Abstract
The brain tumor microenvironment contains numerous distinct types of nonneoplastic cells, which each serve a diverse set of roles relevant to the formation, maintenance, and progression of these central nervous system cancers. While varying in frequencies, monocytes (macrophages, microglia, and myeloid-derived suppressor cells), dendritic cells, natural killer cells, and T lymphocytes represent the most common nonneoplastic cellular constituents in low- and high-grade gliomas (astrocytomas). Although T cells are conventionally thought to target and eliminate neoplastic cells, T cells also exist in other states, characterized by tolerance, ignorance, anergy, and exhaustion. In addition, T cells can function as drivers of brain cancer growth, especially in low-grade gliomas. Since T cells originate in the blood and bone marrow sinuses, their capacity to function as both positive and negative regulators of glioma growth has ignited renewed interest in their deployment as immunotherapeutic agents. In this review, we discuss the roles of T cells in low- and high-grade glioma formation and progression, as well as the potential uses of modified T lymphocytes for brain cancer therapeutics.
Keywords: astrocytoma, glioblastoma, gliomagenesis, microglia, pediatric low-grade glioma, T cells, tumor microenvironment, tumor-associated monocytes
The brain tumor microenvironment (TME) consists of a heterogeneous and dynamic network of interacting immune, vascular, and resident cellular components. In the most common brain tumor encountered in children and adults (gliomas or astrocytomas), these nonneoplastic cell types include macrophages, microglia, myeloid-derived suppressor cells (MDSCs), T lymphocytes, neutrophils, natural killer (NK) cells, dendritic cells (DCs), endothelial cells, neurons, and glial lineage cells (astrocytes, oligodendrocytes).1 Tumor-associated monocytes (TAMs) account for the majority of the stromal cells in both low-grade and high-grade gliomas (LGG and HGG, respectively),2,3 representing either yolk sac-derived brain resident monocytes (microglia) or infiltrating bone marrow-derived macrophages, which each play unique roles in glioma biology.4 Additionally, the stromal content and composition varies depending on the specific tumor grade and histologic subtype with respect to the numbers of myeloid and lymphoid cells, as well as their relative importance to tumor growth.5,6
In contrast to TAMs, comparatively less is known about the contributions of other immune cell populations, specifically T cells, to glioma pathobiology. As such, T lymphocytes can function as both positive and negative regulators of glioma growth.7 These opposing effects could reflect differences in T cell populations (CD4+ versus CD8+ T cells), their particular functional states within the tumor (exhausted versus cytotoxic), or their interactions with other nonneoplastic cell types (neurons, astrocytes, monocytes, and MDSCs).7 Moreover, these differences in T cell-glioma interactions also vary as a consequence of the evolutionary state of the tumor (cancer initiation versus maintenance), exposures to conventional or molecularly targeted therapies, and/or the specific region within the tumor (perivascular space versus the center of the tumor).5,8 Over the course of tumor evolution, CD8+ T cells may efficiently mount a cytotoxic response to block tumor growth, become suppressed by recruited myeloid cells, or be activated by immunostimulatory therapy.9 In addition to temporal heterogeneity, spatial differences exist within tumors, where CD8+ T cells tend to be located in the center of the tumor and CD4+ T cells in the perivascular spaces.5 Due to the heterogeneity of T cells, their complex interactions, and their evolution over time, harnessing these interactions with therapies can be a moving target. However, with the advent of advanced immunologic analytical methods and single cell transcriptomics, the diversity of T cell subtypes and their relevance to glioma formation and growth is becoming clearer. In this review, we discuss the multi-faceted roles of T lymphocytes in glioma biology and their use as therapeutic agents.
The Glioma Tumor Microenvironment
The central nervous system (CNS) was originally considered an immunologically privileged site owing to the presence of the blood–brain barrier (BBB); however, this concept is outdated with the recognition that T cells normally circulate through the healthy brain.10 Moreover, BBB integrity is disrupted during infection, inflammation, or cancer, leading to the accumulation of immune cell infiltrates within the CNS.1,11
Representing as many of 30–50% of the cellular content of gliomas, myeloid cells constitute the majority of all immune cells.2,3 These TAMs, including yolk sac-derived brain resident microglia and bone marrow-derived macrophages, have distinct contributions to glioma formation and growth, which has been extensively reviewed elsewhere.12,13 Bone marrow-derived macrophages are recruited to the TME by a variety of glioma-produced cytokines/chemokines (eg, CCL2, CSF-1, CX3CL1), where they become almost indistinguishable from microglia.13 TAMs may have immunosuppressive properties due to the release of pro- and anti-inflammatory molecules (eg, TGF-β, IL-10, TNF-α), expression of checkpoint inhibitor molecules, interactions with regulatory T cells (Tregs), and/or metabolic effects on T cells.13,14 They can also directly increase tumor growth through the elaboration of soluble factors that enhance tumor proliferation and invasion (eg, ST-1, TGF-β, IL-6, and IL1β).13 Additionally, these TAMs may function in a region-specific manner within HGGs, where blood-derived macrophages predominate in the central portion of the tumor and exert immunosuppressive effects.13 Conversely, microglia can also increase murine LGG growth by producing CCL5, a chemokine necessary and sufficient for mouse LGG formation and maintenance.15 Lastly, some TAMs arise from MDSCs, a heterogeneous population of immunosuppressive cells that arise from myeloid progenitor cells in the bone marrow in response to chronic inflammation.16 These cells exert their immunosuppressive effects by differentiating into TAMs within the tumor or by producing anti-inflammatory cytokines, reactive oxygen species, and/or nitric oxide.16 In some circumstances, MDSCs can interfere with anti-glioma immunotherapy.17,18
Within the lymphoid cell compartment, T cells and NK cells predominate, the majority of which lack anti-tumor activity. In this regard, T cells constitute 1–5% of the total glioma cellular content, including CD8+ cytotoxic T lymphocytes (CTLs), regulatory CD4+ (Tregs), and conventional CD4+ T cells.2,3 In general, CD8+ T cells are “effector” T cells, which are activated by T cell receptor binding to antigens that derive from virally-infected or neoplastic cells. These antigens are bound to major histocompatibility locus (MHC) I proteins expressed on antigen-presenting cells (APCs). Following their activation, CTLs recognize and lyse cells that express those specific antigens.19 Conversely, conventional CD4+ T cells, which are less plentiful, are “helper” T cells, which induce CD8+ T cell activity through cytokine secretion and promote B cell proliferation and differentiation. A subset of these CD4+ T cells is FOXP3+ Tregs,20 which can elicit potent immunosuppressive responses and interfere with anti-HGG immunotherapy.21 For example, HGG-induced hypoxia stimulates Treg activation and the tumor-promoting capabilities of TAMs.22 As conflicting reports exist regarding the prognostic significance of tumoral T cell infiltration, including the relative contribution of CD8+ T cells and Tregs to patient outcomes,23 additional studies on the role of different T cell populations in glioma biology are warranted.
T Cell Recruitment
Several mechanisms have been proposed for glioma T cell recruitment, including disruption of the BBB and chemoattraction by tumor cells and TAMs as a consequence of impaired astrocyte–endothelial cell interactions.24 This disruption can be visualized by magnetic resonance imaging (MRI), where gadolinium-based contrast dye leakage into the tumor indicates impairment of the BBB. In addition, edema in the region surrounding the tumor can result from fluid imbalance between the brain parenchyma and capillaries.11 Further research is needed to determine whether BBB permeabilization is necessary for tumor progression and whether immune cells pass more easily through these areas of BBB disruption.
In addition to passive entry through a leaky BBB, T cells infiltrate brain tumors by chemokine-mediated attraction. In experimental murine models of LGG, T cell trafficking into the tumor is mediated by neoplastic cell production of chemokines (CCL2 and CCL12),25 whereas HGG tumor cells attract T cells by producing indolamine 2,3-dioxygenase (IDO), CCL2 and CCL22.21,26 CD8+ T cells can also be primed by DCs and macrophages, as well as APCs within the deep cervical lymph nodes, which, in addition to tumor-derived T cell migratory cues (eg, chemokines), facilitate CD8+ T cell infiltration.27
The discovery of a functional lymphatic system in the meninges and brain parenchyma has elucidated a pathway by which tumor antigens drain into the cervical lymphatic circulation.28 This lymphatic system can be modified by Vascular Endothelial Growth Factor C (VEGF-C), resulting in T cell-mediated HGG rejection in experimental mouse models.29 Further exploration of the role of lymphatics in LGG and HGG T cell trafficking will help better elucidate the importance of antigen drainage to glioma pathobiology.
T Cell Regulation of Glioma Growth
T cells dynamically interact with both neoplastic (glioma) cells and nonneoplastic stromal cells throughout tumor evolution, sometimes promoting tumor growth and other times inhibiting it. The diversity of T cell-glioma interactions likely reflects differences in T cell and myeloid cell populations within the tumor, the expression of immunomodulatory molecules by the glioma cells, and the particular stage of tumor evolution (Figure 1). In this manner, T cells recruited into the tumor bed by glioma cells can then be “educated” by other nonneoplastic cells, like microglia, neurons, and astrocytes.15,25,30 Since this is an ever-evolving process, an equilibrium can be established between pro-tumoral T cells that increase tumor growth, such as Tregs, and anti-tumoral T cells, like CTLs, that kill tumor cells.31 Alternatively, evolutionary pressures, such as cancer therapy, can disturb this equilibrium and alter the immune content to increase T cell-mediated cytotoxicity and tumor shrinkage.32
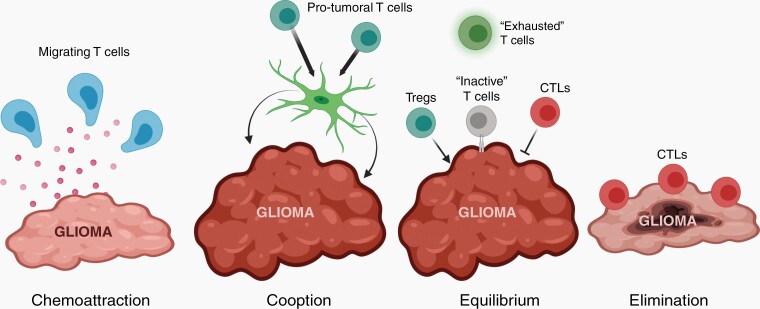
Fig. 1.
T cells shape glioma evolution. Glioma cells recruit T cells into the tumor microenvironment (TME) through the elaboration of chemokines. In addition, T cells produce paracrine factors that can act on other cells in the TME (eg, microglia) to increase tumor growth (“cooption”). Pro-tumoral regulatory T cells (Tregs) can also function to increase glioma growth (“equilibrium”), while cytotoxic T lymphocytes (CTLs) lyse tumor cells by secreting perforin (PFN) and granzyme B (GZMB) or by direct cell-to-cell interaction (“elimination”). Other CTLs can adopt an “exhausted” phenotype or become inactivated by glioma cells through the expression of immune checkpoint proteins. In response to extrinsic or intrinsic pressures, the balance of pro- and anti-tumoral T cells can shift to favor CTLs, resulting in glioma elimination. Created with BioRender.
Once integrated into the glioma TME, “activated” CD8+ T cells recognize and induce tumor apoptosis through the engagement of T cell receptors (TCRs).9 T cell composition and activation status varies among gliomas subtypes and depends, at least in part, upon the tumor grade and associated genetic alterations.6,33 For example, LGGs with Neurofibromatosis type 1 (NF1) mutations exhibit T cell gene enrichment and greater T cell infiltration relative to HGGs.34 In addition, examination of mesenchymal GBM tumors reveals more T cell infiltration than other subtypes, which does not correlate with patient outcome.6,35 Despite conflicting reports,36 cytotoxic T cell infiltration may correlate with increased survival in patients with glioma.37 Further studies will be required to establish a clear anti-tumoral role for glioma-infiltrating CD8+ T cells.
Gliomas can also reprogram T cells into dysfunctional or tumor-promoting lymphocytes through multiple mechanisms. First, gliomas can express Programmed Death Receptor Ligand-1 (PD-L1), which binds to Programmed Death Receptor-1 (PD-1) on T cells, blocking T cell differentiation and activation, culminating in inhibition of CD8+ T cell cytotoxic activity.38 Second, the glioma TME may recruit Tregs to secrete immunosuppressive cytokines (eg, IL-10 and TGF-β) that suppress cytotoxic T cells or directly increase glioma cell survival and self-renewal.39 Third, CD8+ T cell depletion abrogates tumor growth in experimental mouse LGG models.15 In these murine Nf1 optic gliomas, the ability of CD8+ T cells to stimulate murine LGG growth results from neuron-mediated T cell CCL4 production, which, in turn, induces microglia CCL5 secretion and reduced LGG apoptosis.15 The potential for CD8+ T cells to promote glioma growth independent of immune checkpoint expression and through interactions with TAMs suggest that CD8+ T cells may have different roles in LGGs and HGGs. In this regard, mutant Isocitrate Dehydrogenase 1 (IDH1) HGGs epigenetically reprogram their immune microenvironment, resulting in increased Granulocyte Colony Stimulating Factor (G-CSF) production, which reduces the immunosuppressive properties of MDSCs and facilitates CD8+ T cell-mediated glioma cell lysis.17 Additional research focusing on the specific properties of CD8+ T cells in glioma may reveal new targets for immune modulation.
Functional States of Glioma-associated T Cells
The process of T cell activation is a highly regulated process, depending on the nature of the antigen, the surrounding environment, and the duration of the T cell exposure to antigen. The resulting T cell states can vary from an effector T cell state to a hyporesponsive T cell state. The process is typically initiated when naïve CD8+ T cells encounter a foreign antigen presented by APCs, leading to clonal expansion and differentiation into cytotoxic T cells. These expanded antigen-specific T cells then lyse cells expressing that particular antigen.40 It should be appreciated that T cell activation can be interrupted by tumor cells through impaired antigen processing, presentation, or T cell priming, resulting in dysfunctional T cell states that facilitate tumor growth. Below, we will discuss the major T cell dysfunctional states that exist in the glioma immune microenvironment: tolerance, ignorance, anergy, and exhaustion (Figure 2).41
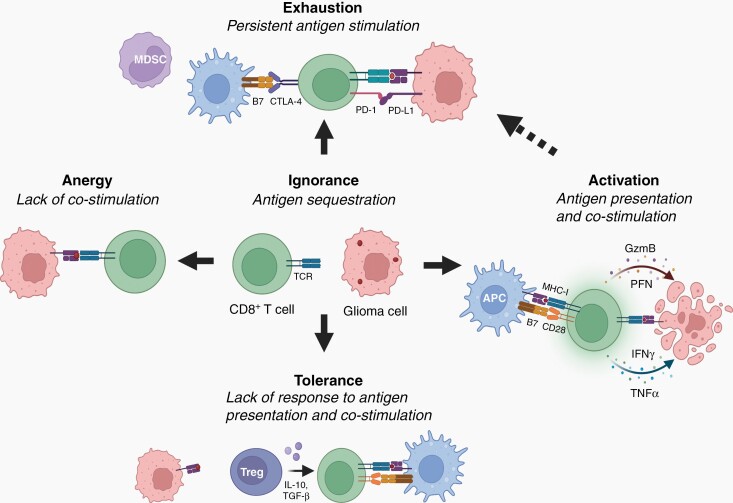
Fig. 2.
Glioma-mediated T-cell dysfunction during gliomagenesis. During early gliomagenesis, CD8+ T cells may remain “ignorant” due to inaccessibility of tumor-associated antigens (lack of Major Histocompatibility I (MHC-I) molecule interactions with T cell receptors (TCRs)). As gliomas progress and sufficient glioma antigen is produced, glioma-specific T cells can exist in different dysfunctional states, depending upon the specific signals from the glioma cells and the immune microenvironment. T cells can also be activated as a consequence of a lack of costimulation (B7-CD28 interactions), inducing a state of T cell “anergy”. Additionally, glioma cells can express immunosuppressive molecules (eg, Programmed Death Ligand-1 (PD-L1)) and recruit immunosuppressive cells (eg, myeloid-derived suppressor cells (MDSCs)) into the tumor microenvironment. Persistent tumor antigen and inhibitory signals can drive T cell “exhaustion”, a state of nontumor reactivity. Finally, in the presence of other immunosuppressive cells (eg, regulatory T cells (Tregs)), T cells can exhibit “tolerance”, in which they do not respond to antigen stimulation due to the presence of immunosuppressive cytokines (eg, Interleukin-10 (IL-10) and transforming growth factor-β (TGF-β)). The goal of immunotherapies is to induce T cell activation, instead of progression to these dysfunctional states. Activated T cells, after interacting with antigen-presenting cells (APCs) function to lyse tumor cells through the release of perforin (PFN), granzyme-B (GZMB), interferon-γ (IFNγ), and tumor necrosis factor-α (TNF-α). Created with BioRender.
Tolerance
T cell tolerance is a major mechanism by which tumor cells escape immune surveillance. T cells become tolerant when they fail to respond to antigen, which can be induced either in the blood or within the tumor itself. Tolerance can be achieved by the elimination of antigen-reactive T cells or by a failure of T cells to become activated following exposure to antigen. The inability of T cells to respond to antigen may result from a lack of positive costimulatory signals or the presence of a negative costimulatory signal (eg, PD-1).42 In the setting of glioma, Treg expansion can also create tolerance by promoting a loss of CD8+ T-cell activation signaling from helper CD4+ T cells.41
Ignorance
T cell ignorance is a state in which fully functional T cells fail to elicit an effective immune response in vivo, distinguishing it from T cell anergy (see below). T cell ignorance can result from a lack of sufficient exposure to antigen (eg, antigen sequestration or insufficient antigen concentration).41 In contrast to T cell tolerance, ignorant T cells are fully functional. In gliomas, tumor-associated antigens (TAAs) are typically concealed from T cell recognition, either by downregulation of TAAs by tumor cells or by anatomical separation of TAAs from immune recognition (eg, in the brain or the tumor center).43 Antigen presentation in gliomas can be further hindered due to an immune suppressive milieu that reduces MHC expression by APCs.41 Additionally, mature T cells can become confined within the bone marrow of patients or mice with GBM to remain in an ignorant state.44
Anergy
T cell activation requires both a main TCR stimulation signal and a costimulatory signal, whereas TCR activation creates T cell anergy in the absence of a costimulatory signal. Gliomas can induce reduced CD80 or CD86 costimulatory ligand expression on APCs, resulting in suboptimal priming of tumor-specific T cells and the development of T cell anergy.45 It is worth noting that “clonal anergy” is distinct from “adaptive tolerance”, reflecting different underlying signal transduction pathways41: Clonal anergy arises from impaired costimulation and de-regulated RAS/MAPK signaling,46,47 whereas adaptive tolerance is caused by chronic low levels of antigen exposure, reduced Zap70 kinase activity, and impaired calcium-induced NFκB signaling.46,47
Exhaustion
T cell exhaustion shares several phenotypic and epigenetic features with T cell anergy. In contrast to T cell anergy, T cell exhaustion results from persistent antigen stimulation of naïve T cells, resulting in a hypofunctional state.43 This term was initially coined in the setting of viral infection, in which chronic infection reduces infiltrating T cell expression of effector molecules, accompanied by altered transcription factor (eg, EOMES, NFAT, TOX, and BALF)48,49 and increased expression of PD-1, T cell immunoglobulin and mucin-domain containing-3 (Tim-3), CD39, T cell immunoreceptor with Ig and ITIM domains (TIGIT), Lymphocyte activating 3 (Lag-3), Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), 2B4, and B and T lymphocyte attenuator (BLTA).50,51 In contrast to other hypofunctional states, T cell exhaustion is an important mechanism to prevent excessive tissue damage in the face of chronic antigen stimulation.52 Glioma cells can increase expression of or directly bind inhibitory immune checkpoint molecules on exhausted T cells, thus limiting their ability to enact cellular programs that lyse tumor cells.53 In this fashion, antibodies that block immune checkpoint protein interactions can reverse this “exhausted” phenotype and induce reactive T cells to participate in anti-tumoral activities (see Immune Checkpoint Therapy section below).
T Cell-targeted Therapies for Glioma
With the recognition that T cells are integral cellular components of the glioma ecosystem and both positively and negatively influence tumor growth, immunotherapies have begun to emerge as adjuvant treatments.54 Five of the most promising T cell-mediated therapies include immune checkpoint inhibitors, chimeric antigen receptor (CAR) T cells, peptide vaccines, oncolytic/immunogenic viruses, and bispecific T cell engager (BiTE) therapy (Figure 3).
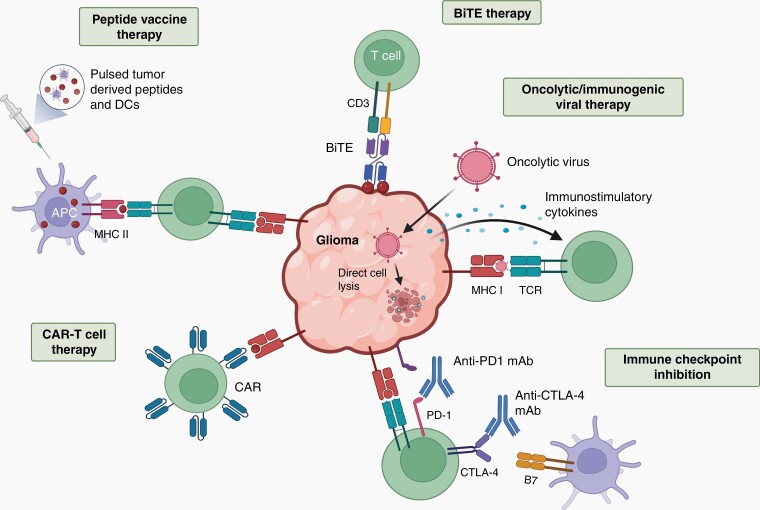
Fig. 3.
T cells as immunotherapeutic agents. T cells can be deployed as therapeutic modalities, serving as cancer vaccine inducers, cytotoxic effectors, or as targets for immune checkpoint de-repression. Bispecific T cell engager (BiTE) therapy uses a bispecific antibody that binds to CD3 and a tumor specific antigen, activating T cells to lyse the tumor. Oncolytic viruses cause direct tumor cell lysis, but can also be designed to stimulate the immune system by producing immunostimulatory cytokines or through tumor cell-mediated viral antigen presentation. Immune checkpoint inhibitors target Programmed Death Receptor-1 (PD-1) or Cytotoxic T-lymphocyte antigen 4 (CTLA-4) receptors for PDL-1 and B7 respectively, on T cells to promote cell cycle arrest and T cell exhaustion. Many tumors express PD-L1, which suppresses cytotoxic T cell function. Chimeric Antigen Receptor (CAR) T cells are autologous or donor T cells primed with an antigen receptor recognizing one or multiple glioma-expressed antigens or neoantigens. Cancer vaccines bolster cytotoxic T cell responses by overloading the immune system with neoantigens (peptides), either alone or presented on dendritic cells (DCs). With an increase in neoantigen content, antigen-presenting cells (APCs) can express these neoantigens on their cell surface, thus enabling T cells to generate more robust anti-tumoral responses. Created with BioRender.
Immune Checkpoint Therapy
Some T cell states in glioma are reversible, and can be rescued by either inhibition of immunosuppressive cells or by blocking “exhausted” T cells.18,55 The goal of inhibitory immune checkpoint pathway therapy is to “release the brakes” on the immune system to enhance anti-tumor immunity, leading to partial or full recovery of functional anti-tumoral CD8+ T cells. In experimental preclinical mouse models, immune checkpoint inhibition in combination with other treatment modalities cause reduced HGG growth.18,55 However, in one clinical trial, Nivolumab, a PD-1 monoclonal antibody, did not improve patient survival in patients with recurrent HGG compared with Bevacizumab monoclonal antibody anti-angiogenesis treatment, or in combination with anti-CTLA-4 antibodies (Ipilimumab).56,57 Currently, forty-three Phase-I/II clinical trials are ongoing to evaluate the effectiveness of immune checkpoint inhibition for glioma, the majority of which are employing monoclonal antibodies targeting PD-1 (Nivolumab, Pembrolizumab, or Cemiplimab) or PD-L1 (Durvalumab, Avelumab, or Atezolizumab) in combination with conventional therapies (NCT02530502, NCT02968940, NCT03743662).
CAR T Cells
CAR T cell therapy entails harnessing autologous T cells from patients to recognize and lyse tumor cells. To generate CAR T cells, patient-derived T cells are transduced to express a CAR harboring an extracellular domain that binds tumor neoantigens or tumor-associated antigens (TAAs). In addition to the antigen binding domain, the CAR has activating and costimulatory cytoplasmic signaling domains to stimulate T cell expansion and activation.58 While CAR T cells have demonstrated striking success for the treatment of lymphomas and leukemias, they have shown little efficacy in HGG, with no completed phase III clinical trials to date.59 Current clinical trials are utilizing CARs that bind to mutated epidermal growth factor receptor (EGFRvIII), interleukin receptor (IL13Ralpha2), or human epidermal growth factor receptor-2 (HER2) using either peripheral or intraventricular delivery.59 While peripherally delivered EGFRvIII CAR T cell therapy given to patients with recurrent HGG reduced tumoral EGFRvIII expression in 7/10 patients, as well as robust CAR T cell engraftment, only one patient remained progression-free after 18 months.60 Similarly, another trial using IL13Ralpha2 CAR T cells showed attenuated tumoral IL13Ralpha2 expression, yet tumors still recurred.61 The reasons for these failures include tumoral heterogeneity, antigen loss, and/or CAR T cell anergy following repeated antigen encounters.59 While CAR T cell therapy represents a promising new treatment modality, it is important to consider the cellular and molecular heterogeneity inherent in these tumors, which will require complementary strategies that focus on limiting the outgrowth of nontargeted tumor cell populations.
To induce stronger immune responses, preclinical studies have combined CAR T cells with immunostimulatory therapies, such as intratumoral delivery of Interleukin-12 (IL-12)62 or the inclusion of a transforming growth factor-beta (TGF-β) “trap” to block the immunosuppressive effect of TGF-β on CTLs.63 Additional phase I clinical trials include the use of novel CAR targets for HGG, such as B7-H3 (NCT04185038) and GD2 (NCT04196413), as well as the deployment of a CAR specifically designed to mimic the binding of chlorotoxin, a component of snake venom (NCT04214392). Other approaches aim to improve existing CAR T cell therapies in combination with immune checkpoint inhibitors by coupling the CAR to stronger intracellular immunostimulatory molecules or by designing them to bind both ubiquitous viruses (eg, cytomegalovirus) and neoantigen.59
Vaccines
Cancer vaccines are designed to trigger tumor-specific T cell responses, often using peptide vaccines where immunogenic peptides phagocytosed by DCs are presented to T cells to trigger their activation and expansion. Alternatively, DC vaccines involve the activation and expansion of DCs in vitro, loading with tumor-expressed peptides, and subsequent reintroduction into patients.64 Early trials have demonstrated some clinical promise for HGG using a mutant IDH1 peptide vaccine in combination with conventional chemotherapy.65 Additionally, interim results from a phase III trial of a whole tumor pulsed DC vaccine combined with combination therapy suggest a survival advantage.66 Unfortunately, other HGG vaccine trials have demonstrated little to no efficacy.67 Current efforts are centered on the use of proteomics to enable high throughput identification of potential neoantigens68 and combination therapies with immune checkpoint inhibitors, CAR T cells, or cytokines.54
Oncolytic Viral Therapy
Another strategy to induce a strong anti-tumoral CD8+ T cell response is the selective infection of tumor cells with a virus to induce tumor cells to present viral antigens. Intratumoral administration of oncolytic viruses, such as adenoviruses,69 herpes simplex virus (targeting ErbB2),70 polio-rhinovirus chimeras (targeting CD155),71 and Zika virus,32 have demonstrated early promise in preclinical and phase I clinical trials. Viral infection triggers APC-mediated tumor antigen presentation and the induction of an adaptive CD8+ T cell response. While these viruses alone exhibit anti-tumoral efficacy, they often require other components to bolster the immune response, such as viral expression of pro-inflammatory cytokines, like IL-12 (NCT02062827) or in combination with an immune checkpoint inhibitor.72
BiTE Therapy
BiTE molecules are composed of two antibodies connected by a linker, one that binds CD3 and another that binds a tumor antigen. This bi-functional molecule activates T cells to target tumor cells, independent of MHC antigen presentation or costimulatory molecules (eg, CD28).73 Based on successful BiTE clinical trials for adults with refractory B cell precursor acute lymphoblastic leukemia,74 phase I trials of BiTE therapies that either bind EGFRvIII (NCT03296696) or EGFRvIII and autologous T cells (NCT04903795) were initiated. Relative to CAR T cells, BiTEs can be manufactured more quickly because they do not require patient-derived cells or MHC matching. They can also be easily modified to target different cell surface antigens. Additionally, the lack of a requirement for MHC-I presentation and costimulation, as well as PD-1 independence, may render them less vulnerable to treatment resistance, although they are still susceptible to antigen downregulation and are dependent upon TME T cell recruitment.73 While antigen-specific T cells are not required for BiTE efficacy, recruitment of T cells that can target other neoantigens may be necessary to overcome tumor cellular and molecular heterogeneity.
Mechanisms of Immunotherapy Resistance
Multiple mechanisms account for immunotherapy resistance in glioma, including both endogenous and exogenous etiologies.75 Endogenous mechanisms are intrinsic to the tumor, such as low mutational load and downregulation of antigens, while exogenous mechanisms rely on other cells within the TME, such as inhibitory immune cells and dysregulated T cell immune checkpoint molecule expression.76
Mutational load is an important factor that determines the efficacy of immune checkpoint inhibitors. Somatic mutations accumulate over the course of tumor development, and can lead to the generation of neoantigens or novel tumor-specific mutant antigens, each capable of evoking CD8+ T cell-mediated anti-tumor responses. High tumor mutational burden is an independent predictor of immunotherapy response across a large variety of non-CNS tumor types.77 While HGGs generally have a relatively low mutational burden, high mutational burden is associated with mismatch repair (MMR) loss, which is more frequently observed upon tumor recurrence.78 Antigen downregulation or alternative splicing of antigens to remove the targeted epitope represents another mechanism of endogenous resistance. In Acute Lymphocytic Leukemia (ALL) clinical trials utilizing CD19 CAR T cells or blinatumomab BiTE therapy targeting CD19, some treatment-resistant patients exhibited novel isoforms of CD19, thus enabling immune escape.79,80
While reducing target peptide levels accounts for some of the therapy resistance observed, increased expression of immune checkpoint molecules on T cells accounts for reduced T cell responses in some patients with high levels of target antigen, while other mechanisms, such as HGG-induced changes in MHC function, poor neoantigen quality, or high levels of tumor heterogeneity, are also operative.75 Rational selection of combination therapies and the use of computational neoantigen prediction tools could improve outcomes.75,81
Extrinsic mechanisms include immune checkpoint molecule upregulation on T cells, infiltration of immunosuppressive cells that block activated T cell function, and impaired migration of activated T cells to the glioma microenvironment. In this regard, treatment of HGG patients with an oncolytic adenovirus vector extended overall survival, as well as reduced TIM-3 checkpoint molecule expression on CD8+ T cells.69 Another approach to potentiate immune checkpoint blockade is targeting the immunosuppressive cells in the TME (TAMs and Tregs), such as inhibiting colony stimulating factor-1 receptor (CSF1R) expression to attenuate tumor growth.82 Because Tregs can both promote tumor growth and exert immunosuppression through TGF-β expression,83 combining immunotherapy with a TGF-β inhibitor may increase efficacy.84 Future combination immunotherapies might be designed to increase tumor neoantigen expression (eg, oncolytic/immunogenic viruses) or activate T cells (eg, immune checkpoint blockade, BiTE, and/or CAR-T cells).
Challenges and Insights
While significant progress has been made, many fundamental aspects of T cell-glioma interactions remain incompletely understood, which should be the focus of future investigations (Figure 4). First, with the application of single cell analyses,1,85,86 it is apparent that numerous T cell populations reside within gliomas, each with potentially different functional roles in glioma biology. As such, unique T cell populations have been identified in HGGs that coexpress genes typically seen in NK cells (KLRB1; CD161), as well as T cells with cytotoxic, interferon-producing, effector memory, or stress-related transcriptomal signatures.33 Moreover, these T cell populations may also be spatially heterogeneous, with TCF1+ T cells located closer to blood vessels and CD103+ resident T cells residing within the tumor parenchyma.5 In addition, the specific type of glioma may also influence its immune microenvironment, where NF1-mutant gliomas harbor more CD8+ than CD4+ T cells15,34 and malignant pediatric gliomas (diffuse intrinsic pontine glioma) lack increased T cell content.87
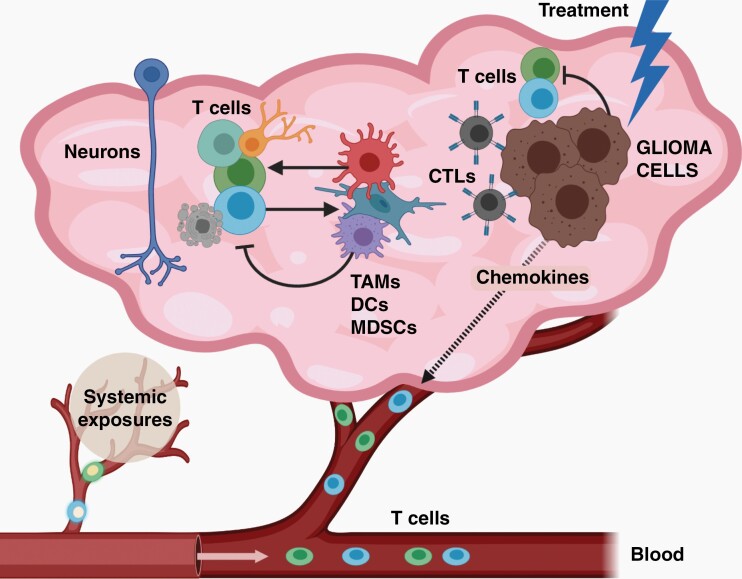
Fig. 4.
The complex and ever-evolving immune microenvironment in glioma. In gliomas, numerous distinct populations of T cells exist, which could either increase or inhibit tumor cell expansion. In addition, both T cell infiltration and function can be controlled by both neoplastic (glioma cells) and nonneoplastic (neurons, TAMs, DCs, MDSCs) cells through paracrine factor signaling. Importantly, T cell function is also influenced by systemic exposures (eg, atopic conditions, like asthma), but also by tumor-directed therapies. Created with BioRender.
Second, it is important to appreciate that T cells exist in distinct functional states, reflecting senescent, immune-tolerant, immune-naïve, and exhausted phenotypes,41 which can change over time and in response to local tumor environmental signals. In this regard, T cell function is sculpted by numerous cues emanating from both neoplastic and nonneoplastic cell types in gliomas. Monocytes induced by tumor cells can attract T cells through chemokine production,88 modify their function through adenosine generation,89 or activate them by functioning as APCs.90 In addition, glioma cells can produce oncometabolites (eg, R-2-hydroxyglutarate) that suppress T cell activity91 or elaborate chemokines that attract T cells,25 while neurons can induce T cell cytokines important for LGG progression.15
Third, additional study of the relationship between tumor mutational burden (TMB) and CD8+ T cell infiltration is warranted. While, one study of adult and pediatric gliomas revealed no association between CD8+ T cell influx and TMB or between tumor grade and TMB,78 another study showed a positive correlation between glioma grade and TMB.92 It is possible that the recruitment and expansion of T cells in the glioma TME is dependent on the type, rather than the quantity, of mutations, such that some mutations might be more immunogenic than others.5 Alternatively, TMB, tumor homogeneity, and MHC expression together may predict T cell infiltration in glioma, as has been reported in lung cancer.93 Elucidating how tumor mutational load impacts CD8+ T cell infiltration may improve our ability to predict which patients will respond to immunotherapies and design peptide vaccines, CAR-T cells, and/or BiTE molecules directed at particularly immunogenic neoantigens.
Finally, as a continually evolving ecosystem, T cell function can be altered by immunomodulatory treatments, such as Zika virus32 or poliovirus71 or by conventional or molecularly targeted chemotherapies.94 Given the seminal role that T cells play in atopic diseases, like asthma and eczema, prior epidemiologic associations between brain tumors and these conditions have been shown.95–97 In this regard, we have recently found that asthma induces the expression of decorin in T cells, which blocks microglia-mediated support of LGGs in experimental mouse models.98 Defining how alterations in T cell function abrogate a permissive TME may lead to new “Trojan Horse” therapeutic approaches that interrupt the glioma ecosystem. As we begin to unravel the unique roles for T cells in glioma pathogenesis and progression, it is highly likely that more tailored and effective immune-based therapies will emerge.
Contributor Information
Elizabeth C Cordell, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri, USA.
Mahmoud S Alghamri, Department of Neurosurgery, University of Michigan, Ann Arbor, Michigan, USA; Department of Cell and Developmental Biology, University of Michigan, Ann Arbor, Michigan, USA.
Maria G Castro, Department of Neurosurgery, University of Michigan, Ann Arbor, Michigan, USA; Department of Cell and Developmental Biology, University of Michigan, Ann Arbor, Michigan, USA.
David H Gutmann, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri, USA.
Funding
D.H.G. is supported by a Research Program Award grant from the National Institutes of Health (1-R35-NS07211-01) and by funding from Schnuck Markets, Inc. E.C.C. is supported by a grant from the DeNardo Research and Education Foundation at the Washington University School of Medicine. M.G.C. is supported by National Institutes of Health Grants R37-NS094804, R01-NS105556, R21-NS107894, RO1 NS122536, R21 NS123879, the Rogel Cancer Center Scholar Award and The Forbes Foundation Award to M.G.C., the Department of Neurosurgery; the Pediatric Brain Tumor Foundation, Leah’s Happy Hearts Foundation, Chad Tough Foundation, Ian’s Friends Foundation and Smiles for Sophie Forever Foundation. M.S.A. was supported by National Institutes of Health/National Cancer Institute T32- CA009676 Post-Doctoral Fellowship funding.
Conflict of interest statement. The authors have no relevant interests to disclosure.
Authorship statement. Writing—E.C.C., M.S.A., M.G.C., D.H.G.; Figures—D.H.G., E.C.C.; Final editing and submission—D.H.G.
References
- 1. Friebel E, Kapolou K, Unger S, et al. Single-cell mapping of human brain cancer reveals tumor-specific instruction of tissue-invading leukocytes. Cell. 2020; 181(7):1626–1642.e20. [DOI] [PubMed] [Google Scholar]
- 2. González-Tablas Pimenta M, Otero A, Arandia Guzman DA, et al. Tumor cell and immune cell profiles in primary human glioblastoma: Impact on patient outcome. Brain Pathol. 2021; 31(2):365–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen J, Sinha N, Cobb O, et al. Immune cell analysis of pilocytic astrocytomas reveals sexually dimorphic brain region-specific differences in T-cell content. Neurooncol Adv. 2021; 3(1):vdab068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gutmann DH, Kettenmann H. Microglia/brain macrophages as central drivers of brain tumor pathobiology. Neuron. 2019; 104(3):442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Robinson MH, Vasquez J, Kaushal A, et al. Subtype and grade-dependent spatial heterogeneity of T-cell infiltration in pediatric glioma. J ImmunoTher Cancer. 2020; 8(2):e001066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hara T, Chanoch-Myers R, Mathewson ND, et al. Interactions between cancer cells and immune cells drive transitions to mesenchymal-like states in glioblastoma. Cancer Cell. 2021; 39(6):779–792.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sampson JH, Gunn MD, Fecci PE, Ashley DM. Brain immunology and immunotherapy in brain tumours. Nat Rev Cancer. 2020; 20(1):12–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitchell K, Troike K, Silver DJ, Lathia JD. The evolution of the cancer stem cell state in glioblastoma: emerging insights into the next generation of functional interactions. Neuro Oncol. 2021; 23(2):199–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019; 234(6):8509–8521. [DOI] [PubMed] [Google Scholar]
- 10. Alves de Lima K, Rustenhoven J, Kipnis J. Meningeal immunity and its function in maintenance of the central nervous system in health and disease. Annu Rev Immunol. 2020; 38:597–620. [DOI] [PubMed] [Google Scholar]
- 11. Sarkaria JN, Hu LS, Parney IF, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. 2018; 20(2):184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wright-Jin EC, Gutmann DH. Microglia as dynamic cellular mediators of brain function. Trends Mol Med. 2019; 25(11):967–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hambardzumyan D, Gutmann DH, Kettenmann H. The role of microglia and macrophages in glioma maintenance and progression. Nat Neurosci. 2016; 19(1):20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020; 11:583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guo X, Pan Y, Xiong M, et al. Midkine activation of CD8(+) T cells establishes a neuron-immune-cancer axis responsible for low-grade glioma growth. Nat Commun. 2020; 11(1):2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018; 19(2):108–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alghamri MS, McClellan BL, Avvari RP, et al. G-CSF secreted by mutant IDH1 glioma stem cells abolishes myeloid cell immunosuppression and enhances the efficacy of immunotherapy. Sci Adv. 2021; 7(40):eabh3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kamran N, Kadiyala P, Saxena M, et al. Immunosuppressive myeloid cells’ blockade in the glioma microenvironment enhances the efficacy of immune-stimulatory gene therapy. Mol Ther J Am Soc Gene Ther. 2017; 25(1):232–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reiser J, Banerjee A. Effector, memory, and dysfunctional CD8(+) T cell fates in the antitumor immune response. J Immunol Res. 2016; 2016:8941260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heimberger AB, Abou-Ghazal M, Reina-Ortiz C, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008; 14(16):5166–5172. [DOI] [PubMed] [Google Scholar]
- 21. Wainwright DA, Balyasnikova IV, Chang AL, et al. IDO expression in brain tumors increases the recruitment of regulatory T cells and negatively impacts survival. Clin Cancer Res. 2012; 18(22):6110–6121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miska J, Lee-Chang C, Rashidi A, et al. HIF-1α is a metabolic switch between glycolytic-driven migration and oxidative phosphorylation-driven immunosuppression of Tregs in glioblastoma. Cell Rep. 2019; 27(1):226–237.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mohme M, Neidert MC. Tumor-specific T cell activation in malignant brain tumors. Front Immunol. 2020;11:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Watkins S, Robel S, Kimbrough IF, et al. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun. 2014; 5:4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guo X, Pan Y, Gutmann DH. Genetic and genomic alterations differentially dictate low-grade glioma growth through cancer stem cell-specific chemokine recruitment of T cells and microglia. Neuro Oncol. 2019; 21(10):1250–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Crane CA, Ahn BJ, Han SJ, Parsa AT. Soluble factors secreted by glioblastoma cell lines facilitate recruitment, survival, and expansion of regulatory T cells: implications for immunotherapy. Neuro Oncol. 2012; 14(5):584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Malo CS, Huggins MA, Goddery EN, et al. Non-equivalent antigen presenting capabilities of dendritic cells and macrophages in generating brain-infiltrating CD8+ T cell responses. Nat Commun. 2018; 9(1):633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015; 523(7560):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Song E, Mao T, Dong H, et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020; 577(7792):689–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. John Lin CC, Yu K, Hatcher A, et al. Identification of diverse astrocyte populations and their malignant analogs. Nat Neurosci. 2017; 20(3):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004; 22:329–360. [DOI] [PubMed] [Google Scholar]
- 32. Nair S, Mazzoccoli L, Jash A, et al. Zika virus oncolytic activity requires CD8+ T cells and is boosted by immune checkpoint blockade. JCI Insight. 2021; 6(1):e144619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mathewson ND, Ashenberg O, Tirosh I, et al. Inhibitory CD161 receptor identified in glioma-infiltrating T cells by single-cell analysis. Cell. 2021; 184(5):1281–1298.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. D’Angelo F, Ceccarelli M, Tala, et al. The molecular landscape of glioma in patients with Neurofibromatosis 1. Nat Med. 2019; 25(1):176–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rutledge WC, Kong J, Gao J, et al. Tumor-infiltrating lymphocytes in glioblastoma are associated with specific genomic alterations and related to transcriptional class. Clin Cancer Res. 2013; 19(18):4951–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Plant AS, Koyama S, Sinai C, et al. Immunophenotyping of pediatric brain tumors: correlating immune infiltrate with histology, mutational load, and survival and assessing clonal T cell response. J Neurooncol. 2018; 137(2):269–278. [DOI] [PubMed] [Google Scholar]
- 37. Lohr J, Ratliff T, Huppertz A, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-β. Clin Cancer Res. 2011; 17(13):4296–4308. [DOI] [PubMed] [Google Scholar]
- 38. Martin AM, Bell WR, Yuan M, et al. PD-L1 expression in pediatric low-grade gliomas is independent of BRAF V600E mutational status. J Neuropathol Exp Neurol. 2020; 79(1):74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu S, Zhang C, Wang B, et al. Regulatory T cells promote glioma cell stemness through TGF-β-NF-κB-IL6-STAT3 signaling. Cancer Immunol Immunother. 2021; 70(9):2601–2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ousman SS, Kubes P. Immune surveillance in the central nervous system. Nat Neurosci. 2012; 15(8):1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Woroniecka KI, Rhodin KE, Chongsathidkiet P, Keith KA, Fecci PE. T-cell dysfunction in glioblastoma: applying a new framework. Clin Cancer Res. 2018; 24(16):3792–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xing Y, Hogquist KA. T-cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol. 2012; 4(6):a006957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Philip M, Schietinger A. CD8+ T cell differentiation and dysfunction in cancer. Nat Rev Immunol. 2021; Advance Online Publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chongsathidkiet P, Jackson C, Koyama S, et al. Sequestration of T cells in bone marrow in the setting of glioblastoma and other intracranial tumors. Nat Med. 2018; 24(9):1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Abe BT, Macian F. Uncovering the mechanisms that regulate tumor-induced T-cell anergy. Oncoimmunology. 2013; 2(2):e22679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chiodetti L, Choi S, Barber DL, Schwartz RH. Adaptive tolerance and clonal anergy are distinct biochemical states. J Immunol (Baltimore, Md.: 1950). 2006; 176(4):2279–2291. [DOI] [PubMed] [Google Scholar]
- 47. Choi S, Schwartz RH. Molecular mechanisms for adaptive tolerance and other T cell anergy models. Semin Immunol. 2007; 19(3):140–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pereira RM, Hogan PG, Rao A, Martinez GJ. Transcriptional and epigenetic regulation of T cell hyporesponsiveness. J Leukoc Biol. 2017; 102(3):601–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015; 15(8):486–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mohme M, Schliffke S, Maire CL, et al. Immunophenotyping of newly diagnosed and recurrent glioblastoma defines distinct immune exhaustion profiles in peripheral and tumor-infiltrating lymphocytes. Clin Cancer Res Off J Am Assoc Cancer Res. 2018; 24(17):4187–4200. [DOI] [PubMed] [Google Scholar]
- 51. Woroniecka K, Chongsathidkiet P, Rhodin K, et al. T-cell exhaustion signatures vary with tumor type and are severe in glioblastoma. Clin Cancer Res Off J Am Assoc Cancer Res. 2018; 24(17):4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Collier JL, Weiss SA, Pauken KE, Sen DR, Sharpe AH. Not-so-opposite ends of the spectrum: CD8+ T cell dysfunction across chronic infection, cancer and autoimmunity. Nat Immunol. 2021; 22(7):809–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mirzaei R, Sarkar S, Yong VW. T cell exhaustion in glioblastoma: intricacies of immune checkpoints. Trends Immunol. 2017; 38(2):104–115. [DOI] [PubMed] [Google Scholar]
- 54. Yap TA, Parkes EE, Peng W, et al. Development of immunotherapy combination strategies in cancer. Cancer Discov. 2021; 11(6):1368–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013; 86(2):343–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol. 2018; 20(5):674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Reardon DA, Omuro A, Brandes AA, et al. OS10.3 Randomized Phase 3 Study evaluating the efficacy and safety of nivolumab vs bevacizumab in patients with recurrent glioblastoma: CheckMate 143. Neuro-Oncology. 2017; 19(suppl_3):iii21–iii21. [Google Scholar]
- 58. Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013; 3(4):388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Land CA, Musich PR, Haydar D, Krenciute G, Xie Q. Chimeric antigen receptor T-cell therapy in glioblastoma: charging the T cells to fight. J Transl Med. 2020; 18(1):428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. O’Rourke DM, Nasrallah MP, Desai A, et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017; 9(399):eaaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brown CE, Alizadeh D, Starr R, et al. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med. 2016; 375(26):2561–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Agliardi G, Liuzzi AR, Hotblack A, et al. Intratumoral IL-12 delivery empowers CAR-T cell immunotherapy in a pre-clinical model of glioblastoma. Nat Commun. 2021; 12(1):444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li Y, Wu H, Chen G, et al. Arming anti-EGFRvIII CAR-T with TGFβ trap improves antitumor efficacy in glioma mouse models. Front Oncol. 2020; 10:1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Perez CR, De Palma M. Engineering dendritic cell vaccines to improve cancer immunotherapy. Nat Commun. 2019; 10(1):5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Platten M, Bunse L, Wick A, et al. A vaccine targeting mutant IDH1 in newly diagnosed glioma. Nature. 2021; 592(7854):463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liau LM, Ashkan K, Tran DD, et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018; 16(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Frederico SC, Hancock JC, Brettschneider EES, et al. Making a cold tumor hot: the role of vaccines in the treatment of glioblastoma. Front Oncol. 2021; 11:672508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen L, Qin D, Guo X, Wang Q, Li J. Putting proteomics into immunotherapy for glioblastoma. Front Immunol. 2021; 12:593255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lang FF, Conrad C, Gomez-Manzano C, et al. Phase I Study of DNX-2401 (Delta-24-RGD) oncolytic adenovirus: replication and immunotherapeutic effects in recurrent malignant glioma. J Clin Oncol. 2018; 36(14):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alessandrini F, Menotti L, Avitabile E, et al. Eradication of glioblastoma by immuno-virotherapy with a retargeted oncolytic HSV in a preclinical model. Oncogene. 2019; 38(23):4467–4479. [DOI] [PubMed] [Google Scholar]
- 71. Mosaheb MM, Dobrikova EY, Brown MC, et al. Genetically stable poliovirus vectors activate dendritic cells and prime antitumor CD8 T cell immunity. Nat Commun. 2020; 11(1):524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hardcastle J, Mills L, Malo CS, et al. Immunovirotherapy with measles virus strains in combination with anti-PD-1 antibody blockade enhances antitumor activity in glioblastoma treatment. Neuro Oncol. 2017; 19(4):493–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Einsele H, Borghaei H, Orlowski RZ, et al. The BiTE (bispecific T-cell engager) platform: development and future potential of a targeted immuno-oncology therapy across tumor types. Cancer. 2020; 126(14):3192–3201. [DOI] [PubMed] [Google Scholar]
- 74. Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017; 376(9):836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Jackson CM, Choi J, Lim M. Mechanisms of immunotherapy resistance: lessons from glioblastoma. Nat Immunol. 2019; 20(9):1100–1109. [DOI] [PubMed] [Google Scholar]
- 76. Pitt JM, Vétizou M, Daillère R, et al. Resistance mechanisms to immune-checkpoint blockade in cancer: tumor-intrinsic and -extrinsic factors. Immunity. 2016; 44(6):1255–1269. [DOI] [PubMed] [Google Scholar]
- 77. Samstein RM, Lee C-H, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019; 51(2):202–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hodges TR, Ott M, Xiu J, et al. Mutational burden, immune checkpoint expression, and mismatch repair in glioma: implications for immune checkpoint immunotherapy. Neuro Oncol. 2017; 19(8):1047–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Fischer J, Paret C, El Malki K, et al. CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017; 40(5):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zhao Y, Aldoss I, Qu C, et al. Tumor-intrinsic and -extrinsic determinants of response to blinatumomab in adults with B-ALL. Blood. 2021; 137(4):471–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Łuksza M, Riaz N, Makarov V, et al. A neoantigen fitness model predicts tumour response to checkpoint blockade immunotherapy. Nature. 2017; 551(7681):517–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pyonteck SM, Akkari L, Schuhmacher AJ, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013; 19(10):1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Löffek S. Transforming of the tumor microenvironment: implications for TGF-β inhibition in the context of immune-checkpoint therapy. J Oncol. 2018; 2018:9732939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lind H, Gameiro SR, Jochems C, et al. Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: status of preclinical and clinical advances. J ImmunoTher Cancer. 2020; 8(1):e000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang Q, Hu B, Hu X, et al. Tumor evolution of glioma-intrinsic gene expression subtypes associates with immunological changes in the microenvironment. Cancer Cell. 2018; 33(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Klemm F, Maas RR, Bowman RL, et al. Interrogation of the microenvironmental landscape in brain tumors reveals disease-specific alterations of immune cells. Cell. 2020; 181(7):1643–1660.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Lieberman NAP, DeGolier K, Kovar HM, et al. Characterization of the immune microenvironment of diffuse intrinsic pontine glioma: implications for development of immunotherapy. Neuro Oncol. 2019; 21(1):83–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chang AL, Miska J, Wainwright DA, et al. CCL2 Produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. 2016; 76(19):5671–5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Takenaka MC, Gabriely G, Rothhammer V, et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat Neurosci. 2019; 22(5):729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Malo CS, Huggins MA, Goddery EN, et al. Non-equivalent antigen presenting capabilities of dendritic cells and macrophages in generating brain-infiltrating CD8. Nat Commun. 2018; 9(1):633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bunse L, Pusch S, Bunse T, et al. Suppression of antitumor T cell immunity by the oncometabolite (R)-2-hydroxyglutarate. Nat Med. 2018; 24(8):1192–1203. [DOI] [PubMed] [Google Scholar]
- 92. Draaisma K, Wijnenga MM, Weenink B, et al. PI3 kinase mutations and mutational load as poor prognostic markers in diffuse glioma patients. Acta Neuropathol Commun. 2015; 3:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. McGranahan N, Furness AJ, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science. 2016; 351(6280):1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Schalper KA, Rodriguez-Ruiz ME, Diez-Valle R, et al. Neoadjuvant nivolumab modifies the tumor immune microenvironment in resectable glioblastoma. Nat Med. 2019; 25(3):470–476. [DOI] [PubMed] [Google Scholar]
- 95. Porcelli B, Zoellner NL, Abadin SS, Gutmann DH, Johnson KJ. Associations between allergic conditions and pediatric brain tumors in Neurofibromatosis type 1. Fam Cancer. 2016; 15(2):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kaur H, Lachance DH, Ryan CS, et al. Asthma and risk of glioma: a population-based case-control study. BMJ Open. 2019; 9(6):e025746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Amirian ES, Zhou R, Wrensch MR, et al. Approaching a scientific consensus on the association between allergies and glioma risk: a report from the Glioma International Case-Control Study. Cancer Epidemiol Biomarkers Prev. 2016; 25(2):282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chatterjee J, Sanapala S, Cobb O, et al. Asthma reduces glioma formation by T cell decorin-mediated inhibition of microglia. Nat Commun. 2021; 12(1):7122. [DOI] [PMC free article] [PubMed] [Google Scholar]