Abstract
Background
Genomic profiling studies of diffuse gliomas have led to new improved classification schemes that better predict patient outcomes compared to conventional histomorphology alone. One example is the recognition that patients with IDH-wildtype diffuse astrocytic gliomas demonstrating lower-grade histologic features but genomic and/or epigenomic profile characteristic of glioblastoma typically have poor outcomes similar to patients with histologically diagnosed glioblastoma. Here we sought to determine the clinical impact of prospective genomic profiling for these IDH-wildtype diffuse astrocytic gliomas lacking high-grade histologic features but with molecular profile of glioblastoma.
Methods
Clinical management and outcomes were analyzed for 38 consecutive adult patients with IDH-wildtype diffuse astrocytic gliomas lacking necrosis or microvascular proliferation on histologic examination that were genomically profiled on a prospective clinical basis revealing criteria for an integrated diagnosis of “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV” per cIMPACT-NOW criteria.
Results
We identified that this diagnosis consists of two divergent clinical scenarios based on integration of radiologic, histologic, and genomic features that we term “early/evolving” and “undersampled” glioblastoma, IDH-wildtype. We found that prospective genomically guided identification of early/evolving and undersampled IDH-wildtype glioblastoma resulted in more aggressive patient management and improved clinical outcomes compared to a biologically matched historical control patient cohort receiving standard-of-care therapy based on histomorphologic diagnosis alone.
Conclusions
These results support routine use of genomic and/or epigenomic profiling to accurately classify glial neoplasms, as these assays not only improve diagnostic classification but critically lead to more appropriate patient management that can improve clinical outcomes.
Keywords: genomic profiling, glioblastoma, molecular diagnostics, molecular neuro-oncology, precision medicine
Key Points.
“Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma” consists of two divergent clinical scenarios: “early/evolving” and “undersampled”.
Prospective identification results in more aggressive therapy and improved outcomes.
Importance of the Study.
While more precise diagnostic classification undoubtedly leads to more accurate prognostication for brain tumor patients, an unresolved question in the neuro-oncology field is whether prospectively using genomic or epigenomic profiling to more accurately classify glial neoplasms will actually lead to improved patient outcomes. Here, we demonstrate that prospectively identifying IDH-wildtype diffuse astrocytic gliomas in adults with lower-grade histologic features but molecular features of glioblastoma results in more aggressive patient management and improved clinical outcomes compared to biologically matched historical control patient cohorts receiving standard-of-care therapy based on histomorphologic diagnosis alone. As such, these results support routine use of genomic and/or epigenomic profiling to accurately classify glial neoplasms, as these assays not only improve diagnostic classification but also lead to altered patient management that improves clinical outcomes.
Genomic profiling of gliomas over the past two decades has led to dramatic advances in tumor classification and treatment for affected patients.1,2 Gliomas are no longer pathologically classified based exclusively on morphologic features, and many brain tumor types now have defining genetic alterations that are considered essential for diagnosis per the 5th edition of the World Health Organization (WHO) Classification of Central Nervous System Tumors.3,4 Examples include demonstrating the presence of IDH1/2 hotspot mutation and chromosomes 1p and 19q whole arm codeletion for the tumor type “oligodendroglioma, IDH-mutant and 1p/19q-codeleted”, and IDH1/2 hotspot mutation with either ATRX inactivation or intact chromosomes 1p and 19q for the tumor type “astrocytoma, IDH-mutant”. This new diagnostic classification schema of diffuse lower-grade gliomas in adults has mostly eliminated the concept of mixed oligoastrocytomas which was previously a frequently used diagnosis for gliomas with ambiguous or overlapping astrocytic and oligodendroglial microscopic features. Such molecular-based diagnostic schemes more accurately predict patient outcomes compared to histomorphology alone and have been widely adopted into clinical practice including by the World Health Organization.
Importantly, we now recognize that IDH-wildtype diffuse astrocytic gliomas of lower histologic grade that harbor molecular alterations frequent in glioblastoma (EGFR amplification, NF1 mutation/deletion, PTEN mutation/deletion, TERT promoter mutation, CDKN2A homozygous deletion) have poor clinical outcomes similar to IDH-wildtype glioblastomas with characteristic histologic features of necrosis and microvascular proliferation.5–8 Such IDH-wildtype diffuse astrocytic gliomas harboring an epigenetic profile aligning with glioblastoma or genetic alterations characteristic of glioblastoma are now thought to biologically represent conventional glioblastoma, rather than diffuse lower-grade astrocytic gliomas.9 Historically, such patients would have been treated less aggressively based on a lower WHO grade pathologic diagnosis relying exclusively on histologic features. Subsequent to the revised 4th edition of the WHO Classification of Central Nervous System Tumors, the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy—Not Official WHO (cIMPACT-NOW) suggested that identification of at least one of three specific molecular criteria could allow for classification of an IDH-wildtype diffuse astrocytoma as a “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV” (so-called “molecular glioblastoma”): combined whole chromosome gain/trisomy of chromosome 7 and loss/monosomy of chromosome 10, focal EGFR gene amplification, and TERT promoter hotspot mutation (either c.-124C > T or c.-146C > T).10 Subsequent retrospective studies have confirmed the prognostic value of this genetic signature in IDH-wildtype diffuse astrocytic gliomas with molecular features of glioblastoma.11–14 As such, these molecular criteria have since been adopted into the 5th edition of WHO Classification of Central Nervous System Tumors as sufficient to qualify for the diagnosis of “glioblastoma, IDH-wildtype, CNS WHO grade 4” even in the absence of high-grade histologic features.4
Since these diagnostic criteria have only recently been standardized, an unresolved question in the neuro-oncology field is whether prospectively using genomic/epigenomic profiling to accurately classify glial neoplasms will lead to improved patient outcomes. Here, we sought to determine if prospective genomic profiling of IDH-wildtype diffuse gliomas in adults lacking histologic features of glioblastoma (ie, necrosis and microvascular proliferation) could improve patient management and lead to improved clinical outcomes. We describe two distinct clinical scenarios where genomic profiling can inform accurate diagnostic classification for IDH-wildtype glioblastoma due to: 1) early/evolving disease, or 2) surgical undersampling. Furthermore, we demonstrate that prospectively using genomic profiling to accurately identify IDH-wildtype glioblastoma amongst diffuse astrocytic gliomas in adults with lower-grade histologic features results in more aggressive patient management and improved clinical outcomes compared to biologically matched historical control patient cohorts receiving standard-of-care therapy based on histomorphologic diagnosis alone.
Methods
Patient Cohort and Tumor Samples
The study cohort consisted of 38 consecutive adult patients over 18 years of age who underwent surgical sampling of an IDH-wildtype diffuse astrocytic glioma lacking necrosis or microvascular proliferation on pathologic examination performed at the University of California, San Francisco between 2015 and 2021 and prospective genomic profiling yielding an integrated diagnosis of “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV” based on cIMPACT-NOW update 3 criteria.10 For 29 of the patients (76%), the genomic profiling was performed immediately following the initial surgical procedure at time of primary diagnosis, and clinical management was based upon this genomically informed integrated diagnosis including maximal safe resection followed by primary adjuvant radiation and chemotherapy as indicated, as well as clinical trial enrollment based on glioblastoma diagnosis in a subset of patients. In the remaining 9 patients (24%), the genomic profiling was performed later during the treatment course, and subsequent therapy decisions were based upon this genomically informed integrated diagnosis. Disease progression was defined based on RANO criteria.15 This study was approved by the institutional review board of the University of California, San Francisco.
Histopathologic Review and Molecularly Integrated Diagnosis
Pathologic review of all tumors was performed at the UCSF Division of Neuropathology. All tumors were composed of glial cells with astrocytic morphology exhibiting a diffuse growth pattern, and uniformly lacked both microvascular proliferation and necrosis. Tumors were substratified into those that would have previously been histologically classified as “diffuse astrocytoma, WHO grade II” versus “anaplastic astrocytoma, WHO grade III” according to the 2016 WHO Classification of Tumors of the Central Nervous System based upon degree of mitotic activity and cytologic anaplasia.16 Prospective genomic evaluation was performed on a clinical basis for all tumors using the UCSF500 NGS Panel as described below, which typically provides greater than 500× sequencing coverage over the IDH1 p.R132 and IDH2 p.R172 mutational hotspots, as well as providing comprehensive assessment of cytogenetic alterations (eg, chromosomes 1p and 19q status, chromosomes 7 and 10 status) and genetic alterations (eg, EGFR, PDGFRA, MET, FGFR3, NF1, BRAF, PIK3CA, PIK3R1, PTEN, CDKN2A, CDK4, CDK6, RB1, TP53, MDM2, MDM4, CIC, FUBP1, TERT [including promoter region], ATRX) critical for glioma diagnostic assessment. All tumors were verified to be IDH-wildtype and contained at least one of the following three definitional alterations: TERT promoter hotspot mutation (either c.-124C > T or c.-146C > T), focal EGFR gene amplification, or combined whole chromosome gain/polysomy of 7 and loss/monosomy of 10. A molecularly integrated diagnosis of “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV” based upon the cIMPACT-NOW update 3 criteria was rendered for all patients.10 All tumors would now qualify for the diagnosis of “glioblastoma, IDH-wildtype, CNS WHO grade 4” per the 2021 WHO Classification of Central Nervous System Tumors.4
Targeted Next-generation Sequencing
Tumor tissue was selectively scraped from unstained slides or punched from formalin-fixed, paraffin-embedded blocks using biopsy punches (Integra Miltex Instruments, cat# 33-31-P/25) to enrich for as high of tumor content as possible. Genomic DNA was extracted from this macrodissected formalin-fixed, paraffin-embedded tumor tissue using the QIAamp DNA FFPE Tissue Kit (Qiagen). Targeted next-generation sequencing was performed using the UCSF500 NGS Panel as previously described.17 Capture-based next-generation DNA sequencing was performed using an assay that targets all coding exons of 479 cancer-related genes, select introns and upstream regulatory regions of 47 genes to enable detection of structural variants including gene fusions, and DNA segments at regular intervals along each chromosome to enable genome-wide copy number and zygosity analysis, with a total sequencing footprint of 2.8 Mb (Supplementary Table S1). Multiplex library preparation was performed using the KAPA Hyper Prep Kit (Roche) according to the manufacturer’s specifications using 250 ng of sample DNA. Hybrid capture of pooled libraries was performed using a custom oligonucleotide library (Nimblegen SeqCap EZ Choice). Captured libraries were sequenced as paired-end reads on an Illumina HiSeq 2500 instrument. Sequence reads were mapped to the reference human genome build GRCh37 (hg19) using the Burrows-Wheeler aligner (BWA). Recalibration and deduplication of reads was performed using the Genome Analysis Toolkit (GATK). Coverage and sequencing statistics were determined using Picard CalculateHsMetrics and Picard CollectInsertSizeMetrics. Single nucleotide variant and small insertion/deletion mutation calling was performed with FreeBayes, Unified Genotyper, and Pindel. Large insertion/deletion and structural alteration calling was performed with Delly. Variant annotation was performed with Annovar. Single nucleotide variants, insertions/deletions, and structural variants were visualized and verified using Integrative Genome Viewer. Genome-wide copy number and zygosity analysis was performed by CNVkit and visualized using Nexus Copy Number (Biodiscovery).
Preoperative Imaging Assessment, Measurement of Tumor Volumes, and Volumetric Extent of Resection
Preoperative imaging studies were reviewed to assess degree of contrast enhancement, and stratified as 1) non-enhancing, 2) wispy or patchy/heterogeneous enhancement, or 3) well-defined ring-enhancement. Preoperative and postoperative tumor volumes were quantified by manual segmentation with the 3D Slicer Medical Image Computing Platform (version 4.8.1).18 MRI scans obtained in close proximity to surgery, typically 24 h prior to surgery and within 72 h postresection, were used for pre- and postoperative evaluation. Total contrast-enhancing (CE) and non-enhancing (NE) tumor volumes were measured at both pre- and postoperative time points. The total CE tumor volume was measured on T1-weighted postcontrast images, and the non-enhancing tumor volume was measured on T2 or FLAIR sequences. Manual segmentation was performed with region-of-interest analysis and volumetric measurements were obtained in a blinded manner in terms of patient outcomes.19
Comparison to Diagnostically Matched Historical Patient Cohorts
Patient demographics, molecular features, treatment regimen, and clinical outcomes were compared against two matched historical control patient cohorts where molecular evaluation was performed on a retrospective research basis and did not alter patient diagnosis or treatment decision making. The first cohort consisted of patients from The Cancer Genome Atlas (TCGA) Research Network study on diffuse lower-grade gliomas,5 and consisted of those 32 adult patients with diffuse lower-grade gliomas (grade II or III histologic features per 2016 WHO Classification criteria) studied by comprehensive genomic profiling that was confirmed to be IDH-wildtype and to harbor TERT promoter hotspot mutation (32/32, 100%), focal EGFR amplification (17/32, 53%), and/or combined trisomy 7/monosomy 10 (23/32, 72%), therefore fulfilling the diagnostic criteria for “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV” per cIMPACT-NOW update 3. The second cohort consisted of patients from the UCSF Adult Glioma Study (AGS) on molecular subgrouping of adult diffuse gliomas,6 and consisted of those 98 adult patients with diffuse lower-grade gliomas (grade II or III histologic features per 2016 WHO Classification criteria) studied by targeted Sanger sequencing and fluorescence in situ hybridization (FISH) that were confirmed to be IDH-wildtype, lacking codeletion of chromosomes 1p and 19q, and harboring TERT promoter hotspot mutation (98/98, 100%) and EGFR amplification in a subset (24/64, 38%), therefore fulfilling the diagnostic criteria for “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV” per cIMPACT-NOW update 3. Patient demographics, tumor histologic features, molecular data, treatment regimen, and clinical outcomes for the TGCA and AGS patient cohorts are listed in Supplementary Tables S2 and S3, respectively.
Statistical Analysis
Patient demographics and tumor characteristics were summarized with descriptive statistics. Student’s t- and χ 2-tests were used to compare continuous and categorical variables between patient cohorts, respectively. Logistic regression was used for multivariate comparisons to estimate odds ratios and 95% confidence intervals (95% CI). Overall survival was defined as the time from initial diagnostic surgical procedure until death or last clinical follow-up visit. Median follow-up was estimated with the reverse Kaplan–Meier method. Differences in survival were determined by log-rank test. Median overall survival times, hazard ratios, and 95% CI were estimated using the Kaplan–Meier method and Cox proportional hazards model. Multivariate models were chosen based on backwards variable selection. All analyses were conducted using the statistical software R version 4.0 (http://www.r-project.org/).
Results
Prospective Glioblastoma Precision Medicine Patient Cohort
This study included 38 consecutive adult patients with IDH-wildtype diffuse astrocytic glioma lacking necrosis or microvascular proliferation on histologic examination that were genomically profiled on a prospective clinical basis revealing criteria for an integrated diagnosis of “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV” per cIMPACT-NOW update 3 criteria.10 Patient demographics and clinical characteristics are summarized in Table 1 and detailed in Supplementary Table S4. The median age at initial diagnosis was 59 years (interquartile range [IQR], 51.5–64.8). 22 patients (58%) were male and 16 (42%) were female. The most frequent presenting symptoms were seizures, headaches, or extremity weakness. Tumors were located in the cerebral hemispheres in 37 patients and the thalamus in 1 patient. Preoperative imaging studies showed no contrast enhancement for 19 patients, wispy, or patchy/heterogeneous enhancement for 14 patients, and well-developed ring-enhancement for 5 patients. 21 patients underwent surgical resection as the initial diagnostic procedure, whereas 17 patients had only a limited diagnostic biopsy performed before initiation of adjuvant therapy. Based on histologic grading criteria per the 2016 WHO Classification,16 17 patients (45%) would have previously been diagnosed as “diffuse astrocytoma, WHO grade II” and 21 patients (55%) as “anaplastic astrocytoma, WHO grade III”.
Table 1.
Clinical Comparison Between the Prospective GPMP Cohort and Retrospective AGS + TCGA Patient Cohorts of “Diffuse Astrocytic Glioma, IDH-wildtype, with Molecular Features of Glioblastoma, WHO Grade IV” Per cIMPACT-NOW Update 3 Criteria
| Grade II Histologic Features Per 2016 WHO Classification | Grade III Histologic Features Per 2016 WHO Classification | Either Grade II or Grade III Histologic Features Combined | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Prospective (N = 17) | Retrospective (N = 28) | P | Prospective (N = 21) | Retrospective (N = 102) | P | Prospective (N = 38) | Retrospective (N = 130) | P | |
| Age at initial diagnosis (yrs) | 0.20 | .32 | .16 | ||||||
| Mean (SD) | 58.8 (9.3) | 54.4 (12.0) | 59.5 (10.7) | 56.7 (11.9) | 59.2 (10.0) | 56.2 (11.9) | |||
| Median | 60.9 | 53.0 | 58.4 | 57.0 | 59.0 | 57.0 | |||
| Q1, Q3 | 48.7, 63.7 | 48.0, 63.5 | 52.6, 65.1 | 51.0, 64.0 | 51.5, 64.8 | 50.0, 64.0 | |||
| Range | 44.0 - 75.4 | 29.0 - 74.0 | 41.7 - 78.4 | 20.0 - 86.0 | 41.7 - 78.4 | 20.0 - 86.0 | |||
| Sex | 0.85 | .60 | .92 | ||||||
| Female | 5 (29.4%) | 9 (32.1%) | 11 (52.4%) | 47 (46.1%) | 16 (42.1%) | 56 (43.1%) | |||
| Male | 12 (70.6%) | 19 (67.9%) | 10 (47.6%) | 55 (53.9%) | 22 (57.9%) | 74 (56.9%) | |||
| Initial surgical procedure | 0.17 | .46 | .07 | ||||||
| Biopsy | 9 (52.9%) | 9 (32.1%) | 7 (33.3%) | 26 (25.5%) | 16 (42.1%) | 35 (26.9%) | |||
| Resection | 8 (47.1%) | 19 (67.9%) | 14 (66.7%) | 76 (74.5%) | 22 (57.9%) | 95 (73.1%) | |||
| Primary adjuvant radiation therapy | 0.69 | .32 | .95 | ||||||
| Yes | 9 (60.0%) | 15 (53.6%) | 19 (95.0%) | 82 (87.2%) | 28 (80.0%) | 97 (79.5%) | |||
| No | 6 (40.0%) | 13 (46.4%) | 1 (5.0%) | 12 (12.8%) | 7 (20.0%) | 25 (20.5%) | |||
| Unknown | 2 | 0 | 1 | 8 | 3 | 8 | |||
| Primary adjuvant chemotherapy | 0.09 | .17 | .06 | ||||||
| Yes | 11 (73.3%) | 13 (46.4%) | 16 (80.0%) | 57 (64.0%) | 27 (77.1%) | 70 (59.8%) | |||
| No | 4 (26.7%) | 15 (53.6%) | 4 (20.0%) | 32 (36.0%) | 8 (22.9%) | 47 (40.2%) | |||
| Unknown | 2 | 0 | 1 | 13 | 3 | 13 | |||
Prospective Genomic Interrogation of IDH-wildtype Diffuse Lower-histologic Grade Astrocytic Glioma
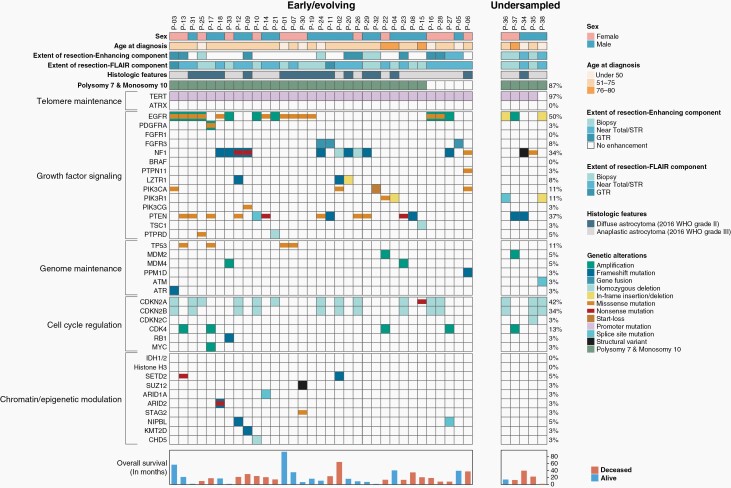
Prospective genomic profiling was performed using the UCSF500 targeted-capture NGS panel as part of the UCSF Glioblastoma Precision Medicine Program (GPMP) to inform accurate diagnostic classification and stratify patients for possible targeted therapy and clinical trial enrollment (Figure 1, Supplementary Tables S5, S6, S7). The 38 tumors were uniformly confirmed to be wildtype for the IDH1 and IDH2 genes, as well as the histone H3 genes (H3F3A, H3F3B, HIST1H3B, and HIST1H3C). Combined whole chromosome gain (polysomy) of chromosome 7 and loss (either monosomy or copy-neutral loss of heterozygosity) of chromosome 10 was found in 33/38 (87%) tumors. TERT promoter hotspot mutation (either c.-124C > T or c.-146C > T) was present in 37/38 (97%) tumors. Focal EGFR gene amplification was found in 11/38 (29%) tumors. Additionally, 8 tumors harbored known activating missense mutations within the extracellular domain or small in-frame insertions within the intracellular tyrosine kinase domain of EGFR in the absence of focal EGFR gene amplification. Additional likely oncogenic alterations known to be common in IDH-wildtype glioblastoma (eg, CDKN2A homozygous deletion, NF1 mutation/deletion, PTEN mutation/deletion, TP53 mutation, FGFR3-TACC3 fusion) were identified in 37/38 tumors. All tumors had a low somatic mutation burden with less than 10 mutations per Mb. MGMT promoter methylation was present in 12 of the 21 evaluated tumors (57%).
Fig. 1.
Oncoprint plot summarizing the genomic alterations identified in the 38 early/evolving or undersampled glioblastoma, IDH-wildtype.
Two Divergent Clinical Scenarios – “Early/evolving” and “Undersampled” Glioblastoma, IDH-wildtype
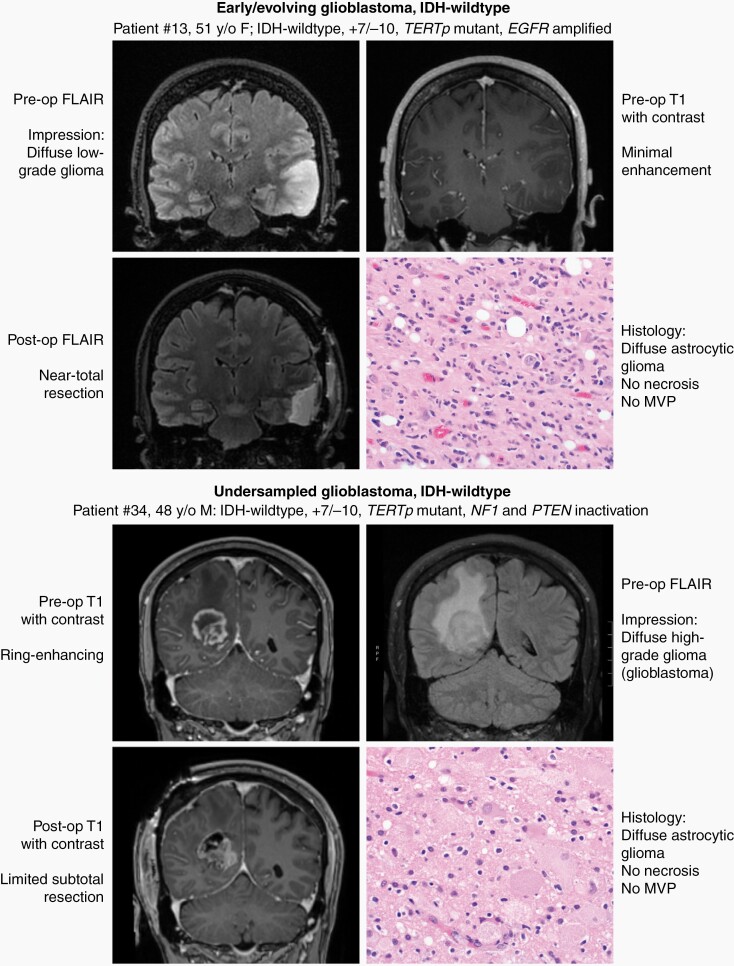
Among diffuse lower-histologic grade astrocytic gliomas fulfilling the new definitional criteria for “glioblastoma, IDH-wildtype” in the 2021 WHO Classification, we identify two divergent clinical scenarios (Figure 2). The first we term “early/evolving glioblastoma, IDH-wildtype”, which we use to designate those patients (33/38) who had surgical sampling of a mass with imaging features suggestive of a diffuse lower-grade glioma (ie, minimal to absent contrast enhancement), which is histologically composed of a diffuse astrocytic glioma without necrosis or microvascular proliferation and proven to be IDH-wildtype but with molecular features of glioblastoma. The second we term “undersampled glioblastoma, IDH-wildtype”, which we use to designate those patients (5/38) who underwent limited surgical sampling of a mass with imaging features suggestive of glioblastoma (ie, ring-enhancement with central necrosis), which is histologically composed of a diffuse astrocytic glioma without necrosis or microvascular proliferation and proven to be IDH-wildtype but with molecular features of glioblastoma. We find that these two clinical scenarios are histologically and molecularly indistinguishable (Figure 1, Supplementary Table S8), and this categorization requires integration with both the preoperative and postoperative imaging findings (eg, assessment of ring-enhancement and extent of surgical sampling). No significant difference in clinical outcomes was appreciated when stratifying the patient cohort by early/evolving versus undersampled disease (Supplementary Figure S1), but this analysis is limited as the cohort only included 5 patients with undersampled glioblastoma.
Fig. 2.
Illustration of two representative patients highlighting the two divergent clinical scenarios where the diagnosis of “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV” based on the cIMPACT-NOW update 3 can be applied. The first (top panel) is “early/evolving” disease where the patient presents with imaging features suggestive of a lower-grade diffuse glioma (ie, minimal to absent contrast enhancement) and histology reveals a diffuse lower-grade astrocytic glioma despite extensive surgical resection. The second (bottom panel) is “undersampled” disease where the patient presents with imaging features of glioblastoma (ie, ring-enhancing mass with central necrosis), but with limited surgical sampling from the infiltrative edge of the tumor whereby histology reveals a diffuse astrocytic glioma without necrosis or microvascular proliferation (MVP) that likely would have been found upon more extensive surgical resection.
Temporal Acquisition of High-grade Radiologic and Histologic Features in Early/evolving Glioblastoma, IDH-wildtype is Accompanied by Genetic Evolution
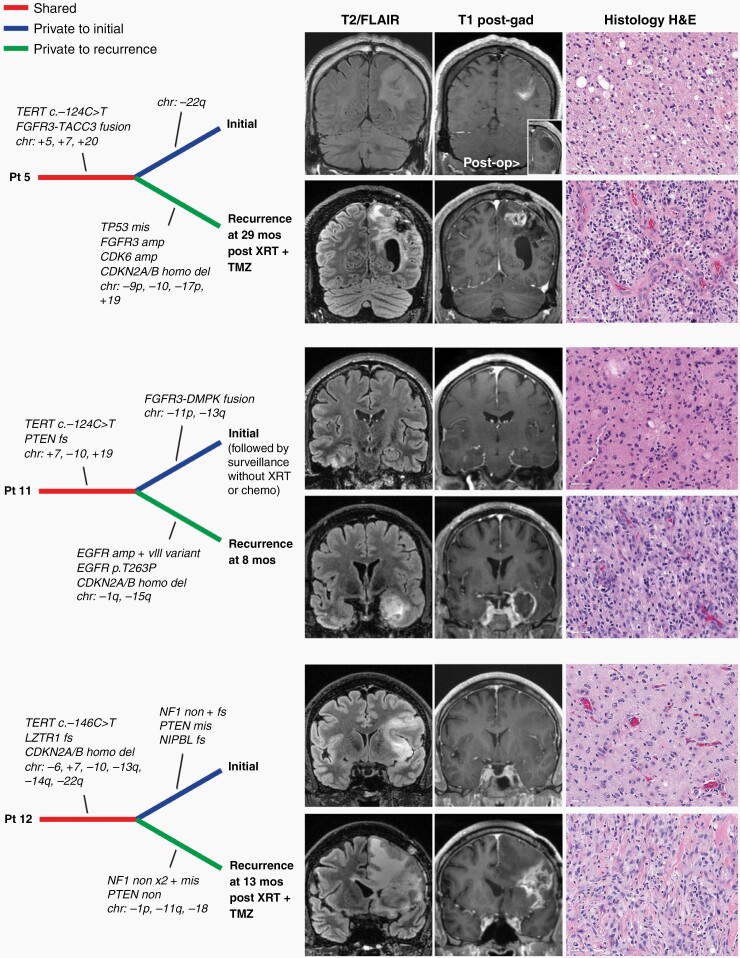
Current speculation is that the tumors we designate here as “early/evolving glioblastoma, IDH-wildtype” are likely to represent an early clinical presentation of glioblastoma that has yet to develop high-grade radiologic and histologic features. In support of this hypothesis, longitudinal observation of these patients over time revealed that disease progression was associated with the development of ring-enhancement and high-grade histologic features including necrosis and microvascular proliferation (Figure 3). Genomic profiling of paired initial and recurrent tumor specimens for three such patients with “early/evolving glioblastoma, IDH-wildtype” demonstrated shared alterations (eg, TERT promoter mutation), as well as genetic divergence including newly acquired alterations at time of recurrence (eg, CDKN2A/B homozygous deletion for patients 5 and 11) accompanying the development of high-grade radiologic and histologic features (Supplementary Table S9).
Fig. 3.
Temporal acquisition of high-grade radiologic and histologic features for early/evolving glioblastoma, IDH-wildtype, is accompanied by genetic evolution. Shown are three patients with IDH-wildtype diffuse astrocytic gliomas lacking ring-enhancement on imaging and necrosis or microvascular proliferation on histology at time of initial surgical procedure. These patients subsequently developed ring-enhancement and histologic features characteristic of glioblastoma (or gliosarcoma for patient 12). Genomic profiling of both initial and recurrent tumor specimens demonstrated shared alterations (eg, TERT promoter mutation), as well as genetic divergence including newly acquired alterations at time of recurrence (eg, CDKN2A/B homozygous deletion for patients 5 and 11) accompanying the development of high-grade radiologic and histologic features.
More Aggressive Adjuvant Treatment of Early/evolving and Undersampled IDH-wildtype Glioblastoma Improves Clinical Outcomes
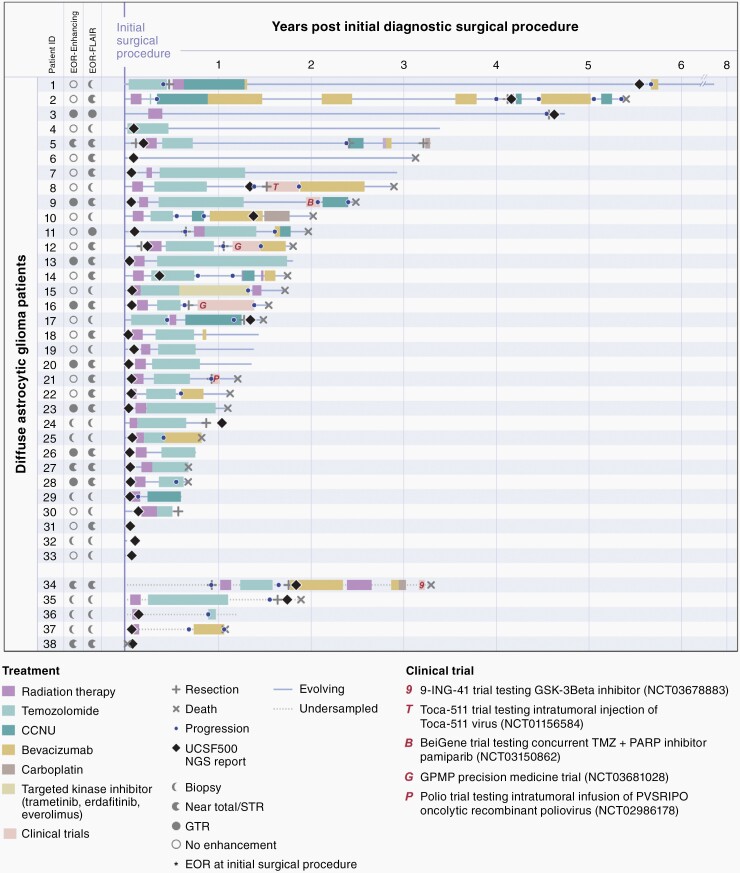
The prospective genomically informed diagnostic classification of these tumors as “glioblastoma, IDH-wildtype” guided aggressive management with most patients receiving adjuvant radiation and chemotherapy despite not meeting traditional histologic criteria for glioblastoma (Figure 4, Supplementary Table S4). To determine the impact of this prospective genomic profiling and more aggressive adjuvant therapy, we compared the clinical outcomes for this prospective GPMP patient cohort against a biologically matched historical control patient cohort where molecular evaluation was performed on a retrospective research basis and did not alter patient diagnosis or treatment decision making. This matched historical control cohort consisted of 130 patients with diffuse lower-grade gliomas (grade II or III histologic features per 2016 WHO Classification criteria) pooled from The Cancer Genome Atlas (n = 32) and the UCSF Adult Glioma Study (n = 98) that were confirmed to be IDH1/2 wildtype and fulfill genetic criteria for “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV” per the cIMPACT-NOW update 3.5,6 Compared to the retrospective cohort, our prospective GPMP cohort had similar age at initial diagnosis (59.2 vs 56.2 years, P = .16) and sex distribution (42% vs 43% female, P = .92), but a greater proportion of tumors with grade II histologic features per 2016 WHO Classification criteria (45% vs 22%). Additionally, a larger proportion of patients in our prospective cohort underwent a diagnostic biopsy only compared to the retrospective cohort (42% vs 27%, P = .07), whereas a greater proportion of patients in our prospective GPMP cohort were treated with adjuvant radiation and chemotherapy given the molecularly integrated diagnosis of IDH-wildtype glioblastoma compared to the retrospective control cohort (Table 1). In a multivariate logistic regression model of membership in the prospective GPMP cohort versus the retrospective control cohort, patient age at diagnosis, histologic grade per 2016 WHO Classification, and primary adjuvant chemotherapy were significantly different (Supplementary Table S10). The odds ratio for primary adjuvant chemotherapy was 3.02 (95% CI: 1.20–7.62), indicating that a patient was approximately 3 times more likely to be from the prospective cohort if they had received primary adjuvant chemotherapy while controlling for age at diagnosis and histologic grade per 2016 WHO Classification.
Fig. 4.
Swimmer’s plot showing timing of genomic results, treatment, and clinical outcomes for the 38 patients with early/evolving or undersampled glioblastoma, IDH-wildtype.
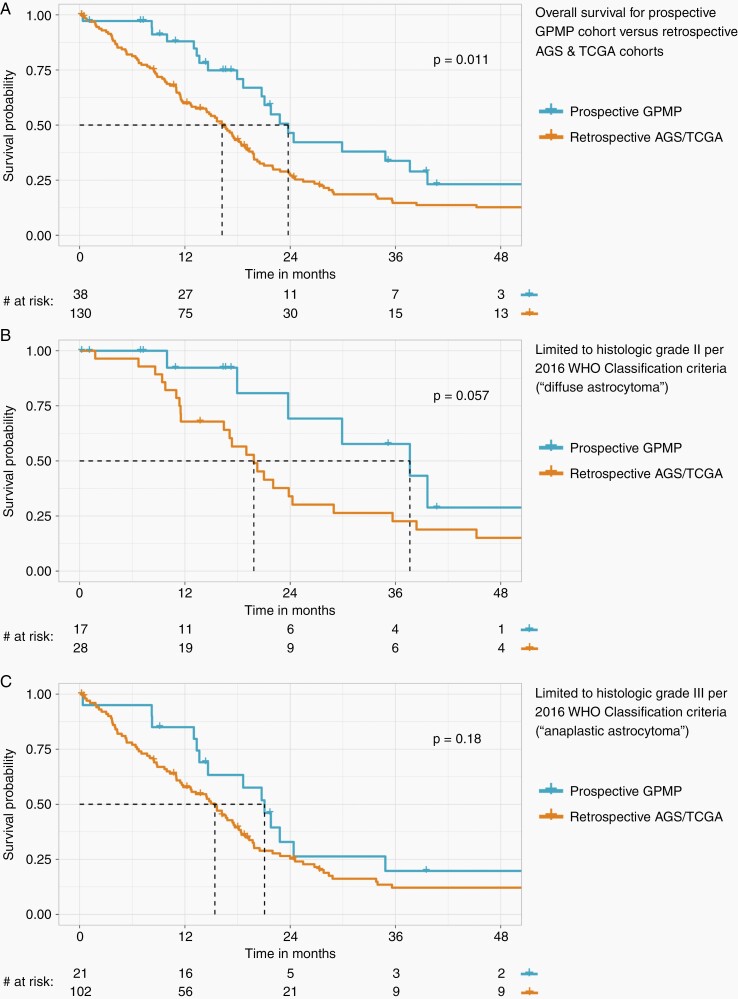
The median overall survival in our prospective GPMP cohort was 23.8 months (95% CI: 20.7–39.6), which was significantly longer when compared to the retrospective control cohort with median survival of 16.2 months (95% CI: 13.2–18.9) (Figure 5A, P = .011 by log-rank test). The hazard ratio (HR) of the retrospective control cohort compared to our prospective GPMP cohort was 1.82 (95% CI: 1.14–2.9, P = .012). When segregated by histologic grade per 2016 WHO Classification, median overall survival was prolonged in the prospective GPMP cohort for both grade II and III subgroups compared to the historical control cohort, most pronounced in the grade II (“diffuse astrocytoma”) tumors (Figure 5B, C). In a multivariate Cox proportional hazard ratio model (Supplementary Table S11), protective factors for overall survival were cohort membership (prospective GPMP vs retrospective control: HR = 0.47, 95% CI: 0.29–0.76), primary adjuvant chemotherapy (yes vs no: HR = 0.53, 95% CI: 0.36–0.78), and initial surgical procedure (resection vs biopsy: HR = 0.45, 95% CI: 0.29–0.69). Adverse risk factors were age at initial diagnosis (per year: HR = 1.02, 95% CI: 1.01–1.04) and primary adjuvant radiotherapy (yes vs no: HR = 2.20, 95% CI: 1.37–3.51). Patient sex and tumor histologic grade per 2016 WHO Classification were not significant. The hazard ratio of 0.47 signifies that if a patient was in the prospective GPMP cohort, they have a 53% reduction in risk of death compared to the retrospective control cohort while controlling for age at diagnosis, initial surgical procedure, primary adjuvant radiotherapy, and primary adjuvant chemotherapy.
Fig. 5.
Kaplan–Meier curves comparing overall survival for the 38 consecutive patients with prospectively identified early/evolving and undersampled IDH-wildtype glioblastoma versus biologically matched historical control patient cohorts receiving standard-of-care therapy based on histomorphologic diagnosis alone (ie, adult diffuse lower-grade astrocytic gliomas where molecular evaluation demonstrating IDH-wildtype status and molecular features of glioblastoma was performed on a retrospective research basis and did not alter patient diagnosis or treatment decision making). (A) Overall survival analysis for all 38 patients from this prospective cohort versus 130 matched historical control patients from The Cancer Genome Atlas (TCGA) and UCSF Adult Glioma Study (AGS). (B) Overall survival analysis limited to those patients with tumors categorized as grade II per the 2016 WHO Classification criteria (“diffuse astrocytoma”). (C) Overall survival analysis limited to those patients with tumors categorized as grade III per the 2016 WHO Classification criteria (“anaplastic astrocytoma”). Patient demographics, tumor histologic features, molecular data, treatment regimen, and clinical outcomes for the TGCA and AGS patient cohorts are listed in Supplementary Tables S2 and S3, respectively.
Discussion
Here, we show that prospective application of a targeted NGS panel can readily identify the molecular alterations characteristic of IDH-wildtype glioblastoma in histologically lower-grade IDH-wildtype diffuse gliomas, thereby improving diagnostic and prognostic classification for affected patients. Importantly, when compared to a biologically matched historical control patient cohort with molecular evaluation performed only on a retrospective research basis, we found that this prospective genomic profiling led to more aggressive treatment including a greater fraction of patients who received primary adjuvant radiation and chemotherapy immediately after initial diagnostic surgical procedure. This was accompanied by prolonged survival times compared to the matched historical control cohort, thereby highlighting the power of a molecularly integrated diagnostic approach to improve outcomes for glioma patients.
Although no significant outcome difference was found between early/evolving and surgically undersampled IDH-wildtype glioblastoma in this cohort potentially due to limited sample size, additional studies are warranted to examine the clinical impact of these two divergent clinical scenarios which have now been included under the diagnosis of “glioblastoma, IDH-wildtype” in the 2021 WHO Classification. We speculate that undersampled tumors are likely to have worse clinical outcomes due to the limited surgical sampling and the known benefit of maximal surgical resection for diffuse glioma patients.19 In contrast, we speculate the tumors we have designated as “early/evolving glioblastoma, IDH-wildtype” likely represent an early clinical presentation of glioblastoma that has yet to develop high-grade radiologic and histologic features. More aggressive therapy for affected patients earlier in the disease course prior to development of high-grade features is likely to be the underlying cause of the prolonged survival for early/evolving glioblastoma patients in our cohort (median overall survival of 23.6 months) compared to conventional glioblastoma, IDH-wildtype. Despite being genetically indistinguishable from conventional glioblastoma, such early/evolving glioblastomas may nonetheless have underlying biologic differences such as immune microenvironment and intact blood-brain barrier that may affect treatment efficacy, for which future studies are warranted. We additionally identified genetic evolution of these early/evolving glioblastomas over time, indicating the need for longitudinal genomic profiling to inform precision medicine treatment approaches for these patients.
While NGS can identify molecular alterations that are suggestive of an IDH-wildtype glioblastoma, these now definitional molecular alterations in isolation are not entirely specific to IDH-wildtype glioblastoma. For example, TERT promoter mutations can be seen in other glial neoplasms such as oligodendroglioma, IDH-mutant and 1p/19q-codeleted, and pleomorphic xanthoastrocytoma (typically those with anaplastic features).20,21 Notably, some studies have demonstrated that diffuse gliomas harboring TERT promoter mutations in the absence of EGFR amplification or combined trisomy 7 and monosomy 10 may follow a more favorable clinical course compared to the broader group of glioblastoma, IDH-wildtype.13,14 In our prospective patient cohort, only two of the 38 patients had diffuse astrocytic gliomas that fulfilled the cIMPACT-NOW criteria for “molecular glioblastoma” based on solitary TERT promoter mutation (ie, lacking concurrent EGFR amplification and combined +7/−10). The first was patient 5, a 42-year-old man whose diffuse astrocytic glioma in the left frontal lobe had patchy enhancement on preoperative imaging, grade III histologic features per 2016 WHO Classification, and harbored TERT promoter mutation, FGFR3-TACC3 fusion, and trisomy 7 (without aberration of chromosome 10). His tumor recurrence at 29 months included development of ring-enhancement and both microvascular proliferation and necrosis histologically, with molecular profiling demonstrating the identical TERT promoter mutation, FGFR3-TACC3 fusion, and trisomy 7, but also with newly acquired monosomy 10, as well as CDKN2A/B homozygous deletion and TP53 mutation (Figure 3). We believe this patient 5 biologically has glioblastoma, IDH-wildtype according to the constellation of radiologic, histologic, and molecular features, and may potentially have experienced longer survival (40 months) due to both the aggressive treatment and FGFR3-TACC3 fusion, which is a known favorable prognostic factor in IDH-wildtype glioblastoma.22,23 The second was patient 6, a 66-year-old woman who had imaging features of “gliomatosis cerebri” with molecular profiling demonstrating TERT promoter mutation, PPM1D exon 6 frameshift mutation, and missense mutations in PIK3CA, NF1, and PTPN11. This patient survived for 38 months and remains enigmatic in terms of definitive diagnostic classification despite fulfilling current criteria for the diagnosis of “Glioblastoma, IDH-wildtype” in the 2021 WHO Classification. Further studies are needed to refine classification and prognosis for IDH-wildtype diffuse gliomas with TERT promoter mutation lacking EGFR amplification and/or combined +7/−10.
We now recognize that not all diffuse gliomas with microvascular proliferation and necrosis are biologically “glioblastoma, IDH-wildtype”. Genomic and epigenomic analysis of histologically high-grade IDH-wildtype gliomas allows for segregation of other distinct CNS tumor entities (eg, diffuse midline gliomas with H3 K27 alteration, diffuse hemispheric gliomas with H3 G34 mutation, high-grade astrocytoma with piloid features, etc) which are each associated with their own unique molecular pathogenesis and natural history.24–27 Even in the setting of an adult-type diffuse astrocytic glioma not aligning with other well-characterized tumor types, the current diagnostic criteria for IDH-wildtype glioblastoma as outlined in the 2021 WHO Classification do not fully encapsulate the broad spectrum of molecular alterations that drive glioblastoma tumorigenesis. This is due to the fact that “glioblastoma, IDH-wildtype” still represents a heterogeneous disease encompassing multiple biologic and epigenetic subtypes with various genetic drivers beyond the combination of TERT promoter mutation, combined +7/−10, or EGFR amplification. While EGFR amplification is present in approximately 50% of currently defined IDH-wildtype glioblastomas, those tumors lacking EGFR amplification can instead have amplification or fusion of other receptor tyrosine kinase genes such as PDGFRA, MET, and FGFR3.7,28,29 We also demonstrate here several tumors with known activating EGFR missense or indel mutations occurring in the absence of EGFR gene amplification (8 of 38 gliomas in this cohort). While one of two recurrent hotspot substitutions (c.-124C > T or c.-146C > T) in the promoter region of the TERT gene is the most frequent mechanism of telomere maintenance in IDH-wildtype glioblastomas,30 other less common genetic alterations can also occur such as TERT gene amplification or rearrangements in the upstream TERT promoter region resulting in “enhancer hijacking”.31 Integrating histologic and molecular features while accounting for any limitations in molecular test results (eg, low tumor content) is now considered best practice for the accurate diagnostic classification of CNS tumors.
Utilizing prospective genomic profiling and refined tumor classification schemes, we have shown that upfront identification and aggressive treatment of early/evolving or undersampled IDH-wildtype glioblastoma extends patient survival compared to prior matched patient cohorts whose treatment was predicated upon histologic grading. While these results are encouraging, additional multi-center studies are necessary to confirm our findings and further support precision medicine-based treatment approaches to CNS tumors.
Supplementary Material
Acknowledgments
We thank the staff of the UCSF Clinical Cancer Genomics Laboratory for assistance with genetic profiling. We also thank our patients (the study participants) and the many providers and clinical staff involved in the multidisciplinary care of these neuro-oncology patients at UCSF Medical Center.
Contributor Information
Yalan Zhang, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Calixto-Hope G Lucas, Department of Pathology, University of California, San Francisco, San Francisco, California, USA.
Jacob S Young, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Ramin A Morshed, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Lucie McCoy, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Nancy Ann Oberheim Bush, Division of Neuro-Oncology, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA; Department of Neurology, University of California, San Francisco, San Francisco, California, USA.
Jennie W Taylor, Division of Neuro-Oncology, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA; Department of Neurology, University of California, San Francisco, San Francisco, California, USA.
Mariza Daras, Division of Neuro-Oncology, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA; Department of Neurology, University of California, San Francisco, San Francisco, California, USA.
Nicholas A Butowski, Division of Neuro-Oncology, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Javier E Villanueva-Meyer, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA; Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA.
Soonmee Cha, Department of Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, California, USA.
Margaret Wrensch, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
John K Wiencke, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Julieann C Lee, Department of Pathology, University of California, San Francisco, San Francisco, California, USA.
Melike Pekmezci, Department of Pathology, University of California, San Francisco, San Francisco, California, USA.
Joanna J Phillips, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA; Department of Pathology, University of California, San Francisco, San Francisco, California, USA.
Arie Perry, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA; Department of Pathology, University of California, San Francisco, San Francisco, California, USA.
Andrew W Bollen, Department of Pathology, University of California, San Francisco, San Francisco, California, USA.
Manish K Aghi, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Philip Theodosopoulos, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Edward F Chang, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Shawn L Hervey-Jumper, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Mitchel S Berger, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Jennifer L Clarke, Division of Neuro-Oncology, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA; Department of Neurology, University of California, San Francisco, San Francisco, California, USA.
Susan M Chang, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA; Division of Neuro-Oncology, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
Annette M Molinaro, Department of Neurological Surgery, University of California, San Francisco, San Francisco, California, USA.
David A Solomon, Department of Pathology, University of California, San Francisco, San Francisco, California, USA.
Funding
This work was supported by the UCSF Glioblastoma Precision Medicine Program, the Panattoni Family Foundation, and the Sullivan family. C.G.L. is supported by the UCSF Training Program in Translational Brain Tumor Research, National Cancer Institute, NIH (T32 CA151022). The UCSF Adult Glioma Study was supported by the National Institutes of Health (R01 CA052689, P50 CA097257, R01 CA126831, R01 CA139020, R25 CA112355, and R01 CA207360), as well as the National Brain Tumor Foundation, the Stanley D. Lewis and Virginia S. Lewis Endowed Chair in Brain Tumor Research, and the Robert Magnin Newman Endowed Chair in Neuro-oncology.
Conflict of interest statement. The authors declare that they have no competing interests related to this report.
Authorship statement. Patient database management and statistical analysis: Y.Z., L.M., M.W., J.K.W., A.M.M. Performed genomic analysis: C.G.L., J.C.L., D.A.S. Performed pathologic assessment: C.G.L., J.C.L., M.P., J.J.P., A.P., A.W.B., D.A.S. Performed radiologic assessment: J.S.Y., R.A.M., J.E.V.M., S.C. Provided neuro-oncologic management: N.A.O.B., J.W.T., M.D., N.A.B., J.L.C., S.M.C. Provided neurosurgical management: J.S.Y., R.A.M., M.K.A., P.T., E.F.C., S.L.H.J., M.S.B. Prepared figures and wrote manuscript: Y.Z., C.G.L., A.M.M., D.A.S. Study supervision: J.L.C., S.M.C., A.M.M., D.A.S. Funding acquisition: M.S.B., J.L.C., S.M.C., A.M.M., D.A.S. All authors critically reviewed the manuscript and approved its submission.
Ethical Approval
This study was approved by the Committee on Human Research of the University of California, San Francisco, with a waiver of patient consent.
Data Availability
Annotated genomic data are available in the electronic Supplementary Material. Raw sequencing data files are available upon request.
References
- 1. Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008; 321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009; 360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis DN, Wesseling P, Aldape K, et al. cIMPACT-NOW update 6: new entity and diagnostic principle recommendations of the cIMPACT-Utrecht meeting on future CNS tumor classification and grading. Brain Pathol. 2020; 30(4):844–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021; 23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brat DJ, Verhaak RGW, Aldape KD, et al. ; Cancer Genome Atlas Research Network. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015; 372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015; 372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ceccarelli M, Barthel FP, Malta TM, et al. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell 2016; 164(3):550–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019; 15(7):405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reuss DE, Kratz A, Sahm F, et al. Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015; 130(3):407–417. [DOI] [PubMed] [Google Scholar]
- 10. Brat DJ, Aldape K, Colman H, et al. cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018; 136(5):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stichel D, Ebrahimi A, Reuss D, et al. Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 2018; 136(5):793–803. [DOI] [PubMed] [Google Scholar]
- 12. Barthel FP, Johnson KC, Varn FS, et al. Longitudinal molecular trajectories of diffuse glioma in adults. Nature. 2019; 576(7785):112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tesileanu CMS, Dirven L, Wijnenga MMJ, et al. Survival of diffuse astrocytic glioma, IDH1/2 wildtype, with molecular features of glioblastoma, WHO grade IV: a confirmation of the cIMPACT-NOW criteria. Neuro Oncol. 2020; 22(4):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Berzero G, Di Stefano AL, Ronchi S, et al. IDH-wildtype lower-grade diffuse gliomas: the importance of histological grade and molecular assessment for prognostic stratification. Neuro Oncol. 2021; 23(6):955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wen PY, Chang SM, Van den Bent MJ, et al. Response assessment in neuro-oncology clinical trials. J Clin Oncol. 2017; 35(21):2439–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016; 131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 17. Kline CN, Joseph NM, Grenert JP, et al. Targeted next-generation sequencing of pediatric neuro-oncology patients improves diagnosis, identifies pathogenic germline mutations, and directs targeted therapy. Neuro Oncol. 2017; 19(5):699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fedorov A, Beichel R, Kalpathy-Cramer J, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012; 30(9):1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Molinaro AM, Hervey-Jumper S, Morshed RA, et al. Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020; 6(4):495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phillips JJ, Gong H, Chen K, et al. The genetic landscape of anaplastic pleomorphic xanthoastrocytoma. Brain Pathol. 2019; 29(1):85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ebrahimi A, Korshunov A, Reifenberger G, et al. Pleomorphic xanthoastrocytoma is a heterogeneous entity with pTERT mutations prognosticating shorter survival. Acta Neuropathol Commun. 2022; 10(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Di Stefano AL, Picca A, Saragoussi E, et al. Clinical, molecular, and radiomic profile of gliomas with FGFR3-TACC3 fusions. Neuro Oncol. 2020; 22(11):1614–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mata DA, Benhamida JK, Lin AL, et al. Genetic and epigenetic landscape of IDH-wildtype glioblastomas with FGFR3-TACC3 fusions. Acta Neuropathol Commun. 2020; 8(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012; 22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 25. Solomon DA, Wood MD, Tihan T, et al. Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2016; 26(5):569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Reinhardt A, Stichel D, Schrimpf D, et al. Anaplastic astrocytoma with piloid features, a novel molecular class of IDH wildtype glioma with recurrent MAPK pathway, CDKN2A/B and ATRX alterations. Acta Neuropathol. 2018; 136(2):273–291. [DOI] [PubMed] [Google Scholar]
- 27. Reinhardt A, Stichel D, Schrimpf D, et al. Tumors diagnosed as cerebellar glioblastoma comprise distinct molecular entities. Acta Neuropathol Commun. 2019; 7(1):163. Published 2019 Oct 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Singh D, Chan JM, Zoppoli P, et al. Transforming fusions of FGFR and TACC genes in human glioblastoma. Science. 2012; 337(6099):1231–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013; 155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Killela PJ, Reitman ZJ, Jiao Y, et al. TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA. 2013; 110(15):6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Diplas BH, He X, Brosnan-Cashman JA, et al. The genomic landscape of TERT promoter wildtype-IDH wildtype glioblastoma. Nat Commun. 2018; 9(1):2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Annotated genomic data are available in the electronic Supplementary Material. Raw sequencing data files are available upon request.