Abstract
Patient: Male, 63-year-old
Final Diagnosis: Neurosarcoidosis
Symptoms: Blurred vision • diplopia • glare • hematuria • hypertension • hypertropia • proteinuria
Medication: —
Clinical Procedure: MRI • renal biopsy • urinalysis
Specialty: Ophthalmology
Objective:
Rare disease
Background:
Sarcoidosis is a systemic granulomatous disease of unknown cause, often affecting the lungs and lymphatic system. Neurologic manifestations of sarcoidosis, called “neurosarcoidosis”, can present as cranial neuropathies and occur in an isolated fashion or alongside other systemic findings. These findings occur in about 5% to 15% of individuals, and mainly in women between the ages of 30 and 40 years. Within those subsets of patients who develop neurologic manifestations, ocular manifestations occur 13% to 79% of the time. Less common presentations include secondary glaucoma, intermediate or posterior inflammation, or other neuro-ophthalmic findings.
Case Report:
A 63-year-old White man initially presented with blurry vision, acute glaucoma, and other symptoms closely simulating hypertensive retinopathy. He later developed diplopia and was not accurately diagnosed by general ophthalmologists and a retina specialist. Due to the unusual presentation, hypertensive retinopathy was the incorrect initial working diagnosis and the patient continued to develop more severe symptoms. A multidisciplinary approach to patient care through a nephrology referral led to the final diagnosis of neurosarcoidosis. Prompt treatment improved renal function and ocular disturbances.
Conclusions:
Retinal cotton-wool spots, glaucoma, and optic nerve swelling are rare presentations of neurosarcoidosis. Unusual vascular symptoms warrant consideration of all vascular diseases and prompts for collaboration through a multidisciplinary team. This case serves to highlight the importance of sarcoidosis as a differential, even in patients with no previous signs of granulomatous disease, and how a team-based approach between multiple specialties improves accuracy, timeliness, and treatment regimen.
Keywords: Glaucoma, Hypertensive Retinopathy, Neurosarcoidosis, Sarcoidosis
Background
Sarcoidosis is a systemic granulomatous disease of unknown cause, often affecting the lungs and lymphatic system. Its incidence is estimated to be 10.9 in 100 000 in Americans of European ancestry and 35.5 in 100 000 in those with African ancestry [1]. Neurologic manifestations of sarcoidosis, called “neurosarcoidosis,” can present as cranial neuropathies and can either occur in an isolated fashion or alongside other systemic findings [2]. These findings occur in about 5% to 15% of individuals, and mainly in women between the ages of 30 and 40 years. Within the subset of patients with neurologic manifestations, ocular manifestations occur anywhere 13% to 79% of the time. Indeed, ocular findings are often the initial presenting symptom in 20% to 30% of patients with neurosarcoidosis, with the most frequent documented finding being anterior uveitis [3]. Anterior uveitis can also be associated with ocular hypertension and secondary glaucoma [4,5]. Other, less common presentations include secondary glaucoma with a 10-year period prevalence of 9.6%, intermediate or posterior inflammation, neuro-ophthalmic findings with a prevalence of 5% to 38% of cases [6–8], eyelid and surface disease, and lacrimal involvement [3,4]. Similar to with general neurosarcoidosis, women are more likely to develop ocular manifestations than men [3]. The wide swath of presenting symptoms in this granulomatous process can make diagnosis difficult. We report a rare case of neurosarcoidosis in a 63-year-old White man who initially presented with symptoms simulating hypertensive retinopathy and was treated by a multispecialty approach. This case serves to highlight the importance of considering sarcoidosis as a differential even in patients with no previous signs of granulomatous disease, and ultimately how a team-based approach between multiple specialties can improve the path towards proper diagnosis, treatment regimen, and contribute positively to patient education.
Case Report
A 63-year-old White man with a past medical history of hyperlipidemia presented to an ophthalmologist in September 2019 with concerns of increasingly blurry vision and glare for 1 month. His only medication was simvastatin. On presentation, visual acuity in his right eye was 20/50 and 20/100 in his left eye; both could be corrected to 20/20. Intraocular pressures were 33 mm Hg and 38 mm Hg by applanation in each eye, respectively, and external examination was normal except for decreased confrontational fields in the left eye. The slit lamp examination was normal, including a clear anterior chamber and vitreous with open angles by gonioscopy in both eyes. The fundus examination demonstrated mild edema of the optic nerves and scattered cotton-wool spots with congestion of retinal vessels bilaterally. In addition, dot-blot hemorrhages were seen in the right retinal periphery. A preliminary diagnosis of bilateral hypertensive retinopathy with optic nerve swelling was made, and magnetic resonance imaging was ordered to rule out intracranial pathology. Additionally, the patient was started on treatment for new-onset open-angle glaucoma with timolol and latanoprost eye drops, and a retina consult was obtained for retinal ischemic changes.
The magnetic resonance imaging results were normal, with no evidence of any intracranial abnormality or mass. Improvement in disc edema was noted during the retinal consultation, and fluorescein angiography identified delayed transit of dye in the vessels, without leakage in both eyes. The working diagnosis of hypertensive retinopathy was established.
Two months later, the patient developed vertical diplopia worsening on downgaze and was referred to an ophthalmologist specializing in strabismus. A review of the patient’s history disclosed no history of high blood pressure, and the patient displayed apathy concerning his medical condition. Visual acuity and intraocular pressure were normal, with the above treatment. A motility examination identified an 8-diopter right hypertropia, suggesting a fourth cranial nerve palsy. Retinal examination demonstrated persistent cotton-wool spots in both eyes. Owing to the history of transient optic nerve swelling, cotton-wool spots, and new onset diplopia, the ophthalmologist suspected a more advanced vascular process. A blood pressure reading taken in the office read 129/80 mm Hg. Owing to the above findings and diagnostic discrepancies, the patient was referred to a nephrologist for further evaluation of the hypertension diagnosis.
The initial nephrology workup included a 24-h blood pressure monitor, serologic testing, urine sediment analysis, urinalysis, and kidney ultrasound. The 24-h blood pressure monitor revealed an average blood pressure of 126/80 mm Hg. The urinalysis results were remarkable for mild hematuria and proteinuria. The urine protein-creatinine ratio was 0.69 (reference range, less than 0.2), and the albumin-creatinine ratio was 22.4 mg/g (reference range, less than 30.0). A urine sediment analysis showed increased urinary B2-microglobulin of 359 mcg/L (reference range, less than 300 mcg/L). The serum creatinine had increased to 2.73 mg/dL from a baseline reading of 1.51 mg/dL, which was obtained 6 months prior. Due to the rapid decline in kidney function, a serologic workup for secondary causes of acute glomerulonephritis was performed. The results were inconclusive, however, with a positive ANA titer of 1: 640 and low complement 3 of 72.4 mg/ dL. The calcium level was in the reference range at 8.6 mg/dL. Serum and urine protein electrophoresis demonstrated a normal pattern. C-reactive protein (CRP) levels were normal at 0.2. The kidney ultrasound results were unremarkable.
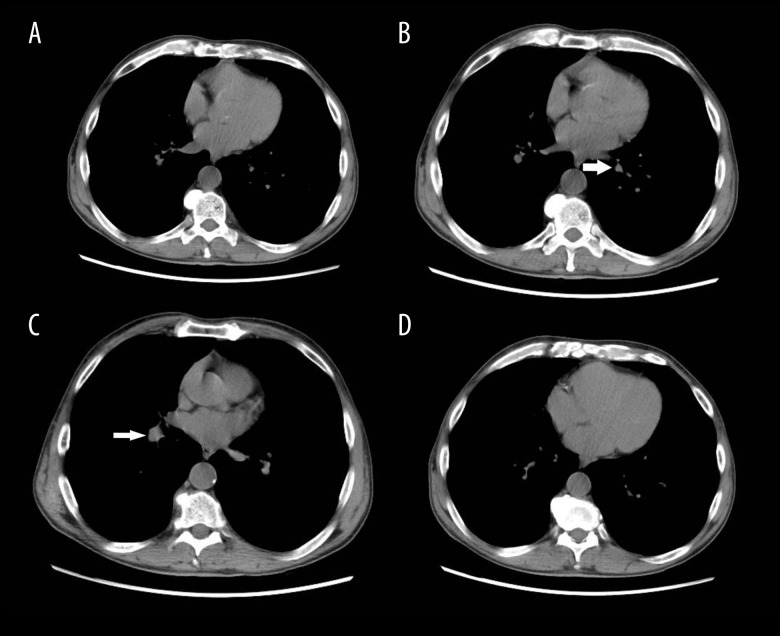
A renal biopsy demonstrated non-caseating granulomatous interstitial nephritis, staining negative for acid fast bacilli and fungi. Based on the biopsy results, the patient’s vitamin D levels were evaluated. The results showed a 1,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 ratio greater than 2 (50 ng/mL: 12.5 ng/mL), indicating extrarenal vitamin D3 production. The angiotensin-converting enzyme level was elevated at 73 U: L (reference range, less than 52 U: L). A chest computed tomography scan disclosed a 4-mm partially calcified nodule in the left lower lobe, a 2.3-cm nodule in the right lung base, and non-specific mediastinal lymphadenopathy (Figure 1).
Figure 1.
Computed tomography chest without contrast. (B) A 4-mm partially calcified nodule in left lower lobe is shown (arrow); (C) a 2.3-cm nodule in right lung base is shown (arrow); (A–D) Non-specific mediastinal lymphadenopathy is shown.
A diagnosis of sarcoidosis was made based upon the renal biopsy findings and the above serological test results. The patient was then immediately started on treatment with oral prednisone 10 mg 4 times a day. Considering the simultaneous neurologic manifestations, including apathy, optic nerve edema, and fourth nerve palsy, the patient was referred to a tertiary center specifically targeting neurosarcoidosis.
Five months later, the patient returned for a follow-up with the nephrologist. His serum creatinine level had improved to 1.72 mg/dL, and the calculated glomerular filtration rate (GFR) had improved from 24 mL/min to 40 mL/min. On ophthalmic assessment, all neurologic symptoms had resolved, although he continued to receive treatment for glaucoma, with normal intraocular pressures of 15 mm Hg and 18 mm Hg, respectively.
Discussion
In this report, a 63-year-old White man with systemic sarcoidosis was correctly diagnosed only when the treating ophthalmologist initiated a multispecialty team approach. The patient’s symptoms of blurry vision brought him to a provider’s office, where the examination soon disclosed significant vascular findings of optic nerve swelling, fourth nerve palsy, and retinal cotton-wool spots. The patient’s apathy, likely secondary to his neurosarcoidosis, as well as the lack of common sarcoid ophthalmic findings allowed his diagnosis and treatment to be delayed. The inaccurate diagnosis of hypertensive retinopathy further delayed treatment. However, that diagnosis did lead to a nephrology consultation and, ultimately, to the correct diagnosis with successful treatment. This case highlights the importance of a multidisciplinary approach in diagnosis and treatment of unusual presentations of any disease.
This patient was an unusual candidate for systemic sarcoidosis for multiple reasons. First, the patient being a White man in his sixties manifesting with neurosarcoidosis was a rare occurrence. According to Rybicki et al, the lifelong risk to develop sarcoidosis is approximately 2.7% for African American women and 2.1% for African American men, but only 1% for White women and even lower at 0.7% for White men [1]. The average age of diagnosis for neurosarcoidosis is between the ages of 33 and 41 years as well; our patient was 2 decades beyond this age group. Furthermore, neurosarcoidosis itself is quite rare, and accounts for only about 5% to 15% of all systemic sarcoidosis cases [9].
Although a patient presenting with visual symptoms is not unusual for sarcoidosis, our patient had atypical ocular findings. The most common ocular presentation for sarcoidosis is anterior uveitis [3]; however, our patient did not have uveitis. If he had, that finding would have triggered a full systemic investigation, including sarcoidosis testing according to the revised International Workshop on Ocular Sarcoidosis (IWOS) criteria for the diagnosis of ocular sarcoidosis (Table 1).
Table 1.
The revised International Workshop on Ocular Sarcoidosis criteria for the diagnosis of ocular sarcoidosis (2017).
Presumed OS: diagnosis not supported by biopsy, but BHL present with two intraocular signs. Probable OS: diagnosis not supported by biopsy and BHL absent, but three intraocular signs and two systemic investigations selected from two to eight are present. |
Reproduced from reference [14] (CC BY-NC-ND 4.0).
Neurosarcoidosis presents with ocular findings only in up to a third of patients [6–8]. Posterior segment involvement in neurosarcoidosis is uncommon and, when identified, commonly manifests as retinal peri-phlebitis. Interestingly, initial examination findings consisted solely of cotton-wool spots and mild optic nerve swelling that ultimately resolved spontaneously. To the best of our knowledge, there have only been 3 other cases reported with cotton-wool spots as an initial clinical finding of sarcoidosis [10].
Finally, this patient’s development of elevated intraocular pressure was not an uncommon finding for ocular sarcoidosis. Previous reports have hypothesized that intraocular pressure rises due to trabecular meshwork dysfunction or obstruction from inflammatory cells. This patient’s lack of anterior inflammation and normal gonioscopy findings was a unique manifestation of his ocular sarcoidosis, without obvious cause [3–5].
There were several limitations to this patient’s case and approach to diagnosis. First, long-term outcomes of neurosarcoidosis have seldom been evaluated, owing to the low prevalence of the disease. Studies encourage early steroid treatment in order to avoid complications, such as polyneuropathies, cranial nerve palsies, myopathy, and central nervous system involvement (Table 2) [10,11]. All of these neurologic processes, left untreated, would prolong the course of the disease. Central nervous system involvement is especially associated with higher morbidity and mortality among presenting patients [12]. Due to the complex and vastly different clinical presentations from patient to patient, neurosarcoidosis has a higher rate of lethality [13]. The lack of typical ophthalmic findings associated with sarcoidosis, which was discussed above, also made diagnosis difficult. Early identification and treatment of neurosarcoidosis is thus critical in the prevention of significant morbidity and mortality.
Table 2.
Table of comparative frequency of clinical neurological manifestations in neurosarcoidosis.
| Neurological features | Frequency | Prognosis* | |
|---|---|---|---|
| Acute | Chornic | ||
| Cranial neuropathy | 50–70% | Good | Good |
| Parenchymal brain lesions | 50% | Fair | Poor |
| Cognitive/behavioral manifestations | 20% | Good | Fair |
| Mentingeal disease | 10–20% | Good | Poor |
| Peripheral neuropathy | 15% | Fair | Fair |
| Seizures** | 5–10% | Good | Good |
| Spinal lesions | 5–10% | Good | Fair |
| Myopathy | 1.4–2.3% | Fair | Poor |
Acute is defined as <3 months; chronic is defined as >3 months.
If there is active brain inflammation seizures may be refractory; if the inflammatory process can be controlled seizures usually respond to standard anti-seizure meditory process can be controlled seizures usually respond to standard anti-seizure medications. Reproduced from reference [15] (CC BY-NC-ND 4.0).
This report highlights the importance of considering sarcoidosis in the differential diagnosis of patients with ophthalmic and neurologic findings, even in the absence of granulomatous disease. Although the lack of recorded ophthalmic imaging on the patient’s initial presentation diminished the effectiveness of this case report slightly, a proper discussion surrounding the symptoms of the presenting illness can still be had. The low prevalence of the illness, along with a growing understanding behind the granulomatous disease, means that patients may present with varied symptoms, depending on how far the disease has progressed.
Furthermore, it is important to confirm that the working diagnosis is thoroughly investigated. The working diagnosis of hypertensive retinopathy in this case could have been quickly eliminated if blood pressure readings were taken at presentation. When the diagnosis and examination findings do not match, a multidisciplinary team approach is critical for appropriate diagnosis. In this case, the consultation between ophthalmology and nephrology teams produced the correct diagnosis.
Conclusions
Neurosarcoidosis, a rare manifestation of systemic sarcoidosis, can occur in an isolated fashion or alongside other, systemic findings. Furthermore, retinal cotton-wool spots, glaucoma, and optic nerve swelling are rare presentations of neurosarcoidosis. Unusual vascular symptoms warrant consideration of all vascular diseases and prompt for collaboration through a multidisciplinary team. This case serves to highlight the importance of sarcoidosis as a differential diagnosis even in patients with no previous signs of granulomatous disease and highlights how a team-based approach between multiple specialties improves accuracy, timeliness, and treatment regimen.
Footnotes
Institution Where Work Was Done
Northeast Ohio Medical University, Ophthalmology, Rootstown, OH, USA.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Rybicki BA, Major M, Popovich J, Jr, et al. Racial differences in sarcoidosis incidence: A 5-year study in a health maintenance organization. Am J Epidemiol. 1997;145(3):234–41. doi: 10.1093/oxfordjournals.aje.a009096. [DOI] [PubMed] [Google Scholar]
- 2.Lacomis D. Neurosarcoidosis. Curr Neuropharmacol. 2011;9(3):429–36. doi: 10.2174/157015911796557975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasadhika S, Rosenbaum JT. Ocular sarcoidosis. Clin Chest Med. 2015;36(4):669–83. doi: 10.1016/j.ccm.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merayo-Lloves J, Power WJ, Rodriguez A, et al. Secondary glaucoma in patients with uveitis. Ophthalmologica. 1999;213(5):300–4. doi: 10.1159/000027443. [DOI] [PubMed] [Google Scholar]
- 5.Mirza S, Saeed T, Murray PI. Ocular hypertension associated with ocular sarcoidosis. Ocul Immunol Inflamm. 2007;15(6):447–49. doi: 10.1080/09273940701732230. [DOI] [PubMed] [Google Scholar]
- 6.Phillips YL, Eggenberger ER. Neuro-ophthalmic sarcoidosis. Curr Opin Ophthalmol. 2010;21(6):423–29. doi: 10.1097/ICU.0b013e32833eae4d. [DOI] [PubMed] [Google Scholar]
- 7.Zajicek JP, Scolding NJ, Foster O, et al. Central nervous system sarcoidosis – diagnosis and management. QJM. 1999;92(2):103–17. doi: 10.1093/qjmed/92.2.103. [DOI] [PubMed] [Google Scholar]
- 8.Baughman RP, Weiss KL, Golnik KC. Neuro-ophthalmic sarcoidosis. Eye Brain. 2012;4:13–25. doi: 10.2147/EB.S29401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prost ME. Retinal Cotton-Wool spots as the first sign of systemic sarcoidosis. Eur J Ophthalmol. 1999;9(3):243–47. doi: 10.1177/112067219900900313. [DOI] [PubMed] [Google Scholar]
- 10.Ferriby D, de Seze J, Stojkovic T, et al. Long-term follow-up of neurosarcoidosis. Neurology. 2001;57(5):927–29. doi: 10.1212/wnl.57.5.927. [DOI] [PubMed] [Google Scholar]
- 11.Chapelon C, Ziza JM, Piette JC, et al. Neurosarcoidosis: Signs, course and treatment in 35 confirmed cases. Medicine. 1990;69(5):261–76. [PubMed] [Google Scholar]
- 12.Oksanen V. Neurosarcoidosis: Clinical presentations and course in 50 patients. Acta Neurol Scand. 2009;73(3):283–90. doi: 10.1111/j.1600-0404.1986.tb03277.x. [DOI] [PubMed] [Google Scholar]
- 13.Blume C, Tuleta I, Nolte K, et al. Neurosarcoidosis as a rare differential diagnosis for single or multiple lesions of the nervous system. Br J Neurosurg. 2020;34(5):495–99. doi: 10.1080/02688697.2018.1506094. [DOI] [PubMed] [Google Scholar]
- 14.Mochizuki M, Smith JR, Takase H, et al. Revised criteria of International Workshop on Ocular Sarcoidosis (IWOS) for the diagnosis of ocular sarcoidosis. Br J Ophthalmol. 2018;103(10):1418–22. doi: 10.1136/bjophthalmol-2018-313356. [DOI] [PubMed] [Google Scholar]
- 15.Stern BJ, Krumholz A, Johns CJ. Neurosarcoidosis. Presentation and management. Ann NY Acad Sci. 1986;465:722–30. doi: 10.1111/j.1749-6632.1986.tb18551.x. [DOI] [PubMed] [Google Scholar]