Abstract
Background
Entrectinib is a TRKA/B/C, ROS1, ALK tyrosine kinase inhibitor approved for the treatment of adults and children aged ≥12 years with NTRK fusion-positive solid tumors and adults with ROS1 fusion-positive non–small-cell lung cancer. We report an analysis of the STARTRK-NG trial, investigating the recommended phase 2 dose (RP2D) and activity of entrectinib in pediatric patients with solid tumors including primary central nervous system tumors.
Methods
STARTRK-NG (NCT02650401) is a phase 1/2 trial. Phase 1, dose-escalation of oral, once-daily entrectinib, enrolled patients aged <22 years with solid tumors with/without target NTRK1/2/3, ROS1, or ALK fusions. Phase 2, basket trial at the RP2D, enrolled patients with intracranial or extracranial solid tumors harboring target fusions or neuroblastoma. Primary endpoints: phase 1, RP2D based on toxicity; phase 2, objective response rate (ORR) in patients harboring target fusions. Safety-evaluable patients: ≥1 dose of entrectinib; response-evaluable patients: measurable/evaluable baseline disease and ≥1 dose at RP2D.
Results
At data cutoff, 43 patients, median age of 7 years, were response-evaluable. In phase 1, 4 patients experienced dose-limiting toxicities. The most common treatment-related adverse event was weight gain (48.8%). Nine patients experienced bone fractures (20.9%). In patients with fusion-positive tumors, ORR was 57.7% (95% CI 36.9-76.7), median duration of response was not reached, and median (interquartile range) duration of treatment was 10.6 months (4.2-18.4).
Conclusions
Entrectinib resulted in rapid and durable responses in pediatric patients with solid tumors harboring NTRK1/2/3 or ROS1 fusions.
Keywords: CNS tumors, entrectinib, pediatric, recommended phase 2 dose, solid tumors
Key Points.
Entrectinib has activity in pediatric solid tumors with NTRK or ROS1 fusions.
The MTD for pediatric patients was identified as 550 mg/m2 daily (F1 formulation).
Based on PK modeling, the pediatric RP2D is 300 mg/m2 daily (F06 formulation).
Importance of the Study.
Entrectinib is a potent TRKA/B/C, ROS1, and ALK tyrosine kinase inhibitor that penetrates the blood-brain barrier. Studies in adults demonstrate activity in TRK inhibitor treatment-naïve patients with NTRK fusion-positive solid tumors and ROS1 inhibitor treatment-naïve patients with ROS1 fusion-positive non–small-cell lung cancer, including patients with central nervous system metastases. Specific evaluation of entrectinib in pediatric patients is necessary to determine the appropriate dose and whether similar safety and efficacy can be achieved in this population. The STARTRK-NG study, including 35 patients aged <12 years, demonstrates that entrectinib is an important therapeutic option for pediatric patients with NTRK or ROS1 fusion-positive intracranial and extracranial solid tumors. Further investigations are ongoing to assess an age-appropriate formulation, determine the optimal duration of therapy, and monitor for resistance and long-term side effects of entrectinib in pediatric patients.
Chromosomal translocations and gene rearrangements resulting in constitutive kinase activation promote carcinogenesis through aberrant signaling via cellular growth pathways.1 Rearrangements in neurotrophic tyrosine receptor kinase (NTRK), ROS proto-oncogene 1 (ROS1), and anaplastic lymphoma kinase (ALK) genes result in fusion proteins that are oncogenic drivers of some childhood cancers.2NTRK1, NTRK2, or NTRK3 fusions, encoding TRKA, TRKB, and TRKC, respectively, occur at high frequency (>90%) in some rare pediatric tumors, including infantile fibrosarcoma (IF), congenital mesoblastic nephroma, and secretory breast carcinoma; at low frequency (5%-25%) in a subset of pediatric gliomas, gastrointestinal stromal tumors, melanoma, and papillary thyroid cancer; and rarely (<5%) in most common tumors including adenocarcinoma, gastrointestinal carcinoma, acute leukemia, or soft tissue sarcoma including inflammatory myofibroblastic tumors (IMT).3–5ROS1 rearrangements have mainly been described in pediatric high-grade gliomas (HGG),6 and a subset of ALK-negative IMTs.7ALK rearrangements occur in non–small-cell lung cancer (NSCLC), anaplastic large-cell lymphoma, renal, breast, and colorectal carcinomas,8 pediatric HGG,6 and IMTs.7ALK gain-of-function mutations are seen in ~14% of patients with newly diagnosed high-risk neuroblastoma.9 TRKA/C and TRKB are overexpressed in non-high-risk and high-risk neuroblastoma, respectively.10
Entrectinib is a potent, central nervous system (CNS)-penetrant inhibitor of TRKA/B/C, ROS1, and ALK.11 Entrectinib was first approved in the United States (2019) for adults and children (≥12 years old) with advanced NTRK fusion-positive solid tumors and adults with ROS1 fusion-positive NSCLC who are TRK- or ROS1 inhibitor treatment-naïve and was subsequently approved in the EU (2020).12,13 The recommended dose in adults is 600 mg daily. Objective response rates (ORR; 95% confidence interval [CI]) were 63.5% (n = 47/74; 51.5-74.4) and 67.1% (n = 108/161; 59.3-74.3) in adults with NTRK and ROS1 fusion-positive tumors, respectively, including a total of 75 patients with baseline intracranial disease (investigator-assessed).14,15
Based on the mechanism of action, safety profile, responses in adults, and the presence of NTRK1/2/3, ROS1, and ALK alterations in pediatric cancers, the STARTRK-NG clinical trial of entrectinib in pediatric patients (NCT02650401) was initiated (2016). Here, we report the determination of the recommended phase 2 dose (RP2D) and efficacy and safety results from the phase 2 expansion as of September 2020.
Methods
Study Design and Participants
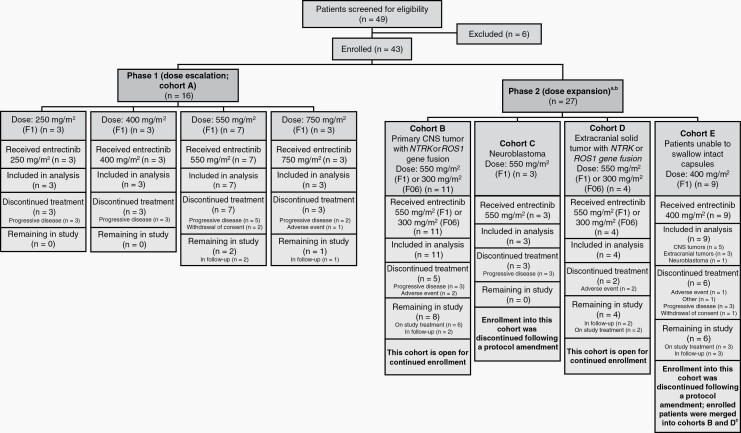
STARTRK-NG is a multicenter, non-randomized, open-label, single-arm phase 1-2 trial of entrectinib in pediatric patients with extracranial solid tumors or primary CNS tumors, with or without target gene fusions in NTRK1/2/3, ROS1, or ALK (Figure 1). Patients were recruited from 34 hospitals in Canada, France, South Korea, Spain, Taiwan, the United Kingdom, and the United States (Supplementary Table S1).
Fig. 1.
CONSORT diagram. aFollowing implementation of protocol version 5 (October 25, 2018), enrollment in cohorts C and E was closed; cohort A was completed following dose-escalation recruitment. Enrollment continued in cohorts B (for patients with primary CNS tumors with gene fusions) and D (for patients with extracranial tumors with gene fusions). Following closure of cohort E, new patients that were unable to swallow capsules were enrolled in cohorts B and D, depending on their tumor type. Molecular testing was required, prior to enrollment in cohorts B or D. bPatients in cohorts B, C, and D received entrectinib at the MTD (F1 formulation at 550 mg/m2 or F06 formulation at 300 mg/m2), and patients in cohort E received entrectinib at 400 mg/m2 (F1 formulation) mixed with soft foods. cPrimary CNS tumors, n = 5; extracranial solid tumors, n = 3; neuroblastoma, n = 1. The patient with neuroblastoma was not merged into cohorts B or D; they were followed up separately. Abbreviations: CNS, central nervous system; F1, F1 formulation; F06, F06 formulation; MTD, maximum tolerated dose; NTRK, neurotrophic tyrosine receptor kinase; ROS1, ROS proto-oncogene 1.
Target gene fusions in NTRK1/2/3, ROS1, or ALK were those predicted to translate into a fusion oncoprotein with a functional kinase domain and without another concomitant oncogenic driver. Target gene fusions were identified by validated nucleic acid-based diagnostic assays performed in a Clinical Laboratory Improvement Amendments or equivalently certified central or site laboratory. Following the implementation of protocol version 5 (October 25, 2018), for patients enrolled based on a biomarker-eligible result from local molecular testing, submission of an archival tumor tissue sample from diagnosis or relapsed disease has been required for central standardized biomarker testing. Phase 1 was an all-comer solid tumor trial, based on entrectinib being a multitargeted kinase inhibitor.11 No responses were seen in patients with tumors lacking target gene fusions and therefore protocol version 6 (May 21, 2019) restricted enrollment to patients with tumors harboring NTRK1/2/3 or ROS1 gene fusions.
Eligible patients were aged <22 years with relapsed or refractory (failed to respond to frontline curative therapy) disease and had: Lansky or Karnofsky performance score of ≥60%; life expectancy of ≥4 weeks; adequate bone marrow, liver, renal, cardiac, and neurologic function; ability to swallow entrectinib capsules intact (if unable they were enrolled into phase 2, cohort E and permitted to mix the contents [F1 formulation] with soft foods); and sufficiently recovered from acute toxic effects of previous therapy to meet the defined eligibility criteria (Supplementary Table S2). Patients previously treated with TRK, ROS1, or ALK inhibitors were only eligible for phase 1. Following protocol amendment 8 (December 17, 2019), enrollment was restricted to patients aged <18 years and included patients without satisfactory treatment options or where surgical resection was likely to result in severe morbidity, including newly diagnosed patients.
Patients were required to have measurable (or evaluable; Supplementary file, page 1) disease at baseline by Response Evaluation Criteria in Solid Tumors (RECIST) v1.116 (phase 1; phase 2, cohorts C, D, and E [extracranial tumors]), Response Assessment in Neuro-Oncology criteria17 (RANO; phase 2, cohorts B and E [primary CNS tumors]), or evaluable disease by Curie score18 (phase 2, cohort C [neuroblastoma with metaiodobenzylguanidine (MIBG)-evaluable disease]). Patients were excluded if they had active infections or were pregnant, breastfeeding, or receiving enzyme-inducing antiepileptic medication. Details on the different cohorts in this study are provided in Figure 1.
The study protocol and all amendments were approved by institutional review boards at all participating institutions. The study was conducted according to the Declaration of Helsinki and International Council for Harmonisation guidelines for Good Clinical Practice. Signed informed consent and assent were obtained according to regulatory and institutional guidelines.
Drug Formulation and Pharmacokinetics
Drug formulation administration is detailed in the Supplementary file, page 1. First dose pharmacokinetic (PK) evaluations were performed in phase 1 and cohort E of phase 2. PK parameters were estimated using a non-compartmental analysis (WinNonlin Certara, Princeton, NJ, USA), and summary statistics were calculated.
Procedures
In phase 1, patients were enrolled using a 3 + 3 design to evaluate up to 4 entrectinib doses (250, 400, 550, 750 mg/m2) and define the maximum tolerated dose (MTD). Entrectinib was administered orally with food, once daily in 28-day cycles, and adherence to protocol therapy was documented in a medication diary. Up to 2 dose reductions for treatment-related adverse events (TRAEs) were permitted, prescribed by a dosing nomogram (Supplementary Table S3). Upon completion of phase 1, phase 2 accrual was initiated (Figure 1).
The MTD was administered to patients enrolled in phase 2 cohorts B-D. Patients in cohort E received 1 dose level below the MTD during cycle 1 and could then escalate to the MTD. In the absence of progressive disease (PD) or toxicity requiring treatment discontinuation, there was no maximum number of treatment cycles. Following protocol amendment 5, patients able to swallow intact capsules received the F06 entrectinib formulation; cohorts C and E were closed, and patients in cohort E were merged into cohorts B or D (Figure 1).
Physical examinations and routine laboratory panels were conducted weekly during cycle 1, every 2 weeks during cycles 2-6, and at the start of each subsequent cycle. Electrocardiograms were performed during screening, before and after the first dose on cycle 1, pre-dose on cycle 1, day 22 and day 1 of cycles 2-6, and at the end of treatment. Treatment continued until evidence of PD (and beyond if sufficient evidence of clinical benefit) or failure to resolve a dose-limiting toxicity (DLT) to grade ≤2 or baseline within 21 days of drug discontinuation. Toxicities were graded according to National Cancer Institute Common Toxicity Criteria for Adverse Events (v4.03) or pediatric-specific criteria for peripheral neuropathy.19 Safety follow-up was conducted until 25-35 days following the last entrectinib dose.
In phase 1 and phase 2 cohort E, response assessments were performed at screening, every 2 cycles x3, every 3 cycles x2, every 4 cycles x3, then once every 6 cycles. In phase 2 cohorts B, C, and D, response assessments were performed at screening and every 8 weeks thereafter. Objective response (complete response [CR] or partial response [PR]) was assessed using disease-specific criteria. Confirmed objective responses were those confirmed by subsequent assessment ≥28 days after initial response; patients without either a confirmed objective response or post-baseline tumor assessments were considered non-responders. Blinded independent central review (BICR) was performed for patients with a target gene fusion.
Outcomes
The phase 1 primary objective was to determine the MTD and the RP2D of entrectinib in pediatric patients with relapsed or refractory solid tumors. The phase 2 primary objective was to evaluate the ORR in patients with primary CNS or extracranial solid tumors harboring NTRK1/2/3 or ROS1 gene fusions by BICR.
Key secondary objectives were: in all patients receiving entrectinib, safety, duration of response (DoR), time to response (TtR), clinical benefit rate, progression-free survival (PFS), and overall survival (OS); in phase 1 and phase 2 cohort E, PK of entrectinib (all formulations) in plasma; in patients with measurable primary or secondary CNS disease (using RANO or RANO-brain metastases, as applicable), intracranial tumor response, DoR, TtR, and CNS-PFS.
Statistical Analysis
In phase 1, patients were evaluable for toxicity and MTD determination if they received ≥75% of the prescribed dose, experienced a DLT, or discontinued treatment due to toxicity during cycle 1. Patients without a DLT who discontinued treatment due to PD or reasons unrelated to toxicity were replaced if they received <75% of the prescribed dose during cycle 1. Using the 3 + 3 dose-escalation schema, the MTD was defined as the dose level immediately below that at which ≥2 patients from a cohort of 3-6 patients experienced a DLT. Sample size was based on 3 + 3 design requirements (6-30 patients).
Phase 2 cohorts B and D utilized a 2-stage sequential design. A minimum of 10 patients were planned for initial enrollment in the expansion cohorts (B or D; excluding patients transferred from cohort E) and followed for at least 6 months. If ≥40% ORR (target response) was observed at the interim efficacy analysis, approximately 10 additional patients could be further enrolled in the expansion cohorts B or D. The efficacy endpoint for each cohort will be considered met if ORR of ≥40% is observed following additional expansion. Baseline characteristics and TRAE frequencies were summarized using descriptive statistics. Safety-evaluable population comprised patients who received any dose of entrectinib. Response-evaluable patients received ≥1 dose of entrectinib at the MTD (F1 formulation) or RP2D (F06 formulation) and had measurable/evaluable disease at baseline. All-comer ORR was calculated for all response-evaluable patients. ORR per BICR was the proportion of responders (PR and CR) among patients with tumors harboring target gene fusions. TtR (time from first dose to documentation of objective response), DoR (time from first objective response to radiographic disease progression), and PFS were calculated using the Kaplan-Meier method.
Following protocol amendment 5, interim analyses of efficacy data were specified following enrollment of ≥10 patients and completion of 6-month follow-up for each cohort. Data cutoff for this analysis was September 17, 2020.
Results
Patient Characteristics
Between May 2016 and September 2020, 43 patients were enrolled (Table 1). Phase 1 dose-escalation enrolled 16 patients, including 7 patients treated at 550 mg/m2 dose (F1 formulation) and included in phase 2. Phase 2 expansion enrolled 27 additional patients (Figure 1). Twenty-six patients had tumors with target gene fusions (phase 1, n = 3; phase 2, n = 23).
Table 1.
Patient Demographic and Baseline Characteristics
| Phase 1 Dose-Escalation (n = 16) | Phase 2 | All Patients | ||||
|---|---|---|---|---|---|---|
| Characteristic | 250 mg/m2 | 400 mg/m2 | 550 mg/m2 | 750 mg/m2 | ||
| (n = 3) | (n = 3) | (n = 7) | (n = 3) | (n = 27) | (n = 43) | |
| Median age, years (range) | 9 (7-13) | 15 (6-20) | 7 (6-17) | 10 (4-16) | 5 (2 months-19 years)a | 7 (2 months-20 years) |
| Sex, n (%) | ||||||
| Male | 2 (66.7) | 1 (33.3) | 5 (71.4) | 2 (66.7) | 12 (44.4) | 22 (51.2) |
| Female | 1 (33.3) | 2 (66.7) | 2 (28.6) | 1 (33.3) | 15 (55.6) | 21 (48.8) |
| Race, n (%) | ||||||
| White | 2 (66.7) | 2 (66.7) | 6 (85.7) | 3 (100.0) | 24 (88.9) | 37 (86.0) |
| Black or African American | 1 (33.3) | 1 (33.3) | 1 (14.3) | 0 | 2 (7.4) | 5 (11.6) |
| Other | 0 | 0 | 0 | 0 | 1 (3.7) | 1 (2.3) |
| Ethnicity, n (%) | ||||||
| Hispanic or Latino | 0 | 1 (33.3) | 0 | 0 | 3 (11.1) | 4 (9.3) |
| Non-Hispanic or Latino | 3 (100.0) | 2 (66.7) | 6 (85.7) | 2 (66.7) | 23 (85.2) | 36 (83.7) |
| Not stated | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (2.3) |
| Unknown | 0 | 0 | 1 (14.3) | 0 | 1 (3.7) | 2 (4.7) |
| Karnofsky/Lansky score, n (%)b | ||||||
| 60 | 0 | 0 | 0 | 0 | 2 (7.4) | 2 (4.8) |
| 70 | 0 | 0 | 1 (16.7) | 0 | 1 (3.7) | 2 (4.8) |
| 80 | 0 | 1 (33.3) | 0 | 1 (33.3) | 8 (29.6) | 10 (23.8) |
| 90 | 0 | 1 (33.3) | 4 (66.7) | 2 (66.7) | 6 (22.2) | 13 (31.0) |
| 100 | 3 (100.0) | 1 (33.3) | 1 (16.7) | 0 | 10 (37.0) | 15 (35.7) |
| Prior systemic therapies, n (%) | ||||||
| Chemotherapy | 3 (100.0) | 3 (100.0) | 5 (71.4) | 3 (100.0) | 19 (70.4) | 33 (76.7) |
| Immunotherapy | 0 | 2 (66.7) | 4 (57.1) | 1 (33.3) | 4 (14.8) | 11 (25.6) |
| Targeted therapy | 0 | 2 (66.7) | 1 (14.3) | 0 | 0 | 3 (7.0) |
| Monoclonal antibody | 0 | 3 (100.0) | 2 (28.6) | 3 (100.0) | 4 (14.8) | 12 (27.9) |
| Radiation | 3 (100.0) | 3 (100.0) | 5 (71.4) | 2 (66.7) | 11 (40.7) | 24 (55.8) |
| Tumor type/histology, n (%) | ||||||
| Extracranial solid tumor | 1 (33.3) | 1 (33.3) | 2 (28.6) | 1 (33.3) | 7 (25.9) | 12 (27.9) |
| Infantile fibrosarcoma | 0 | 0 | 0 | 1 (33.3) | 1 (3.7) | 2 (4.7) |
| IMT | 0 | 0 | 2 (28.6) | 0 | 3 (11.1) | 5 (11.6) |
| Melanoma | 0 | 0 | 0 | 0 | 1 (3.7) | 1 (2.3) |
| Salivary gland adenocarcinoma | 1 (33.3) | 0 | 0 | 0 | 0 | 1 (2.3) |
| Spindle cell sarcoma | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (2.3) |
| Myofibroblastic tumor | 0 | 0 | 0 | 0 | 1 (3.7) | 1 (2.3) |
| Infantile myofibroma | 0 | 0 | 0 | 0 | 1 (3.7) | 1 (2.3) |
| Primary CNS (brain) tumor | 0 | 0 | 0 | 0 | 16 (59.3) | 16 (37.2) |
| Glioblastoma | 0 | 0 | 0 | 0 | 3 (11.1) | 3 (7.0) |
| Astrocytoma | 0 | 0 | 0 | 0 | 4 (14.8) | 4 (9.3) |
| Ganglioglioma | 0 | 0 | 0 | 0 | 2 (7.4) | 2 (4.7) |
| Epithelioid glial neoplasm | 0 | 0 | 0 | 0 | 1 (3.7) | 1 (2.3) |
| Medulloblastoma | 0 | 0 | 0 | 0 | 1 (3.7) | 1 (3.7) |
| High-grade glioma NOS | 0 | 0 | 0 | 0 | 3 (11.1) | 3 (7.0) |
| Glioma NOS | 0 | 0 | 0 | 0 | 1 (3.7) | 1 (2.3) |
| Ganglioneuroblastoma | 0 | 0 | 0 | 0 | 1 (3.7) | 1 (2.3) |
| Neuroblastoma | 2 (66.7) | 2 (66.7) | 5 (71.4) | 2 (66.7) | 4 (14.8) | 15 (34.9) |
| Target gene fusion, n (%) | ||||||
| NTRK1/2/3 | 0 | 0 | 0 | 1 (33.3) | 14 (51.9) | 15 (34.9) |
| ROS1 | 0 | 0 | 1 (14.3) | 0 | 7 (25.9) | 8 (18.6) |
| ALK | 0 | 0 | 1 (14.3) | 0 | 2 (7.4) | 3 (7.0) |
| Non-fusion target gene alteration, n (%) | ||||||
| NTRK1c | 0 | 1 (33.3)d | 1 (14.3)d | 0 | 0 | 2 (4.7) |
| ROS1 | 0 | 0 | 0 | 0 | 0 | 0 |
| ALK | 0 | 0 | 1 (14.3) | 0 | 1 (3.7) | 2 (4.7) |
| Any target gene alteration, n (%) | ||||||
| NTRK1/2/3 | 0 | 1 (33.3) | 1 (14.3) | 1 (33.3) | 14 (51.9) | 17 (39.5) |
| ROS1 | 0 | 0 | 1 (14.3) | 0 | 7 (25.9) | 8 (18.6) |
| ALK | 0 | 0 | 2 (28.6) | 0 | 3 (11.1) | 5 (11.6) |
Abbreviations: ALK, anaplastic lymphoma kinase; CNS, central nervous system; IMT, inflammatory myofibroblastic tumor; NTRK, neurotrophic tyrosine receptor kinase; NOS, not otherwise specified; ROS1, ROS proto-oncogene 1.
aPatient aged 2 months received 250 mg/m2 dosage.
bn = 42; 1 patient in 550 mg/m2 dose group was excluded from Karnofsky/Lansky score category due to incorrect performance score scale for age.
cNo non-fusion gene alterations were identified in NTRK2 or 3.
dConsidered variants of unknown significance.
Phase 1 Determination of MTD
Sixteen patients were enrolled in phase 1, of whom 15 were evaluable for toxicity. One patient was non-evaluable as they had PD and received <75% of the prescribed dose (F1 formulation). Four patients (550 mg/m2, n = 1; 750 mg/m2, n = 3) experienced 5 DLTs (Supplementary Table S4). DLTs were reversible following dose reduction (dysgeusia and pulmonary edema) and/or interruption (increased creatinine). Daily entrectinib 550 mg/m2 (F1 formulation) was established as the MTD in pediatric patients.
Phase 1 and Phase 2 Safety
All patients reported ≥1 adverse event (AE) (43/43; 100%) and 76.7% experienced grade 3/4 events (Supplementary Table S5). Dizziness (any cause; any grade) was reported in 6 patients (14%; Supplementary Table S5). Any grade TRAEs occurred in 97.7% (42/43) of patients and grade 3/4 events in 53.5% (Table 2). The most common TRAEs included weight gain (48.8%), anemia (39.5%), increased blood creatinine (39.5%), and nausea (34.9%). Of the 17 patients who had increased blood creatinine, 13 (76.5%) had a resolved event. All events were grade 1 or 2 and 7 (41.2%) of the patients who had a reported event had more than 1 episode of blood creatinine increased. Other notable TRAEs included neurological effects, such as somnolence (9.3%) and paresthesia (4.7%), and bone fractures. Nine patients (20.9%) experienced 13 bone fracture events, of which maximum severity was grade 2 (non-operative) for 8/13 and grade 3 (operative intervention required) for 5/13 (Supplementary Table S6). Two patients with primary CNS tumors receiving the MTD had bilateral femoral fractures (cycle 4; cycle 8); both had rapid weight gain. Eight fracture events (61.5%) were considered related to entrectinib; of which, 2 had not resolved at data cutoff. Eleven of the 13 (84.6%) fractures had recovered/resolved with or without sequelae by data cutoff. These had been managed by dose interruption or withdrawal, where necessary.
Table 2.
Summary of the Most Common Grade 1/2 Treatment-Related Adverse Events During Phases 1 and 2 (>10% Incidence in Total Safety-Evaluable Population) and Any Grade 3/4 Treatment-Related Adverse Events (All Treatment Cycles)
| Phase 1 Dose Escalation, mg/m2 (n = 16) | Phase 2 (n = 27) | Total (n = 43) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 250 (n = 3) | 400 (n = 3) | 550 (n = 7) | 750 (n = 3) | ||||||||||
| G1/2 | G3/4 | G1/2 | G3/4 | G1/2 | G3/4 | G1/2 | G3/4 | G1/2 | G3/4 | G1/2 | G3/4 | Any G | |
| Any TRAE | 3 (100) | 0 | 2 (66.7) | 1 (33.3) | 7 (100) | 0 | 1 (33.3) | 2 (66.7) | 6 (22.2) | 20 (74.1) | 19 (44.2) | 23 (53.5) | 42 (97.7) |
| Weight gain | 0 | 0 | 0 | 0 | 4 (57.1) | 0 | 1 (33.3) | 0 | 9 (33.3) | 7 (25.9) | 14 (32.6) | 7 (16.3) | 21 (48.8) |
| Anemia | 1 (33.3) | 0 | 0 | 0 | 2 (28.6) | 0 | 2 (66.7) | 0 | 11 (40.7) | 1 (3.7) | 16 (37.2) | 1 (2.3) | 17 (39.5) |
| Blood creatinine increased | 2 (66.7) | 0 | 2 (66.7) | 0 | 2 (28.6) | 0 | 2 (66.7) | 0 | 9 (33.3) | 0 | 17 (39.5) | 0 | 17 (39.5) |
| Nausea | 3 (100) | 0 | 1 (33.3) | 0 | 2 (28.6) | 0 | 1 (33.3) | 0 | 8 (29.6) | 0 | 15 (34.9) | 0 | 15 (34.9) |
| Constipation | 1 (33.3) | 0 | 0 | 0 | 4 (57.1) | 0 | 1 (33.3) | 0 | 7 (25.9) | 0 | 13 (30.2) | 0 | 13 (30.2) |
| ALT increased | 0 | 0 | 1 (33.3) | 0 | 3 (42.9) | 0 | 2 (66.7) | 0 | 4 (14.8) | 2 (7.4) | 10 (23.3) | 2 (4.7) | 12 (27.9) |
| AST increased | 2 (66.7) | 0 | 2 (66.7) | 0 | 1 (14.3) | 0 | 2 (66.7) | 0 | 3 (11.1) | 1 (3.7) | 10 (23.3) | 1 (2.3) | 11 (25.6) |
| Neutrophil count decreased | 0 | 0 | 0 | 1 (33.3) | 1 (14.3) | 0 | 0 | 1 (33.3) | 2 (7.4) | 5 (18.5) | 3 (7.0) | 7 (16.3) | 10 (23.3) |
| White blood cell decreased | 0 | 0 | 0 | 0 | 0 | 0 | 2 (66.7) | 0 | 5 (18.5) | 2 (7.4) | 7 (16.3) | 2 (4.7) | 9 (20.9) |
| Vomiting | 0 | 0 | 0 | 0 | 2 (28.6) | 0 | 0 | 0 | 7 (25.9) | 0 | 9 (20.9) | 0 | 9 (20.9) |
| Diarrhea | 0 | 0 | 1 (33.3) | 0 | 2 (28.6) | 0 | 0 | 0 | 5 (18.5) | 0 | 8 (18.6) | 0 | 8 (18.6) |
| Dysgeusia | 0 | 0 | 1 (33.3) | 0 | 2 (28.6) | 0 | 2 (66.7) | 0 | 3 (11.1) | 0 | 8 (18.6) | 0 | 8 (18.6) |
| Fatigue | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 5 (18.5) | 1 (3.7) | 7 (16.3) | 1 (2.3) | 8 (18.6) |
| Flatulence | 0 | 0 | 0 | 0 | 2 (28.6) | 0 | 2 (66.7) | 0 | 3 (11.1) | 0 | 7 (16.3) | 0 | 7 (16.3) |
| Urinary incontinence | 0 | 0 | 0 | 0 | 1 (14.3) | 0 | 1 (33.3) | 0 | 5 (18.5) | 0 | 7 (16.3) | 0 | 7 (16.3) |
| Hypernatremia | 1 (33.3) | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 4 (14.8) | 0 | 6 (14.0) | 0 | 6 (14.0) |
| Fracture (combined)a | 0 | 0 | 0 | 0 | 1 (14.3) | 0 | 0 | 0 | 3 (11.1) | 2 (7.4) | 4 (9.3) | 2 (4.7) | 6 (14.0) a |
| Headache | 0 | 0 | 0 | 0 | 1 (14.3) | 0 | 1 (33.3) | 0 | 3 (11.1) | 0 | 5 (11.6) | 0 | 5 (11.6) |
| Abdominal pain | 0 | 0 | 0 | 0 | 2 (28.6) | 0 | 0 | 0 | 3 (11.1) | 0 | 5 (11.6) | 0 | 5 (11.6) |
| Pain in extremity | 1 (33.3) | 0 | 0 | 0 | 1 (14.3) | 0 | 0 | 0 | 3 (11.1) | 0 | 5 (11.6) | 0 | 5 (11.6) |
| Increased appetite | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 (18.5) | 0 | 5 (11.6) | 0 | 5 (11.6) |
| Platelet count decreased | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (33.3) | 2 (7.4) | 0 | 3 (7.0) | 1 (2.3) | 4 (9.3) |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.7) | 3 (11.1) | 1 (2.3) | 3 (7.0) | 4 (9.3) |
| Lymphocyte count decreased | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 1 (3.7) | 1 (3.7) | 2 (4.7) | 1 (2.3) | 3 (7.0) |
| Dyspnea | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 1 (3.7) | 0 | 1 (2.3) | 1 (2.3) | 2 (4.7) |
| Syncope | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 1 (3.7) | 1 (2.3) | 1 (2.3) | 2 (4.7) |
| Neutrophil percentage decreased | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.7) | 0 | 1 (2.3) | 1 (2.3) |
| Pancreatitis | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (3.7) | 0 | 1 (2.3) | 1 (2.3) |
| Pulmonary edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (2.3) | 1 (2.3) |
| Pneumonia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (2.3) | 1 (2.3) |
| Respiratory failure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (2.3) | 1 (2.3) |
| Hypoxia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (33.3) | 0 | 0 | 0 | 1 (2.3) | 1 (2.3) |
Abbreviations: AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TRAE, treatment-related adverse event.
AEs were encoded using Medical Dictionary for Regulatory Activities (version 21.0).
Values for the total population (Phase 1 and Phase 2) are shown in bold for emphasis.
aIncludes preferred terms fibula fracture, femur fracture, fracture, tibia fracture, pathological fracture, and stress fracture. Values correspond to the number (%) of patients, not events. See Supplementary Table S6 for all fracture events (all causality).
AEs led to dose reduction in 16 patients (37.2%) (weight gain [n = 5]; increased blood creatinine [n = 2]; prolonged corrected QT interval, ataxia, dysgeusia, headache, fatigue, intermittent falling episodes, neutrophil count decreased, bilateral femur fracture, and pulmonary edema [all n = 1]). Eight patients (18.6%) discontinued entrectinib due to AEs (fractures [n = 3]; dyspnea, encephalitis, pancreatitis, increased alanine aminotransferase, and pulmonary edema [all n = 1]). Twenty-one patients (48.8%) experienced dose interruptions. Across all patients, median dose intensity was 98.8% (95% CI 78.8-100.0). Among 34 patients treated at the MTD/RP2D (all formulations), 13 (38.2%) had an initial dose reduction due to an AE a median of 149 days after first entrectinib dose; 7 (20.6%) required discontinuation. No AE or TRAE led to death.
Pharmacokinetics and Dose-Finding
PK data for the F1 formulation can be found in the Supplementary file, page 1 and Supplementary Table S7. The dose rationale for the F1 formulation of 550 mg/m2 was based on the MTD, but the F1 formulation was not suitable for commercial use and was therefore discontinued.
The RP2D of 300 mg/m2 (F06 formulation) was determined by 2 modeling approaches: (1) the population pharmacokinetic (PopPK) modeling, a top-down (semi-empirical) approach,20 and (2) physiologically based pharmacokinetic (PBPK) modeling in both Simcyp21 and Gastroplus22 (bottom-up approach; more physiologic and considering organ maturation). These models consistently predicted similar entrectinib PK exposures in pediatric and adult patients with a recommended dose of 300 mg/m2. Individual PK estimates were calculated using the PopPK model for all available pediatric patients dosed with the F1 formulation. These individual values were used to estimate what systemic exposures would have been achieved if the patients had been given the 300 mg/m2 dose with the F06 formulation (Supplementary Figure S1A). The data show that systemic exposure across all pediatric ages with the F06 formulation is within the efficacious and tolerable range that has been established in the adult population (Supplementary Figure S1B). Supplementary Figure S2 shows PK data from the first pediatric patient dosed with 300 mg/m2 at the F06 formulation; the systemic exposure of this patient was well within the range of the adult exposure.
Phase 1 and Phase 2 Efficacy
As of September 17, 2020, 43 patients were enrolled and evaluated for response and 11 patients remained on treatment. For all-comer efficacy, refer to Supplementary file, page 1.
Efficacy according to the presence or absence of target gene fusion.
—The target fusion population (n = 26) comprised 16 patients with primary CNS tumors and 10 with extracranial solid tumors; confirmed ORR was 57.7% (15/26; 95% CI 36.9-76.7), including 7 CRs (26.9%) and 8 PRs (30.8%; Table 3). In the non-fusion population (n = 17), 1 patient with neuroblastoma harboring an ALK F1174L point mutation achieved a CR (investigator-assessed Curie score); investigator-assessed ORR was 5.9% (1/17; 95% CI 0.15-28.7; Supplementary Figure S3).
Table 3.
Summary of BICR-Assessed Best Overall Confirmed Responses in Patients With Tumors Harboring Target Gene Fusions, According to Fusion Kinase and Tumor Type
| Response, n (%) | Fusion Kinase | Tumor Type | Total (n = 26) | |||
|---|---|---|---|---|---|---|
| NTRK1/2/3 (n = 15) | ROS1 (n = 8) | ALK (n = 3) | Primary CNS (n = 16) | Extracranial Solid (n = 10) | ||
| Objective response rate, % (95% CI) | 60.0 (32.3, 83.7) | 62.5 (24.5, 91.5) | 33.3 (0.84, 90.6) | 50.0 (24.7, 75.4) | 70.0 (34.8, 93.3) | 57.7 (36.9, 76.7) |
| Complete response | 5 (33.3) | 1 (12.5) | 1 (33.3) | 4 (25.0) | 3 (30.0) | 7 (26.9) |
| Partial response | 4 (26.7) | 4 (50.0) | 0 | 4 (25.0) | 4 (40.0) | 8 (30.8) |
| Stable disease | 4 (26.7) | 2 (25.0) | 1 (33.3) | 5 (31.3) | 2 (20.0) | 7 (26.9) |
| Progressive disease | 1 (6.7) | 0 | 1 (33.3) | 2 (12.5) | 0 | 2 (7.7) |
| Missing/unevaluable | 1 (6.7) | 1 (12.5) | 0 | 1 (6.3) | 1 (10.0) | 2 (7.7) |
Abbreviations: ALK, anaplastic lymphoma kinase; BICR, blinded independent central review; CI, confidence interval; CNS, central nervous system; CR, complete response; NTRK, neurotrophic tyrosine receptor kinase; PD, progressive disease; ROS1, ROS proto-oncogene 1.
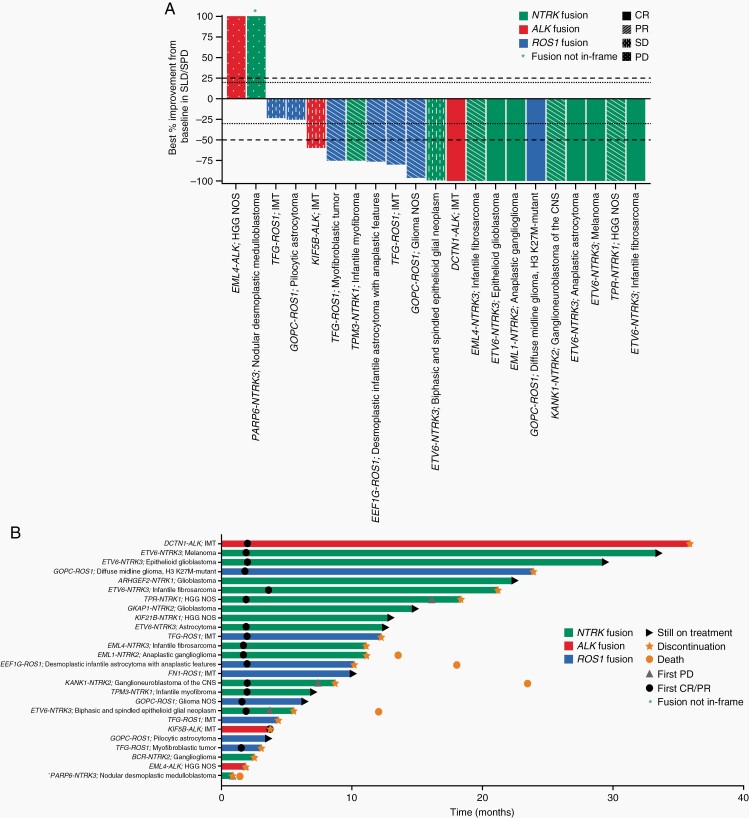
In the target fusion population, reductions in measurable target lesions were observed in 17/21 (80.9%) patients; 2 patients did not meet the definition of confirmed objective response at the time of data cutoff, as they were not measured on 2 separate evaluations >4 weeks apart) (Figure 2A). Of the 15 confirmed objective responses (BICR-assessed), 14 (93.3%) were observed at the first evaluation (end of cycle 2; Figure 2B). Median DoR (95% CI) among responders in the target fusion population (BICR-assessed) was not reached (14.3 months-not evaluable [NE]), due to patients still receiving therapy. Median duration of treatment (interquartile range) at data cutoff was 10.6 months (4.2-18.4) in the target fusion population and 1.8 months (1.7-2.1) in the non-fusion population. Median PFS (95% CI) was not reached (12.8 months-NE) in the target fusion population and 1.9 months (1.7-5.7) in the non-fusion population (P < .0001; Supplementary Figure S4).
Fig. 2.
Responses to entrectinib as assessed by BICR in patients with tumors harboring target gene fusions (n = 26). (A) Waterfall plot of BICR-assessed maximum percentage change in tumor size from baseline as measured by RECIST or RANO in patients with measurable target lesions. Plot includes 21 patients with both baseline and post-baseline measurements available for SLD or SPD. Five patients were excluded due to the presence of non-target lesions only (n = 3) or non-evaluable response (n = 2). Best overall confirmed responses per BICR assessment are also indicated; note that confirmed response does not align with best percentage improvement from baseline in SLD/SPD in 5 patients due to consideration of response in non-target lesions (n = 2), development of new lesions (n = 1), and requirement for confirmation of response after ≥28 days (n = 2; initial PR unconfirmed due to subsequent surgical resection [n = 1] or new lesion [n = 1]). (B) Swimmer plot of BICR-assessed best overall response from start of therapy to time of last therapy. Abbreviations: ALK, anaplastic lymphoma kinase; BICR, blinded independent central review; CNS, central nervous system; CR, complete response; HGG, high-grade glioma; IMT, inflammatory myofibroblastic tumors; NOS, not otherwise specified; NTRK, neurotrophic tyrosine receptor kinase; PD, progressive disease; PR, partial response; RANO, Response Assessment in Neuro-Oncology; RECIST, Response Evaluation Criteria in Solid Tumors; ROS1, ROS proto-oncogene 1; SD, stable disease; SLD, sum of longest diameter; SPD, sum of products of diameters.
Efficacy according to type of target gene fusion.
—The target fusion population (n = 26) comprised 15 patients with NTRK1/2/3 fusions, 8 patients with ROS1 fusions, and 3 patients with ALK fusions. Confirmed ORR was 60.0% (9/15; 95% CI 32.3-83.7), 62.5% (5/8; 95% CI 24.5-91.5), and 33.3% (1/3; 95% CI 0.84-90.6) in patients with NTRK1/2/3, ROS1, and ALK fusions, respectively (Table 3).
Efficacy in CNS tumors.
—In phase 2, 16 patients with primary CNS tumors and target gene fusions were response-evaluable (cohort B, n = 11; cohort E, n = 5; Supplementary Table S8). Confirmed objective responses by BICR were observed in 8 patients ([4 CRs; 4 PRs]; ORR, 50.0%; 95% CI 24.7-75.4; Figure 2; Table 3). Patients in cohort B (phase 2; n = 11) showed an ORR per BICR of 54.5% (n = 6/11; 95% CI 23.4-83.3), which met the threshold of 40% for the interim efficacy analysis.
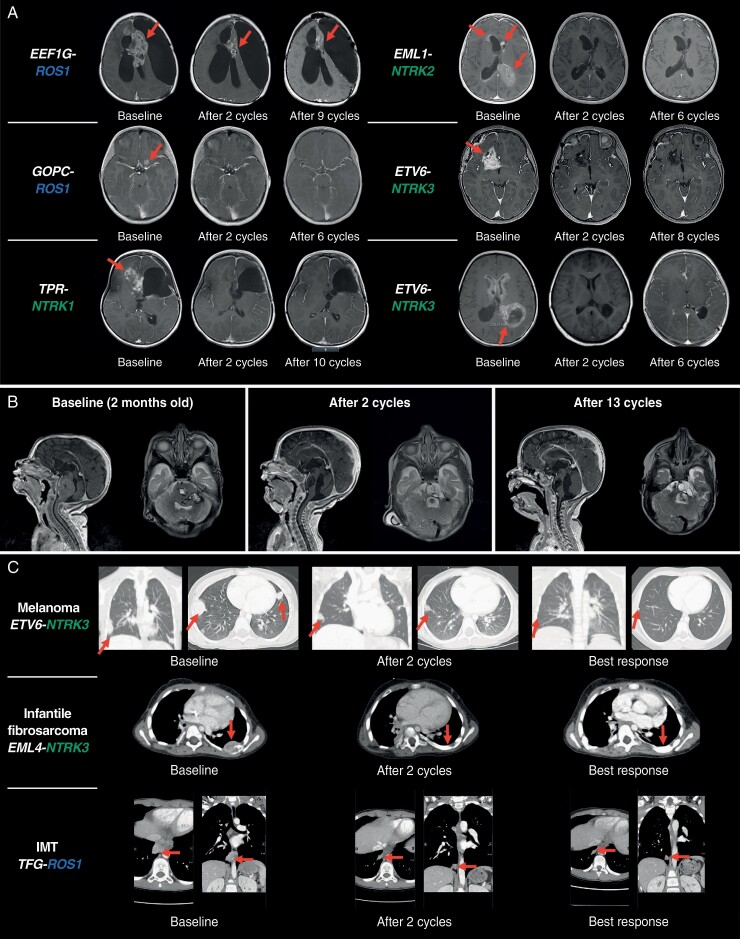
Figure 3A presents responses in selected patients with primary CNS tumors and target gene fusions. One patient who did not respond at first disease evaluation on therapy enrolled with a relapsed medulloblastoma harboring a purported PARP6-NTRK3 fusion was noted as out-of-frame. A patient who achieved confirmed response was an infant diagnosed aged 2 weeks with a large pontine tumor (a presumed fatal diffuse intrinsic pontine glioma); subsequent diagnostic tumor biopsy revealed an ETV6-NTRK3 fusion. Entrectinib treatment was initiated aged 2 months and resulted in a CR; the patient remains on therapy after 1 year (Figure 3B). In total, 7/16 patients remain on treatment; 9 discontinued treatment due to PD (n = 6) or an AE (n = 3; bone fracture, tibia fracture, or pancreatitis [all n = 1]).
Fig. 3.
Example MRI scans showing measurable and durable responses to entrectinib. (A) Primary CNS tumors. (B) An infant with a primary CNS tumor determined to be an anaplastic astrocytoma harboring an ETV6-NTRK3 fusion. Patient remains on treatment after 1 year. (C) Extracranial solid tumors. Per protocol, entrectinib was administered in 28-day cycles. Abbreviations: CNS, central nervous system; IMT, inflammatory myofibroblastic tumor; MRI, magnetic resonance imaging; NTRK, neurotrophic tyrosine receptor kinase; ROS1, ROS proto-oncogene 1.
Efficacy in extracranial solid tumors.
—Ten patients enrolled with extracranial solid tumors and target gene fusions were evaluable for response (Supplementary Table S9); 3 were enrolled in phase 1 (550 mg/m2, n = 2; 750 mg/m2, n = 1) and 7 in phase 2 (cohort D, n = 4; cohort E, n = 3). Confirmed objective responses were observed in 7 patients ([3 CRs; 4 PRs]; 70.0%; 95% CI 34.8-93.3; Figure 2; Table 3). ORR for cohort D (phase 2) alone could not be calculated yet, as only 4 patients had been enrolled in this cohort at the data cutoff. Per the protocol, ORR for the interim analysis will be evaluated when another 6 patients have been enrolled in this cohort.
Figure 3C presents responses in selected patients with extracranial solid tumors and target gene fusions. Three patients remain on treatment; 7 discontinued due to AEs (n = 3; femur fracture, increased alanine and aspartate aminotransferase, or pulmonary edema [all n = 1]) study withdrawal (n = 2), persistent toxicities (n = 1), and prolonged non-evaluable disease status (n = 1).
Fifteen patients with neuroblastoma were evaluable for response (phase 1, n = 11; phase 2, n = 4). One patient with neuroblastoma harboring an ALK point mutation (F1174L) had a CR by Curie score (Supplementary Figure S3), and 3 patients had stable MIBG-evaluable disease by Curie score, and 8 patients had PD. Three patients had missing data/unevaluable responses.
Discussion
In this phase 1/2 study, entrectinib showed a positive benefit-risk profile in pediatric patients with solid tumors, including CNS tumors, harboring target NTRK1/2/3 or ROS1 fusions. Frequent TRAEs were grade 1 or 2 weight gain, anemia, or gastrointestinal side effects, including nausea or constipation. In phase 1, reversible grade 3 pulmonary edema and persistent grade 2 increased creatinine, fatigue, and dysgeusia were DLTs that established 550 mg/m2 (F1 formulation) as the MTD. In phase 2, TRAEs were similar to those reported in adults,1 and consistent with the on-target effects of entrectinib. Dysgeusia and ataxia may be related to the role of TRKA or TRKC in proprioception and sensation.5 Weight gain, possibly associated with TRKB-mediated effects on appetite, and neurocognitive side effects have been reported with other ALK/ROS1 inhibitors.23 For patients with weight gain, dietary modifications and counseling are recommended. To assess the effects of entrectinib on cognitive development in children, the neurocognitive examination was incorporated into the safety assessment schedule following protocol amendment 8.
Nine patients experienced 13 bone fracture events, of which 8 were considered related to entrectinib and 11 had recovered/resolved at data cutoff. An association between fractures and TRK inhibitors has not yet been established; however, neurotrophins and TRK receptors may be involved in bone formation and healing.24 No gender, histology type, tumor location, or gene fusion patterns have been observed to date to be associated with a higher incidence of fractures. Protocol amendments have implemented close monitoring and biomarker measurement (bone mineral density and markers of bone formation, resorption, and calcium metabolism) to increase understanding of fracture etiology and risk.
Exposure to entrectinib (F1 formulation) increased with dose but was highly variable, due to F1 formulation sensitivity to gastric conditions.22 Three patients received a different formulation (F2B), adding to the observed variability. Overall, systemic exposure in some pediatric patients receiving the F1 formulation was lower than that seen in adults receiving the 600 mg dose (F06 formulation), due to the sensitivity of the F1 formulation to gastric environmental conditions.22,25,26 Pediatric patients in this trial experienced a high frequency of TRAEs (grade 3/4, 53.5%), resulting in discontinuation in 14.0% of patients; however, median dose intensity was high (98.8%). The MTD of 550 mg/m2 (F1 formulation) was superseded by the RP2D of 300 mg/m2 (F06 formulation) based on PK modeling approaches.20,21 Ongoing studies will evaluate the safety and efficacy of the F06 formulation (300 mg/m2), and a pediatric age-appropriate formulation (minitablets) in patients unable to swallow intact capsules.25,26
Entrectinib is efficacious against solid tumors with NTRK1/2/3 and ROS1 gene fusions. Responses occurred in patients with tumors harboring target gene fusions irrespective of histology or location (intracranial or extracranial). Responses similar to those observed with larotrectinib (TRK inhibitor) were achieved in patients with IF and metastatic melanoma.27 Patients with ROS1 or ALK fusion-positive IMTs and a patient with neuroblastoma harboring an ALK point mutation (F1174L) showed responses, demonstrating the multi-target inhibition profile of entrectinib. A patient with an unresectable extracranial solid tumor (IMT) at enrollment underwent complete surgical resection after a PR with entrectinib, reflecting the potential application of entrectinib early in the disease course. Patients with neuroblastoma were included without biomarker selection based on preclinical data,28 likely contributing to the limited response in this population.
Eight patients with primary CNS tumors and target fusions achieved objective responses with entrectinib. These patients ranged in age from 2 months to 9 years with a variety of tumor histologies, which was expected as pediatric gliomas present in very young children and histologic classification is challenging.6,29 Infant HGGs are poorly understood and often fatal in the event of relapse.30 Rapid objective responses in this population indicate that entrectinib treatment before surgical excision may reduce morbidity and spare or delay cranial radiation in very young children. As demonstrated by the infant with brainstem glioma enrolled in this trial, molecular testing may facilitate access to entrectinib treatment for patients where target gene fusions are not suspected.31,32
In conclusion, entrectinib demonstrated rapid and durable activity against pediatric intracranial and extracranial solid tumors with target gene fusions in NTRK1/2/3 or ROS1. Although tumors that harbor these fusions remain rare, the profound effect of this single agent on these pediatric patients with life-threatening diseases makes it invaluable to this population. Further studies are ongoing to assess an age-appropriate formulation, determine the optimal treatment duration, and monitor for resistance and long-term side effects in pediatric patients.
Supplementary Material
Acknowledgments
The authors thank the patients, their families, and the participating study centers. F. Hoffmann-La Roche Ltd. was involved in the study design, data analysis and interpretation, writing of the manuscript, and the decision to submit it for publication. Support was also provided by Alex’s Lemonade Stand Foundation Center of Excellence Grant (to E.F., A.V.D., A.J.S., J.F., S.S.), Cancer Research Foundation (to A.V.D.), United-4 A Cure-Riviera Country Club and Sports Center (to A.V.D.), Ted Mullin Fund (to A.V.D.), and National Cancer Institute Award (to A.J.S.). Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Emily Morton, BSc and Charlotte Kennerley, PhD, of Ashfield MedComms, an Ashfield Health company, and was funded by F. Hoffmann-La Roche Ltd.
Contributor Information
Ami V Desai, Department of Pediatrics, Section of Hematology/Oncology/Stem Cell Transplantation, University of Chicago Medical Center, Chicago, Illinois, USA.
Giles W Robinson, Division of Neuro-Oncology, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Karen Gauvain, Pediatric Neuro-Oncology, Washington University School of Medicine, St. Louis, Missouri, USA.
Ellen M Basu, Department of Pediatrics, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Margaret E Macy, Pediatric Hematology-Oncology, Children’s Hospital Colorado, University of Colorado Anschutz Medical Campus, Aurora, Colorado, USA.
Luke Maese, Department of Pediatrics, Division of Hematology/Oncology, University of Utah/Huntsman Cancer Institute, Salt Lake City, Utah, USA.
Nicholas S Whipple, Pediatric Hematology-Oncology, University of Utah, Salt Lake City, Utah, USA.
Amit J Sabnis, Division of Pediatric Oncology, Department of Pediatrics, University of California, San Francisco, California, USA.
Jennifer H Foster, Department of Pediatrics, Hematology-Oncology, Texas Children’s Hospital, Houston, Texas, USA.
Suzanne Shusterman, Pediatric Hematology and Oncology, Dana Farber Cancer Institute/Children’s Cancer and Blood Disorders Center, Boston, Massachusetts, USA.
Janet Yoon, Department of Pediatrics, University of California San Diego, San Diego, California, USA.
Brian D Weiss, Department of Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Mohamed S Abdelbaki, Division of Hematology & Oncology, Nationwide Children’s Hospital, Columbus, Ohio, USA.
Amy E Armstrong, Division of Pediatric Hematology/Oncology, Washington University School of Medicine, St. Louis, Missouri, USA.
Thomas Cash, Pediatric Hematology/Oncology, Aflac Cancer & Blood Disorders Center, Children’s Healthcare of Atlanta, Emory University School of Medicine, Atlanta, Georgia, USA.
Christine A Pratilas, Department of Oncology, Division of Pediatric Oncology, Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins University School of Medicine, Baltimore, Maryland, USA.
Nadège Corradini, Department of Pediatric Hematology and Oncology, Institute of Pediatric Hematology and Oncology (IHOPe), Léon Bérard Cancer Centre, Lyon, France.
Lynley V Marshall, Children and Young People’s Unit, The Royal Marsden Hospital and The Institute of Cancer Research, London, UK.
Mufiza Farid-Kapadia, Biometrics Department, F. Hoffmann-La Roche Ltd., Mississauga, Ontario, Canada.
Saibah Chohan, PDD Data & Statistical Sciences, F. Hoffmann-La Roche Ltd., Mississauga, Ontario, Canada.
Clare Devlin, Pharma Development Oncology and Hematology, Roche Products Ltd., Welwyn Garden City, UK.
Georgina Meneses-Lorente, Pharma Research and Early Development, Roche Products Ltd., Welwyn Garden City, UK.
Alison Cardenas, Clinical Safety, Genentech, Inc., South San Francisco, California, USA.
Katherine E Hutchinson, Oncology Biomarker Development, Genentech, Inc., South San Francisco, California, USA.
Guillaume Bergthold, Product Development Oncology, F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Hubert Caron, Product Development Oncology, F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Edna Chow Maneval, Clinical Development, Ignyta, Inc., San Diego, California, USA.
Amar Gajjar, Division of Neuro-Oncology, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Elizabeth Fox, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, Tennessee, USA.
Funding
This work was supported by F. Hoffmann-La Roche Ltd., Basel, Switzerland (NCT02650401).
Conflict of interest statement. A.V.D. has stock and ownership interests in Pfizer and Viatris; has received consultancy/advisory fees from Merck, Ology Medical Education, and Y-mAbs Therapeutics, Inc.; has received institutional research funding from Merck, F. Hoffmann-La Roche Ltd./Ignyta, Jubilant Draximage, Y-mAbs Therapeutics, Inc., Eli Lilly, Actuate Therapeutics, GlaxoSmithKline plc; and has received travel or accommodation expenses from GlaxoSmithKline plc. G.W.R. has received consultancy fees from Eli Lilly and F. Hoffmann-La Roche Ltd./Genentech, Inc.; has received research funding from Novartis and F. Hoffmann-La Roche Ltd./Genentech, Inc. K.G. has received consultancy fees from Bayer Pharmaceuticals. M.E.M. has received research funding from Bayer, institutional research funding from F. Hoffmann-La Roche Ltd./Genentech, Inc., Bayer, Pfizer, Merck, AbbVie, and Epizyme; received advisory board fees from Y-mAbs Therapeutics, Inc.; has stock and ownership interests in Johnson and Johnson. L.M. has received consulting fees and travel expenses from Jazz Pharmaceuticals. N.S.W. received consultancy fees from Bayer Pharmaceuticals. J.H.F. has received advisory board fees from BTG International, Cellectar, and EUSA Pharma. J.Y. received consultancy fees from Bayer Pharmaceuticals. B.D.W. declares associated research funding from Novartis, Exelixis, and F. Hoffmann-La Roche Ltd. in the past 2 years. M.S.A. reports he is the co-chair of a trial receiving drug supply from Novartis and research funding from the PLGAA Foundation; and has received funding from CancerFree Kids. A.E.A. has received consultancy fees from SpringWorks Therapeutics and through EM Partners. T.C. has received research funding from Celgene and institutional research funding from F. Hoffmann-La Roche Ltd./Genentech, Inc.; has received advisory board/consultancy fees from EUSA Pharma, Inc. and Y-mAbs Therapeutics, Inc.; is study chair of a clinical trial that receives funding from United Therapeutics. C.P. has received consultation fees from F. Hoffmann-La Roche Ltd./Genentech, Inc.; receives research funding from Kura Oncology and Novartis. N.C. has received advisory board fees from BMS International. L.V.M. has received consultancy fees from Bayer Pharmaceuticals; received advisory board fees from BMS and Day One Therapeutics; and is on external Data Monitoring Committees for Eisai & Merck. M.F.-K. and S.C. are employees of F. Hoffmann-La Roche Ltd. C.D. is an employee of, and holds shares in, F. Hoffmann-La Roche Ltd. G.M.-L., A.C., and G.B. are employees of F. Hoffmann-La Roche Ltd./Genentech, Inc. K.E.H. has stock and ownership interests and is an employee of F. Hoffmann-La Roche Ltd./Genentech, Inc. H.C. is an employee of, and holds shares in, F. Hoffmann-La Roche Ltd. E.C.M. is a former employee of F. Hoffmann-La Roche Ltd./Ignyta. A.G. has received research funding from Genentech, Inc., and Kazia Therapeutics, received advisory board fees from QED Pharmaceuticals and forms a participant of the Data and Safety Monitoring Board for DOT 1 Therapeutics. E.M.B., A.J.S., S.S., and E.F. declare no conflicts of interest.
Authorship statement. Conception and design: A.V.D., G.W.R., E.F., G.B.; Patient recruitment: A.V.D., G.W.R., K.G., E.M.B., M.E.M., L.M., N.S.W., A.J.S., J.H.F., S.S., J.Y., B.D.W., M.S.A., A.E.A., T.C., C.P., N.C., L.V.M., A.G., E.F.; Principal investigators at contributing sites: A.V.D., K.G., E.M.B., M.E.M., L.M., N.S.W., A.J.S., S.S., J.Y., B.D.W., M.S.A., A.E.A., T.C., C.P., N.C., L.V.M., A.G., E.F.; Data collection: all authors; Data analysis: A.V.D., G.W.R., E.F., A.G., M.F.-K., A.C., S.C., C.D., K.E.H., E.C.M.; Data interpretation, writing of the report, and approval of the report: all authors.
Data Availability
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli. https://vivli.org/ourmember/roche/. For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than 1 data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.
References
- 1. Dziadziuszko R, Krebs MG, De Braud F, et al. Updated integrated analysis of the efficacy and safety of entrectinib in locally advanced or metastatic ROS1 fusion-positive non–small-cell lung cancer. J Clin Oncol. 2021;39(11):1253–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dupain C, Harttrampf AC, Urbinati G, Geoerger B, Massaad-Massade L. Relevance of fusion genes in pediatric cancers: toward precision medicine. Mol Ther Nucleic Acids. 2017;6:315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Okamura R, Boichard A, Kato S, et al. Analysis of NTRK alterations in pan-cancer adult and pediatric malignancies: implications for NTRK-targeted therapeutics. JCO Precis Oncol. 2018;2:PO.18.00183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Albert CM, Davis JL, Federman N, Casanova M, Laetsch TW. TRK fusion cancers in children: a clinical review and recommendations for screening. J Clin Oncol. 2019;37(6):513–524. [DOI] [PubMed] [Google Scholar]
- 5. Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Guerreiro Stucklin AS, Ryall S, Fukuoka K, et al. Alterations in ALK/ROS1/NTRK/MET drive a group of infantile hemispheric gliomas. Nat Commun. 2019;10(1):4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamamoto H, Yoshida A, Taguchi K, et al. ALK, ROS1 and NTRK3 gene rearrangements in inflammatory myofibroblastic tumours. Histopathology. 2016;69(1):72–83. [DOI] [PubMed] [Google Scholar]
- 8. Ross JS, Ali SM, Fasan O, et al. ALK fusions in a wide variety of tumor types respond to anti-ALK targeted therapy. Oncologist. 2017;22(12):1444–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trigg RM, Turner SD. ALK in neuroblastoma: biological and therapeutic implications. Cancers (Basel). 2018;10(4):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Brodeur GM, Minturn JE, Ho R, et al. Trk receptor expression and inhibition in neuroblastomas. Clin Cancer Res. 2009;15(10):3244–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fischer H, Ullah M, de la Cruz CC, et al. Entrectinib, a TRK/ROS1 inhibitor with anti-CNS tumor activity: differentiation from other inhibitors in its class due to weak interaction with P-glycoprotein. Neuro Oncol. 2020;22(6):819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. EMA. ROZLYTREK (Entrectinib). 2020. Available at https://www.ema.europa.eu/en/medicines/human/summaries-opinion/rozlytrek. Accessed January 17, 2022.
- 13. FDA. ROZLYTREK Prescribing Information. 2019. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212725s000lbl.pdf. Accessed January 17, 2022.
- 14. Doebele RC, Drilon A, Paz-Ares L, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):271–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drilon A, Siena S, Dziadziuszko R, et al. Entrectinib in ROS1 fusion-positive non–small-cell lung cancer: integrated analysis of three phase 1–2 trials. Lancet Oncol. 2020;21(2):261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. [DOI] [PubMed] [Google Scholar]
- 17. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 18. Ady N, Zucker JM, Asselain B, et al. A new 123I-MIBG whole body scan scoring method—application to the prediction of the response of metastases to induction chemotherapy in stage IV neuroblastoma. Eur J Cancer. 1995;31A(2):256–261. [DOI] [PubMed] [Google Scholar]
- 19. Fox E, Maris JM, Widemann BC, et al. A phase I study of ABT-751, an orally bioavailable tubulin inhibitor, administered daily for 21 days every 28 days in pediatric patients with solid tumors. Clin Cancer Res. 2008;14(4):1111–1115. [DOI] [PubMed] [Google Scholar]
- 20. Gonzalez-Sales M, Djebli N, Meneses-Lorente G, et al. Population pharmacokinetic analysis of entrectinib in pediatric and adult patients with advanced/metastatic solid tumors: support of new drug application submission. Cancer Chemother Pharmacol. 2021;88(6):997–1007. [DOI] [PubMed] [Google Scholar]
- 21. Djebli N, Buchheit V, Parrott N, et al. Physiologically-based pharmacokinetic modelling of entrectinib parent and active metabolite to support regulatory decision-making. Eur J Drug Metab Pharmacokinet. 2021;46(6):779–791. [DOI] [PubMed] [Google Scholar]
- 22. Parrott N, Stillhart C, Lindenberg M, et al. Physiologically based absorption modelling to explore the impact of food and gastric pH changes on the pharmacokinetics of entrectinib. AAPS J. 2020;22(4):78. [DOI] [PubMed] [Google Scholar]
- 23. Shaw AT, Solomon BJ, Chiari R, et al. Lorlatinib in advanced ROS1-positive non–small-cell lung cancer: a multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol. 2019;20(12):1691–1701. [DOI] [PubMed] [Google Scholar]
- 24. Su Y, Zhou X, Foster B, et al. Roles of neurotrophins in skeletal tissue formation and healing. J Cell Physiol. 2018;233(3):2133–2145. [DOI] [PubMed] [Google Scholar]
- 25. Meneses-Lorente G, Bentley D, Guerini E. Characterization of the pharmacokinetics of entrectinib and its active M5 metabolite in healthy volunteers and patients with solid tumors. Invest New Drugs. 2021;39(3):803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Djebli N, Parrot N, Mercier F, et al. Combined use of population and physiologically-based PK to support pediatric dose recommendation of entrectinib. Presented at the American Society for Clinical Pharmacology and Therapeutics 2020 (Poster P1-020). 2020. Available at https://medically.gene.com/global/en/unrestricted/oncology/ASCPT-2021/ascpt-2021-poster-djebli-combined-use-of-population-and.html. Accessed 13 April 2022.
- 27. Hong DS, DuBois SG, Kummar S, et al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21(4):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pacenta HL, Macy ME. Entrectinib and other ALK/TRK inhibitors for the treatment of neuroblastoma. Drug Des Devel Ther. 2018;12:3549–3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sturm D, Pfister SM, Jones DTW. Pediatric gliomas: current concepts on diagnosis, biology, and clinical management. J Clin Oncol. 2017;35(21):2370–2377. [DOI] [PubMed] [Google Scholar]
- 30. Karremann M, Gielen GH, Hoffmann M, et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol. 2018;20(1):123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao X, Kotch C, Fox E, et al. NTRK fusions identified in pediatric tumors: the frequency, fusion partners, and clinical outcome. JCO Precis Oncol. 2021;5:204–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clarke M, Mackay A, Ismer B, et al. Infant high-grade gliomas comprise multiple subgroups characterized by novel targetable gene fusions and favorable outcomes. Cancer Discov. 2020;10(7):942–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For eligible studies, qualified researchers may request access to individual patient-level clinical data through a data request platform. At the time of writing, this request platform is Vivli. https://vivli.org/ourmember/roche/. For up-to-date details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here: https://go.roche.com/data_sharing. Anonymized records for individual patients across more than 1 data source external to Roche cannot, and should not, be linked due to a potential increase in risk of patient re-identification.