Abstract
BACKGROUND
African Americans have a higher prevalence of resistant hypertension compared with Caucasians. Racial differences in obstructive sleep apnea (OSA) and increased aldosterone level may explain the racial disparity in resistant hypertension prevalence. Therefore, the purpose of this study is to investigate if aldosterone level and hypertension status differ by risks for OSA (e.g., obesity, loud snoring, and daytime sleepiness) and how aldosterone level varies with hypertension severity and control among African Americans.
METHODS
A cross-sectional analysis was performed using baseline data on 5,052 African American adults in the Jackson Heart Study to investigate the relationships of interest using multivariable linear and multinomial logistic regression models adjusted for potential confounders. Risks for OSA were defined by a “risk score” consisting of the number of risks for OSA.
RESULTS
Of the 5,052 participants, 623 had no risks for OSA. Body mass index was the highest among those with a risk score of 6. About 39% of the sample had no hypertension, 29% had controlled hypertension, 26% had uncontrolled hypertension, and 6% had resistant hypertension. Higher odds of having uncontrolled hypertension or resistant hypertension were present in those with a higher risk score compared with those without risks for OSA. Log-aldosterone level increased with each additional risk for OSA (P-trend <0.05). Similarly, log-aldosterone also increased with more severe hypertension (P-trend <0.001). The highest aldosterone level was found in those with resistant hypertension that was inadequately controlled with medications.
CONCLUSIONS
Risks for OSA were positively associated with resistant hypertension and higher aldosterone level in African American adults.
Keywords: African Americans, aldosterone, blood pressure, hypertension, obstructive sleep apnea, resistant hypertension
Graphical Abstract
Graphical Abstract.
African Americans have a high prevalence of hypertension and cardiovascular comorbidities.1 Hypertension is more common among African Americans compared with Caucasians.2 Moreover, hypertension among African Americans may be more resistant to treatment, requiring either higher medication dosages or alternative drug class combinations, compared with Caucasians.3,4
Resistant hypertension is defined as blood pressure (BP) that is uncontrolled despite being onthree optimally dosed antihypertensive medications of different classes including a long-acting calcium channel blocker (CCB), a renin–angiotensin system blocker, and a diuretic. The definition also includes the patients who achieve target BP on ≥4 antihypertensive medications.5 Obstructive sleep apnea (OSA) is an independent risk factor for hypertension and associated cardiovascular disease.6,7 Based on previous studies, racial differences in OSA may explain why African Americans have a higher prevalence of resistant hypertension than Caucasians.
Specifically, previous studies have shown that resistant hypertension is associated with a greater risk of OSA compared with less severe hypertension8; and African Americans have more frequent symptoms associated with OSA and more severe sleep-disordered breathing compared with Caucasians.9–13 The mechanism of resistant hypertension involving OSA may be that OSA serves to activate the renin–angiotensin–aldosterone system (RAAS) via increased sympathetic activity due to repetitive hypoxemia and hypercapnia during episodes of apnea.14 The activation of RAAS results in aldosterone secretion, which is mediated by the release of renin into the blood which converts angiotensin I to angiotensin II.
Studies have shown that patients with OSA and hypertension had higher angiotensin II and aldosterone levels compared with patients without OSA or hypertension; hyperaldosteronism was associated with resistant hypertension.15–17 Aldosterone level was also shown to correlate with OSA severity in subjects with resistant hypertension.18 However, some studies did not confirm these associations.19,20
The purpose of this study is to investigate if aldosterone level and hypertension status differ by risks for OSA and how aldosterone level varies with hypertension severity and control among African Americans.
METHODS
Study population
The Jackson Heart Study (JHS) is an observational study of African Americans from Jackson, Mississippi. Its purpose is to study cardiovascular disease among African Americans and address health disparities experienced by this population. The study design and process of recruitment were described elsewhere.21,22 Participants were noninstitutionalized African Americans adults who were 21–94 years old. Between 2000 and 2004, 5,306 African Americans were enrolled. Participants completed baseline questionnaires and physical examinations at the time of enrollment.
A cross-sectional analysis of baseline data was performed to examine the relationship between risks for OSA, hypertension, and aldosterone level. Participants included in the analytic sample were those who answered all of the modified Berlin questionnaire for OSA,23 had aldosterone measured, and had all necessary covariate information at the baseline visit. Of the abovementioned 5,306 African Americans, 254 (4.79%) were excluded from the analytic sample—186 were excluded due to missing data (age, gender, systolic or diastolic BP, aldosterone measurements, or any OSA screening question), and 68 were excluded due to being prescribed a mineralocorticoid receptor antagonist which may substantially vary aldosterone levels. Those who were taking angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) were not excluded because these medications only exhibit a minor effect on aldosterone level,24 and as will be discussed later, African Americans have a low renin state.
The JHS adhered to the guidelines set forth by the Declaration of Helsinki and was approved by institutional review boards of participating institutions. All participants provided written informed consent.
Exposures
Risks for obstructive sleep apnea
Obesity, loud snoring, breathing cessation during sleep (apnea), daytime sleepiness, unrefreshing sleep, and sleepiness while driving predict OSA.11,23 Obesity was defined as having a body mass index (BMI) ≥30 kg/m2.25 Continuous BMI was categorized into a binary variable, indicating the presence or absence of obesity. Loud snoring, breathing cessation during sleep (apnea), daytime sleepiness, unrefreshing sleep, and sleepiness while driving were evaluated based on responses to the following statements: “You are told that you snore loudly and bother others.”; “You are told that you stop breathing (‘hold your breath’) in sleep.”; “You fall asleep during the day, particularly when not busy”; “You are tired after sleeping”; and “You feel sleepy or fall asleep while driving.”, respectively. The response options for all 5 statements were categorized into 2 groups. Participants who answered “Never” or “Seldom” were categorized as not having the risk factor. Those who answered “Sometimes,” “Often,” or “Almost always” were categorized as having the risk factor.
A surrogate measurement for OSA using a “risk score” was created based on factors that are strongly associated with OSA. The risk score include the following risk factors: BMI ≥ 30 kg/m2, loud snoring ≥ “sometimes,” presence of apnea during sleep ≥ “sometimes,” daytime sleepiness ≥ “sometimes,” unrefreshing sleep ≥ “sometimes,” and falling asleep while driving ≥ “sometimes.” A summary score, expressed as a dummy variable, was determined based on the presence or absence of these 6 risk factors (0 = no risk factors, 1 = one risk factor, etc.). The minimum score was 0, and the maximum score was 6.
Hypertension
The diagnosis of nonresistant hypertension (henceforth called hypertension) or resistant hypertension was defined based on the number and type of antihypertensive medications a participant was taking at the baseline visit. Participants brought with them antihypertensive medications that they have taken in the past 2 weeks. The medication names were transcribed and coded by a pharmacist. Medication classes included alpha-receptor blocker, ACE inhibitor, ARB, beta-receptor blocker, calcium channel blocker, thiazide, loop, and potassium-sparing diuretic excluding mineralocorticoid receptor antagonist, and miscellaneous agents (vasodilator, central-acting agents, and others).
Hypertension was categorized based on the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7).26 Systolic and diastolic BPs were recorded as means of 2 readings.27 A cutoff of ≥140/90 mm Hg was used to indicate presence of hypertension or uncontrolled hypertension in those who were taking antihypertensive medications. Hypertension and resistant hypertension are defined based on the number of antihypertensive medications and BP control. A 5-level variable was used to examine the relationship between categories of hypertension, risks for OSA, and aldosterone level. The levels are defined as follows:
No hypertension—no antihypertensive medications and BP <140/90 mm Hg.
Controlled hypertension—3 or fewer medications and BP <140/90 mm Hg.
Uncontrolled hypertension—no medications or fewer than 3 medications along with BP ≥140/90 mm Hg.
Controlled resistant hypertension—4 or more medications and BP <140/90 mm Hg.
Uncontrolled resistant hypertension—3 or more medications and BP ≥140/90 mm Hg.
Outcomes
Aldosterone
Aldosterone level was collected at the baseline visit between 2000 and 2004. Fasting blood samples were drawn and then separated into plasma and serum by sedimentation in a refrigerated centrifuge. The blood samples were sent to University of Minnesota’s central laboratories for storage. Radioimmunoassay was used to measure serum aldosterone (ng/dl).21,28 Aldosterone level was analyzed as a continuous variable.
Hypertension
The abovementioned 5-level hypertension variable was used to examine the relationship between risks for OSA and distinct categories of hypertension.
Covariates
Covariates included baseline age and gender which have previously been shown to be associated with OSA and the renin–angiotensin–aldosterone system.29–31 Age was considered as a continuous variable. Gender was categorized as a binary variable (male vs. female) based on self-report.
Statistical analysis
In univariable analyses, descriptive statistics were used to examine the distribution of the exposures, outcomes, and covariates at baseline. Continuous variables that followed a normal distribution were presented as means with their respective SDs. Continuous variables that did not follow a normal distribution were presented as medians with their respective interquartile ranges. Categorical variables were presented as counts and percentages. For bivariable analyses, analysis of variance (ANOVA) and χ 2 tests were used to examine relationships between risks for OSA and normally distributed continuous variables and categorical variables. For nonnormally distributed continuous variables, Kruskal–Wallis tests were used.
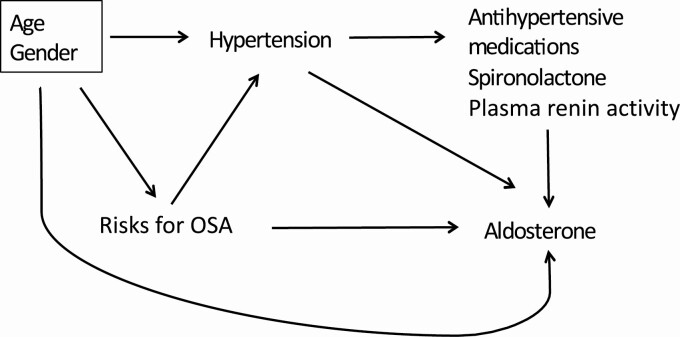
Unadjusted and adjusted linear and multinomial logistic regression models were used to examine relationships between risks for OSA, hypertension, and aldosterone. Adjusted models accounted for potential confounders based on a directed acyclic graph (Figure 1). First, unadjusted and adjusted linear regression models were fit to examine the relationship between risks for OSA (risk score) and log-aldosterone level. Second, unadjusted and adjusted multinomial logistic regression models were fit to examine the odds of having one of the categories of hypertension compared with not having hypertension as a function of the risk score. Finally, unadjusted and adjusted linear regression models were fit to examine the relationship between the categories of hypertension and log-aldosterone level. To assess improvement in model fit, adjusted linear and logistic regression models that used restricted cubic spline transformation vs. linear terms to control for continuous potential confounders were compared.32
Figure 1.
Direct acyclic graph (DAG) representing hypothesized relationships between risks for obstructive sleep apnea (OSA), hypertension, and aldosterone.
Logistic regression was used to further delineate which risk for OSA in the risk score specifically contribute most to aldosterone level. The adjusted model accounted for each risk for OSA along with age and gender. A Forest plot of the regression estimate for each risk for OSA was constructed.
In the group with uncontrolled hypertension, there may be misclassification of participants who were hypertensive on presentation but not on any medications. Elevation of BP may be due to white-coat hypertension or other nonchronic presentations, such as heavy exertion prior to the visit. Therefore, a sensitivity analysis was performed to examine the impact of the aforementioned misclassification. Participants in the uncontrolled hypertension group who had BP readings of ≥140/90 mm Hg were reclassified as having no hypertension. The previously described modeling was then repeated as part of sensitivity analyses. All statistical analyses were performed using SAS, version 9.4 with an α = 0.05 (SAS Institute, Cary, NC).
RESULTS
Univariable analyses
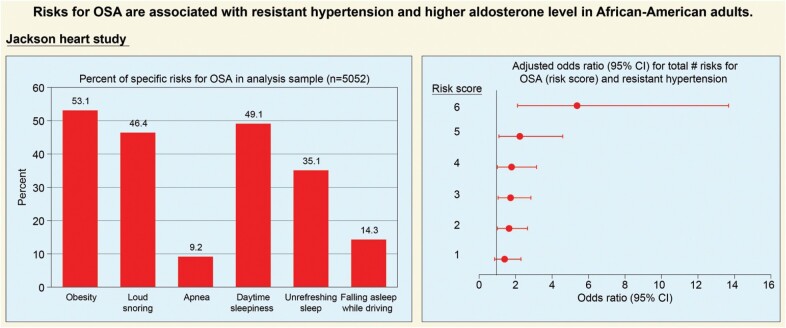
Characteristics of the study population at baseline are presented in Table 1. The median (interquartile range) aldosterone concentration was 4.30 (4.50) ng/dl. The study population had a mean (SD) age of 55 (12.83) years and consisted primarily of females (63.06%). In relation to the risks for OSA, approximately half of the population was obese and reported loud snoring and daytime sleepiness. Notably, subjects reported having the following: apneas during sleep (9.16%), unrefreshing sleep (35.10%), and falling asleep while driving (14.31%).
Table 1.
Covariates, hypertension, risks for obstructive sleep apnea (OSA), and aldosterone overall and by the number of risks for OSA at baseline
| Variablesa | Number of risks for OSAb | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (n = 5,052) | 0 (n = 623) | 1 (n = 1,223) | 2 (n = 1,419) | 3 (n = 1,027) | 4 (n = 525) | 5 (n = 187) | 6 (n = 48) | P-value | |
| Age (y) | 55.30 (12.83) | 55.66 (13.24) | 56.83 (13.44) | 56.00 (13.07) | 54.51 (12.59) | 52.72 (11.04) | 51.44 (10.03) | 50.70 (8.54) | <0.001 |
| Gender | 0.003 | ||||||||
| Female | 3,186 (63.06) | 377 (60.51) | 746 (61.00) | 896 (63.14) | 686 (66.80) | 350 (66.67) | 101 (54.01) | 30 (62.50) | |
| Male | 1,866 (36.94) | 246 (39.49) | 477 (39.00) | 523 (36.86) | 341 (33.20) | 175 (33.33) | 86 (45.99) | 18 (37.50) | |
| BMI (kg/m2) | 31.72 (7.22) | 25.68 (2.98) | 29.56 (6.02) | 32.03 (6.89) | 34.06 (7.36) | 35.95 (7.33) | 36.70 (6.63) | 40.17 (7.78) | <0.001 |
| Hypertension categories | <0.001 | ||||||||
| No hypertension | 1,992 (39.4) | 319 (51.2) | 508 (41.5) | 538 (37.9) | 363 (35.4) | 194 (37.0) | 56 (30.0) | 14 (29.2) | |
| Controlled hypertension | 1,468 (29.1) | 135 (21.7) | 323 (26.4) | 447 (31.5) | 328 (31.9) | 162 (30.9) | 60 (32.1) | 13 (27.1) | |
| Uncontrolled hypertension | 1,306 (25.9) | 147 (23.6) | 327 (26.7) | 348 (24.5) | 273 (26.6) | 139 (26.5) | 58 (31.0) | 14 (29.2) | |
| Resistant hypertension | 286 (5.6) | 22 (3.5) | 65 (5.3) | 86 (6.1) | 63 (6.1) | 30 (5.7) | 13 (7.0) | 7 (14.6) | |
| Hypertension control categories | <0.001 | ||||||||
| No hypertension | 1,992 (39.4) | 319 (51.2) | 508 (41.5) | 538 (37.9) | 363 (35.4) | 194 (37.0) | 56 (30.0) | 14 (29.2) | |
| Controlled hypertension | 1,468 (29.1) | 135 (21.7) | 323 (26.4) | 447 (31.5) | 328 (31.9) | 162 (30.9) | 60 (32.1) | 13 (27.1) | |
| Uncontrolled hypertension | 1,306 (25.9) | 147 (23.6) | 327 (26.7) | 348 (24.5) | 273 (26.6) | 139 (26.5) | 58 (31.0) | 14 (29.2) | |
| Controlled resistant HTN | 174 (3.4) | 15 (2.4) | 41 (3.4) | 47 (3.3) | 38 (3.7) | 21 (4.0) | 8 (4.3) | 4 (8.3) | |
| Uncontrolled resistant HTN | 112 (2.2) | 7 (1.1) | 24 (2.0) | 39 (2.8) | 25 (2.4) | 9 (1.7) | 5 (2.7) | 3 (6.3) | |
| Aldosterone level (ng/dl) | 4.30 (4.50) | 3.90 (3.90) | 4.20 (4.60) | 4.40 (4.30) | 4.50 (4.80) | 4.50 (4.60) | 5.20 (6.10) | 4.35 (5.35) | 0.001 |
| Risks for OSA | |||||||||
| Obesity | 2,682 (53.09) | ||||||||
| Loud snoring | 2,344 (46.40) | ||||||||
| Apnea | 463 (9.16) | ||||||||
| Daytime sleepiness | 2,480 (49.09) | ||||||||
| Unrefreshing sleep | 1,773 (35.10) | ||||||||
| Falling asleep while driving | 723 (14.31) |
aMean (SD), median (interquartile range), or n (%) are reported accordingly. Significant differences across groups were determined using analysis of variance (ANOVA) for normally distributed continuous variables, Kruskal-Wallis for nonnormally distributed continuous variables, and chi square for categorical variables.
bRisks for OSA included obesity, loud snoring, apnea, daytime sleepiness, unrefreshing sleep, and falling asleep while driving.
The prevalence of hypertension was approximately 60%. Of the participants who had hypertension, 29.1% had controlled hypertension and 25.9% had uncontrolled hypertension. Of note, a small proportion of participants had resistant hypertension (5.6%).
Bivariable analyses
As shown in Table 1, across the risk score, participants with more risks for OSA were younger. The distribution of gender was not similar among the groups. In those with risk score ≥2, about 30% have controlled hypertension, 25%–30% have uncontrolled hypertension, and 6%–15% have resistant hypertension. The prevalence of hypertension and resistant hypertension appeared higher in those with risks for OSA compared with no risks. Aldosterone level was also higher in those with a greater risk score compared with having no risks.
Contribution of each risk for OSA to aldosterone level and resistant hypertension
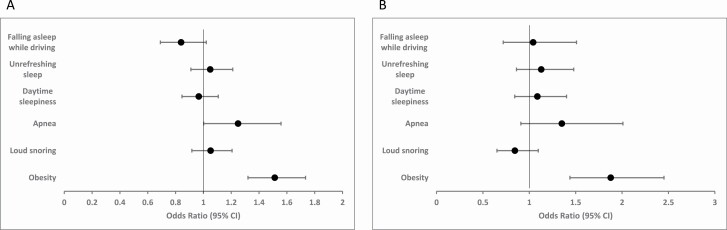
Risks for OSA in the score are presented as the absolute number of risks present. The contribution of each risk for OSA to the subsequent analyses is presented in Figure 2. Obesity (OR 1.51, 95% CI 1.32–1.73) and witnessed apneas (OR 1.25, 95% CI 1.002–1.56) contributed most to the aldosterone level adjusting for each risk, age, and gender while only obesity contributed most to resistant hypertension.
Figure 2.
Adjusted odds ratio (95% CI) for specific risks for obstructive sleep apnea (OSA) and highest quartile of aldosterone (a) and resistant hypertension (b).
Adjusted analyses—risks for OSA and aldosterone level
The relationship between risks for OSA and log-aldosterone level is presented in Table 2. Adjusting for age and gender, log-aldosterone level remained higher in participants with risks for OSA compared with those without risks. Log-aldosterone level incrementally increased with each additional risk for OSA and was the highest in those with the highest risk score (P-trend 0.049).
Table 2.
Linear regression models of risks for obstructive sleep apnea (OSA) (risk score) and log-aldosterone level
| Exposure | Unadjusted model | Adjusted modela | ||||
|---|---|---|---|---|---|---|
| Risk score | Log-aldosterone | Confidence interval | P-trend | Log-aldosterone | Confidence interval | P-trend |
| 0 | 1.40 | 1.35–1.46 | 0.040 | 1.40 | 1.35–1.45 | 0.049 |
| 1 | 1.49 | 1.45–1.52 | 1.49 | 1.45–1.52 | ||
| 2 | 1.50 | 1.47–1.54 | 1.50 | 1.47–1.54 | ||
| 3 | 1.54 | 1.50–1.58 | 1.54 | 1.50–1.58 | ||
| 4 | 1.52 | 1.47–1.58 | 1.52 | 1.47–1.58 | ||
| 5 | 1.65 | 1.55–1.74 | 1.64 | 1.55–1.73 | ||
| 6 | 1.61 | 1.43–1.80 | 1.61 | 1.42–1.79 |
aModel adjusted for age and gender.
Adjusted analyses—risks for OSA and hypertension
The relationship between risks for OSA and hypertension is presented in Table 3. The results indicated that the odds of having hypertension or resistant hypertension compared with not having hypertension increased with each additional risk for OSA compared with no risks after adjusting for age and gender. For example, comparing a risk score of 1 to no risks, the odds ratios for having uncontrolled hypertension or resistant hypertension to not having hypertension ranged from 1.5 to 2 in both the unadjusted and adjusted models. In the adjusted model, those with a risk score of 4–5 compared with those without risks had approximately 3–7 times the odds of having uncontrolled hypertension or resistant hypertension compared with not having hypertension. The odds of having uncontrolled hypertension or resistant hypertension in those with a risk score of 6 compared with those without risks were approximately 3 and 18 times higher, respectively, compared with the odds of not having hypertension. There was a dose-response increase in odds of having hypertension or resistant hypertension with increasing risk score (P-trend <0.001).
Table 3.
Multinomial logistic regression models examining the odds of having specified hypertension as a function of the number of risks for obstructive sleep apnea (OSA) (risk score) where no hypertension and no risks were the referent groups
| Unadjusted model | Adjusted modela | |||||
|---|---|---|---|---|---|---|
| Odds ratio | Confidence interval | P-valueb | Odds ratio | Confidence interval | P-valueb | |
| Controlled hypertension | ||||||
| 1 | 1.50 | 1.18–1.92 | 0.001 | 1.52 | 1.16–1.97 | 0.002 |
| 2 | 1.96 | 1.55–2.49 | <0.0001 | 2.16 | 1.67–2.78 | <0.0001 |
| 3 | 2.14 | 1.66–2.74 | <0.0001 | 2.67 | 2.03–3.50 | <0.0001 |
| 4 | 1.97 | 1.48–2.64 | <0.0001 | 2.74 | 2.00–3.74 | <0.0001 |
| 5 | 2.53 | 1.67–3.84 | <0.0001 | 4.24 | 2.73–6.59 | <0.0001 |
| 6 | 2.19 | 1.01–4.79 | 0.049 | 3.65 | 1.62–8.21 | 0.002 |
| Uncontrolled hypertension | ||||||
| 1 | 1.40 | 1.10–1.78 | 0.006 | 1.39 | 1.08–1.80 | 0.011 |
| 2 | 1.40 | 1.11–1.78 | 0.005 | 1.53 | 1.19–1.96 | 0.001 |
| 3 | 1.63 | 1.27–2.10 | 0.000 | 2.03 | 1.56–2.65 | <0.0001 |
| 4 | 1.56 | 1.16–2.08 | 0.003 | 2.09 | 1.54–2.85 | <0.0001 |
| 5 | 2.25 | 1.48–3.41 | 0.000 | 3.27 | 2.12–5.06 | <0.0001 |
| 6 | 2.17 | 1.01–4.67 | 0.048 | 3.31 | 1.51–7.29 | 0.003 |
| Resistant hypertension—controlled | ||||||
| 1 | 1.72 | 0.94–3.15 | 0.082 | 1.72 | 0.93–3.20 | 0.084 |
| 2 | 1.86 | 1.02–3.38 | 0.042 | 2.06 | 1.12–3.78 | 0.019 |
| 3 | 2.23 | 1.20–4.12 | 0.011 | 2.87 | 1.53–5.38 | 0.001 |
| 4 | 2.30 | 1.16–4.57 | 0.017 | 3.38 | 1.67–6.81 | 0.001 |
| 5 | 3.04 | 1.23–7.50 | 0.016 | 5.45 | 2.16–13.73 | 0.000 |
| 6 | 6.08 | 1.78–20.70 | 0.004 | 11.05 | 3.15–38.76 | 0.000 |
| Resistant hypertension—uncontrolled | ||||||
| 1 | 2.15 | 0.92–5.06 | 0.078 | 2.15 | 0.91–5.08 | 0.082 |
| 2 | 3.30 | 1.46–7.47 | 0.004 | 3.69 | 1.62–8.42 | 0.002 |
| 3 | 3.14 | 1.34–7.35 | 0.009 | 4.16 | 1.76–9.83 | 0.001 |
| 4 | 2.11 | 0.78–5.77 | 0.144 | 3.23 | 1.17–8.93 | 0.024 |
| 5 | 4.07 | 1.25–13.27 | 0.020 | 7.53 | 2.26–25.06 | 0.001 |
| 6 | 9.77 | 2.28–41.82 | 0.002 | 18.86 | 4.27–83.29 | 0.000 |
aModel adjusted for age and gender.
bTrend P-value within each hypertension level for risks for OSA <0.001.
Adjusted analyses—association of hypertension and aldosterone level
The relationship between hypertension and aldosterone level is presented in Table 4. Log-aldosterone level was higher in participants with hypertension compared with no hypertension. Additionally, log-aldosterone level was higher in those with resistant hypertension compared with those with hypertension. There was direct relationship in log-aldosterone level across hypertension severity (P-trend <0.001) with the highest level in those with uncontrolled resistant hypertension.
Table 4.
Linear regression models of hypertension control and log-aldosterone level
| Hypertension control categories | Unadjusted model | Adjusted modela | ||||
|---|---|---|---|---|---|---|
| Log-aldosterone | Confidence interval | P-trend | Log-aldosterone | Confidence interval | P-trend | |
| No hypertension | 1.34 | 1.31–1.37 | <0.001 | 1.32 | 1.28–1.36 | <0.001 |
| Controlled hypertension | 1.63 | 1.59–1.66 | 1.67 | 1.62–1.71 | ||
| Uncontrolled hypertension | 1.55 | 1.52–1.58 | 1.57 | 1.52–1.61 | ||
| Controlled resistant hypertension | 1.79 | 1.70–1.89 | 1.83 | 1.74–1.93 | ||
| Uncontrolled resistant hypertension | 1.79 | 1.67–1.91 | 1.83 | 1.71–1.95 |
aModel adjusted for age, gender, and risks for obstructive sleep apnea (OSA).
Sensitivity analyses examining misclassification
Participants who were hypertensive and not on antihypertensive medications were redistributed to the “no hypertension” group. Apparent log-aldosterone level differences were not found across hypertension severity except in the uncontrolled hypertension group where the aldosterone level is higher compared with the original distribution. This may be due to misclassification as these participants have elevated BP due to a nonchronic presentation. An increasing number of risks for OSA led to a higher effect estimate in the odds of having uncontrolled hypertension compared with no risks in the multinomial regression models. Again, this finding supports the possibility of misclassification of hypertensive participants who are not on antihypertensive medications (see Appendix).
DISCUSSION
We examined the relationships between risks for OSA, hypertension, and aldosterone level in 5,052 African American adults in the JHS based on data collected at baseline between 2000 and 2004. To summarize our findings, risks for OSA were significantly associated with both hypertension and resistant hypertension and aldosterone levels with obesity and apneas during sleep contributing most to the associations. Specifically, each additional risk for OSA increased aldosterone level. Participants who had a higher number of risks for OSA had significantly increased odds of having resistant hypertension. Aldosterone level was highest in those with uncontrolled resistant hypertension. Therefore, our findings are consistent with aldosterone playing a role in resistant hypertension in African Americans with risks for OSA.
The limited availability of studies provided the motivation to explore our inquiry regarding the relationship between risks for OSA, resistant hypertension, and aldosterone in African Americans. Furthermore, establishing this relationship is important because previous studies have shown that inhibiting aldosterone activity improves BP control and OSA severity (reduce aldosterone-induced fluid retention which decreases pharyngeal edema and airway resistance).33,34 Along with continuous positive airway pressure therapy, it may provide more consistent and effective BP control.35,36
Although we found that the aldosterone level increased with greater number of risks for OSA, the median aldosterone level obtained for each risk factor category was in the normal range. This could be explained from a previous study showing that African Americans have a lower plasma renin activity due to the reduction in renin secretion rate compared with whites, resulting in lower aldosterone levels.37 Due to low renin, ACE inhibitors, and ARB may not effectively lower BP in this population.38
Although the aldosterone level is low, due to the increased sensitivity to aldosterone in African Americans, small variations in aldosterone concentration may affect BP control. Also, obesity (increased visceral adiposity) may enhance the sensitivity to aldosterone-mediated mineralocorticoid receptor activation.39 Note, normal aldosterone level in African Americans does not preclude the use of a mineralocorticoid receptor antagonist to treat resistant hypertension. Nishizaka et al.40 demonstrated that spironolactone, a mineralocorticoid receptor antagonist, effectively lowered BP independent of aldosterone levels.
Limitations and strengths
Our study is the first to demonstrate associations between risks for OSA and aldosterone levels among African Americans. In addition, we provided data on the relationship between these risks and distinct categories of hypertension, which for our study population was important because of the higher prevalence of resistant hypertension. Our results confirmed previous studies on OSA and hypertension being associated with higher aldosterone levels and OSA may be a significant contributing factor to resistant hypertension regardless of control. Moller et al.15 demonstrated that subjects with OSA had higher BP, angiotensin II, and aldosterone compared with control subjects. Calhoun et al.16 utilized the Berlin questionnaire and found that subjects with high probability of OSA and resistant hypertension had increased aldosterone excretion.
Our study had a few limitations. First, we employed a cross-sectional study design. We were not able to determine if the duration of having any of the risks for OSA or hypertension played a role in altering the aldosterone levels in our study population. Additionally, we could not determine if participants with resistant hypertension would remain resistant if they had the opportunity to be treated for a longer duration. Similarly, participants who are on 1 or 2 medications and have uncontrolled hypertension may be resistant if followed for a longer period of time. However, our study provided insight into the risks for OSA potentially affecting aldosterone level and being associated with resistant hypertension.
Second, the JHS cohort only included African Americans from Jackson, Mississippi. African Americans from this region may not represent the general African American population. Therefore, generalizability of the results may be limited. However, our finding of low-normal aldosterone levels was consistent with previous studies. Most importantly, clinicians should strongly consider OSA being the contributing factor to a patient’s hypertension when the risks for OSA are detected.
Third, our definition of hypertension based on the BP and number of antihypertensive medications may be biased due to misclassification of the participants in the uncontrolled hypertension group. About 15% of those in the group had BP readings of ≥140/90 mm Hg and not on any antihypertensive medications. Some of the participants may have white-coat hypertension or elevated BP due to other nonchronic presentations. We examined this with a sensitivity analysis, reclassifying them to the “no hypertension” group which confirmed nonchronic elevation of BP in some of the hypertensive participants.
Fourth, the confidence intervals for some of the effect estimates especially in the multinomial logistic regression models are wide, indicating a small sample size for the comparisons. Many patients do not have all the risks for OSA unless their sleep-disordered breathing is severe. Some may underreport symptoms such as snoring especially when they do not have a bed partner. Although some of the confidence intervals are wide, the trend or dose-response is significant and reflects biological plausibility.
Finally, the diagnosis of OSA, which is assessed with overnight polysomnography, was not available in the JHS. Nevertheless, we used the risks for OSA obtained from a validated questionnaire to study their associations with hypertension and aldosterone. The use of a validated questionnaire is applicable to a “real world” scenario, where a primary care provider screens a patient at risk for sleep-disordered breathing for OSA.
African Americans have an increased risk of having resistant hypertension compared with Caucasians. Undiagnosed OSA may in part explain this disparity in at-risk individuals with resistant hypertension, and it is in need of more clinical attention. Furthermore, the presence of resistant hypertension regardless of BP control should instigate a clinician to consider further investigation of sleep-disordered breathing.
The mechanism of resistant hypertension may be due to elevated aldosterone level from RAAS activation during apnea-induced periods of hypoxemia and hypercapnia. We found that risks for OSA were associated resistant hypertension and aldosterone level. The highest levels of aldosterone were found in those with the greatest number of risks for OSA and with resistant hypertension. Increased sensitivity to aldosterone rather than aldosterone level may elucidate the relationship between OSA and resistant hypertension. Future studies using a longitudinal and prospective study design are needed to confirm the relationship between OSA and aldosterone level in African Americans with resistant hypertension and determine if diagnosing and treating OSA and/or adding an aldosterone receptor antagonist in African Americans would result in achieving BP control with fewer medications.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank the Jackson Heart Study participants, clinical sites, investigators, and staff for their efforts in providing and collecting data. We would also like to thank Mary B. Roberts at the Center for Primary Care and Prevention, Alpert Medical School of Brown University, Pawtucket, Rhode Island for her wonderful help and expertise in statistical programming and analysis. The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Contributor Information
Patrick Koo, Department of Respiratory, Critical Care, and Sleep Medicine, Erlanger Baroness Hospital, University of Tennessee Health Science Center, Chattanooga, Tennessee, USA.
Paul Muntner, Department of Epidemiology, University of Alabama at Birmingham School of Public Health, Birmingham, Alabama, USA.
Michael E Hall, Division of Cardiovascular Diseases, University of Mississippi Medical Center, Jackson, Mississippi, USA.
Annie Gjelsvik, Department of Epidemiology, Brown School of Public Health, Providence, Rhode Island, USA.
Franklin Dennis McCool, Division of Pulmonary, Critical Care, and Sleep Medicine, Memorial Hospital of Rhode Island, Alpert Medical School of Brown University, Providence, Rhode Island, USA.
Charles B Eaton, Department of Family Medicine and Epidemiology, Memorial Hospital of Rhode Island, Alpert Medical School of Brown University and Brown School of Public Health, Providence, Rhode Island, USA.
FUNDING
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I), and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I, and HHSN268201800012I) contracts from the National Heart, Lung, and Blood Institute (NHLBI) and the National Institute on Minority Health and Health Disparities (NIMHD).
DISCLOSURE
The authors declared no conflict of interest.
This manuscript was sent to Guest Editor, Hillel W. Cohen, MPH, DrPH for editorial handling and final disposition.
REFERENCES
- 1. Saunders E. Managing hypertension in African-American patients. J Clin Hypertens (Greenwich) 2004; 6:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension, prevalence, awareness, and management. Arch Intern Med 2005; 165:2098–2104. [DOI] [PubMed] [Google Scholar]
- 3. Wright JT Jr, Agodoa L, Contreras G, Greene T, Douglas JG, Lash J, Randall O, Rogers N, Smith MC, Massry S; African American Study of Kidney Disease and Hypertension Study Group. Successful blood pressure control in the African American study of kidney disease and hypertension. Arch Intern Med 2002; 162:1636–1643. [DOI] [PubMed] [Google Scholar]
- 4. Flack JM, Sica DA, Bakris G, Brown AL, Ferdinand KC, Grimm RH Jr, Hall WD, Jones WE, Kountz DS, Lea JP, Nasser S, Nesbitt SD, Saunders E, Scisney-Matlock M, Jamerson KA; International Society on Hypertension in Blacks. Management of high blood pressure in Blacks: an update of the International Society on Hypertension in Blacks consensus statement. Hypertension 2010; 56:780–800. [DOI] [PubMed] [Google Scholar]
- 5. Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB; American Heart Association Professional/Public Education and Publications Committee of the Council on Hypertension; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Genomic and Precision Medicine; Council on Peripheral Vascular Disease; Council on Quality of Care and Outcomes Research; and Stroke Council. Resistant hypertension: detection, evaluation, and management: a scientific statement from the American Heart Association. Hypertension 2018; 72:e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shahar E, Whitney CW, Redline S, Lee ET, Newman AB, Nieto FJ, O’Connor GT, Boland LL, Schwartz JE, Samet JM. Sleep-disordered breathing and cardiovascular disease: cross-sectional results of the Sleep Heart Health Study. Am J Respir Crit Care Med 2001; 163:19–25. [DOI] [PubMed] [Google Scholar]
- 7. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med 2000; 342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 8. Demede M, Pandey A, Zizi F, Bachmann R, Donat M, McFarlane SI, Jean-Louis G, Ogedegbe G. Resistant hypertension and obstructive sleep apnea in the primary-care setting. Int J Hypertens 2011; 2011:340929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. O’Connor GT, Lind BK, Lee ET, Nieto FJ, Redline S, Samet JM, Boland LL, Walsleben JA, Foster GL; Sleep Heart Health Study Investigators. Variation in symptoms of sleep-disordered breathing with race and ethnicity: the Sleep Heart Health study. Sleep 2003; 26:74–79. [PubMed] [Google Scholar]
- 10. Kripke DF, Ancoli-Israel S, Klauber MR, Wingard DL, Mason WJ, Mullaney DJ. Prevalance of sleep-disordered breathing in ages 40–64 years: a population-based survey. Sleep 1997; 20:65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, Walsleben JA, Finn L, Enright P, Samet JM; Sleep Heart Health Study Research Group. Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health study. Arch Intern Med 2002; 162:893–900. [DOI] [PubMed] [Google Scholar]
- 12. Redline S, Tishler PV, Hans MG, Tosteson TD, Strohl KP, Spry K. Racial differences in sleep-disordered breathing in African-Americans and Caucasians. Am J Respir Crit Care Med 1997; 155:186–192. [DOI] [PubMed] [Google Scholar]
- 13. Ancoli-Israel S, Klauber MR, Stepnowsky C, Estline E, Chinn A, Fell R. Sleep-disordered breathing in African-American elderly. Am J Respir Crit Care Med 1995; 152:1946–1949. [DOI] [PubMed] [Google Scholar]
- 14. Narkiewicz K, Somers VK. The sympathetic nervous system and obstructive sleep apnea: implications for hypertension. J Hypertens 1997; 15:1613–1619. [DOI] [PubMed] [Google Scholar]
- 15. Moller DS, Lind P, Strunge B, Pedersen EB. Abnormal vasoactive hormones and 24-hour blood pressure in obstructive sleep apnea. Am J Hypertens 2003; 16:274–280. [DOI] [PubMed] [Google Scholar]
- 16. Calhoun DA, Nishizaka MK, Zaman MA, Harding SM. Aldosterone excretion among subjects with resistant hypertension and symptoms of sleep apnea. Chest 2004; 125:112–117. [DOI] [PubMed] [Google Scholar]
- 17. Calhoun DA, Nishizaka MK, Zaman MA, Thakkar RB, Weissmann P. Hyperaldosteronism among black and white subjects with resistant hypertension. Hypertension 2002; 40:892–896. [DOI] [PubMed] [Google Scholar]
- 18. Pratt-Ubunama MN, Nishizaka MK, Boedefeld RL, Cofield SS, Harding SM, Calhoun DA. Plasma aldosterone is related to severity of obstructive sleep apnea in subjects with resistant hypertension. Chest 2007; 131:453–459. [DOI] [PubMed] [Google Scholar]
- 19. Gjorup PH, Sadauskiene L, Wessels J, Nyvad O, Strunge B, Pedersen EB. Abnormally increased endothelin-1 in plasma during the night in obstructive sleep apnea: relation to blood pressure and severity of disease. Am J Hypertens 2007; 20:44–52. [DOI] [PubMed] [Google Scholar]
- 20. Svatikova A, Olson LJ, Wolk R, Phillips BG, Adachi T, Schwartz GL, Somers VK. Obstructive sleep apnea and aldosterone. Sleep 2009; 32:1589–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Taylor HA Jr, Wilson JG, Jones DW, Sarpong DF, Srinivasan A, Garrison RJ, Nelson C, Wyatt SB. Toward resolution of cardiovascular health disparities in African Americans: design and methods of the Jackson Heart study. Ethn Dis 2005; 15:S6-4-17. [PubMed] [Google Scholar]
- 22. Fuqua SR, Wyatt SB, Andrew ME, Sarpong DF, Henderson FR, Cunningham MF, Taylor HA Jr.. Recruiting African-American research participation in the Jackson Heart Study: methods, response rates, and sample description. Ethn Dis 2005; 15:S6-18-29. [PubMed] [Google Scholar]
- 23. Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med 1999; 131:485–491. [DOI] [PubMed] [Google Scholar]
- 24. Seifarth C, Trenkel S, Schobel H, Hahn EG, Hensen J. Influence of antihypertensive medication on aldosterone and renin concentration in the differential diagnosis of essential hypertension and primary aldosteronism. . Clin Endocrinol (Oxf) 2002; 57:457–465. [DOI] [PubMed] [Google Scholar]
- 25. Weir CB, Jan A. BMI classification percentile and cut off points. In StatPearls [Internet]. StatPearls Publishing: Treasure Island, FL, 2022. [PubMed] [Google Scholar]
- 26. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA 2003; 289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 27. Wyatt SB, Akylbekova EL, Wofford MR, Coady SA, Walker ER, Andrew ME, Keahey WJ, Taylor HA, Jones DW. Prevalence, awareness, treatment, and control of hypertension in the Jackson Heart Sstudy. Hypertension 2008; 51:650–656. [DOI] [PubMed] [Google Scholar]
- 28. Carpenter MA, Crow R, Steffes M, Rock W, Heilbraun J, Evans G, Skelton T, Jensen R, Sarpong D. Laboratory, reading center, and coordinating center data management methods in the Jackson Heart Study. Am J Med Sci 2004; 328:131–144. [DOI] [PubMed] [Google Scholar]
- 29. Komukai K, Mochizuki S, Yoshimura M. Gender and the renin-angiotensin-aldosterone system. Fundam Clin Pharmacol 2010; 24:687–698. [DOI] [PubMed] [Google Scholar]
- 30. Conti S, Cassis P, Benigni A. Aging and the renin-angiotensin system. Hypertension 2012; 60:878–883. [DOI] [PubMed] [Google Scholar]
- 31. Gabbay IE, Lavie P. Age- and gender-related characteristics of obstructive sleep apnea. Sleep Breath 2012; 16:453–460. [DOI] [PubMed] [Google Scholar]
- 32. Devlin TF, Weeks BJ. Spline functions for logistic regression modeling. In Proc 11th Annual SAS Users Group International Conference, Cary, NC, 1986, pp. 646–649. https://support.sas.com/resources/papers/proceedings-archive/SUGI86/Sugi-11-119%20Devlin%20Weeks.pdf.
- 33. Wang Y, Li CX, Lin YN, Zhang LY, Li SQ, Zhang L, Yan YR, Lu FY, Li N, Li QY. The role of aldosterone in OSA and OSA-related hypertension. Front Endocrinol (Lausanne) 2022; 12:801689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea, and aldosterone. J Hum Hypertens 2012; 26:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barceló A, Piérola J, Esquinas C, de la Peña M, Arqué M, Alonso-Fernández A, Bauçà JM, Robles J, Barceló B, Barbé F. Relationship between aldosterone and the metabolic syndrome in patients with obstructive sleep apnea hypopnea syndrome: effect of continuous positive airway pressure treatment. PLoS One 2014; 9:e84362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lloberes P, Sampol G, Espinel E, Segarra A, Ramon MA, Romero O, Ferrer R, Martínez-Garcia MA, Tovar JL. A randomized controlled study of CPAP effect on plasma aldosterone concentration in patients with resistant hypertension and obstructive sleep apnea. J Hypertens 2014; 32:1650–1657; discussion 1657. [DOI] [PubMed] [Google Scholar]
- 37. Sagnella GA. Why is plasma renin activity lower in populations of African origin? J Hum Hypertens 2001; 15:17–25. [DOI] [PubMed] [Google Scholar]
- 38. Wright JT Jr, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, Haywood LJ, Leenen FH, Margolis KL, Papademetriou V, Probstfield JL, Whelton PK, Habib GB; ALLHAT Collaborative Research Group. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA 2005; 293:1595–1608. [DOI] [PubMed] [Google Scholar]
- 39. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. . Cir Res 2015; 116:991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nishizaka ML, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens 2003; 16:925–930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.