Abstract
Introduction:
The incidence of infection and scarring in intraoral raw wounds are decreased when wounds are grafted with biological materials. The favourable results of many studies about amniotic membrane in wound healing inspired us to investigate the effects of lyophilised amniotic membrane in intaoral surgical defects. The aim of this study was to evaluate the healing of oral mucosal defects after application of lyophilised amniotic membrane (AM).
Methods:
Fifteen patients with oral precancerous lesions were included in this study. Lyophilised amniotic membrane was applied to the intraoral surgical defect, after wide excision of the lesion. The effectiveness of the lyophilised AM was evaluated by scoring the following parameters operability, haemostatic status, pain, feeding situation, epithelialisation, change in mouth opening, mucosal suppleness and safety.
Results:
The lyophilised amniotic membrane has been found to be effective in this study after evaluation of the parameters. No infection or allergic reaction was noticed after application of the lyophilised amniotic membrane in intraoral surgical defects.
Discussion:
In our study, the size and site of the surgical defect influenced the scar contracture so we suggest lyophilised AM may not prevent scarring for extensive surgical defects. All other findings regarding the effectiveness of lyophilised amniotic membrane in oral wound healing are in accordance with the findings of other studies conducted on hyperdry and cryopreserved AM.
Conclusion:
Within the limitations of the study, the results showed that the lyophilised amniotic membrane is a cost effective material for immediate coverage of the intraoral surgical defects.
Keywords: Amniotic membrane, autograft, oral mucosa, precancerous conditions, wound healing
INTRODUCTION
Intraoral raw wounds heal by granulation and epithelialisation. The incidence of infection and scarring is decreased when wounds are grafted with biological materials rather than left uncovered or covered by nonbiological materials. Although autografts are immunologically ideal, there are certain drawbacks associated with autografts, such as - a separate surgical procedure, donor-site morbidity and limitations in mucosal grafts and low mobility, colour and texture difference of skin grafts.[1,2]
Bovine collagen has been used under various clinical situations. Screening tests of donors for pathogenic microorganisms and more severe processing to remove or inactivate zoonotic pathogens may increase the product cost and also decrease the desirable biological activities of collagen.[2]
Amniotic membrane (AM) is the innermost layer lining the amniotic cavity. With a thickness of 0.02–0.5 mm, the amnion is a translucent tissue devoid of any vasculature.[3] AM has several properties including secondary epithelialisation and angiogenesis, adhesiveness, bacteriostatic properties, wound protection, pain reduction, and lack of immunogenicity and stem cell-like property.[4,5,6,7,8,9,10,11,12,13,14] It is also inexpensive and readily available in large amounts.
Davis published the first scientific description concerning skin transplantation with these membranes in 1910. Following this first use, amnions have been used widely to treat skin burn, ulcer, fistula, and in ocular surface reconstruction, among other wounds.[15,16]
Kothary first used AM in maxillofacial surgery to restore surgical defects of the floor of the mouth.[17] Zohar et al. treated flap necrosis after neck dissection and tumour surgery with AM.[18] Lai et al. used amnion in the surgical treatment of oral submucous fibrosis.[19] Amnion has been used in mandibular vestibuloplasty.[20] Since its first application in the oral cavity, this membrane has been extensively used in the field of oral surgery.[21]
Glycerol preservation, cryopreservation, lyophilisation, gamma irradiation, and hyperdrying are commonly used methods of preservation and sterilisation for AM.
Arai et al.[2] and Kar et al.[22] evaluated hyperdried and cryopreserved AM in surgical defects of the oral mucosa.
The favourable results of these studies inspired us to investigate the effects of lyophilised AM in intraoral surgical defects. The lyophilised AM is available at low cost and it may serve as a good substitute for other materials which are difficult to obtain or available at a high cost. The purpose of this study was to evaluate the effect of lyophilised AM in the healing of intraoral surgical defects.
MATERIALS AND METHODS
This was a prospective clinical study conducted in the Department of Oral and Maxillofacial Surgery of the institutional hospital located in Guwahati, Assam, between March 2015–March 2020. A team of four maxillofacial surgeons (two senior faculties, two residents) operated upon the cases.
This study was performed with the approval of the institutional ethics committee (Ethical committee clearance number-RDC/29/2011/605). All procedures performed in the study were conducted in accordance with the ethics standards given in the 1964 Declaration of Helsinki as revised in 2013. All patients were treated with grafting of the lyophilised AM after obtaining informed and written consent.
Fifteen patients with oral precancerous lesions were included in this study [Table 1]. Inclusion criteria were patients with secondary surgical defects of oral mucosa that are sufficiently large and cannot be closed primarily, which occur after excision of the lesion. Patients with severe systemic disease, HIV, HBV, and HCV infection were excluded from this study. Detailed descriptions of age, gender, site, and size of the surgical defect are also listed in Table 1.
Table 1.
Total number of patients with a detailed description of age, gender, and site and size of the surgical defect
| Case | Age (year) | Gender | Site (defect) | Size (cm) |
|---|---|---|---|---|
| 1 | 65 | Male | BM | 3×2 |
| 2 | 47 | Female | BM | 4×3 |
| 3 | 40 | Male | LA, BM | 5×3 |
| 4 | 65 | Male | LA, BM | 4×4 |
| 5 | 55 | Male | BM | 3×2 |
| 6 | 38 | Male | LA, BM | 5×4 |
| 7 | 35 | Male | LA, BM | 4×3 |
| 8 | 40 | Male | LA, BM, lip | 9×4 |
| 9 | 60 | Female | LA, BM | 5×4 |
| 10 | 32 | Female | BM | 3×2 |
| 11 | 62 | Male | BM | 4×4 |
| 12 | 43 | Male | LA, BM, lip | 8×3 |
| 13 | 52 | Male | BM | 3×2 |
| 14 | 42 | Male | BM | 5×3 |
| 15 | 38 | Female | UA, BM | 4×3 |
LA=Lower alveolus; BM=Buccal mucosa; UA=Upper alveolus
Lyophilised AM was obtained from Tata Hospital Tissue Bank, Mumbai. The donors were routinely screened for HIV, hepatitis B and C, and syphilis. After cleaning, AM is pasteurised at 60°C, treated with 70% ethanol, and finally freeze dried to remove 95% of the moisture. The packed and sealed AM is sterilised by 25 kGy gamma radiation.[23]
After wide excision of the lesion, a lyophilised AM was used to cover the surgical defects which cannot be closed primarily. A membrane little larger than the actual wound was soaked in saline for 1 min and then placed directly on the wound. The membrane was stabilised with Vicryl suture to the surrounding mucosa at the periphery of the defect [Figures 1-4].
Figure 1.
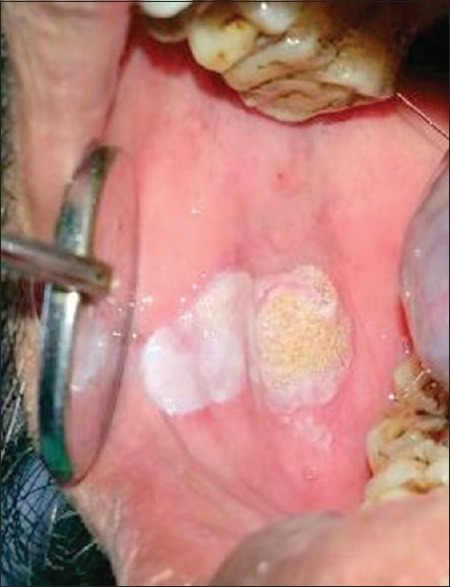
Preoperative photograph of leukoplakia in buccal mucosa
Figure 4.
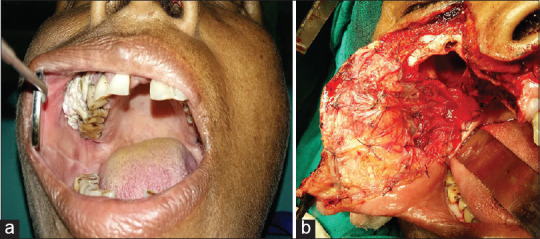
(a) Preoperative photograph of leukoplakia of upper alveolus and buccal mucosa (b) Application of lyophilised amniotic membrane over the surgical defect after wide excision with subtotal maxillectomy
Figure 2.
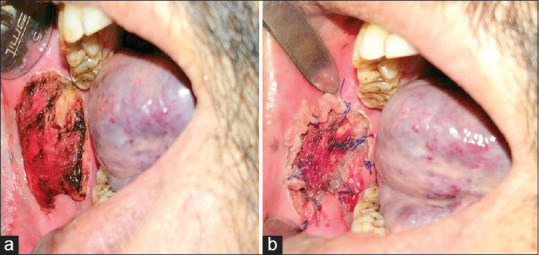
(a) Intraoperative photograph of the surgical defect in the buccal mucosa. (b). Placement of the lyophilised amniotic membrane on the defect
The effectiveness and usefulness of the lyophilised AM were evaluated by scoring different parameters intra- and postoperatively. The scoring pattern was determined by the criteria described by Rastogi et al.,[1] Arai et al.,[2] and Kar et al.[22] The criteria for the judgment in this study are presented in Table 2. The result of each parameter was evaluated by two senior oral and maxillofacial surgeons, each with more than 15 years of clinical experience, as good (2 points), fair (1point), or poor (0 point).
Table 2.
Criteria for judgment of lyophilised amniotic membrane
| Parameter | Definition | Score |
|---|---|---|
| Operability | Easy to use | 2 (good) |
| Acceptable | 1 (fair) | |
| Impractical | 0 (poor) | |
| Haemostasis | No bleeding | 2 (good) |
| Slight bleeding, no haemostasis required | 1 (fair) | |
| Bleeding that required haemostasis | 0 (poor) | |
| Pain | None to mild | 2 (good) |
| Moderate | 1 (fair) | |
| Severe requiring analgesics | 0 (poor) | |
| Feeding | Oral feeding throughout or oral feeding and normal diet within 2 weeks | 2 (good) |
| Oral feeding and normal diet within 4 weeks | 1 (fair) | |
| Tube diet even after 4 weeks | 0 (poor) | |
| Epithelialisation | Entire wound | 2 (good) |
| Nearly entire wound | 1 (fair) | |
| Inadequate | 0 (poor) | |
| Change in mouth opening | None or little (<25%) | 2 (good) |
| Moderate (25%-50%) | 1 (fair) | |
| Serious (>50%) | 0 (poor) | |
| Mucosal suppleness | Similar on both sides | 2 (good) |
| Slightly altered | 1 (fair) | |
| Contracture | 0 (poor) | |
| Safety | No reaction | 2 (good) |
| Mild reactions | 1 (fair) | |
| Reactions requiring intervention | 0 (poor) | |
| Effectiveness | Very effective | >15 |
| effective | 11-15 | |
| Ineffective | <11 |
The operability of the lyophilised AM was based on the surgeon’s experience regarding its handling properties (easy to use = 2, acceptable = 1, and impractical = 0).
Haemostasis was assessed on the 1st postoperative day (no bleeding = 2, slight bleeding = 1, and bleeding requiring haemostasis = 0).
The pain was categorised based on the patient’s own words (mild = 2, moderate = 1, severe requiring analgesics = 0), on the 3rd postoperative day when the patient was no longer taking analgesic medications.
Feeding situation was assessed according to the time and oral feeding commenced (oral feeding throughout or oral feeding and normal diet within 2 weeks = 2, oral feeding and normal diet within 4 weeks = 1, and tube diet even after 4 weeks = 0).
Epithelialisation was evaluated at 1 month postoperatively (entire wound = 2, nearly entire wound = 1, inadequate = 0).
Scar contracture of the wound was assessed 2 months postoperatively. Changes in mouth opening and mucosal suppleness were taken as the criteria for contracture. Change in mouth opening was assessed by calculating the difference between pre- and postoperative mouth opening (none or little = 2, moderate = 1, and serious = 0). Mucosal suppleness was evaluated by comparing the suppleness of the operated side to the opposite side (similar on both sides = 2, slightly altered = 1, and contracture = 0). The change in site and size of the defects would influence the scar contracture, and hence, it was considered as a confounding factor in this study.
The parameter of epithelialisation was the primary variable in our study, rest of the parameters were secondary variables.
The safety of membrane use was assessed depending on any allergic reaction (no reaction = 2, mild reactions = 1, and reactions requiring intervention = 0).
Finally, the effectiveness was evaluated summing up the total scores of these eight parameters. A score of >15 was considered as very effective, 11–15 points as effective, and <11 points as ineffective.
Statistical analysis was done using Chi-square test. P value remained <0.05 for all the parameters, which is statistically significant.
RESULTS
Fifteen patients were included in our study; 11 (73%) were male and 4 (27%) were female [Table 1]. The age of the patients ranged from 32 to 65 years. Outcomes of the cases treated with lyophilised AM are listed in Table 3. Operability of lyophilised AM was rated as good in all patients. Haemostasis was rated as good in 5 cases and fair in 10 cases. Pain control was recorded as good in 8 cases and fair in 7 cases. Feeding situation was rated as fair in all the patients. Epithelialisation was rated as good in 13 cases and fair in 2 cases. Change in mouth opening was evaluated as none or little in 13 patients, and serious in two patients. Mucosal suppleness was rated as fair in all the patients. No wound infection or allergic reaction was noticed in any of the patients. The lyophilised AM was found to be effective in all patients in the present study.
Table 3.
Outcomes of cases treated with the lyophilised amniotic membrane
| Case | Operability | Haemostasis | Pain | Feeding | Epithelialisation | Scar contracture | Safety | Total | Effectiveness | |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Mouth opening | Mucosal suppleness | |||||||||
| 1 | 2 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 13 | Effective |
| 2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 13 | Effective |
| 3 | 2 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 13 | Effective |
| 4 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 12 | Effective |
| 5 | 2 | 1 | 2 | 1 | 2 | 0 | 1 | 2 | 11 | Effective |
| 6 | 2 | 2 | 2 | 1 | 2 | 0 | 1 | 2 | 12 | Effective |
| 7 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 14 | Effective |
| 8 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 13 | Effective |
| 9 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 12 | Effective |
| 10 | 2 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 12 | Effective |
| 11 | 2 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 11 | Effective |
| 12 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 12 | Effective |
| 13 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 12 | Effective |
| 14 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 14 | Effective |
| 15 | 2 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 13 | Effective |
DISCUSSION
AM has been investigated and found to be beneficial for wound healing in different surgical fields. It facilitates epithelialisation.[4,5,6,7] It has anti-scarring property.[8] Amniotic cells release cytokines including platelet-derived growth factor, vascular endothelial growth factor, angiogenin, transforming growth factor-beta 2 (TGF-b2), and tissue inhibitor of metalloproteinase-1,2.[9] Amniotic cells possess pluripotency and are able to differentiate into adipocytes, osteocytes, chondrocytes, myocytes, cardiomyocytes, hepatocytes, neurocytes, and vascular endothelial cells.[10]
Different methods of preparation, preservation, and sterilisation of AM have been developed over the years and these methods may alter some of its properties. Lyophilised AM is easily available and found to be cost-effective. Lyophilised AM in the dry state is a semitransparent, fragile, paper-thin membrane. We have evaluated the histology of lyophilised AM [Figure 5]. It showed mainly monolayered epithelial cells, basement membrane, and underlying stroma which looks acellular, probably because of preservation procedure.
Figure 5.
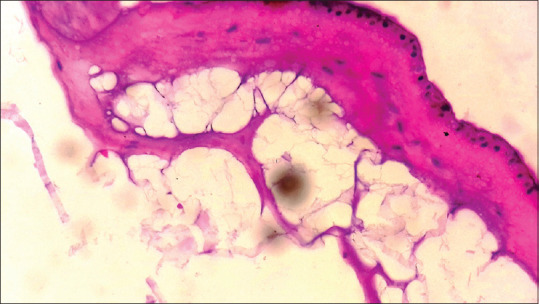
Histology of lyophilised amniotic membrane shows monolayered epithelial cells, basement membrane, and underlying stroma
It was easy to cut and shape with scissors according to the defect size and shape. We have observed that after soaking it in normal saline for 1 min, it thickens and becomes flabby. Once placed over the surgical wound, AM always showed good adherence. The adherence of the AM was thought to be the result of a fibrin–collagen interaction or due to the presence of laminin-5 adhesive glycoprotein.[2,22] However in this study, good adherence of AM with scanty stromal layer suggests that this adhesion is more of a mechanical type than a real chemical interaction. The adhesion of AM prevents wound surface drying, which accelerates wound healing.[13] The antimicrobial effect of AM is also due to their adherence to the wound surface.[14] Relative to autograft, the self-adherent nature of the amnion reduces surgical time.
The haemostatic effect is believed to be the outcome of its antiangiogenic property. However, the pro- or anti-angiogenic effects of AM are controversial.[9] In most of our patients, slight oozing was noticed under the membrane, although it never required any haemostatic measure.
Hajiiski and Anatassov[13] suggested that the adhesion of AM facilitates coverage of the dermal nerve endings which results in significant pain relief in burns. Pain control was fair to good but never nil in our patients.
The observation regarding the changes during epithelialisation is consistent with the findings of Samandari et al.[20] and Kar et al.[22] We observed a white necrotic slough in the 1st week [Figure 3], a slight hyperaemic mucosal tissue in 2nd week, and a completely epithelialised wound after 1 month. The newly epithelialised area appeared smooth and glossy [Figure 3]. The favourable result in epithelialisation is attributable to its properties such as epithelial cell migration,[4] adhesion of basal epithelial cells,[5] promotion of epithelial differentiation,[6] and prevention of epithelial apoptosis.[7] As suggested by the histology [Figure 5], lyophilised AM consists of an intact epithelial layer which may preserve these properties.
Figure 3.
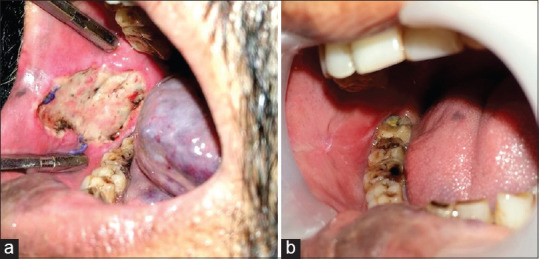
(a) One-week postoperative view showing white necrotic slough (b). Postoperative view after 1 month shows the site of operation resembles surrounding mucosa
Size and site of the defects influenced the scar contracture. In the two cases where the change in mouth opening was evaluated as serious, the lesions were extensive. Therefore, we suggest that lyophilised AM may not prevent scarring for extensive surgical defects. Mucosal suppleness was slightly altered in all the patients. The minimal anti-scarring property of AM is probably due to its downregulation of the TGF-b signaling system and subsequent myofibroblast differentiation.[8]
No infection or allergic reaction was noticed after the application of lyophilised AM. The success rate of AM grafting is attributable to its immunosuppressive property by its ability to suppress T-lymphocyte.[12]
Recent studies have reported encouraging results of AM in temporomandibular joint arthroplasty, bone regeneration, tissue engineering, and cell therapy of cancer.[24,25,26,27,28,29,30,31,32,33,34] In our literature search, we have noticed that only a few comparative studies have reported the use of AM with local flaps or autografts in intraoral surgical defects.[35,35,36,37,38] Mostafa et al. reported the use AM and buccal fat pad in intraoral surgical defects after excision of leukoplakia. They observed that the epithelialisation after 1 month was similar in both groups, but fibrosis was more in the AM group than that of the buccal fat pad group.[35] Babaki et al. reported significantly faster postoperative healing onset with acellular dermal matrix than with cryopreserved human AM on the periosteum.[38]
The lyophilised AM has been found to be effective for the healing of intraoral surgical defects in our study. Our findings regarding its effectiveness in oral wound healing are in accordance with the findings of similar studies conducted on hyperdry and cryopreserved AM.[2,22,39] Although we admit the limitations in this study such as small sample size and lack of control group, the results of this study could serve as an initial report for future studies on the use of lyophilised AM.
CONCLUSION
The lyophilised AM used in this study is found to be distinctively cost-effective. It served as an effective and useful material for immediate coverage of the surgical defects with added benefits from its intact epithelial layer. From our experience, we suggest that this material may not prevent scar contracture for extensive surgical defects, hence favouring its use only for small size defects intraorally.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rastogi S, Modi M, Sathian B. The efficacy of collagen membrane as a biodegradable wound dressing material for surgical defects of oral mucosa:A prospective study. J Oral Maxillofac Surg. 2009;67:1600–6. doi: 10.1016/j.joms.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 2.Arai N, Tsuno H, Okabe M, Yoshida T, Koike C, Noguchi M, et al. Clinical application of a hyperdry amniotic membrane on surgical defects of the oral mucosa. J Oral Maxillofac Surg. 2012;70:2221–8. doi: 10.1016/j.joms.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 3.Danforth D, Hull RW. The microscopic anatomy of the fetal membranes with particular reference to the detailed structure of the amnion. Am J Obstet Gynecol. 1958;75:536–47. doi: 10.1016/0002-9378(58)90610-0. [DOI] [PubMed] [Google Scholar]
- 4.Tseng SC, Prabhasawat P, Lee SH. Amniotic membrane transplantation for conjunctival surface reconstruction. Am J Ophthalmol. 1997;124:765–74. doi: 10.1016/s0002-9394(14)71693-9. [DOI] [PubMed] [Google Scholar]
- 5.Shimazaki J, Shinozaki N, Tsubota K. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br J Ophthalmol. 1998;82:235–40. doi: 10.1136/bjo.82.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo M, Grinnell F. Basement membrane and human epidermal differentiation in vitro . J Invest Dermatol. 1989;93:372–8. [PubMed] [Google Scholar]
- 7.Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–3. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179:325–35. doi: 10.1002/(SICI)1097-4652(199906)179:3<325::AID-JCP10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 9.Koizumi NJ, Inatomi TJ, Sotozono CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000;20:173–7. [PubMed] [Google Scholar]
- 10.Toda A, Okabe M, Yoshida T, Nikaido T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci. 2007;105:215–28. doi: 10.1254/jphs.cr0070034. [DOI] [PubMed] [Google Scholar]
- 11.Miki T. Stem cell characteristics and the therapeutic potential of amniotic epithelial cells. Am J Reprod Immunol. 2018;80:e13003. doi: 10.1111/aji.13003. [DOI] [PubMed] [Google Scholar]
- 12.Ueta M, Kweon MN, Sano Y, Sotozono C, Yamada J, Koizumi N, et al. Immunosuppressive properties of human amniotic membrane for mixed lymphocyte reaction. Clin Exp Immunol. 2002;129:464–70. doi: 10.1046/j.1365-2249.2002.01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hajiiski O, Anatassov N. Amniotic membranes for temporary burn coverage. Ann Burns Fire Disasters. 1990;9:88–92. [Google Scholar]
- 14.Talmi YP, Sigler L, Inge E, Finkelstein Y, Zohar Y. Antibacterial properties of human amniotic membranes. Placenta. 1991;12:285–8. doi: 10.1016/0143-4004(91)90010-d. [DOI] [PubMed] [Google Scholar]
- 15.Stern M. The grafting of preserved amniotic membrane to burned and ulcerated skin surfaces, substituting skin grafts. JAMA. 1913;60:973. [Google Scholar]
- 16.Kogan S, Sood A, Granick MS. Amniotic membrane adjuncts and clinical applications in wound healing:A review of the literature. Wounds. 2018;30:168–73. [PubMed] [Google Scholar]
- 17.Kothary PM. Preliminary report on the use of amniotic membrane as a graft after extensive oropharyngeal surgery. Indian J Med Sci. 1969;23:329. [Google Scholar]
- 18.Zohar Y, Talmi YP, Finkelstein Y, Shvili Y, Sadov R, Laurian N. Use of human amniotic membrane in otolaryngologic practice. Laryngoscope. 1987;97:978–80. [PubMed] [Google Scholar]
- 19.Lai DR, Chen HR, Lin LM, Huang YL, Tsai CC. Clinical evaluation of different treatment methods for oral submucous fibrosis. A 10 year experience with 150 cases. J Oral Pathol Med. 1995;24:402–6. doi: 10.1111/j.1600-0714.1995.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 20.Samandari MH, Yaghmaei M, Ejlali M, Moshref M, Saffar AS. Use of amnion as a graft material in vestibuloplasty:A preliminary report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:574–8. doi: 10.1016/S107921040400006X. [DOI] [PubMed] [Google Scholar]
- 21.Fénelon M, Catros S, Fricain JC. What is the benefit of using amniotic membrane in oral surgery?A comprehensive review of clinical studies. Clin Oral Investig. 2018;22:1881–91. doi: 10.1007/s00784-018-2457-3. [DOI] [PubMed] [Google Scholar]
- 22.Kar IB, Singh AK, Mohapatra PC, Mohanty PK, Misra S. Repair of oral mucosal defects with cryopreserved human amniotic grafts:Prospective clinical study. Int J Oral Maxillofac Surg. 2014;43:1339–44. doi: 10.1016/j.ijom.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Gajiwala AL. Setting up a Tissue Bank in India:The Tata Memorial Hospital Experience. Cell Tissue Bank. 2003;4:193–201. doi: 10.1023/B:CATB.0000007026.00604.97. [DOI] [PubMed] [Google Scholar]
- 24.Akhlaghi F, Hesami N, Rad MR, Nazeman P, Fahimipour F, Khojasteh A. Improved bone regeneration through amniotic membrane loaded with buccal fat pad-derived MSCs as an adjuvant in maxillomandibular reconstruction. J Craniomaxillofac Surg. 2019;47:1266–73. doi: 10.1016/j.jcms.2019.03.030. [DOI] [PubMed] [Google Scholar]
- 25.Etchebarne M, Fricain JC, Kerdjoudj H, Di Pietro R, Wolbank S, Gindraux F, et al. Use of amniotic membrane and its derived products for bone regeneration:A systematic review. Front Bioeng Biotechnol. 2021;9:661332. doi: 10.3389/fbioe.2021.661332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dziedzic DS, Mogharbel BF, Irioda AC, Stricker PE, Perussolo MC, Franco CR, et al. Adipose-derived stromal cells and mineralized extracellular matrix delivery by a human decellularized amniotic membrane in periodontal tissue engineering. Membranes (Basel) 2021;11:606. doi: 10.3390/membranes11080606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fénelon M, Catros S, Meyer C, Fricain JC, Obert L, Auber F, et al. Applications of human amniotic membrane for tissue engineering. Membranes (Basel) 2021;11:387. doi: 10.3390/membranes11060387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guarda-Nardini L, Trojan D, Montagner G, Cogliati E, Bendini M, Manfredini D. Human amniotic membrane positioning in the surgical treatment of temporomandibular joint degenerative disorder. Case Rep Surg. 2019;2019:6037191. doi: 10.1155/2019/6037191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Zhou Z, Wen J, Jiang F, Xia Y. Human amniotic mesenchymal stem cells promote endogenous bone regeneration. Front Endocrinol (Lausanne) 2020;11:543623. doi: 10.3389/fendo.2020.543623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragazzo M, Val M, Montagner G, Trojan D, Fusetti S, Guarda Nardini L. Human amniotic membrane:An improvement in the treatment of Medication-related osteonecrosis of the jaw (MRONJ)?A case-control study. Cell Tissue Bank. 2022;23:129–41. doi: 10.1007/s10561-021-09922-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Niknejad H, Yazdanpanah G, Ahmadiani A. Induction of apoptosis, stimulation of cell-cycle arrest and inhibition of angiogenesis make human amnion-derived cells promising sources for cell therapy of cancer. Cell Tissue Res. 2016;363:599–608. doi: 10.1007/s00441-016-2364-3. [DOI] [PubMed] [Google Scholar]
- 32.Modaresifar K, Azizian S, Zolghadr M, Moravvej H, Ahmadiani A, Niknejad H. The effect of cryopreservation on anti-cancer activity of human amniotic membrane. Cryobiology. 2017;74:61–7. doi: 10.1016/j.cryobiol.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Jafari A, Niknejad H, Rezaei-Tavirani M, Zali H. The biological mechanism involved in anticancer properties of amniotic membrane. Oncol Rev. 2020;14:429. doi: 10.4081/oncol.2020.429. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Jafari A, Rezaei-Tavirani M, Niknejad H, Zali H. Tumor targeting by conditioned medium derived from human amniotic membrane:New insight in breast cancer therapy. Technol Cancer Res Treat. 2021;20:1–12. doi: 10.1177/15330338211036318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mostafa A, Mohammad KA, Khan SR, Ahmed AU. Fresh frozen amniotic membrane and buccal pad of fat for reconstruction of oral mucosal defect after surgical excision of leukoplakia. Pesqui Bras Odontopediatria Clín Integr. 2019;19:1–8. [Google Scholar]
- 36.Chopra R, Bhate K, Gadkari N, Kakodkar P, Kulkarni D. Evaluation and comparison of human chorionic amniotic membrane and platelet-rich fibrin in achieving bone formation and soft tissue healing in extraction sockets indicated for rehabilitation with implants:A preliminary study. Int J Oral Maxillofac Implants. 2021;36:341–5. doi: 10.11607/jomi.8344. [DOI] [PubMed] [Google Scholar]
- 37.Gajul M, Bhate K, Awate S, Kakodkar P, Shah S. Comparative evaluation of the efficacy of wound healing with and without dehydrated human amniotic/chorionic membrane in alveoloplasty:A pilot study. J Korean Assoc Oral Maxillofac Surg. 2021;47:279–85. doi: 10.5125/jkaoms.2021.47.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Babaki D, Khoshsimaybargard M, Yaghoubi S, Gholami M. Comparison of vestibular depth relapse and wound healing after reconstructive preprosthetic surgery using cryopreserved amniotic membrane and acellular dermal matrix –A comparative study. Ann Maxillofac Surg. 2021;11:12–6. doi: 10.4103/ams.ams_322_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fujiwara K, Tsuno H, Okabe M, Yoshida T, Imaue S, Tomihara K, et al. Clinical application of hyperdry amniotic membrane in cleft palate repair. Cleft Palate Craniofac J. 2022 doi: 10.1177/10556656221075937. doi:10.1177/10556656221075937. [DOI] [PubMed] [Google Scholar]


