Abstract
Dynamin 2 mutations are associated with Charcot-Marie-Tooth neuropathy. We report two siblings with a novel missense heterozygous point mutation (c.1609 G>A) in the highly conserved pleckstrin homology domain in exon 15 of Dynamin 2 presenting with progressive length-dependent sensorimotor polyneuropathy with mixed demyelinating and axonal features on electrodiagnostic studies. The previously unrecognized missense point mutation, which was inherited from their symptomatic but previously undiagnosed mother, was determined to be likely pathogenic based on a non-conservative amino acid substitution (p.Gly537Ser) that is predicted to damage secondary protein structure or function. This report emphasizes the importance of recognizing inherited neuropathies in clinical practice and evaluating suspected pathogenic gene variants initially classified to be of undetermined clinical significance in family cohorts. These cases add to the spectrum of pathogenic Dynamin 2 mutations associated with dominant-intermediate Charcot-Marie-Tooth neuropathy.
Keywords: case report, Charcot-Marie-Tooth neuropathy, Dynamin 2, familial genetic studies, pathogenic gene mutations
Introduction
Charcot-Marie-Tooth (CMT) neuropathy is a heterogeneous group of hereditary sensory and motor neuropathies that typically present with a phenotype of foot deformities such as pes cavus (abnormally high plantar longitudinal foot arches) and hammertoes, progressive distal muscle weakness and atrophy that preferentially involves the peroneal muscles early in the disease course, multimodal sensory loss, and decreased or absent myotactic stretch reflexes. Different gene mutations that impair Schwann cell, myelin, and axonal structure or function cause CMT neuropathy.1-3 Over 60 genes associated with CMT neuropathy have been discovered to date. The most common subclass, CMT Type 1, or hereditary motor and sensory neuropathy type 1 (HMSN 1) consists of primary demyelinating polyneuropathies with diffuse reduction in motor nerve conduction velocities (NCV; <38 m/s in the upper limbs). Axonal CMT or CMT2 is characterized by preservation or mild reduction of NCVs with reduced motor amplitudes. There are also subclasses with histopathological evidence of demyelination and prominent axonal degeneration on nerve biopsies with mildly decreased (25-45 m/s) or normal median NCV, classified as dominant-intermediate (DI)-CMT.1-3
Dynamin 2 (DNM2) is a guanosine triphosphatase (GTPase) that mediates vesicle budding, organelle fission and fusion, and clathrin-coated endocytosis and acts with other proteins such as actin, endophilin, and amphiphysin. It is composed of 5 domains: (1) N-terminal GTPase domain, (2) middle domain, (3) the lipophilic pleckstrin homology [PH] domain that interacts with membrane phosphatidylinositol 4,5-bisphosphate, (4) GTPase effector domain, and (5) proline/arginine-rich domain at the C-terminus.4-7 DNM2 mutations are commonly associated with axonal CMT neuropathy type 2M (CMT2M; inherited in an autosomal dominant pattern) or DI-CMT neuropathy type B (DI-CMTB),1-3,7-12 as well as centronuclear myopathy (CNM), lethal congenital contractures syndrome type 5, and hereditary spastic paraplegia,7,8,13,14 implying phenotypic variability in inherited neuromuscular disorders.
We report 2 cases of a progressive sensorimotor polyneuropathy in adult siblings harboring a previously unrecognized DNM2 mutation that was inherited from their symptomatic but previously undiagnosed mother, consistent with DI-CMTB. This report further emphasizes the importance of recognizing CMT neuropathy to provide genetic counseling while excluding other chronic neuropathies for which treatment options are available. Performing familial genetic studies in clinical practice to evaluate variants of undetermined significance in potentially relevant genes that are hypothesized to alter transcribed protein structure or function is important to confirm a CMT neuropathy diagnosis.
Case Report
Case 1 (Proband)
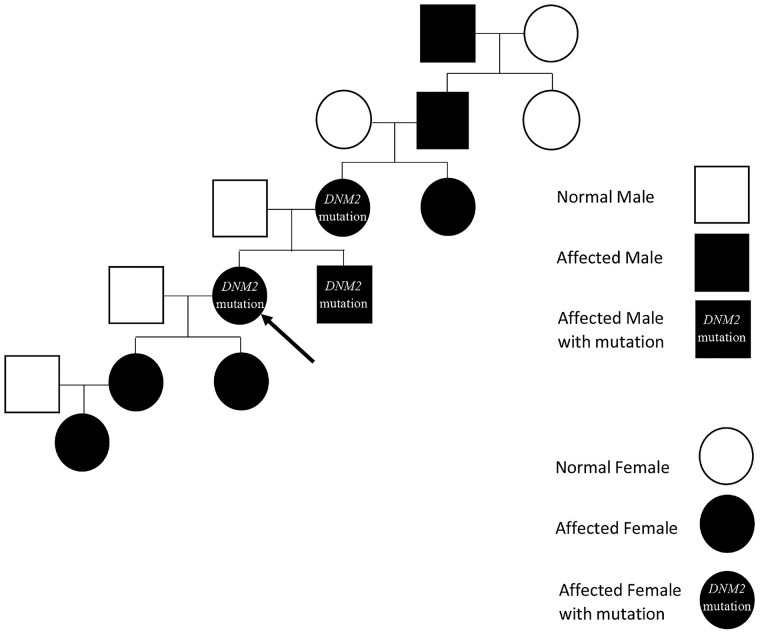
This is a 59-year-old woman diagnosed with CMT neuropathy at 16 years of age based on clinical assessment and electrodiagnostic (EDX) studies performed in 1979. Data from that clinical assessment were not available for review. She returned to the neuromuscular clinic after a long absence requesting confirmatory genetic testing. She reported a family history of presumed CMT neuropathy based on foot drop and intrinsic hand weakness in her maternal grandfather, mother, brother, two daughters, and one granddaughter, although genetic testing or EDX studies had not been performed on extended family members (Figure 1). She complained of progressive weakness, numbness, and tingling in her hands and feet, associated with pain and swelling in her feet, shoulders, and arms. She had been using ankle-foot orthoses (AFOs) since age 19 and denied any falls or need for a cane or walker while using the AFOs.
Figure 1.
Family pedigree. The Proband’s family pedigree demonstrates an autosomal dominant inheritance pattern in the genetically confirmed and clinically suspected individuals indicated.
Neurologic examination showed muscle atrophy in the hands and feet, with finger flexion contractures. Confrontational strength examination (based on the Medical Research Council [MRC] scale) showed normal proximal upper and lower limb muscle strength with wrist and finger extension 3/5, finger abduction and adduction 1/5, ankle dorsiflexion 1/5, and plantarflexion 3/5 weakness bilaterally. Sensation to pinprick, proprioception, and vibration were all subjectively decreased from feet to mid-shins. Myotactic stretch reflexes were absent at the knees and ankles, diminished at brachioradialis and normal at the biceps and triceps on both sides.
Case 2
The Proband’s brother is a 58-year-old man who presented with appendicular and low back pain with progressive reduced manual dexterity and imbalance since childhood. Due to work-related difficulties and a clinical suspicion of CMT neuropathy, he was referred to the neuromuscular clinic for specialist evaluation. He had elective carpal tunnel decompression surgeries at a private clinical facility (data not available for review) and underwent magnetic resonance imaging of the lumbar spine with and without contrast at an external facility that showed broad-based disk bulge, bilateral facet joint degeneration changes, and mild-to-moderate bilateral neuroforaminal narrowing at the L4-L5 and L5-S1 levels, associated with Grade 1 L5-S1 anterior spondylolisthesis. Prior to his initial neuromuscular clinic evaluation, he underwent lumbar epidural spinal injections at a private practice pain clinic that improved the pain and numbness in his feet. He mentioned that his older sister had been diagnosed with CMT neuropathy as a teenager.
Examination revealed muscle atrophy of the hands, feet, and distal legs with high arched feet and high steppage gait. Confrontational strength testing based on the MRC scale showed finger abduction, adduction and extension (4/5), ankle plantarflexion (3/5), and ankle dorsiflexion (2/5) weakness bilaterally. All other muscle groups were normal. Sensory examination revealed subjectively decreased proprioception and absent vibratory sense at the hallux with decreased light touch and pinprick sensation below the knees bilaterally. Myotactic stretch reflexes were absent at the ankles and normal elsewhere.
Comprehensive laboratory tests for acquired causes of polyneuropathy (comprehensive metabolic panel; complete blood count with differentials; hemoglobin A1c; thyroid function tests; vitamins B1, B6, B12, and D levels; serum electrophoresis; serum immunofixation; Lyme disease serology; autoimmune neuropathy panel [antiganglioside IgG/IgM, anti-myelin-associated glycoprotein IgM; and anti-sulfate-3-glucuronyl paragloboside IgM antibodies]), and 24-hour urine heavy metal screen was normal. EDX studies (Table 1) demonstrated a moderately severe, chronic length-dependent predominantly axonal sensorimotor polyneuropathy with some demyelinating features and no conduction block, as seen in DI-CMTB.
Table 1.
Case 2 Nerve Conduction Study Data.
| Side/nerve | Stimulation site | Recording site | Distal latency (ms) | Amplitude (mV) | Normal amplitude (mV) | Negative waveform duration (ms) | Conduction velocity (m/s) |
|---|---|---|---|---|---|---|---|
| Motor nerve conduction studies | |||||||
| R Median | Wrist | APB | 4.4 | 0.1 | >7 | 17.9 | |
| Elbow | APB | 12.6 | 0.1 | 29.3 | 30 a (≥50) | ||
| R Ulnar | Wrist | ADM | 3.2 | 10.1 | >7 | 15.4 | |
| Below elbow | ADM | 8.9 | 9.4 | 22.7 | 37 a (≥50) | ||
| Above elbow | ADM | 12.1 | 8.5 | 22.7 | 31 | ||
| R Peroneal | Ankle | EDB | NR | >2.5 | |||
| Fibula | EDB | NR | NR (≥40) | ||||
| R Peroneal | Fibula | TA | 4.2 | 2.4 | >4 | 45.8 | |
| Popliteal fossa | TA | 6.3 | 2.4 | 43.7 | 38 (≥40) | ||
| R Tibial | Ankle | AHB | 5.2 | 0.2 | >4 | 24.8 | |
| Popliteal fossa | AHB | NR | NR (≥40) | ||||
| Sensory nerve conduction studies | |||||||
| R Median | Wrist | Digit II | 4.1 | 3.0 | >15 | 1.2 | 30 a (≥50) |
| R Ulnar | Wrist | Digit V | 3.6 | 4.0 | >12 | 1.6 | 28 a (≥50) |
| R Radial | Forearm | Snuff box | 3.0 | 4.0 | >15 | 1.8 | 33 a (≥50) |
| R Sural | Foreleg | Ankle | NR | >3 | NR (≥40) | ||
| R Superficial Peroneal | Foreleg | Ankle | NR | >3 | NR (≥40) | ||
Motor and sensory nerve conduction data from case 2 demonstrate a moderately severe, chronic length-dependent predominantly axonal sensorimotor polyneuropathy with several conduction velocities in the demyelinating range without conduction block, consistent with intermediate forms of CMT neuropathy. Abnormal values indicated in bold type. Brackets indicate normal conduction velocity values.
Abbreviations: R, Right; APB, abductor pollicis brevis; ADM, abductor digiti minimi; EDB, extensor digitorum brevis; NR, not recordable; TA, tibialis anterior; AHB, abductor hallucis brevis; CMT, Charcot-Marie-Tooth.
Demyelinating range conduction velocity.
To evaluate a clinical suspicion of autosomal dominant CMT neuropathy in our patients, the Hereditary Neuropathy genetic test panel was performed by GeneDx (Gaithersburg, Maryland) using oral rinse specimens. Using genomic DNA from the submitted specimens, the coding regions and splice junctions of the genes in the panel were enriched via GeneDx’s proprietary-targeted capture system. These targeted regions were sequenced simultaneously using massively parallel (NextGen) sequencing on an Illumina platform using pair-end reads. Bi-directional sequence was assembled, aligned to reference gene sequences based on human genome build GRCh37/UCSC hg19, and analyzed for sequence variants.
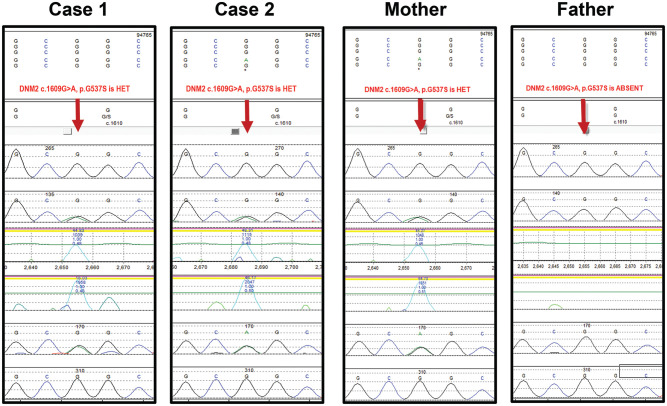
Genetic testing identified a heterozygous missense point mutation in exon 15 of the DNM2 gene (c.1609 G>A) on chromosome 19, resulting in substitution of glycine 537 to serine (p.Gly537Ser) in the Proband and her brother (see arrow, Figure 2). This is a non-conservative amino acid substitution, which likely impacts the highly conserved PH domain protein structure and function based on GeneDx’s in silico analysis. Importantly, a different amino acid substitution at the same position, c.1609 G>T (p.Gly573Cys), 15 and the same point mutation in a nearby residue, c.1597 G>T (p.Gly533Cys), 10 are associated with DNM2-related disorders. As the mutation had neither been previously published as a pathologic variant nor reported as a benign variant, it was initially classified as a variant of undetermined significance.
Figure 2.
DNA electropherograms. The clinically likely pathogenic heterozygous DNM2 mutation (c.1609 G>A) is demonstrated in the Proband (case 1), her brother (case 2), and their clinically affected mother. The normal allele is present in their unaffected father, indicating maternal dominant gene transmission. HET = Heterozygous.
Due to our high index of suspicion that this mutation was pathogenically significant based on the clinical phenotypes that were consistent with CMT neuropathy and the potential impact of the mutation on secondary protein structure or function, the patients’ undiagnosed symptomatic mother and asymptomatic father underwent targeted genetic testing. Their mother possessed the same heterozygous missense DNM2 point mutation which was absent in their father (Figure 2), supporting our inference that DNM2 (c.1609 G>A) is causative of DI-CMTB in this family cohort.
Clinical Follow-up
During follow-up neuromuscular clinic visits over the next 2-3 years, the Proband (case 1) reported increased falls despite wearing AFOs; worsening pain in multiple joints, including her hips and knees; progressive weakness in her hands with reduced dexterity; and persistent paresthesias in her feet, adequately treated with Gabapentin. Her brother (case 2) reported worsening hand and leg strength and progressive difficulty standing for prolonged periods and walking. He decided against using AFOs due to perceived discomfort and walked cautiously to prevent falls without using ambulatory aids.
Discussion
We report a novel heterozygous DNM2 c.1609 G>A mutation that results in amino acid substitution, p.Gly537Ser, in three family members that expands the repertoire of known mutations associated with autosomal dominant CMT neuropathy. Previously identified pathogenic or likely pathogenic DMN2 mutations are accessible via the United States of America National Library of Medicine National Center for Biotechnology Information ClinVar website (accessible at https://www.ncbi.nlm.nih.gov/clinvar).
The EDX study in case 2 demonstrated absent or markedly low lower limb sensory and distal motor responses with the upper limbs involved but less affected, in keeping with a length-dependent axonopathy. However, minor features of demyelination were noted in the ulnar motor nerve conduction study with a normal compound motor action potential and conduction velocity slowing below the cutoff value of 38 m/s expected in CMT2. In addition, the right median motor, right ulnar sensory, and right superficial radial sensory conduction velocities were in the demyelinating range. The clinical history and physical examination findings, EDX data, and genetic testing were most consistent with DI-CMTB.
Pathogenic DNM2 mutations, including those associated with DI-CMTB and CMT2M, commonly alter the conserved PH domain,10,12-15 consistent with the patients’ mutation. These mutations may alter Schwann cell fission, endocytosis, and transport by disrupting the cytoskeleton and Schwann cell and axon interactions6,7,12 but could directly affect peripheral nerve axons with or without Schwann cell membrane instability. 4
Conclusions
In summary, we report a novel heterozygous missense point mutation c.1609 G>A that results in p.Gly537Ser in the highly conserved DNM2 PH domain in two adult siblings with a CMT neuropathy phenotype, consistent with a diagnosis of DI-CMTB. Identical gene expression in their symptomatic mother, implying an autosomal dominant inheritance pattern in this family, supports that this mutation is pathogenic. This case report emphasizes the importance of pursuing potentially causative gene variants of undetermined significance by targeted familial genetic testing in symptomatic and asymptomatic individuals in clinical practice. Furthermore, the case report adds to the spectrum of clinically likely pathologic DNM2 mutations associated with DI-CMTB. Functional studies using relevant in vitro or animal model systems would further confirm the significance and pathologic relevance of this mutation.
Acknowledgments
We would like to thank Taylor Zuck, MS, CGC, Lead Genetic Counselor at GeneDx, for providing the DNA electropherograms. Special thanks to our patients who agreed to allow scientific publication of their family pedigree and de-identified clinical data in a publicly accessible scientific journal.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iDs: Kenkichi Nozaki  https://orcid.org/0000-0002-0992-1362
https://orcid.org/0000-0002-0992-1362
Eroboghene E. Ubogu  https://orcid.org/0000-0002-8307-9994
https://orcid.org/0000-0002-8307-9994
References
- 1. Pipis M, Rossor AM, Laura M, Reilly MM. Next-generation sequencing in Charcot-Marie-Tooth disease: opportunities and challenges. Nat Rev Neurol. 2019;15:644-656. doi: 10.1038/s41582-019-0254-5. [DOI] [PubMed] [Google Scholar]
- 2. Laura M, Pipis M, Rossor AM, Reilly MM. Charcot-Marie-Tooth disease and related disorders: an evolving landscape. Curr Opin Neurol. 2019;32:641-650. doi: 10.1097/WCO.0000000000000735. [DOI] [PubMed] [Google Scholar]
- 3. Klein CJ. Charcot-Marie-Tooth disease and other hereditary neuropathies. Continuum (Minneap Minn). 2020;26:1224-1256. doi: 10.1212/CON.0000000000000927. [DOI] [PubMed] [Google Scholar]
- 4. Faelber K, Gao S, Held M, et al. Oligomerization of dynamin superfamily proteins in health and disease. Prog Mol Biol Transl Sci. 2013;117:411-443. doi: 10.1016/B978-0-12-386931-9.00015-5. [DOI] [PubMed] [Google Scholar]
- 5. Gu C, Chang J, Shchedrina VA, et al. Regulation of dynamin oligomerization in cells: the role of dynamin-actin interactions and its GTPase activity. Traffic. 2014;15:819-838. doi: 10.1111/tra.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heymann JA, Hinshaw JE. Dynamins at a glance. J Cell Sci. 2009;122:3427-3431. doi: 10.1242/jcs.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Durieux AC, Prudhon B, Guicheney P, et al. Dynamin 2 and human diseases. J Mol Med (Berl). 2010;88:339-350. doi: 10.1007/s00109-009-0587-4. [DOI] [PubMed] [Google Scholar]
- 8. Chen S, Huang P, Qiu Y, et al. Phenotype variability and histopathological findings in patients with a novel DNM2 mutation. Neuropathology. 2018;38:34-40. doi: 10.1111/neup.12432. [DOI] [PubMed] [Google Scholar]
- 9. Claeys KG, Zuchner S, Kennerson M, et al. Phenotypic spectrum of dynamin 2 mutations in Charcot-Marie-Tooth neuropathy. Brain. 2009;132:1741-1752. doi: 10.1093/brain/awp115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fabrizi GM, Ferrarini M, Cavallaro T, et al. Two novel mutations in dynamin-2 cause axonal Charcot-Marie-Tooth disease. Neurology. 2007;69:291-295. doi: 10.1212/01.wnl.0000265820.51075.61. [DOI] [PubMed] [Google Scholar]
- 11. Sidiropoulos PN, Miehe M, Bock T, et al. Dynamin 2 mutations in Charcot-Marie-Tooth neuropathy highlight the importance of clathrin-mediated endocytosis in myelination. Brain. 2012;135:1395-1411. doi: 10.1093/brain/aws061. [DOI] [PubMed] [Google Scholar]
- 12. Tanabe K, Takei K. Dynamin 2 in Charcot-Marie-Tooth disease. Acta Med Okayama. 2012;66:183-190. doi: 10.18926/AMO/48557. [DOI] [PubMed] [Google Scholar]
- 13. Bitoun M, Bevilacqua JA, Prudhon B, et al. Dynamin 2 mutations cause sporadic centronuclear myopathy with neonatal onset. Ann Neurol. 2007;62:666-670. doi: 10.1002/ana.21235. [DOI] [PubMed] [Google Scholar]
- 14. Bohm J, Biancalana V, Dechene ET, et al. Mutation spectrum in the large GTPase dynamin 2, and genotype-phenotype correlation in autosomal dominant centronuclear myopathy. Hum Mutat. 2012;33:949-959. doi: 10.1002/humu.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stenson PD, Mort M, Ball EV, Shaw K, Phillips A, Cooper DN. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Hum Genet. 2014;133:1-9. doi: 10.1007/s00439-013-1358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]