Abstract
Reducing the risk of dementia can halt the worldwide increase of affected people. The multifactorial and heterogeneous nature of late-onset dementia, including Alzheimer’s disease (AD), indicates a potential impact of multidomain lifestyle interventions on risk reduction. The positive results of the landmark multidomain Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) support such an approach. The World-Wide FINGERS (WW-FINGERS), launched in 2017 and including over 25 countries, is the first global network of multidomain lifestyle intervention trials for dementia risk reduction and prevention. WW-FINGERS aims to adapt, test, and optimize the FINGER model to reduce risk across the spectrum of cognitive decline—from at-risk asymptomatic states to early symptomatic stages—in different geographical, cultural, and economic settings. WW-FINGERS aims to harmonize and adapt multidomain interventions across various countries and settings, to facilitate data sharing and analysis across studies, and to promote international joint initiatives to identify globally implementable and effective preventive strategies.
Keywords: Alzheimer’s disease, cognitive impairment, dementia, lifestyle, multidomain intervention, prevention, randomized controlled trial, World-Wide FINGERS
1 |. INTRODUCTION
Worldwide, nearly 50 million people are living with dementia. Alzheimer’s Disease International (ADI) predicts this number will reach nearly 82 million in 2030 and over 152 million in 20501 unless interventions are identified and implemented to prevent or delay onset, slow progression, or stop Alzheimer’s disease (AD) and other disorders that cause dementia. Indeed, delaying the onset of AD by only a few years could substantially reduce its prevalence and related human and economic burdens.2
Several studies published in recent years suggest a decline in the age-adjusted incidence of dementia, with stable or reduced prevalence, in Western countries including the United States,3,4 Sweden,5,6 the United Kingdom,7 and The Netherlands.8 Other studies, however, indicate an increased incidence in some Asian countries, such as China and Japan.9–12 Factors that may have contributed to a lower occurrence of dementia in some countries relate to changes in risk factor profiles, including improved treatment for hypertension, diabetes, and other vascular risk factors,13 as well as increased educational opportunities.3 A meta-analysis of population-based observational studies from the United States and Europe concluded that about 30% of AD cases may be attributable to seven potentially modifiable risk factors for AD—diabetes, midlife hypertension, midlife obesity, physical inactivity, depression, smoking, and low educational attainment.14 This study and others have concluded that targeting these risk factors may represent a powerful strategy for risk reduction of AD. Given the complex, multifactorial, and heterogeneous nature of late-onset AD and dementia, interventions targeting several risk factors and mechanisms simultaneously may be required to achieve optimal preventive effects.15
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER, ClinicalTrials.gov: NCT01041989) represents the first large, long-term randomized controlled trial (RCT) demonstrating that a multidomain lifestyle intervention can improve cognitive function in older adults from the general population who are at elevated risk of developing dementia.16 Participants in the trial were randomized to either a group that received general health advice or a group to be enrolled in a 2-year multidomain intervention that incorporated dietary counseling, physical exercise, cognitive training, and vascular and metabolic risk monitoring.17 Improvement in global cognition after 24 months, assessed using the comprehensive Neuropsychological Test Battery (NTB total score), was 25% higher in the multidomain intervention group than in the general health advice group (P = .03). Performance was improved in all cognitive sub-domains including executive function, processing speed, and complex memory tasks.16 Further analyses showed that the FINGER multidomain intervention benefited cognition regardless of sociodemographic and socioeconomic factors or other baseline characteristics, supporting the potential benefits of the FINGER model for large at-risk populations.18 Individuals with genetic susceptibility (apolipoprotein E gene (APOE) ε4 carriers) showed cognitive benefits from the intervention.19 The FINGER intervention also reduced the risk of developing new chronic diseases.20 Extended 5- and 7-year follow-up assessments have been completed recently and data analysis is ongoing to measure the long-term effects of the intervention. Although the FINGER trial results were encouraging, two other large multidomain RCTs—the French Multidomain Alzheimer Preventive Trial (MAPT) and the Dutch Prevention of Dementia by Intensive Vascular Care (PreDIVA)—reported lack of effect on the primary outcomes.21,22 Remarkably, exploratory subgroup analyses in both studies provided evidence that interventions yielded cognitive benefits in subpopulations of participants with increased risk of dementia,21–23 highlighting the importance of methodological issues such as selection of at-risk individuals, adequate timing and intensity of the interventions, and selection of appropriate sensitive tools to detect changes in cognition. Overall, the potential of multidomain interventions for dementia risk reduction and prevention needs further validation, and there is a need to test and adapt these approaches in diverse geographical, economic, and cultural settings.
The Lancet Commission on Dementia Prevention, Intervention, and Care proposed a life-course model of dementia risk that reflects how lifestyle factors across the lifespan contribute to dementia risk. They estimated that interventions across the lifespan could theoretically prevent more than a third of dementia cases. These interventions include increasing access to and quality of education in early life; treating or reducing hypertension and obesity in midlife; addressing hearing loss; and reducing smoking, depression, physical inactivity, social isolation, and diabetes in late life.24
In 2017, the National Institute on Aging (NIA) asked the National Academies of Science, Engineering, and Medicine to convene a committee to explore evidence regarding interventions with the potential to prevent or slow cognitive decline and dementia. Although it found insufficient high-strength evidence to justify a public health campaign to encourage people to adopt lifestyle interventions to prevent dementia, the committee acknowledged that this may reflect methodologic inconsistencies across clinical studies. They also cited results from the FINGER trial as providing promising data from a large and long-duration trial of a multidomain intervention, and they suggested replication of FINGER through multiple independent studies testing the same components.25 In addition, dementia risk reduction is one of the strategic action areas of World Health Organization’s (WHO’s) Global action plan on the public health response to dementia 2017–2025. The recently published WHO Guidelines for risk reduction of cognitive decline and dementia provide evidence-based recommendations to support countries as they develop approaches to delay or prevent the onset of dementia. The guidelines highlight the importance of further research on the efficacy of multidomain interventions that are adjusted to specific geographical and cultural contexts.26
In line with these indications, the launch of the World-Wide FINGERS (WW-FINGERS) Network was announced at the 2017 Alzheimer’s Association International Conference (AAIC) in London.27 The Network aims to test the FINGER multidomain lifestyle model in various populations and settings. Harmonization across trials is paramount, yet adaptations will be needed to ensure acceptance of and adherence to interventions, as well as assessments among populations that are appropriate in terms of language, ethnicity, culture, environment, and risk of AD and dementia. The WW-FINGERS Network held two face-to-face meetings in connection with AAIC: the first on July 20, 2018, in Chicago, and the second on July 12, 2019, in Los Angeles. Periodic teleconferences and ancillary meetings have been also held regularly to move forward with the Network activities. This report summarizes the proceedings of the first two face-to-face meetings, which are now repeated yearly in connection with AAIC.
2 |. WW-FINGERS NETWORK STUDIES
WW-FINGERS was established to support and convene global multidomain dementia prevention trials, share experiences and data, as well as harmonize methods. The aim is not to replicate the original FINGER intervention, but rather to adapt and optimize it to various settings, to test whether FINGER-based protocols are feasible and effective in various populations. Methodological pillars of WW-FINGERS trials include: assessment of a multidomain intervention aiming to ameliorate vascular, metabolic, and lifestyle-related factors; delivery of the intervention through individual and group sessions to optimize the intervention at an individual level but also stimulate social interaction and peer support; prospective harmonization of cognitive outcomes (ie, assessment of cognitive changes) as well as other outcomes; and use of randomization to support balanced comparisons among intervention conditions. The network includes investigator teams from around the world, with the aim of building on “lessons learned” from previous studies and expand the knowledge on feasibility and efficacy of multidomain interventions for risk reduction and prevention of dementia in diverse populations.27 At the time of the WW-FINGERS Network meeting in 2019, over 25 countries had joined, and WW-FINGERS trials had been planned in several European countries, USA, China, Singapore, South Korea, Japan, and Australia. Currently, similar efforts are ongoing in Central and South America, Canada, India, and Malaysia (Table 1 and Figure 1).
TABLE 1.
WW-FINGERS Network Studies
| Study | Population | Interventions | Trial duration | Primary outcome | Other assessments | Study status |
|---|---|---|---|---|---|---|
| FINGER (Finland) | 1260 at-risk (CAIDE score) adults aged 60–77 | 1. Regular health advice 2. Intensive multidomain intervention |
2 years intervention, extended follow-ups | Global cognitive composite | Cognitive domains; functioning; vascular and metabolic risk factors, morbidity and mortality; disability; depressive symptoms; quality of life; change in lifestyle habits; utilization of health resources; blood markers (eg, APOE, GWAS, telomere length, metabolomics); brain imaging sub-study | Ongoing extended follow-up |
| U.S. POINTER (USA) | 2000 at-risk, cognitively normal adults age 60–79 | 1. Self-guided lifestyle intervention 2. Structured lifestyle intervention |
2 years intervention | Global cognitive composite, cognitive domain-specific composites (executive function, episodic memory) | Vascular and metabolic status and events; Walk Test and physical function; depressive symptoms; quality of life; change in lifestyle habits; APOE genotype Sub-studies: brain imaging (MRI, amyloid and tau PET); sleep; microbiome; vascular function and structure |
Ongoing |
| MIND-CHINA (China) | 3000 non-demented adults age 60–79 at 52 villages in Western Shandong province | 1. Regular health care services 2. Vascular intervention 3. Multidomain intervention |
2 years intervention, extended follow-ups | Global cognitive composite | Physical function, incident MCI and dementia at 5 years, cardiovascular events Brain MRI sub-study (3DT1, T2, FLAIR, SWI, fMRI, MRA) |
Ongoing |
| MYB (Australia) | 6236 adults age 55–77 with two or more dementia risk factors | 1. Non-interactive web-based advice via internet 2. Personalized modular internet-based multidomain coaching in four thematic areas |
3 years | Global cognitive composite score | Dementia incidence; change in dementia risk score (ANU-ADRI-SF); change in cognitive domain scores and individual cognitive tests; risk factor reduction (BMI, hip-waist ratio, level of physical activity; physical functional level, new chronic health conditions, adherence to Mediterranean diet, alcohol and smoking status, mental activity levels, psychological distress); service utilization (hospital admissions, medical and social care services, prescribed medications); study adherence; module expectations, adverse events | Ongoing |
| AU-ARROW (Australia) | 900 adults age 55–75 at two sites | 1. Passive health education and support 2. Active health education and support 3. Multidomain lifestyle intervention |
2 years | Global cognitive composite score | Blood and CSF biomarkers, brain and retinal imaging | Funded, recruitment pending |
| MIND-AD (Sweden, Finland, France, Germany) | 120 adults with prodromal AD plus vascular and lifestyle risk factors | 1. Usual care 2. Multidomain lifestyle intervention 3. Multidomain lifestyle intervention + medical food |
6 months + optional 6-month extension | Feasibility, adherence | Vascular and metabolic factors; depressive, anxiety and stress symptoms; health-related quality of life; physical performance; blood markers; cognition; functioning; microbiome and brain imaging sub-studies | Ongoing |
| SINGER (Singapore) | 70 seniors, age >65 with mild-to-moderate frailty and/or cognitive impairment | 1. Original FINGER intervention; 2. Culturally adapted multidomain intervention |
6 months intervention | Feasibility, adherence | Physical activity and fitness, cognitive performance, changes in body weight, BMI, hip-waist-ratio, blood pressure, fasting blood glucose and lipid, changes in blood pressure management and medications | Ongoing |
| SUPERBRAIN (Korea) | 150 adults, cognitively normal, with at least one modifiable risk factor for cognitive impairment Age 60–79 | 1. Facility-based multidomain intervention 2. Home-based multidomain intervention 3. Regular health advice (subjects will receive the multidomain intervention after the end of the study) |
6 months intervention | Feasibility, adherence | Global cognition and memory, functional status, depressive symptoms, quality of life, nutritional status, change in levels of motivation, physical function. Biological studies on underlying mechanisms: neurotrophic, neurodegeneration and neuroinflammation factors, gut microbiome, telomere length, electroencephalography, neuroimaging | Ongoing |
| J-MINT (Japan) | 440 adults with cognitive impairment (age-adjusted cognitive scores <1.5 SD from the reference value in selected cognitive domains) aged 65–85 | 1. Usual care 2. Multidomain intervention |
18 months intervention | Global cognitive composites | Cognitive domains; functioning Geriatric assessment (frailty, polypharmacy, comorbidities, lifestyle, subjective cognitive complaints, poor sleep, depressive symptom, social isolation, hearing impairment, nutritional status/appetite) Blood biomarkers for AD pathology and neurodegeneration, and omics analysis Brain MRI for structural analysi |
Ongoing |
| GOIZ-ZAINDU (Spain) | 125 at-risk (CAIDE score) adults aged 60+ years | 1. Regular health services 2. Intensive multidomain intervention |
1 year (pilot intervention) | Feasibility, adherence | Global cognitive composite. Vascular and metabolic risk factors (CAIDE score). 6 minutes walk test. Change in life habits. Adherence to Mediterranean Diet. Quality of life. | Ongoing |
| PENSA (Spain) | 200 adults meeting criteria of subjective cognitive decline plus, carriers of the APOE4 allele, 60+ years | 1. Multidomain lifestyle intervention + EGCG 2. Multidomain lifestyle intervention + placebo EGCG 3. Usual care + EGCG 4. Usual care + placebo EGCG |
1-year (pilot) intervention and 3 months follow-up after discontinuing intervention | Global cognitive composite (ADCS-PACC-Plus-exe) | MRI and fMRI; microbiota; neuroinflammation biomarkers; metabolomics; blood, brain-derived exosomes and CSF neuropathological biomarkers Physical activity, dietary assessments, cognitive training performance and, quality-of-life records |
Recruiting |
| Can Thumbs Up (Canada) | 2024 non-demented adults aged 60–85 | 1. Directed educational effort toward brain health 2. Selected candidate lifestyle interventions 3. Selected pharmacological interventions |
Up to 5 years with intermediate futility analyses | Global cognitive composite | Surveys and remote assessments. IADL, NPIQ, Geriatric Depression Scale, SF-36, Gait, Single and Dual Task, PSQI, Research Satisfaction Blood-based biomarkers Actigraphy cranial MRI |
Protocol development |
| LATAM-FINGER (Argentina, Brazil, Bolivia, Chile, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, Mexico, Paraguay, Peru, Puerto Rico, and Uruguay) | 1400 at-risk adults aged 60–77 | 1. Systematic multidomain lifestyle intervention 2. Flexible lifestyle intervention |
1-year intervention | Feasibility and global cognitive composite (harmonized with FINGER and U.S. POINTER) | Cognitive domains Subgroup analysis by APOE status, brain MRI sub-study Blood biobank established to test biomarkers |
Protocol submitted |
Key features of the WW-FINGERS Network studies in all levels that were presented during the face-to-face meetings. Additional studies around the world are in various stages of planning and initiation. Abbreviations: AD, Alzheimer’s disease; ADCS-PACC, Alzheimer Disease Cooperative Study Preclinical Alzheimer Cognitive Composite; ANU-ADRI-SF, Australian National University Alzheimer’s Disease Risk Index short form; APOE, apolipoprotein E gene; BMI, body mass index; CAIDE, Cardiovascular Risk Factors, Aging, and Incidence of Dementia; CSF, cerebrospinal fluid; EGCG, epigallocatechin gallate; fMRI, resting state functional magnetic resonance imaging; GWAS, genome-wide association study; IADL, instrumental activities of daily living; MCI, mild cognitive impairment; MRA, Magnetic resonance angiography; MRI, magnetic resonance imaging; NPI-Q, Neuropsychiatric Inventory–Questionnaire; PET, positron emission tomography; PSQI, Pittsburgh Sleep Quality Index; SD, standard deviation; SF-36, Short Form Health Survey.
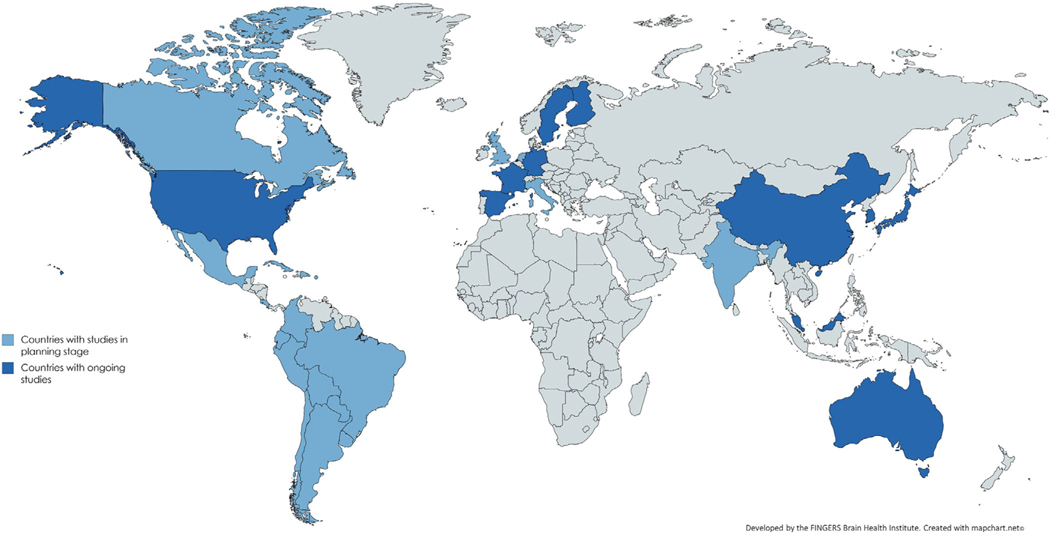
FIGURE 1.
World map with countries that are involved in the World-Wide FINGERS Network. Dark blue indicates involvement in ongoing World-Wide FINGERS studies. Studies are currently planned in countries marked with light blue
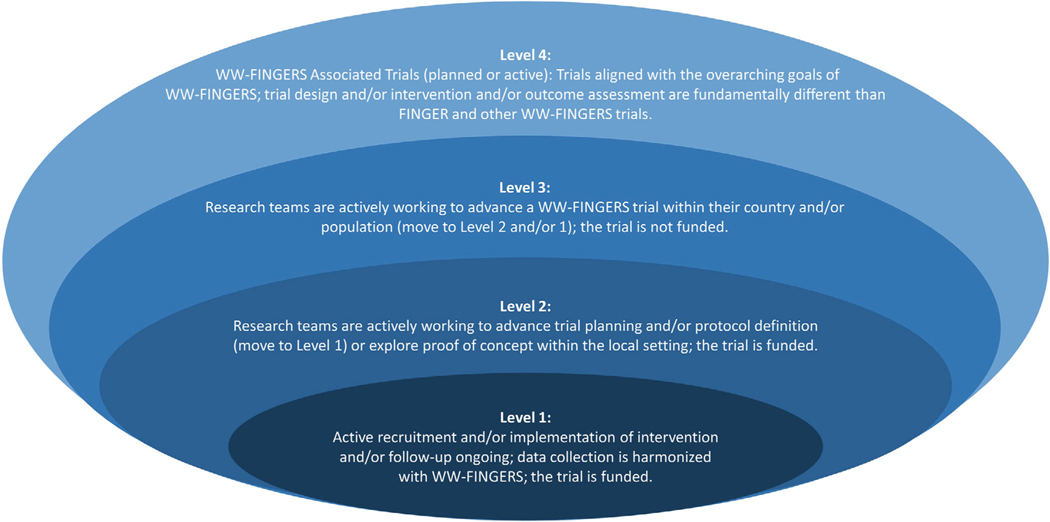
WW-FINGERS comprises studies at different stages of implementation and with varying gradations of alignment to the FINGER trial (Table 1). For instance, in the U.S. study to Protect Brain Health through a Lifestyle Intervention to Reduce Risk (U.S. POINTER), the interventions and outcomes are harmonized with FINGER. This consistency will allow for numerous cross-study comparisons as data become available in U.S. POINTER. A number of feasibility studies are evaluating the ability to conduct such a trial in a particular country and/or population, whereas other studies are in the initial stages of discussion and/or implementation (Table 1 and Figure 1). To help classify and understand the status of the WW-FINGERS trials, the network has agreed on the definition of four main levels, which reflect the trials’ key features, including current status (eg, from RCTs still in the planning stage to those in which follow-up of participants is available); funding availability; and degree of alignment with the FINGER RCT, in terms of design, intervention being tested, and assessment of cognitive outcomes (Figure 2). Each study in the WW-FINGERS Network can be assigned to a level, and movement among levels is expected.
FIGURE 2.
Levels defined for the trials participating in the World-Wide FINGERS Network
The next sections summarize the WW-FINGERS Network studies (see also Table 1), followed by a section discussing the key issue of data sharing and harmonization, and a final section on future steps needed to advance the Network activity.
2.1 |. U.S. POINTER
In the United States, the U.S. POINTER study (ClinicalTrials.gov: NCT03688126) will enroll approximately 2000 cognitively normal adults who are at an increased risk for cognitive decline and dementia in later life. Increased risk is defined by sedentary lifestyle, poor diet, and other factors such as suboptimal cardiovascular health status and first-degree family history of significant memory impairment. At five sites across the country, participants will be randomly assigned to a self-guided or structured lifestyle intervention program focused on increasing physical exercise, consuming a healthy diet, intellectual stimulation and social engagement, and health monitoring to manage cardiovascular risk factors. The primary outcome is change in global cognition, assessed by a composite score that is harmonized with the primary outcome in FINGER. Other measures will include separate composite measures of episodic memory, executive function, and processing speed; self-reported changes in mood, sleep, and lifestyle practices; walking speed; and cardiometabolic health metrics. Intervention effects will be examined by subgroups based on APOE ε4 genotype and baseline cognitive performance. Blood samples are collected and stored for future investigations of potential mechanisms underlying intervention effects. Sub-studies will be conducted to collect magnetic resonance imaging (MRI) and amyloid and tau positron emission tomography (PET); objective measures of sleep quality; and microbiome data. U.S. POINTER initiated recruitment in January 2019 for the vanguard site in North Carolina. The other four sites include Northern California, Chicago (IL), Houston (TX), and Rhode Island. The Alzheimer’s Association funds the study and collaborates with the U.S. POINTER Coordinating Center at Wake Forest School of Medicine (Winston-Salem, NC, USA) to assist in leading the study.
2.2 |. MIND-CHINA
The randomized controlled Multimodal INtervention to delay Dementia and disability in rural China (MIND-CHINA, Chinese Clinical Trial Registry: ChiCTR1800017758) study targets residents from 52 villages in rural areas of Western Shandong Province (Yanlou Town of Yanggu county). In March-October 2018, baseline assessments and screenings for participants were completed, during which over 5700 subjects who were 60 years of age or older were examined. Starting from March 2019, non-demented and non-disabled residents aged 60 to 79 are being enrolled into the MIND-CHINA Study and randomly divided by village (a method known as cluster randomization) into one of three groups: a group that will receive regular health care services provided by government; a vascular intervention group that will receive intensive medical treatment to improve the control of three major vascular risk factors (high blood pressure, high blood glucose, and dyslipidemia); or a multidomain intervention group that will receive guidelines of healthy lifestyle and diet, physical exercise, promotion of personalized leisure activities, as well as cognitive training, in addition to intensive management of major vascular risk factors. MIND-CHINA aims to recruit 3000 participants. A cognitive composite measure that involves memory, language, information processing speed, and executive function similar to FINGER’s NTB will be used as the primary outcome. Secondary outcomes will include physical function, incident mild cognitive impairment (MCI) and dementia (after 5 years of follow-up), and occurrence of cardiovascular events. In addition, a brain MRI sub-study is planned within MIND-CHINA, with core MRI sequences including magnetic resonance angiography, structural MRI measures, and resting-state functional MRI. MIND-CHINA is supported by the grant from the National Key R&D Program of China and the Sino-Sweden joint research project grants from the National Natural Science Foundation of China and the Swedish Research Council.
2.3 |. Australia—AU-ARROW and Maintain Your Brain
Australia includes two lifestyle intervention studies that comprise different interventions and outcomes. One is the AUstralian-Multidomain Approach to Reduce Dementia Risk by PrOtecting Brain Health with Lifestyle intervention (AU-ARROW), which will randomize 900 participants ages 55 to 75 into one of three groups: a usual care group; a group that will receive health education and support; and a multidomain intervention group. The trial will run for 2 years, with an additional 6-month follow-up. As with other FINGER studies, change in cognition is the primary outcome. Secondary outcomes will include blood and cerebrospinal fluid (CSF) biomarkers and brain imaging—amyloid and fluorodeoxyglucose PET, MRI, and retinal imaging.
A second Australian study, called Maintain Your Brain (MYB, Australian New Zealand Clinical Trials Registry: ACTRN12618000851268) is ongoing and tests an internet-based multidomain intervention, which if successful could provide a relatively low-cost strategy to deliver potentially preventive interventions at a population level and in particular to geographically isolated people. To qualify for the study, individuals were 55- to 77-years-old and had two or more lifestyle-related risk factors for dementia, including physical inactivity, cognitive inactivity, depression/anxiety, overweight or obesity, and poor dietary habits.28 Participants (n = 6236) were randomized to receive a personalized multidomain intervention or non-interactive web-based health advice and information. A unique feature of the MYB trial is that the intervention is targeted to improve participant-specific risk factors. The interventions are delivered in the form of coaching modules across four thematic areas: physical activity, diet and nutrition, cognitive training, and mood, and participants do not need to receive the intervention in all four areas. Modules are delivered quarterly in Year 1 followed by monthly boosters until end of Year 3. Primary outcomes include a cognitive composite measure, in synergy with FINGER and U.S. POINTER.
2.4 |. Europe—MIND-AD
The FINGER protocol is also tested in Europe (Sweden, Finland, Germany, France) in the Multimodal Prevention Trial for Alzheimer’s Disease (MIND-ADmini, ClinicalTrials.gov: NCT03249688). MIND-ADmini is an ongoing pilot trial evaluating the feasibility of the FINGER multidomain lifestyle intervention in prodromal AD defined according to the International Working Group (IWG)-1 criteria.29 Participants also have modifiable vascular or lifestyle-related risk factors.
Conducting a feasibility trial in people who are already in the early stages of AD and have some cognitive impairment is important because of the potential need for additional support with healthy lifestyle maintenance. The duration of the trial is 6 months, with an optional 6-month extension. Participants are randomized into three groups: multidomain lifestyle intervention based on the FINGER model; multidomain lifestyle intervention plus the medical food Fortasyn Connect; or standard care. The rationale for combining a multidomain intervention with medical food is suggested by studies that evaluate the synergistic effects between different intervention components (eg, omega-3 fatty acids and physical activity). Fortasyn Connect is a multinutrient including omega-3 fatty acids, phospholipids, choline, vitamins, and minerals. It has been selected for MIND-AD because prior results from another phase-2 RCT in prodromal AD suggest that a combination of interventions should be evaluated.30
The main aims of MIND-ADmini are to evaluate the feasibility of and adherence to the multidomain intervention. Exploratory aims include intervention effects on changes in vascular and metabolic risk factors; depressive, anxiety, and stress symptoms; cognition; health-related quality of life; and physical performance. Blood samples are collected for exploring potential mechanisms and mediating pathways of the multidomain intervention. There is also a qualitative interview study focusing specifically on the experiences of the trial participants. Results will be used in the planning of future larger trials and will serve as a model for combining non-pharmacological and pharmacological interventions.
2.5 |. Singapore—SINGER
The SINGapore GERiatric intervention study to reduce physical frailty and cognitive decline (SINGER) is a pilot study aiming to evaluate culturally appropriate adaptations of the FINGER interventions, and the feasibility of implementing this protocol in 70 seniors, aged >65 years, with mild-to-moderate frailty and/or cognitive impairment over a 6-month period (Table 1). The main outcome of this pilot study is the feasibility of and adherence to the multidomain intervention, as concerns have been raised particularly about the dietary and cognitive interventions, which could require novel and culturally appropriate approaches. The aim is to develop scalable digital platforms and build partnerships in order to conduct a larger confirmatory RCT in 1200 elderly community-dwelling Singaporeans who are at risk of cognitive impairment and dementia. This larger study, comparing self-guided lifestyle management versus a structured multidomain lifestyle intervention, will also incorporate neuroimaging and blood biomarkers to investigate mechanisms of action.
2.6 |. South Korea—SUPERBRAIN
The SoUth Korean study to PrEvent cognitive impaiRment and protect BRAIN health through lifestyle intervention in at-risk elderly people (SUPERBRAIN, ClinicalTrials.gov: NCT03980392) is a multicenter feasibility study enrolling seniors (60 to 79 years) with at least one modifiable risk factor for dementia. The study includes two intervention arms—facility-based intervention and home-based intervention—as well as a group receiving regular health advice. The 24-weekmultidomain intervention has been derived by the FINGER model and includes five components: monitoring and management of metabolic and vascular risk factors; cognitive training and social activity; physical exercise; nutritional guidance; and motivational enhancement. The trial aims to assess the feasibility of the multidomain intervention, and includes as secondary outcomes disability, depressive symptoms, quality of life, vascular risk factors, physical performance, nutritional assessment, and a motivation questionnaire. To investigate mechanisms underlying the intervention, neurotrophic, neurodegeneration, and neuroinflammation factors, gut microbiome, telomere length, electroencephalography, and neuroimaging measures will be evaluated. Based on the study results, a large-scale RCT will be launched to assess the effects of the multidomain intervention on cognition.
2.7 |. Japan—J-MINT
The Japan-multimodal intervention Trial for prevention of dementia (J-MINT, UMIN Clinical Trials Registry: UMIN000038671) is an RCT that tests the effect of a multidomain intervention in subjects with some degree of cognitive impairment (Table 1). The study will recruit 440 older people (65- to 85-years-old) with a high risk of dementia, defined as decreased cognitive performance in at least one of the four cognitive functional domains of memory, attention, executive function, and processing speed. Subjects will be randomized to either a group that receives usual care or a group that participates in an 18-month multidomain intervention, consisting of exercise, nutritional counseling (visits and telephone follow-ups by health consultants with expertise in nutrition), and cognitive training using the “Brain HQ” program. In the exercise program, the intervention group participates in activities described as “cognicise,” from the combination of the words “cognition” and “exercise”—a program developed at the National Center for Geriatrics and Gerontology, NCGG in Japan, which combines physical exercise and cognitive tasks. In addition, diabetes, hypertension, and dyslipidemia are treated in accordance with the Japanese guidelines for older patients.
The primary outcome of J-MINT is cognition, whereas the secondary outcomes include functional status, blood-based biomarkers reflecting amyloid beta (Aβ) accumulation in the brain, omics analysis, and neuroimaging (MRI). Evidence from this research is expected to inform a large-scale, national implementation of multidomain intervention programs for older people at high risk of dementia.
2.8 |. Spain—GOIZ-ZAINDU and PENSA
GOIZ-ZAINDU (Basque words for “caring early”) is an ongoing pilot RCT that tests the feasibility of the FINGER multidomain intervention model in the Basque population in Spain. Participants, age 60+ years, have been recruited within the GOIZ-Alzheimer cognitive decline early detection program, using inclusion criteria similar to those used in the FINGER RCT: increased risk of dementia, based on a CAIDE (Cardiovascular Risk Factors, Aging, and Incidence of Dementia) Dementia Risk score of six points or higher,31 and lower-than-expected cognitive performance in at least one of three brief cognitive screening tests (AD8 questionnaire, Fototest, memory alteration test).32–34 Since the RCT started in June 2018, over 250 participants have been screened, and 125 fulfilled the inclusion criteria. Stratification by cognitive status (MCI and normal cognition) and age (75 or older) has been performed before randomization to a multidomain intervention or to a group receiving standard health advice. The multidomain intervention lasts 1 year and includes nutritional counseling, physical activity, cognitive training, and monitoring of vascular risk factors. The study will be completed by June 2020, and the outcomes include adherence to the intervention, change in the CAIDE risk score, and global cognition, measured with the NTB. Results from this pilot study will pave the way to larger efficacy trials in the Basque country to provide knowledge for public health strategies on healthy-active aging and dementia prevention. The GOIZ-ZAINDU project is performed and funded by the CITA-Alzheimer Foundation in collaboration with the Municipality of Beasain and Osakidetza (the public Basque Health System).
Another study in Spain, the “Prevention of cognitive decline after a multimodal intervention combined with epigallocatechin gallate in APOE4 carriers with subjective cognitive decline” (PENSA study: in Catalan PENSA means “think about”; ClinicalTrials.gov: NCT03978052) is an ongoing project in Barcelona, performed in collaboration with the Hospital del Mar Medical Research Institute (IMIM) and the BarcelonaBeta Brain Research Center (BBRC). PENSA will randomize 200 individuals meeting criteria of subjective cognitive decline (SCD), fulfilling four SCD plus features, including subjects older than 60 years and APOE4 carriers. The project combines a multidomain lifestyle intervention including diet, physical activity, and cognitive stimulation and training and the administration as dietary supplement of the flavanol from green tea, epigallocatechin gallate (EGCG, 5 to 6 mg/kg up to 532 mg/day). The intervention will last 12 months. Participants will be randomized into one of four study arms (Table 1), and the primary outcome is global cognition measured with the ADCS Preclinical Alzheimer Cognitive Composite-Plusexe. Secondary and exploratory outcomes include neuroimaging (structural and functional MRI); microbiota; neuroinflammation biomarkers; metabolomics; blood, brain-derived exosomes, and CSF neuropathological biomarkers; physical activity, dietary assessments, cognitive training performance, and quality-of-life records. The project is primarily funded by the Alzheimer’s Association, with additional support from the Spanish Ministry of Science, Innovation and Universities (Instituto Carlos III).
2.9 |. CANADA—CAN-Thumbs-UP
In Canada, the Canadian Therapeutics Platform Trial for Multidomain Interventions to Prevent Dementia (CAN-Thumbs-Up) is in development and will include the recruitment of a platform trial-ready cohort of participants identified as being at increased risk of dementia. This cohort will initially participate in an online Brain Health Support Program (BHSP) for up to 1 year, aimed at improving dementia literacy, self-efficacy, and engagement. It will include remote assessments during the BHSP that will enable evaluation of compliance, and changes in lifestyle through this educational intervention. The platform trial-ready cohort data will support the modeling of clinical and biomarker trajectories of the high-risk participants, who initially will either be cognitively normal or have MCI. These data will inform development of the master trial protocol as well as permitting identification and tailoring of interventions to high risk study groups. The open platform trial will particularly focus on testing multidomain interventions, including treatments of lifestyle, pharmaceuticals, and other types of combination treatments in periods of up to 3 years. The primary outcome for the platform trial is expected to be a global cognitive composite, with a range of secondary and exploratory measures including blood-based and digital biomarkers.
2.10 |. South and Central America—LatAm FINGER
LatAm-FINGERS is a Latin American initiative gathering 14 countries— Argentina, Bolivia, Brazil, Chile, Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, Mexico, Paraguay, Peru, Puerto Rico, and Uruguay—and is open to incorporating other regional initiatives. The consortium is developing a multidomain RCT that will enroll 1400 individuals (100 from each country) aged 60 to 77 years, who are at a high risk of cognitive deterioration due to sedentary lifestyle and to suboptimal metabolic-cardiovascular profile. Subjects will be randomized to either a Systematic Lifestyle Intervention (SLI) or a Flexible Lifestyle intervention (FLI). The SLI group will receive dietary counseling, physical exercise, cognitive training, and control of cardiovascular risk factors. The FLI group will receive regular health advice. Outcomes will be measured every 6 months and will include clinical and neuropsychological assessment harmonized with the original FINGER and with the U.S. POINTER. The protocol also includes collection of blood samples and MRI data for the creation of a biobank for future research. The LatAm FINGERS has two main objectives: first, to evaluate the feasibility of the FINGER multidomain lifestyle intervention in the Latin American context; second, to evaluate the efficacy of the SLI, primarily on a global cognition composite score as well as on specific cognitive domains: episodic memory, executive function, and processing speed. Analyses of the intervention effects will consider the participants’ baseline cognition and APOE genotyping status. LatAm-FINGERS will allow to share, compare, and harmonize data across the participating centers, thus consolidating methods throughout Latin America for future collaborative research in dementia.
2.11 |. India FINGER
Two longitudinal cohort studies of aging—one in an urban population and one in a rural population—are being carried out in India. In urban Bangalore, adults 45 years or older are enrolled in a study funded by the Tata Trusts, while in the rural Kolar district, adults 45 years or older are enrolled in the Srinivaspura Aging, Neuro Senescence and COGnition (SANSCOG) study. Similar assessments are used in both studies, including a cognitive battery comparable to that used in FINGER and adapted for culture and education across India, brain MRI and PET, biochemical tests with a focus on micronutrient deficiency and vascular risk factors, carotid Doppler ultrasonography, complete genetics, and activity monitoring with wearables. The contrasts observed between the urban and rural populations will help inform a future FINGER-type intervention study. These populations differ across many domains, including education, language, socialization, prevalence of cardiovascular risk factors, and nutritional deficiencies. For example, rural residents may be reluctant to accept dietary modification and structured exercise programs, but there may be more acceptance of yoga as a physical activity. Thus, a 6-month pilot study in 100 participants will investigate whether the practice of yoga affects cognitive and imaging end points. These observational studies are paramount so that population level data can be collected before moving into an intervention phase.
2.12 |. Other WW-FINGERS studies
In Europe, a joint EURO-FINGERS protocol is currently being developed to aid various European countries (eg, The Netherlands, Spain, the UK, Italy, Sweden) in establishing FINGER-like studies. Some of the new trial design elements that may be tested include, for example, utilizing e-health and mobile-health (m-health) tools and tailoring the interventions to take into account specific risk profiles and combining a multidomain lifestyle-based intervention with pharmacological interventions. Encouraging results, in terms of acceptability and efficacy of e-health interventions for dementia risk reduction, have been recently reported in the European Healthy Aging Through Internet Counselling in the Elderly (HATICE, ISRCTN48151589). In this 18 month multinational RCT, a coach-supported internet-based multidomain intervention for self-management of vascular and metabolic risk factors was tested in 2724 seniors, and the intervention was associated with a modest improvement of the risk profile.35,36
Still in Europe, Germany is assessing key issues related to the implementation of the FINGER model, in a pragmatic 2-year trial (AgeWell.de, German Clinical Trials Register DRKS00013555) enrolling 1152 at-risk seniors through general practitioners.37 In addition, Malaysia is starting the Multidomain Intervention for Reversal of Cognitive Frailty (AGELESS) study.
3 |. HARMONIZATION OF TRIALS, DATA SHARING, AND ALIGNMENT
WW-FINGERS promotes the prospective harmonization of methods and outcomes across studies and responsible sharing of data. This will enable joint analyses and comparisons across studies and will generate robust evidence to inform dementia prevention strategies. The power of WW-FINGERS can only be realized through data sharing. In the AD field, open and responsible data sharing has fueled tremendous progress in the Alzheimer’s Disease Neuroimaging Initiative (ADNI), as well as in four prevention studies that came together under the umbrella of the Collaboration for Alzheimer’s Prevention (CAP).38 Recommendations to promote responsible data sharing were also adopted by a European task force through a consensus-building process.39
A vision for data sharing in WW-FINGERS has been adapted from the Institute of Medicine (IOM) report on sharing clinical trial data.40 These principles include (1) establishing a culture of sharing with incentives for sharing data; (2) building a global platform for sharing data; (3) establishing best practices for data sharing; (4) providing adequate financial support for the sharing of data and allocating the costs among all stakeholders; and (5) minimizing the risks and reducing disincentives for data sharing.
Integrating data from different RCTs and cohort studies into a shared database requires that data are harmonized using existing and accepted data standards, templates, and common data elements. Common data elements enable linkage of data across multiple studies, increase the consistency and quality of data, facilitate comparisons, drive efficiencies, and promote interoperability. In WW-FINGERS, harmonization will also be promoted in study design, enrollment criteria, selection of interventions and outcomes, and monitoring of intervention adherence. Members of the WW-FINGERS Network recognize the need to establish rigorous standards and harmonize these elements before the studies begin data collection. Prospective harmonization of the WW-FINGERS trials is being detailed in the WW-FINGERS Harmonization Protocol, which is currently under development. Among the key points, the Protocol specifies that in all trials the main principles of the intervention are based on the experiences from the FINGER study. It is recommended that all the basic components of the multidomain intervention available in the FINGER study should be implemented; that is, dietary counseling, physical exercise, cognitive training, and vascular and metabolic risk monitoring. At the same time, local and cultural adaptations of the content and delivery method of the intervention are encouraged. For instance, dietary counseling will follow national recommendations while considering country- or region-specific habits. In MIND-China, for example, dietary guidance puts special emphasis on reducing salt intake, which is a key dietary challenge in China. In India, yoga might be promoted to increase physical exercise, as it is commonly practiced in some regions. In addition, pharmacological vascular risk factor management, when applicable, will be based on national care guidelines. This is essential to improve engagement and adherence, and subsequently, to facilitate the effective and sustainable implementation of preventive strategies.
The Harmonization Protocol indicates that similar tools to detect cognitive changes are recommended in all new WW-FINGERS trials. When this is not possible, test batteries similar to the original but validated in the local settings can be used. There is also the possibility for new cognitive outcome scores to be added (eg, in the U.S. POINTER, some cognitive computer-based measures have been added as exploratory outcomes). Change in a global measure of cognitive function is the primary efficacy outcome in WW-FINGERS trials, but direct translation of cognitive assessments tools into different languages and in a manner that is relevant to different cultures and populations may not always be possible or feasible. In addition, education can influence factors for cognitive assessment and performance. It may be possible, however, to harmonize cognitive outcomes across disparate populations with respect to the cognitive domains tested. In the FINGER study, the major domains assessed included processing speed, executive function, and episodic memory. Given cultural differences in the WW-FINGERS Network, assessment of these domains may involve administration of different cognitive tests. Another approach might be to administer a test that assesses everyday functional memory (as opposed to rote memory) in ways that are more culturally relevant to individuals under study.
It should be emphasized that the WW-FINGERS is not a single study with multiple centers around the world, with unified strict criteria on harmonized outcomes and interventions. Instead, the aim is to test the adaptation of the FINGER study in different populations regarding feasibility and effectiveness. Thus, the studies in the WW-FINGERS are separate studies with their own research questions with pooled data analyses enabled through prospective harmonization, which increases power and permits subgroup analyses. Indeed, one of the key goals in the WW-FINGERS is to incorporate diversity, and inclusion of historically underrepresented groups (eg, sex, race, ethnicity, education, socioeconomic status) is highly encouraged. Pooled data from the WW-FINGERS Network can provide insight into the potential of multidomain lifestyle interventions to impact cognitive health and decline across widely diverse populations.
While full alignment of outcome measures across all WW-FINGERS trials could be challenging regarding cognition and lifestyle, it might be easier for brain imaging, blood and CSF biomarkers, and clinical measures, for which substantial work toward standardization has been done. Omics and genome-wide association study data are relatively new in clinical trials and thus there has been less progress on harmonization. The WW-FINGERS Network will work toward establishing global standards for sample collection and explore the potential of a WW-FINGERS biorepository.
Regarding the need for a data sharing platform, the WW-FINGERS Network will build upon prior work, as well as search for innovative ICT-solutions (ie, Information and communications technology), generated by the rapid expansion of data sharing technologies and tools. For instance, U.S. POINTER will use the Global Alzheimer’s Association Interactive Network (GAAIN, gaain.org) as a platform for global data sharing, as GAAIN can link diverse data sets. ICT solutions can help overcome the challenges of having data cross country borders, harmonizing data sets, and allowing for aggregate interrogation in a secure framework. As the WW-FINGERS Network expands and trials are launched, data-sharing tools will be assessed as it will be important for the various initiatives to align so that trial data are analyzed, interpreted, and validated in a consistent manner and that privacy and confidentiality are maintained. From the European perspective, given the current changes in regulation regarding data protection (ie, European General Data Protection Regulation, GDPR),41 sharing individual level data with countries outside of the European Union is difficult, even with proper pseudonymization.42 Therefore, instead of sharing individual level data across the borders, the aim within the WW-FINGERS Network is to create a federated database platform enabling pooled data analyses remotely.43 Overall, WW-FINGERS aims to optimize harnessing of data, while accounting for global variations and increasing requirements for data protection and sharing.
4 |. NEXT STEPS
WW-FINGERS is the first global network of multidomain lifestyle intervention trials for dementia risk reduction and prevention, and aims to adapt, test, and optimize the FINGER model to prevent dementia across the entire spectrum of cognitive decline—from at-risk asymptomatic states to early symptomatic stages, such as prodromal AD— and in different geographical, cultural, and economic settings. Multiple WW-FINGERS studies are in the early stages of initiation in several countries, targeting subjects with different risk profiles, either without cognitive impairment or with pre-dementia symptomatic cognitive changes. Multidomain strategies have already been proved effective and are internationally implemented in guidelines for the prevention of cardiovascular disease and diabetes mellitus. This supports the scalability that is being developed through WW-FINGERS.
Overall aims of the WW-FINGERS Network are aligned with the recent WHO guidelines for risk reduction of cognitive decline and dementia,26 in which the WHO recommends conducting multidomain intervention trials similar to FINGER to define evidence-based approaches to public health preventive interventions. This is especially crucial for low- and middle-income countries (LMICs), which lack robust evidence on risk and protective factors for dementia and AD, and yet are expected to face the highest rise in the number of dementia cases by 2050.1 Toward this end, the Network has started working to engage and support LMICs as well as other countries (eg, Middle East: Israel, Iran; Africa: Cameroon, South Africa; Russia, Hungary), and the close collaboration with the WHO can help disseminating study findings in these areas and potentially expand the WW-FINGERS Network.
The WHO recognizes the need for research coordination and harmonization. In this regard, WW-FINGERS will provide insight on the challenges that need to be overcome to drive global collaborative efforts and the implications for research, ethics, and policy. A WW-FINGERS Harmonization Protocol is being compiled, to ensure that country-specific WW-FINGERS trials are harmonized with the core features of the FINGER study, allowing for local adaptations to be introduced. An Oversight Committee, described in the protocol, plans to oversee its proper implementation and guide the development of a set of common data elements, the synchronization of outcomes across studies engaging diverse populations, and the building of an infrastructure to support a network-wide data sharing.
In WW-FINGERS, the focus is on individual-based preventive approaches in persons with increased risk of dementia. Although WW-FINGERS is not designed to incorporate broader health care and social policies, the pooled analyses will provide the opportunity to explore the feasibility and effectiveness of interventions in different health care and social settings.
Results from WW-FINGERS can be used to potentially update the WHO recommendations specifically relating to multidomain interventions, and findings from WW-FINGERS trials can be disseminated though the WHO’s Global Dementia Observatory (GDO), a data and knowledge exchange platform that offers easy access to key dementia data from Member States across the following three domains: policies, service delivery, and information and research. The GDO supports countries in measuring progress on dementia actions outlined in the Global Dementia Action Plan on the Public Health Response to Dementia 2017–2025 and assists them in strengthening policies, service planning, and health and social care systems for dementia.44
WW-FINGERS will offer numerous opportunities for hypotheses testing, in addition to those assessing the efficacy of multidomain interventions to slow cognitive decline. For example, several studies are planning to investigate the relationship of vascular risk factors and vascular disease with dementia. In addition, the rich neuroimaging and biorepository collected will enable studies on mediating pathways and underlying mechanisms of the multidomain interventions, as well as contribute to identifying risk profiles that may be more responsive to specific interventions. Together with the introduction of pharmacological components within the multidomain lifestyle model, this approach can facilitate the definition of preventive approaches within the frame-work of precision prevention.
By collectively convening several multidisciplinary research teams, WW-FINGERS will provide a forum to establish harmonization and encourage adaptation of the multidomain lifestyle intervention across various countries and settings, facilitate responsible data sharing and analysis across studies, establish opportunities for joint initiatives across country borders, and strengthen the potential evidence base for multidomain lifestyle interventions to prevent cognitive decline and dementia. The WW-FINGERS Network of studies will provide important new knowledge that will facilitate the development of effective interventions and contribute to identifying globally applicable strategies to prevent or delay the onset and progression of late-life cognitive impairment, AD, and dementia. Risk factor modifications by public health interventions have had a remarkable impact on the occurrence of cardiovascular disease, and the same can be expected for dementia.
RESEARCH IN CONTEXT.
1. Systematic review:
The Authors met in 2 occasions (plus additional teleconferences and smaller working-groups meetings) to discuss adaptation and testing of the FINGER multidomain prevention model in different settings and populations. The development of a scientific methodology was supported also by search (PubMed, Authors’ own reference catalogues) for RCTs to prevent cognitive impairment or dementia, which target multiple lifestyle factors simultaneously. Ongoing and completed trials were identified and discussed. These studies are appropriately cited.
2. Interpretation:
The FINGER model has shown beneficial effects on cognition in at-risk seniors. The model needs to be further tested, adapted and optimized in diverse geographical and cultural settings. Within the World-Wide FINGERS Network, a scientific methodology has been outlined towards this goal.
3. Future directions:
Ongoing and planned trials within the World-Wide FINGERS Network will investigate feasibility and efficacy of the FINGER intervention in different populations, and generate data for evidence-based knowledge for globally implementable and effective preventive strategies for cognitive impairment, dementia and AD.
HIGHLIGHTS.
Prevention is pivotal to reduce the occurrence of dementia worldwide
The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) trial model supports the benefits of multidomain lifestyle interventions
The World-Wide FINGERS (WW-FINGERS) network is testing and adapting the FINGER model globally
WW-FINGERS is a unique network supporting data harmonization and sharing
WW-FINGERS can lead to effective and feasible dementia preventive strategies
ACKNOWLEDGMENTS
This work was supported by the Academy of Finland; Joint Program of Neurodegenerative Disorders—prevention (MIND-AD) grant through the following funding organizations under the aegis of JPND—www.jpnd.eu: Finland, Suomen Akatemia (Academy of Finland, 291803); Sweden, Vetenskapsrådet (VR) (Swedish Research Council, 529-2014-7503) France, French National Agency for Research (ANR), ANR-14-JPPS-0001-02]; Swedish Research Council grant 2017-06105; Juho Vainio Foundation, Finnish Medical Foundation; Finnish Social Insurance Institution; Ministry of Education and Culture Research Grant; Finnish Cultural Foundation; Knut and Alice Wallenberg Foundation Sweden; Center for Innovative Medicine (CIMED) at Karolinska Institutet Sweden; Stiftelsen Stockholms sjukhem Sweden; Konung Gustaf V:s och Drottning Victorias Frimurarstiftelse Sweden; af Jochnick Foundation Sweden; Alzheimer’s Research and Prevention Foundation; the European Research Council Starting Grant (ERC-804371), Alzheimerfonden Sweden; Region Stockholm (ALF and NSV grants), Sweden. U.S. POINTER trial is supported by the Alzheimer’s Association POINTER-19-611541 and POINTER-19-612024 (PIs: Laura Baker, Mark Espeland, Miia Kivipelto, Rachel Whitmer, and ALZ PIs: Maria C. Carrillo and Heather M. Snyder), and ancillary Neuroimaging study to U.S. POINTER with National Institutes of Health (NIH) grant number R01AG062689 (PI: S Landau). Additional support was provided by the Wake Forest Alzheimer’s Disease Core Center (P30AG049638-01A1). The MYB trial is funded by a National Health and Medical Research Council (NHMRC) Boosting Dementia Research Team Grant (APP1095097). The SINGER study was supported by Centre For Healthy Ageing, National University Health System, Singapore. The J-MINT study is supported by Japan Agency for Medical Research and Development. The Canadian Thumbs Up Platform Trial is part of the Canadian Consortium on Neurodegeneration in Aging (CCNA) Phase 2, which is funded by the Canadian Institutes of Health Research (CIHR #201901CNA-417847-CAN-ABPI-32054) and a consortium of partners including the Alzheimer Society of Canada. The work in India is made possible by funding from Centre for Brain Research, Bengaluru, India. The PENSA study is funded by the Alzheimer’s Association (18PTC-R-592192) and the Instituto Carlos III (PI17/00223). MIND-CHINA was supported by the National Key R&D Program of China from the Ministry of Science and Technology of China (grant no.: 2017YFC1310100), and additional grants were received from the Shandong Taishan Scholar Program, Shandong, China; the Sino-Sweden joint research project grants from the National Natural Science Foundation of China (NSFC grant no.: 81861138008) and the Swedish Research Council (grants no.: 2017-00740 and 2017-05819); and the Joint China-Sweden Mobility Programme grants from the National Natural Science Foundation of China NSFC (grant no.: 8191101618) and the Swedish Foundation for International Cooperation in Research and Higher Education (STINT, grant no.: CH2019-8320). GOIZ ZAINDU project has been partially financed by the Health Department of the Basque Government (File No. 2017111120). Paulo Caramelli holds a senior research fellowship (bolsa de produtividade em pesquisa) from CNPq, Brazil. SUPERBRAIN was supported by the grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI18C0479).
The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
The WW-FINGERS Network acknowledges the dedication and the commitment of all participants and volunteers for the studies taking place around the world.
Funding information
Swedish Research Council, Grant/Award Number: 529-2014-7503; French National Agency for Research, Grant/Award Number: ANR-14-JPPS-0001-02; Swedish Research Council, Grant/Award Number: 2017-06105; Juho Vainio Foundation; Finnish Medical Foundation; Finnish Social Insurance Institution; Ministry of Education and Culture Research Grant; Finnish Cultural Foundation; Knut and Alice Wallenberg Foundation; Center for Innovative Medicine; Karolinska Institutet Sweden; Stiftelsen Stockholms sjukhem Sweden; Konung Gustaf V:s och Drottning Victorias Frimurarstiftelse Sweden; af Jochnick Foundation Sweden; Alzheimer’s Research and Prevention Foundation; European Research Council Starting, Grant/Award Number: ERC-804371; Alzheimerfonden Sweden; Region Stockholm; National Institutes of Health, Grant/Award Number: R01AG062689; Wake Forest Alzheimer’s Disease Core Center, Grant/Award Number: P30AG049638-01A1; National Health and Medical Research, Grant/Award Number: APP1095097; Centre For Healthy Ageing; National University Health System; Japan Agency for Medical Research and Development; Canadian Institutes of Health Research, Grant/Award Number: #201901CNA-417847-CAN-ABPI-32054; Centre for Brain Research; Alzheimer’s Association, Grant/Award Number: 18PTC-R-592192; Instituto Carlos III, Grant/Award Number: PI17/00223; National Key R&D Program of China; Ministry of Science and Technology of China, Grant/Award Number: 2017YFC1310100; National Natural Science Foundation of China, Grant/Award Number: 81861138008; Swedish Research Council, Grant/Award Numbers: 2017-00740, 2017-05819; Joint China-Sweden Mobility Programme grants from the National Natural Science Foundation of China; NSFC, Grant/Award Number: 8191101618; Swedish Foundation for International Cooperation; Research and Higher Education, Grant/Award Number: CH2019-8320
Footnotes
CONFLICTS OF INTEREST
Maria C. Carrillo and Heather M. Snyder are full-time employees of the Alzheimer’s Association. Henry Brodaty (MYB) is on the Advisory Board for Nutricia Australia. Rafael De la Torre and José L. Molinuevo are full-time employees of the Hospital del Mar Medical Research Institute and the Barcelonabeta Brain Research Institute, respectively. All the other co-authors declare no conflicts of interest in connection with this work.
REFERENCES
- 1.ADI. Alzheimer’s Disease International. World Alzheimer Report 2018: the state of the art of dementia research: new frontiers. 2018. [Google Scholar]
- 2.Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88:1337–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langa KM, Larson EB, Crimmins EM, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of dementia over three decades in the Framingham Heart Study. N EnglJ Med. 2016;374:523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qiu C, von Strauss E, Backman L, Winblad B, Fratiglioni L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology. 2013;80:1888–1894. [DOI] [PubMed] [Google Scholar]
- 6.Ding M, Qiu C, Rizzuto D, Grande G, Fratiglioni L. Tracing temporal trends in dementia incidence over 25 years in central Stockholm, Sweden. Alzheimers Dement. 2020;16:770–778. [DOI] [PubMed] [Google Scholar]
- 7.Matthews FE, Arthur A, Barnes LE, et al. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet. 2013;382:1405–1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schrijvers EM, Verhaaren BF, Koudstaal PJ, Hofman A, Ikram MA, Breteler MM. Is dementia incidence declining?: Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78:1456–1463. [DOI] [PubMed] [Google Scholar]
- 9.Chan KY, Wang W, Wu JJ, et al. Epidemiology of Alzheimer’s disease and other forms of dementia in China, 1990–2010: a systematic review and analysis. Lancet. 2013;381:2016–2023. [DOI] [PubMed] [Google Scholar]
- 10.Gao S, Ogunniyi A, Hall KS, et al. Dementia incidence declined in African-Americans but not in Yoruba. Alzheimers Dement. 2016;12:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ohara T, Hata J, Yoshida D, et al. Trends in dementia prevalence, incidence, and survival rate in a Japanese community. Neurology. 2017;88:1925–1932. [DOI] [PubMed] [Google Scholar]
- 12.Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time—current evidence. Nat Rev Neurol. 2017;13:327–339. [DOI] [PubMed] [Google Scholar]
- 13.Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N EnglJ Med. 2013;369:2275–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol. 2014;13:788–794. [DOI] [PubMed] [Google Scholar]
- 15.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol. 2018;14:653–666. [DOI] [PubMed] [Google Scholar]
- 16.Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255–2263. [DOI] [PubMed] [Google Scholar]
- 17.Kivipelto M, Solomon A, Ahtiluoto S, et al. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimers Dement. 2013;9:657–665. [DOI] [PubMed] [Google Scholar]
- 18.Rosenberg A, Ngandu T, Rusanen M, et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimers Dement. 2018;14:263–270. [DOI] [PubMed] [Google Scholar]
- 19.Solomon A, Turunen H, Ngandu T, et al. Effect of the apolipoprotein E genotype on cognitive change during a multidomain lifestyle intervention: a subgroup analysis of a randomized clinical trial. JAMA Neurol. 2018;75:462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marengoni A, Rizzuto D, Fratiglioni L, et al. The effect of a 2-year intervention consisting of diet, physical exercise, cognitive training, and monitoring of vascular risk on chronic morbidity-the FINGER randomized controlled trial. J Am Med Dir Assoc. 2018;19:355–360.e1. [DOI] [PubMed] [Google Scholar]
- 21.Andrieu S, Guyonnet S, Coley N, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16:377–389. [DOI] [PubMed] [Google Scholar]
- 22.Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388:797–805. [DOI] [PubMed] [Google Scholar]
- 23.Chhetri JK, de Souto Barreto P, Cantet C, et al. Effects of a 3-year multi-domain intervention with or without Omega-3 supplementation on cognitive functions in older subjects with increased CAIDE dementia scores. J Alzheimers Dis. 2018;64:71–78. [DOI] [PubMed] [Google Scholar]
- 24.Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. [DOI] [PubMed] [Google Scholar]
- 25.National Academies of Sciences, Engineering, and Medicine. Leshner, Alan I, Landis, Story, Stroud, Clare & Downey, Autumn, Preventing Cognitive Decline and Dementia: A Way Forward. Washington, DC: National Academies Press; 2017. 10.17226/24782. [DOI] [PubMed] [Google Scholar]
- 26.WHO. Risk Reduction of Cognitive Decline and Dementia: World Health Organization Guidelines. Geneva: World Health Organization; 2019.https://www.who.int/mental_health/neurology/dementia/guidelines_risk_reduction/en/. [PubMed] [Google Scholar]
- 27.Kivipelto M, Mangialasche F, Ngandu T, World Wide Fingers N. World Wide Fingers will advance dementia prevention. Lancet Neurol. 2018;17:27. [DOI] [PubMed] [Google Scholar]
- 28.Heffernan M, Andrews G, Fiatarone Singh MA, et al. Maintain your brain: protocol of a 3-year randomized controlled trial of a personalized multi-modal digital health intervention to prevent cognitive decline among community dwelling 55 to 77 year olds. J Alzheimers Dis. 2019;70:S221–S237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. [DOI] [PubMed] [Google Scholar]
- 30.Soininen H, Solomon A, Visser PJ, et al. 24-month intervention with a specific multinutrient in people with prodromal Alzheimer’s disease (LipiDiDiet): a randomised, double-blind, controlled trial. Lancet Neurol. 2017;16:965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735–741. [DOI] [PubMed] [Google Scholar]
- 32.Carnero-Pardo C, Espejo-Martinez B, Lopez-Alcalde S, et al. Diagnostic accuracy, effectiveness and cost for cognitive impairment and dementia screening of three short cognitive tests applicable to illiterates. PLoS One. 2011;6:e27069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65:559–564. [DOI] [PubMed] [Google Scholar]
- 34.Rami L, Molinuevo JL, Sanchez-Valle R, Bosch B, Villar A. Screening for amnestic mild cognitive impairment and early Alzheimer’s disease with M@T (Memory Alteration Test) in the primary care population. Int J Geriatr Psychiatry. 2007;22:294–304. [DOI] [PubMed] [Google Scholar]
- 35.Barbera M, Mangialasche F, Jongstra S, et al. Designing an internet-based multidomain intervention for the prevention of cardiovascular disease and cognitive impairment in older adults: the HATICE trial. J Alzheimers Dis. 2018;62:649–663. [DOI] [PubMed] [Google Scholar]
- 36.Richard E, Moll van Charante EP, Hoevenaar-Blom MP, et al. Healthy ageing through internet counselling in the elderly (HATICE): a multinational, randomised controlled trial. Lancet Digital Health. 2019;1:PE424-E34. [DOI] [PubMed] [Google Scholar]
- 37.Zulke A, Luck T, Pabst A, et al. AgeWell.de—study protocol of a pragmatic multi-center cluster-randomized controlled prevention trial against cognitive decline in older primary care patients. BMC Geriatr. 2019;19:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weninger S, Carrillo MC, Dunn B, et al. Collaboration for Alzheimer’s Prevention: principles to guide data and sample sharing in preclinical Alzheimer’s disease trials. Alzheimers Dement. 2016;12:631–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohmann C, Banzi R, Canham S, et al. Sharing and reuse of individual participant data from clinical trials: principles and recommendations. BMJ Open. 2017;7:e018647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Institute of Medicine (IOM). Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk. Washington, DC: The National Academies Press; 2015. 10.17226/18998. [DOI] [PubMed] [Google Scholar]
- 41.EU. Data protection in the EU. The General Data Protection Regulation (GDPR). 2018. Available at: https://ec.europa.eu/info/law/law-topic/data-protection/data-protection-eu_en Accessed 10 March 2020.
- 42.Ursin G, Stenbeck M, Chang-Claude J, et al. Data must be shared-also with researchers outside of Europe. Lancet. 2019;394:1902–1903. [DOI] [PubMed] [Google Scholar]
- 43.Wolfson M, Wallace SE, Masca N, et al. DataSHIELD: resolving a conflict in contemporary bioscience—performing a pooled analysis of individual-level data without sharing the data. Int J Epidemiol. 2010;39:1372–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO. World Health Organization. Global Dementia Observatory (GDO). https://www.who.int/mental_health/neurology/dementia/Global_Observatory/en/ last accessed 10 March 2020.