Abstract
Background
The association between inflammatory bowel disease (IBD) and dementia remains uncertain. We aim to investigate whether IBD is associated with higher dementia risk.
Methods
Using multivariable Cox regression models, we analyzed the onset of all-cause dementia among 497,775 participants, including 5778 IBD patients in the UK Biobank as primary analysis. In secondary analysis, we further examined the difference in brain structure and cognitive function changes between IBD and non-IBD individuals. The diagnosis of IBD and dementia was confirmed with combination of primary care data, hospital inpatient data, death registry, and self-report data. Brain structure was measured by brain MRI as anatomic and tissue-specific volumes; cognitive function was tested in terms of reaction, visual episodic memory, verbal-numerical reasoning, and prospective memory.
Results
During a mean follow-up of 11.58 years, 100 and 6709 incident all-cause dementia with or without IBD were documented, respectively. In multivariable Cox regression model, hazard ratio for incident dementia among IBD patients was 1.14 (95% confidence interval [CI], 0.94-1.39; P=.182) comparing with non-IBD participants; no statistically significant difference was observed in their brain MRI measures of anatomic and tissue-specific volumes, whereas IBD patients had a significantly increased reaction time (β=12.32; 95% CI, 1.97, 22.67; P = .020). Results of subgroup and sensitivity analyses were consistent with the main analysis.
Conclusions
Our study does not support a significant association between IBD and dementia. Further studies with better design and longer follow-up are needed to elucidate the association.
Keywords: inflammatory bowel disease, dementia, longitudinal cohort
Introduction
Inflammatory bowel disease (IBD), which primarily comprises Crohn’s disease (CD) and ulcerative colitis (UC), is an emerging chronic immune-mediated disease triggering gastrointestinal tract inflammation with major symptoms such as abdominal pain and diarrhea.1,2 Over the period of 1990-2017, the global burden of IBD increased 85.1% (from 3.7 million to 6.8 million), and it is expected that the prevalence will continue to increase worldwide.3 Meanwhile, as a protracted course of disease with no known cure and limited excess mortality, the prevalence of IBD is predominantly high in the elderly, accounting for approximately one-third of IBD patients.4
Several neurodegenerative complications such as Parkinson’s disease, multiple sclerosis, and dementia have been reported in IBD patients.5-10 It is hypothesized that gut dysbiosis may increase inflammation and disrupt the blood-brain barriers in IBD patients, leading to neuroinflammation via gut-brain axis and, consequently, increase the risk of such diseases.11 Under the context of aging, dementia has been a common and overwhelming neurodegenerative disorder that affects the elderly by deteriorating the attained cognitive level, ability of daily living, and social functioning.12 Elucidating whether IBD is associated with high risk of dementia is therefore of clinical importance to formulate targeted intervention to slow cognitive deterioration and reduce the disease burden of dementia particularly in IBD patients.
There is a limited number of epidemiological studies exploring the association between IBD and dementia, and their findings are mixed and controversial.8-10,13-14 Prior studies on the topic were mostly based on record-linkage cohorts, with lack of adjustment for some well-established confounders, particulary environmental risk factors. Moreover, some of these studies aimed to explore the comorbidities of IBD and dementia, and they did not account for chronological order of disease development.8,9 Therefore, in this study, we performed a longitudinal cohort study with a large sample size to assess the impact of IBD as a potential risk factor for subsequent development of dementia in the UK Biobank.
Materials and Methods
Data Set and Study Population
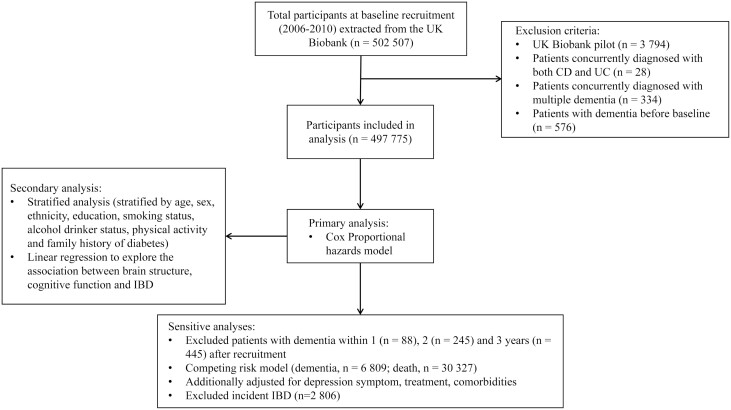
The UK Biobank is a prospective cohort study with more than half a million participants recruited from 2006 to 2010. It collected extensive health-related information via questionnaires, physical measures, and medical records. Data were accessed until February 28 2021. Ethical approval of the UK Biobank was obtained from National Health System Northwest Multicenter Research Ethics Committee.15 The current study included 497,775 individuals after excluding the participants who were with dementia at baseline (Figure 1).
Figure 1.
Flowchart of the cohort study.
Exposure and Outcomes
Inflammatory bowel disease, composed of CD and UC, was ascertained by self-report data and data linkage with primary care, hospital admissions, and death registry records based on the International Classification of Disease ninth and tenth editions coding system. Specifically, all-cause dementia records were documented including Alzheimer’s disease, vascular disease, dementia in other disease classified elsewhere, and unspecified dementia through data linkage with primary care, hospital admissions, and death registry records using ICD-9 and 10. Of note, self-reported dementia at recruitment was excluded from study population (details see Table S1).When a participant had multiple diagnosis records, we used the date of the first confirmed diagnosis to estimate the time to event, and additional records were referred to classify the specific subtypes of UC and CD.
Due to limited follow-up years and insidious nature of dementia, we additionally included brain structure obtained from brain magnetic resonance imaging (MRI) and cognitive function assessed by cognitive tests as 2 secondary outcomes. Brain MRI imaging was done by UK Biobank, along with its ongoing multimodal imaging study.16 All brain MRI data were acquired on a Siemens Skyra 3 T scanner with a standard protocol (http://www.fmrib.ox.ac.uk/ukbiobank/protocol/V4_23092014.pdf). Structural MRI measures of brain anatomy were documented as tissue-specific and structure volumes, including total volume of white matter hyperintensities,17 volume of white matter,18 volume of gray matter,19 volume of brain,20 total volume of hippocampus,21 and volume of gray matter in hippocampus.22 We also used computed change in cognitive function tests derived from baseline and 2014 onwards. The cognitive tests covering 4 domains covering reaction, visual episodic memory, verbal-numerical reasoning, and prospective memory demonstrated modest to-good concurrent validity and moderate to high test-retest reliability (details in Table S2).23 We used the cognitive tests as intermediate surrogate traits of dementia because they have proved to aid prediction to incident dementia (added up to 5% to the discriminative accuracy in UK Biobank dementia-free sample at baseline during 3-to-8-year follow-up).24
Covariates
A wide range of sociodemographic factors, lifestyle factors, and family history of dementia were considered as covariates to adjust for any potential confounding. The covariates of sociodemographic factors included age, sex, race (White, non-White), education (university/college degree, upper secondary, lower secondary, vocational, other), body mass index (BMI), Townsend deprivation index (TDI, an indicator to represent socioeconomic status and lower value indicating higher socioeconomic status). Lifestyle factors included smoking and alcohol drinking status (never, previous, current), physical activity (assessed by international physical activity questionnaire and was categorized as low, moderate and high intensity). Family history of dementia was considered as a binary categorical variable, dependent on the presence or absence of at least 1 first-degree relative affected by dementia at the time of recruitment. Inflammatory bowel disease treatments in combination with IBD-related medication (5-aminosalicylic acid, glucocorticoid, immunosuppressants, and monoclonal)25,26 and bowel resection surgery (detailed definition Table S6), symptoms of depression assessed with validated 2-item Patients Health Questionnaire (PHQ-2),27 and widely adjusted dementia-related comorbidities (cardiovascular diseases, type 2 diabetes mellitus, hypertension, and hypothyroidism) were additionally considered as potential confounders.
Statistical Analysis
Baseline characteristics are presented as mean (SD) or counts (percentages). Analysis of variance (ANOVA) and χ2 test were performed for group comparisons. In the primary and subgroup analysis, contributed person-time was calculated from baseline until the date of the first diagnosis of all-cause dementia, date of death, date of loss, or end of follow-up, whichever came first. Cumulative incidence curves were depicted to feature the development of dementia during follow-up period. Multivariable Cox proportional hazard models were applied after verifying proportional hazard assumption through Schoenfeld residuals. Covariates were adjusted progressively in 3 models: 1) model 1 with basic adjustment for age, age-square, sex, and race; 2) model 2 with additional adjustment for education and TDI; 3) model 3 with additional adjustment for BMI, smoking status, alcohol drinking status, physical activity, and family history of dementia. Meanwhile, the hazard ratios (HRs) and 95% confidence intervals (CIs) of all-cause dementia in IBD, CD, and UC patients were estimated in all the 3 models, respectively. Subgroup analyses stratified by each covariate were also performed.
We additionally conducted a series of sensitivity analyses to test robustness of primary analysis results. First, considering that dementia may be underdiagnosed at the time of IBD diagnosis due to its insidious feature,13 we excluded dementia cases diagnosed within the first 3 years after recruitment to minimize the possibility of reverse association. Second, we repeated the analyses after the exclusion of newly developed IBD during follow-up to ensure dementia diagnosed after IBD diagnosis. Third, to reduce the possibility of potential confounding, further adjustments were performed for IBD treatments, symptoms of depression, and dementia-related comorbidities mentioned previously. Fourth, we additionally performed stratified Cox regression model by categorizing patients with IBD into receipt of IBD-related medications, bowel resection surgery, both, or none (Table S6). Fifth, considering dementia is relatively late-onset, we applied competing risk model to account for the competing risk of death (referring to death as competing event).
In secondary analysis, to further explore the association between IBD and cognitive health, we additionally assessed the relationship of IBD with brain structure and cognitive function using linear regression models by fully adjusting for all covariates. All analyses were performed with R software (version 4.0.3), and statistically significant 2-tailed P value was defined as < 0.05.
Results
Baseline characteristics stratified by IBD subtypes are shown in Table 1 and Table 2. Among 497,775 participants (mean age 57.0 [SD: 8.1] and 54.4% female), 5,778 IBD patients were documented at the baseline. During a mean follow-up of 11.58 years (comprising 791,368 person months), 6809 individuals developed dementia, of which 30 cases were from CD patients and 70 cases were from UC patients.
Table 1.
Baseline characteristics by inflammatory bowel disease category in the UK Biobank (n = 497 775).
| Overall (n = 497,775) | Non-IBD (n = 491,997) | CD (n = 1826) | UC (n = 3952) | P a | |
|---|---|---|---|---|---|
| Age at baseline, years, mean(SD) | 57.0 (8.1) | 57.0 (8.1) | 56.9 (8.1) | 58.1 (7.8) | <0.001 |
| Female, n (%) | 270894 (54.4) | 267861 (54.4) | 1024 (56.1) | 2009 (50.8) | <0.001 |
| White, n (%) | 467652 (94.5) | 462129 (94.4) | 1765 (97.0) | 3758 (95.6) | <0.001 |
| Education, n (%) | <0.001 | ||||
| University/College degree | 160967 (32.8) | 159344 (32.8) | 489 (27.1) | 1134 (29.0) | |
| Upper secondary | 55250 (11.2) | 54671 (11.3) | 190 (10.5) | 389 (9.9) | |
| Lower secondary | 131844 (26.8) | 130249 (26.8) | 508 (28.1) | 1087 (27.8) | |
| Vocational | 58413 (11.9) | 57680 (11.9) | 244 (13.5) | 489 (12.5) | |
| Other | 84989 (17.3) | 83799 (17.3) | 376 (20.8) | 814 (20.8) | |
| BMI, kg/m2,mean(SD) | 27.43 (4.80) | 27.43 (4.80) | 26.89 (4.77) | 27.30 (4.65) | <0.001 |
| Townsend deprivation index, mean(SD) | −1.30 (3.09) | −1.30 (3.09) | −1.11 (3.21) | −1.32 (3.04) | 0.025 |
| Smoking status, n (%) | <0.001 | ||||
| Never | 271127 (54.8) | 268402 (54.9) | 814 (44.7) | 1911 (48.6) | |
| Previous | 171428 (34.6) | 168959 (34.5) | 726 (39.9) | 1743 (44.3) | |
| Current | 52321 (10.6) | 51764 (10.6) | 279 (15.3) | 278 (7.1) | |
| Alcohol drink status, n (%) | |||||
| Never | 22175 (4.5) | 21889 (4.5) | 98 (5.4) | 188 (4.8) | <0.001 |
| Previous | 17870 (3.6) | 17571 (3.6) | 106 (5.8) | 193 (4.9) | |
| Current | 456113 (91.9) | 450939 (92.0) | 1616 (88.8) | 3558 (90.3) | |
| Physical activity, n (%) | |||||
| Low | 76000 (18.9) | 74996 (18.9) | 345 (24.2) | 659 (21.0) | <0.001 |
| Moderate | 163720 (40.8) | 161892 (40.8) | 551 (38.7) | 1277 (40.6) | |
| High | 161891 (40.3) | 160153 (40.3) | 529 (37.1) | 1209 (38.4) | |
| Family history of dementia b, n(%) | 48291 (9.7) | 47712 (9.7) | 157 (8.6) | 422 (10.7) | 0.033 |
| Symptoms of depression, n (%) | 27565 (6.0) | 27144 (5.9) | 159 (9.3) | 262 (7.1) | <0.001 |
| Treatments, n (%) | <0.001 | ||||
| Medication | 10722 (2.2) | 8597 (1.7) | 571 (31.3) | 1554 (39.3) | |
| Resection of bowel | 10959 (2.2) | 10427 (2.1) | 198 (10.8) | 334 (8.5) | |
| Both | 610 (0.1) | 276 (0.1) | 170 (9.3) | 164 (4.1) | |
| None | 475484 (95.5) | 472697 (96.1) | 887 (48.6) | 1900 (48.1) | |
| Cardiovascular disease, n (%) | 34161 (6.9) | 33625 (6.8) | 150 (8.2) | 386 (9.8) | <0.001 |
| Type-2 diabetes mellitus, n (%) | 13452 (2.7) | 13185 (2.7) | 76 (4.2) | 191 (4.8) | <0.001 |
| Hypertension, n (%) | 137576 (27.6) | 135851 (27.6) | 539 (29.5) | 1186 (30.0) | 0.001 |
| Hypothyroidism, n (%) | 33929 (6.8) | 33526 (6.8) | 125 (6.8) | 278 (7.0) | 0.860 |
Abbreviations: IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; SD, standard deviation; BMI, body mass index.
P values were calculated by χ2 tests or analysis of variance.
Family history of dementia included mother’s and father’s dementia.
Table 2.
Hazard ratios (95% CIs) for developing dementia stratified by IBD.
| Person-months | No. Events | Model 1 a | p | Model 2 b | p | Model 3 c | p | |
|---|---|---|---|---|---|---|---|---|
| Non-IBD | 68190768 | 6709 | Ref | Ref | Ref | |||
| IBD | 791368 | 100 | 1.21 (0.99-1.47) | 0.062 | 1.17 (0.96-1.43) | 0.111 | 1.14 (0.94-1.39) | 0.182 |
| CD | 247416 | 30 | 1.31 (0.92-1.88) | 0.139 | 1.26 (0.88-1.80) | 0.210 | 1.20 (0.84-1.71) | 0.329 |
| UC | 543952 | 70 | 1.17 (0.92-1.48) | 0.199 | 1.15 (0.91-1.45) | 0.255 | 1.12 (0.89-1.42) | 0.334 |
Model 1 adjusted for age, age-square, sex, race.
Model 2 adjusted for age, age-square, sex, race, education, Townsend deprivation index.
Model 3 adjusted for age, age-square, sex, race, education, Townsend deprivation index, body mass index, alcohol drinker status, smoking status, physical activity, family history of dementia.
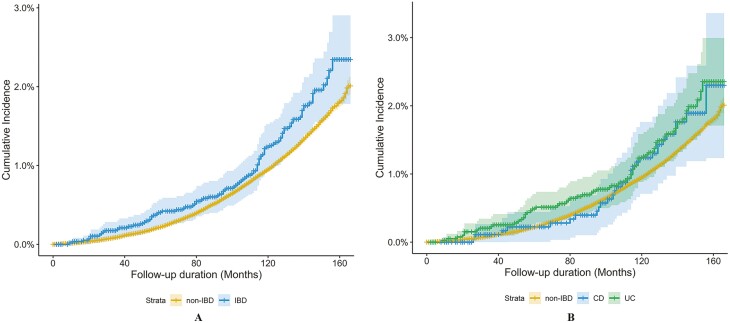
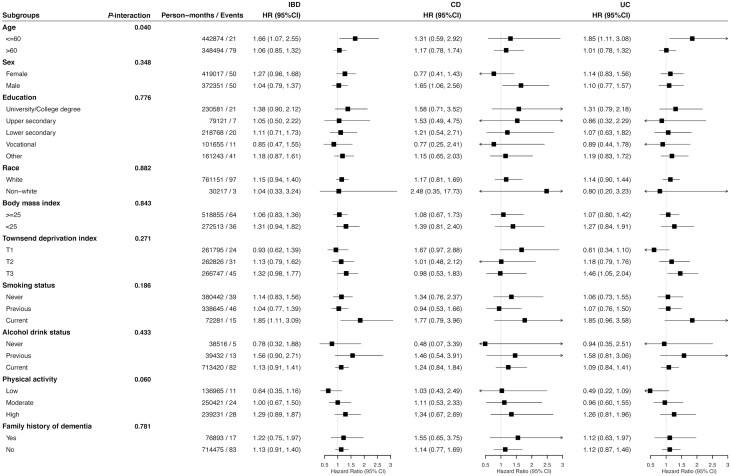
As shown in Figure 2, the accumulative incidence of dementia among IBD patients was slightly higher than that of non-IBD participants over time. However, in any of the 3 models, subsequent dementia risk was not significantly associated with either IBD (HR, 1.14; 95% CI, 0.94-1.39; P = .182) or its subtypes of CD (HR, 1.20; 95% CI, 0.84-1.71; P = .329) and UC (HR, 1.12; 95% CI, 0.89-1.42; P = .334; Table 2). Results of subgroup analyses are shown in Figure 3. Significant effect modification was observed for different age groups (P value for interaction = .040). Specifically, IBD (HR, 1.66; 95% CI, 1.07-2.55; P = .022) and UC (HR, 1.85, 95% CI, 1.11-3.08; P = .018) were significantly associated with incident dementia only among patients aged younger or equal to 60 years old.
Figure 2.
Cumulative incidence curve of risk of incident dementia stratified by IBD. The P value from the test of the proportional hazard assumption based on weighted residuals for a Cox regression model fit was 0.83.
Figure 3.
Hazard ratios (95% CIs) for developing dementia among patients with and without inflammatory bowel disease stratified by covariates. Hazard ratios were calculated adjusting for age, age-square, sex, race, education, Townsend deprivation index, BMI, alcohol drinker status, smoking status, physical activity, family history of dementia; Townsend deprivation index (TDI) was grouped by tertiles (T1-T3, from low to high).
Consistently, null risk estimates were reported in the sensitivity analyses after excluding patients diagnosed with dementia within 1, 2, and 3 years after baseline (Table S4), and incidence IBD (Table S5). Meanwhile, additional adjustment for IBD treatments, symptoms of depression, and a number of dementia-related comorbidities (Table S6), or involving death as competing risk (Table S8) furtherly comfirmed the results in primary analysis with no notable change in estimators. Cox-regression models stratified IBD treatments also yielded consistent null findings (Table S7). Moreover, no significant difference was observed in the measures of brain structure as well. However, in terms of cognitive tests performance, IBD patients had a significant increase in reaction time than non-IBD participants (β=12.32; 95% CI, 1.97 to 22.67; P = .020; Table 3).
Table 3.
Association between brain structure, cognitive function and IBD. (Linear regression)
| Variable | Participants | IBD patients | Beta (95%CI) a | p |
|---|---|---|---|---|
| Brain structure | ||||
| Total volume of white matter hyperintensities | 19847 | 194 | −311.80 (−1103.85 to 480.24) | 0.440 |
| Volume of white matter | 21039 | 205 | −239.99 (−5622.71 to 5142.73) | 0.930 |
| Volume of gray matter | 21039 | 205 | −1042.44 (−5992.91 to 3908.04) | 0.680 |
| Volume of brain | 21039 | 205 | −1282.46 (−9567.11 to 7002.20) | 0.762 |
| Volume of hippocampus (total) | 21022 | 205 | −61.10 (−171.85 to 49.64) | 0.280 |
| Volume of gray matter in hippocampus (total) | 21034 | 205 | −78.81 (−179.39 to 21.78) | 0.125 |
| Cognitive function | ||||
| Reaction time change | 44908 | 408 | 12.32 (1.97, 22.67) | 0.020 |
| Visual memory change | 45316 | 410 | 0.16 (−0.23 to 0.56) | 0.414 |
| Verbal-numerical reasoning change | 15098 | 136 | −0.09 (−0.38 to 0.20) | 0.545 |
| Prospective memory change | 15467 | 139 | 0.04 (−0.05 to 0.12) | 0.397 |
Adjusted for age, age-square, sex, race, education, Townsend deprivation index, body mass index, alcohol drinker status, smoking status, physical activity, family history of dementia.
Discussion
With a mean follow-up of 11.58 years, we examined the subsequent dementia risk among 497,775 participants with or without IBD. One hundred out of 5778 patients with IBD developed dementia during follow-up period. To our knowledge, this is the largest longitudinal cohort study with long follow-up observations focusing on the risk of developing dementia among patients with IBD. Due to the insidious nature of dementia, we also included measures of brain structure and cognitive performance as intermediate surrogate traits of dementia. Our study found limited evidence in support of any significant association between IBD and subsequent dementia incidence, with the HR for dementia in patients with IBD at 1.14 (95% CI, 0.94-1.39; P = .182).
To date, a few epidemiology studies examining the association between IBD and dementia were published.8-10,13-14 Contrary to present findings that did not detect any direct significant association between IBD and the subsequent development of dementia, several published studies found significantly elevated risk. Wotton et al reported a rate ratio (RR) of 1.10 (95% CI, 1.05-1.15) and 1.06 (95% CI, 1.03-1.10) for dementia after admission for UC and CD respectively.8 Another study based on the Taiwan National Health Insurance Research Database reported a 2.54-fold (95% CI, 1.91-3.37) increased risk of developing dementia among IBD patients,13 and the study using the IQVIA Disease Analyzer database in Germany also revealed 22% (95% CI, 1.07-1.39) higher risk to develop dementia in IBD patients. However, referring to IBD subtypes, significantly elevated dementia risk was only detected among UC (HR, 1.25; 95% CI, 1.07-1.46) rather than CD (HR, 1.17; 95% CI, 0.93-1.47) patients.14 Nevertheless, the magnitude of effect estimates in our study is similar to these reported by a very recent published study, which used longitudinal data from all Manitobans with IBD and matched controls and reported null association in UC (HR, 1.01; 95% CI, 0.85, 1.19) but an increased risk of dementia in patients with CD (HR, 1.44; 95% CI, 1.16-1.78).10 In line with a Danish study examining comorbidities experienced by IBD patients, they also reported a nonsignificant association between Alzheimer and UC (odds ratio, [OR],1.05; 95% CI, 0.57-1.94) and CD (OR,1.08; 95% CI, 0.79-1.49).9 The discrepant conclusions of different studies should be interpreted with caution, considering the possibility of residue confounding and selection bias. For example, some of the important covariates like education, lifestyle, or socioeconomic factors that were identified as the most important modifiable risk factors of dementia were not properly adjusted in some of the studies. Additionally, on contrary to our cohort study, several of these studies that reported positive associations used matched case-control design; however, the selection of matched controls against IBD patients could be extensively diverse according to the matched variables. Thus, selection bias was prone to happen, especially in a large database with a relatively low incidence of IBD.
Interestingly, when restricting to individuals younger than 60 years at entry, we found that IBD patients had a significantly higher risk of developing dementia. In line with a study that examined comorbid disease with IBD, they also found increased HR for dementia diminished with age advancing (age 25-50 years: HR, 1.58, 95% CI, 1.02-2.46; age 50-65 years: HR, 1.19, 95% CI, 0.87-1.62; age 65 and older: HR, 1.14, 95% CI, 1.00-1.30).10 It could be that although dementia is an insidious and late-onset disease that occurs mainly among people aged over 65 years,12 IBD may accelerate its manifestation earlier than expected compared with relative reduction in dementia among younger general population. Moreover, there could be survival bias. As IBD has been linked to an elevated mortality,28 older IBD patients may not have survived long enough to be diagnosed with dementia. However, due to the small number of cases in each age group, the results should be interpreted cautiously.
With regard to the significantly slower reaction time in IBD patients rather than the other 3 cognitive tests, it raises the possibility that cognitive function of IBD patients may deteriorate in more strategic aspects such as reaction times. To our knowledge, we are the first to investigate the longitudinal change in cognitive function among patients with IBD, thus we are limited to make a comparison. However, our findings have similar implications with a recent meta-analysis.29 The research reported no evident difference between IBD and non-IBD population in multidomain cognitive screening tools (Mini Mental State Examination and Montreal Cognitive Assessment; standard mean difference [SMD],−0.27; 95% CI −0.68 to 0.08), but they reported obvious specific cognitive deficits in response time (SMD=−0.53; 95% CI, −1.03 to −0.04) comparing Stroop test performance. Another research revealed that those who suffered from CD had slower than normal cognitive response times across all time points but no difference in rate of making errors compared with their well-matched healthy counterparts, indicating their cognitive dysfunction in the signal processing speed rather than in decision-making.30 Therefore in view of most of the studies examining particular cognitive function in IBD, patients were small in scale, and few studies have ever tested the cognitive function changes prospectively. Thus, further studies with detailed cognitive tests targeting specific cognitive function domains of IBD patients should be conducted to validate the findings of delayed reaction times in IBD patients.
Strengths and Limitations
This study expanded our knowledge on the association between IBD and dementia and highlighted the need for further longitudinal studies. We did a series of rigorous statistical analyses and one of the first to include intermediate surrogate traits of dementia, brain structure, and cognitive tests performance into prospective analyses for further exploration. Although strengthened by large sample size and adequate follow-up period in UK Biobank cohort which allowed us to capture the association between IBD and age-associated dementia, we may still have potentially underestimated the effect size, as some participants might develop dementia at an older age. Likewise, the cognitive function tests were derived from baseline and onwards. Because of the relatively short time measurement interval, measurement errors may happen, and the results are possible to change over time while aging. In addition, because of the relatively small number of cases, we were unable to assess any association with dementia subtypes. Moreover, although pooled statistics showed that estimators generated from UK Biobank were similar to population-representative cohorts,31 selection bias may exist and restrict the generalizability of the study findings. Finally, although we adjusted for a wide range of potential confounders, residual confounding may still exist.
Conclusions
We conclude that the association between IBD and dementia remains uncertain, whereas heterogeneity in disease definition and study design may partially explain these inconsistent results. Further well-designed studies are needed to elucidate the association.
Supplementary Material
Acknowledgments
This project has been conducted using the UK Biobank Resource under Application 66354. We also thank Prof. Yunxian Yu (Zhejiang University) for helping with statistics.
Contributor Information
Yuhao Sun, Center for Global Health, Zhejiang University, Hangzhou, China.
Jiawei Geng, Center for Global Health, Zhejiang University, Hangzhou, China.
Xuejie Chen, Department of Gastroenterology, The Third Xiangya Hospital of Central South University, Changsha, China.
Hui Chen, School of Public Health, Zhejiang University School of Medicine, Hangzhou, China.
Xiaoyan Wang, Department of Gastroenterology, The Third Xiangya Hospital of Central South University, Changsha, China.
Jie Chen, Center for Global Health, Zhejiang University, Hangzhou, China; Department of Gastroenterology, The Third Xiangya Hospital of Central South University, Changsha, China.
Xue Li, School of Public Health and the Second Affiliated Hospital, Zhejiang University, Hangzhou, China; Centre for Global Health Research, Usher Institute, University of Edinburgh, Edinburgh, United Kingdom.
Therese Hesketh, Center for Global Health, Zhejiang University, Hangzhou, China; Institute for Global Health, University College London, London, UK.
Author Contributions
J.C., X.L., and X.Y.W. conceptualized the project. Y.H..S, J.W.G., and X.J.C. performed the analyses and wrote the first draft. X.J.C., H.C., and T.H. helped with the review and editing. J.C., X.L., and X.Y.W. revised the final version. All authors have read and agreed to the final version of the article.
Funding
This project is funded by National Natural Science Foundation of China (81970494) and Key Project of Research and Development Plan of Hunan Province (2019SK2041).
Conflicts of Interest
We declare that we have no conflicts of interest, either financial or non-financial.
Data Availability
Data used in this study was from the UK Biobank and are available to researchers through an access procedure described at (https://www.ukbiobank.ac.uk/enable-your-research).
References
- 1. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol. 2015;12:720–727. [DOI] [PubMed] [Google Scholar]
- 2. Ng SC, Shi HY, Hamidi N, et al. . Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–2778. [DOI] [PubMed] [Google Scholar]
- 3. Alatab S, Sepanlou SG, Ikuta K, et al. . The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Faye AS, Colombel J-F.. Aging and IBD: a new challenge for clinicians and researchers. Inflamm Bowel Dis. 2022;28:126–132. [DOI] [PubMed] [Google Scholar]
- 5. Brudek T. Inflammatory bowel diseases and Parkinson’s Disease. J Parkinsons Dis. 2019;9:S331–S344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu F, Li C, Gong J, et al. . The risk of Parkinson’s disease in inflammatory bowel disease: a systematic review and meta-analysis. Dig Liver Dis. 2019;51:38–42. [DOI] [PubMed] [Google Scholar]
- 7. Kosmidou M, Katsanos AH, Katsanos KH, et al. . Multiple sclerosis and inflammatory bowel diseases: a systematic review and meta-analysis. J Neurol. 2017;264:254–259. [DOI] [PubMed] [Google Scholar]
- 8. Wotton CJ, Goldacre MJ.. Associations between specific autoimmune diseases and subsequent dementia: retrospective record-linkage cohort study, UK. J Epidemiol Community Health. 2017;71:576–583. [DOI] [PubMed] [Google Scholar]
- 9. Vadstrup K, Alulis S, Borsi A, et al. . Extraintestinal manifestations and other comorbidities in ulcerative colitis and Crohn’s disease: a danish nationwide registry study 2003–2016. Crohn’s & Colitis 360. 2020;2:otaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernstein CN, Nugent Z, Shaffer S, et al. . Comorbidity before and after a diagnosis of inflammatory bowel disease. Aliment Pharmacol Ther. 2021;54:637–651. [DOI] [PubMed] [Google Scholar]
- 11. Roy Sarkar S, Banerjee S.. Gut microbiota in neurodegenerative disorders. J Neuroimmunol. 2019;328:98–104. [DOI] [PubMed] [Google Scholar]
- 12. Livingston G, Sommerlad A, Orgeta V, et al. . Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 13. Zhang B, Wang HE, Bai YM, et al. . Inflammatory bowel disease is associated with higher dementia risk: a nationwide longitudinal study. Gut. 2021;70:85–91. [DOI] [PubMed] [Google Scholar]
- 14. Zingel R, Bohlken J, Kostev K.. Association between inflammatory bowel disease and dementia: a retrospective cohort study. J Alzheimer’s Dis. 2021;80:1471–1478. [DOI] [PubMed] [Google Scholar]
- 15. Sudlow C, Gallacher J, Allen N, et al. . UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. Plos Med. 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miller KL, Alfaro-Almagro F, Bangerter NK, et al. . Multimodal population brain imaging in the UK Biobank prospective epidemiological study. Nat Neurosci. 2016;19:1523–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu HY, Ou YN, Shen XN, et al. . White matter hyperintensities and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 36 prospective studies. Neurosci Biobehav Rev. 2021;120:16–27. [DOI] [PubMed] [Google Scholar]
- 18. Dadar M, Maranzano J, Ducharme S, Collins DL; Alzheimer’s Disease Neuroimaging Initiative . White matter in different regions evolves differently during progression to dementia. Neurobiol Aging. 2019;76:71–79. [DOI] [PubMed] [Google Scholar]
- 19. Last N, Tufts E, Auger LE.. The effects of meditation on grey matter atrophy and neurodegeneration: a systematic review. J Alzheimer’s Dis. 2017;56:275–286. [DOI] [PubMed] [Google Scholar]
- 20. Walker KA, Hoogeveen RC, Folsom AR, et al. . Midlife systemic inflammatory markers are associated with late-life brain volume: the ARIC study. Neurology. 2017;89:2262–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Evans TE, Adams HHH, Licher S, et al. . Subregional volumes of the hippocampus in relation to cognitive function and risk of dementia. Neuroimage. 2018;178:129–135. [DOI] [PubMed] [Google Scholar]
- 22. Suzuki H, Venkataraman AV, Bai W, et al. ; Alzheimer’s Disease Neuroimaging Initiative . Associations of regional brain structural differences with aging, modifiable risk factors for dementia, and cognitive performance. JAMA Netw Open. 2019;2:e1917257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fawns-Ritchie C, Deary IJ.. Reliability and validity of the UK Biobank cognitive tests. Plos One. 2020;15:e0231627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calvin CM, Wilkinson T, Starr JM, et al. . Predicting incident dementia 3-8 years after brief cognitive tests in the UK Biobank prospective study of 500,000 people. Alzheimer’s Dement. 2019;15:1546–1557. [DOI] [PubMed] [Google Scholar]
- 25. Lichtenstein GR, Loftus EV, Isaacs KL, et al. . ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113:481–517. [DOI] [PubMed] [Google Scholar]
- 26. Rubin DT, Ananthakrishnan AN, Siegel CA, et al. . ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol. 2019;114:384–413. [DOI] [PubMed] [Google Scholar]
- 27. Kroenke K, Spitzer RL, Williams JB.. The patient health questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. [DOI] [PubMed] [Google Scholar]
- 28. Olén O, Askling J, Sachs MC, et al. . Mortality in adult-onset and elderly-onset IBD: a nationwide register-based cohort study 1964-2014. Gut. 2020;69:453–461. [DOI] [PubMed] [Google Scholar]
- 29. Hopkins CWP, Powell N, Norton C, et al. . Cognitive impairment in adult inflammatory bowel disease: a systematic review and meta-analysis. J Acad Consult Liaison Psychiatry. 2021;62:387–403. [DOI] [PubMed] [Google Scholar]
- 30. van Langenberg DR, Yelland GW, Robinson SR, Gibson PR.. Cognitive impairment in Crohn’s disease is associated with systemic inflammation, symptom burden and sleep disturbance. United European Gastroenterol J. 2017;5:579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Batty GD, Gale CR, Kivimäki M, et al. . Comparison of risk factor associations in UK Biobank against representative, general population based studies with conventional response rates: prospective cohort study and individual participant meta-analysis. Bmj. 2020;368:m131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this study was from the UK Biobank and are available to researchers through an access procedure described at (https://www.ukbiobank.ac.uk/enable-your-research).