(7):416–418. [DOI] [PubMed] [Google Scholar]
- 93. Baethge C. The languages of medicine. Dtsch Arzteblatt Int. 2008; 105(3):37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.