Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), are chronic immune-mediated inflammatory diseases that share epidemiologic and genetic associations with other autoimmune diseases, including multiple sclerosis (MS).1,2 However, IBD phenotype and clinical outcomes among patients with MS who develop new-onset IBD (MS-IBD) have not been well studied. Furthermore, antitumor necrosis factor therapy (anti-TNF), a cornerstone for treating aggressive IBD, can induce demyelination and may be associated with relapses of MS. Consequently, patients with MS-IBD may be less likely to receive anti-TNF therapy and may suffer from suboptimal IBD outcomes.3,4
We present a longitudinal, retrospective cohort composed of 28 patients with MS who developed new-onset IBD (MS-IBD) and were matched 1:4 to 112 patients with isolated IBD. We evaluated demographic and phenotypic characteristics of IBD in MS-IBD. Additionally, we examined the use of IBD-directed therapies and clinical IBD outcomes among MS-IBD patients compared with IBD patients alone.
Materials and Methods
We performed a longitudinal retrospective cohort study of patients with MS and IBD from 3 hospitals in the Partners Health Care system in the greater Boston area. Patients with MS-IBD were identified using the International Classification of Diseases, 9th or 10th edition codes for CD, UC, and MS. Patients with MS-IBD must have had an existing diagnosis of MS prior to a new-onset diagnosis of IBD. Patients with MS-IBD were matched 1:4 to IBD controls in respect to sex, age of diagnosis, and IBD phenotype (CD or UC). Disease extent and distribution were defined by Montreal classification within 3 months of diagnosis. An IBD-related surgery was defined as either incision and drainage, seton insertion, fistulotomy, small bowel resection, partial colonic resection, or colectomy. Immunosuppressives were defined as any immunomodulators, biologics, Jak kinase inhibitors, or sphingosine 1 phosphate receptor (S1p) modulators. Follow-up time was defined as date of IBD diagnosis to last clinic follow-up date.
We determined the (1) demographics and phenotype of IBD (4), the cumulative risk of exposure to IBD-directed immunosuppressives, and (3) the risk of progression to IBD-related surgery among patients with MS-IBD compared with IBD. Covariates included family history of inflammatory disease, age of IBD diagnosis, IBD behavior, and extent. Continuous variables were summarized with means and standard deviations, whereas categorical variables were described using proportions. Categorical variables were analyzed using χ2 or Fisher exact test to calculate odds ratios and 95% confidence intervals (CIs). Non-normal continuous data were analyzed with the Mann-Whitney U test. Time-to-event survival analysis was performed with log-rank tests, and multivariate cox proportional hazard analysis was performed with disease extent and age at diagnosis as covariates. Statistical significance was defined as P < .05. All analyses were performed using R studio (Version 1.1.456). Ethical approval was obtained from the Partners Institutional Review Board, Boston, MA, and anonymity of patient data was carefully protected.
Results
Demographics of MS-IBD
Twenty-eight patients with MS who developed new-onset IBD and 112 matched patients with isolated IBD were identified. Patients with MS-IBD were initially diagnosed with MS and developed IBD within a median of 6 years (interquartile range [IQR], 4-13 yrs). Age of IBD diagnosis was higher among those with MS-IBD compared with IBD alone (39 vs 32, P < .005). Frequencies of stricturing or fistulizing CD (17.9% vs 17.9%), ileocolonic CD (17.9% vs 29.5%) and pan-UC (21.4% vs 22.3%) were comparable between MS-IBD and IBD. Other autoimmune disorders were more common in MS-IBD patients compared with isolated IBD (25% vs 8.9%; P < .05; Table 1). Follow-up time was comparable between MS-IBD and isolated IBD patients (median 9 yrs vs 15 yrs, P = .07)
Table 1.
Demographic and Phenotypic Characteristics of MS-IBD vs IBD.
| MS-IBD | IBD | OR | P | ||
|---|---|---|---|---|---|
| n | 28 | 112 | |||
| Crohn’s disease (%) | 14 (50) | 55 (49.1) | |||
| Ulcerative colitis (%) | 14 (50) | 57 (50.9) | |||
| Fam Hx IBD (%) | 8 (28.6) | 37 (33.0) | 0.85 | .822 | |
| Fam Hx Autoim (%) | 13 (46.4) | 50 (44.6) | 1.15 | 1.000 | |
| Age (SD) | 51.7 (11.8) | 49 (13.5) | 0.291 | ||
| Age of IBD diagnosis (SD) | 39.4 (10.6) | 32.3 (12.8) | < 0.005 | ||
| Other co-autoimmune disorders | 7 (25%) | 10 (8.9%) | < 0.05 | ||
| Montreal Classification | A1 | 0 | 7 (12.7) | 0 | 0.330 |
| A2 | 10 (71.4) | 38 (69.1) | 1.49 | 1.000 | |
| A3 | 4 (28.6) | 10 (18.2) | 1.35 | 0.460 | |
| L1 | 5 (35.7) | 11 (20) | 1.78 | 0.287 | |
| L2 | 4 (28.6) | 9 (16.4) | 2.27 | 0.443 | |
| L3 | 5 (35.7) | 33 (60) | 0.42 | 0.136 | |
| B1 | 9 (54.3) | 32 (58.2) | 1.15 | 0.767 | |
| B2 | 3 (21.4) | 17 (30.9) | 0.67 | 0.743 | |
| B3 | 2 (14.3) | 17 (29.8) | 0.64 | 0.324 | |
| E1 | 6 (42.9) | 25 (43.9) | 0.71 | 1.000 | |
| E2 | 2 (14.3) | 17 (29.8) | 0.64 | 0.324 | |
| E3 | 6 (42.9) | 25 (43.9) | 0.71 | 1.000 | |
| Thiopurine use (%) | 9 (32.1) | 59 (52.7) | 0.45 | 0.059 | |
| Anti-TNF (%) | 2 (7.1) | 55 (49.1) | 0.08 | < 0.005 | |
| Natalizumab (%) | 7 (25.0) | 2 (1.8) | 15.71 | < 0.005 | |
| Anti-CD20 (%) | 6 (21.4) | 0 | - | - | |
| Ustekinumab | 0 | 3 (2.7) | - | - | |
| Frequency of Surgery | 6 (21.4) | 31 (27.7) | 0.59 | 0.634 | |
Immunosuppressive and Biologic Utilization in MS-IBD
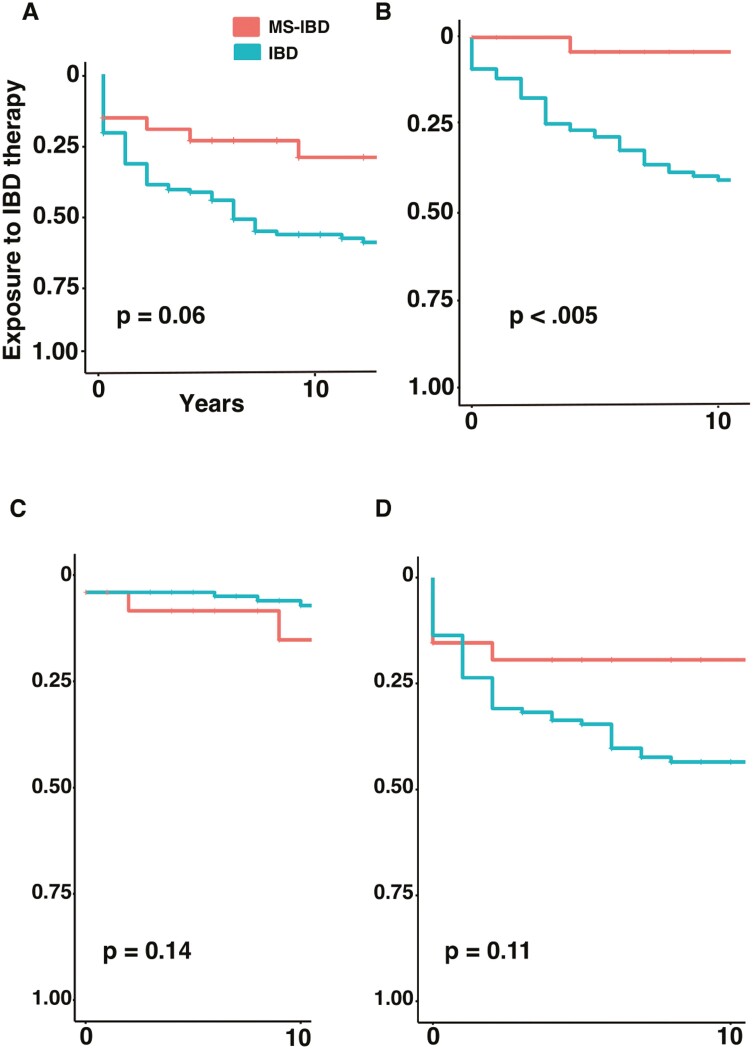
Frequency of immunosuppressive use was similar between MS-IBD and IBD (81.5% vs 78.9%). The cumulative risk of starting immunosuppressives was comparable in MS-IBD compared with IBD (1 yr cumulative risk, 15.4% vs 31.7%, hazard ratio [HR] 53, 95% CI, 0.27-1.03; and 3-yr cumulative risk, 19.4% vs 40.8%, Figure 1A).
Figure 1.
Cumulative risk of exposure to IBD-directed therapy among patients with MS-IBD (red) and IBD (green) including time to initiation of (A) any immunosuppressive, (B) tumor necrosis factor inhibitors, (C) vedolizumab, or (D) thiopurines.
The frequency of anti-TNF therapy use was significantly lower in MS-IBD (7.1% vs 49.1%, P < .005). The cumulative risk of starting anti-TNF therapy after IBD diagnosis in patients with MS-IBD was significantly lower than in patients with IBD alone (1 yr cumulative risk, 0 vs 11.8%, HR 14, 95% CI, 0.03-0.59; and 3 yr cumulative risk, 0 vs 24.6%; Figure 1B).
Rates of vedolizumab use were similar in MS-IBD compared with IBD patients alone (11.1% vs 6.3%). The cumulative risk of starting vedolizumab therapy was low and similar between the 2 groups (1 yr cumulative risk was 0% for both groups, HR 2.69, 95% CI 0.10-1.45; and 3 yr cumulative risk, 4.4% vs 0%; Figure 1C).
Frequency of thiopurine use was similar in patients with MS-IBD (32.1% vs 52.7%). The cumulative risk of starting thiopurine therapy was similar between the 2 groups (1 yr cumulative risk 15.4% vs 23.6%, HR 0.56, 95% CI 0.85-3.75; and 3 yr cumulative risk, 19.4% vs 31.8%; Figure 1D).
Patients with MS who developed new-onset IBD were more likely to be started on natalizumab (25% vs 1.8%, P < .005). Ustekinumab was not utilized in MS-IBD, although it was used in IBD alone at low rates (0% vs 2.7%). Anti-CD20 therapies were frequently used in MS-IBD, including rituximab and ocrelizumab (21.4%), as was S1p modulation. Most patients with MS-IBD were exposed to interferon therapy (78.6%) during their MS course.
We performed a Cox multivariate regression analysis; and after adjusting for age of IBD diagnosis, disease behavior, and disease extent, we did not find that an MS diagnosis conferred an independent risk for time to initiate immunosuppressive use (HR 0.63, 95% CI 0.80-3.13, Supplementary Table 1).
Of the 2 MS-IBD patients for whom anti-TNFs were used, anti-TNF therapy did not worsen MS. In the first case, a 45-year-old woman with MS was started on 6-mercaptopurine and infliximab for Crohn’s disease, with neurologic consultation and follow-up neuroimaging. There was no progression of MS while on infliximab. In the second case, a 27-year-old woman with MS was treated with azathioprine and infliximab for her Crohn’s disease. Following loss of response to infliximab, she was switched to natalizumab. There was no progression of MS during her anti-TNF therapy.
Surgical Outcomes
Patients with MS-IBD and IBD patients had similar rates of IBD-related surgery (21.4% vs 27.7%, P < 1; Table 1). Patients with MS-IBD had a similar rate of progression to IBD-related surgery compared with IBD patients alone, with a 1-year cumulative risk of IBD-related surgery of 4.2% vs 9.2% and a 3-year cumulative risk of 4.2% vs 11.9% (HR, 0.65; 95% CI, 0.54-4.40). After adjusting for age of IBD diagnosis, disease behavior, and disease extent, a Cox multivariate regression analysis showed that an MS diagnosis did not confer an independent risk for time to IBD-related surgery (HR, 0.96; 95% CI, 0.35-3.06; Supplementary Table 2).
Discussion
In this study, we utilized a longitudinal cohort of 140 patients to examine new-onset IBD among patients with MS. First, we found that patients with MS-IBD shared a similar IBD phenotype to those with IBD in isolation, with comparable frequencies of stricturing or fistulizing CD or pancolonic UC. Second, despite similar rates of moderate to severe IBD in both groups, patients with MS-IBD had a reduced cumulative risk of initiating anti-TNF therapy (0% at 1 and 3 years following diagnosis). Instead, MS-IBD patients were more likely to be started on natalizumab, whereas rates of thiopurine and vedolizumab use were similar between both groups. Third, while anti-TNF use was reduced in MS-IBD, avoiding anti-TNF therapy did not increase the risk of IBD-related surgery in MS-IBD.
We did not find that an MS diagnosis conferred an independent risk factor for use of immunosuppressives despite the lower utilization of anti-TNF therapy in MS-IBD, likely because alternatives to anti-TNF therapy were available. Provider knowledge that anti-TNF therapy may worsen CNS inflammation may have led to use of other agents to treat both diseases. Higher rates of natalizumab in MS-IBD is likely secondary to the drug having moderate efficacy at treating MS and IBD simultaneously, although use of the drug in this context follows a restricted distribution program due to risk of progressive multifocal leukoencephalopathy.5 Vedolizumab and ustekinumab may prove useful in treating IBD alongside MS-directed therapies. Emerging therapies including recently approved S1p modulators (ozanimod) may have utility in treating both disorders.6,7
The strengths of this study include the use of a longitudinal and matched cohort of patients. Although Zephir et al examined IBD outcomes in MS-IBD,8 their study was cross-sectional and examined only 2 time points. Additionally, they studied patients who developed MS and IBD in either order of diagnosis, rather than evaluating new-onset IBD among patients with MS. Furthermore, they did not examine the use of biologics across drug classes, nor did they perform time-to-event analyses. One of the limitations of this study is that we did not ascertain severity of IBD through biochemical markers, although we did find the frequency of aggressive and extensive IBD to be similar between both groups.
Conclusion
We find that the phenotype of IBD in MS-IBD does not appreciably vary compared with IBD in isolation. However, patients with IBD in MS-IBD were less likely to initiate anti-TNF therapy, more likely to be prescribed natalizumab, and equally as likely to be given thiopurines or vedolizumab. Despite avoidance of anti-TNF therapy, patients with MS-IBD were not more likely to suffer surgical complications. Consequently, we underline continued avoidance of anti-TNF therapy in patients with MS-IBD.
Supplementary Material
Contributor Information
Shiv Gandhi, Division of Gastroenterology, Tufts Medical Center, Boston, MA, United States.
Sara Zelman, Division of Gastroenterology, Tufts Medical Center, Boston, MA, United States.
Ricardo Eduardo De Armas, Division of Gastroenterology, Brigham and Women’s Hospital, Boston, MA, United States.
Christopher Hemond, Division of Neurology, University of Massachusetts Medical School, Worcester, MA, United States.
Alexander N Levy, Division of Gastroenterology, Tufts Medical Center, Boston, MA, United States.
Siddharth Singh, Division of Gastroenterology, University of California, La Jolla, CA, United States.
Joshua Korzenik, Division of Gastroenterology, Brigham and Women’s Hospital, Boston, MA, United States.
Sushrut Jangi, Division of Gastroenterology, Tufts Medical Center, Boston, MA, United States.
Funding
None
Conflicts of Interest
S.S. is supported by NIH/NIDDK K23DK117058 and R03DK129631. He has received research grants from AbbVie and Janssen in the last 24 months and personal fees from Takeda and Pfizer. All other authors declare no conflicts of interest.
References
- 1. Schreiber S, Colombel JF, Feagan BG, et al. Incidence rates of inflammatory bowel disease in patients with psoriasis, psoriatic arthritis and ankylosing spondylitis treated with secukinumab: a retrospective analysis of pooled data from 21 clinical trials. Ann Rheum Dis. 2019;78(4):473–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lees CW, Barrett JC, Parkes M, et al. New IBD genetics: common pathways with other diseases. Gut. 2011;60(12):1739–1753. [DOI] [PubMed] [Google Scholar]
- 3. Thomas CW, Jr., Weinshenker BG, Sandborn WJ.. Demyelination during anti-tumor necrosis factor alpha therapy with infliximab for Crohn’s disease. Inflamm Bowel Dis. 2004;10(1):28–31. [DOI] [PubMed] [Google Scholar]
- 4. Singh S, Kumar N, LoftusEV, Jr, et al. Neurologic complications in patients with inflammatory bowel disease: increasing relevance in the era of biologics. Inflamm Bowel Dis. 2013;19(4):864–872. [DOI] [PubMed] [Google Scholar]
- 5. Honey K. The comeback kid: TYSABRI now FDA approved for Crohn disease. J Clin Invest. 2008;118(3):825–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sandborn WJ, Feagan BG, Hanauer S, et al. Long-term efficacy and safety of ozanimod in moderate-to-severe ulcerative colitis: results from the open-label extension of the randomized, phase 2 touchstone study. J Crohns Colitis. 2021;15(7):1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Comi G, Kappos L, Selmaj KW, et al. Safety and efficacy of ozanimod versus interferon beta-1a in relapsing multiple sclerosis (SUNBEAM): a multicentre, randomised, minimum 12-month, phase 3 trial. Lancet Neurol. 2019;18(11):1009–1020. [DOI] [PubMed] [Google Scholar]
- 8. Zephir H, Gower-Rousseau C, Salleron J, et al. Milder multiple sclerosis course in patients with concomitant inflammatory bowel disease. Mult Scler. 2014;20(8):1135–1139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.