Abstract
Introduction:
Endometriosis is associated with systemic inflammation and increased risk of cardiovascular disease (CVD). Endothelial dysfunction is one of the first manifestations of CVD but is unexplored in women with endometriosis. HMG-CoA-reductase inhibitors (statins) exert potent anti-inflammatory effects, and have been proposed as an adjunctive therapy in women with endometriosis. We hypothesized that microvascular endothelial function would be impaired in otherwise healthy women with endometriosis mediated by reduced nitric oxide (NO)-dependent dilation and that short term statin administration would improve endothelial function.
Methods:
In 8 healthy control (HC: 33±9 yr) and 8 women with endometriosis (EN: 34±9 yr), laser-Doppler flux (LDF) was measured continuously during graded intradermal microdialysis perfusion of the endothelium-dependent agonist acetylcholine (Ach: 10−10-10−1M) alone and in combination with the NO synthase inhibitor (L-NAME: 0.015M). 6 EN repeated the microdialysis experiment following 7 days of oral atorvastatin treatment (10mg). Cutaneous vascular conductance was calculated (CVC=LDF*mmHg−1) and normalized to site-specific maximum (28mM sodium nitroprusside, 43°C). The NO-dependent dilation was calculated as the difference between the areas under the dose response curves.
Results:
Ach-induced vasodilation was blunted in women with endometriosis (main effect p<0.01), indicating impaired endothelial function. NO-dependent vasodilation was also reduced in women with endometriosis (HC: 217±120.3 AUC vs. EN: 88±97 AUC, p=0.03). Oral atorvastatin improved Ach-induced (main effect p<0.01) and NO-dependent (295±153 AUC; p=0.05) vasodilation in women with endometriosis.
Conclusion:
Microcirculatory endothelium-dependent vasodilation is impaired in women with endometriosis, mediated in part by reductions in NO. Short-term oral atorvastatin improved endothelium-dependent vasodilation, suggesting that statin therapy may be a viable intervention strategy to mitigate accelerated CVD risk in women with endometriosis.
INTRODUCTION
Endometriosis derives from the presence of endometrium-like tissue in sites outside the uterine cavity. This estrogen-dependent disorder affects up to 1 in 10 women of reproductive age and is associated with chronic pelvic pain and persistent systemic inflammation (1–8). Cardiovascular disease (CVD) is the leading cause of death in women (9). Epidemiologic data demonstrate a clear association between endometriosis and accelerated CVD risk (5, 9–11). The chronic, systemic inflammation associated with endometriosis appears to impact cardiovascular function though several mechanisms, leading to vascular endothelial cell dysfunction (12).
Endothelial dysfunction may be the first functional manifestation of atherosclerosis and a primary causative event in the development of overt CVD (13, 14). Endothelial dysfunction is also predictive of long-term morbidity and mortality (15). Otherwise healthy women with endometriosis have impaired conduit artery endothelial function, but the precise mechanisms mediating this are unresolved (16, 17). 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors (statins) have been identified as a potential treatment for endometriosis (3, 18, 19). In both murine and primate models of endometriosis, treatment with simvastatin reduces the number and size of endometriotic lesions, and exerts potent systemic anti-inflammatory effects (3, 18, 19). A large body of clinical literature demonstrates that statins have cholesterol-independent effects on the vasculature and improve endothelial NO bioavailability through a variety of mechanisms (20–22). Our published data demonstrate that low dose statin treatment improves endothelial function by improving eNOS coupling in the microvasculature of patients with hypercholesterolemia (23–25). However, the impact of statin treatment on endothelial function in otherwise healthy women with endometriosis is unknown.
Given the elevated CVD risk associated with endometriosis and the potential role for statin therapy in improving CVD and endometriosis outcomes, in a proof of concept study we sought to: (1) examine microvascular endothelial dysfunction in women with endometriosis and (2) determine if short-term statin therapy was effective at improving microvascular endothelial-dependent vasodilation in these women. We hypothesized that women with endometriosis would have impaired microvascular endothelial function mediated by a reduction in NO-dependent vasodilation and that short-term statin therapy would improve endothelial function. Using the cutaneous microcirculation, we pharmacologically assessed endothelial function in the cutaneous microcirculation, which is thought to be a validated bioassay for systemic microvascular dysfunction and has been used to examine the mechanisms underlying microvascular dysfunction in multiple pathologies (8, 12, 23, 29, 51).
METHODS
The Institutional Review Board at The Pennsylvania State University approved all experimental procedures and protocols. A Food and Drug Administration Investigational Drug Number was obtained for all protocols (IND 78,954). Verbal and written informed consent were voluntarily obtained from all participants before participation and in accordance with the guidelines set forth by the Declaration of Helsinki.
Participants
All participants were screened by clinical staff. Screening included a physical examination, medical health history questionnaire, and a blood chemistry analysis (Chem 24, Quest Diagnostics, Pittsburgh, PA). All participants were free from stage II hypertension, as well as renal, pulmonary, neurological, or dermatological diseases, and had never experienced an adverse cardiovascular disease event. Participants did not use tobacco products. One woman with endometriosis was taking a selective serotonin reuptake inhibitor. All other participants were not taking over-the-counter or prescription medications with primary or secondary cardiovascular effects (e.g., antihypertensives, hormonal therapy, statins, anticoagulants, antidepressants) at the time of testing. All women were premenopausal. Healthy control participants had regularly occurring menstrual cycles (28–32 days, Table 1) but we did not control for menstrual cycle phase during the study visit (26). All endometriosis patients had a confirmed diagnosis prior to enrollment via laparoscopy.
Table 1.
Participant Characteristics
| Mean ± SD (min-max) | Healthy Control (n=8) | Endometriosis (n=8) |
|---|---|---|
| Age (yrs) | 33 ± 9 (20–48) | 34 ± 9 (21–45) |
| Height (in) | 65 ± 2 (62–68) | 66 ± 2 (64–69) |
| Weight (kg) | 65 ± 10 (53–84) | 70 ± 17 (52–101) |
| BMI (kg/m2) | 28 ± 11 (20–28) | 25 ± 6 (19–36) |
| SBP (mmHg) | 111 ± 8 (101–124) | 111 ± 13 (96–135) |
| DBP (mmHg) | 73 ± 7 (60–86) | 69 ± 11 (58–88) |
| MAP (mmHg) | 86 ± 7 (74–99) | 83 ± 11 (71–104) |
| HbA1c (%) | 5.1 ± 0.27 (4.8–5.6) | 4.8 ± 0.3 (4.3–5.3) |
| Total CHO (mg/dL) | 155 ± 12 (115–222) | 168 ± 30 (132–230) |
| HDL (mg/dL) | 55 ± 4 (45–78) | 54 ± 8 (41–64) |
| LDL (mg/dL) | 89 ± 10 (52–139) | 95 ± 27 (69–155) |
| Comorbidities | ||
| Elevated blood pressure | 1 | 0 |
| Stage 1 Hypertension | 1 | 2 |
| Hypercholesterolemia | 0 | 1 |
| Medications | ||
| OCP | 1 | 2 |
| IUD | 0 | 1 |
| Menstrual Cycle Status | ||
| Regular Cycles | 8 | 3 |
| Irregular Cycles | 0 | 2 |
| Partial hysterectomy (has ovaries) | 0 | 1 |
| Full hysterectomy (no cervix/uterus) | 0 | 1 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; CHO, cholesterol; HDL, high density lipoprotein cholesterol; LDL, low density lipoprotein cholesterol; OCP, oral contraceptive; IUD, intrauterine device. Data are mean ± standard deviation (minimum-maximum)
Microvascular Function Assessment
All participants underwent a cutaneous microdialysis experiment to assess microvascular endothelial function. Participants were instructed to abstain from caffeine and alcohol for 12 hours and strenuous physical activity for 24 hours prior to the experimental visit. Two intradermal microdialysis fibers (10mm, 55kDa, CMA Linear 31 probe, Harvard Apparatus, Holliston, MA) were inserted into the ventral forearm skin for the local delivery of either lactated Ringer’s solution alone (Control), or with Ringer’s solution and 0.015 M NG-nitro-l-arginine methyl ester (l-NAME; Calbiochem, EMD Millipore, Billerica, MA) to non-selectively inhibit NO synthase. In endometriosis patients only, a third intradermal microdialysis fiber was placed for the local delivery of 0.00002 M atorvastatin (statin; USP, Rockville, MD).
After microdialysis fiber insertion, 60–90 min were allowed for hyperemia resolution during which site-specific pharmacological agents were perfused (2 μL/min; Hive controller and microinfusion pumps; BASi, West Lafayette, IN). Cutaneous red blood cell flux was continuously measured directly over each microdialysis site with an integrated laser-Doppler flowmeter probe placed in a local heating unit (VP12 and VHP2; Moor Instruments, Wilmington, DE) set to a thermoneutral 33°C. After baseline measurements, progressively increasing concentrations of the endothelium-dependent agonist acetylcholine (ACh; 10−10 to 10−1 M; USP, Rockville, MD) were co-perfused with lactated Ringer’s, l-NAME, or statin (endometriosis patients only) sequentially for 5 min each. At the conclusion of the dose-response protocols, 0.028 M sodium nitroprusside (USP) was perfused and the local skin temperature was increased to 43°C to elicit maximal cutaneous vasodilation (27–30). Automated brachial blood pressure (Connex Spot Monitor, Welch Allyn, Skaneateles Falls, NY) was measured every 5 min throughout the protocol.
Systemic Statin Intervention
A subset (n=6) of endometriosis patients participated in a seven-day oral atorvastatin (Lipitor®) therapy (10mg/day). These subjects returned to the laboratory on day 7, having taken the final dose that morning. We repeated the microdialysis experimental visit as described above.
Data and Statistical Analysis
Intradermal microdialysis data collection procedures and the pharmacological efficacy of the site-specific inhibitors have been previously reported (27–32). Data were recorded at 1000 Hz and stored for offline analysis (Powerlab and LabChart, ADInstruments, Bella Vista, NSW, Australia). Average values for red cell flux (perfusion units) were obtained during 5 min of baseline, during the last minute of each dose, and at maximum. Cutaneous vascular conductance (CVC) was calculated as red cell flux divided by mean arterial pressure. Due to the heterogeneity of capillary density at each microdialysis site, CVC was normalized as a percentage of the site-specific maximum (CVC%max), which is a standard normalization procedure for data derived from laser-Doppler flowmetry (27–32). Area under the dose-response curve (AUC) was calculated using the trapezoid rule (Prism v8.1, GraphPad Software, La Jolla, CA). The NO-dependent contributions were calculated as the difference between the AUC of the control site and the L-NAME sites (27, 28, 33). All data were analyzed with either a one- or two-way repeated measures ANOVA (Prism v8.1, GraphPad Software, La Jolla, CA; IBM Corp. Released 2019. IBM SPSS Statistics for Windows, v. 26.0. Armonk, NY: IBM Corp; SAS v. 9.4; Cary, NC). When appropriate, post hoc Tukey–Kramer corrections were applied to account for multiple comparisons. Linear regression analyses were performed using a Pearson’s correlation coefficient. Significance was set a priori at α < 0.05. Text and table results are presented as mean ± SD. Figure results are presented as means ± SE for visual clarity of main effects.
Assuming an effect size of d = 0.68 (27), we determined a priori (power = 0.80, α = 0.05) a sample size of n = 8 would be sufficient to detect a significant difference in microvascular function between groups (healthy control vs. endometriosis patients). Based on previous data (28), assuming an effect size of d = 0.65 we determined a priori (power = 0.80, α = 0.05) a sample size of n = 8 would be sufficient to determine a significant difference between pre- and post-intervention (7-day systemic statin treatment). We originally recruited 8 women with endometriosis to participate in the intervention; however, 2 participants dropped out (n=1 developed COVID-19, n=1 received surgical treatment for endometriosis). However, the effects pre- to post-intervention were large (NO contribution: d= 1.49), despite 2 drop-outs.
RESULTS
A total of 8 endometriosis patients and 8 age- and body mass index-matched women (controls) without endometriosis participated in the study. There were no differences in characteristics between groups (Table 1). In the control group, 6 were normotensive, 1 had elevated blood pressure (122/78 mmHg), and 1 had stage 1 hypertension (124/86 mmHg). In the endometriosis group, 6 were normotensive, and 2 had stage 1 hypertension (120/81 and 135/88 mmHg) according to the 2017 AHA/ACC guidelines (34). With the exceptions of 1 endometriosis patient who had hypercholesterolemia (LDL: 155mg/dl) and was obese (body mass index = 36 kg/m2), all women were otherwise heathy (Table 1). A breakdown of medications and menstrual cycle status is presented in Table 1.
ACh-induced vasodilation was attenuated in the endometriosis group (Figure 1, main effect p=0.03). In controls the local co-perfusion of L-NAME decreased the vasodilator response to ACh at the highest six doses of ACh (Figure 1, all p<0.01). In women with endometriosis, the vasodilatory response was only attenuated at the highest dose of ACh (Figure 1, p<0.01). The NO-mediated contribution to ACh-induced vasodilation was greater in the control group compared to the endometriosis group (217±120 vs. 88±97 AU, p=0.03).
Figure 1.
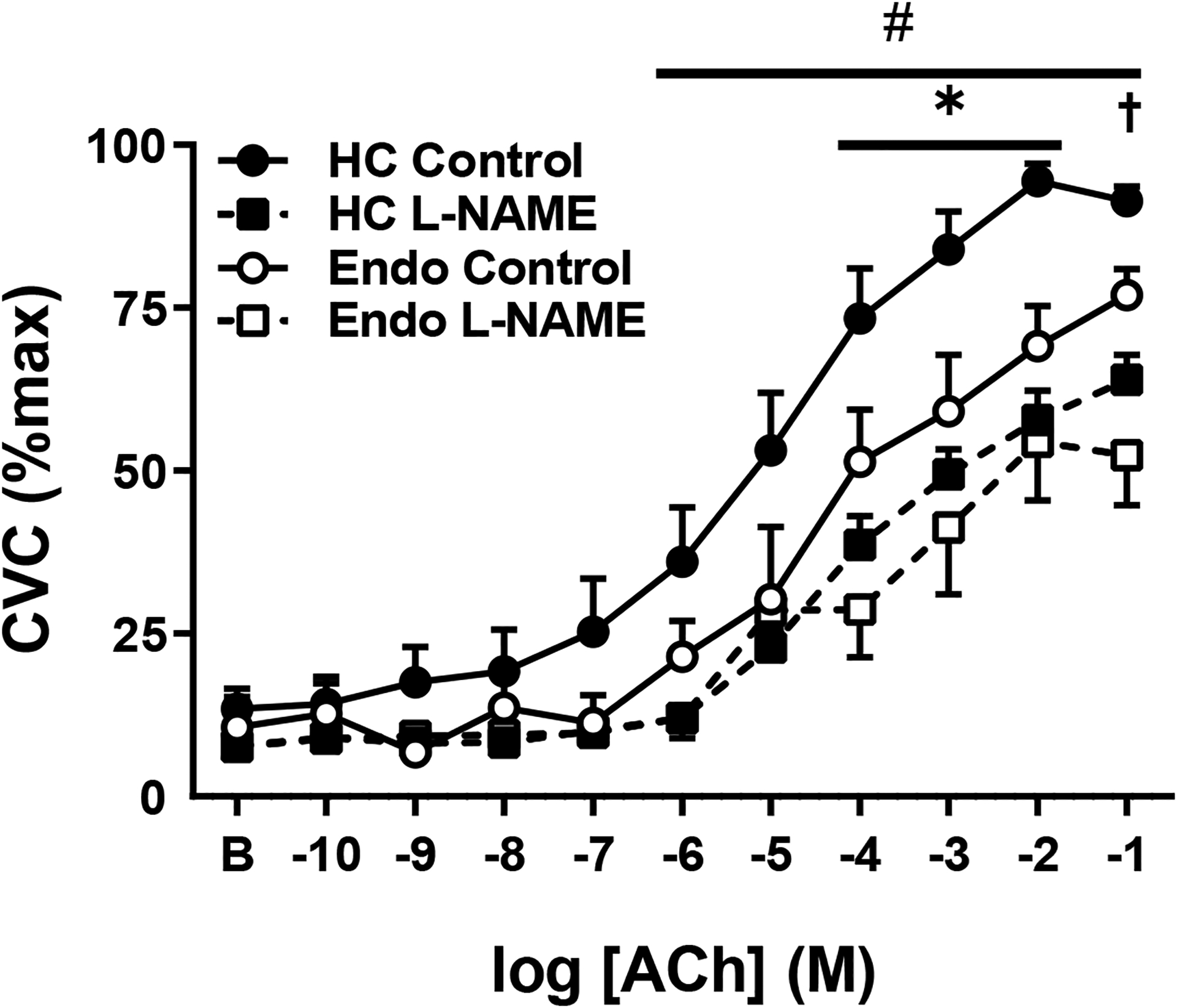
Acetylcholine (ACh)-induced vasodilation in healthy controls (HC, closed symbols) and endometriosis patients (Endo, open symbols) during co-infusion of Ringer’s (Control, circles) or NOS-inhibition (L-NAME, squares). The Endo group had an attenuated ACh-induced vasodilatory response. Co-infusion with L-NAME attenuated ACh-induced vasodilation at the highest 6 doses in the HC group; however, in the Endo group L-NAME only attenuated ACh-vasodilation at the highest dose of ACh. Data are mean ± standard error. Some error bars are not visible as they are smaller than the representative symbol.*p<0.05 HC Control vs. Endo Control; #p<0.01 HC Control vs. HC L-NAME; †p<0.01 Endo Control vs. Endo L-NAME
6 of the 8 women with endometriosis completed the seven-day systemic atorvastatin intervention. Seven days of systemic atorvastatin intervention lowered total cholesterol (Figure 2A, p<0.01) and low-density lipoprotein cholesterol (Figure 2C, p<0.01); but did not impact high-density lipoprotein cholesterol (Figure 2B, p=0.43) or mean arterial pressure (84±13 vs. 83±10 mmHg, p=0.76). The oral statin treatment increased the ACh-induced vasodilatory response (Figure 3A; main effect p<0.01). After the oral statin treatment, the local co-perfusion of L-NAME attenuated the ACh-induced response (Figure 3A, p <0.01). The NO-mediated contribution increased pre- to post-intervention (Figure 3B, p=0.05). Prior to oral statin treatment, local statin co-perfusion (intradermal microdialysis) augmented ACh-induced vasodilation (Figure 4A, p<0.01). After statin treatment, local statin co-perfusion attenuated ACh-induced vasodilation (Figure 4B, p=0.01). There were no associations between the change in cholesterol and the change in vasodilatory function (all p>0.05).
Figure 2.

Total (panel A), high-density lipoprotein (HDL, panel B), and low-density lipoprotein (LDL, panel C) cholesterol (CHO) in endometriosis patients before and after seven-days of systemic statin treatment. Total and LDL, but not HDL, cholesterol was significantly lower following treatment. Dashed lines indicate cut-offs for normal cholesterol. *p<0.01 vs. Pre-treatment.
Figure 3.

Acetylcholine (ACh)-induced vasodilation during co-infusion of Ringer’s (Control, circles) or NOS-inhibition (L-NAME, squares) in endometriosis patients (n=6) before (closed symbols) and after (open circles) seven-days of systemic statin treatment (Panel A). Systemic statin treatment increased ACh-induced dilation (main effect p<0.01) and lowered the response to L-NAME co-infusion (main effect p<0.01). Before statin treatment, L-NAME reduced the vasodilatory response at the highest dose of ACh. After treatment, co-infusion with L-NAME attenuated ACh-induced vasodilation at the five highest doses of ACh. The nitric oxide (NO)-contribution to acetylcholine induced vasodilation increased after treatment (Panel B). The dashed line indicates average NO-contribution in healthy controls (derived from Figure 1). Data are mean ± standard error. †p<0.05 Pre Control vs. Pre L-NAME; *p<0.05 Post Control vs. Post-L-NAME; ǂ p<0.05 Pre- vs. Post-treatment.
Figure 4.

Acetylcholine (ACh)-induced vasodilation during co-infusion of Ringer’s (Control, circles) or atorvastatin (Statin, triangles) in endometriosis patients (n=6) before (open symbols, Panel A) and after (closed symbols, Panel B) seven-day systemic statin treatment. Before statin treatment, local statin co-infusion augmented ACh-induced vasodilation. After statin treatment, local statin co-infusion attenuated ACh-induced vasodilation. Data in figure are mean ± standard error. *p<0.05 Control vs. L-NAME.
DISCUSSION
The results of this proof-of-concept trial demonstrate that women with endometriosis have impaired endothelial-dependent microvascular function, mediated in part through reduced NO-dependent mechanisms. Localized statin perfusion directly to the cutaneous microvasculature prior to systemic statin treatment modestly increased microvascular endothelial-dependent vasodilation. Seven days of oral statin improved endothelium-dependent dilation and NO-dependent vasodilation. These data suggest that short term oral statin treatment improved microvascular endothelium-dependent vasodilation and that the local impact of statin also increased microvascular function potentially through anti-inflammatory, anti-oxidant, or through the acute inhibition of the lectin-like oxidized LDL (LOX-1) receptor mechanisms (20–22, 35–37). These data provide compelling support for future randomized clinical trials investigating statin therapy interventions for the treatment of endometriosis-associated microvascular dysfunction.
Santoro et al., (2012) observed ~4.6% lower flow-mediated vasodilation (FMD) in women with endometriosis compared to healthy controls; however, no differences in carotid intima-media thickness were observed between groups suggesting that women with endometriosis have impaired conduit artery vascular function despite no alterations in vascular structure (17). Impaired endothelial function is indicative of early changes in the atherosclerotic disease process putatively mediated by chronic systemic inflammation in women with endometriosis (3, 8, 17). Interestingly, FMD was improved in women with endometriosis 2 years after surgical excision of endometrial lesions such that there was no longer a difference between healthy controls and women with endometriosis (16). FMD is a gold standard for assessing conduit artery vasodilator capacity to a shear stimulus. The underlying mechanisms of FMD include both endothelial and vascular smooth muscle pathways, as well as NO-mediated and non-NO-mediated mechanisms (e.g. endothelial derived hyperpolarizing factors, prostaglandins) (38). In the present study we used the cutaneous circulation to interrogate endothelial function in the microcirculation where decrements in vascular function often precede or evolve in parallel with conduit artery endothelial function (13, 14). Using this approach, we pharmaco-dissected the NO-mediated endothelial dependent vasodilatory pathway using the endothelium-dependent agonist acetylcholine paired with direct nitric oxide synthase (NOS) blockade. Our results indicate that women with endometriosis display impaired microvascular endothelial function mediated, in part, by reductions in NO bioavailability. Our results are novel because we demonstrate reductions in NO-dependent mechanisms in the microcirculation where previous studies have inferred these results based on indirect measures in conduit arteries.
Statins are a cornerstone treatment for the management of atherosclerotic disease risk. We have demonstrated that statins are effective at improving microvascular NO-dependent endothelial function in middle-aged adults with dyslipidemia (23–25). In the current study, seven days of low-dose oral statins improved microvascular function in women with endometriosis. Importantly, the majority of the subject cohort did not have clinically significant hypercholesterolemia. One participant had elevated cholesterol, but removing this participant from the analysis did not change the overall result. We had originally designed the study postulating that plasma cholesterol concentrations would not significantly change after this short-term treatment of the lowest dose of atorvastatin. While there was no relation between the change in cholesterol and changes in microvascular function, we cannot exclude the possibility that reductions in plasma lipids may have impacted our results.
Statins mediate their beneficial effects on the vasculature in a number of different ways including directly reducing cholesterol synthesis, and indirectly through exerting non-specific antioxidant and anti-inflammatory effect (20–22, 35, 36), and antagonizing the LOX-1 receptor (20, 37). LOX-1 is a ubiquitously expressed scavenger receptor that is directly agonized by ox-LDL and upregulated by inflammatory cytokines that are known to be elevated in women with endometriosis (39). LOX-1 is an upstream signaling initiator that promotes increased oxidant production, and reduced NO metabolism (39) resulting in pronounced endothelial dysfunction. Our results suggest that LOX-1 is a potential target mediating endothelial dysfunction in women with endometriosis. While the pleotropic effects of lipophilic statins are well documented in cohorts with traditional cardiovascular disease risk factors (36), their precise mechanisms in those with non-traditional cardiovascular disease risk including endometriosis have not been explored. Interestingly, statins decrease endometrial lesional load in animal models of endometriosis through modulating the expression of genes encoding for estrogen receptors and decreasing inflammation (19).
In order to examine the direct of effects of statins on the microcirculation, thus eliminating the systemic effect such as reducing cholesterol, we locally perfused atorvastatin via intradermal microdialysis. The local infusion of atorvastatin moderately augmented the vasodilatory responses to the endothelium-dependent agonist acetylcholine. We postulate that this improvement in endothelial microvascular function is through antioxidant mechanisms and/or attributable to direct LOX-1 antagonism. Statins inhibit LOX-1 receptor activation through direct steric hindrance properties and induce destabilization of the dimerized LOX-1 receptor (20, 37). It was hypothesized that the addition of the local statin infusion after oral treatment would result in a ceiling effect, such that no additional improvement in microvascular function would be observed. However, after seven days of oral statins, the addition of localized statin modestly attenuated the endothelial-dependent vasodilation. We speculate that this may be due to the high antioxidant load, which can induce variable responses, including reductions in NO-dependent vasodilation in healthy subjects (40–43).
Clinical Perspectives
While endometriosis is a local inflammatory syndrome, the inflammatory process is systemic (3) and underlies many of the co-morbidities associated with this devastating disease. Endometriosis and atherosclerotic CVD are both inflammation-induced diseases (8). Robust epidemiologic data demonstrate a clear association between endometriosis, inflammation and CVD risk (5, 9–11, 44), the leading cause of death in women worldwide (9). Investigations into the mechanistic links and putative therapies are necessary for reducing cardiovascular disease risk in women with endometriosis. Statins have been shown to effectively reduce endometriotic lesions in primate and murine models (3, 18, 19). Statins have also been suggested for off label use to reduce systemic inflammation and estrogen receptor expression after excision surgery (45). Lowering cholesterol through dietary manipulation is also recommended for endometriosis patients to relieve endometriosis-related symptoms (46), although direct mechanistic evidence is lacking. Randomized control trials are needed to investigate statins as a component for the management of endometriosis including the long term mitigation of elevated CVD risk in women with endometriosis.
Limitations
As a proof-of-concept-study, the small sample size is a limitation to the interpretation and translatability of the results. However, our repeated measures design provided an appropriately statistically powered approach for determining the impact of oral statin treatment on microvascular function in women with endometriosis. Several participants had elevated and stage one hypertension according to the 2017 AHA/ACC guidelines (34). This elevated CVD risk profile is common in women with endometriosis in this age range (9). We have previously observed no decrements in endothelial-dependent microvascular function in those with stage 1 HTN (27). Nevertheless, the severity of endothelial dysfunction in this group of women with endometriosis indicates a clear risk for future atherosclerotic CVD, as documented in the epidemiological data (13, 14). Finally, we did not control for phase of the menstrual cycle because this was a small proof-of-concept study involving short term statin therapy. In naturally menstruating women, hormone exposure would change over the course of 7 days. Future randomized control trials should aim to control for reproductive hormone exposure. Interestingly, in the present study we observed consistent results among participants, despite the differences of experiment timing, suggesting menstrual cycle phase may not have had a large impact on the current results.
Conclusions
In conclusion, the results of this initial proof-of-concept study suggest that short term systemic treatment with a statin improved cutaneous microvascular endothelium-dependent vasodilation in women with endometriosis, via an augmentation of NO-dependent pathways. As such, intervention strategies specifically targeting lowering of cholesterol, reducing systemic inflammation and/or LOX-1 receptor inhibition should be considered for mitigating vascular dysfunction in women with endometriosis.
Acknowledgements.
We acknowledge the time and effort given by the study volunteers. We would also like to thank Sue Slimak, R.N. for her assistance.
Funding.
NIH T-32-5T32AG049676 (GAD, CS), NIH R01 HL-161000-01 (LMA, NS)
Footnotes
Conflict(s) of Interest/Disclosures: None
REFERENCES
- 1.Donnez J, Binda MM, Donnez O, and Dolmans M-M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertility and Sterility 106: 1011–1017, 2016. [DOI] [PubMed] [Google Scholar]
- 2.Bulun SE. Endometriosis. The New England journal of medicine 360: 268–279, 2009. [DOI] [PubMed] [Google Scholar]
- 3.Alderman MH 3rd, Yoder N, and Taylor HS. The Systemic Effects of Endometriosis. Seminars in reproductive medicine 35: 263–270, 2017. [DOI] [PubMed] [Google Scholar]
- 4.Burney RO, and Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril 98: 511–519, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Marshall LM, and Hunter DJ. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol 160: 784–796, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Nematian SE, Mamillapalli R, Kadakia TS, Majidi Zolbin M, Moustafa S, and Taylor HS. Systemic Inflammation Induced by microRNAs: Endometriosis-Derived Alterations in Circulating microRNA 125b-5p and Let-7b-5p Regulate Macrophage Cytokine Production. The Journal of clinical endocrinology and metabolism 103: 64–74, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Seifer BJ, Su D, and Taylor HS. Circulating miRNAs in Murine Experimental Endometriosis. Reproductive sciences (Thousand Oaks, Calif) 24: 376–381, 2017. [DOI] [PubMed] [Google Scholar]
- 8.Taskin O, Rikhraj K, Tan J, Sedlak T, Rowe TC, and Bedaiwy MA. Link between Endometriosis, Atherosclerotic Cardiovascular Disease, and the Health of Women Midlife. Journal of minimally invasive gynecology 26: 781–784, 2019. [DOI] [PubMed] [Google Scholar]
- 9.Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Forman JP, and Missmer SA. Association Between Endometriosis and Hypercholesterolemia or Hypertension. Hypertension (Dallas, Tex : 1979) 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.May KE, Conduit-Hulbert SA, Villar J, Kirtley S, Kennedy SH, and Becker CM. Peripheral biomarkers of endometriosis: a systematic review. Hum Reprod Update 16: 651–674, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agic A, Xu H, Finas D, Banz C, Diedrich K, and Hornung D. Is endometriosis associated with systemic subclinical inflammation? Gynecol Obstet Invest 62: 139–147, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Ma L, Guo X, and Chen W. Inhibitory effects of oleoylethanolamide (OEA) on H(2)O(2)-induced human umbilical vein endothelial cell (HUVEC) injury and apolipoprotein E knockout (ApoE−/−) atherosclerotic mice. Int J Clin Exp Pathol 8: 6301–6311, 2015. [PMC free article] [PubMed] [Google Scholar]
- 13.Jung F, Pindur G, Ohlmann P, Spitzer G, Sternitzky R, Franke RP, Leithäuser B, Wolf S, and Park JW. Microcirculation in hypertensive patients. Biorheology 50: 241–255, 2013. [DOI] [PubMed] [Google Scholar]
- 14.Kenney WL. Edward F. Adolph Distinguished Lecture: Skin-deep insights into vascular aging. Journal of applied physiology (Bethesda, Md : 1985) 123: 1024–1038, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Waard GA, Fahrni G, de Wit D, Kitabata H, Williams R, Patel N, Teunissen PF, van de Ven PM, Umman S, Knaapen P, Perera D, Akasaka T, Sezer M, Kharbanda RK, and van Royen N. Hyperaemic microvascular resistance predicts clinical outcome and microvascular injury after myocardial infarction. Heart (British Cardiac Society) 104: 127–134, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Santoro L, D’Onofrio F, Campo S, Ferraro PM, Flex A, Angelini F, Forni F, Nicolardi E, Campo V, Mascilini F, Landolfi R, Tondi P, and Santoliquido A. Regression of endothelial dysfunction in patients with endometriosis after surgical treatment: a 2-year follow-up study. Hum Reprod 29: 1205–1210, 2014. [DOI] [PubMed] [Google Scholar]
- 17.Santoro L, D’Onofrio F, Campo S, Ferraro PM, Tondi P, Campo V, Flex A, Gasbarrini A, and Santoliquido A. Endothelial dysfunction but not increased carotid intima-media thickness in young European women with endometriosis. Hum Reprod 27: 1320–1326, 2012. [DOI] [PubMed] [Google Scholar]
- 18.Cosar E, and Mamillapalli R. Serum MicroRNA Biomarkers Regulated by Simvastatin in a Primate Model of Endometriosis. 26: 1343–1350, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor HS, Alderman Iii M, D’Hooghe TM, Fazleabas AT, and Duleba AJ. Effect of simvastatin on baboon endometriosis. Biology of reproduction 97: 32–38, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puccetti L, Sawamura T, Pasqui AL, Pastorelli M, Auteri A, and Bruni F. Atorvastatin reduces platelet-oxidized-LDL receptor expression in hypercholesterolaemic patients. Eur J Clin Invest 35: 47–51, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Biocca S, Iacovelli F, Matarazzo S, Vindigni G, Oteri F, Desideri A, and Falconi M. Molecular mechanism of statin-mediated LOX-1 inhibition. Cell cycle (Georgetown, Tex) 14: 1583–1595, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehta JL, Li DY, Chen HJ, Joseph J, and Romeo F. Inhibition of LOX-1 by statins may relate to upregulation of eNOS. Biochemical and biophysical research communications 289: 857–861, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Holowatz LA, and Kenney WL. Oral atorvastatin therapy increases nitric oxide-dependent cutaneous vasodilation in humans by decreasing ascorbate-sensitive oxidants. American journal of physiology Regulatory, integrative and comparative physiology 301: R763–768, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holowatz LA, and Kenney WL. Acute localized administration of tetrahydrobiopterin and chronic systemic atorvastatin treatment restore cutaneous microvascular function in hypercholesterolaemic humans. The Journal of physiology 589: 4787–4797, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holowatz LA, Santhanam L, Webb A, Berkowitz DE, and Kenney WL. Oral atorvastatin therapy restores cutaneous microvascular function by decreasing arginase activity in hypercholesterolaemic humans. The Journal of physiology 589: 2093–2103, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stanhewicz AE, and Wong BJ. Counterpoint: Investigators should not control for menstrual cycle phase when performing studies of vascular control that include women. 129: 1117–1119, 2020. [DOI] [PubMed] [Google Scholar]
- 27.Dillon GA, Greaney JL, Shank S, Leuenberger UA, and Alexander LM. AHA/ACC-defined stage 1 hypertensive adults do not display cutaneous microvascular endothelial dysfunction. American journal of physiology Heart and circulatory physiology 319: H539–h546, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dillon GA, Stanhewicz AE, Serviente C, Greaney JL, and Alexander LM. Hydrogen sulfide-dependent microvascular vasodilation is improved following chronic sulfhydryl-donating antihypertensive pharmacotherapy in adults with hypertension. American Journal of Physiology-Heart and Circulatory Physiology 321: H728–H734, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dillon GA, Wolf ST, and Alexander LM. Nitric Oxide-mediated Cutaneous Microvascular Function is not Altered in Young Adults Following Mild-to-Moderate SARS CoV-2 Infection. American journal of physiology Heart and circulatory physiology 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolf ST, and Jablonski NG. Four weeks of vitamin D supplementation improves nitric oxide-mediated microvascular function in college-aged African Americans. 319: H906–h914, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greaney JL, Kutz JL, Shank SW, Jandu S, Santhanam L, and Alexander LM. Impaired Hydrogen Sulfide-Mediated Vasodilation Contributes to Microvascular Endothelial Dysfunction in Hypertensive Adults. Hypertension (Dallas, Tex : 1979) 69: 902–909, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith CJ, Santhanam L, Bruning RS, Stanhewicz A, Berkowitz DE, and Holowatz LA. Upregulation of inducible nitric oxide synthase contributes to attenuated cutaneous vasodilation in essential hypertensive humans. Hypertension (Dallas, Tex : 1979) 58: 935–942, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greaney JL, Saunders EFH, Santhanam L, and Alexander LM. Oxidative Stress Contributes to Microvascular Endothelial Dysfunction in Men and Women With Major Depressive Disorder. Circulation research 124: 564–574, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD, and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 138: e426–e483, 2018. [DOI] [PubMed] [Google Scholar]
- 35.Satny M, Hubacek JA, and Vrablik M. Statins and Inflammation. 23: 80, 2021. [DOI] [PubMed] [Google Scholar]
- 36.Davignon J Beneficial cardiovascular pleiotropic effects of statins. Circulation 109: Iii39–43, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Matarazzo S, Quitadamo MC, Mango R, Ciccone S, Novelli G, and Biocca S. Cholesterol-lowering drugs inhibit lectin-like oxidized low-density lipoprotein-1 receptor function by membrane raft disruption. Molecular pharmacology 82: 246–254, 2012. [DOI] [PubMed] [Google Scholar]
- 38.Thijssen DHJ, Black MA, Pyke KE, Padilla J, Atkinson G, Harris RA, Parker B, Widlansky ME, Tschakovsky ME, and Green DJ. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. American journal of physiology Heart and circulatory physiology 300: H2–H12, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta JL, Chen J, Hermonat PL, Romeo F, and Novelli G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc Res 69: 36–45, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Holowatz LA, and Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. American journal of physiology Heart and circulatory physiology 293: H1090–1096, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Moreau KL, DePaulis AR, Gavin KM, and Seals DR. Oxidative stress contributes to chronic leg vasoconstriction in estrogen-deficient postmenopausal women. Journal of applied physiology (Bethesda, Md : 1985) 102: 890–895, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Moreau KL, Gavin KM, Plum AE, and Seals DR. Oxidative stress explains differences in large elastic artery compliance between sedentary and habitually exercising postmenopausal women. Menopause (New York, NY) 13: 951–958, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Moreau KL, Gavin KM, Plum AE, and Seals DR. Ascorbic acid selectively improves large elastic artery compliance in postmenopausal women. Hypertension (Dallas, Tex : 1979) 45: 1107–1112, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Teng SW, Horng HC, Ho CH, Yen MS, Chao HT, Wang PH, and Taiwan Association of Gynecology Systematic Review G. Women with endometriosis have higher comorbidities: Analysis of domestic data in Taiwan. J Chin Med Assoc 79: 577–582, 2016. [DOI] [PubMed] [Google Scholar]
- 45.Almassinokiani F, Mehdizadeh A, Sariri E, Rezaei M, Almasi A, Akbari H, Pazooki A, Solaymani-Dodaran M, Asadollah S, Amirkhani J, Chaichian S, Vahdat M, Moosavi A, Ashouri M, and Tamannaei Z. Effects of simvastatin in prevention of pain recurrences after surgery for endometriosis. Med Sci Monit 19: 534–539, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halpern G, Schor E, and Kopelman A. Nutritional aspects related to endometriosis. Revista da Associacao Medica Brasileira (1992) 61: 519–523, 2015. [DOI] [PubMed] [Google Scholar]


