Abstract
Brain metastases occur commonly in patients with advanced solid malignancies. Yet, less is known about brain metastases than cancer-related entities of similar incidence. Advances in oncologic care have heightened the importance of intracranial management. Here, in this consensus review supported by the Society for Neuro-Oncology (SNO), we review the landscape of brain metastases with particular attention to management approaches and ongoing efforts with potential to shape future paradigms of care. Each coauthor carried an area of expertise within the field of brain metastases and initially composed, edited, or reviewed their specific subsection of interest. After each subsection was accordingly written, multiple drafts of the manuscript were circulated to the entire list of authors for group discussion and feedback. The hope is that the these consensus guidelines will accelerate progress in the understanding and management of patients with brain metastases, and highlight key areas in need of further exploration that will lead to dedicated trials and other research investigations designed to advance the field.
Keywords: brain metastases, consensus, expert, guidelines, recommendations, treatment
Brain metastases are common among patients with advanced solid malignancies and represent a significant source of morbidity and mortality.1–3 Although the true incidence of brain metastases has been challenging to determine, it is estimated that approximately 10–40% of patients with solid tumors will develop brain metastases, translating to an estimated incidence of brain metastases in the United States of 70 000–400 000 cases per year.1,4 With expanding availability and utilization of magnetic resonance imaging (MRI), as well as improving systemic therapy for extracranial control with lagging intracranial efficacy, the incidence of brain metastases has increased over time.1,5
Given the heterogeneous penetration of most systemic therapies into the microenvironment of brain metastases, the historical management of brain metastases has largely consisted of local, brain-directed therapy involving stereotactic radiation or large field radiation therapy and, when indicated, neurosurgical resection.6,7 In recent years, advances in systemic therapy have led to a paradigm shift for certain patients with brain metastases, with systemic therapy as monotherapy now a first-line consideration for subgroups of asymptomatic patients.8–10Although the prognosis for many patients with brain metastases remains guarded, it does seem to be improving.2,11
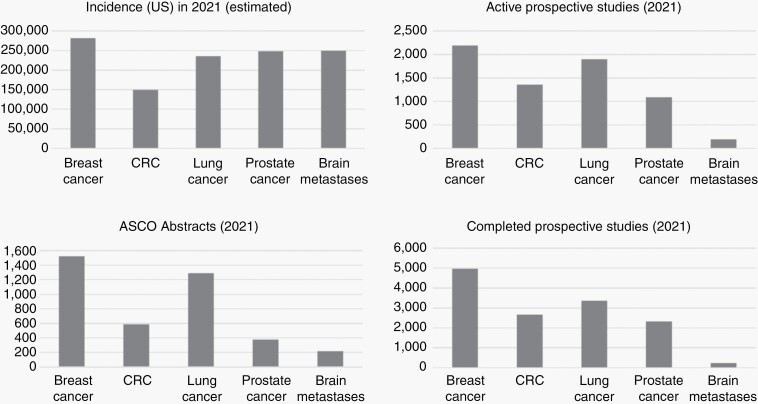
Despite the high incidence of brain metastases, which rivals that of primary breast, colorectal, lung, and prostate cancer in the United States, a relative dearth of oncologic research devoted to patients with brain metastases exists (Figure 1). As a result, heterogeneity in practice exists among different centers and individuals.12 The focus of this consensus statement is to provide a comprehensive outline regarding the epidemiology, pathogenesis, diagnosis, and treatment of brain metastases, as well as highlight future directions in investigative efforts and clinical care that may improve the outlook and management of patients with brain metastases. This work is part of a series of articles supported by SNO seeking to provide context regarding optimal oncologic treatment and highlight areas of needed research of common neurologic entities; this article complements recently published recommendations from ASCO-SNO-ASTRO.13 Of note, leptomeningeal disease will be addressed by a separate, future effort.
Fig. 1.
Incidence and research output as measured by ASCO abstracts (annual meeting) and active/completed prospective trials based on clinicaltrials.gov among patients with brain metastases versus other oncologic entities of similar incidence. (Abbreviations: ASCO, American Society of Clinical Oncology; CRC, colorectal cancer; US, United States).
Epidemiology
Incidence
The exact incidence of brain metastases has historically been difficult to elucidate. This has, in part, been due to a lack of mandated reporting of brain metastases to local and federal registries, such as the Central Brain Tumor Registry of the United States.14,15 In 2016, the Surveillance Epidemiology and End Results (SEER) program released data regarding the presence versus absence of brain metastases among patients with extracranial primaries at the time of primary cancer diagnosis.16 Thereafter, Cagney et al. reported the first population-based epidemiologic study of brain metastases in the United States using SEER data (Table 1).16 However, because most consensus guidelines, such as those published by the National Comprehensive Cancer Network (NCCN), only favor screening imaging of the brain for specific malignancies/stages of disease, including select patients with small cell lung cancer (SCLC), non-small cell lung cancer (NSCLC), melanoma, testicular cancer, alveolar soft parts sarcoma, angiosarcoma, and left-sided cardiac sarcoma, but not other cancers such as breast cancer, renal cancer, gastrointestinal primaries, head/neck cancer, and most other cancers, the reported incidence for unscreened malignancies may be underestimated.17
Table 1.
Incidence Proportion of Brain Metastases in the United States at Diagnosis of Malignancy by Primary Site. Reproduced with permission from Lamba et al., Neuro-Oncology, 20213
| Primary Site | Sub-Site | Incidence Proportion Among Entire Cohorta | Incidence Proportion Among Subset with Metastatic Diseaseb |
|---|---|---|---|
| Breast | |||
| HR+/HER2- | 0.22 | 5.46 | |
| HR+/HER2+ | 0.61 | 7.98 | |
| HR-/HER2+ | 1.09 | 11.45 | |
| Triple negative | 0.68 | 11.37 | |
| Head and Neck | All | 0.20 | 5.06 |
| Gastrointestinal | |||
| Esophagus | 1.66 | 5.31 | |
| Gastric | 0.64 | 1.96 | |
| Hepatobiliary | 0.36 | 1.77 | |
| Pancreatic | 0.41 | 0.82 | |
| Colorectal | 0.27 | 1.36 | |
| Anal | 0.11 | 1.58 | |
| Other gastrointestinal | 0.68 | 2.08 | |
| Genitourinary | |||
| Renal | 1.48 | 10.84 | |
| Bladder | 0.25 | 3.45 | |
| Prostate | 0.07 | 1.47 | |
| Testicular | 0.88 | 7.61 | |
| Other genitourinary | 0.23 | 2.88 | |
| Gynecologic | |||
| Ovarian | 0.24 | 0.94 | |
| Endometrial | 0.22 | 3.40 | |
| Cervical | 0.38 | 2.94 | |
| Other gynecologic | 0.21 | 2.19 | |
| Lung | |||
| Small cell | 15.83 | 23.46 | |
| Squamous cell | 5.29 | 15.86 | |
| Adenocarcinoma | 14.44 | 26.82 | |
| Bronchioloalveolar | 2.31 | 15.47 | |
| Nonsmall cell not otherwise specified | 12.81 | 25.56 | |
| Melanoma | Any | 0.65 | 28.16 |
| Sarcoma | Any | 0.74 | 4.44 |
| Thyroid | Thyroid | 0.12 | 5.86 |
| All others | All others | 1.73 | 9.94 |
aIncidence proportion was defined as the number of patients diagnosed with brain metastases and a specific primary cancer divided by the total number of individuals diagnosed with that primary cancer.
bIncidence proportion was defined as the number of patients diagnosed with brain metastases and a specific primary cancer divided by patients with de novo metastatic disease to any distant site.
A significant proportion of patients free from brain metastases at initial diagnosis will develop intracranial disease later in their clinical course.18,19 Unfortunately, recurrence-based information after initial management is not available in SEER, although promising claims-based techniques to abstract such data now exist.20 Data depicting the cumulative incidence of brain metastases can be obtained for select patients from other sources. For example, among patients in the adjuvant HERA trial who died, approximately 50% of patients with HER2-positive breast cancer develop intracranial metastases by the time of death.18 These data led to the activation of prospective studies evaluating the role of screening MRI of the brain in patients with advanced, metastatic, or inflammatory breast cancer (NCT04030507, NCT03881605). Among patients with SCLC, autopsy series have indicated that approximately 80% of patients will develop brain metastases.21 For many cancers, the role of surveillance imaging of the brain after an initially unremarkable scan has not been elucidated by prior investigations or delineated by consensus guidelines. Given the high proportion of patients at risk for development of intracranial disease after initial cancer diagnosis, characterization of the incidence/outcomes of brain metastases among this population is critical.
Prognosis
Prior studies of prognosis in patients with brain metastases led to the development, validation, and widespread utilization of two major prognostic indices, namely the recursive partitioning analysis (RPA) and the more contemporary diagnosis-specific graded prognostic assessment (DS-GPA).22–26 The DS-GPA is based on aggregated data from patients with brain metastases across multiple institutions and has identified significant prognostic factors, within each major primary tumor site, including Karnofsky performance status (lung, melanoma, renal cell, breast, and gastrointestinal primaries), age (lung, breast), presence of extracranial metastases (lung), and number of brain metastases (lung, melanoma, and renal cell).22,27–30 More recent versions of these scores have also included molecular covariates, such as EGFR and ALK alterations in lung adenocarcinoma (lung-molGPA),31 estrogen/progesterone and HER2-receptor status for breast cancer (breast-GPA),29 and BRAF status in melanoma (melanoma-molGPA).30
Compared to GPA-based indices, population-based delineations of survival among patients with brain metastases, using validated claims-based techniques,20 have displayed more concerning prognostic estimates (Table 2). A SEER-Medicare study of 9882 older patients with brain metastases demonstrated median survival times of <4 months across all primary sites (lung, breast, melanoma, kidney, esophageal, and colorectal) with the exception of a smaller cohort of patients with ovarian primaries.11 In a separate SEER study of adult patients of all ages harboring brain metastases at the time of diagnosis of the primary, a median survival of ≤12 months across nearly all primary sites was noted.16
Table 2.
Prognosis of Brain Metastases in the United States by Primary Site as Derived from SEER, SEER-Medicare, and GPA-based Data.a,b Reproduced with permission from Lamba et al., Neuro-Oncology, 20213
| Primary Site | Sub-Site | Median Survival (Months) Based on SEER data | Median Survival (Months) in Older Patients Based on SEER-Medicare Datac | Median Survival (Months) Based on GPA Data |
|---|---|---|---|---|
| Breast | 2.1-4.5 | 16 | ||
| HR+/HER2− | 14.0 | 2.0–4.9 | ||
| HR+/HER2+ | 21.0 | 2.5–6.4 | ||
| HR−/HER2+ | 10.0 | |||
| Triple negative | 6.0 | 2.3–3.4 | ||
| Head and Neck | All | 5.0 | ||
| Gastrointestinal | 8 | |||
| Esophagus | 4.0 | 2.3–4.0 | ||
| Gastric | 4.0 | |||
| Hepatobiliary | 3.0 | |||
| Pancreatic | 2.0 | |||
| Colorectal | 6.0 | 2.5–3.0 | ||
| Anal | 7.0 | |||
| Other gastrointestinal | 4.0 | |||
| Genitourinary | ||||
| Renal | 5.0 | 1.8–3.5 | 12 | |
| Bladder | 4.0 | |||
| Prostate | 12.0 | |||
| Testicular | Not reached | |||
| Other genitourinary | 7.0 | |||
| Gynecologic | ||||
| Ovarian | 5.0 | 7.5–7.7 | ||
| Endometrial | 4.0 | |||
| Cervical | 4.0 | |||
| Other gynecologic | Not reached | |||
| Lung | 2.9–3.3 | |||
| Small cell | 6.0 | 3.0–3.6 | ||
| Squamous cell | 4.0 | 2.2–2.8 | ||
| Adenocarcinoma | 6.0 | 3.7–3.8 | 15 | |
| Bronchioloalveolar | 10.0 | |||
| Nonsmall cell not otherwise specified | 4.0 | 1.9–2.7 | ||
| Melanoma | Any | 6.0 | 2.8–3.0 | 10 |
| Sarcoma | Any | 4.0 | ||
| Thyroid | Thyroid | 5.0 | ||
| All others | All others | 3.0 |
Abbreviations: GPA, Graded Prognostic Assessment; HER2, Human Epidermal Growth Factor receptor 2; HR, Hormone Receptor; SEER, Surveillance Epidemiology and End Results.
aEmpty cells reflect missing data.
bFor patients with brain metastases at the time of diagnosis of primary malignancy.
cRange reflects estimates for synchronous (present at diagnosis of systemic malignancy) and metachronous (developed after diagnosis of systemic malignancy) brain metastases; limited to patients ≥65 years of age.
Epidemiological studies of brain metastases are essential to characterize evolving trends in incidence, identify at-risk populations, inform screening paradigms, guide treatment strategies, and facilitate trial design. Efforts should be made to collect this data more routinely in national and state registries.
Biology and Molecular Pathogenesis
Pathogenesis
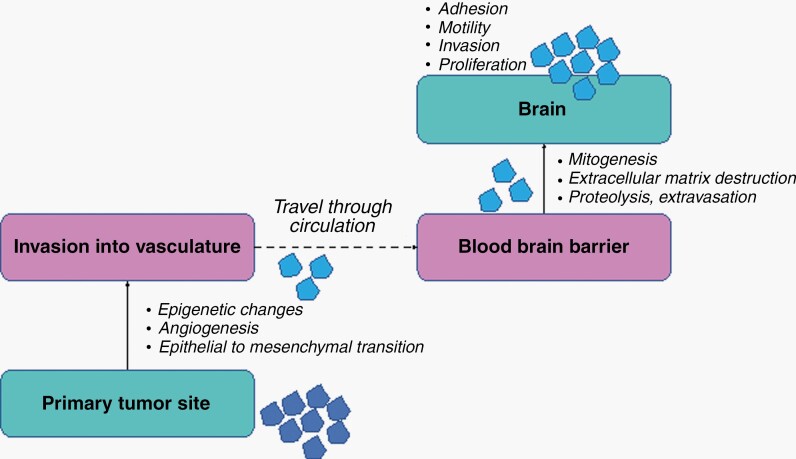
Multiple theories regarding the pathophysiology of intracranial metastatic disease have been postulated. Cancer cells spread as a result of epigenetic and proliferative changes, including growth of preexisting or development of new blood vessels,32,33 followed by vascular invasion.34 Cancer cells reaching the brain must traverse the blood–brain barrier (BBB) and adhere to brain endothelia potentially via upregulation of genes related to mitogenesis and extracellular matrix destruction, such as vascular endothelial growth factor and matrix metalloproteinases, activation of signaling pathways that permeabilize the BBB, and increased expression of proteins allowing for proteolysis, extravasation, and tumor cell colonization.4,33,35–37 Once inside the brain, tumor cell interactions with brain endothelia can promote adhesion within the intracranial parenchyma, via upregulation of particular cell surface proteins and growth factors.38,39 Finally, complex interactions between the tumor cells and brain cells, including formation of tumor-astrocyte gap junctions and subsequent secretion of inflammatory chemokines, promote tumor cell motility, invasion, and survival (Figure 2).4 Examples of specific metastasis–brain interactions underlying the pathogenesis of brain metastases demonstrated via in vitro and mouse models include estradiol-induced activation of brain-derived neurotrophic factor in astrocytes interacting with TrkB receptors on breast tumor cells, synaptic formation between cancer cells and glutamergic neurons allowing for N-methyl-D-aspartate receptor (NMDAR) activation, loss of PTEN expression among cancer cells once in the brain microenvironment, driven by signals from resident astrocytes and leading to chemokine-mediated proliferation of the metastatic cells, and cancer cell cooption of a pro-metastatic program driven by STAT3 in reactive astrocytes.40–43 Targeting of these pathways offers substantial promise in inhibiting the successful proliferation of brain metastases.
Fig. 2.
Pathogenesis of brain metastases. The development of brain metastases depends on a complex interplay of factors involving tumor cells migrating from an extracranial site into the vasculature via a series of epigenetic changes, proliferation of blood vessels, and an epithelial-to-mesenchymal transition. Once the tumor cells reach the brain, they traverse the blood brain barrier, which involves upregulation of genes and proteins involved in proteolysis, extracellular matrix destruction, and mitogenesis/growth. Once inside the brain, the cells must adhere to the brain endothelia and undergo further stimulatory processes to allow for proliferation.
Although a similar set of processes must occur regardless of primary tumor type, studies have implicated distinct genes involved with brain metastasis formation by primary site. For example, cyclooxygenase 2 (COX2), epidermal growth factor (EGFR) ligand HBEGF, and the membrane glycosyltransferase ST6GALNAC5 facilitate the development of breast cancer brain metastases, while lymphoid enhancing-binding factor 1 (LEF1), Cadherin 2, and Kinesin Family Member C1 (KIFC1) are strongly associated with lung cancer brain metastases.33,38,44,45 Activation/alteration of the PI3K/AKT and cyclin-dependent kinase (CDK) pathways have also been associated with increased risk of multiple tumors to metastasize to the brain.10,46–50 Copy number changes may also lead to the formation of brain metastases; for example, amplifications in YAP1 and MMP13 contribute to brain metastases in lung adenocarcinoma.50 Characterization of the site-specific genes that must be upregulated for brain metastasis formation may allow for novel therapeutic strategies to prevent development of intracranial disease. However, further exploration is needed to determine the relationship between molecular changes, intracranial involvement/outcomes and specificity for driving brain metastases or metastatic disease in general.
Heterogeneity
Recent data has demonstrated genomic heterogeneity between brain metastases and respective primary tumors, suggesting that specific transformations allowing cells to metastasize to the brain occur, and moreover, that genomic diversity of brain metastases may contribute to occasionally differing intracranial and extracranial responses to systemic therapy.10,51 The significance of genetic heterogeneity between primary/extracranial and intracranial sites of disease is an area of active clinical investigation.
Non-small cell lung cancer
Molecular characterization is vital to the management of patients with lung adenocarcinoma. In contrast, among patients with SCLC or squamous NSCLC, molecular studies may be less impactful due to a far lower incidence of targetable mutations.52,53 In patients with lung adenocarcinomas, activating EGFR mutations are present in approximately 15–20% and 40–50% of Caucasian and Asian patients, respectively, with most patients harboring EGFR L858R point mutations or exon 19 deletions targetable by EGFR tyrosine kinase inhibitors (TKI).54 While some less-common mutations also are sensitive to classical EGFR TKIs, others, such as most EGFR exon 20 insertion mutations, are resistant.55 Most patients receiving older (1st and 2nd generation) EGFR TKIs eventually acquire resistance through development of the exon 20 point mutation T790M; however, multiple other on-target and off-target resistance mechanisms exist.56 A recent Phase III study and post hoc analyses from two Phase II studies assessing intracranial response to osimertinib, a third-generation TKI with coverage of T790M, demonstrated high intracranial response rates in patients with, at least, extracranial evidence of T790M manifesting on prior 1st and 2nd generation TKI therapy.57,58 Mechanisms of resistance to osimertinib include both on-target EGFR tertiary mutations/amplifications, such as exon 20 C797S, as well as off-target/second driver mechanisms, such as RAS-MAPK or PI3K pathway activation, MET amplification, HER2 amplification, small-cell transformation, and RET or ALK gene rearrangements.59
Less common than EGFR mutations are anaplastic lymphoma kinase (ALK) rearrangements, which are found in 4–5% of all NSCLC patients and confer sensitivity to ALK TKIs.60,61 Beyond EGFR and ALK-related changes, approximately 25–30% of patients with lung adenocarcinomas harbor a mutation/rearrangement/fusion in a potentially targetable abnormality, including ROS1 rearrangements, MET mutations/amplifications, RET rearrangements, HER2 mutations, BRAF V600E mutations, NTRK rearrangements, and KRAS G12C mutations.62 In addition to broad-based genetic testing, an assessment of PD-L1 status and potentially other markers of immunotherapy-responsiveness are essential for optimizing treatment choice for patients with NSCLC, especially for those lacking a targetable abnormality.
Breast cancer
The risk of intracranial dissemination among patients with breast cancer varies by subtype, with lower rates observed in hormone receptor-positive/HER2-negative disease and higher rates noted in HER2-positive or triple negative breast cancer.41 Of note, receptor status can change between extracranial and intracranial sites.63,64 In a meta-analysis of 29 studies assessing receptor conversion from primaries to paired distant sites, estrogen-receptor discordance was notably high among brain metastases at 20.8%.65 In addition, HER2-positivity can be gained or lost, with significant associated therapeutic impact.
Melanoma
Several studies have implicated activation of the PI3K-AKT pathway in melanoma brain metastases. An initial protein-based interrogation of signaling pathways identified increased expression of activation markers in the PI3K-AKT pathway in melanoma brain metastases compared to extracranial sites.66 Subsequently, analysis of BRAF mutations, NRAS mutations, and loss of PTEN (which results in activation of the PI3K-AKT pathway) in stage III patients showed that PTEN loss predicted increased risk of brain metastasis, a finding also seen in the RCAS-TVA mouse model of melanoma.49 Another preclinical study demonstrated that PI3K-AKT pathway activation is required for early colonization of the brain by melanoma cells.67 Two additional studies analyzing melanoma patients with paired extracranial and intracranial tissue identified increased activation of the PI3K-AKT pathway in all brain metastases.68,69 More recently, global analysis of gene expression in brain metastases by RNAseq demonstrated increased expression of genes in the oxidative phosphorylation (OXPHOS) metabolic pathway and decreased infiltration by multiple immune cell populations, including T cells and B cells, compared to patient-matched extracranial metastases.70 Notably, this metabolic change in confirmatory preclinical models was observed with cells that were directly injected into brain tissue, thus precluding significant selective pressure as an explanation, and instead suggesting that induction of OXPHOS may stem from interactions of tumor cells with the tumor microenvironment of the central nervous system (CNS). Consistent with this hypothesis, analysis of brain metastases and patient-matched non-CNS tumors in breast, lung, and kidney cancer cohorts also identified increased OXPHOS, increased PI3K-AKT activation, and decreased immune infiltrates in brain metastases.71 Another study also demonstrated unique metabolic features and dependencies of brain metastases from melanoma and breast cancer, namely upregulation of 3-phosphoglycerate dehydrogenase (PHGDH), the rate limiting enzyme for glucose-derived serine synthesis, to compensate for the very low levels of nucleotides available in the brain tumor microenvironment.72 Importantly, inhibitors of OXPHOS and PHGDH exhibited greater anti-tumor activity against brain metastases than against metastases in extracranial sites.70–72
The PI3K-AKT pathway in brain metastases has also been implicated in other studies. Preclinical studies have demonstrated that chemokine receptor 4 (CCR4) is overexpressed in melanoma cells that metastasize to the brain via increased activity of the PI3K/AKT pathway.73 Further, whole exome sequencing of brain metastases across primary sites identified new mutations in the PI3K-AKT pathway in 40–50% of patients.10 However, such frequent differences were not seen in studies comparing brain metastases to extracranial metastases.10,74 Both global and targeted sequencing have confirmed high concordance for BRAF mutations between brain metastases and non-CNS tumors. Interestingly, increased OXPHOS metabolism, increased PI3K-AKT pathway activation, and decreased immune infiltration have all been implicated in decreased responsiveness to BRAF and MEK inhibitors.75–79 Each of these pathways/features has also been implicated in resistance to anti-PD-1-based immunotherapy.80,81
Tumor Sampling and Future Directions
Although the data presented above demonstrates the promise of tailoring systemic therapies for patients with brain metastases based on molecular characterization of intracranial disease, one of the inherent challenges is obtaining tissue. However, non/less-invasive methods that allow for genomic profiling of cancers, such as the utilization of circulating tumor DNA (ctDNA), may prove promising,82 although brain-derived, as opposed to extracranially-derived, plasma ctDNA levels can be low.83,84 Cerebrospinal fluid (CSF) offers potential regarding detection of relevant intracranial mutations via ctDNA analyses,83,85,86 suggesting the utility of CSF-ctDNA for identification of potential genetic targets and mechanisms of resistance, but few institutions can perform CSF-based molecular analyses in a reliable and reproducible manner; development and implementation of such assays has significant potential to advance care. In addition, imaging measures to noninvasively assess molecular status in patients with brain metastases carry promise, such as the use of HER2-targeting PET tracers in breast cancer.87,88
Imaging
Background
Imaging represents an essential component of the diagnosis and management of brain metastases, as brain tumor-directed biopsies are typically not indicated. For certain patients at high risk of developing brain metastases, such as those with SCLC, advanced NSCLC, and advanced melanoma, the initial oncologic work-up includes a screening brain MRI.89,90 For most other primary disease sites, intracranial imaging is often reserved for the setting of neurologic symptomatology. While MRI is preferred, computed tomography (CT) may be initially performed emergently to exclude acute changes.91,92
For optimal evaluation of brain metastases, MRIs should incorporate IV gadolinium-based contrast. Lesion conspicuity/detection can be enhanced using stronger magnetic fields (3T vs 1.5T), contrast agents with greater relativity, higher contrast doses, delays between injection and image acquisition, and use of T1-weighted postcontrast imaging with thin sections/volumetric imaging.93,94 While inversion recovery gradient echo pulse sequences like MPRAGE give exquisite anatomic detail and spatial resolution, with 1 mm isotropic voxels very achievable, postgadolinium 3D T1-weighted fast spin echo pulse sequences like SPACE, CUBE, or VISTA may be superior for detection of small metastases.95–99 Further details on consensus-recommended imaging for brain metastases, both for clinical trials and for routine clinical use, have recently been published.95
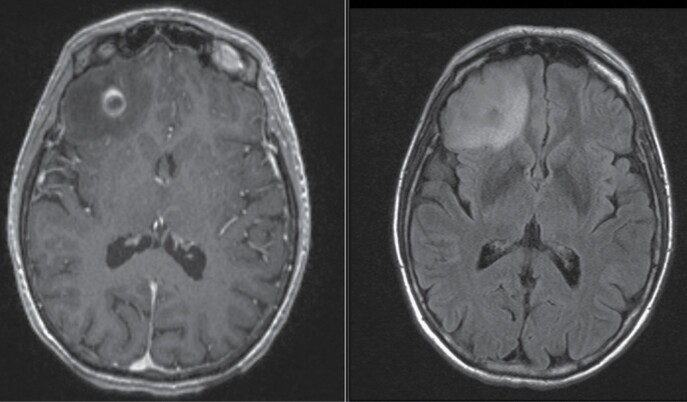
Brain metastases commonly appear as well-demarcated, contrast-enhancing lesions at the subcortical gray–white junction; peritumoral vasogenic edema is commonly present.93,100 Typically, brain metastases appear iso- or hypointense on precontrast T1-weighted images; however, the presence of associated hemorrhage, common in melanoma and renal/ovarian/thyroid primaries, can yield hyperintensity on T1- and T2-weighted images, and loss of signal on T2*- or susceptibility-weighted images (Figure 3).93,95,100 As lesions grow, they may become centrally hypointense on T1, with enhancement surrounding a nonenhancing core (ie a “cystic” metastasis); in other lesions, enhancement often remains homogeneous (ie a “solid” metastasis).101,102 Cystic versus solid designation can affect radiosensitivity/response to oncologic treatment.103,104 It is important to delineate cystic from necrotic brain metastases as the response-based principles above may not translate to necrotic lesions. In this regard, cystic metastases display thin/smooth ring enhancement with a uniformly spherical or elliptical T1 hypointense center, while necrotic lesions have irregular enhancing walls with non-uniform/irregular centrally T1 hypointense regions; such delineations can be challenging however. Also of note, brain metastases from SCLC, as well as occasional lesions from other underlying primaries, can restrict diffusion, with relatively low apparent diffusion coefficients (ADC), due to densely cellular histologies.93,105
Fig. 3.
Characteristic MRI of a brain metastasis. T1-weighted postgadolinium MRI of a right frontal brain metastasis displaying characteristic rim enhancement (left) and associated T2-weighted FLAIR sequence showing extensive surrounding vasogenic edema (right). (Abbreviations: FLAIR, Fluid Attenuated Inversion Recovery; MRI, Magnetic Resonance Imaging).
Differential Diagnosis
Multiple intracranial lesions
The presence of multiple, enhancing intracranial lesions in a patient with a preceding cancer diagnosis should raise concern for brain metastases. Yet, mimickers of brain metastases exist, including primary CNS neoplasms such as multicentric glioma and CNS lymphoma, infection/abscess (eg fungal/atypical infections, septic emboli), vascular disease, and/or inflammatory processes such as multiple sclerosis, acute disseminated encephalomyelitis, or sarcoidosis.106 Like brain metastases, abscesses can also be rim-enhancing. However, unlike most brain metastases, the central, nonenhancing, necrotic portion of abscesses tends to restrict diffusion107,108; cystic brain metastases may have restricted water diffusion in their relatively hypercellular walls but typically not centrally. A history/exam can also be useful in distinguishing these entities. Subacute ischemic infarcts can also mimic brain metastasis since infarcted tissues frequently begin to enhance following the acute phase.109 Infarction with enhancement can often be distinguished from metastasis by its wedge-like (nonnodular) shape involving white matter and often overlying cortex, and the lack of surrounding vasogenic edema in the acute phase. Surveillance imaging can often differentiate infarct (enhancement regresses with time) and tumor (enhancement increases with time without effective treatment).
Single intracranial lesion
Approximately 20–40% of patients with brain metastases present with a single intracranial lesion,32 and brain metastases should remain high on the differential diagnosis for patients with a single/solitary focus of enhancement in the setting of a known extracranial primary. Other etiologies to consider, however, include primary CNS neoplasms, such as gliomas, primary CNS lymphomas, meningiomas, abscesses, and vascular malformations.
Follow-up Imaging Regimens
Patients with brain metastases require close radiographic follow-up. Generally, it is recommended that brain MRIs be performed every 2–3 months for the first 1–2 years after initial treatment, although new/worsening symptomatology or a history of rapid disease progression should prompt earlier scans; continuing brain MRIs regularly beyond 1–2 years after initial treatment seems prudent and patients with active disease or necrosis well beyond the initial treatment period often require very close radiographic follow-up long-term.110 In addition, the high resolution imaging provided by 3D T1-weighted postcontrast sequences allows for assessments of systemic therapy efficacy at an earlier timepoint, often within a few weeks of initiation of a new regimen, facilitating earlier implementation of salvage therapy if necessary.111
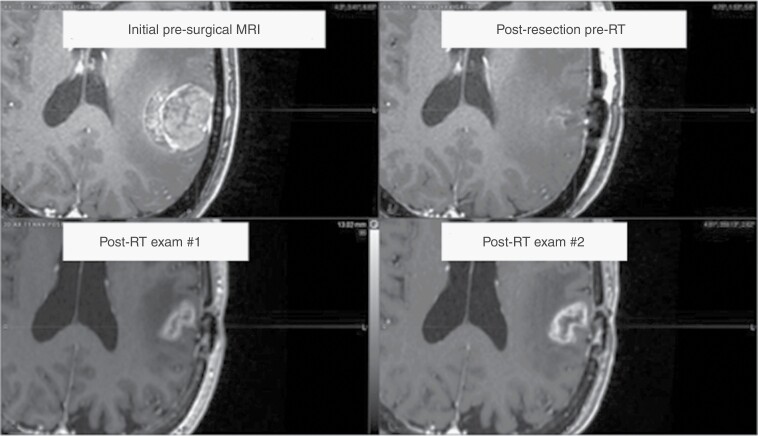
Imaging after stereotactic radiation therapy may show adverse radiation effects, which can be challenging to differentiate from tumor progression (Figure 4). In these situations, advanced imaging techniques, such as magnetic resonance spectroscopy (MRS), Dynamic Susceptibility Contrast (DSC) and Dynamic Contrast Enhanced (DCE) perfusion MRI, treatment response assessment maps/contrast clearance imaging, and FDG (Fluorodeoxyglucose) and amino acid positron-emission tomography (PET), among others, can be considered, or closer interval imaging may be warranted.112–118 However, the Response Assessment in Neuro-Oncology Brain Metastasis group states that while advanced imaging techniques may provide value, the current medical literature is “insufficiently robust” to routinely recommend any one particular modality or approach.119 In clinical practice, serial routine imaging, as well as correlation with a patient’s clinical status, are often relied upon prior to performing such specialized imaging.
Fig. 4.
MRI-based appearance of radiation necrosis. A left parietal metastasis is shown prior to resection (top left) and postresection, preadjuvant stereotactic radiation (top right). Five years later, the patient developed enhancement at the treated site (bottom left), which enlarged with time (bottom right). The patient was taken to the operating room for resection, and the lesion proved to be radiation necrosis. (Abbreviations: MRI, Magnetic Resonance Imaging).
Neuro-oncologic Management
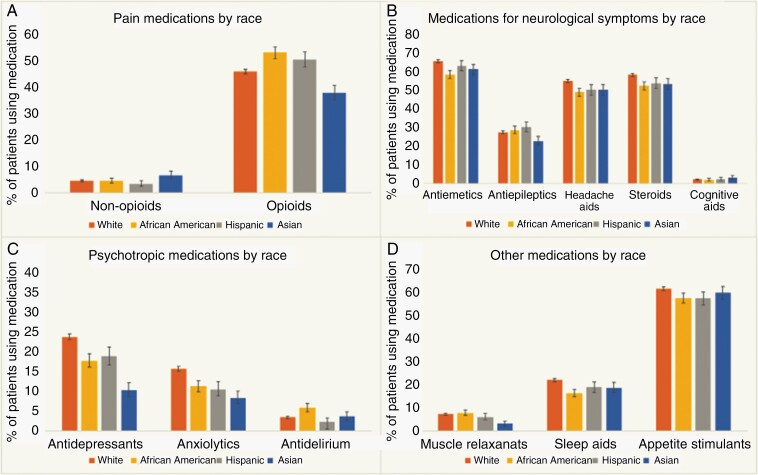
Neuro-oncologic management of patients with brain metastases is multi-faceted.120,121 Patients with brain metastases are often affected by significant neurologic symptomatology from both underlying intracranial disease as well as treatment-related sequelae.122–125 In addition, metastases may differentially impact patients neurologically based on their location, with the motor strip, brainstem, and thalamus being especially sensitive to the impact of brain metastases. Such symptomatology often necessitates careful medication management, including steroids, antiepileptic drugs, analgesics, and other supportive medications (Figure 5).126–129 Here, we focus on common brain metastases-related neuro-oncologic issues, including symptomatic vasogenic edema, seizures, venous thromboembolism, radiation necrosis, and neurocognitive decline.130,131
Fig. 5.
Supportive medication utilization among patients with brain metastases. Retrospective data from a population-based study of 17 957 patients with brain metastases demonstrating the high prevalence of supportive medication use in the first 30 days following a diagnosis of brain metastases (as stratified by race and medication class). Opioids, anti-emetics, headache aids, and appetite stimulants were among the most frequently utilized medications among this patient population. Reproduced with permission from Lamba et al., Neuro-Oncology, 2020.126
Vasogenic Edema
Systemic glucocorticoids play an integral role in the management of patients with symptomatic brain metastases.132–135 Dexamethasone is typically preferred given its relative lack of mineralocorticoid activity.136 For asymptomatic patients, prophylactic corticosteroids are usually not indicated,137 although in patients receiving potentially edema-exacerbating local therapy, short-term preventative corticosteroids are reasonable.138 For moderately symptomatic patients, dexamethasone in the 4–8 mg/day range given once or twice daily (eg with breakfast and lunch) seems appropriate, consistent with prior randomized studies suggesting that the therapeutic benefit of dexamethasone wanes beyond 4–8 mg/day while toxicity increases somewhat linearly.139 For patients with marked symptomatology, mass effect, elevated intracranial pressure, and/or impending herniation, higher doses of dexamethasone (eg 16 mg/day) may be warranted.140 Corticosteroids therapy duration should be minimized to prevent long-term sequealae141 and generally should be tapered rather than abruptly discontinued given the gradual improvement of edema with oncologic therapy and the potential for adrenal insufficiency in patients having received corticosteroids for prolonged periods.142 For patients requiring prolonged steroid courses (typically >4 weeks), consideration of prophylaxis against pneumocystis jirovecii pneumonia with trimethoprim/sulfamethoxazole, atovaquone, or pentamidine may be prudent; prophylaxis against steroid-mediated gastritis with a proton-pump inhibitor may also be appropriate.143 Although studies are mixed regarding steroid-mediated inhibition of immunotherapy efficacy, more recent data suggests potential for concern, particularly in patients with brain metastases.144–147 As such, it seems prudent to restrict/minimize steroids among patients receiving immunotherapy where possible, as even doses of dexamethasone ≤4 mg/day have been associated with worse outcomes.146,148–150
Seizures
Seizures reflect a complication associated with both brain metastases, local treatment, and occasionally systemic therapy.151,152 Approximately 10–20% of patients with brain metastases present with seizures at diagnosis of intracranial involvement.153 In addition, population-based and institutional data indicate that approximately 10–11% of patients free from seizures at diagnosis subsequently develop seizures, with greater risk noted in patients with melanoma and/or a larger burden of untreated supratentorial disease.152 Current guidelines suggest that anti-seizure medications should not be used as primary prophylaxis among patients with brain metastases.154–156 These guidelines are based on a limited number of randomized studies typically involving smaller (N ≤ 100), heterogeneous cohorts of patients with brain tumors managed with older anti-seizure medications that did not identify significant differences between patients randomized to anti-seizure medications versus not.157,158 Whether primary prophylactic anti-seizure medications reduce seizure development among certain high-risk subgroups is not clear and should be explored in randomized studies. A potential exception to the guideline of avoiding prophylactic anti-seizure medications exists in the shorter-term among patients undergoing local therapy with epileptogenic potential, such as stereotactic radiation, neurosurgical resection, and laser interstitial thermal therapy (LITT), although data are conflicting.138,159–163
When anti-seizure medications are utilized, agents that do not significantly impact hepatic metabolizing enzymes, such as levetiracetam, lacosamide, or lamotrigine are generally preferred. There is no compelling evidence to select one drug over another.164 Patients with seizures, particularly if recent or uncontrolled, should not drive; specific laws regarding seizures and driving vary by region. In addition, factors that stimulate seizure development should be minimized/managed. In this regard, sleep hygiene, avoidance of drugs/alcohol, and minimization of stress are prudent; medically, metabolic derangements should be addressed, intracranial pressure should be controlled, and growing intracranial tumors should be definitively managed.165–167
Venous Thromboembolism
Patients with advanced malignancy commonly develop venous thromboembolism (VTE) with some studies suggesting an especially high risk of VTE among patients with brain metastases, potentially due to mobility issues or overlap between the tumor types with propensity to both spread to the brain and increase the likelihood of VTE.168,169 Anticoagulation with either low-molecular weight heparin or direct oral anticoagulants constitutes the mainstay of management for patients with cancer and VTE, although concerns regarding intracranial hemorrhage (ICH) exist in patients with brain metastases.170–173 No dedicated trials randomizing patients with brain metastases to anticoagulation versus not have been published and few prior retrospective studies on this topic exist. In addition, published studies evaluating ICH-risk in patients with brain metastases receiving anticoagulation versus not are subject to selection bias, which may account for the general conclusion that anticoagulation is safe in such patients.174–178 A recent study by Wood et al., however, used a propensity score-based matching algorithm, as well as multivariable modeling, and a careful pre versus postanticoagulation analysis to demonstrate an association with modestly increased ICH-risk in patients with brain metastases who receive anticoagulation, particularly among those with melanoma or prior intracranial bleeds; anticoagulation should be used cautiously in such patients.179 Ultimately, until prospective studies are conducted, the indication for anticoagulation should be carefully weighed against the risk of ICH in patients with brain metastases when deciding whether to employ anticoagulation versus not.
Radiation Necrosis
A subset of patients undergoing radiosurgery will develop adverse radiation effects, most commonly radiation necrosis, which involves inflammation or injury to the brain. Rates of radiation necrosis, although variable, range from 0% to 30% across studies; the range reflects variation in the definition of radiation necrosis and whether neurologic symptomatology is present versus absent.180–185 The initial management of imaging changes related to radiation effects often entails observation given that progression of radiation-related changes typically ceases, sometimes before symptoms develop. In the setting of radiation necrosis, the first-line therapy in symptomatic patients involves glucocorticoids, although prolonged courses/tapers are often required. If corticosteroids prove unsuccessful in stabilizing radiation necrosis or yield unacceptable side effects, definitive therapy such as neurosurgical resection and/or laser interstitial thermal therapy (LITT) can be employed.185–188 Another approach is bevacizumab, which is supported by two small randomized studies including a smaller, 14 patient crossover study randomizing patients with radiation necrosis to bevacizumab 7.5 mg/kg every 3 weeks (for two initial doses, with two additional doses administered if benefit was seen) versus placebo. All patients in the intervention arm, as well as control patients who subsequently crossed over to the intervention arm, displayed improvement of both imaging findings and symptomatology.189 A larger study of bevacizumab (5 mg/kg every 2 weeks for 4 doses) versus methylprednisolone (500 mg IV daily for three days followed by a prednisone taper for approximately 2 months) for radiation necrosis developing after treatment for nasopharyngeal cancer showed better radiographic/clinical control with bevacizumab than corticosteroids.190 Practical limitations of bevacizumab include diagnostic uncertainty in delineating necrosis from tumor progression (corticosteroids may be appropriate for either scenario; bevacizumab is often only appropriate for necrosis, although this varies by underlying primary), toxicities of bevacizumab, and concerns regarding wound healing should patients require resection. Other, less-common management approaches for radiation necrosis include anticoagulation, hyperbaric oxygen, vitamin E, and pentoxifylline, but robust supporting data are lacking.191–193 Ultimately, further prospective studies evaluating therapeutic options/sequencing for radiation necrosis are warranted.
Neurocognitive Decline
Decline in neurocognitive function (NCF) occurs in up to 90% of patients with brain metastases,194 affecting quality of life by interference with job function, relationships, motor vehicle operation,195 and self-care.196–198 Brain metastases can directly cause NCF-deficits but side effects of treatments including resection,199 radiation,200 chemotherapy,201 and immunotherapy202 also contribute significantly.203
Treatments for cognitive symptoms in patients with brain metastases have typically been conducted in patients with different types of brain tumors, with mixed results.204 An acetylcholinesterase-inhibiting medication (donepezil) has been evaluated in various brain tumor populations, though only one study included a substantial proportion of brain metastases.205 Administered after radiation, slight benefits on one metric of recognition memory compared with placebo were noted, suggesting efficacy for patients with a specific pattern of cognitive impairment (eg recent memory). Studies of agents that enhance attention (methylphenidate) or wakefulness (modafinil) in brain tumor patients also included few patients with brain metastases. Early single-arm open-label studies suggested some improvement in attention,206 but randomized placebo-controlled trials found no improvement in fatigue.207 Memantine is reviewed in the radiation therapy section. Cognitive rehabilitation, a nonpharmacological intervention, is well-established in patients with brain tumors208,209 and involves development of compensatory strategies and “cognitive exercise” paradigms; such approaches have shown positive results.210,211
As treatments for cancer continue to improve survival, simultaneous advances in the prevention and management of neurocognitive deficits are important. Further refinements in radiation techniques (eg sparing the genu of the corpus callosum [NCT03223922] and using SRS for up to 15-20 brain metastases [NCT03075072, NCT03550391]) are being investigated, as are trials of novel neuroprotectant agents (eg porphyrin BMX-001 [NCT03608020] and Ganglioside-Monsialic Acid [NCT04395339]) and neuroplasticity techniques.212
Surgical Resection and Laser Interstitial Therapy Therapy
Surgical resection plays an important role in the management of patients with brain metastases. Standardly-accepted indications for craniotomy include: (1) diagnostic uncertainty, based on imaging, where observation is not viable, (2) brain metastases causing symptoms refractory to steroids, (3) bulky metastases (typically >3–4 cm in maximal unidimensional size), and (4) solitary brain metastases (ie one brain metastasis in the absence of extracranial disease).213,214 Considerations regarding diagnostic uncertainty are provided in the imaging section. Among most patients with neurologic symptoms caused by brain metastases despite the use of steroids, neither radiation nor systemic therapy can reliably and quickly decompress affected areas and improve symptomatology before more permanent sequelae/decline develop. Consequently, surgery is typically indicated. However, it should be noted that for radiosensitive tumors such as SCLC, select germ cells tumors, select liquid malignancies, and Merkel cell carcinoma, radiation in lieu of surgery can be considered. Similarly, for select cancer subtypes with a known, targetable driver mutation for which effective CNS-penetrant systemic therapies exist, such as EGFR-mutant NSCLC, ALK-rearranged NSCLC, or BRAF-mutant melanoma, systemic therapy, with initial deferral of surgery and close monitoring, may prove to be viable.215–217 For patients harboring bulky tumors, the stereotactic radiation dose that can be safely administered is limited due to constraints imposed by the surrounding brain,181,218 often compromising control; therefore, surgery is typically indicated. Lastly, among patients with solitary brain metastases, neurosurgical resection may prove helpful, as retrospective/hypothesis-generating evidence suggests associations between combined modality local therapy (ie resection and cavity radiation) and longer-term survival relative to stereotactic radiation alone even among small foci of disease, although prospective studies are lacking.214
Three randomized studies have assessed the role of neurosurgical resection in addition to WBRT versus WBRT alone in patients with a single brain metastasis (Table 3).6,219–221 Two of the three studies showed an overall survival advantage with neurosurgical resection; subset analyses of these studies suggested that the benefit associated with neurosurgical resection may be most significant among patients with stable/controlled extracranial disease given a lower competing risk.6,219–221 In the modern era, adjuvant WBRT has been largely replaced by adjuvant stereotactic radiosurgery for patients with a limited number of brain metastases.222–225
Table 3.
Randomized Studies Comparing Neurosurgical Resection + Whole Brain Radiation Relative to Whole Brain Radiation Alone in the Management of a Single Brain Metastasis
| Study | Years of Enrollment | N | Arms | Local Recurrence | Overall Survival | Functional Status |
|---|---|---|---|---|---|---|
| United States6 | 1985–1988 | 48 | WBRT + biopsy vs WBRT + surgery | Surgery better | Surgery better | Surgery better |
| Dutch213 | 1985–1990 | 66 | WBRT +/− surgery | N/A | Surgery better | Trend to favoring surgery |
| Canadian215 | 1989–1993 | 84 | WBRT +/– surgery | N/A | No difference | No difference |
Abbreviations: N, Number; N/A, Not Applicable; WBRT, Whole Brain Radiation Therapy.
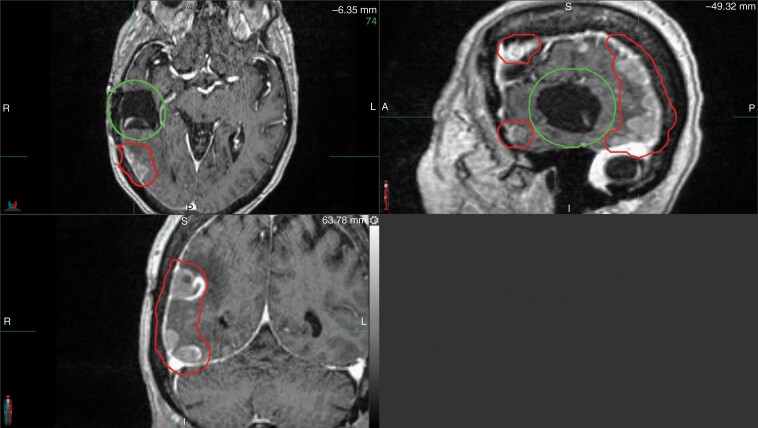
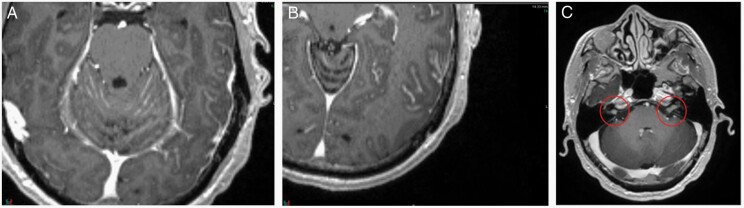
One adverse effect associated with craniotomy is pachymeningeal seeding (also called nodular leptomeningeal disease), a phenomenon in which tumor cells dispersed by surgical intervention recur along the pachymeninges beyond the adjuvant stereotactic radiation field (Figure 6).226,227 Pachymeningeal seeding after resection was not commonly seen in the adjuvant WBRT era given the potential for WBRT to control micrometastatic disease. In the postoperative stereotactic era, however, approximately 6–12% of craniotomies are complicated by pachymeningeal seeding.226 Multiple publications have now described this phenomenon.226,227 Unlike classical leptomeningeal disease, hydrocephalus requiring CSF diversion is uncommon in pachymeningeal seeding and either WBRT or stereotactic radiosurgery can be employed as salvage (as pachymeningeal seeding does not involve the CSF), with little role for intrathecal chemotherapy given the penetration concerns into lesions greater than several millimeters.228 Conversely, in classical leptomeningeal disease, imaging typically shows linear subarachnoid deposits along cranial nerves, cerebellar folia, supratentorial sulci, and/or ventricular surfaces (Figure 7).229 In addition, hydrocephalus risk is greater, and WBRT/intrathecal chemotherapy can be considered while there is little role for stereotactic radiation. Consequently, clinical/radiographic delineation of pachymeningeal seeding from classical leptomeningeal disease is critical (Table 4).
Fig. 6.
Pachymeningeal seeding. Pachymeningeal seeding after neurosurgical resection of a brain metastasis. Note the multifocal pachymeningeal recurrences (red) occurring in the absence of a cavity recurrence (green).
Fig. 7.
Classical leptomeningeal disease. Classical leptomeningeal disease as noted by linear enhancement along the cerebellar folia (A), supratentorial sulci (B), and cranial nerves VII/VIII bilaterally (C, red circles).
Table 4.
Etiology, Natural History, and Management of Pachymeningeal Seeding Relative to Classical Leptomeningeal Disease in Patients with Brain Metastases
| Pachymeningeal Seeding | Classical Leptomeningeal Disease | |
|---|---|---|
| Etiology | Postsurgical | Usually not surgical |
| Natural history | Likely reflective of a one-time event | Ongoing process |
| Imaging | Pachymeningeal/dural nodular recurrences often near surgical cavity and potentially in more distant sites | Linear subarachnoid deposits along cranial nerves, cerebellar folia, supratentorial sulci, and/or ventricular surfaces |
| Hydrocephalus risk | Usually not | Sometimes |
| Whole brain radiation utilized for management | Sometimes | Often |
| Stereotactic radiation utilized for management | Sometimes | Usually not |
| Intrathecal chemotherapy utilized for management | Usually not | Sometimes |
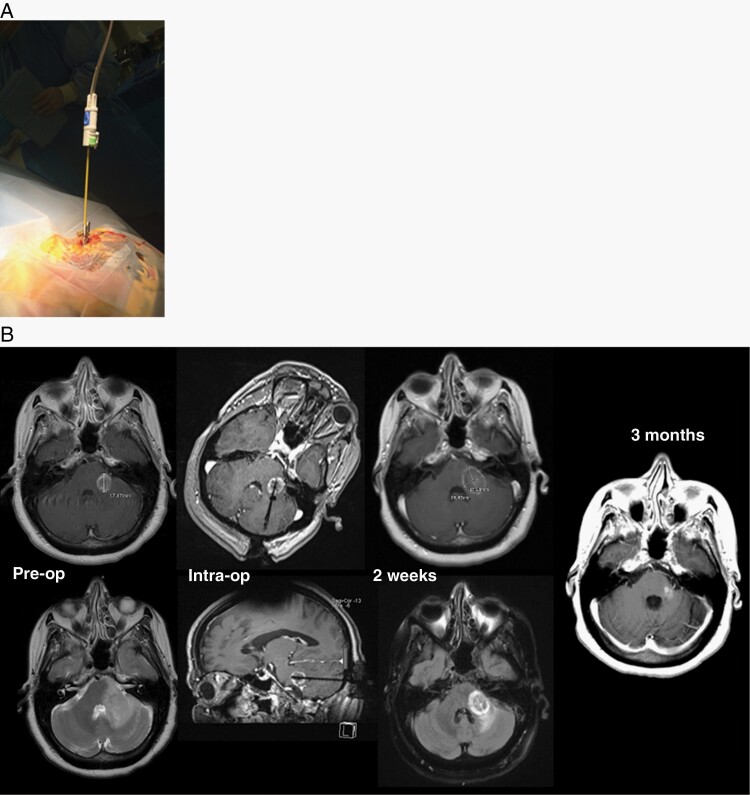
MRI-guided laser interstitial thermal therapy (LITT) is a minimally-invasive surgical technique with efficacy in treating brain lesions (Figure 8).230,231 LITT involves a 3–5 mm twist drill hole in the skull through which a catheter is navigated to the target; laser-derived thermal energy is used for ablation. The most established role of LITT is for radiation necrosis although LITT-based treatment for recurrent tumor after prior radiation is a consideration as well. A prospective, single-arm, multicenter study involving postradiation LITT demonstrated 12 week local progression-free survival of 100% versus 54% in patients with necrosis versus recurrent tumor.187 Neurological complications of LITT were seen in 7/42 patients, mainly relating to weakness/hemiparesis, neglect, headache, and hemorrhage. Consequently, among patients with radiation necrosis and perhaps recurrent tumor who are not candidates for other approaches, LITT can often achieve intracranial disease control with a reasonable toxicity profile.
Fig. 8.
Laser interstitial therapy. LITT technique showing stereotactic laser fiber insertion to the target through a skull anchoring bolt (A) and MRI-based assessments before, during, and after LITT procedure (B). In Part (B), images reveal: (left) T1 and T2-weighted MRI showing regrowth of brain metastasis 12 months after radiosurgery; (left middle) intraoperative images displaying laser inserted into lesion; (right middle) 2-week post-LITT MRI showing postlaser ablation lesion increased in size but FLAIR signal improved; (right) MRI revealing resolution of lesion at 3 months post-LITT. (Abbreviations: FLAIR, Fluid Attenuated Inversion Recovery; LITT, Laser Interstitial Thermal Therapy; MRI, Magnetic Resonance Imaging).
Future studies relating to surgical resection and LITT should investigate surgical techniques that may decrease the likelihood of pachymeningeal seeding. In addition, ongoing clinical trials exploring preoperative, rather than postoperative, stereotactic management and the risk of pachymeningeal seeding, among other outcomes, are being pursued (NCT03741673, NCT04422639, NCT04474925, NCT03750227). Immunogenic effects of LITT are being explored; in this regard, a study combining LITT with PD-1 inhibition is ongoing (NCT04187872). Finally, emerging techniques such as focused ultrasound may have potential applications for novel drug delivery, sonodynamic therapy, and immunomodulation (NCT04559685).232
Radiation
Radiation therapy represents the historical mainstay of therapy for patients with brain metastases given concerns regarding the limited intracranial efficacy of many systemic therapies. External beam radiation encompasses two primary forms of treatment: (1) WBRT with or without hippocampal avoidance and (2) stereotactic radiation. Nonstereotactic partial brain radiation is less commonly utilized and will not be reviewed.
Whole Brain Radiation Therapy
Historically, WBRT represented the primary management modality for patients with multifocal intracranial disease. An older, small randomized study in the pre-CT/MRI era suggested a 4-week survival advantage with WBRT over steroids alone.233 Subsequent seminal work in the 1990s established a role for adjuvant WBRT among patients with a single, resected brain metastasis, demonstrating a significant improvement in intracranial recurrence rates and neurologic death with surgical resection plus adjuvant WBRT compared to surgical resection alone.234 Although effective against both visible and microscopic intracranial disease, WBRT carries a significant short and long-term toxicity profile, including fatigue, anorexia, xerostomia, nausea, and alopecia in the short term, and cognitive dysfunction, balance problems, and hearing loss in the longer term.235,236 It is important to note that WBRT should be used cautiously in patients with a significant extracranial disease burden and limited systemic options given the decreased likelihood that intracranial management will impact prognosis, as seen in the QUARTZ study.237
Multiple strategies to mitigate the neurocognitive impact of WBRT exist. In RTOG 0614, memantine (used for 24 weeks including a 4-week uptitration period), an N-methyl-D-aspartate (NMDA) receptor antagonist, was evaluated against placebo in a randomized trial among patients receiving WBRT,238 with a trend towards preservation in delayed recall at 24 weeks (primary endpoint) and significantly longer time to any measure of cognitive decline noted among patients receiving memantine.238 Consequently, memantine serves as a useful adjunctive therapy for patients receiving WBRT.
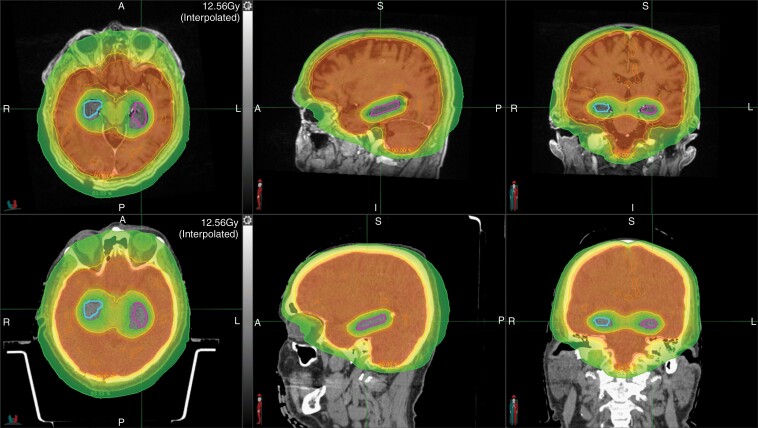
The observation that a potential driver of radiation-induced cognitive toxicity from WBRT, specifically memory loss, was dose deposition to neural stem cells within the subgranular zone of the hippocampal dentate gyrus239,240 led to development of hippocampal-avoidance WBRT (HA-WBRT), Figure 9.241–243 NRG CC001 demonstrated lower rates of cognitive failure with HA-WBRT and memantine as opposed to traditional WBRT and memantine, establishing HA-WBRT and memantine as standard in most patients devoid of brain metastases in/near (5 mm) the hippocampi or leptomeningeal disease.244,245 Of note, relapse rates near the hippocampus were comparable with 11 and 16 relapses in the HA region in the HA-WBRT and traditional WBRT arms, respectively, dispelling the concern that hippocampal avoidance would substantially increase the risk of peri-hippocampal recurrence.
Fig. 9.
Hippocampal-sparing whole brain radiation in a patient with brain metastases. The right and left hippocampi are contoured in blue and pink, respectively. The top and bottom panel shows the planning magnetic resonance imaging and computed tomography scans, respectively. The red, orange, yellow, and green dose-based shading depict the 33 Gy, 30 Gy, 27 Gy, and 16 Gy isodose lines, respectively.
Stereotactic Radiation Therapy
Stereotactic radiation therapy can be delivered as either a single fraction of highly conformal, high-dose treatment (stereotactic radiosurgery [SRS], generally 18–24 Gy) or as multiple, moderately-dosed fractions (stereotactic radiotherapy [SRT], commonly 20 Gy in 2 fractions, 24–27 Gy in 3 fractions, or 25–40 Gy in 5 fractions, also known as fractionated SRS), with a planning target volume (margin for uncertainty) of 0–2 mm.133 Of note, the nomenclature used to describe such treatment has varied in the literature; we will use the terminology/abbreviations noted above for consistency throughout the manuscript. Randomized studies of SRS alone versus SRS plus WBRT in patients with 1–4 metastases have been conducted (Table 5), collectively demonstrating higher intracranial failure (both locally and in the uninvolved brain) with SRS alone, but without an overall survival benefit with WBRT. Contemporary trials utilizing sensitive cognitive batteries have demonstrated worse neurocognition with the addition of WBRT.222–224,247 Based on these results, stereotactic radiation alone as initial treatment, with close MRI surveillance, salvage SRS/SRT for limited distant intracranial failure, and deferral of WBRT for widespread intracranial recurrence has become widely adopted for patients with limited intracranial metastases.
Table 5.
Randomized Trials Evaluating Local Treatment with/Involving Whole Brain Radiation Therapy vs Local Treatment Alone in the Management of Brain Metastases
| Study | N | Number of BM | Arms | Years of Accrual | Intracranial Recurrence with WBRT | OS with WBRT | QoL with WBRT | Neurocognition with WBRT |
|---|---|---|---|---|---|---|---|---|
| Patchell/multi-center246 | 95 | 1 | 1. Surgery 2. Surgery + WBRT |
1989–1997 | Lower | No difference | N/A | N/A |
| Japanese Radiation Oncology Study Group 99-1236 | 132 | 1–4 | 1. SRS 2. SRS + WBRT |
1999–2003 | Lower | No difference | N/A | Limited data |
| MD Anderson Cancer Center216 | 58 | 1–3 | 1. SRS 2. SRS + WBRT |
2001–2007 | Lower | Worse | No difference identified | Worse |
| European Organization for Research and Treatment of Cancer 22952217 | 359 | 1–3 | 1. Local Therapy 2. Local Therapy + WBRT |
1996–2007 | Lower | No difference | Trend to worse | N/A |
| North Central Cancer Treatment Group N0574218 | 213 | 1–3 | 1. SRS 2. SRS + WBRT |
2002–2013 | Lower | No difference | Worse | Worse |
| Japan Clinical Oncology Group 0504240 | 271 | 1–4 | 1. Resection + Salvage SRS 2. Resection + WBRT |
2006–2014 | Lower | No difference | N/A | Generally worse in some domains |
| Polish241 | 59 | 1 | 1. Resection + SRS 2. Resection + WBRT |
2011–2015 | No difference | Better | Mixed results | No difference |
| Alliance/North Central Cancer Treatment Group N107C242 | 194 | 1–4 | 1. Resection + SRS 2. Resection + SRS + WBRT |
2011–2015 | Lower | No difference | No significant difference | Worse |
| MD Anderson Cancer Center238 | 72 | 4–15 | 1. SRS 2. WBRT |
2012–2019 | – | No difference | – | Worse (possible trend) |
Abbreviations: N, Number; N/A, Not Applicable; OS, Overall Survival; QoL, Quality of Life; SRS, Stereotactic Radiosurgery; WBRT, Whole Brain Radiation Therapy.
Stereotactic Radiation Among Patients with >4 Brain Metastases
The lower rate of cognitive loss associated with SRS/SRT without a decrement in survival compared to WBRT led to interest in exploring stereotactic approaches for patients with >4 brain metastases. A large, multi-institutional observational experience of SRS in patients with up to 10 brain metastases suggested relatively comparable outcomes among patients receiving SRS for 2–4 as opposed to 5–10 lesions, although fewer than expected patients with 5–10 brain metastases enrolled, as noted by the authors, raising the possibility of selection bias.248 There are now multiple ongoing phase III studies comparing HA-WBRT to SRS among patients with >4 brain metastases (NCT03075072; NCT04277403; NCT03550391), all with potential to significantly impact care.
Recent data relating to immune checkpoint inhibitors and receptor tyrosine kinase inhibitors have demonstrated relatively high intracranial response rates in select subpopulations, often leading to an approach of deferring SRS/SRT in certain subpopulations. The broad justification for this approach stems from the contention that SRS/SRT may not improve survival in patients with >1 lesion,7 and that it can always be employed as salvage. This may be reasonable in some subsets, but prospective randomized data supporting this practice are lacking.
Adjuvant Stereotactic Radiation in Patients with Resected Brain Metastases
Among patients with resected brain metastases, the historical standard of care was to utilize adjuvant WBRT but randomized data now support the use of SRS/SRT, ideally within 4 weeks of surgery, in patients with a limited number of additional brain metastases.223,225,249–252 However, the 1-year cavity recurrence rate in prospective/randomized studies after surgery plus stereotactic radiation remains high, ranging from 28% to 40%; in contrast, cavity recurrence rates with adjuvant WBRT are substantially lower.223,225,234,249 This underscores the need for significant improvement in stereotactic approach/technique. In this regard, cavity SRS is being compared to cavity SRT in an ongoing randomized trial (NCT04114981). In addition, contouring guidelines may improve delineation of the target.253 Of note, pachymeningeal relapse can occur in a significant percentage of patients managed with adjuvant SRS/SRT, with impact on patient morbidity/mortality.226,254 Postoperative SRS/SRT is also associated with a relatively high rate of necrosis, given the typically large associated volumes, generous expansions, and interdigitation of the target with normal brain.254 Consequently, multiple prospective trials are evaluating the role of preoperative stereotactic radiation prior to resection of a brain metastasis (NCT03741673, NCT04422639, NCT04474925, NCT03750227) with the objective of decreasing recurrence, pachymeningeal seeding, and necrosis rates.
Stereotactic Radiation Therapy in Patients with Small Cell Lung Cancer
Historically, WBRT has been the mainstay of treatment for patients with SCLC and brain metastases given concern for widespread micrometastic intracranial disease. Patients with SCLC have been excluded from nearly all prospective evaluations of omission of WBRT.222–225,234,247 However, SRS/SRT-based outcomes in SCLC appear encouraging.255,256 As such, ongoing prospective trials (NCT03391362, NCT04516070, NRG CC009) are evaluating the viability of stereotactic approaches among patients with SCLC and a limited burden of intracranial disease. The treatment paradigm for brain metastases in patients with SCLC will likely continue to evolve as the results of ongoing trials become available.
Toxicities Associated with Stereotactic Radiation Therapy
Given the limited radiation fields/volume inherently associated with SRS/SRT, relative to WBRT, acute side effects secondary to SRS/SRT tend to be more modest in nature. However, the higher biologic dose of radiation utilized can lead to posttreatment inflammatory changes among other rare effects, as well as long-term adverse radiation effects, including radiation necrosis.163,257 Generally developing 3 months to 3 years after treatment,183,258 radiation necrosis (when confirmed histopathologically) involves inflammation or injury to the brain approximating the SRS/SRT site. Given the variation in SRS/SRT delivery patterns across institutions and inconsistent diagnostic criteria, the reported incidence of radiation necrosis after SRS/SRT varies considerably, ranging from 5% to 35% in various retrospective series; the wide range may reflect inconsistencies in the definition of radiation necrosis and that some investigators combine radiation necrosis with more transient, imaging-based adverse radiation effects.182,258–260 Factors predictive of radiation necrosis include a larger volume of brain receiving doses >10–12 Gy (V10/V12), prior brain radiation, use of concurrent systemic therapies including immunotherapy, tumor histology, and inherent radiosensitivity.181,261–263 In terms of location, the brainstem, thalamus, and optics display the greatest risk of radiographic to symptomatic conversion.261 The diagnosis and management of radiation necrosis is reviewed in the imaging and neuro-oncology sections, respectively.
Deciding Between Whole Brain Versus Stereotactic Radiation
Historically, the majority of patients with brain metastases received WBRT; upfront SRS/SRT has now become the predominant upfront strategy, especially for patients with limited intracranial disease.153 This shift has been motivated by cognitive dysfunction associated with WBRT, as measured by sensitive neurocognitive test batteries in prospective clinical trials.224,225
Other factors may influence the decision to proceed with SRS/SRT versus HA-WBRT/WBRT as well. In patients with extensive extracranial disease with effective therapeutic options, SRS/SRT permits a more rapid transiition back to systemic therapy.237 Geriatric patients may experience greater cognitive decline with WBRT/HA-WBRT, potentially supporting stereotactic approaches in this patient group.264 Patients with so called “radioresistant” tumors (eg melanoma) may benefit from SRS/SRT, due to greater fractional cell kill from higher-dose radiation delivered with each treatment. Conversely, in patients with significant intracranial disease burden driven by a larger number of brain metastases (eg >4 tumors based on published randomized data but particularly >10–20 tumors), as well as those with a greater brain metastasis velocity (BMV, ie the cumulative number of new brain metastases that develop over time after SRS/SRT),265 WBRT/HA-WBRT remains more standard, in part given the limited randomized data for SRS.222–225 Ultimately, until further prospective trials are published, such as NCT03075072 and NCT03550391, selection of SRS/SRT versus WBRT may depend upon the above factors as well as a nuanced patient-provider discussion.
Brachytherapy
Brachytherapy involves the intraoperative placement of radioactive isotopes within a resection bed, allowing for highly conformal delivery of high-dose radiation.266 While brachytherapy is uncommonly utilized in patients with brain metastases, it represents an effective salvage therapy for some patients with multiply (locally) recurrent tumors.267 A recent review of 23 studies evaluating brachytherapy in the treatment of brain metastases demonstrated local control rates of 80–100% across most studies, although follow-up was <12 months in the majority of investigations.268 Despite promising local control rates, the use of brachytherapy for brain metastases has been limited, primarily because it is resource intensive and more subject to specialized expertise in comparison to external beam techniques. Further, concerns regarding radiation necrosis, observed in legacy glioblastoma brachytherapy trials, have tempered enthusiasm.268 Randomized studies to more definitively evaluate the role of brachytherapy in the management of resected brain metastases are underway (NCT04690348).
Systemic Therapy
The evolving landscape of systemic therapy has significantly changed the management of brain metastases. Historically, the limited activity of systemic agents in the CNS effectively mandated local brain-directed therapy for nearly all patients.269 However, increasingly effective systemic agents now exist for many extracranial malignancies.270,271 In addition, unlike in glioma, where tumor cells infiltrate the underlying brain, brain metastases tend to form discrete tumor masses with potential breakdown of the BBB, allowing for some penetration of systemic agents into the tumor microenvironment, even if minimal penetration across an intact BBB is possible. As a result, for some patients with untreated, asymptomatic, or minimally symptomatic brain metastases, or those with progressive brain metastases despite local therapy, systemic therapy alone, with close intracranial surveillance, may prove reasonable. Here, we review systemic therapy considerations among the extracranial primaries that harbor a predisposition for intracranial dissemination, but which also have potentially viable systemic options, namely NSCLC, breast cancer, and melanoma. When relevant, the integration of systemic therapy and local, brain-directed therapy is discussed.
Non-small Cell Lung Cancer
Increasingly, the systemic management of brain metastases in patients with NSCLC, particularly adenocarcinomas, is contingent on the presence versus absence of targetable mutations or gene rearrangements, as many current small-molecule, targeted drugs achieve some degree of intracranial penetration (Table 6). Approximately 33–45% of lung adenocarcinomas harbor such genetic changes in the United States, with even higher rates seen in nonsmokers.9,272–280 It should be noted that, even in the absence of targetable genetic changes, anti-PD1 agents, particularly in patients who are PD-L1 positive or who harbor other biomarkers for immunogenicity, or pemetrexed (among patients with adenocarcinomas) may yield intracranial disease control in some patients, although responses can be limited in magnitude and duration.281–285 Of note, intervention studies stipulating local, brain-directed therapy prior to administration of novel systemic agents can make estimates of intracranial efficacy difficult to determine.286
Table 6.
Incidence Proportions and Potentially Viable Systemic Options for Targetable Alterations in Non-small Cell Lung Cancer
| Alteration | Incidence Proportion Among Non-small Cell Lung Cancer (Adenocarcinoma) in United States | Potential Therapeutic Agents |
|---|---|---|
| ALK rearrangements | 4–5% | Alectinib, ceritinib, brigatinib, lorlatinib, crizotinib |
| BRAF V600E mutations | 1–3% | Dabrafenib+trametinib |
| EGFR (common and uncommon mutations) | 15–20% | Osimertinib, erlotinib, gefitinib, afatinib, dacomitinib |
| EGFR exon 20 insertion mutations | 1–2% | Amivantamab |
| HER2 Exon 20 insertion mutations | 1–3% | Trastuzumab, afatinib, ado-trastuzumab emtamsine, trastuzumab deruxtecan |
| KRAS G12C mutations | 10–12% | Sotorasib |
| MET exon 14 skip mutations/high-level amplification | 2–3% | Capmatinib, tepotinib, crizotinib |
| NTRK rearrangements | 0–1% | Larotrectinib, entrectinib |
| RET rearrangements | 1–2% | Selpercatinib, pralsetinib |
| ROS1 rearrangements | 1–2% | Entrectinib, crizotinib, lorlatinib, ceritinib, |
Most patients with EGFR alterations harbor exon 19 deletions or exon 21 L858R substitutions, which account for approximately 90% of mutations in EGFR-mutated NSCLC; such patients are sensitive to EGFR-targeting TKIs.287 Patients with uncommon EGFR mutations may also respond to licensed 1st–3rd generation EGFR TKIs; however, most exon 20 insertions are not sensitive to such agents.288 Among patients with common and uncommon EGFR mutations and brain metastases, prospective studies involving erlotinib, gefitinib, or afatinib, mainly in patients with asymptomatic brain metastases, have generally indicated intracranial response rates between 70% and 88%.28,289–291 However, the current standard of care for patients with brain metastases secondary to EGFR-mutant lung cancer is the 3rd generation inhibitor, osimertinib. In this regard, the first-line FLAURA study comparing the first-generation EGFR TKIs erlotinib and gefitinib to osimeritinib demonstrated prolonged progression-free and overall survival with osimertinib.292 In addition, a trend to improved intracranial response rate with osimertinib was noted (68% vs 91%, respectively).292 Osimertinib can also yield intracranial responses in patients who received other EGFR-TKI therapy and manifest a T790M resistance mutation.57,58Table 7 displays the intracranial efficacy of osimertinib. It is notable that most patients in these studies had stable, asymptomatic, and/or radiated brain metastases. For patients with EGFR exon 20 insertions, amivantamab, a bispecific monoclonal antibody has recently been licensed, although data relating to intracranial efficacy are lacking.288 Of note, neratinib, a dual inhibitor of HER2 and EGFR, may have activity in select patients with EGFR mutations.293
Table 7.
Intracranial Response and Duration of Efficacy for Patients with EGFR-Mutant Non-small Cell Lung Cancer Managed with Osimertinib on Prospective Trialsa
| Study | Drug | Higher Level Entry Criteria | Years of Accrual | N | Median Intracranial Response Duration | CR | PR | SD | PD | N/A |
|---|---|---|---|---|---|---|---|---|---|---|
| AURA extension/AURA256 | Osimertinib | Asymptomatic, stable BM with prior EGFR therapy, T790M mutant | 2014–2015 | 50 | Not reached | 12% | 42% | 38% | 6% | 2% |
| AURA355 | Osimertinib | Asymptomatic, stable BM with prior EGFR therapy, T790M mutant | 2014–2015 | 30 | 8.9 months | 7% | 63% | 23% | 3% | 3% |
| FLAURA262,284 | Osimertinib | Asymptomatic, stable, or symptomatic/ unstable but radiated BM | 2014–2016 | 22 | 15.2 months | 23% | 68% | 5% | 0% | 5% |
Abbreviations: BM, Brain Metastases; CR, Complete Response; N, Number; N/A, Not Applicable/Available; PD, Progressive Disease; PR, Partial Response; SD, Stable Disease.
aWhere possible table focuses on patients with measurable disease.
Patients with ALK-rearranged NSCLC also have multiple systemic options for intracranial disease control. With the possible exception of the first-generation drug crizotinib, ALK targeting agents such as alectinib, ceritinib, brigatinib, and lorlatinib have generally displayed high rates of intracranial disease control in prospective trials (Table 8).272,294,296–306 Lorlatinib may also be effective after progression on a second-generation ALK inhibitor, such as ceritinib, alectinib, or brigatinib.307 The unique side effect profile of lorlatinib, which includes hyperlipidemia, central nervous system effects such as mood, cognitive, and speech changes, weight gain, edema, peripheral neuropathy, and gastrointestinal effects, and its potential for utility in later-line settings, has proven challenging with regard to first-line use among patients with ALK rearrangements.308–310
Table 8.
Intracranial Response and Duration of Efficacy for Patients with ALK-Rearranged Non-small Cell Lung Cancer Managed with Targeted Agents on Prospective Trialsa
| Study | Drug | Higher Level Entry Criteria | Years of Accrual | N | Median Intracranial Response Duration | CR | PR | SD | PD | N/A |
|---|---|---|---|---|---|---|---|---|---|---|
| ASCEND-1287 | Ceritinib | Asymptomatic, stable BM, mostly pretreated with ALK inhibitor | 2011–2013 | 36 | 8–11 monthsb | 0% | 42% | 19% | 17% | 22% |
| ASCEND-2288 | Ceritinib | Asymptomatic, stable BM, prior platinum + crizotinib | 2012–2013 | 20 | N/A | 10% | 35% | 35% | 15% | 5% |
| ASCEND-4289 | Ceritinib | Asymptomatic/stable BM, largely chemo naïve | 2013–2015 | 35 | 17 monthsc | 11% | 60% | 17% | 6% | 6% |
| AF-002JG292 | Alectinib | Asymptomatic BM, prior crizotinib | 2012–2013 | 9 | N/A | 0% | 56% | 22% | 22% | 0% |
| Multisite global290 | Alectinib | Stable or asymptomatic BM, prior crizotinib | 2013–2014 | 35 | 10 months | 20% | 37% | 29% | 9% | 3% |
| Multisite North American291 | Alectinib | Stable, asymptomatic BM, prior crizotinib | 2013–2014 | 16 | 11 months | 25% | 50% | 25% | 0% | 0% |
| ALEX292 | Alectinib | Asymptomatic BM | 2014–2017 | 15 | Not reached | 33% | 20% | 27% | 13% | 7% |
| ALUR294 | Alectinib | Asymptomatic or symptomatic but ineligible for radiation | N/A | 24 | Not reached | 4% | 50% | 25% | 13% | 8% |
| Multisite American/ Spanish293 | Brigatinib | Stable BM, largely pretreated with crizotinib | 2011–2014 | 15 | 19 months | 7% | 47% | 33% | 13% | 0% |
| ALTA295 | Brigatinib | Asymptomatic, stable BM, prior crizotinib | 2014–2015 | 44 | Not reached | 5% | 48% | 32% | N/A | N/A |
| ATLA-1L296 | Brigatinib | Asymptomatic or stable BM | 2016–2017 | 18 | Not reached | 28% | 50% | N/A | N/A | N/A |
| Multisite Global297 | Lorlatinib | Asymptomatic BM | 2015–2016 | 81d | 14.5 months | 20% | 43% | 25% | 9% | 4% |
| CROWN263 | Lorlatinib | Asymptomatic BM | 2017–2019 | 17 | Not reached | 71% | 12% | N/A | N/A | N/A |
Abbreviations: BM, Brain Metastases; CR, Complete Response; N, Number; N/A, Not Applicable/Available; PD, Progressive Disease; PR, Partial Response; SD, Stable Disease.
aWhere possible, table focuses on patients with measurable disease.
bVariable based on receipt of ALK therapy vs not.
cAmong patients with unirradiated brain metastases.
dExcludes treatment-naïve patients (N = 3).
Whether to add radiation to the management of patients with targetable NSCLC remains an area of active controversy. Select multi-institution retrospective and hypothesis-generating data may support combined modality therapy,311 but biases inherent to retrospective designs can impact such analyses. Randomized studies evaluating the role of local brain-directed radiation in patients receiving TKI-based therapy for targetable NSCLC are ongoing (NCT03769103, NCT04634110). However, as consolidation radiation at the point of maximal response to systemic therapy becomes more established for extracranial disease in advanced NSCLC, in order to limit development of acquired resistance, the same logic in the CNS may also need to be formally explored in addition to simply studying the role of drugs in deferring CNS radiation.
Breast Cancer
Viable systemic options vary by breast cancer subtype, with HER2+ patients having the most options for intracranial disease control. The backbone of first-line systemic management for extracranial disease involves trastuzumab, pertuzumab, and either paclitaxel or docetaxel (THP).17 Despite excellent systemic efficacy, the intracranial penetration and disease control of THP is guarded, even when high doses of trastuzumab are used.312 The PATRICIA trial demonstrated an intracranial response rate of 11% with high dose trastuzumab (6 mg/kg) and pertuzumab in patients who had progressed on prior trastuzumab as well as radiotherapy, although 51% achieved clinical benefit at 6 months. Trastuzumab emtansine (T-DM1) offers more potential for intracranial disease control. In the KAMILLA study of T-DM1 in patients with advanced or metastatic HER2+ breast cancer in which patients had received prior HER2-based therapy along with chemotherapy, the best overall response (CNS and extracranial disease) was 21%; CNS objective responses were observed in 33% of patients who had most recently received CNS-directed radiation ≥30 days prior, and in 49% of radiation-naïve patients.313 Among responders, the median duration of exposure to T-DM1 was 9.5 months. Lapatinib, a small dual tyrosine kinase inhibitor of HER1/HER2, when used with capecitabine in newly diagnosed, radiation-naïve patients, demonstrated an intracranial response rate of 66% in the LANDSCAPE study.314 The CNS response rates were lower (~20%) in patients who had progressed after radiotherapy.315 Neratinib, an irreversible pan-HER TKI, displayed intracranial response rates of 33–49% (based on prior lapatinib versus not) when used with capecitabine, in a population who had largely progressed after radiation.316 More recently, regimens based on tucatinib (a more selective HER2-targeting TKI) have demonstrated more promise for intracranial disease control in patients with HER2+ breast cancer and brain metastases. The HER2CLIMB study, which randomized patients who previously received trastuzumab, pertuzumab, and T-DM1 to the regimen of tucatinib, capecitabine, and trastuzumab versus capecitabine and trastuzumab alone, identified an overall intracranial response rate with tucatinib, among patients with both measurable and active brain metastases, of 47% with a median duration of response of 6.8 months; respective estimates in patients only receiving capecitabine and trastuzumab were 20% and 3.0 months, respectively. Overall survival, PFS, and CNS–PFS were all significantly better with tucatinib.317 Early data suggesting CNS efficacy of the novel antibody-drug-conjugate, trastuzumab deruxtecan, also exists. In the DESTINY-Breast01, in heavily pretreated patients (median = 6 prior regimens), 24 patients with treated/asymptomatic brain metastases were included, with an overall response rate of 58% and CNS response rate of 41%. Importantly, the median duration of response was 18.1 months.318 An ongoing study (DESTINY-Breast12) will enroll up to 250 patients with stable or progressive HER2+ breast cancer brain metastases to further define the intracranial activity of trastuzumab deruxtecan.
The optimal sequencing of HER2-targeted agents in patients with or without brain metastases remains an area of active investigation, as does the utility of dual-agent HER2-based therapy. In this regard, ongoing clinical trials are testing novel combinations of HER2-targeting systemic agents (such as tucatinib plus T-DM1 [NCT03975647], T-DM1 plus neratinib [NCT01494662], and trastuzumab deruxtecan plus tucatinib [NCT04539938]).
Among patients with triple negative breast cancer (TNBC) and brain metastases, systemic options for intracranial disease control are limited. Few prospective trials of systemic agents exist. Sacituzumab govitecan, an antibody-drug conjugate consisting of an anti-Trop-2 antibody linked with an active metabolite of irinotecan, SN-38, was evaluated in the ASCENT study of patients with TNBC, including those with stable brain metastases. Despite systemic efficacy, the intracranial response rate and clinical benefit rate were 3% and 9%, respectively, though interpretation of these results is limited by the exclusion of patients with active brain metastases.319 An ongoing SWOG trial is evaluating the CNS activity of sacituzumab specifically in patients with active brain metastases (NCT04647916). Immunotherapeutic agents such as atezolizumab, which is often combined with nab-paclitaxel or carboplatin/gemcitabine in patients with PD-L1 positive TNBC, may have potential activity against brain metastases in TNBC but supporting data are lacking.320
Patients with hormone receptor-positive, HER2-negative breast cancer also have limited options for intracranial management. Although CDK 4/6 inhibition combined with hormonal therapy represents the first-line approach for patients with metastatic disease, intracranial responses are guarded; a phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer identified a response rate of 5% (HER2-negative patients) and 0% (HER2-positive patients), although the clinical benefit rate was 11–24%.321 In patients with PIK3CA mutations, alpelisib may have intracranial efficacy based on a smaller case series322; consequently molecular profiling of this population may be important.
Among patients with any subtype of breast cancer, BRCA mutations, and brain metastases, limited reports supporting the potential viability of PARP inhibitors have been published but prospective studies demonstrating significant intracranial efficacy are limited.323 Carboplatin/bevacizumab may have some utility in patients with breast cancer and brain metastases, although regulatory approval for bevacizumab in metastatic breast cancer is lacking in the United States.324 Other potential options in patients with HER2-negative breast cancer include eribulin,325 capecitabine,326 anthracyclines, and other hormonal (if hormone receptor-positive) and conventional chemotherapeutic agents, although supporting data and efficacy remain guarded. Consequently, for patients with HER2-negative breast cancer and brain metastases, utilization of local therapies for intracranial disease control remains an important consideration. Ultimately, in all subtypes of breast cancer, but particularly within triple negative or hormone receptor-positive/HER2 negative subsets, development of more promising systemic agents with potential for intracranial efficacy represents a key priority.
Melanoma
Advances in systemic approaches, including immunotherapy and, for patients with actionable mutations (particularly BRAF), targeted therapy, have revolutionized the management and prognosis of patients with melanoma and brain metastases, with a significant percentage achieving durable disease control/cure with immunotherapeutic approaches. The optimal systemic management of patients with melanoma and brain metastases is dependent on numerous considerations including age, comorbidities, performance status, intracranial/extracranial disease burden, trajectory of disease pretreatment, prior treatment, steroid requirements, and the presence of targetable mutations in genes such as BRAF, NRAS, and KIT, among other factors. Among the 40–50% of melanoma patients harboring a BRAF mutation, those presenting with an intracranial or visceral oncologic crisis secondary to bulky or rapidly progressive disease may benefit from upfront BRAF/MEK inhibitors (in addition to possible local therapy) due to the possibility of a rapid and profound initial response to these agents.327 In a prospective study of dabrafenib plus trametinib in patients with BRAF V600 mutant melanoma (COMBI-MB study), the intracranial response ranged from 44% to 59% based on subset; the intracranial disease control rate was as high as 75–88%. However, the median intracranial progression-free survival (PFS) ranged from 4.2 to 7.2 months and was reduced almost by half compared to reported PFS in patients without brain metastases (11.1 months).327,328 Other BRAF/MEK regimens, including encorafenib plus binimetinib or vemurafenib plus cobimetinib, may be viable options for patients as well, although prospective data in this population are lacking.329,330 Of note, for patients with melanoma, brain metastases, and NRAS mutations, limited data support the use of MEK inhibitors such as binimetinib in achieving a limited and sometimes shorter term response.331 Toxicities of BRAF/MEK inhibition include fevers, diarrhea, asthenia, headache, nausea, diarrhea, arthralgias, myalgias, elevated liver enzymes, musculoskeletal toxicities, and dermatologic toxicities, including rashes, cardiomyopathies, and QTc prolongation.327,332
In patients with a targetable BRAF mutation harboring a less-pressing need to achieve rapid disease control, immunotherapeutic approaches have appeal, given concerns regarding the sustainability/durability of responses to targeted therapy and challenges in converting BRAF/MEK therapy to immunotherapy given the possibility of rapidly progressive disease after cessation of BRAF/MEK agents.111 Immunotherapy also represents the first-line systemic treatment in patients lacking a BRAF alteration. Early data regarding the role of immunotherapy in the management of patients with melanoma and intracranial involvement stemmed from a single-arm phase 2 trial involving ipilimumab in which disease control was achieved in 24% and 10% of patients who were devoid of neurologic symptoms/steroid requirements versus not, respectively.147 More impressively however, relatively few patients who were alive at 12 months died thereafter, indicating durability of response. Subsequent trials involving PD-1 inhibition as monotherapy also yielded promising results, with intracranial response rates of approximately 20%, which was notably lower than the extracranial response rate of 35–40%.281,333
The currently preferred immunotherapeutic regimen for patients with melanoma and brain metastases involves concurrent ipilimumab and nivolumab followed by maintenance nivolumab. Data supporting dual-agent immunotherapy stem from two landmark trials (Table 9): (1) CheckMate 204, a single-arm phase II study of combination ipilimumab/nivolumab in patients with melanoma and active/unirradiated brain metastases who were devoid of (Cohort A) or who harbored (Cohort B) symptoms/steroid requirements and (2) the ABC study, a randomized study of patients with asymptomatic/unirradiated brain metastases secondary to melanoma assessing ipilimumab/nivolumab (Cohort A) versus nivolumab (Cohort B), along with a single-arm cohort (Cohort C) of patients with progressive disease after local therapy, leptomeningeal disease, or neurologic symptoms managed with nivolumab monotherapy.8,146,333,334 In patients on Cohort A from either CheckMate 204 or the ABC study, the intracranial response/clinical benefit rate was approximately 50–60%, with an encouraging duration of response noted in addition to significant correlation between intracranial and extracranial efficacy. As a result, ipilimumab plus nivolumab forms the backbone of management for patients with melanoma and asymptomatic brain metastases. Unfortunately, intracranial responses with dual-agent immunotherapy in patients who are either symptomatic or on significant doses of steroids are more limited; CheckMate 204 identified an intracranial response rate of only 16.7% in such patients (Cohort B) and, concerningly, patients were only permitted to receive a maximum daily dose of dexamethasone (or equivalent) of 4 mg, suggesting the potential for even lower response rates in patients with greater steroid requirements.145,333,335 It is important to note that immunotherapy can carry a significant risk of side effects. In CheckMate 204, 55% of patients experienced a grade 3–4 adverse effect that was felt to be related to study treatment.8 Adverse effects of dual-agent immunotherapy include immune-related pneumonitis, hepatitis, colitis, nephritis, pancreatitis, arthritis, myositits, dermatologic changes, neurologic toxicities, cardiac inflammation, decreased blood counts, fatigue, and infusion reactions.336 It should be noted however, that the toxicity profile in patients with brain metastases was identical to that in patients without brain metastases, and there were no unique or novel side effects observed in this population.
Table 9.
Prospective Trials of Dual-agent Immunotherapy in the Management of Patients with Melanoma and Active Brain Metastases
| CheckMate 204 (Cohort A)8,144 | CheckMate 204 (Cohort B)8,144 | ABC Study (Cohort A)324 | |
|---|---|---|---|
| High-level eligibility | ≥1 unirradiated brain metastasis (0.5–3.0cm in size), asymptomatic | ≥1 unirradiated brain metastasis (0.5–3.0cm in size), symptomatic or on steroidsa | Asymptomatic brain metastasis, naïve to local, brain-directed therapy |
| Sample size | 101 | 18 | 35 |
| Median follow-up (months) | 20.6 | 5.2 | 14 |
| Years of enrollment | 2015–2017 | 2015–2017 | 2014–2017 |
| Treatment | Ipilimumab + nivolumab | Ipilimumab + nivolumab | Ipilimumab + nivolumab |
| Intracranial response rate | 54% | 22% | 46% |
| Intracranial PFS, median (months) | Not reached | 1.2 | Not reached |
| Extracranial response rate | 49% | 22% | 63% |
| Extracranial PFS, median (months) | Not reached | 2.2 | 14 |
| Overall survival, median (months) | Not reached | 8.7 | Not reached |
Abbreviations: PFS, Progression-Free Survival.
a≤4 mg/day of dexamethasone (or equivalent).
Whether to combine local, brain-directed therapy along with dual-agent immunotherapy remains unresolved. Multi-modality therapy offers potential synergy between immunotherapeutic, surgical, and radiotherapeutic approaches.337,338 Radiation, for example, appears to be more effective when concurrent immunotherapy is administered, although supporting evidence remains largely retrospective.338 In addition, neurosurgical management can quickly decompress bulky or symptomatic brain metastases, potentially rendering patients less steroid-dependent and facilitating effective combination immunotherapy.339 Consequently, in patients who are symptomatic or steroid-dependent, interdigitation of local, brain-directed therapy along with dual-agent immunotherapy seems reasonable. Concerns about multi-modality therapy largely center around toxicity, including pachymeningeal seeding in patients undergoing a craniotomy and radiation necrosis in patients undergoing stereotactic, brain-directed radiation.226 Consequently, the role of local, brain-directed therapy in patients with asymptomatic disease remains unclear.185,226 A randomized study of ipilimumab plus nivolumab with or without brain-directed stereotactic radiation among patients with melanoma and asymptomatic brain metastases is underway (NCT03340129).
Future directions in the management of melanoma and brain metastases include evaluating combinations such as novel immunotherapeutic agents with or without bevacizumab or VEGF-targeting TKIs and the development of biomarkers to identify which patients are most likely to respond to immunotherapy. In addition, among patients with targetable BRAF mutations, combination studies of BRAF-targeted and immunotherapeutic approaches are being conducted (NCT04511013); such approaches may allow patients to benefit from the typically rapid responses to targeted therapy while also providing the opportunity to attain long-term disease control via immunotherapy. Furthermore, evaluation of BRAF/MEK inhibitors with improved CNS penetration are now being explored in phase I studies (NCT04543188, NCT03332589, NCT04190628).
Advancing Clinical Trials
Compared with other oncologic entities of similar incidence, such as breast, prostate, lung and colorectal cancer, relatively few ongoing or completed brain metastases-related prospective studies exist (Figure 1). In addition, brain metastasis trials are often difficult to execute, complete, and interpret, in part due to heterogeneous patient populations, selection bias, effect of prior treatment, dropout, and difficulties with selection of a suitable primary endpoint. With regard to patient heterogeneity, study participants often vary with regard to underlying cancer type (particularly for local therapy studies), molecular alterations, underlying disease trajectory, intracranial and extracranial disease burdens, prior therapy, viable systemic options, prognosis, and functional status.223,340,341 Patient heterogeneity contributes to effect modification and challenges in real-world application. For trials of novel systemic therapies among patients with brain metastases, patients with neurologic symptomatology or bulky intracranial disease have often been excluded, leading to estimates of response and recurrence that may only apply to patients with stable/treated brain metastases, asymptomatic disease, and a modest intracranial disease burden.9,299 For example, in CheckMate 204, the intracranial response rate to dual-agent immunotherapy in nonsteroid dependent patients with asymptomatic brain metastases secondary to melanoma was 54%; the respective rate in an expansion cohort consisting of symptomatic or steroid-dependent patients was only 22%.146 In addition, prior treatment, notably radiation, can confound interpretation of systemic response given the potential for radiation to durably control intracranial disease, and due to complexities in ascertaining which intracranial lesions have been radiated versus not. Moreover, obtaining Food and Drug Administration (FDA) approval for novel systemic therapies can ultimately be challenging given the historical need to show both intracranial and extracranial efficacy. Conversely, in local, brain-directed therapy studies, patient dropout can be significant,238 leading to decreased power, difficulty obtaining study assessments, and a potentially compromised opportunity to identify a statistically significant benefit related to a novel intervention.
Regarding primary endpoint generation, the competing risk of systemic death and salvage options available for intracranial progression can compromise the viability of selecting overall survival as a primary endpoint, particularly for local therapy studies. Progression-free survival may also be of limited utility; for example, in studies examining omission of WBRT in lieu of stereotactic approaches among patients with a limited number of brain metastases, progression-free survival was nearly always better with WBRT even though stereotactic approaches now constitute the preferred approach.223–225,247 The use of alternative survival-based outcome measures, such as neurologic survival, can be difficult to apply and interpret, in part due to a lack of a validated/accepted definition of this endpoint. In addition, local response and local recurrence can be challenging to delineate in the context of immunotherapy-related pseudoprogression,342 bevacizumab/TKI-mediated blunting of contrast enhancement,343 or necrosis related to stereotactic radiation,187,262 each of which can cloud such delineations. Investigations centered on quality of life or neurocognitive function offer promise in characterizing key functional endpoints relevant to patients with brain metastases; although such outcomes may be especially relevant given that many patients cannot be cured and that a primary goal of treatment relates to maintaining/sustaining function and quality of life, such assessments can be especially challenging to obtain in patients who are impacted most by neurologic symptomatology, oncologic disease, and/or treatment leading to bias with regard to missing data. As a result, for some studies related to brain metastases, there may not be a “best” primary outcome measure.
Efforts to improve clinical trials relating to brain metastases are underway. The Response Assessment in Neuro-Oncology-Brain Metastasis (RANO-BM) working group has advanced the assessment of intracranial tumor response and delineation of study-related end points, including among patients managed with immunotherapy and local, brain-directed therapy (Table 10).119,344,345 RANO-BM criteria is distinguished from RECIST by establishing the brain as a separate compartment and accounting for steroid use and clinical deterioration346; in addition to use within the domain of clinical trials, RANO-BM criteria have applicability for routine clinical care.347 With regard to imaging, consensus guidelines outlining standardization of imaging protocols for patients in brain tumor-related trials have been published.95 Moreover, the Food and Drug Administration has recently released recommendations regarding clinical trial eligibility for patients with brain metastases, emphasizing the importance of including patients with brain metastases on trials where feasible, defining enrollment parameters based on extent of CNS disease (treated/stable vs active vs leptomeningeal disease), and proposing specific situations where exclusion may be justified.348,349 In addition, systemic therapy-based trials are enrolling patients with active/unirradiated brain metastases.8,317,334,350–352 Lastly, quality measures to monitor and improve the care of patients with brain metastases have been proposed.353
Table 10.
RANO-BM Criteria for Response Assessment
| Parameter | Complete Response | Partial Response | Stable Disease | Progressive Disease |
|---|---|---|---|---|
| Target lesions | No evidence of disease | ≥30% decrease in sum of longest diameter of target lesions | Response between <30% decrease to <20% increase in sum of longest diameter of target lesions | ≥20% in sum of longest diameter of target lesions |
| Nontarget lesions | No evidence of disease | Stable/improved | Stable/improved | Unequivocal radiographic progression |
| Steroids | None | Stable/reduced | Stable/reduced | Not applicable |
| Clinical status | Stable/improved | Stable/improved | Stable/improved | Deterioration |
Abbreviations: RANO-BM, Response Assessment in Neuro-Oncology - Brain Metastases.
Multiple ongoing or recently completed prospective studies are pushing the historical boundaries of brain metastasis-related investigations. For example, increasingly, brain metastases-related trials are being focused on select subsets of patients, often linked by a histology or even specific molecular alterations. In this regard, NCT03994796 is a cooperative group study in which patients with specific mutations (CDK, PI3K/mTOR, NTRK/ROS1) are stratified and then receive alteration-specific targeted agents. A recently published randomized trial of tucatinib, capecitabine, and trastuzumab compared to capecitabine, trastuzumab, and placebo among patients with HER2+ breast cancer demonstrated an overall survival benefit to the tucatinib arm; this study enrolled patients with active intracranial disease, representing a shift from many prior studies.317 The use of neurologic death as a primary endpoint is being explored via a tribunal approach in NCT03391362 (a phase II study of stereotactic radiation for SCLC) with neurologic death defined as marked, progressive radiographic progression in the brain accompanied by corresponding neurologic symptomatology in the absence of systemic disease progression/systemic symptoms of a life-threatening nature; this study will determine whether a consistent definition of neurologic death can be reproducibly applied among independent reviewers. In addition, all MRIs for the clinical course of a given patient are registered to minimize error related to patient positioning and generate greater inference regarding necrosis versus progression-based delineations (Supplemental Figure 1).
Although significant progress has been made with regard to the design and implementation of brain metastases-related prospective trials, numerous hurdles persist, as noted above. However, increasing recognition of the impact of brain metastases on a patient- and systems-level has reinvigorated efforts to advance the care of this population in need. Minimization of patient heterogeneity, refinement of eligibility criteria, improvements in primary endpoint generation, and advances in study conduct have potential to transform the management of patients with brain metastases.
Summary
Brain metastases have garnered increasing attention among patients, providers, investigators, and health care systems, resulting in significant progress in management over recent years. Yet, significant challenges remain. Ongoing efforts have potential to further improve outcomes for this increasingly relevant population of patients.
Supplementary Material
Contributor Information
Ayal A Aizer, Department of Radiation Oncology, Dana-Farber Cancer Institute, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Nayan Lamba, Department of Radiation Oncology, Dana-Farber Cancer Institute, Brigham and Women’s Hospital, Boston, Massachusetts, USA; Harvard Radiation Oncology Program, Boston, Massachusetts, USA.
Manmeet S Ahluwalia, Department of Medical Oncology, Miami Cancer Institute, Miami, Florida, USA.
Kenneth Aldape, Laboratory of Pathology, National Cancer Institute, Bethesda, Maryland, USA.
Adrienne Boire, Department of Neurology, Human Oncology and Pathogenesis Program, Memorial Sloan Kettering Cancer Center, New York, New York, USA.
Priscilla K Brastianos, Departments of Neuro-Oncology and Medical Oncology, Massachusetts General Hospital, Boston, Massachusetts, USA.
Paul D Brown, Department of Radiation Oncology, Mayo Clinic, Rochester, Minnesota, USA.
D Ross Camidge, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, USA.
Veronica L Chiang, Departments of Neurosurgery and Radiation Oncology, Yale School of Medicine, New Haven, Connecticut, USA.
Michael A Davies, Department of Melanoma Medical Oncology, MD Anderson Cancer Center, Houston, Texas, USA.
Leland S Hu, Department of Radiology, Neuroradiology Division, Mayo Clinic, Phoenix, Arizona, USA.
Raymond Y Huang, Department of Radiology, Brigham and Women’s Hospital, Boston, Massachusetts, USA.
Timothy Kaufmann, Department of Radiology, Mayo Clinic, Rochester, Minnesota, USA.
Priya Kumthekar, Department of Neurology at The Feinberg School of Medicine at Northwestern University and The Malnati Brain Tumor Institute at the Robert H. Lurie Comprehensive Cancer Center of Northwestern University, Chicago, Illinois, USA.
Keng Lam, Department of Neurology, Kaiser Permanente, Los Angeles Medical Center, Los Angeles, California, USA.
Eudocia Q Lee, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, USA.
Nancy U Lin, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, USA.
Minesh Mehta, Department of Radiation Oncology, Miami Cancer Institute, Miami, Florida, USA.
Michael Parsons, Departments of Oncology and Psychiatry, Massachusetts General Hospital, Boston, Massachusetts, USA.
David A Reardon, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, USA.
Jason Sheehan, Department of Neurosurgery, University of Virginia, Charlottesville, Virginia, USA.
Riccardo Soffietti, Division of Neuro-Oncology, Department of Neuroscience Rita Levi Montalcini, University of Turin, Turin, Italy.
Hussein Tawbi, Department of Melanoma Medical Oncology, MD Anderson Cancer Center, Houston, Texas, USA.
Michael Weller, Department of Neurology, University Hospital and University of Zurich, Zurich, Switzerland.
Patrick Y Wen, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, USA.
Funding
No funding was required for this manuscript.
Conflict of interest statement. Dr. Aizer reports research funding from Varian Medical Systems and NH TherAguix and consulting/advisory board fees from Novartis and Seagen. Dr. Ahluwalia reports grants/research support from Astrazeneca, Abbvie, BMS, Bayer, Incyte, Pharmacyclics, Novocure, Merck, Mimivax, Novartis, Roswell Park Cancer Foundation, and Velosano as well as stock shareholdership for Cytodyn, Doctible, Mimivax, and Medinnovate Advisors LLC, in addition to honoria/consulting fees from Abbvie, AstraZeneca, Bayer, Cellularity, Elsevier, Forma therapeutics. GSK, Insightec, karyopharm, Kiyatec, Novocure Inc, Tocagen, VBI Vaccines, Wiley, Xoft, Nuvation, SDP Oncology, Apollomics, Prelude, Janssen, Anheart Therapeutics, Viewray, Voyager Therapeutics, Caris Lifesciences, Pyramid Bio, and Theraguix. Dr. Ahluwalia also reports conflicts relating to Caris lifesciences and Varian medical systems. Dr. Brastianos has consulted for Angiochem, Genentech- Roche, Lilly, Tesaro, ElevateBio, Pfizer (Array), Dantari, SK Life Sciences, Voyager Therapeutics, and Sintetica and received grant/research support to MGH from Merck, BMS, Mirati and Lilly and honoraria from Merck. Dr. Brown reports personal fees from UpToDate (contributor) outside the submitted work. Dr. Camidge reports consulting/advisory board participation with Abbvie, Achilles, Amgen, Anchiarno, Apollomics, Archer, AstraZeneca, BeyondSpring, Bio-Thera, Blueprint, BMS, CBT Pharmaceuticals, Daiichi-Sankyo, Eisai, Elevation (14ner/Elevation), Eli Lilly, EMD Serono, G1 Therapeutics, GSK, Helsinn, Janssen, Kestrel, Nuvalent, Onkure, Mersana, Medtronic, Pfizer, Puma, Qilu, Ribon, Roche, Sanofi, Seattle Genetics, Takeda, and Turning Point; he has received research funding from Inivata. He has received company sponsored trials in the PI role from: Abbvie, AstraZeneca, Dizal, Inhibrx, Karyopharm, Pfizer, Phosplatin, Psioxus, Rain, Roche/Genentech, Seattle Genetics, Takeda, Turning Point. Dr. Chiang is a paid consultant for Monteris Medical Inc. Dr. Davies is supported by the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation, the AIM at Melanoma Foundation, the NIH/NCI (1 P50 CA221703-02), the American Cancer Society and the Melanoma Research Alliance, Cancer Fighters of Houston, the Anne and John Mendelsohn Chair for Cancer Research, and philanthropic contributions to the Melanoma Moon Shots Program of MD Anderson; he has been a consultant to Roche/Genentech, Array, Pfizer, Novartis, BMS, GSK, Sanofi-Aventis, Vaccinex, Apexigen, Eisai, and ABM Therapeutics, and he has been the PI of research grants to MD Anderson by Roche/Genentech, GSK, Sanofi-Aventis, Merck, Myriad, and Oncothyreon. Dr. Hu reports the following grant funding (NS082609, CA221938, CA220378, CA250481, Mayo Clinic Foundation, Arizona Biomedical Research Commission) as well as medical advisory board participation for Imaging Biometrics, a co-founding role for Precision Oncology Insights, a paid speakership for Bayer Pharmaceutical, and holding US patent 10,909,675. Dr. Huang reports research support from Agios Pharmaceuticals and BMS as well as consulting from Nuvation Bio Inc and scientific advisory board participation for Vysioneer. Dr. Kumthekar reports consulting Fees (e.g., advisory board and medical advising) from Janssen, Orbus Therapeutics, Novocure, Celularity, SDP Oncology, Biocept, Affinia, Sintetica, Angiochem, EnClear and reports Data Safety Monitoring Committee forBerg Oncology. Dr. Mehta reports consulting for Karyopharm, Sapience, Zap, Mevion, Xoft. Dr. Mehta reports stock ownership in Chimerix. Dr. Wen reports research support Astra Zeneca/Medimmune, Beigene, Celgene, Chimerix, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Nuvation Bio, Puma, Servier, Vascular Biogenics, VBI Vaccines. Dr. Wen is on the Advisory Boards for Astra Zeneca, Bayer, Black Diamond, Boehringer Ingelheim, Boston Pharmaceuticals, Celularity, Chimerix, Day One Bio, Genenta, Glaxo Smith Kline, Karyopharm, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience, Servier, Sagimet, Vascular Biogenics, VBI Vaccines. Dr. Lin reports institutional research support (to DFCI) from Genentech, Pfizer, Merck, Seattle Genetics, Zion Pharmaceuticals, Olema Pharmaceuticals, AstraZeneca. Dr. Lin reports consulting honoraria relating to Puma, Seattle Genetics, Daichii-Sankyo, AstraZeneca, Denali Therapeutics, Prelude Therapeutics, Olema Pharmaceuticals, Aleta BioPharma, Affinia Therapeutics, Voyager Therapeutics. Dr. Lin reports royalties from Up-to-date (royalties from book chapter) and stock/ownership interests in Artera Inc. Dr. Reardon reports research support from Acerta Phamaceuticals, Agenus, Celldex, EMD Serono, Incyte, Inovio, Omniox, Tragara; he has been a advisory/consultation to Abbvie, Advantagene, Agenus, Amgen, Bayer, Bristol-Myers Squibb, Celldex, DelMar, EMD Serono, Genentech/Roche, Imvax, Inovio, Medicenna Biopharma, Inc., Merck, Merck KGaA, Monteris, Novocure, Oncorus, Oxigene, Regeneron, Stemline, Sumitono Dainippon Pharma, Taiho Oncology, Inc.; and honoraria for Abbvie, Advantagene, Agenus, Bristol-Myers Squibb, Celldex, EMD Serono, Genentech/Roche, Imvax, Inovio, Medicenna Biopharma, Inc., Merck, Merck KGaA, Monteris, Novocure, Oncorus, Oxigene, Regeneron, Stemline, Sumitono Dainippon Pharma, Taiho Oncology, Inc. Dr. Tawbi reports consulting for BMS, Merck, Novartis, Genentech, Eisai, Iovance, Karyopharm, Boxer Capital, and Pfizer Dr. Weller has received research grants from Apogenix, Merck, Sharp & Dohme, Merck (EMD), Philogen and Quercis, and honoraria for lectures or advisory board participation or consulting from Adastra, Bayer, Bristol Meyer Squibb, Medac, Merck, Sharp & Dohme, Merck (EMD), Nerviano Medical Sciences, Novartis, Orbus, Philogen and yMabs. The remaining authors declare no conflicts of interest.
Authorship statement. Conception/Design: all authors; Data Collection/Analysis/Interpretation: n/a; Statistical Analysis: n/a; Drafting of the Manuscript: AA, NL, PW; Manuscript Editing/Critical Revision of the Manuscript: All Authors; Supervision: PW, AA.
References
- 1. Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012; 14(1):48–54. [DOI] [PubMed] [Google Scholar]
- 2. Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004; 22(14):2865–2872. [DOI] [PubMed] [Google Scholar]
- 3. Lamba N, Wen PY, Aizer AA. Epidemiology of brain metastases and leptomeningeal disease. Neuro Oncol. 2021; 23(9):1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Achrol AS, Rennert RC, Anders C, et al. Brain metastases. Nat Rev Dis Primers. 2019; 5(1):5. [DOI] [PubMed] [Google Scholar]
- 5. Fabi A, Felici A, Metro G, et al. Brain metastases from solid tumors: disease outcome according to type of treatment and therapeutic resources of the treating center. J Exp Clin Cancer Res. 2011; 30(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990; 322(8):494–500. [DOI] [PubMed] [Google Scholar]
- 7. Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004; 363(9422):1665–1672. [DOI] [PubMed] [Google Scholar]
- 8. Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018; 379(8):722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018; 378(2):113–125. [DOI] [PubMed] [Google Scholar]
- 10. Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015; 5(11):1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamba N, Kearney RB, Catalano PJ, et al. Population-based estimates of survival among elderly patients with brain metastases. Neuro Oncol. 2021; 23(4):661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim AE, Wang GM, Waite KA, et al. Cross-sectional survey of patients, caregivers, and physicians on diagnosis and treatment of brain metastases. Neurooncol Pract. 2021; 8(6):662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vogelbaum MA, Brown PD, Messersmith H, et al. Treatment for brain metastases: ASCO-SNO-ASTRO guideline. J Clin Oncol. 2021; 40(5):JCO2102314. [DOI] [PubMed] [Google Scholar]
- 14. Ostrom QT, Wright CH, Barnholtz-Sloan JS. Brain metastases: epidemiology. Handb Clin Neurol. 2018; 149:27–42. [DOI] [PubMed] [Google Scholar]
- 15. Sacks P, Rahman M. Epidemiology of brain metastases. Neurosurg Clin N Am. 2020; 31(4):481–488. [DOI] [PubMed] [Google Scholar]
- 16. Cagney DN, Martin AM, Catalano PJ, et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: a population-based study. Neuro Oncol. 2017; 19(11):1511–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Breast cancer. Version 2; 2017. www.nccn.org.
- 18. Pestalozzi BC, Holmes E, de Azambuja E, et al. CNS relapses in patients with HER2-positive early breast cancer who have and have not received adjuvant trastuzumab: a retrospective substudy of the HERA trial (BIG 1-01). Lancet Oncol. 2013; 14(3):244–248. [DOI] [PubMed] [Google Scholar]
- 19. Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012; 14(9):1171–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lamba N, Kearney RB, Mehanna E, et al. Utility of claims data for identification of date of diagnosis of brain metastases. Neuro Oncol. 2020; 22(4):575–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nugent JL, BunnPA, Jr., Matthews MJ, et al. CNS metastases in small cell bronchogenic carcinoma: increasing frequency and changing pattern with lengthening survival. Cancer. 1979; 44(5):1885–1893. [DOI] [PubMed] [Google Scholar]
- 22. Sperduto PW, Chao ST, Sneed PK, et al. Diagnosis-specific prognostic factors, indexes, and treatment outcomes for patients with newly diagnosed brain metastases: a multi-institutional analysis of 4,259 patients. Int J Radiat Oncol Biol Phys. 2010; 77(3):655–661. [DOI] [PubMed] [Google Scholar]
- 23. Gaspar L, Scott C, Rotman M, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997; 37(4):745–751. [DOI] [PubMed] [Google Scholar]
- 24. Sperduto PW, Berkey B, Gaspar LE, Mehta M, Curran W. A new prognostic index and comparison to three other indices for patients with brain metastases: an analysis of 1,960 patients in the RTOG database. Int J Radiat Oncol Biol Phys. 2008; 70(2):510–514. [DOI] [PubMed] [Google Scholar]
- 25. Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000; 47(4):1001–1006. [DOI] [PubMed] [Google Scholar]
- 26. Sperduto CM, Watanabe Y, Mullan J, et al. A validation study of a new prognostic index for patients with brain metastases: the Graded Prognostic Assessment. J Neurosurg. 2008; 10(9 Suppl):87–89. [DOI] [PubMed] [Google Scholar]
- 27. Sperduto PW, Yang TJ, Beal K, et al. The effect of gene alterations and tyrosine kinase inhibition on survival and cause of death in patients with adenocarcinoma of the lung and brain metastases. Int J Radiat Oncol Biol Phys. 2016; 96(2):406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Park SJ, Kim HT, Lee DH, et al. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012; 77(3):556–560. [DOI] [PubMed] [Google Scholar]
- 29. Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012; 82(5):2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sperduto PW, Jiang W, Brown PD, et al. Estimating survival in melanoma patients with brain metastases: an update of the graded prognostic assessment for melanoma using molecular markers (melanoma-molGPA). Int J Radiat Oncol Biol Phys. 2017; 99(4):812–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sperduto PW, Yang TJ, Beal K, et al. Estimating survival in patients with lung cancer and brain metastases: an update of the graded prognostic assessment for lung cancer using molecular markers (lung-molGPA). JAMA Oncol. 2017; 3(6):827–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005; 75(1):5–14. [DOI] [PubMed] [Google Scholar]
- 33. Eichler AF, Chung E, Kodack DP, et al. The biology of brain metastases-translation to new therapies. Nat Rev Clin Oncol. 2011; 8(6):344–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu Q, Zhang H, Jiang X, et al. Factors involved in cancer metastasis: a better understanding to “seed and soil” hypothesis. Mol Cancer. 2017; 16(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fan J, Cai B, Zeng M, et al. Integrin β4 signaling promotes mammary tumor cell adhesion to brain microvascular endothelium by inducing ErbB2-mediated secretion of VEGF. Ann Biomed Eng. 2011; 39(8):2223–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Küsters B, Leenders WP, Wesseling P, et al. Vascular endothelial growth factor-A(165) induces progression of melanoma brain metastases without induction of sprouting angiogenesis. Cancer Res. 2002; 62(2):341–345. [PubMed] [Google Scholar]
- 37. Boire A, Brastianos PK, Garzia L, Valiente M. Brain metastasis. Nat Rev Cancer. 2020; 20(1):4–11. [DOI] [PubMed] [Google Scholar]
- 38. Bos PD, Zhang XH, Nadal C, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009; 459(7249):1005–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wrobel JK, Toborek M. Blood-brain barrier remodeling during brain metastasis formation. Mol Med. 2016; 22:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Priego N, Zhu L, Monteiro C, et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat Med. 2018; 24(7):1024–1035. [DOI] [PubMed] [Google Scholar]
- 41. Zhang L, Zhang S, Yao J, et al. Microenvironment-induced PTEN loss by exosomal microRNA primes brain metastasis outgrowth. Nature. 2015; 527(7576):100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zeng Q, Michael IP, Zhang P, et al. Synaptic proximity enables NMDAR signalling to promote brain metastasis. Nature. 2019; 573(7775):526–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Contreras-Zarate MJ, Day NL, Ormond DR, et al. Estradiol induces BDNF/TrkB signaling in triple-negative breast cancer to promote brain metastases. Oncogene. 2019; 38(24):4685–4699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Grinberg-Rashi H, Ofek E, Perelman M, et al. The expression of three genes in primary non-small cell lung cancer is associated with metastatic spread to the brain. Clin Cancer Res. 2009; 15(5):1755–1761. [DOI] [PubMed] [Google Scholar]
- 45. Nguyen DX, Chiang AC, Zhang XH, et al. WNT/TCF signaling through LEF1 and HOXB9 mediates lung adenocarcinoma metastasis. Cell. 2009; 138(1):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ippen FM, Grosch JK, Subramanian M, et al. Targeting the PI3K/Akt/mTOR pathway with the pan-Akt inhibitor GDC-0068 in PIK3CA-mutant breast cancer brain metastases. Neuro Oncol. 2019; 21(11):1401–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Valiente M, Ahluwalia MS, Boire A, et al. The evolving landscape of brain metastasis. Trends Cancer. 2018; 4(3):176–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bucheit AD, Chen G, Siroy A, et al. Complete loss of PTEN protein expression correlates with shorter time to brain metastasis and survival in stage IIIB/C melanoma patients with BRAFV600 mutations. Clin Cancer Res. 2014; 20(21):5527–5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cho JH, Robinson JP, Arave RA, et al. AKT1 activation promotes development of melanoma metastases. Cell Rep. 2015; 13(5):898–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shih DJH, Nayyar N, Bihun I, et al. Genomic characterization of human brain metastases identifies drivers of metastatic lung adenocarcinoma. Nat Genet. 2020; 52(4):371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Berghoff AS, Brastianos PK. Toward precision medicine in brain metastases. Semin Neurol. 2018; 38(1):95–103. [DOI] [PubMed] [Google Scholar]
- 52. Heist RS, Sequist LV, Engelman JA. Genetic changes in squamous cell lung cancer: a review. J Thorac Oncol. 2012; 7(5):924–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wistuba II, Gazdar AF, Minna JD. Molecular genetics of small cell lung carcinoma. Semin Oncol. 2001; 28(2 Suppl 4):3–13. [PubMed] [Google Scholar]
- 54. Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII). Am J Cancer Res. 2015; 5(9):2892–2911. [PMC free article] [PubMed] [Google Scholar]
- 55. Arcila ME, Nafa K, Chaft JE, et al. EGFR exon 20 insertion mutations in lung adenocarcinomas: prevalence, molecular heterogeneity, and clinicopathologic characteristics. Mol Cancer Ther. 2013; 12(2):220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Jänne PA. Challenges of detecting EGFR T790M in gefitinib/erlotinib-resistant tumours. Lung Cancer. 2008; 60(Suppl 2):S3–S9. [DOI] [PubMed] [Google Scholar]
- 57. Wu YL, Ahn MJ, Garassino MC, et al. CNS efficacy of osimertinib in patients with T790M-positive advanced non-small-cell lung cancer: data from a randomized phase III trial (AURA3). J Clin Oncol. 2018; 36(26):2702–2709. [DOI] [PubMed] [Google Scholar]
- 58. Goss G, Tsai CM, Shepherd FA, et al. CNS response to osimertinib in patients with T790M-positive advanced NSCLC: pooled data from two phase II trials. Ann Oncol. 2018; 29(3):687–693. [DOI] [PubMed] [Google Scholar]
- 59. Leonetti A, Sharma S, Minari R, et al. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br J Cancer. 2019; 121(9):725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014; 371(23):2167–2177. [DOI] [PubMed] [Google Scholar]
- 61. Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013; 368(25):2385–2394. [DOI] [PubMed] [Google Scholar]
- 62. Calvayrac O, Pradines A, Pons E, Mazières J, Guibert N. Molecular biomarkers for lung adenocarcinoma. Eur Respir J. 2017; 49(4):1601734. [DOI] [PubMed] [Google Scholar]
- 63. Bachmann C, Grischke EM, Fehm T, et al. CNS metastases of breast cancer show discordant immunohistochemical phenotype compared to primary. J Cancer Res Clin Oncol. 2013; 139(4):551–556. [DOI] [PubMed] [Google Scholar]
- 64. Hulsbergen AFC, Claes A, Kavouridis VK, et al. Subtype switching in breast cancer brain metastases: a multicenter analysis. Neuro Oncol. 2020; 22(8):1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schrijver WAME, Suijkerbuijk KPM, van Gils CH, et al. Receptor conversion in distant breast cancer metastases: a systematic review and meta-analysis. J Natl Cancer Inst. 2018; 110(6):568–580. [DOI] [PubMed] [Google Scholar]
- 66. Davies MA, Stemke-Hale K, Lin E, et al. Integrated molecular and clinical analysis of AKT activation in metastatic melanoma. Clin Cancer Res. 2009; 15(24):7538–7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tehranian C, Fankhauser L, Harter PN, et al. The PI3K/Akt/mTOR pathway as a preventive target in melanoma brain metastasis. Neuro Oncol. 2021; 24(2):213–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen G, Chakravarti N, Aardalen K, et al. Molecular profiling of patient-matched brain and extracranial melanoma metastases implicates the PI3K pathway as a therapeutic target. Clin Cancer Res. 2014; 20(21):5537–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Niessner H, Forschner A, Klumpp B, et al. Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases. Cancer Med. 2013; 2(1):76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fischer GM, Jalali A, Kircher DA, et al. Molecular profiling reveals unique immune and metabolic features of melanoma brain metastases. Cancer Discov. 2019; 9(5):628–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fukumura K, Malgulwar PB, Fischer GM, et al. Multi-omic molecular profiling reveals potentially targetable abnormalities shared across multiple histologies of brain metastasis. Acta Neuropathol. 2021; 141(2):303–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ngo B, Kim E, Osorio-Vasquez V, et al. Limited environmental serine and glycine confer brain metastasis sensitivity to PHGDH inhibition. Cancer Discov. 2020; 10(9):1352–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Klein A, Sagi-Assif O, Meshel T, et al. CCR4 is a determinant of melanoma brain metastasis. Oncotarget. 2017; 8(19):31079–31091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Varaljai R, Horn S, Sucker A, et al. Integrative genomic analyses of patient-matched intracranial and extracranial metastases reveal a novel brain-specific landscape of genetic variants in driver genes of malignant melanoma. Cancers (Basel). 2021; 13(4):731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kwong LN, Boland GM, Frederick DT, et al. Co-clinical assessment identifies patterns of BRAF inhibitor resistance in melanoma. J Clin Invest. 2015; 125(4):1459–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Trunzer K, Pavlick AC, Schuchter L, et al. Pharmacodynamic effects and mechanisms of resistance to vemurafenib in patients with metastatic melanoma. J Clin Oncol. 2013; 31(14):1767–1774. [DOI] [PubMed] [Google Scholar]
- 77. Kwong LN, Davies MA. Navigating the therapeutic complexity of PI3K pathway inhibition in melanoma. Clin Cancer Res. 2013; 19(19):5310–5319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gopal YN, Rizos H, Chen G, et al. Inhibition of mTORC1/2 overcomes resistance to MAPK pathway inhibitors mediated by PGC1alpha and oxidative phosphorylation in melanoma. Cancer Res. 2014; 74(23):7037–7047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Roesch A, Vultur A, Bogeski I, et al. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell. 2013; 23(6):811–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen PL, Roh W, Reuben A, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 2016; 6(8):827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Peng W, Chen JQ, Liu C, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 2016; 6(2):202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ramkissoon LA, Pegram W, Haberberger J, et al. Genomic profiling of circulating tumor DNA from cerebrospinal fluid to guide clinical decision making for patients with primary and metastatic brain tumors. Front Neurol. 2020; 11:544680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. De Mattos-Arruda L, Mayor R, Ng CKY, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015; 6:8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lee JH, Menzies AM, Carlino MS, et al. Longitudinal monitoring of ctDNA in patients with melanoma and brain metastases treated with immune checkpoint inhibitors. Clin Cancer Res. 2020; 26(15):4064–4071. [DOI] [PubMed] [Google Scholar]
- 85. Ma C, Yang X, Xing W, et al. Detection of circulating tumor DNA from non-small cell lung cancer brain metastasis in cerebrospinal fluid samples. Thorac Cancer. 2020; 11(3):588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Boire A, Brandsma D, Brastianos PK, et al. Liquid biopsy in central nervous system metastases: a RANO review and proposals for clinical applications. Neuro Oncol. 2019; 21(5):571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Henry KE, Ulaner GA, Lewis JS. Human epidermal growth factor receptor 2-targeted PET/single- photon emission computed tomography imaging of breast cancer: noninvasive measurement of a biomarker integral to tumor treatment and prognosis. PET Clin. 2017; 12(3):269–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Pereira PMR, Ragupathi A, Shmuel S, et al. HER2-targeted PET imaging and therapy of hyaluronan-masked HER2-overexpressing breast cancer. Mol Pharm. 2020; 17(1):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Network NCC. Small cell lung cancer. Version 3.2021. https://www.nccn.org/professionals/physician_gls/pdf/sclc.pdf. Accessed August 8, 2021.
- 90. Network NCC. Melanoma: cutaenous. Version 2.2021. https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf. Accessed August 8, 2021.
- 91. Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. J Neurooncol. 1999; 44(3):275–281. [DOI] [PubMed] [Google Scholar]
- 92. Yokoi K, Kamiya N, Matsuguma H, et al. Detection of brain metastasis in potentially operable non-small cell lung cancer: a comparison of CT and MRI. Chest. 1999; 115(3):714–719. [DOI] [PubMed] [Google Scholar]
- 93. Fink KR, Fink JR. Imaging of brain metastases. Surg Neurol Int. 2013; 4(Suppl 4):S209–S219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Balériaux D, Colosimo C, Ruscalleda J, et al. Magnetic resonance imaging of metastatic disease to the brain with gadobenate dimeglumine. Neuroradiology. 2002; 44(3):191–203. [DOI] [PubMed] [Google Scholar]
- 95. Kaufmann TJ, Smits M, Boxerman J, et al. Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases. Neuro Oncol. 2020; 22(6):757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Danieli L, Riccitelli GC, Distefano D, et al. Brain tumor-enhancement visualization and morphometric assessment: a comparison of MPRAGE, SPACE, and VIBE MRI techniques. AJNR Am J Neuroradiol. 2019; 40(7):1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Suh CH, Jung SC, Kim KW, Pyo J. The detectability of brain metastases using contrast-enhanced spin-echo or gradient-echo images: a systematic review and meta-analysis. J Neurooncol. 2016; 129(2):363–371. [DOI] [PubMed] [Google Scholar]
- 98. Komada T, Naganawa S, Ogawa H, et al. Contrast-enhanced MR imaging of metastatic brain tumor at 3 tesla: utility of T(1)-weighted SPACE compared with 2D spin echo and 3D gradient echo sequence. Magn Reson Med Sci. 2008; 7(1):13–21. [DOI] [PubMed] [Google Scholar]
- 99. Kato Y, Higano S, Tamura H, et al. Usefulness of contrast-enhanced T1-weighted sampling perfection with application-optimized contrasts by using different flip angle evolutions in detection of small brain metastasis at 3T MR imaging: comparison with magnetization-prepared rapid acquisition of gradient echo imaging. AJNR Am J Neuroradiol. 2009; 30(5):923–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Pope WB. Brain metastases: neuroimaging. Handb Clin Neurol. 2018; 149:89–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Smirniotopoulos JG, Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. Radiographics. 2007; 27(2):525–551. [DOI] [PubMed] [Google Scholar]
- 102. Lignelli A, Khandji AG. Review of imaging techniques in the diagnosis and management of brain metastases. Neurosurg Clin N Am. 2011; 22(1):15–25, v. [DOI] [PubMed] [Google Scholar]
- 103. Brigell RH, Cagney DN, Martin AM, et al. Local control after brain-directed radiation in patients with cystic versus solid brain metastases. J Neurooncol. 2019; 142(2):355–363. [DOI] [PubMed] [Google Scholar]
- 104. Marta GN, da Cunha Colombo Bonadio RR, Martins RE, Zuppani HB, de Castro GJ. Cystic brain metastases in ALK-rearranged non-small cell lung cancer. Ecancermedicalscience. 2018; 12:818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Al-Okaili RN, Krejza J, Wang S, Woo JH, Melhem ER. Advanced MR imaging techniques in the diagnosis of intraaxial brain tumors in adults. Radiographics. 2006; 26(Suppl 1):S173–S189. [DOI] [PubMed] [Google Scholar]
- 106. Garg RK, Sinha MK. Multiple ring-enhancing lesions of the brain. J Postgrad Med. 2010; 56(4):307–316. [DOI] [PubMed] [Google Scholar]
- 107. Cartes-Zumelzu FW, Stavrou I, Castillo M, et al. Diffusion-weighted imaging in the assessment of brain abscesses therapy. AJNR Am J Neuroradiol. 2004; 25(8):1310–1317. [PMC free article] [PubMed] [Google Scholar]
- 108. Chang SC, Lai PH, Chen WL, et al. Diffusion-weighted MRI features of brain abscess and cystic or necrotic brain tumors: comparison with conventional MRI. Clin Imaging. 2002; 26(4):227–236. [DOI] [PubMed] [Google Scholar]
- 109. Augustin M, Bammer R, Simbrunner J, et al. Diffusion-weighted imaging of patients with subacute cerebral ischemia: comparison with conventional and contrast-enhanced MR imaging. AJNR Am J Neuroradiol. 2000; 21(9):1596–1602. [PMC free article] [PubMed] [Google Scholar]
- 110. National Comprehensive Cancer Network. Central nervous system cancers (Version 1.2021). [DOI] [PubMed]
- 111. Cagney DN, Alexander BM, Hodi FS, et al. Rapid progression of intracranial melanoma metastases controlled with combined BRAF/MEK inhibition after discontinuation of therapy: a clinical challenge. J Neurooncol. 2016; 129(3):389–393. [DOI] [PubMed] [Google Scholar]
- 112. Horky LL, Hsiao EM, Weiss SE, Drappatz J, Gerbaudo VH. Dual phase FDG-PET imaging of brain metastases provides superior assessment of recurrence versus post-treatment necrosis. J Neurooncol. 2011; 103(1):137–146. [DOI] [PubMed] [Google Scholar]
- 113. Mitsuya K, Nakasu Y, Horiguchi S, et al. Perfusion weighted magnetic resonance imaging to distinguish the recurrence of metastatic brain tumors from radiation necrosis after stereotactic radiosurgery. J Neurooncol. 2010; 99(1):81–88. [DOI] [PubMed] [Google Scholar]
- 114. Rock JP, Scarpace L, Hearshen D, et al. Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery. 2004; 54(5):1111–1117; discussion 1117-1119. [DOI] [PubMed] [Google Scholar]
- 115. Galldiks N, Langen KJ, Albert NL, et al. PET imaging in patients with brain metastasis-report of the RANO/PET group. Neuro Oncol. 2019; 21(5):585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zach L, Guez D, Last D, et al. Delayed contrast extravasation MRI: a new paradigm in neuro-oncology. Neuro Oncol. 2015; 17(3):457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Barajas RF, Chang JS, Sneed PK, et al. Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol. 2009; 30(2):367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kaufmann TJ, Smits M, Boxerman J, et al. Consensus recommendations for a standardized brain tumor imaging protocol for clinical trials in brain metastases (BTIP-BM). Neuro Oncol. 2020; 22(6):757–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lin NU, Lee EQ, Aoyama H, et al. Response assessment criteria for brain metastases: proposal from the RANO group. Lancet Oncol. 2015; 16(6):e270–e278. [DOI] [PubMed] [Google Scholar]
- 120. Schiff D, Lee EQ, Nayak L, et al. Medical management of brain tumors and the sequelae of treatment. Neuro Oncol. 2015; 17(4):488–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Soffietti R, Ahluwalia M, Lin N, Ruda R. Management of brain metastases according to molecular subtypes. Nat Rev Neurol. 2020; 16(10):557–574. [DOI] [PubMed] [Google Scholar]
- 122. Bezjak A, Adam J, Barton R, et al. Symptom response after palliative radiotherapy for patients with brain metastases. Eur J Cancer. 2002; 38(4):487–496. [DOI] [PubMed] [Google Scholar]
- 123. Chow E, Fan G, Hadi S, et al. Symptom clusters in cancer patients with brain metastases. Clin Oncol (R Coll Radiol). 2008; 20(1):76–82. [DOI] [PubMed] [Google Scholar]
- 124. Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Radiosurgery with or without whole-brain radiotherapy for brain metastases: the patients’ perspective regarding complications. Am J Clin Oncol. 2005; 28(2):173–179. [DOI] [PubMed] [Google Scholar]
- 125. Soffietti R, Kocher M, Abacioglu UM, et al. A European Organisation for Research and Treatment of Cancer phase III trial of adjuvant whole-brain radiotherapy versus observation in patients with one to three brain metastases from solid tumors after surgical resection or radiosurgery: quality-of-life results. J Clin Oncol. 2013; 31(1):65–72. [DOI] [PubMed] [Google Scholar]
- 126. Lamba N, Mehanna E, Kearney RB, et al. Racial disparities in supportive medication use among older patients with brain metastases: a population-based analysis. Neuro Oncol. 2020; 22(9):1339–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Le Rhun E, Preusser M, van den Bent M, Andratschke N, Weller M. How we treat patients with leptomeningeal metastases. ESMO Open. 2019; 4(Suppl 2):e000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Le Rhun E, Devos P, Weller J, et al. Prognostic validation and clinical implications of the EANO ESMO classification of leptomeningeal metastasis from solid tumors. Neuro Oncol. 2021; 23(7):1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Pellerino A, Brastianos PK, Ruda R, Soffietti R. Leptomeningeal metastases from solid tumors: recent advances in diagnosis and molecular approaches. Cancers (Basel). 2021; 13(12):2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Roth P, Pace A, Le Rhun E, et al. Neurological and vascular complications of primary and secondary brain tumours: EANO-ESMO Clinical Practice Guidelines for prophylaxis, diagnosis, treatment and follow-up. Ann Oncol. 2021; 32(2):171–182. [DOI] [PubMed] [Google Scholar]
- 131. Le Rhun E, Guckenberger M, Smits M, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol. 2021; 32(11):1332–1347. [DOI] [PubMed] [Google Scholar]
- 132. Dixit KS, Kumthekar PU. Optimal management of corticosteroids in patients with intracranial malignancies. Curr Treat Options Oncol. 2020; 21(9):77. [DOI] [PubMed] [Google Scholar]
- 133. Aizer AA, Lee EQ. Brain metastases. Neurol Clin. 2018; 36(3):557–577. [DOI] [PubMed] [Google Scholar]
- 134. Ly KI, Wen PY. Clinical relevance of steroid use in neuro-oncology. Curr Neurol Neurosci Rep. 2017; 17(1):5. [DOI] [PubMed] [Google Scholar]
- 135. Arvold ND, Armstrong TS, Warren KE, et al. Corticosteroid use endpoints in neuro-oncology: Response Assessment in Neuro-Oncology Working Group. Neuro Oncol. 2018; 20(7):897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chang SM, Messersmith H, Ahluwalia M, et al. Anticonvulsant prophylaxis and steroid use in adults with metastatic brain tumors: summary of SNO and ASCO endorsement of the Congress of Neurological Surgeons guidelines. Neuro Oncol. 2019; 21(4):424–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Lee EQ, Wen PY. Corticosteroids for peritumoral edema: time to overcome our addiction? Neuro Oncol. 2016; 18(9):1191–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Arvold ND, Pinnell NE, Mahadevan A, et al. Steroid and anticonvulsant prophylaxis for stereotactic radiosurgery: large variation in physician recommendations. Pract Radiat Oncol. 2016; 6(4):e89–e96. [DOI] [PubMed] [Google Scholar]
- 139. Vecht CJ, Hovestadt A, Verbiest HB, van Vliet JJ, van Putten WL. Dose-effect relationship of dexamethasone on Karnofsky performance in metastatic brain tumors: a randomized study of doses of 4, 8, and 16 mg per day. Neurology. 1994; 44(4):675–680. [DOI] [PubMed] [Google Scholar]
- 140. Ryken TC, Kuo JS, Prabhu RS, et al. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of steroids in the treatment of adults with metastatic brain tumors. Neurosurgery. 2019; 84(3):E189–E191. [DOI] [PubMed] [Google Scholar]
- 141. Swartz SL, Dluhy RG. Corticosteroids: clinical pharmacology and therapeutic use. Drugs. 1978; 16(3):238–255. [DOI] [PubMed] [Google Scholar]
- 142. Spiegel RJ, Vigersky RA, Oliff AI, et al. Adrenal suppression after short-term corticosteroid therapy. Lancet. 1979; 1(8117):630–633. [DOI] [PubMed] [Google Scholar]
- 143. Cooley L, Dendle C, Wolf J, et al. Consensus guidelines for diagnosis, prophylaxis and management of Pneumocystis jirovecii pneumonia in patients with haematological and solid malignancies, 2014. Intern Med J. 2014; 44(12b):1350–1363. [DOI] [PubMed] [Google Scholar]
- 144. Maslov DV, Tawagi K, Kc M, et al. Timing of steroid initiation and response rates to immune checkpoint inhibitors in metastatic cancer. J ImmunoTher Cancer. 2021; 9(7):e002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Tawbi HA, Forsyth PA, Hodi FS, et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): final results of an open-label, multicentre, phase 2 study. Lancet Oncol. 2021; 22(12):1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tawbi HA, Forsyth PA, Hodi FS, et al. Safety and efficacy of the combination of nivolumab plus ipilimumab in patients with melanoma and asymptomatic or symptomatic brain metastases (CheckMate 204). Neuro Oncol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012; 13(5):459–465. [DOI] [PubMed] [Google Scholar]
- 148. Arbour KC, Mezquita L, Long N, et al. Impact of baseline steroids on efficacy of programmed cell death-1 and programmed death-ligand 1 blockade in patients with non-small-cell lung cancer. J Clin Oncol. 2018; 36(28):2872–2878. [DOI] [PubMed] [Google Scholar]
- 149. Ricciuti B, Dahlberg SE, Adeni A, et al. Immune checkpoint inhibitor outcomes for patients with non-small-cell lung cancer receiving baseline corticosteroids for palliative versus nonpalliative indications. J Clin Oncol. 2019; 37(22):1927–1934. [DOI] [PubMed] [Google Scholar]
- 150. Lauko A, Thapa B, Sharma M, et al. Neutrophil to lymphocyte ratio influences impact of steroids on efficacy of immune checkpoint inhibitors in lung cancer brain metastases. Sci Rep. 2021; 11(1):7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wolpert F, Lareida A, Terziev R, et al. Risk factors for the development of epilepsy in patients with brain metastases. Neuro Oncol. 2020; 22(5):718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lamba N, Catalano PJ, Cagney DN, et al. Seizures among patients with brain metastases: a population- and institutional-level analysis. Neurology. 2021; 96(8):e1237–e1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Cagney DN, Martin AM, Catalano PJ, et al. Implications of screening for brain metastases in patients with breast cancer and non-small cell lung cancer. JAMA Oncol. 2018; 4(7):1001–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Glantz MJ, Cole BF, Forsyth PA, et al. Practice parameter: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000; 54(10):1886–1893. [DOI] [PubMed] [Google Scholar]
- 155. Chen CC, Rennert RC, Olson JJ. Congress of neurological surgeons systematic review and evidence-based guidelines on the role of prophylactic anticonvulsants in the treatment of adults with metastatic brain tumors. Neurosurgery. 2019; 84(3):E195–E197. [DOI] [PubMed] [Google Scholar]
- 156. Walbert T, Harrison RA, Schiff D, et al. SNO and EANO practice guideline update: anticonvulsant prophylaxis in patients with newly diagnosed brain tumors. Neuro Oncol. 2021; 23(11):1835–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Glantz MJ, Cole BF, Friedberg MH, et al. A randomized, blinded, placebo-controlled trial of divalproex sodium prophylaxis in adults with newly diagnosed brain tumors. Neurology. 1996; 46(4):985–991. [DOI] [PubMed] [Google Scholar]
- 158. Forsyth PA, Weaver S, Fulton D, et al. Prophylactic anticonvulsants in patients with brain tumour. Can J Neurol Sci. 2003; 30(2):106–112. [DOI] [PubMed] [Google Scholar]
- 159. Wu AS, Trinh VT, Suki D, et al. A prospective randomized trial of perioperative seizure prophylaxis in patients with intraparenchymal brain tumors. J Neurosurg. 2013; 118(4):873–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Franceschetti S, Binelli S, Casazza M, et al. Influence of surgery and antiepileptic drugs on seizures symptomatic of cerebral tumours. Acta Neurochir (Wien). 1990; 103(1-2):47–51. [DOI] [PubMed] [Google Scholar]
- 161. North JB, Penhall RK, Hanieh A, et al. Postoperative epilepsy: a double-blind trial of phenytoin after craniotomy. Lancet. 1980; 1(8165):384–386. [DOI] [PubMed] [Google Scholar]
- 162. Iuchi T, Kuwabara K, Matsumoto M, et al. Levetiracetam versus phenytoin for seizure prophylaxis during and early after craniotomy for brain tumours: a phase II prospective, randomised study. J Neurol Neurosurg Psychiatry. 2015; 86(10):1158–1162. [DOI] [PubMed] [Google Scholar]
- 163. Wali AR, Rennert RC, Wang SG, Chen CC. Evidence-based recommendations for seizure prophylaxis in patients with brain metastases undergoing stereotactic radiosurgery. Acta Neurochir Suppl. 2021; 128:51–55. [DOI] [PubMed] [Google Scholar]
- 164. Ruda R, Mo F, Pellerino A. Epilepsy in brain metastasis: an emerging entity. Curr Treat Options Neurol. 2020; 22(2):6. [DOI] [PubMed] [Google Scholar]
- 165. Malow BA. Sleep deprivation and epilepsy. Epilepsy Curr. 2004; 4(5):193–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Rathlev NK, Ulrich AS, Delanty N, D’Onofrio G. Alcohol-related seizures. J Emerg Med. 2006; 31(2):157–163. [DOI] [PubMed] [Google Scholar]
- 167. Gunn BG, Baram TZ. Stress and seizures: space, time and hippocampal circuits. Trends Neurosci. 2017; 40(11):667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, MeltonLJ, 3rd. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med. 2000; 160(6):809–15. [DOI] [PubMed] [Google Scholar]
- 169. Wolpert F, Berghoff AS, Grossenbacher B, et al. Venous thromboembolic events in patients with brain metastases: the PICOS score. Eur J Cancer. 2020; 134:75–85. [DOI] [PubMed] [Google Scholar]
- 170. Farge D, Debourdeau P, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of venous thromboembolism in patients with cancer. J Thromb Haemost. 2013; 11(1):56–70. [DOI] [PubMed] [Google Scholar]
- 171. Raskob GE, van Es N, Verhamme P, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018; 378(7):615–624. [DOI] [PubMed] [Google Scholar]
- 172. Lyman GH, Bohlke K, Khorana AA, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update 2014. J Clin Oncol. 2015; 33(6):654–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Young AM, Marshall A, Thirlwall J, et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D). J Clin Oncol. 2018; 36(20):2017–2023. [DOI] [PubMed] [Google Scholar]
- 174. Hunter BD, Minichiello T, Bent S. Anticoagulation for the treatment of venous thromboembolism in patients with brain metastases: a meta-analysis and systematic review. J Thromb Thrombolysis. 2017; 44(3):392–398. [DOI] [PubMed] [Google Scholar]
- 175. Zwicker JI, Karp Leaf R, Carrier M. A meta-analysis of intracranial hemorrhage in patients with brain tumors receiving therapeutic anticoagulation. J Thromb Haemost. 2016; 14(9):1736–1740. [DOI] [PubMed] [Google Scholar]
- 176. Donato J, Campigotto F, Uhlmann EJ, et al. Intracranial hemorrhage in patients with brain metastases treated with therapeutic enoxaparin: a matched cohort study. Blood. 2015; 126(4):494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Alvarado G, Noor R, Bassett R, et al. Risk of intracranial hemorrhage with anticoagulation therapy in melanoma patients with brain metastases. Melanoma Res. 2012; 22(4):310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Horstman H, Gruhl J, Smith L, Ganti AK, Shonka NA. Safety of long-term anticoagulation in patients with brain metastases. Med Oncol. 2018; 35(4):43. [DOI] [PubMed] [Google Scholar]
- 179. Wood P, Boyer G, Mehanna E, et al. Intracerebral haemorrhage in patients with brain metastases receiving therapeutic anticoagulation. J Neurol Neurosurg Psychiatry. 2021:jnnp-2020–324488. [DOI] [PubMed] [Google Scholar]
- 180. Cagney DN, Martin AM, Catalano PJ, et al. Impact of pemetrexed on intracranial disease control and radiation necrosis in patients with brain metastases from non-small cell lung cancer receiving stereotactic radiation. Radiother Oncol. 2018; 126(3):511–518. [DOI] [PubMed] [Google Scholar]
- 181. Blonigen BJ, Steinmetz RD, Levin L, et al. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010; 77(4):996–1001. [DOI] [PubMed] [Google Scholar]
- 182. Donovan EK, Parpia S, Greenspoon JN. Incidence of radionecrosis in single-fraction radiosurgery compared with fractionated radiotherapy in the treatment of brain metastasis. Curr Oncol. 2019; 26(3):e328–e333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Giglio P, Gilbert MR. Cerebral radiation necrosis. Neurologist. 2003; 9(4):180–188. [DOI] [PubMed] [Google Scholar]
- 184. Kaidar-Person O, Zagar TM, Deal A, et al. The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases: the potential impact of immunotherapy. Anticancer Drugs. 2017; 28(6):669–675. [DOI] [PubMed] [Google Scholar]
- 185. Shi DD, Arnaout O, Bi WL, et al. Severe radiation necrosis refractory to surgical resection in patients with melanoma and brain metastases managed with ipilimumab/nivolumab and brain-directed stereotactic radiation therapy. World Neurosurg. 2020; 139:226–231. [DOI] [PubMed] [Google Scholar]
- 186. Rahman R, Alexander BM, Wen PY. Neurologic complications of cranial radiation therapy and strategies to prevent or reduce radiation toxicity. Curr Neurol Neurosci Rep. 2020; 20(8):34. [DOI] [PubMed] [Google Scholar]
- 187. Ahluwalia M, Barnett GH, Deng D, et al. Laser ablation after stereotactic radiosurgery: a multicenter prospective study in patients with metastatic brain tumors and radiation necrosis. J Neurosurg. 2018; 130(3):804–811. [DOI] [PubMed] [Google Scholar]
- 188. Le Rhun E, Wolpert F, Fialek M, et al. Response assessment and outcome of combining immunotherapy and radiosurgery for brain metastasis from malignant melanoma. ESMO Open. 2020; 5(4):e000763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Levin VA, Bidaut L, Hou P, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011; 79(5):1487–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Xu Y, Rong X, Hu W, et al. Bevacizumab monotherapy reduces radiation-induced brain necrosis in nasopharyngeal carcinoma patients: a randomized controlled trial. Int J Radiat Oncol Biol Phys. 2018; 101(5):1087–1095. [DOI] [PubMed] [Google Scholar]
- 191. Glantz MJ, Burger PC, Friedman AH, et al. Treatment of radiation-induced nervous system injury with heparin and warfarin. Neurology. 1994; 44(11):2020–2027. [DOI] [PubMed] [Google Scholar]
- 192. Co J, De Moraes MV, Katznelson R, et al. Hyperbaric oxygen for radiation necrosis of the brain. Can J Neurol Sci. 2019; 47(1):92–99. [DOI] [PubMed] [Google Scholar]
- 193. Williamson R, Kondziolka D, Kanaan H, Lunsford LD, Flickinger JC. Adverse radiation effects after radiosurgery may benefit from oral vitamin E and pentoxifylline therapy: a pilot study. Stereotact Funct Neurosurg. 2008; 86(6):359–366. [DOI] [PubMed] [Google Scholar]
- 194. Meyers CA, Smith JA, Bezjak A, et al. Neurocognitive function and progression in patients with brain metastases treated with whole-brain radiation and motexafin gadolinium: results of a randomized phase III trial. J Clin Oncol. 2004; 22(1):157–165. [DOI] [PubMed] [Google Scholar]
- 195. Louie AV, Chan E, Hanna M, et al. Assessing fitness to drive in brain tumour patients: a grey matter of law, ethics, and medicine. Curr Oncol. 2013; 20(2):90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Withaar FK, Brouwer WH, van Zomeren AH. Fitness to drive in older drivers with cognitive impairment. J Int Neuropsychol Soc. 2000; 6(4):480–490. [DOI] [PubMed] [Google Scholar]
- 197. Ashendorf L, Alosco ML, Bing-Canar H, et al. Clinical utility of select neuropsychological assessment battery tests in predicting functional abilities in dementia. Arch Clin Neuropsychol. 2017; 33(5):530–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Li J, Bentzen SM, Li J, Renschler M, Mehta MP. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008; 71(1):64–70. [DOI] [PubMed] [Google Scholar]
- 199. Noll KR, Weinberg JS, Ziu M, et al. Neurocognitive changes associated with surgical resection of left and right temporal lobe glioma. Neurosurgery. 2015; 77(5):777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Wilke C, Grosshans D, Duman J, Brown P, Li J. Radiation-induced cognitive toxicity: pathophysiology and interventions to reduce toxicity in adults. Neuro Oncol. 2018; 20(5):597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015; 65(2):123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Velasco R, Villagran M, Jove M, et al. Encephalitis induced by immune checkpoint inhibitors: a systematic review. JAMA Neurol. 2021; 78(7):864–873. [DOI] [PubMed] [Google Scholar]
- 203. Wefel JS, Parsons MW, Gondi V, Brown PD. Neurocognitive aspects of brain metastasis. Handb Clin Neurol. 2018; 149:155–165. [DOI] [PubMed] [Google Scholar]
- 204. Karschnia P, Parsons MW, Dietrich J. Pharmacologic management of cognitive impairment induced by cancer therapy. Lancet Oncol. 2019; 20(2):e92–e102. [DOI] [PubMed] [Google Scholar]
- 205. Rapp SR, Case LD, Peiffer A, et al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015; 33(15):1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Meyers CA, Weitzner MA, Valentine AD, Levin VA. Methylphenidate therapy improves cognition, mood, and function of brain tumor patients. J Clin Oncol. 1998; 16(7):2522–2527. [DOI] [PubMed] [Google Scholar]
- 207. Butler JM, Jr., Case LD, Atkins J, et al. A phase III, double-blind, placebo-controlled prospective randomized clinical trial of d-threo-methylphenidate HCl in brain tumor patients receiving radiation therapy. Int J Radiat Oncol Biol Phys. 2007; 69(5):1496–1501. [DOI] [PubMed] [Google Scholar]
- 208. Coomans MB, van der Linden SD, Gehring K, Taphoorn MJB. Treatment of cognitive deficits in brain tumour patients: current status and future directions. Curr Opin Oncol. 2019; 31(6):540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. van Lonkhuizen PJC, Klaver KM, Wefel JS, et al. Interventions for cognitive problems in adults with brain cancer: a narrative review. Eur J Cancer Care (Engl). 2019; 28(3):e13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Yang S, Chun MH, Son YR. Effect of virtual reality on cognitive dysfunction in patients with brain tumor. Ann Rehabil Med. 2014; 38(6):726–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Maschio M, Dinapoli L, Fabi A, Giannarelli D, Cantelmi T. Cognitive rehabilitation training in patients with brain tumor-related epilepsy and cognitive deficits: a pilot study. J Neurooncol. 2015; 125(2):419–426. [DOI] [PubMed] [Google Scholar]
- 212. Parsons MW, Peters KB, Floyd SR, Brown P, Wefel J. Preservation of neurocognitive function in the treatment of brain metastases. Neuro-Oncol Adv. 2021; 3( 5):v96–v107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Ratnaike TE, Das S, Gregson BA, Mendelow AD. A review of brain abscess surgical treatment--78 years: aspiration versus excision. World Neurosurg. 2011; 76(5):431–436. [DOI] [PubMed] [Google Scholar]
- 214. Lamba N, Cagney DN, Brigell RH, et al. Neurosurgical resection and stereotactic radiation versus stereotactic radiation alone in patients with a single or solitary brain metastasis. World Neurosurg. 2019; 122:e1557–e1561. [DOI] [PubMed] [Google Scholar]
- 215. Mishra MV, Louie AV, Gondi V, Slotman B. The evolving role of radiotherapy in the management of small cell lung cancer. J Thorac Dis. 2018; 10(Suppl 21):S2545–S2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Jonska-Gmyrek J, Peczkowski P, Michalski W, et al. Radiotherapy in testicular germ cell tumours - a literature review. Contemp Oncol (Pozn). 2017; 21(3):203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015; 13:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Minniti G, Clarke E, Lanzetta G, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011; 6:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Vecht CJ, Haaxma-Reiche H, Noordijk EM, et al. Treatment of single brain metastasis: radiotherapy alone or combined with neurosurgery? Ann Neurol. 1993; 33(6):583–590. [DOI] [PubMed] [Google Scholar]
- 220. Noordijk EM, Vecht CJ, Haaxma-Reiche H, et al. The choice of treatment of single brain metastasis should be based on extracranial tumor activity and age. Int J Radiat Oncol Biol Phys. 1994; 29(4):711–717. [DOI] [PubMed] [Google Scholar]
- 221. Mintz AH, Kestle J, Rathbone MP, et al. A randomized trial to assess the efficacy of surgery in addition to radiotherapy in patients with a single cerebral metastasis. Cancer. 1996; 78(7):1470–1476. [DOI] [PubMed] [Google Scholar]
- 222. Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009; 10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 223. Kocher M, Soffietti R, Abacioglu U, et al. Adjuvant whole-brain radiotherapy versus observation after radiosurgery or surgical resection of one to three cerebral metastases: results of the EORTC 22952-26001 study. J Clin Oncol. 2011; 29(2):134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Brown PD, Jaeckle K, Ballman KV, et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: a randomized clinical trial. JAMA. 2016; 316(4):401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Brown PD, Ballman KV, Cerhan JH, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017; 18(8):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Cagney DN, Lamba N, Sinha S, et al. Association of neurosurgical resection with development of pachymeningeal seeding in patients with brain metastases. JAMA Oncol. 2019; 5(5):703–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Prabhu RS, Turner BE, Asher AL, et al. A multi-institutional analysis of presentation and outcomes for leptomeningeal disease recurrence after surgical resection and radiosurgery for brain metastases. Neuro Oncol. 2019; 21(8):1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Peak S, Abrey LE. Chemotherapy and the treatment of brain metastases. Hematol Oncol Clin North Am. 2006; 20(6):1287–1295. [DOI] [PubMed] [Google Scholar]
- 229. Chamberlain M, Junck L, Brandsma D, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2017; 19(4):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Torres-Reveron J, Tomasiewicz HC, Shetty A, Amankulor NM, Chiang VL. Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol. 2013; 113(3):495–503. [DOI] [PubMed] [Google Scholar]
- 231. Hong CS, Kundishora AJ, Elsamadicy AA, Chiang VL. Laser interstitial thermal therapy in neuro-oncology applications. Surg Neurol Int. 2020; 11:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Meng Y, Hynynen K, Lipsman N. Applications of focused ultrasound in the brain: from thermoablation to drug delivery. Nat Rev Neurol. 2021; 17(1):7–22. [DOI] [PubMed] [Google Scholar]
- 233. Horton J, Baxter DH, Olson KB. The management of metastases to the brain by irradiation and corticosteroids. Am J Roentgenol Radium Ther Nucl Med. 1971; 111(2):334–336. [DOI] [PubMed] [Google Scholar]
- 234. Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA 1998; 280(17):1485–1489. [DOI] [PubMed] [Google Scholar]
- 235. Kotecha R, Gondi V, Ahluwalia MS, Brastianos PK, Mehta MP. Recent advances in managing brain metastasis. F1000Res. 2018; 7:F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Mehta MP. The controversy surrounding the use of whole-brain radiotherapy in brain metastases patients. Neuro Oncol. 2015; 17(7):919–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Mulvenna P, Nankivell M, Barton R, et al. Dexamethasone and supportive care with or without whole brain radiotherapy in treating patients with non-small cell lung cancer with brain metastases unsuitable for resection or stereotactic radiotherapy (QUARTZ): results from a phase 3, non-inferiority, randomised trial. Lancet. 2016; 388(10055):2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Brown PD, Pugh S, Laack NN, et al. Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: a randomized, double-blind, placebo-controlled trial. Neuro Oncol. 2013; 15(10):1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Raber J, Rola R, LeFevour A, et al. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiat Res. 2004; 162(1):39–47. [DOI] [PubMed] [Google Scholar]
- 240. Monje ML, Mizumatsu S, Fike JR, Palmer TD. Irradiation induces neural precursor-cell dysfunction. Nat Med. 2002; 8(9):955–962. [DOI] [PubMed] [Google Scholar]
- 241. Gondi V, Tomé WA, Mehta MP. Why avoid the hippocampus? A comprehensive review. Radiother Oncol. 2010; 97(3):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Gondi V, Tolakanahalli R, Mehta MP, et al. Hippocampal-sparing whole-brain radiotherapy: a “how-to” technique using helical tomotherapy and linear accelerator-based intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010; 78(4):1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014; 32(34):3810–3816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Gondi V, Pugh S, Brown P D, et al. NCOG-01. Preservation of neurocognitive function (NCF) with hippocampal avoidance during whole-brain radiotherapy (WBRT) for brain metastases: preliminary results of phase III trial NRG oncology CC001. Neuro-Oncology. 2018; 20(Suppl 6):vi172–vi172. [Google Scholar]
- 245. Brown PD, Gondi V, Pugh S, et al. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol. 2020; 38(10):1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Caruso JP, Moosa S, Fezeu F, Ramesh A, Sheehan JP. A cost comparative study of Gamma Knife radiosurgery versus open surgery for intracranial pathology. J Clin Neurosci. 2015; 22(1):184–188. [DOI] [PubMed] [Google Scholar]
- 247. Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006; 295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 248. Yamamoto M, Serizawa T, Shuto T, et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): a multi-institutional prospective observational study. Lancet Oncol. 2014; 15(4):387–395. [DOI] [PubMed] [Google Scholar]
- 249. Mahajan A, Ahmed S, McAleer MF, et al. Post-operative stereotactic radiosurgery versus observation for completely resected brain metastases: a single-centre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017; 18(8):1040–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Kayama T, Sato S, Sakurada K, et al. Effects of surgery with salvage stereotactic radiosurgery versus surgery with whole-brain radiation therapy in patients with one to four brain metastases (JCOG0504): a phase III, noninferiority, randomized controlled trial. J Clin Oncol. 2018; JCO2018786186. [DOI] [PubMed] [Google Scholar]
- 251. Kepka L, Tyc-Szczepaniak D, Bujko K, et al. Stereotactic radiotherapy of the tumor bed compared to whole brain radiotherapy after surgery of single brain metastasis: results from a randomized trial. Radiother Oncol. 2016; 121(2):217–224. [DOI] [PubMed] [Google Scholar]
- 252. Roth O’Brien DA, Kaye SM, Poppas PJ, et al. Time to administration of stereotactic radiosurgery to the cavity after surgery for brain metastases: a real-world analysis. J Neurosurg. 2021; 1–11. [DOI] [PubMed] [Google Scholar]
- 253. Soliman H, Ruschin M, Angelov L, et al. Consensus contouring guidelines for postoperative completely resected cavity stereotactic radiosurgery for brain metastases. Int J Radiat Oncol Biol Phys. 2018; 100(2):436–442. [DOI] [PubMed] [Google Scholar]
- 254. Prabhu RS, Patel KR, Press RH, et al. Preoperative vs postoperative radiosurgery for resected brain metastases: a review. Neurosurgery. 2019; 84(1):19–29. [DOI] [PubMed] [Google Scholar]
- 255. Robin TP, Jones BL, Amini A, et al. Radiosurgery alone is associated with favorable outcomes for brain metastases from small-cell lung cancer. Lung Cancer. 2018; 120:88–90. [DOI] [PubMed] [Google Scholar]
- 256. Rusthoven CG, Yamamoto M, Bernhardt D, et al. Evaluation of first-line radiosurgery vs whole-brain radiotherapy for small cell lung cancer brain metastases: The FIRE-SCLC Cohort Study. JAMA Oncol. 2020; 6(7):1028–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Werner-Wasik M, Rudoler S, Preston PE, et al. Immediate side effects of stereotactic radiotherapy and radiosurgery. Int J Radiat Oncol Biol Phys. 1999; 43(2):299–304. [DOI] [PubMed] [Google Scholar]
- 258. Ohguri T, Imada H, Kohshi K, et al. Effect of prophylactic hyperbaric oxygen treatment for radiation-induced brain injury after stereotactic radiosurgery of brain metastases. Int J Radiat Oncol Biol Phys. 2007; 67(1):248–255. [DOI] [PubMed] [Google Scholar]
- 259. Chao ST, Ahluwalia MS, Barnett GH, et al. Challenges with the diagnosis and treatment of cerebral radiation necrosis. Int J Radiat Oncol Biol Phys. 2013; 87(3):449–457. [DOI] [PubMed] [Google Scholar]
- 260. Vellayappan B, Tan CL, Yong C, et al. Diagnosis and management of radiation necrosis in patients with brain metastases. Front Oncol. 2018; 8:395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Flickinger JC, Kondziolka D, Lunsford LD, et al. Development of a model to predict permanent symptomatic postradiosurgery injury for arteriovenous malformation patients. Arteriovenous Malformation Radiosurgery Study Group. Int J Radiat Oncol Biol Phys. 2000; 46(5):1143–1148. [DOI] [PubMed] [Google Scholar]
- 262. Martin AM, Cagney DN, Catalano PJ, et al. Immunotherapy and symptomatic radiation necrosis in patients with brain metastases treated with stereotactic radiation. JAMA Oncol. 2018; 4(8):1123–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Colaco RJ, Martin P, Kluger HM, Yu JB, Chiang VL. Does immunotherapy increase the rate of radiation necrosis after radiosurgical treatment of brain metastases? J Neurosurg. 2016; 125(1):17–23. [DOI] [PubMed] [Google Scholar]
- 264. Minniti G, Filippi AR, Osti MF, Ricardi U. Radiation therapy for older patients with brain tumors. Radiat Oncol. 2017; 12(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Farris M, McTyre ER, Cramer CK, et al. Brain metastasis velocity: a novel prognostic metric predictive of overall survival and freedom from whole-brain radiation therapy after distant brain failure following upfront radiosurgery alone. Int J Radiat Oncol Biol Phys. 2017; 98(1):131–141. [DOI] [PubMed] [Google Scholar]
- 266. Suh JH, Barnett GH. Brachytherapy for brain tumor. Hematol Oncol Clin North Am. 1999; 13(3):635–650, viii–ix. [DOI] [PubMed] [Google Scholar]
- 267. Ruge MI, Kickingereder P, Grau S, et al. Stereotactic biopsy combined with stereotactic (125)iodine brachytherapy for diagnosis and treatment of locally recurrent single brain metastases. J Neurooncol. 2011; 105(1):109–118. [DOI] [PubMed] [Google Scholar]
- 268. Chitti B, Goyal S, Sherman JH, et al. The role of brachytherapy in the management of brain metastases: a systematic review. J Contemp Brachytherapy. 2020; 12(1):67–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007; 25(16):2295–2305. [DOI] [PubMed] [Google Scholar]
- 270. Reungwetwattana T, Nakagawa K, Cho BC, et al. CNS response to osimertinib versus standard epidermal growth factor receptor tyrosine kinase inhibitors in patients with untreated EGFR-mutated advanced non-small-cell lung cancer. J Clin Oncol. 2018; 36(33):JCO2018783118. [DOI] [PubMed] [Google Scholar]
- 271. Shaw AT, Bauer TM, de Marinis F, et al. First-line lorlatinib or crizotinib in advanced ALK-positive lung cancer. N Engl J Med. 2020;383(21):2018–2029. [DOI] [PubMed] [Google Scholar]
- 272. Gadgeel SM, Gandhi L, Riely GJ, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014; 15(10):1119–1128. [DOI] [PubMed] [Google Scholar]
- 273. Shaw AT, Felip E, Bauer TM, et al. Lorlatinib in non-small-cell lung cancer with ALK or ROS1 rearrangement: an international, multicentre, open-label, single-arm first-in-man phase 1 trial. Lancet Oncol. 2017; 18(12):1590–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Planchard D, Besse B, Groen HJ, et al. Dabrafenib plus trametinib in patients with previously treated BRAF(V600E)-mutant metastatic non-small cell lung cancer: an open-label, multicentre phase 2 trial. Lancet Oncol. 2016; 17(7):984–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Capmatinib could alter NSCLC treatment landscape. Cancer Discov. 2020; 10(6):OF4. [DOI] [PubMed] [Google Scholar]
- 276. Guo R, Schreyer M, Chang JC, et al. Response to selective RET inhibition with LOXO-292 in a patient with RET fusion-positive lung cancer with leptomeningeal metastases. JCO Precis Oncol. 2019;3:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Offin M, Feldman D, Ni A, et al. Frequency and outcomes of brain metastases in patients with HER2-mutant lung cancers. Cancer. 2019; 125(24):4380–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Canon J, Rex K, Saiki AY, et al. The clinical KRAS(G12C) inhibitor AMG 510 drives anti-tumour immunity. Nature. 2019; 575(7781):217–223. [DOI] [PubMed] [Google Scholar]
- 279. Janne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015; 372(18):1689–1699. [DOI] [PubMed] [Google Scholar]
- 280. Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: AURA Study Phase II Extension Component. J Clin Oncol. 2017; 35(12):1288–1296. [DOI] [PubMed] [Google Scholar]
- 281. Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016; 17(7):976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Bearz A, Garassino I, Tiseo M, et al. Activity of Pemetrexed on brain metastases from Non-Small Cell Lung Cancer. Lung Cancer. 2010; 68(2):264–268. [DOI] [PubMed] [Google Scholar]
- 283. He Q, Bi X, Ren C, et al. Phase II study of the efficacy and safety of high-dose pemetrexed in combination with cisplatin versus temozolomide for the treatment of non-small cell lung cancer with brain metastases. Anticancer Res. 2017; 37(8):4711–4716. [DOI] [PubMed] [Google Scholar]
- 284. Goldberg SB, Schalper KA, Gettinger SN, et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020; 21(5):655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Paz-Ares LG, Ciuleanu T-E, Lee J-S, et al. Nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first-line (1L) treatment for advanced non-small cell lung cancer (NSCLC): 4-year update from CheckMate 227. J Clin Oncol. 2021; 39(15_suppl):9016–9016. [Google Scholar]
- 286. Li S, Zhang H, Liu T, Chen J, Dang J. The effect of asymptomatic and/or treated brain metastases on efficacy of immune checkpoint inhibitors in metastatic non-small cell lung cancer: a meta-analysis. Front Oncol. 2021; 11:702924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Zhang T, Wan B, Zhao Y, et al. Treatment of uncommon EGFR mutations in non-small cell lung cancer: new evidence and treatment. Transl Lung Cancer Res. 2019; 8(3):302–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Yun J, Lee SH, Kim SY, et al. Antitumor activity of amivantamab (JNJ-61186372), an EGFR-MET bispecific antibody, in diverse models of EGFR Exon 20 insertion-driven NSCLC. Cancer Discov. 2020; 10(8):1194–1209. [DOI] [PubMed] [Google Scholar]
- 289. Iuchi T, Shingyoji M, Sakaida T, et al. Phase II trial of gefitinib alone without radiation therapy for Japanese patients with brain metastases from EGFR-mutant lung adenocarcinoma. Lung Cancer. 2013; 82(2):282–287. [DOI] [PubMed] [Google Scholar]
- 290. Wu YL, Zhou C, Cheng Y, et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann Oncol. 2013; 24(4):993–999. [DOI] [PubMed] [Google Scholar]
- 291. Schuler M, Wu YL, Hirsh V, et al. First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J Thorac Oncol. 2016; 11(3):380–390. [DOI] [PubMed] [Google Scholar]
- 292. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 2020; 382(1):41–50. [DOI] [PubMed] [Google Scholar]
- 293. Goldman JW, Ramirez SV, Mahipal A, et al. Neratinib efficacy in a subgroup of patients with EGFR exon 18-mutant non-small cell lung cancer (NSCLC) and central nervous system (CNS) involvement: findings from the SUMMIT basket trial. J Clin Oncol. 2021; 39(15_suppl):9068–9068. [Google Scholar]
- 294. Ou SH, Ahn JS, De Petris L, et al. Alectinib in crizotinib-refractory ALK-rearranged non-small-cell lung cancer: a phase II global study. J Clin Oncol. 2016; 34(7):661–668. [DOI] [PubMed] [Google Scholar]
- 295. Tsui DCC, Camidge DR. Molecular profiling of the cerebrospinal fluid in leptomeningeal NSCLC: the shape of things to come? J Thorac Oncol. 2021; 16(2):194–196. [DOI] [PubMed] [Google Scholar]
- 296. Crino L, Ahn MJ, De Marinis F, et al. Multicenter phase II study of whole-body and intracranial activity with ceritinib in patients with ALK-rearranged non-small-cell lung cancer previously treated with chemotherapy and crizotinib: results from ASCEND-2. J Clin Oncol. 2016; 34(24):2866–2873. [DOI] [PubMed] [Google Scholar]
- 297. Kim DW, Mehra R, Tan DS, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016; 17(4):452–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Soria JC, Tan DSW, Chiari R, et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): a randomised, open-label, phase 3 study. Lancet. 2017; 389(10072):917–929. [DOI] [PubMed] [Google Scholar]
- 299. Shaw AT, Gandhi L, Gadgeel S, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016; 17(2):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Gadgeel S, Peters S, Mok T, et al. Alectinib versus crizotinib in treatment-naive anaplastic lymphoma kinase-positive (ALK+) non-small-cell lung cancer: CNS efficacy results from the ALEX study. Ann Oncol. 2018; 29(11):2214–2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Gettinger SN, Bazhenova LA, Langer CJ, et al. Activity and safety of brigatinib in ALK-rearranged non-small-cell lung cancer and other malignancies: a single-arm, open-label, phase 1/2 trial. Lancet Oncol. 2016; 17(12):1683–1696. [DOI] [PubMed] [Google Scholar]
- 302. Kim DW, Tiseo M, Ahn MJ, et al. Brigatinib in patients with crizotinib-refractory anaplastic lymphoma kinase-positive non-small-cell lung cancer: a randomized, multicenter phase II trial. J Clin Oncol. 2017; 35(22):2490–2498. [DOI] [PubMed] [Google Scholar]
- 303. Solomon BJ, Besse B, Bauer TM, et al. Lorlatinib in patients with ALK-positive non-small-cell lung cancer: results from a global phase 2 study. Lancet Oncol. 2018; 19(12):1654–1667. [DOI] [PubMed] [Google Scholar]
- 304. Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in advanced ALK inhibitor-naive ALK-positive non-small cell lung cancer: second interim analysis of the phase III ALTA-1L trial. J Clin Oncol. 2020; 38(31):3592–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Camidge DR, Kim HR, Ahn MJ, et al. Brigatinib versus crizotinib in ALK-positive non-small-cell lung cancer. N Engl J Med. 2018; 379(21):2027–2039. [DOI] [PubMed] [Google Scholar]
- 306. Novello S, Mazieres J, Oh IJ, et al. Alectinib versus chemotherapy in crizotinib-pretreated anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer: results from the phase III ALUR study. Ann Oncol. 2018; 29(6):1409–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Lin JJ, Riely GJ, Shaw AT. Targeting ALK: precision medicine takes on drug resistance. Cancer Discov. 2017; 7(2):137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Camidge DR. Lorlatinib should not be considered as the preferred first-line option in patients with advanced ALK rearranged NSCLC. J Thorac Oncol. 2021; 16(4):528–531. [DOI] [PubMed] [Google Scholar]
- 309. Pacheco JM, Gao D, Smith D, et al. Natural history and factors associated with overall survival in stage IV ALK-rearranged non-small cell lung cancer. J Thorac Oncol. 2019; 14(4):691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Bauer TM, Felip E, Solomon BJ, et al. Clinical management of adverse events associated with lorlatinib. Oncologist. 2019; 24(8):1103–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor-naive epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017; 35(10):1070–1077. [DOI] [PubMed] [Google Scholar]
- 312. Lin NU, Pegram M, Sahebjam S, et al. Pertuzumab plus high-dose trastuzumab in patients with progressive brain metastases and HER2-positive metastatic breast cancer: primary analysis of a phase II study. J Clin Oncol. 2021; 39(24):2667–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Montemurro F, Delaloge S, Barrios CH, et al. Trastuzumab emtansine (T-DM1) in patients with HER2-positive metastatic breast cancer and brain metastases: exploratory final analysis of cohort 1 from KAMILLA, a single-arm phase IIIb clinical trial. Ann Oncol. 2020; 31(10):1350–1358. [DOI] [PubMed] [Google Scholar]
- 314. Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013; 14(1):64–71. [DOI] [PubMed] [Google Scholar]
- 315. Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009; 15(4):1452–1459. [DOI] [PubMed] [Google Scholar]
- 316. Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019; 37(13):1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317. Lin NU, Borges V, Anders C, et al. Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol. 2020; 38(23):2610–2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Jerusalem GHM, Park YH, Yamashita T, et al. Trastuzumab deruxtecan (T-DXd) in patients with HER2+ metastatic breast cancer with brain metastases: a subgroup analysis of the DESTINY-Breast01 trial. J Clin Oncol. 2021; 39(15_suppl):526–526. [Google Scholar]
- 319. Diéras VWR, Tolaney S, et al. Subgroup analysis of patients with brain metastases from the phase 3 ASCENT study of sacituzumab govitecan versus chemotherapy in metastatic triple-negative breast cancer [abstract]. In: Proceedings of the 2020 San Antonio Breast Cancer Virtual Symposium; 2020 Dec 8-11; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2021;81(4 Suppl):Abstract nr PD13-07.
- 320. Kim JS, Kim IA. Evolving treatment strategies of brain metastases from breast cancer: current status and future direction. Ther Adv Med Oncol. 2020; 12:1758835920936117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Tolaney SM, Sahebjam S, Le Rhun E, et al. A phase II study of abemaciclib in patients with brain metastases secondary to hormone receptor-positive breast cancer. Clin Cancer Res. 2020; 26(20):5310–5319. [DOI] [PubMed] [Google Scholar]
- 322. Batalini F, Moulder SL, Winer EP, et al. Response of brain metastases from PIK3CA-mutant breast cancer to alpelisib. JCO Precis Oncol. 2020; 4:572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323. Exman P, Mallery RM, Lin NU, Parsons HA. Response to olaparib in a patient with germline BRCA2 mutation and breast cancer leptomeningeal carcinomatosis. NPJ Breast Cancer. 2019; 5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Leone JP, Emblem KE, Weitz M, et al. Phase II trial of carboplatin and bevacizumab in patients with breast cancer brain metastases. Breast Cancer Res. 2020; 22(1):131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Fabi A, Terrenato I, Vidiri A, et al. Eribulin in brain metastases of breast cancer: outcomes of the EBRAIM prospective observational trial. Future Oncol. 2021; 17(26):3445–3456. [DOI] [PubMed] [Google Scholar]
- 326. Ekenel M, Hormigo AM, Peak S, Deangelis LM, Abrey LE. Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol. 2007; 85(2):223–227. [DOI] [PubMed] [Google Scholar]
- 327. Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017; 18(7):863–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Robert C, Grob JJ, Stroyakovskiy D, et al. Five-year outcomes with dabrafenib plus trametinib in metastatic melanoma. N Engl J Med. 2019; 381(7):626–636. [DOI] [PubMed] [Google Scholar]
- 329. Drago JZ, Lawrence D, Livingstone E, et al. Clinical experience with combination BRAF/MEK inhibitors for melanoma with brain metastases: a real-life multicenter study. Melanoma Res. 2019; 29(1):65–69. [DOI] [PubMed] [Google Scholar]
- 330. Holbrook K, Lutzky J, Davies MA, et al. Intracranial antitumor activity with encorafenib plus binimetinib in patients with melanoma brain metastases: a case series. Cancer. 2020; 126(3):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Goldinger SM, Valeska Matter A, Urner-Bloch U, et al. Binimetinib in heavily pretreated patients with NRAS-mutant melanoma with brain metastases. Br J Dermatol. 2020; 182(2):488–490. [DOI] [PubMed] [Google Scholar]
- 332. Welsh SJ, Corrie PG. Management of BRAF and MEK inhibitor toxicities in patients with metastatic melanoma. Ther Adv Med Oncol. 2015; 7(2):122–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Long GV, Atkinson V, Lo S, et al. Five-year overall survival from the anti-PD1 brain collaboration (ABC Study): randomized phase 2 study of nivolumab (nivo) or nivo+ipilimumab (ipi) in patients (pts) with melanoma brain metastases (mets). J Clin Oncol. 2021; 39(15_suppl):9508–9508. [Google Scholar]
- 334. Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018; 19(5):672–681. [DOI] [PubMed] [Google Scholar]
- 335. Tawbi HA, Forsyth PA, Hodi FS, et al. Long-term outcomes of patients with active melanoma brain metastases treated with combination nivolumab plus ipilimumab (CheckMate 204): final results of an open-label, multicentre, phase 2 study. Lancet Oncol. 2021; 22(12):1692–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2018; 36(17):1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Amin S, Baine MJ, Meza JL, Lin C. Association of immunotherapy with survival among patients with brain metastases whose cancer was managed with definitive surgery of the primary tumor. JAMA Netw Open. 2020; 3(9):e2015444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Qian JM, Martin AM, Martin K, et al. Response rate and local recurrence after concurrent immune checkpoint therapy and radiotherapy for non-small cell lung cancer and melanoma brain metastases. Cancer. 2020; 126(24):5274–5282. [DOI] [PubMed] [Google Scholar]
- 339. Alvarez-Breckenridge C, Giobbie-Hurder A, Gill CM, et al. Upfront surgical resection of melanoma brain metastases provides a bridge toward immunotherapy-mediated systemic control. Oncologist. 2019; 24(5):671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Lin NU, Wefel JS, Lee EQ, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol. 2013; 14(10):e407–e416. [DOI] [PubMed] [Google Scholar]
- 341. Lin NU, Lee EQ, Aoyama H, et al. Challenges relating to solid tumour brain metastases in clinical trials, part 1: patient population, response, and progression. A report from the RANO group. Lancet Oncol. 2013; 14(10):e396–e406. [DOI] [PubMed] [Google Scholar]
- 342. Galldiks N, Kocher M, Ceccon G, et al. Imaging challenges of immunotherapy and targeted therapy in patients with brain metastases: response, progression, and pseudoprogression. Neuro Oncol. 2020; 22(1):17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343. Thompson EM, Frenkel EP, Neuwelt EA. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology. 2011; 76(1):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Alexander BM, Brown PD, Ahluwalia MS, et al. Clinical trial design for local therapies for brain metastases: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018; 19(1):e33–e42. [DOI] [PubMed] [Google Scholar]
- 345. Okada H, Weller M, Huang R, et al. Immunotherapy response assessment in neuro-oncology: a report of the RANO working group. Lancet Oncol. 2015; 16(15):e534–e542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Camidge DR, Lee EQ, Lin NU, et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018; 19(1):e20–e32. [DOI] [PubMed] [Google Scholar]
- 347. Chukwueke UN, Wen PY. Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol. 2019; 8(1):CNS28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Excellence USDoHaHSFaDAOCo. Cancer Clinical Trial Eligibility Criteria: Brain Metastases. Silver Spring, MD: Guidance for Industry; 2020. [Google Scholar]
- 349. Excellence USDoHaHSFaDAOCo. Evaluating Cancer Drugs in Patients with Central Nervous System Metastases. Silver Spring, MD: Guidance for Industry; 2021. [DOI] [PubMed] [Google Scholar]
- 350. Yang JCH, Kim SW, Kim DW, et al. Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: The BLOOM Study. J Clin Oncol. 2020; 38(6):538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Ahluwalia MS, Chao ST, Parsons MW, et al. Phase II trial of sunitinib as adjuvant therapy after stereotactic radiosurgery in patients with 1-3 newly diagnosed brain metastases. J Neurooncol. 2015; 124(3):485–491. [DOI] [PubMed] [Google Scholar]
- 352. Lin NU, Prowell T, Tan AR, et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology-Friends of Cancer Research Brain Metastases Working Group. J Clin Oncol. 2017; 35(33):3760–3773. [DOI] [PubMed] [Google Scholar]
- 353. Silvestre J, Gosse T, Read P, et al. Genesis of quality measurements to improve the care delivered to patients with brain metastases. JCO Oncol Pract. 2021; 17(3):e397–e405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.