Abstract
The contraction of cardiac muscle underlying the pumping action of the heart is mediated by the process of excitation-contraction coupling (ECC). While triggered by Ca2+ entry across the sarcolemma during the action potential, it is the release of Ca2+ from the sarcoplasmic reticulum (SR) intracellular Ca2+ store via ryanodine receptors (RyRs) that plays the major role in induction of contraction. Ca2+ also acts as a key intracellular messenger regulating transcription underlying hypertrophic growth. Although Ca2+ release via RyRs is by far the greatest contributor to the generation of Ca2+ transients in the cardiomyocyte, Ca2+ is also released from the SR via inositol 1,4,5-trisphosphate (InsP3) receptors (InsP3Rs). This InsP3-induced Ca2+ release modifies Ca2+ transients during ECC, participates in directing Ca2+ to the mitochondria, and stimulates the transcription of genes underlying hypertrophic growth. Central to these specific actions of InsP3Rs is their localization to responsible signalling microdomains, the dyad, the SR-mitochondrial interface and the nucleus. In this review, the various roles of InsP3R in cardiac (patho)physiology and the mechanisms by which InsP3 signalling selectively influences the different cardiomyocyte cell processes in which it is involved will be presented.
This article is part of the theme issue ‘The cardiomyocyte: new revelations on the interplay between architecture and function in growth, health, and disease’.
Keywords: calcium signalling, cardiomyocyte, excitation contraction coupling, InsP3R, cardiac hypertrophy, calcium microdomains
1. Ca2+ and the heart
Ca2+ is a pleiotropic intracellular messenger controlling key aspects of cardiac biology [1]. Of particular importance is its role in the physiology of the cardiomyocyte, where global increases in its intracellular concentration couple electrical depolarization of the sarcolemma during excitation-contraction coupling (ECC) with contraction [2,3]. Supporting this role in ECC and other cell processes, Ca2+ is taken up into the mitochondria to stimulate metabolism, generate ATP required for contraction and mediate Ca2+ clearance from the cytosol during relaxation. Ca2+ transients underlying contraction are acutely tuned to the cardiovascular needs of the organism, being augmented in amplitude and kinetics under periods of increased sympathetic drive, such as during the fight-or-flight response. Further, and consistent with this role in coupling cardiac output with haemodynamic requirements, via stimulation of gene expression, alterations in Ca2+ induce hypertrophic growth of the heart required for sustained increases in demand. Such hypertrophic growth occurs during developmental growth, pregnancy and during disease processes such as in response to cardiac damage following an infarct. When dysregulated, Ca2+ is involved in cardiomyocyte cell death processes and importantly in cardiac pathologies including in mediating arrhythmic activity and in the reduction in cardiac output during heart failure. The diversity of these functions of Ca2+ in the cardiomyocyte suggests the requirement for complex mechanisms for Ca2+ signal modulation to ensure discrete encoding of its involved cell processes.
2. Inositol 1,4,5-trisphosphate signalling in the heart
During ECC, the cell-wide increase in [Ca2+]i required for induction of contraction is generated by the Ca2+-dependent activation of ryanodine receptors (RyR) Ca2+ release channels located on the sarcoplasmic reticulum (SR) intracellular Ca2+ store by Ca2+ entering the cell through sarcolemmal L-type voltage-gated Ca2+ channels (LTCC) [3,4]. During this process of Ca2+-induced Ca2+ release (CICR), Ca2+ release from the SR dominates over Ca2+ entry by approximately 10 : 1 and is thus primarily responsible for cardiomyocyte contraction. In addition to RyRs, cardiomyocytes also express inositol 1,4,5-trisphosphate receptors (InsP3R) Ca2+ release channels that are also located on the SR Ca2+ store. While RyRs play a central role in the generation of Ca2+ signals underlying ECC, the contribution of Ca2+ release from InsP3Rs to cardiomyocyte physiology is not so clear. In contrast to RyRs, which are activated and inhibited by Ca2+ [5], InsP3Rs require both InsP3 and Ca2+ for full activation [6–8]. As in other cell types, InsP3 is generated in cardiomyocytes via phospholipase C (PLC)-mediated hydrolysis of phosphatidyl inositol 4,5-bisphosphate (PtdIns4,5P2). The mechanism of PLC activation is dependent on the isoform involved. PLCβ isoforms are activated by Gαq following engagement of G-protein coupled receptors (GPCR), PLCγ by recruitment to receptor tyrosine kinases (RTK) and PLCε by Rho, Ras and Rap pathways activated downstream of GPCR and RTK (figure 1a). Cardiomyocytes express a number of GPCRs that respond to an array of locally produced or circulating neurohormones and peptides including α1-adrenoceptors, angiotensin (AT), endothelin and purinergic receptors liganded by catecholamines (norepeinephrine and epinephrine or synthetic α1-AR ligand phenylephrine), angiotensin II (Ang II), endothelin-1 (ET-1) and ATP, respectively [10]. These ligands and their receptors are engaged during both physiology and pathophysiology where they play roles in stress adaptation and tissue remodeling. Despite expression of these receptors in cardiomyocytes, levels of InsP3 produced following their engagement is by comparison with other cell types, relatively low [11]. The effects of receptor engagement may also be long lasting owing to continued activity subsequent to receptor endocytosis [12,13]. Growth factor receptors such as the insulin-like growth factor 1 receptor (IGF1R) (from the family of tyrosine kinase receptors) via activation of GPCRs docked to a pertussis toxin-sensitive heterotetrameric Gi protein, also stimulate the generation of InsP3 upon their engagement [14]. Nuclear anchored PLCε activated downstream of Ras-MAPK and cAMP signalling produces InsP3 locally [15]. Significantly, PLCε may be activated by G12-13-dependent Rho, cAMP-Epac and Ras pathways engaged downstream of ETA receptors, β-adrenoceptors and IGF1Rs, respectively.
Figure 1.
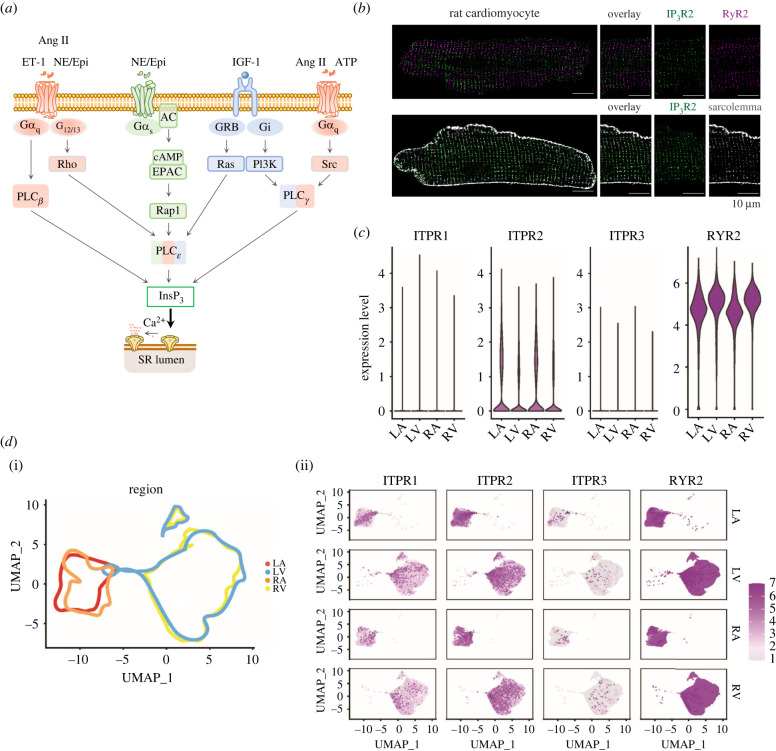
InsP3R expression in the heart. (a) InsP3 is generated by activated phospholipase C (PLC) following the engagement of G-protein coupled receptors (GPCRs) liganded by either angiotensin II (Ang II; angiotensin receptor, AT1), endothelin-1 (ET-1; endothelin receptor, ET receptors), adenosine triphosphate (ATP; purinergic receptors, P2Y), the catecholamines (CA; α and β-adrenoreceptors, α-AR and β-AR) epinephrine (Epi) and norepinephrine (NE) and insulin-like growth factor 1 (IGF-1; IGF-1 receptor, IGF-1R). After diffusion into the cytosol or the nucleus, InsP3 binds to each subunit within the InsP3R tetramer leading to channel opening and release of Ca2+ from intracellular Ca2+ storage sites. (b) InsP3R2 localization relative to RyR2 (top) and t-tubules (bottom). InsP3Rs are stained in green, RyRs are stained in purple and the t-tubules (Caveolin (Cav3)/NCX) are in grey. A 4× zoom of the white square is shown. (c) Log normalized expression of the genes encoding the three inositol 1,4,5-trisphosphate receptors (ITPR1-3) and the gene encoding the type 2 RyR (RYR2) in the cardiomyocyte single nucleus RNA-Seq from each of the four heart chambers. (d) (i) Uniform Manifold Approximation and Projection for dimension reduction (UMAP) embedding of the cardiomyocytes from the four heart chambers including 14 772 nuclei from left atrium (LA), 41 699 nuclei from left ventricle (LV), 8711 nuclei from right atrium (RA) and 30 915 nuclei from right ventricle (RV). These data are from a recent publication by Litviňuková et al. [9], which included transcriptomes of cardiomyocyte nuclei harvested from 14 individuals from two main sources (Harvard Medical School and Wellcome Sanger Institute) and that were processed using Chromium Controller (10× Genomics). Dots representing the nuclei within the UMAP are removed and lines encompassing the nuclei per heart chamber are retained to illustrate the distribution of the nuclei from each heart region relative to other regions in the UMAP. (ii) Distribution of the ITPR
/RyR2 expressing cardiomyocytes across the separated heart regions (colour intensity is binned according to the maximum log normalized value of RyR2 expression).
Expression of all three InsP3R isoforms is reported in cardiomyocytes. The type 2 InsP3R (InsP3R2) is however most prevalent, albeit at varying levels with an approximately sixfold greater abundance in atria than in the ventricles [16,17]. The type 1 InsP3R (InsP3R1) is most abundant in the fetal heart, although both type 1 and 3 InsP3Rs have also been detected in the adult. In line with these previous reports, our interrogation of the cardiomyocyte compartment (identified by their transcriptomes) in a published dataset of single nucleus RNA-sequencing data from different regions of the human heart also show predominance of InsP3R2 in cardiomyocytes from all heart regions (figure 1c–d) [9]. Expression of InsP3R1 is also detected in the heart, especially in the atria albeit at a substantially lower level than InsP3R2, while InsP3R3 is almost absent. The biophysical properties of InsP3R2 make it most appropriate for its function in cardiomyocytes. It exhibits the greatest InsP3 sensitivity of the three isoforms (InsP3R2 > InsP3R1 > InsP3R3) and thus can be activated by the low InsP3 concentrations (10–30 nM) produced following neurohormonal stimulation in cardiomyocytes [11,18]. In contrast to the clear role of RyRs in generating Ca2+ signals during ECC, the function of InsP3Rs in cardiomyocytes is not as defined and is less consistent between studies. This is perhaps not surprising considering the lower level of InsP3R expression and the less obvious contribution of Ca2+ release via these receptors to Ca2+ handling [16,19]. These observations raise the question whether InsP3Rs are required for normal cardiomyocyte function in the adult or whether their existence is simply a vestige of an earlier developmental stage. Indeed, Ca2+ release via InsP3Rs underlies the first heart beat [20–22] and InsP3Rs are more highly expressed during early development than in the adult [23]. Moreover, InsP3Rs are required for compaction of the myocardium and valve formation during development [24,25]. Further, the lack of an overt heart phenotype in InsP3R2 knockout adult mice would suggest that InsP3Rs are not required for the normal physiological function of cardiomyocytes during adulthood [26]. By contrast, in contexts of greater InsP3R2 expression such as in the atria or in the diseased ventricle, where InsP3R expression is heightened, a clearer picture of InsP3R biology is emerging. For example, InsP3R2 knockout protects against ET-1-induced arrhythmias in atrial cardiomyocytes and improves cardiac function in ischemic heart disease respectively [26,27]. Supporting the additional role of InsP3R2 in regulation of cardiac hypertrophy, transgenic mice engineered to selectively overexpress InsP3R2 in cardiomyocytes develop mild hypertrophy and exhibit increased arrhythmias [28,29]. Despite some reports showing a minor or no involvement of InsP3Rs in the actions of neurohormonal stimuli, the weight of evidence would indicate that InsP3Rs contribute to intracellular signalling evoked by neurohormonal stimulation of cardiomyocytes [26,30–32]. Moreover, through localization to subcellular compartments and responsiveness to InsP3 generated in cardiomyocytes, InsP3-induced Ca2+ release (IICR) is now established as a unique signal involved in the regulation of diverse cell functions including ECC, metabolism and gene expression. How InsP3 signalling contributes to, and selectively influences various cell processes in cardiomyocytes is discussed in the following sections.
3. Inositol 1,4,5-trisphosphate in excitation-contraction coupling
By mediating Ca2+ release from the SR, a role for InsP3Rs in ECC can be explained [16]. The contribution of Ca2+ release via this mechanism to ECC is however inconsistent between studies, ranging from no effect to altered dynamics of Ca2+ transients and increased propensity of spontaneous Ca2+ release events [28,31,33–35]. These divergent effects may be ascribed to differences in expression, intracellular localization and activity of InsP3Rs associated with the heart region, animal model and developmental stage. Indeed, in immature [20–22], atrial [32,35–38] and diseased adult ventricular cardiomyocytes [31,39,40] where InsP3R expression is greatest, an influence of InsP3Rs on ECC is consistently observed. Early studies revealed a much greater InsP3R abundance and hence an effect of InsP3-generating stimuli on the contractility of atrial preparations than upon ventricular counterparts [16,36]. To influence ECC, the location of InsP3Rs is important. In both atrial and ventricular cardiomyocytes, InsP3Rs substantially co-locate with junctional RyRs [33,41]. InsP3Rs are thus observed in a striated pattern along the Z-lines coinciding with RyRs. In ventricular cardiomyocytes, owing to the presence of t-tubules (TTs), InsP3Rs are thus located in specialized structures termed dyads [33,42,43]. In atrial cells, while co-located with RyRs along the Z-lines, InsP3Rs are also enriched at sub-sarcolemmal regions, where they co-localise with RyRs [16,41,44].
(a) . Atria
Both type 1 and 2, InsP3Rs are expressed in atrial cardiomyocytes [16,45,46]. In these cells, irrespective of species analysed, InsP3R activation results in increased Ca2+ mobilization from the SR [36–38,47]. Specifically, InsP3R activation leads to an increased amplitude of Ca2+ transients in the sub-sarcolemmal region and in regions distal to the periphery, thereby augmenting the magnitude of cell-wide Ca2+ transient [41,48,49] (figure 2, atria). In addition to effects on the electrically-evoked Ca2+ transient during ECC, activation of InsP3 signalling contributes to an increase in the incidence of extra-systolic Ca2+ elevations and spontaneous contractions in atrial cardiomyocytes exposed to ET-1 and Ang II [36,41,48]. Supporting the involvement of InsP3R2 in these pro-arrhythmic effects of GPCR ligands, spontaneous Ca2+ elevations are absent in atrial cardiomyocytes from InsP3R2 knock-out mice [26]. Underlying and likely contributing to these arrhythmic events induced by GPCR agonists or InsP3 is a substantial increase in occurrence of Ca2+ sparks [16,36,37,41,48]. As a consequence of this increased Ca2+ spark frequency, diastolic Ca2+ levels have also been reported in atrial cardiomyocytes under conditions of GPCR or InsP3 stimulation. The aforementioned effects are particularly pronounced in atrial cardiomyocytes from hypertrophic hearts, in which InsP3R expression is greater [45,46,48,50]. The greater InsP3R abundance may have dual consequences however. While initially, increased InsP3R activity enhances atrial contractility to augment their capacity to propel blood into the ventricle, the constitutive activation of InsP3Rs is deleterious. Specifically, through more frequent spontaneous Ca2+ releases and/or greater SR Ca2+ leak, constitutive activation of InsP3Rs leads to a reduction in SR load with associated suppression of Ca2+ transients as well as to an augmentation of inward Na+/CA2+ exchanger (NCX) current and membrane depolarization, that if of sufficient magnitude can trigger delayed after-depolarization or action potentials (APs) [48]. This increased abundance and activity of InsP3Rs in pathology potentially combines with RyRs sensitized by hyperactive Ca2+/calmodulin-dependent protein kinase II (CaMKII) and protein kinase A (PKA) to create a perfect storm of dysregulated Ca2+ release that generates cell-wide and tissue arrhythmia [51].
Figure 2.
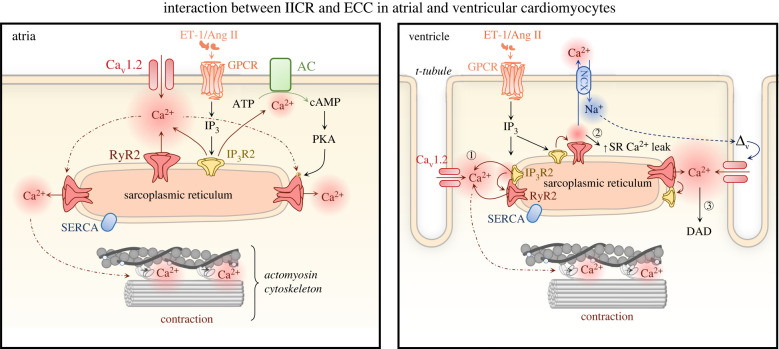
Mechanisms of InsP3-mediated regulation of ECC in atrial and ventricular cardiomyocytes. Atria: GPCRs activated by ET-1 or Ang II produce InsP3 that stimulates Ca2+ release via InsP3 receptors type 1 or 2 (InsP3R1/2). This InsP3 mediated Ca2+ release in turn acts either via priming of proximal RyRs for Ca2+ release or via activation of Ca2+-sensitive adenylyl cyclases (AC1 or AC8) and activation of PKA by cAMP, which then phosphorylates RyRs, modulates Ca2+ transients and hence strength of contraction. Ventricle: Ca2+ release via InsP3Rs facilitates RyR opening and enhances their recruitment during ECC (1). However, the enhanced activity of RyRs leads also to enhanced SR Ca2+ leak (2), which reduces the Ca2+ load in the SR and can lead to activation of NCX. If the SR Ca2+ leak is of sufficient amplitude, via NCX, it can trigger substantial Na+ influx into the cell leading to membrane depolarization manifest as a delayed after-depolarisation (DAD) and potentially AP generation (3). AC, adenylyl cyclase; Ang II, angiotensin II; ATP, adenosine-5′-triphosphate; cAMP, cyclic adenosine monophosphate, Cav1.2, α1C, subunit of voltage-gated L-type calcium channel; DAD, delayed after-depolarizations; ET-1, endothelin 1; GPCR, G protein-coupled receptor; IP3, inositol 1,4,5-trisphosphate; IP3R1/2, inositol trisphosphate receptor type 1/2; NCX, sodium-calcium exchanger; PKA, protein kinase A; RyR2, ryanodine receptor type 2; SERCA, sarco-endoplasmic reticulum Ca2+-ATPase; SR, sarcoplasmic reticulum. (Online version in colour.)
Increasing evidence supports the notion that Ca2+ release via InsP3Rs shapes global Ca2+ transients and thereby atrial cardiomyocyte contractility via functional crosstalk with RyRs, whereby SR Ca2+ flux via InsP3Rs primes proximal RyRs for Ca2+ release [36,40]. Support for this mechanism comes from experiments, where IICR is monitored under conditions of RyR inhibition and from the use of advanced imaging methodologies. A recent study from the Egger group imaged facilitation of RyR opening by a preceding Ca2+ puff (elementary Ca2+ release events through InsP3Rs) [40]. Unlike for RyR-mediated Ca2+ sparks, the direct detection of events solely arising from InsP3Rs is experimentally challenging owing to their smaller magnitude and a lower probability of occurrence. Moreover, conditions under which expression of InsP3Rs is elevated maybe required [33,36,40]. The reports of an absence of discrete events in the presence of RyR inhibition but enhancement of RyR-mediated Ca2+ sparks has led to the coining of the term ‘eventless Ca2+ release via InsP3Rs' [37]. This form of Ca2+ release from the SR is uncovered under conditions of RyR and NCX inhibition preventing Ca2+ extrusion from the cell, thereby allowing Ca2+ released via InsP3Rs to accumulate in the cytosol [52]. The eventless Ca2+ release via InsP3Rs is proposed to recruit neighbouring RyR clusters through increased [Ca2+]i in their vicinity. Besides functional interactions with RyR, IICR has recently been reported to regulate atrial Ca2+ transients through a mechanism involving activation of the Ca2+ stimulated adenylyl cyclases, AC1 and AC8 [44] (figure 2, atria). As for coupling to RyRs, IICR engagement of ACs is possible owing to the proximity of the involved proteins, whereby InsP3Rs are co-localized with AC8 and are in the vicinity of AC1 in the sub-sarcolemmal region. At this location, IICR activation of AC8 or AC1 and consequent generation of cAMP leads to activation of PKA, which in turn affects Ca2+ handling.
(b) . Ventricle
In line with their lower expression in cardiomyocytes of this heart region, the influence of InsP3R on ECC in the healthy ventricle is substantially less than in the atria [34,35]. Despite this low expression however, InsP3Rs elicit a surprisingly potent effect on ECC [28,31,33]. Probably owing to this lower expression, reported effects on ECC are not consistent (table 1). Subsequent analysis of Ca2+ dynamics in cardiomyocytes exposed to InsP3, either introduced through cell permeabilization, via a patch pipette or using a cell permeant form of InsP3—revealed complex effects on Ca2+ handling [33,35,42,48] (figure 2, ventricle). These include increased amplitude of electrically-evoked Ca2+ transient, greater propensity of ectopic Ca2+ elevations and increased frequency of Ca2+ sparks. Notably, the magnitude of the increase in Ca2+ transient amplitude elicited by InsP3 is not substantial, ranging between a 1.2 and 1.5-fold increase [33,48]. As in atrial cardiomyocytes, Gαq-coupled GPCR engagement by ET-1, Ang II or catecholamines represents the physiological mechanism by which InsP3 levels are elevated in ventricular cardiomyocytes. Although these agonists often induce an increase in systolic Ca2+ transient amplitude as well as a positive inotropic response, the contribution of InsP3 signalling to the action of these GPCRs is often variable or not conclusively established. Indeed, InsP3-mediated Ca2+ signals are reported to contribute to the inotropic effects of ET-1 in the rabbit [35], but not in the rat [33,34]. Elsewhere, both in human and mouse cardiomyocytes, activation of the GPCR/InsP3/InsP3R axis causes enhancement of pacing-evoked Ca2+ transients and cell contraction [31]. In these aforementioned studies, however, the contribution of InsP3Rs to the effects of GPCR activation is not fully established, particularly in humans. Such species discrepancies are likely due to the differences in ET, AT or α1-adrenoceptor density and downstream activated signalling pathways. Variability between the effects of agonists may also arise owing to their limited capacity to acutely elevate InsP3 levels and thus an effect on Ca2+ handling is only observed after prolonged exposure to the GPCR agonist.
Table 1.
Differential effect of InsP3 signalling on cardiomyocyte contractility and Ca2+ handling. (Ang II, angiotensin II; ATP, adenosine triphosphate; CaT, Ca2+ transient; CMs, cardiomyocytes; ET-1, endothelin-1; IP3, inositol 1,4,5-trisphosphate; NRVMS, neonatal rat ventricular cardiomyocytes; PE, phenylephrine; SHR, spontaneously hypertensive rat; SR, sarcoplasmic reticulum; WKY, Wistar-Kyoto strain of rat.)
| species | cell type | agonist | observations | reference |
|---|---|---|---|---|
| rat | NRVMs | PE | ↑ frequency of spontaneous CaT in cytosol | [53] |
| IP3 | ↑ Ca2+ spark frequency in cytosol/nucleus | |||
| ↑ number of Ca2+ waves in nucleus | ||||
| mouse | ventricular CMs | Ang II | ↑ CaT | [54] |
| mouse | ventricular CMs | ET-1 | ↑ CaT | [55] |
| human (healthy and failing) | ventricular CMs | ↑ contractility | [31] | |
| ↑ CaT | ||||
| ↑ frequency of extra-systolic Ca2+ elevations | ||||
| ATP | ↑ after-contractions during resting period | |||
| ET-1 | ↑ rare spontaneous/sustained Ca2+ elevations | |||
| ↓ resting membrane potential | ||||
| ↑ duration of the action potential | ||||
| ↑ frequency of early after-depolarization | ||||
| mouse | ventricular CMs | ET-1 | ↑ contractility | |
| ATP | ↑ CaT | |||
| Ang II | ↑ diastolic [Ca2+]i | |||
| PE | ↑ after-contractions and prolonged contractures | |||
| ↑ extra-systolic and sustained Ca2+ elevations | ||||
| ↓ resting membrane potential | ||||
| ↑ duration of the action potential | ||||
| ↑ frequency of early after-depolarization | ||||
| rat (WKY and SHR) | ventricular CMs | IP3 ester | ↑ contractility | [33] |
| ET-1 | ↑ CaT | |||
| ↑ frequency of extra-systolic Ca2+ elevations | ||||
| ↑ rate of rise of CaT | ||||
| ↑ diastolic [Ca2+]i | ||||
| ↑ frequency of Ca2+ sparks in the cytosol | ||||
| rat | ventricular CMs | ET-1 | ↑ CaT | [30] |
| IP3 ester | ↑ frequency of extra-systolic Ca2+ elevations | |||
| rat | ventricular CMs | ET-1 | ↓ contractility (2 min post-stimulation) | [34] |
| ↑ contractility (20 min post-stimulation) | ||||
| ↑ rate of contraction (20 min post-stimulation) | ||||
| ↑ CaT (amplitude; rate of rise) (20 min post-stimulation) | ||||
| rabbit | ventricular CMs | IP3 | ↑ Ca2+ leak in the presence of ruthenium red | [52] |
| rabbit | ventricular CMs | IP3 | ↑ frequency of Ca2+ sparks (immediately observed) | [35] |
| ET-1 | ↓ CaT (2 min post-stimulation) | |||
| ↑ CaT (15 min post-stimulation) | ||||
| mouse (healthy and failing) | ventricular CMs | Ang II | ↑ diastolic [Ca2+]i | [56] |
| ↑ CaT | ||||
| mouse (IP3 overexpression) | ventricular CMs | ET-1 | wild-type: | [28] |
| ↑ CaT | ||||
| ↑ SR Ca2+ load | ||||
| ↑ probability of Ca2+ wave occurrence | ||||
| IP3-overexpression: | ||||
| ↓ probability of Ca2+ wave occurrence sustained SR Ca2+ leak | ||||
| IP3-salt | wild-type: | |||
| ↑ frequency of Ca2+ sparks | ||||
| ↓ SR Ca2+ load | ||||
| IP3-overexpression: | ||||
| ↓ frequency of Ca2+ sparks | ||||
| ↓ SR Ca2+ load | ||||
| no Ca2+ puffs were detected | ||||
| unaltered properties of Ca2+ sparks | ||||
| dog (atrial fibrillation) | atrial CMs | ATP | ↑ CaT | [50] |
| ↑ number of Ca2+ transients | ||||
| rat | atrial CMs | ET-1 | ↓ contractility (4 min post-stimulation) | [49] |
| ↑ contractility (as from 8 min post-stimulation) | ||||
| ↓ CaT (4 min post-stimulation) | ||||
| IP3 ester | ↑ CaT (as from 8 min post-stimulation) | |||
| ↑ frequency of extra-systolic Ca2+ elevations | ||||
| ↑ frequency of Ca2+ sparks | ||||
| rabbit | atrial CMs | ET-1 | ↑ CaT | [32] |
| ↓ decay τ of CaT | ||||
| ↑ time to peak of CaT | ||||
| dog (atrial fibrillation) | atrial CMs | ET-1 | ↑ CaT (in nucleus of diseased animals) | [46] |
| IP3 | ↑ diastolic [Ca2+]i | |||
| rat | atrial CMs | IP3 ester | ↑ CaT | [16] |
| ↑ frequency of Ca2+ sparks | ||||
| ↑ frequency of extra-systolic Ca2+ elevations | ||||
| mouse | atrial CMs | ET-1 | ↑ CaT | [57] |
| ↑ diastolic [Ca2+]i | ||||
| ↑ frequency of Ca2+ sparks | ||||
| ↑ frequency of extra-systolic Ca2+ elevations | ||||
| cat | atrial CMs | ET-1 | ↑ CaT (4 min post-stimulation) | [36] |
| ↑ diastolic [Ca2+]i | ||||
| ↑ frequency of Ca2+ sparks (immediately observed) | ||||
| ↑ frequency of extra-systolic Ca2+ elevations | ||||
| atrial/ventricular CMs | IP3 | atrial CMs: | ||
| ↑ frequency of Ca2+ sparks (immediately | ||||
| adenophostin | observed) | |||
| ↑ diastolic [Ca2+]i | ||||
| ↑ diastolic [Ca2+]i and frequency of Ca2+ puffs in the presence of tetracaine | ||||
| ventricular CMs: | ||||
| ⇔ frequency or properties of Ca2+ sparks | ||||
| cat | atrial CMs | IP3 | ↑ diastolic [Ca2+]i | [58] |
| adenophostin | ↑ frequency of Ca2+ sparks | |||
| cat | atrial CMs | PE | ↑ L-type Ca2+ current | [59] |
| rabbit | atrial CMs | caged-IP3 | ↑ CaT | [38] |
| IP3 ester | ↑ diastolic [Ca2+]i | |||
| ↑ frequency of Ca2+ puffs in the presence of tetracaine | ||||
| rabbit (healthy and failing) | atrial/ventricular CMs | Ang II | failing CMs: | [48] |
| ↑ diastolic [Ca2+]i | ||||
| ↓ CaT | ||||
| ↓ SR Ca2+ load | ||||
| healthy CMs: | ||||
| ↑ diastolic [Ca2+]i | ||||
| ↑ CaT | ||||
| caged-IP3 | failing CMs: | |||
| ↓ CaT (in failing CMs) | ||||
| ↑ diastolic [Ca2+]i | ||||
| healthy CMs: | ||||
| ↑ diastolic [Ca2+]i | ||||
| ↑ CaT | ||||
| tetracaine + IP3 | ↑ frequency of Ca2+ puffs in healthy and failing CMs | |||
| mouse | atrial CMs | ET-1 | ↑ frequency of Ca2+ sparks | [37] |
| ↑ SR Ca2+ leak | ||||
| caged-IP3 | ↑ frequency of Ca2+ sparks | |||
| mouse (IP3 overexpression) | atrial CMs | ET-1 | ↑ frequency of Ca2+ sparks | [40] |
| PE | ↑ occurrence of Ca2+ waves |
IICR subsequent to GPCR stimulation is reported to modulate Ca2+ signalling during ECC either by directly mediating Ca2+ release from the SR or through increasing diastolic [Ca2+]i, thereby facilitating SR Ca2+ release through RyRs [33,35]. By tracking Ca2+ responses at individual dyads with a genetically-encoded Ca2+ indicator targeted to these sites, we recently demonstrated in paced rat ventricular cardiomyocytes that IICR underlies an increased recruitment of dyads and enhanced SR Ca2+ flux at them following ET-1 stimulation [42]. While this effect can be beneficial contributing to acceleration of the Ca2+ transient and robust contraction [42], the sensitization of RyRs by IICR can increase the propensity for spontaneous Ca2+ releases and the potential for arrhythmogenic Ca2+ signals. Indeed, the most consistent effects of IICR observed in ventricular cardiomyocytes across species, including in humans, are induction of arrhythmogenic Ca2+ release [30,31,33,60–62] and increased occurrence of Ca2+ sparks [28,33,35]. Furthermore, eventless InsP3R-dependent Ca2+ release that reduces SR Ca2+ content (via contribution to SR leak) during ET-1 stimulation is also described in ventricular cardiomyocytes of mice [28]. This InsP3-dependent reduction in SR Ca2+ content likely results in diminished contraction but is also proposed to protect against arrhythmias [28].
InsP3R2 expression is often elevated in ventricular cardiomyocytes of hearts undergoing hypertrophic remodeling or that are in heart failure subsequent to pathological stressors, such as those associated with myocardial infarction or pressure overload [31,45,48,63–66]. The increase in InsP3R2 expression parallels that of the re-activated fetal gene programme that is associated with and used as an index of hypertrophic remodeling. Indeed, InsP3R2 expression is higher in neonatal hearts and is downregulated with adult maturation [22]. During disease, this increase in InsP3R expression has been shown to be mediated by the transcription factor NFATc1 [67] and via post-transcriptional regulation by the hypertrophy-associated microRNAs (miRNA) (e.g. miR-133 regulation of InsP3R2 and miRNA-26a of InsP3R1 in ventricular and atrial cardiomyocytes respectively) [46,64]. Exacerbating the effect of increased InsP3R expression in disease and contributing to an increased function, circulating and local levels of neurohormones and expression of their cardiomyocyte cognate receptors are also upregulated with pathology. As a consequence, the impact of InsP3Rs on ECC becomes more important during disease [31,64,65]. Particular effects observed include increased amplitude of systolic Ca2+ transients, elevated diastolic Ca2+ levels, more frequent arrhythmic events, remodeling of resting membrane potential and prolonged duration of the AP [31,33,65]. Notably, the sufficiency of increased InsP3R expression for these effects is demonstrated by the augmented Ca2+ release and arrhythmogenic activity observed in InsP3R2 overexpressing transgenic mice [28,29]. Augmented InsP3 signalling and its generation of elevated diastolic Ca2+ levels is also proposed to contribute to the rhythm disturbances and conduction defects in Chagas disease patients (a disease caused by the parasite Trypansoma cruzi endemic to latin American countries) [68]. While it is not clear whether InsP3R expression is altered in cardiomyocytes from these patients, levels of InsP3 are elevated. Inappropriate InsP3R signalling leading to Ca2+ elevations that do not track the AP-stimulated electrical depolarization of the cardiomyocyte is not in itself sufficient to induce arrhythmias or alter cardiac function. For these ectopic Ca2+ elevations to have a wider pro-arrhythmic effect, the Ca2+ signal must induce a cellular depolarization sufficient to generate an AP that propagates to neighbouring cells. In this regard, interaction between IICR and NCX has been described in which the increase in intracellular Ca2+ generated following InsP3R engagement leads to enhanced forward mode NCX activity, thereby augmenting Na+ entry into the cell and a slow membrane depolarization that increases the propensity for arrhythmic events [31]. Further supporting this notion, InsP3Rs are reported to localize proximally to NCX-enriched domains in the sarcolemma [69].
The almost complete absence or presence of fewer and smaller elementary Ca2+ release events in cardiomyocytes in which RyRs are inhibited with tetracaine supports the limited activity of InsP3Rs as well as their lower capacity to generate Ca2+ signals [28,33,35]. Furthermore, Ca2+ release via RyRs was necessary for the full activation of InsP3Rs [40]. Since InsP3R-mediated elementary events (Ca2+ puffs) arise from clusters of two or more InsP3Rs channels [70], the poorly detectable nature of IICR could suggest that InsP3Rs are not appropriately organized in clusters and are diffusely spread across the SR. This however does not appear to be the case. The suppression of the effects of InsP3R activation by inhibition of RyRs has led to the proposal of a mechanism whereby Ca2+ release via InsP3Rs facilitates RyR opening and enhances their recruitment to generate Ca2+ sparks [28,33,35,42]. Further supporting this conclusion, Ca2+ puffs that contribute to increased frequency of Ca2+ sparks or that trigger RyR opening have been reported [33,40]. The augmentation of RyR-mediated sparks by IICR would require InsP3Rs to lie immediately adjacent to RyR clusters [33]. Supporting this hypothesis, we recently demonstrated the presence of approximately 30% and approximately 50% fraction of InsP3Rs in the dyad and overlapping with RyRs, respectively, thereby enabling Ca2+ signals through InsP3R to influence Ca2+ release via RyR clusters [42]. At the dyads, Ca2+ release via InsP3Rs facilitated RyR activation. This action of IICR is likely mediated in two ways - either by direct activation (by CICR) of immediately adjacent RyRs or through increasing dyadic Ca2+ thereby bringing RyR in this microdomain closer to threshold for activation, which could then be exploited by stochastically opening RyRs to fully engage the cluster and/or to prevent its detrimental shutdown. In disease, this enhanced IICR-RyR crosstalk may serve to rescue the diminished coupling between LTCC and RyRs and the disrupted Ca2+ release due to TT atrophy.
How InsP3Rs are targeted to the dyadic region is not fully resolved but the loss of targeting in Ankyrin-deficient mice would suggest that this protein may be involved [69]. The observed selective increase in dyadic InsP3Rs relative to nuclear InsP3Rs would also suggest that targeting of these two InsP3Rs populations is independently regulated [33]. Alternatively, nuclear InsP3R expression is invariable and maintained at this location via a separate anchoring protein. InsP3Rs were also suggested to lie on regions of the SR devoid of RyRs, perhaps akin to the rogue RyRs proposed to contribute to sparkless leak [71]. This population of InsP3Rs has been proposed to elicit its effect via regulation of NCX and/or membrane potential [31].
4. Inositol 1,4,5-trisphosphate receptors and nuclear Ca2+ regulation
In both atrial and ventricular cardiomyocytes, InsP3 signalling potently affects nuclear Ca2+ levels [32,33]. Indeed, increases in the amplitude and rate of rise as well as a prolongation of the decay phase of the Ca2+ transient is reported in response to stimulation with GPCR agonists or cell-permeant forms of InsP3 [32,39,72]. In the absence of Ca2+ transients, InsP3 promotes nuclear-localized Ca2+ elevations, manifested as increased frequency of nuclear and perinuclear Ca2+ sparks [39,53]. An elevation in basal levels of nuclear Ca2+ also contributes to increased Ca2+-dependent gene expression underlying cardiomyocyte hypertrophy [56].
The relatively potent effect of InsP3 on Ca2+ changes in the nuclear region is likely owing to the greater enrichment of InsP3Rs in this region. The nucleus is bounded by the nuclear envelope that serves to separate the genome from the processes in bulk cytosol. The nuclear envelope is densely populated with nuclear pores that permeate entry of proteins less than 30 kDa, ATP and ions including Ca2+ to the nucleus. Owing to these properties, the nuclear envelope is not generally considered a barrier to Ca2+. As a consequence, cardiomyocyte nuclei are flooded with Ca2+ during every Ca2+ transient. The nuclear envelope is contiguous with the SR that together form the Ca2+ storage compartment of the cardiomyocyte [73]. Furthermore, the nuclear envelope forms invaginations (termed the nucleoplasmic reticulum) that penetrates deep into the nucleoplasm and function as a Ca2+ store capable of releasing and removing Ca2+ owing to Ca2+ release channels and sarco-endoplasmic reticulum Ca2+-ATPase (SERCA) pumps that are localized to it, respectively [56,74]. Owing to these properties of the nuclear envelope, nuclear Ca2+ signals are subject to regulation by both cytoplasmic Ca2+ and local Ca2+ signals originating from RyRs and InsP3Rs [75,76].
Functional and structural evidence indicate that InsP3Rs can mediate the generation of nuclear Ca2+ signals independently of the Ca2+ transients arising owing to ECC [58]. The subcellular localization of InsP3R is a crucial determinants of the generation of highly localized Ca2+ signals that modulate the activity of Ca2+-dependent transcription factors and regulators, which govern the expression of genes underlying hypertrophic remodelling [39,53,57,77,78] (figure 3). While InsP3 was considered a highly diffusible messenger, recent data suggests otherwise, thus raising the importance of proximity of the site of InsP3 generation with its target receptor [79]. In this regard, GPCRs are found to reside on the nuclear membrane and on TTs that penetrate the cytosol close to the nuclear envelope [80–82]. Additionally, InsP3 produced downstream of GPCRs located on the plasma membrane can also diffuse to the nucleus and activate InsP3Rs [11].
Figure 3.
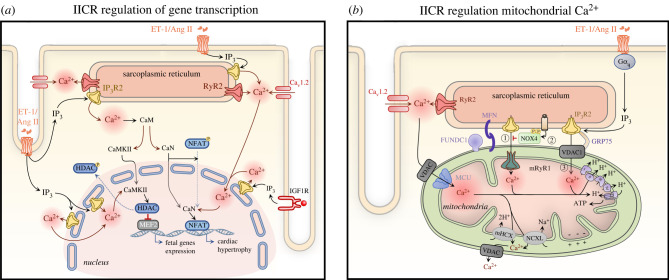
InsP3-mediated signalling in regulation of gene expression and mitochondrial function. (a) Nuclear and cytosolic Ca2+ increases generated by Ca2+ release from InsP3Rs regulate gene expression underlying cardiomyocyte hypertrophic remodelling. Ca2+ released from InsP3Rs binds to calmodulin (CaM), which then activates CaM-dependent kinase II (CaMKII) and calcineurin (CaN). Activated CaMKII phosphorylates the inhibitory factor histone deacetylase (HDAC) and induces its export from the nucleus, resulting in MEF2 de-repression and induction of hypertrophic gene expression. Meanwhile, CaN dephosphorylates the nuclear factor of activated T-cells (NFAT) promoting its nuclear translocation and hypertrophic gene transcription. Ang II, angiotensin II; CaM, calmodulin; CaMKII, Ca2+/calmodulin-dependent protein kinase II; Cav1.2, α1C, subunit of voltage-gated L-type calcium channel; CaN - calcineurin; ET-1, endothelin-1; HDAC, histone deacetylase; IGF1R, insulin-like growth factor 1 receptor; IP3, inositol 1,4,5-trisphosphate; IP3R2, inositol trisphosphate receptor type 2; MEF2, myocyte enhancer factor-2; NFAT, nuclear factor of activated T cells, RyR2, ryanodine receptor type 2. (b) Mitochondrial Ca2+ uptake sites are closely localized to Ca2+ release sites at the junctional SR forming ‘hotspots’ with the help of tethers MFN and FUNDC1. Ca2+ released from the SR via RyRs is taken up via the voltage-gated anion channel (VDAC) associated with the mitochondrial Ca2+ uniporter (MCU) (1). In mitochondria, Ca2+ controls ATP production and apoptosis. Ca2+ is extruded from the mitochondria through Na+/Li+/Ca2+ exchanger (NCXL) and Ca2+/H+ exchanger (mHCX). Upon stimulation of Gαq by ET-1, Ang II or NE, InsP3 activates Ca2+ release from the SR leading to its uptake into the mitochondrial matrix through either VDAC (2) or mRyR1 (4). While Ca2+ transfer via VDAC1—GRP75—InsP3R results in induction of cell apoptosis (2), when taken up through mRyR1 it is associated with increased ATP production (4). To counterbalance InsP3-mediated mitochondrial Ca2+ overload during cellular stress, NOX4 augments the level of active phosphorylated AKT, which in turn phosphorylates and suppresses InsP3Rs thereby inhibiting Ca2+ flux from the SR to mitochondria (3). Akt, protein kinase B; Ang II, angiotensin II; ET-1, endothelin-1; FUNDC1, FUN14 domain-containing protein 1; GRP75, chaperone 75 kDa glucose-regulated protein; MCU, mitochondrial Ca2+ uniporter; MFN, mitofusin; mHCX, mitochondrial Ca2+/H+ exchanger; NCXL, Na+/Li+/Ca2+ exchanger; NE, norepinephrine; NOX4, NADPH oxidase 4; VDAC, voltage-gated anion channel. (Online version in colour.)
InsP3Rs are located on both the inner and outer membrane of the nuclear envelope, as well as in the perinuclear region and on the nucleoplasmic reticulum [38,56,83]. While the expression of InsP3Rs on the inner membrane of the nuclear envelope has been debated for some time, the presence of functional InsP3Rs facing towards the nucleoplasm is now widely accepted. Indeed, using an elegant approach in which a fluorescent immobile Ca2+ buffer was entrapped in the nucleoplasm or in the cytosol, Zima et al. demonstrated InsP3-dependent Ca2+ release into the nucleoplasm [58]. Further supporting this finding, electron microscopy studies show InsP3Rs localized to the inner leaflet of the nuclear envelope [56]. InsP3Rs on the outer surface of the nuclear envelope release Ca2+ into the cytosol that through diffusion may enter the nucleus via nuclear pores [53]. Owing to the lower buffering capacity of the nucleus, diffusion is anisotropic resulting in a greater influence of this Ca2+ release on free Ca2+ levels in the nucleus than in the cytosol [75,84]. Together, these data clearly support the capacity for InsP3R activation to induce alterations in nuclear Ca2+ signalling.
Nucleoplasmic Ca2+ homeostasis is altered in cardiac hypertrophy and failure and is thus considered to be associated with the underlying transcriptional changes. During disease, nuclear volume is increased and the density of nuclear invaginations decreased contributing to elevated nuclear [Ca2+] at diastole and slower kinetics of nuclear Ca2+ transients [46,56]. These effects may in part be owing to the lower surface to volume ratio of the nucleoplasmic reticulum to nucleoplasm, which would reduce the effectiveness of Ca2+ clearance mechanisms. Changes in the expression levels of perinuclear InsP3Rs, RyRs, SERCA and proteins of the nuclear pore complex are also reported, thereby influencing nuclear Ca2+ dynamics [56]. Significantly, upregulation of InsP3Rs (particularly type 1 and 2) appears to be central to the aforementioned changes in nuclear Ca2+ handling and subsequent transcriptional regulation [46,54,56,85,86].
5. Inositol 1,4,5-trisphosphate receptors in transcriptional regulation during cardiac remodelling
Increases in InsP3R expression, as well as the activity of mechanisms responsible for InsP3 generation, are observed in hypertrophy, heart failure and atrial fibrillation.
In addition to influencing ECC, IICR also has a signalling function in the heart, which is involved in stimulating hypertrophic remodelling of cardiomyocytes. The significance of this mechanism is further reinforced by the observation that expression of InsP3R and associated Ca2+ fluxes are upregulated in different animal models of cardiac hypertrophy and in human heart failure. Indeed, in human and animal models of heart failure and atrial fibrillation (AF), the increased expression of InsP3R2 (heart failure) and InsP3R1 (AF) in the nuclear/perinuclear regions was observed and associated elevated nuclear resting Ca2+ levels has been assumed to enhance activity of transcriptional factors that regulate pro-hypertrophic gene expression [46,56]. Given its dominant expression in both atria and the ventricle and the effects observed in gain and loss of function studies, a role for the InsP3R2 in promoting hypertrophic remodelling is supported [29,39,50,56,64]. While less reported, a role in hypertrophy induction for all three InsP3R isoforms in hypertrophic remodelling has been proposed [17]. For instance, InsP3R1 is invoked in driving atrial remodeling during AF [46]. Interestingly, overexpression of the InsP3R2 in the heart was shown to be sufficient for inducing cardiac hypertrophy in transgenic mice that can be further exacerbated by isoproterenol infusion (β-adrenergic stimulation) and exercise stimulation [29]. The activation of nuclear InsP3-induced Ca2+ signalling followed by induction of hypertrophy is also well-established in vitro in response to autocrine/paracrine neuroendocrine factors (ET-1, Ang II, catecholamines and ATP) acting via Gαq/11 and IGF-1 induced Gαi-PLC-InsP3 signalling [39,77,81,87]. While gating of the tetrameric InsP3R requires binding of InsP3 and Ca2+ [7], the further responsiveness of this channel is regulated by post-transcriptional modifications and via association with regulatory proteins [43,88,89], which may also determine, as in the case for the K-Ras associated protein, whether the receptor is susceptible or licensed for activation [90]. In cardiomyocytes, neuronal calcium sensor-1 associates with InsP3Rs enhancing Ca2+ release, leading to the triggering of cardiac hypertrophy through engagement of both CaMKII and calcineurin (CaN) pathways [91]. Chromogranin B, which is a Ca2+ binding protein forming a complex with InsP3R, is also detected in cardiomyocytes, where its expression is upregulated during Ang II-induced hypertrophy [92]. Upon Ca2+ binding chromogranin B modifies the magnitude and velocity of IICR and promotes fetal gene expression via the transcription factor nuclear factor κB [92]. InsP3R2 gating can also be negatively regulated by CaMKII-mediated phosphorylation, thereby providing a feedback mechanism for InsP3R activation [43].
While many studies show a key role of nuclear InsP3R in inducing hypertrophy, the contribution of cytosolically-located InsP3Rs to this process remains to be fully resolved. Ca2+ signals promote gene expression changes required for hypertrophy via modulation of both nuclear and cytosolic Ca2+ which lead to the activation of Ca2+-dependent transcriptional regulatory pathways, including the CaN/nuclear factor of activated T cells (NFAT) and CaMKII/histone deacetylase 4 and 5 (HDAC)/myocyte enhance factor 2 (MEF2) signalling pathway [39,46,77,87]. Although these pathways can be engaged by global changes in Ca2+, this mechanism would not allow the cell to discriminate between changes in Ca2+ involved in contraction, which are enhanced during periods of stress. To ensure only appropriate activation of hypertrophic gene expression, a number of mechanisms have been proposed that allow selective encoding of hypertrophy. These include, alterations in the frequency, amplitude, duration (the duty cycle) and location of the Ca2+ signal. By modifying Ca2+ transients associated with ECC and by having the capacity to influence nuclear Ca2+ in a selective manner, IICR may contribute to regulation of transcription in several ways, which may be required in toto for the maximal effects to be manifest. In support of a mechanism involving the generation of spatially localized and regulated nuclear Ca2+ signalling microdomains independent of cytosolic Ca2+ release, nuclear-specific expression of either Ca2+-buffering proteins or InsP3 chelators abrogate hypertrophic remodelling in isolated cardiomyocytes [39,72]. Further, enhancement of the magnitude of global Ca2+ signals is not sufficient to induce hypertrophy [14,39,46,72,77]. Using a computational modelling approach, Hunt et al. proposed that InsP3R activation in the cytosol drives NFAT nuclear translocation via modulation of the global Ca2+ transient in a way that extends the time when Ca2+ levels are above the threshold required for NFAT activation [93]. Active CaN dephosphorylates and complexes with NFAT in the cytosol, although cardiomyocytes also express CaN in the nucleus [54]. The CaN-NFAT complex then translocates to the nucleus [94,95]. Elevated nuclear Ca2+ levels subsequently maintain the integrity of the CaN-NFAT complex necessary for sustained NFAT dephosphorylation and nuclear residence required for its full transcriptional activity [94,95]. While activation of CaN appears to be InsP3-dependent, the precise mechanism is not yet known [87]. CaN engagement is however activated by sustained local elevations in resting [Ca2+]i [54,96]. In cardiomyocytes, this Ca2+ signal can be produced either via InsP3-mediated increased Ca2+ leak from the SR [37,42] or via induction of store-operated Ca2+ entry [97]. During hypertrophy and heart failure, elevated Ca2+ levels in the nucleus generated via InsP3R2 has been consistently shown, thereby providing a mechanism for sustained CaN/NFAT signalling independent from cytosolic Ca2+ [39,54,56].
Ca2+ release through InsP3R2 can induce hypertrophic gene expression additionally via activation of CaMKIIδB in the nucleus [77]. In mediating atrial remodelling associated with atrial fibrillation, Ca2+ signals arising from InsP3R1 also engages CaMKII. CaMKII regulates transcriptional processes via phosphorylation of transcription factors (e.g. MEF2, CREB, Nkx2–5, GATA4, etc.) and epigenetic regulators and histone deacetylases (e.g. HDAC4, 5, 7 and 9) [98]. Of particular importance to the regulation of cardiac gene expression, CaMKII phosphorylates class II HDACs leading to de-repression of gene expression mediated by the hypertrophy-related transcription factor MEF2 [99]. To bring about this effect, phosphorylation of HDACs by nuclear CaMKIIδB induces association with 14-3-3 proteins, resulting in nuclear export of the complex, while phosphorylation by cytosolic CaMKIIδC blocks HDAC nuclear import [77,100]. To date, the Ca2+ source leading to activation of cytosolic CaMKIIδC in the cardiomyocytes is not fully known. However, a recent study by Qi et al. demonstrated that autophosphorylation of CaMKIIδC was prevented by knockdown of ITPR1 [46].
While cues for physiological and pathological hypertrophy are considered to engage distinct pathways leading to different outcomes, InsP3-mediated Ca2+ signalling has also been shown to be required for the hypertrophic response to IGF-1 stimulation [72]. The downstream effectors of IICR generated downstream of IGF-1-induced PLC activation are however largely unknown. IGF-1 stimulation was shown to activate MEF2C in an InsP3- and nuclear Ca2+-dependent manner [101]. Whether this effect is mediated via activation of CaMKII-HDAC or CaN-NFAT pathways remains to be elucidated.
6. Role of inositol 1,4,5-trisphosphate receptors in the regulation of mitochondrial Ca2+
ECC has a high energetic cost consuming the largest part of ATP produced in cardiomyocytes [3]. The ATP required for cardiac contraction is primarily generated by oxidative phosphorylation in the mitochondria [102,103]. While fatty acids are the major substrate for ATP generation by cardiomyocytes, other sources of energy (e.g. glucose, ketones, amino acids) are also used when available [104]. These alternate sources are particularly used in the ageing or failing heart to compensate for the metabolic insufficiency and loss of ATP generation owing to mitochondrial dysfunction [105,106]. In engaging these alternate energy sources, Ca2+ signalling from InsP3Rs mediates increased cellular glucose uptake in response to insulin stimulation through GLUT4 translocation and fusion with the sarcolemma [107].
Mitochondria accumulate Ca2+ via the voltage-dependent anion channel (VDAC) on the outer mitochondrial membrane [108] and the mitochondrial uniporter located on the inner mitochondrial membrane in a Ca2+-regulated manner [109]. Increased mitochondrial Ca2+ in turn stimulates Ca2+-dependent dehydrogenases involved in oxidative phosphorylation [110,111] (figure 3b(1)). Through this mechanism, intracellular Ca2+ levels are coupled with mitochondrial metabolism. For example, under conditions of increased cytosolic Ca2+ fluxes (exercise, β-adrenergic stimulation), mitochondrial Ca2+ accumulation is enhanced, thereby boosting ATP production to provide for the increased demands of ATP consuming pumps [112]. Under pathological conditions, over-accumulation of Ca2+ within mitochondria leads to activation of programmed cell death pathways and increased oxidative stress [113]. Ca2+ overload brings about this effect through activating the mitochondrial chaperone cyclophilin D (CypD) that induces opening of permeability transition pore (mPTP) [114]. Mitochondrial Ca2+ uptake also contributes to shaping cytosolic Ca2+ dynamics [115,116].
Ca2+ uptake by mitochondria occurs at membrane contact sites known as mitochondrial-associated membranes (MAMs) [117]. In line with other cell types and tissues, SR-mitochondrial Ca2+ flux involving InsP3Rs at MAMs is supported and regulated by association with the VDAC1 and the mitochondrial stress 70 protein (chaperone 75 kDa glucose-regulated protein; GRP75) in a macromolecular complex [114,118] (figure 3b(2)). In this context, the InsP3R1 is invoked and together with VDAC1 are the channels involved in Ca2+ transfer between SR and mitochondria respectively, while GRP75 links both channels through binding to their cytosolically facing regions. The importance of this pathway in the induction of mitochondrial permeability during stress is underlined by the increased interaction between CypD and the InsP3R-GRP75-VDAC1 complex under conditions of greater mitochondrial Ca2+ content [114]. In line with the widely reported involvement of InsP3Rs in endoplasmic reticulum (ER)-mitochondrial signalling and cell death induction, augmented InsP3-mediated Ca2+ fluxes to the mitochondria also have a pro-apoptotic effect in ischaemia-reperfusion (IR) injury [119,120]. During IR injury, this increase in InsP3R-mitochondria transfer is brought about by glycogen synthase kinase 3β (GSK3β) mediated phosphorylation of InsP3R1 [120]. Linking stress and mitochondrial Ca2+ homeostasis and metabolism, mitochondrial Epac1 complex interacts with and promotes InsP3R-GRP75-VDAC1 complex formation under IR conditions, leading to mitochondrial Ca2+ overload and opening of the mPTP [121]. First described in a cancer cell context [122–124], phosphorylation of InsP3Rs by the protein kinase Akt has also been shown to suppress ER-mitochondrial Ca2+ transfer and cell death in the heart. Following IR injury, the abundance of Nox4 at MAMs is increased where it, through generation of reactive oxygen species activates Akt, which in turn phosphorylates and inhibits InsP3Rs [119] (figure 3b(3)). Whether and in what context anti-apoptotic members of the Bcl-2 family of proteins interact with and suppress Ca2+ transfer via InsP3Rs to the mitochondria in cardiomyocytes, as they do in other cell types, remains to be fully explored [125]. By contrast, Seidlmayer et al. reported that Ca2+ released from the SR via InsP3Rs, activated downstream of ET-1 stimulation, is taken up by mitochondria via mitochondrial type 1 RyR (mRyR1) resulting in increased ATP generation in both quiescent and electrically stimulated cells (figure 3b(4)) [126].
Potentially contributing to the diverging effects of InsP3 signalling on mitochondrial function in the heart is the presence of different mitochondrial populations that have distinct roles in cardiac physiology. Particularly, interfibrillar mitochondria, which provide ATP for contraction, subsarcolemmal mitochondria, which provide ATP for active transport processes across the sarcolemma, and perinuclear mitochondria, which generate ATP necessary for nuclear processes [127]. Remarkably, Ca2+ uptake by interfibrillar and perinuclear mitochondria following InsP3-mediated Ca2+ release is twice that taken up by subsarcolemmal mitochondria [127].
Further influencing local Ca2+ delivery to the mitochondria are electron dense physical linkages called tethers. In cardiomyocytes, these tethers comprise either mitofusin 2 (MFN2) [127] or FUN14 domain-containing protein 1 (FUNDC1) [128]. Ablation of either MFN2 or FUNDC1 was shown to disrupt the association between mitochondria and ER/SR, impair Ca2+ uptake into mitochondria and consequently suppress mitochondrial respiration and apoptosis. While both MFN2 and FUNDC1 regulate InsP3R-mitochondria crosstalk by maintaining the integrity of MAMs, FUNDC1 was also shown to directly interact with InsP3R2 and regulate its stability [128]. Remarkably, FUNDC1 expression is reduced in hearts from patients with dilated cardiomyopathy and in mice with acute myocardial infarction [128].
7. Inositol 1,4,5-trisphosphate receptors in cardiac rhythm and conduction
In addition to their role in contractile atrial and ventricular cardiomyocytes, InsP3Rs are also functionally expressed in sino-atrial node (SAN) and Purkinje cells. In these cell types, InsP3R activation contributes to rhythm regulation and AP propagation, which given the influence of these cells on contraction of the myocardium has significant consequences for cardiac function. In SAN and Purkinje cells, InsP3Rs are relatively more abundant than in atrial and ventricular cardiomyocytes. As shown for atrial and ventricular cardiomyocytes, InsP3R activation in both of these cell types is amplified by Ca2+ release via RyRs leading to more substantial consequences for cellular Ca2+ signalling [129,130]. In SAN cells, spontaneous SR Ca2+ release events underlie a Ca2+ clock involved in pacemaker activity [131]. By stimulating the activity of the electrogenic NCX and membrane potential, this intracellular Ca2+ clock interacts with a membrane clock centred on the funny current (If) conveyed by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels [131,132]. Ca2+ release via InsP3R2 increases the frequency of the spontaneous SR Ca2+ release events and Ca2+ waves, which by stimulating NCX activity, accelerates membrane depolarization resulting in the threshold for AP generation being more rapidly reached [129,133]. This potent effect of IICR is lost in mice deficient in InsP3R2 and under conditions of InsP3R inhibition [133]. Further, experiments in knockout mice revealed that augmentation of spontaneous Ca2+ wave frequency (the Ca2+ clock) by IICR was independent of NCX, thereby demonstrating the importance of IICR in heart rhythm regulation [129]. Through this mechanism IICR contributes to the actions of α-adrenergic, as well as other relevant GPCR agonists [44,129,133]. In addition to this mechanism for regulation of pacemaker activity by IICR, a more recent study from Terrar and colleagues proposed a mechanism whereby IICR elicits its effects via cAMP generated through stimulation of proximally located Ca2+-sensitive adenylate cyclases (AC1 and 8) [44]. The cAMP generated acts in turn either directly on HCN channels that underlie the funny current (If), or via PKA and its modulation of the Ca2+ handling machinery [44]. The localization of InsP3Rs to sub-sarcolemmal regions of the SR in the vicinity of sarcolemmal HCN channels makes this mechanism possible [129,133]. In contrast to the mouse studies of Ju et al. and Kapoor et al., baseline pacemaking activity was not influenced by loss of IICR in the murine atrial preparations used by Capel et al. The reason for this discrepancy is not clear but could be owing to preparation (cells versus tissue), or the species studied. In Purkinje cells in which the InsP3R1 is predominant [130,134], InsP3R activation is proposed to contribute to the generation of pathological arrhythmias [135]. This mechanism has been reported in Purkinje cells that survive in the infarcted heart [135]. In these cells, through engagement of RyRs, Ca2+ release via InsP3Rs leads to the generation of Ca2+ waves emanating from the nuclear and sub-sarcolemmal regions of the cell where InsP3R expression is enriched [130]. Through engaging NCX, these Ca2+ waves may then give rise to triggered activity [136].
8. Concluding remarks and future challenges
The diversity of functions that Ca2+ controls in the cardiomyocyte raises a significant problem for its ability to concurrently and specifically regulate them. Key to the overlapping and non-overlapping functions of InsP3 signalling in the heart is the localization of InsP3Rs to cellular Ca2+ microdomains coupled with the involved effectors — the dyad with RyRs, the nucleus with associated transcription factors and their regulators, and at MAMs with mitochondria and the Ca2+ uptake machinery. The capacity for cardiomyocytes to function in the absence of InsP3Rs despite their involvement in multiple cardiomyocyte functions is not clear. Compensation for InsP3R2 by upregulation of other InsP3R isoforms is one possibility. This potential redundancy is however overcome by use of genetically encoded inhibitors of InsP3 signalling such as of the InsP3 5-phosphatase or of a high affinity version of the ligand binding domain of InsP3R1 (InsP3 sponge) [137], which has the added advantage that it may be targeted to subcellular domains of interest. Both the InsP3 sponge and the InsP3 5-phosphatase have been applied to analysis of hypertrophic signalling, and in the case of the InsP3 sponge, proven to be effective in in vivo studies [29,39]. Whether cardiomyocytes tolerate long term expression of these constructs necessary to test the lifelong role of InsP3 signalling, including through cardiac development has not however been determined. To allow acute analysis of the role of InsP3Rs in cardiomyocyte physiology without the cell culture required for adenoviral-mediated expression of InsP3 probes or interfering RNAs or for transgenesis, improved drugs that target the InsP3R are required. For inhibition of InsP3Rs, a toolbox including Heparin [138], Xestospongins B, C and D [139], derived from the marine sponge Xestospongia exigua and 2-aminoethoxydiphenyl borate (2-APB) is relied upon [140,141]. While heparin is an effective antagonist of the InsP3R [138], it is not cell permeant and has effects on RyRs, GPCR coupling and the InsP3 3-kinase [142]. Xestospongins and 2-APB are however cell permeant and can thus be employed in intact cells and tissues. However, these agents have several drawbacks, which should be considered when interpreting studies in which they are used. Xestospongin C for example, in addition to its reported antagonism of the InsP3R, equally suppresses SERCA pump activity leading to Ca2+ store depletion [142]. This effect of Xestospongins can lead to a misinterpretation of data showing a loss of the Ca2+ mobilizing activity of InsP3 or of an InsP3 generating agonist in experiments in which it is applied. Specifically, IICR may be lost owing to store depletion rather than InsP3R inhibition. Xestospongin B was reported to elicit a more selective effect on InsP3R without the off target effects on SERCA [139]. Xestospongin D also lacks effects on SERCA but sensitises Ca2+ release via RyR [143]. In a more recent study, in experiments designed to directly examine IICR release [138], no inhibitory effect of Xestospongin C or Xestospongin D on InsP3Rs was detected, thereby further supporting an indirect mechanism of action for these agents on IICR. Of the InsP3R inhibitors used in cardiomyocytes, 2-APB is most reliable and widely used. Like the aforementioned inhibitors, off target effects of 2-APB on store-operated Ca2+ entry, mitochondria and SERCA pumps have however been reported. Careful titration of 2-APB in cardiomyocytes, showed that when applied at a low concentration of ∼2 µM, selective inhibition of InsP3Rs is achieved with no effects on Ca2+ transients or SR store loading detected [30,33,144]. Caffeine also inhibits InsP3Rs but owing to its potent activation of RyRs has limited use in cardiomyocytes [138]. As a complement to experiments involving InsP3R inhibition, InsP3R may also be activated pharmacologically. To these ends, cell permeant forms of InsP3 or caged derivatives are employed in intact cells and InsP3 salts and caged derivatives introduced via patch pipettes [33,36,37]. The oxidizing agent thimerosol has also been used in cardiomyocytes to induce InsP3R activation [31]. While this mercury based agent may sensitise InsP3Rs, it has multiple other targets including induction of Zn release from cellular stores [145]. The issues raised above highlight the need for future development and application of improved InsP3R probes such as those involving modified versions of InsP3 [146] or of the carbon ring on which it is based [147]. These new tools should be used together with advanced imaging approaches to selectively interrogate the localization and function of InsP3Rs. Development of strategies to selectively modulate InsP3Rs in distinct cellular microdomains and to relocalize InsP3Rs to different cellular microdomains to selectively influence discrete functions may also prove of use — for example to prevent arrhythmogenic Ca2+ signals. Moreover, application of these approaches in large preclinical models of cardiac disease as well as in human cardiomyocytes is necessary to fill our gap in knowledge regarding the role of InsP3R signalling in human pathology.
Acknowledgements
We wish to thank members of the Laboratory of Experimental Cardiology and Profs J. Goldhaber and G. Bultynck for discussion. We also thank Guillaume Gilbert for assistance with figures.
Ethics
Data presented are extracted from a previous publication. As that publication used human samples, ethical permission was required and is quoted in the original publication.
Data accessibility
We have analysed previously published data. The data are freely available in the previous publication.
Authors' contributions
K.D.: conceptualization, visualization, writing—original draft, writing—review and editing; S.E.-T.: investigation, visualization, writing—review and editing; H.L.R.: conceptualization, funding acquisition, project administration, resources, supervision, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Research in the HLR laboratory related to this work is supported by grants from the FWO (Flemish Research Foundation; project grant no. G08861N and Odysseus project no. 90663) and KULeuven (iBOF-21-12 to H.L.R. and PhD funding to K.D.).
References
- 1.Berridge MJ, Bootman MD, Roderick HL. 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517-529. ( 10.1038/NRM1155) [DOI] [PubMed] [Google Scholar]
- 2.Gilbert G, Demydenko K, Dries E, Puertas RD, Jin X, Sipido K, Roderick HL. 2020. Calcium signaling in cardiomyocyte function. Cold Spring Harb. Perspect. Biol. 12, a035428. ( 10.1101/cshperspect.a035428) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bers DM. 2002. Cardiac excitation-contraction coupling. Nature 415, 198-205. ( 10.1038/415198a) [DOI] [PubMed] [Google Scholar]
- 4.Roderick HL, Berridge MJ, Bootman MD. 2003. Calcium-induced calcium release. Curr. Biol. 13, R425. ( 10.1016/S0960-9822(03)00358-0) [DOI] [PubMed] [Google Scholar]
- 5.Ching LL, Williams AJ, Sitsapesan R. 2000. Evidence for Ca2+ activation and inactivation sites on the luminal side of the cardiac ryanodine receptor complex. Circ. Res. 87, 201-206. ( 10.1161/01.RES.87.3.201) [DOI] [PubMed] [Google Scholar]
- 6.Alzayady KJ, Wang L, Chandrasekhar R, Wagner LE II, Van Petegem F, Yule DI. 2016. Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release. Sci Signal. 9, ra35. ( 10.1126/scisignal.aad6281) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor CW, Konieczny V. 2016. IP3 receptors: take four IP3 to open. Sci. Signal. 9, pe 1. ( 10.1126/scisignal.aaf6029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paknejad N, Hite RK. 2018. Structural basis for the regulation of inositol trisphosphate receptors by Ca2+ and IP3. Nat. Struct. Mol. Biol. 25, 660-668. ( 10.1038/s41594-018-0089-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litviňuková M, et al. 2020. Cells of the adult human heart. Nature 588, 466-472. ( 10.1038/s41586-020-2797-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Gareri C, Rockman HA. 2018. G-protein-coupled receptors in heart disease. Circ. Res. 123, 716-735. ( 10.1161/CIRCRESAHA.118.311403) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Remus TP, Zima AV, Bossuyt J, Bare DJ, Martin JL, Blatter LA, Bers DM, Mignery GA. 2006. Biosensors to measure inositol 1,4,5-trisphosphate concentration in living cells with spatiotemporal resolution. J. Biol. Chem. 281, 608-616. ( 10.1074/jbc.M509645200) [DOI] [PubMed] [Google Scholar]
- 12.Hilal-Dandan R, Villegas S, Gonzalez A, Brunton LL. 1997. The quasi-irreversible nature of endothelin binding and g protein-linked signaling in cardiac myocytes. J. Pharmacol. Exp. Ther. 281, 267-273. [PubMed] [Google Scholar]
- 13.Archer CR, Robinson EL, Drawnel FM, Roderick HL. 2017. Endothelin-1 promotes hypertrophic remodelling of cardiac myocytes by activating sustained signalling and transcription downstream of endothelin type A receptors. Cell. Signal. 36, 240-254. ( 10.1016/j.cellsig.2017.04.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibarra C, Estrada M, Carrasco L, Chiong M, Liberona JL, Cardenas C, Díaz-Araya G, Jaimovich E, Lavandero S. 2004. Insulin-like growth factor-1 induces an inositol 1,4,5-trisphosphate-dependent increase in nuclear and cytosolic calcium in cultured rat cardiac myocytes. J. Biol. Chem. 279, 7554-7565. ( 10.1074/jbc.M311604200) [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Malik S, Kelley GG, Kapiloff MS, Smrcka AV. 2011. Phospholipase Cε scaffolds to muscle-specific A kinase anchoring protein (mAKAPβ) and integrates multiple hypertrophic stimuli in cardiac myocytes. J. Biol. Chem. 286, 23 012-23 021. ( 10.1074/jbc.M111.231993) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, Bootman MD. 2000. Functional InsP3 receptors that may modulate excitation – contraction coupling in the heart. Curr. Biol. 10, 939-942. ( 10.1016/s0960-9822(00)00624-2) [DOI] [PubMed] [Google Scholar]
- 17.Garcia MI, Karlstaedt A, Chen JJ, Amione-Guerra J, Youker KA, Taegtmeyer H, Boehning D. 2017. Functionally redundant control of cardiac hypertrophic signaling by inositol 1,4,5-trisphosphate receptors. J. Mol. Cell. Cardiol. 112, 95-103. ( 10.1016/j.yjmcc.2017.09.006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foskett JK, White C, Cheung K-H, Mak D-OD. 2007. Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 87, 593-658. ( 10.1152/physrev.00035.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moschella MC, Marks AR. 1993. Inositol 1,4,5-trisphosphate receptor expression in cardiac myocytes. J. Cell Biol. 120, 1137-1146. ( 10.1083/jcb.120.5.1137) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sasse P, Zhang J, Cleemann L, Morad M, Hescheler J, Fleischmann BK. 2007. Intracellular Ca2+ oscillations, a potential pacemaking mechanism in early embryonic heart cells. J. Gen. Physiol. 130, 133-144. ( 10.1085/jgp.200609575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Méry A, Aimond F, Ménard C, Mikoshiba K, Michalak M, Pucéat M. 2005. Initiation of embryonic cardiac pacemaker activity by inositol 1,4,5-trisphosphate–dependent calcium signaling. Mol. Biol. Cell 16, 2414-2423. ( 10.1091/MBC.E04-10-0883) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schroder EA, Wei Y, Satin J. 2006. The developing cardiac myocyte: maturation of excitability and excitation-contraction coupling. Ann. NY Acad. Sci. 1080, 63-75. ( 10.1196/ANNALS.1380.006) [DOI] [PubMed] [Google Scholar]
- 23.Rosemblit N, Moschella MC, Ondriašová E, Gutstein DE, Ondriaš K, Marks AR. 1999. Intracellular calcium release channel expression during embryogenesis. Dev. Biol. 206, 163-177. ( 10.1006/DBIO.1998.9120) [DOI] [PubMed] [Google Scholar]
- 24.Uchida K, et al. 2010. Gene knock-outs of inositol 1,4,5-trisphosphate receptors types 1 and 2 result in perturbation of cardiogenesis. PLoS ONE 5, e12500. ( 10.1371/journal.pone.0012500) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang F, et al. 2020. Inositol 1,4,5-trisphosphate receptors are essential for fetal-maternal connection and embryo viability. PLoS Genet. 16, e1008739. ( 10.1371/JOURNAL.PGEN.1008739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Zima AV, Sheikh F, Blatter LA, Chen J. 2005. Endothelin-1–induced arrhythmogenic Ca2+ signaling is abolished in atrial myocytes of inositol-1,4,5- trisphosphate (IP3)–receptor type 2–deficient mice. Circ. Res. 96, 1274-1281. ( 10.1161/01.RES.0000172556.05576.4c) [DOI] [PubMed] [Google Scholar]
- 27.Cooley N, et al. 2013. No contribution of IP3-R(2) to disease phenotype in models of dilated cardiomyopathy or pressure overload hypertrophy. Circ. Heart Fail. 6, 318-325. ( 10.1161/CIRCHEARTFAILURE.112.972158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blanch i Salvador J, Egger M. 2018. Obstruction of ventricular Ca2+-dependent arrhythmogenicity by inositol 1,4,5-trisphosphate-triggered sarcoplasmic reticulum Ca2+ release. J. Physiol. 596, 4323-4340. ( 10.1113/JP276319) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama H, et al. 2010. The IP3 receptor regulates cardiac hypertrophy in response to select stimuli. Circ. Res. 107, 659-666. ( 10.1161/CIRCRESAHA.110.220038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proven A, Roderick HL, Conway SJ, Berridge MJ, Horton JK, Capper SJ, Bootman MD. 2006. Inositol 1,4,5-trisphosphate supports the arrhythmogenic action of endothelin-1 on ventricular cardiac myocytes. J. Cell Sci. 119, 3363-3375. ( 10.1242/jcs.03073) [DOI] [PubMed] [Google Scholar]
- 31.Signore S, et al. 2013. Inositol 1, 4, 5-trisphosphate receptors and human left ventricular myocytes. Circulation 128, 1286-1297. ( 10.1161/CIRCULATIONAHA.113.002764) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kockskämper J, Seidlmayer L, Walther S, Hellenkamp K, Maier LS, Pieske B. 2008. Endothelin-1 enhances nuclear Ca2+ transients in atrial myocytes through Ins(1,4,5)P3-dependent Ca2+ release from perinuclear Ca2+ stores. J. Cell Sci. 121, 186-195. ( 10.1242/jcs.021386) [DOI] [PubMed] [Google Scholar]
- 33.Harzheim D, Movassagh M, Foo RS-Y, Ritter O, Tashfeen A, Conway SJ, Bootman MD, Roderick HL. 2009. Increased InsP3Rs in the junctional sarcoplasmic reticulum augment Ca2+ transients and arrhythmias associated with cardiac hypertrophy. Proc. Natl Acad. Sci. USA 106, 11 406-11 411. ( 10.1073/pnas.0905485106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyrnias I, et al. 2018. Contractile responses to endothelin-1 are regulated by PKC phosphorylation of cardiac myosin binding protein-C in rat ventricular myocytes. J. Mol. Cell. Cardiol. 117, 1-18. ( 10.1016/J.YJMCC.2018.02.012) [DOI] [PubMed] [Google Scholar]
- 35.Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. 2008. IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am. J. Physiol. Heart Circ. Physiol. 294, H596-H604. ( 10.1152/ajpheart.01155.2007) [DOI] [PubMed] [Google Scholar]
- 36.Zima AV, Blatter LA. 2004. Inositol-1,4,5-trisphosphate-dependent Ca2+ signalling in cat atrial excitation-contraction coupling and arrhythmias. J. Physiol. 555, 607-615. ( 10.1113/jphysiol.2003.058529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horn T, Ullrich ND, Egger M. 2013. ‘Eventless’ InsP3-dependent SR-Ca2+ release affecting atrial Ca2+ sparks. J. Physiol. 591, 2103-2111. ( 10.1113/jphysiol.2012.247288) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hohendanner F, Maxwell JT, Blatter LA. 2015. Cytosolic and nuclear calcium signaling in atrial myocytes: IP3-mediated calcium release and the role of mitochondria. Channels (Austin) 9, 129-138. ( 10.1080/19336950.2015.1040966) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Higazi DR, et al. 2009. Endothelin-1-stimulated InsP3-induced Ca2+ release is a nexus for hypertrophic signaling in cardiac myocytes. Mol. Cell 33, 472-482. ( 10.1016/j.molcel.2009.02.005) [DOI] [PubMed] [Google Scholar]
- 40.Wullschleger M, Blanch J, Egger M. 2017. Functional local crosstalk of inositol 1, 4, 5- trisphosphate receptor- and ryanodine receptor-dependent Ca2+ release in atrial cardiomyocytes. Cardiovasc. Res. 113, 542-552. ( 10.1093/cvr/cvx020) [DOI] [PubMed] [Google Scholar]
- 41.Mackenzie L, Bootman MD, Berridge MJ, Lipp P. 2001. Predetermined recruitment of calcium release sites underlies excitation-contraction coupling in rat atrial myocytes. J. Physiol. 530, 417-429. ( 10.1111/j.1469-7793.2001.0417k.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demydenko K, Sipido KR, Roderick HL. 2021. Ca2+ release via InsP3Rs enhances RyR recruitment during Ca2+ transients by increasing dyadic [Ca2+] in cardiomyocytes. J. Cell Sci. 134, jcs258671. ( 10.1242/JCS.258671) [DOI] [PubMed] [Google Scholar]
- 43.Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. 2005. Cardiac type 2 inositol 1,4,5-trisphosphate receptor: interaction and modulation by calcium/calmodulin-dependent protein kinase II. J. Biol. Chem. 280, 15 912-15 920. ( 10.1074/jbc.M414212200) [DOI] [PubMed] [Google Scholar]
- 44.Capel RA, Bose SJ, Collins TP, Rajasundaram S, Ayagama T, Zaccolo M, Burton R-AB, Terrar DA. 2021. IP3-mediated Ca2+ release regulates atrial Ca2+ transients and pacemaker function by stimulation of adenylyl cyclases. Am. J. Physiol. Heart Circ. Physiol. 320, H95-107. ( 10.1152/ajpheart.00380.2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamada J, Ohkusa T, Nao T, Ueyama T, Yano M, Kobayashi S, Hamano K, Esato K, Matsuzaki M. 2001. Up-regulation of inositol 1,4,5 trisphosphate receptor expression in atrial tissue in patients with chronic atrial fibrillation. J. Am. Coll. Cardiol. 37, 1111-1119. ( 10.1016/S0735-1097(01)01144-5) [DOI] [PubMed] [Google Scholar]
- 46.Qi X-Y, et al. 2021. Inositol trisphosphate receptors and nuclear calcium in atrial fibrillation. Circ. Res. 128, 619-635. ( 10.1161/CIRCRESAHA.120.317768) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mackenzie L, Bootman MD, Laine M, Berridge MJ, Holmes A, Li W-H, Lipp P. 2002. The role of inositol 1,4,5-trisphosphate receptors in Ca2+ signalling and the generation of arrhythmias in rat atrial myocytes. J. Physiol. 541, 395-409. ( 10.1113/jphysiol.2001.013411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hohendanner F, Walther S, Maxwell JT, Kettlewell S, Awad S, Smith GL, Lonchyna VA, Blatter LA. 2015. Inositol-1,4,5-trisphosphate induced Ca2+ release and excitation-contraction coupling in atrial myocytes from normal and failing hearts. J. Physiol. 593, 1459-1477. ( 10.1113/jphysiol.2014.283226) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bootman MD, Harzheim D, Smyrnias I, Conway SJ, Roderick HL. 2007. Temporal changes in atrial EC-coupling during prolonged stimulation with endothelin-1. Cell Calcium 42, 489-501. ( 10.1016/j.ceca.2007.05.004) [DOI] [PubMed] [Google Scholar]
- 50.Zhao Z-H, Zhang H-C, Xu Y, Zhang P, Li X-B, Liu Y-S, Guo J-H. 2007. Inositol-1,4,5-trisphosphate and ryanodine-dependent Ca2+ signaling in a chronic dog model of atrial fibrillation. Cardiology 107, 269-276. ( 10.1159/000095517) [DOI] [PubMed] [Google Scholar]
- 51.Niggli E, Ullrich ND, Gutierrez D, Kyrychenko S, Poláková E, Shirokova N. 2013. Posttranslational modifications of cardiac ryanodine receptors: Ca2+ signaling and EC-coupling. Biochim. Biophys. Acta 1833, 866-875. ( 10.1016/j.bbamcr.2012.08.016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zima AV, Bovo E, Bers DM, Blatter LA. 2010. Ca2+ spark-dependent and -independent sarcoplasmic reticulum Ca2+ leak in normal and failing rabbit ventricular myocytes. J. Physiol. 588(Pt 23), 4743-4757. ( 10.1113/jphysiol.2010.197913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luo D, et al. 2008. Nuclear Ca2+ sparks and waves mediated by inositol 1,4,5-trisphosphate receptors in neonatal rat cardiomyocytes. Cell Calcium 43, 165-174. ( 10.1016/j.ceca.2007.04.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olivares-Florez S, et al. 2018. Nuclear calcineurin is a sensor for detecting Ca2+ release from the nuclear envelope via IP3R. J. Mol. Med. (Berl). 96, 1239-1249. ( 10.1007/s00109-018-1701-2) [DOI] [PubMed] [Google Scholar]
- 55.Chen M, Xu D, Wu AZ, Kranias E, Lin S-F, Chen P-S, Chen Z. 2018. Phospholamban regulates nuclear Ca2+ stores and inositol 1,4,5-trisphosphate mediated nuclear Ca2+ cycling in cardiomyocytes. J. Mol. Cell. Cardiol. 123, 185-197. ( 10.1016/J.YJMCC.2018.09.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ljubojevic S, et al. 2014. Early remodeling of perinuclear Ca2+ stores and nucleoplasmic Ca2+ signaling during the development of hypertrophy and heart failure. Circulation 130, 244-255. ( 10.1161/CIRCULATIONAHA.114.008927) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luo D, Gao J, Lan X, Wang G, Wei S, Xiao R, Han Q. 2006. Role of inositol 1,4,5-trisphosphate receptors in α1-adrenergic receptor-induced cardiomyocyte hypertrophy. Acta Pharmacol. Sin. 27, 895-900. ( 10.1111/j.1745-7254.2006.00382.x) [DOI] [PubMed] [Google Scholar]
- 58.Zima AV, Bare DJ, Mignery GA, Blatter LA. 2007. IP3-dependent nuclear Ca2+ signalling in the mammalian heart. J. Physiol. 584, 601-611. ( 10.1113/jphysiol.2007.140731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang YG, Dedkova EN, Ji X, Blatter LA, Lipsius SL. 2005. Phenylephrine acts via IP3-dependent intracellular NO release to stimulate L-type Ca2+ current in cat atrial myocytes. J. Physiol. 567, 143-157. ( 10.1113/jphysiol.2005.090035) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woodcock EA, Arthur JF, Matkovich SJ. 2000. Inositol 1,4,5-trisphosphate and reperfusion arrhythmias. Clin. Exp. Pharmacol. Physiol. 27, 734-737. ( 10.1046/j.1440-1681.2000.03326.x) [DOI] [PubMed] [Google Scholar]
- 61.Gilbert JC, Shirayama T, Pappano AJ. 1991. Inositol trisphosphate promotes Na-Ca exchange current by releasing calcium from sarcoplasmic reticulum in cardiac myocytes. Circ. Res. 69, 1632-1639. ( 10.1161/01.res.69.6.1632) [DOI] [PubMed] [Google Scholar]
- 62.Amirahmadi F, Turnbull L, Du XJ, Graham RM, Woodcock EA. 2008. Heightened α1A-adrenergic receptor activity suppresses ischaemia/reperfusion-induced Ins(1,4,5)P3 generation in the mouse heart: a comparison with ischaemic preconditioning. Clin. Sci. (Lond). 114, 157-164. ( 10.1042/CS20070110) [DOI] [PubMed] [Google Scholar]
- 63.Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR. 1995. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J. Clin. Invest. 95, 888-894. ( 10.1172/JCI117739) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drawnel FM, et al. 2012. Mutual antagonism between IP3RII and miRNA-133a regulates calcium signals and cardiac hypertrophy. J. Cell Biol. 199, 783-798. ( 10.1083/jcb.201111095) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harzheim D, Talasila A, Movassagh M, Foo RS, Figg N, Bootman MD, Roderick HL. 2010. Elevated InsP3R expression underlies enhanced calcium fluxes and spontaneous extra-systolic calcium release events in hypertrophic cardiac myocytes. Channels (Austin) 4, 67-71. ( 10.4161/chan.4.1.11537) [DOI] [PubMed] [Google Scholar]
- 66.Jacobsen AN, Du XJ, Dart AM, Woodcock EA. 1997. Ins(1,4,5)P3 and arrhythmogenic responses during myocardial reperfusion: evidence for receptor specificity. Am. J. Physiol. 273, H1119-H1125. ( 10.1152/ajpheart.1997.273.3.H1119) [DOI] [PubMed] [Google Scholar]
- 67.Sankar N, Pieter P, Mignery GA. 2014. Calcineurin-NFATc regulates type 2 inositol 1,4,5- trisphosphate receptor (InsP3R2) expression during cardiac remodeling. J. Biol. Chem. 289, 6188-6198. ( 10.1074/jbc.M113.495242) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mijares A, Espinosa R, Adams J, Lopez JR. 2020. Increases in [IP3]i aggravates diastolic [Ca2+] and contractile dysfunction in Chagas' human cardiomyocytes. PLoS Negl. Trop. Dis. 14, e0008162. ( 10.1371/JOURNAL.PNTD.0008162) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mohler PJ, Davis JQ, Bennett V. 2005. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-Tubule/SR microdomain. PLoS Biol. 3, e423. ( 10.1371/journal.pbio.0030423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dickinson GD, Swaminathan D, Parker I. 2012. The probability of triggering calcium puffs is linearly related to the number of inositol trisphosphate receptors in a cluster. Biophys. J. 102, 1826-1836. ( 10.1016/j.bpj.2012.03.029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kolstad TR, et al. 2018. Ryanodine receptor dispersion disrupts Ca2+ release in failing cardiac myocytes. Elife 7, e39427. ( 10.7554/eLife.39427) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Arantes LAM, et al. 2012. Nuclear inositol 1,4,5-trisphosphate is a necessary and conserved signal for the induction of both pathological and physiological cardiomyocyte hypertrophy. J. Mol. Cell. Cardiol. 53, 475-486. ( 10.1016/j.yjmcc.2012.06.017) [DOI] [PubMed] [Google Scholar]
- 73.Wu X, Bers DM. 2006. Sarcoplasmic reticulum and nuclear envelope are one highly interconnected Ca2+ store throughout cardiac myocyte. Circ. Res. 99, 283-291. ( 10.1161/01.RES.0000233386.02708.72) [DOI] [PubMed] [Google Scholar]
- 74.Echevarria W, Leite MF, Guerra MT, Zipfel WR, Nathanson MH. 2003. Regulation of calcium signals in the nucleus by a nucleoplasmic reticulum. Nat. Cell Biol. 5, 440-446. ( 10.1038/ncb980) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lipp P, Thomas D, Berridge MJ, Bootman MD. 1997. Nuclear calcium signalling by individual cytoplasmic calcium puffs. EMBO J. 16, 7166-7173. ( 10.1093/emboj/16.23.7166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bootman MD, Fearnley C, Smyrnias I, MacDonald F, Roderick HL. 2009. An update on nuclear calcium signalling. J. Cell Sci. 122, 2337-2350. ( 10.1242/JCS.028100) [DOI] [PubMed] [Google Scholar]
- 77.Wu X, et al. 2006. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J. Clin. Invest. 116, 675-682. ( 10.1172/JCI27374) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Molkentin JD. 2006. Dichotomy of Ca2+ in the heart: contraction versus intracellular signaling. J. Clin. Invest. 116, 623-626. ( 10.1172/JCI27824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thillaiappan NB, Chakraborty P, Hasan G, Taylor CW. 2019. IP3 receptors and Ca2+ entry. Biochim. Biophys. Acta Mol. Cell Res. 1866, 1092-1100. ( 10.1016/j.bbamcr.2018.11.007) [DOI] [PubMed] [Google Scholar]
- 80.Bers DM. 2013. Membrane receptor neighborhoods: snuggling up to the nucleus. Circ. Res. 112, 224-226. ( 10.1161/CIRCRESAHA.112.300494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ibarra C, et al. 2013. Local control of nuclear calcium signaling in cardiac myocytes by perinuclear microdomains of sarcolemmal insulin-like growth factor 1 receptors. Circ. Res. 112, 236-245. ( 10.1161/CIRCRESAHA.112.273839) [DOI] [PubMed] [Google Scholar]
- 82.Tadevosyan A, Maguy A, Villeneuve LR, Babin J, Bonnefoy A, Allen BG, Nattel S. 2010. Nuclear-delimited angiotensin receptor-mediated signaling regulates cardiomyocyte gene expression. J. Biol. Chem. 285, 22 338-22 349. ( 10.1074/jbc.M110.121749) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Escobar M, Cardenas C, Colavita K, Petrenko NB, Franzini-Armstrong C. 2011. Structural evidence for perinuclear calcium microdomains in cardiac myocytes. J. Mol. Cell. Cardiol. 50, 451-459. ( 10.1016/j.yjmcc.2010.11.021) [DOI] [PubMed] [Google Scholar]
- 84.Ljubojević S, Walther S, Asgarzoei M, Sedej S, Pieske B, Kockskämper J. 2011. In situ calibration of nucleoplasmic versus cytoplasmic Ca2+ concentration in adult cardiomyocytes. Biophys. J. 100, 2356-2366. ( 10.1016/j.bpj.2011.03.060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tarazón E, et al. 2012. Heart failure induces significant changes in nuclear pore complex of human cardiomyocytes. PLoS ONE 7, e48957. ( 10.1371/journal.pone.0048957) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Plačkić J, Preissl S, Nikonova Y, Pluteanu F, Hein L, Kockskämper J. 2016. Enhanced nucleoplasmic Ca2+ signaling in ventricular myocytes from young hypertensive rats. J. Mol. Cell. Cardiol. 101, 58-68. ( 10.1016/j.yjmcc.2016.11.001) [DOI] [PubMed] [Google Scholar]
- 87.Rinne A, Blatter LA. 2010. Activation of NFATc1 is directly mediated by IP3 in adult cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 299, H1701-H1707. ( 10.1152/ajpheart.00470.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nakao S, Wakabayashi S, Nakamura TY. 2015. Stimulus-dependent regulation of nuclear Ca2+ signaling in cardiomyocytes: a role of neuronal calcium sensor-1. PLoS ONE 10, e0125050. ( 10.1371/journal.pone.0125050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roderick HL, Bootman MD. 2003. Bi-directional signalling from the InsP3 receptor: regulation by calcium and accessory factors. Biochem. Soc. Trans. 31, 950-953. ( 10.1042/BST0310950) [DOI] [PubMed] [Google Scholar]
- 90.Thillaiappan NB, Smith HA, Atakpa-Adaji P, Taylor CW. 2021. KRAP tethers IP3 receptors to actin and licenses them to evoke cytosolic Ca2+ signals. Nat. Commun. 12, 4514. ( 10.1038/s41467-021-24739-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakamura TY, Jeromin A, Mikoshiba K, Wakabayashi S. 2011. Neuronal calcium sensor-1 promotes immature heart function and hypertrophy by enhancing Ca2+ signals. Circ. Res. 109, 512-523. ( 10.1161/CIRCRESAHA.111.248864) [DOI] [PubMed] [Google Scholar]
- 92.Heidrich FM, Zhang K, Estrada M, Huang Y, Giordano FJ, Ehrlich BE. 2008. Chromogranin B regulates calcium signaling, nuclear factor κB activity, and brain natriuretic peptide production in cardiomyocytes. Circ. Res. 102, 1230-1238. ( 10.1161/CIRCRESAHA.107.166033) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hunt H, Bass G, Soeller C, Llewelyn Roderick H, Rajagopal V, Crampin EJ. 2020. Ca2+ release via IP3 receptors shapes cytosolic Ca2+ transients for hypertrophic signalling in ventricular cardiomyocytes. Biophys. J. 119, 1178-1192. ( 10.1016/j.bpj.2020.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhu J, McKeon F. 1999. NF-AT activation requires suppression of Crm1-dependent export by calcineurin. Nature 398, 256-260. ( 10.1038/18473) [DOI] [PubMed] [Google Scholar]
- 95.Hallhuber M, Burkard N, Wu R, Buch MH, Engelhardt S, Hein L, Neyses L, Schuh K, Ritter O. 2006. Inhibition of nuclear import of calcineurin prevents myocardial hypertrophy. Circ. Res. 99, 626-635. ( 10.1161/01.RES.0000243208.59795.d8) [DOI] [PubMed] [Google Scholar]
- 96.Kar P, Mirams GR, Christian HC, Parekh AB. 2016. Control of NFAT isoform activation and NFAT-dependent gene expression through two coincident and spatially segregated intracellular Ca2+ signals. Mol. Cell 649, 746-759. ( 10.1016/j.molcel.2016.11.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ohba T, et al. 2007. Upregulation of TRPC1 in the development of cardiac hypertrophy. J. Mol. Cell. Cardiol. 42, 498-507. ( 10.1016/j.yjmcc.2006.10.020) [DOI] [PubMed] [Google Scholar]
- 98.Dewenter M, von der Lieth A, Katus HA, Backs J. 2017. Calcium signaling and transcriptional regulation in cardiomyocytes. Circ. Res. 121, 1000-1020. ( 10.1161/CIRCRESAHA.117.310355) [DOI] [PubMed] [Google Scholar]
- 99.Kim Y, et al. 2008. The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice. J. Clin. Invest. 118, 124-132. ( 10.1172/JCI33255) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang T, et al. 2007. CaMKIIδ isoforms differentially affect calcium handling but similarly regulate HDAC/MEF2 transcriptional responses. J. Biol. Chem. 282, 35 078-35 087. ( 10.1074/jbc.M707083200) [DOI] [PubMed] [Google Scholar]
- 101.Ibarra C, Vicencio JM, Varas-Godoy M, Jaimovich E, Rothermel BA, Uhlén P, Hill JA, Lavandero S. 2014. An integrated mechanism of cardiomyocyte nuclear Ca2+ signaling. J. Mol. Cell. Cardiol. 75, 40-48. ( 10.1016/j.yjmcc.2014.06.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Territo PR, French SA, Dunleavy MC, Evans FJ, Balaban RS. 2001. Calcium activation of heart mitochondrial oxidative phosphorylation: rapid kinetics of mVO2, NADH, and light scattering. J. Biol. Chem. 276, 2586-2599. ( 10.1074/JBC.M002923200) [DOI] [PubMed] [Google Scholar]
- 103.Territo PR, Mootha VK, French SA, Balaban RS. 2000. Ca2+ activation of heart mitochondrial oxidative phosphorylation: role of the F0/F1-ATPase. Am. J. Physiol. Cell Physiol. 278, C423-C435. ( 10.1152/AJPCELL.2000.278.2.C423) [DOI] [PubMed] [Google Scholar]
- 104.Bing RJ, Siegel A, Ungar I, Gilbert M. 1954. Metabolism of the human heart. II. Studies on fat, ketone and amino acid metabolism. Am. J. Med. 16, 504-515. ( 10.1016/0002-9343(54)90365-4) [DOI] [PubMed] [Google Scholar]
- 105.Tocchi A, Quarles EK, Basisty N, Gitari L, Rabinovitch PS. 2015. Mitochondrial dysfunction in cardiac aging. Biochim. Biophys. Acta 1847, 1424-1433. ( 10.1016/j.bbabio.2015.07.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rosca MG, Hoppel CL. 2013. Mitochondrial dysfunction in heart failure. Heart Fail. Rev. 18, 607-622. ( 10.1007/s10741-012-9340-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Contreras-Ferrat AE, et al. 2010. An inositol 1,4,5-triphosphate (IP3)-IP3 receptor pathway is required for insulin-stimulated glucose transporter 4 translocation and glucose uptake in cardiomyocytes. Endocrinology 151, 4665-4677. ( 10.1210/en.2010-0116) [DOI] [PubMed] [Google Scholar]
- 108.Shimizu H, et al. 2015. Mitochondrial Ca2+ uptake by the voltage-dependent anion channel 2 regulates cardiac rhythmicity. Elife 4, e04801. ( 10.7554/ELIFE.04801) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kirichok Y, Krapivinsky G, Clapham DE. 2004. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360-364. ( 10.1038/NATURE02246) [DOI] [PubMed] [Google Scholar]
- 110.Balaban RS. 2009. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim. Biophys. Acta 1787, 1334-1341. ( 10.1016/J.BBABIO.2009.05.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Denton RM, McCormack JG. 1985. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am. J. Physiol. 249, E543-E554. ( 10.1152/AJPENDO.1985.249.6.E543) [DOI] [PubMed] [Google Scholar]
- 112.Kwong JQ, et al. 2015. The mitochondrial calcium uniporter selectively matches metabolic output to acute contractile stress in the heart. Cell Rep. 12, 15-22. ( 10.1016/J.CELREP.2015.06.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pinton P, Romagnoli A, Rizzuto R, Giorgi C. 2008. Ca2+ signaling, mitochondria and cell death. Curr. Mol. Med. 8, 119-130. ( 10.2174/156652408783769571) [DOI] [PubMed] [Google Scholar]
- 114.Paillard M, et al. 2013. Depressing mitochondria-reticulum interactions protects cardiomyocytes from lethal hypoxia-reoxygenation injury. Circulation 128, 1555-1565. ( 10.1161/CIRCULATIONAHA.113.001225) [DOI] [PubMed] [Google Scholar]
- 115.Rizzuto R, Pozzan T. 2006. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86, 369-408. ( 10.1152/PHYSREV.00004.2005) [DOI] [PubMed] [Google Scholar]
- 116.Drago I, De Stefani D, Rizzuto R, Pozzan T. 2012. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc. Natl Acad. Sci. USA 109, 12 986-12 991. ( 10.1073/PNAS.1210718109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Csordás G, Weaver D, Hajnóczky G. 2018. Endoplasmic reticulum-mitochondrial contact-ology: structure and signaling functions. Trends Cell Biol. 28, 523-540. ( 10.1016/J.TCB.2018.02.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lu F, et al. 2010. Calcium-sensing receptors regulate cardiomyocyte Ca2+ signaling via the sarcoplasmic reticulum-mitochondrion interface during hypoxia/reoxygenation. J. Biomed. Sci. 17, 50. ( 10.1186/1423-0127-17-50) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Beretta M, et al. 2020. Nox4 regulates InsP3 receptor-dependent Ca2+ release into mitochondria to promote cell survival. EMBO J. 39, e103530. ( 10.15252/embj.2019103530) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gomez L, et al. 2016. The SR/ER-mitochondria calcium crosstalk is regulated by GSK3β during reperfusion injury. Cell Death Differ. 23, 313-322. ( 10.1038/cdd.2015.101) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fazal et al. 2017. Multifunctional mitochondrial Epac1 controls myocardial cell death. Circ. Res. 120, 645-657. ( 10.1161/CIRCRESAHA.116.309859) [DOI] [PubMed] [Google Scholar]
- 122.Khan MT, Wagner L, Yule DI, Bhanumathy C, Joseph SK. 2006. Akt kinase phosphorylation of inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 281, 3731-3737. ( 10.1074/JBC.M509262200) [DOI] [PubMed] [Google Scholar]
- 123.Marchi S, Marinello M, Bononi A, Bonora M, Giorgi C, Rimessi A, Pinton P. 2012. Selective modulation of subtype III IP₃R by Akt regulates ER Ca2+ release and apoptosis. Cell Death Dis. 3, e304. ( 10.1038/CDDIS.2012.45) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Szado T, et al. 2008. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc. Natl Acad. Sci. USA 105, 2427-2432. ( 10.1073/PNAS.0711324105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Loncke J, Kaasik A, Bezprozvanny I, Parys JB, Kerkhofs M, Bultynck G. 2021. Balancing ER-mitochondrial Ca2+ fluxes in health and disease. Trends Cell Biol. 31, 598-612. ( 10.1016/J.TCB.2021.02.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Seidlmayer LK, et al. 2016. Inositol 1,4,5-trisphosphate-mediated sarcoplasmic reticulum–mitochondrial crosstalk influences adenosine triphosphate production via mitochondrial Ca2+ uptake through the mitochondrial ryanodine receptor in cardiacmyocytes. Cardiovasc. Res. 112, 491-501. ( 10.1093/cvr/cvw185) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seidlmayer LK, et al. 2019. Mitofusin 2 is essential for IP3-mediated SR/mitochondria metabolic feedback. Front. Physiol. 10, 733. ( 10.3389/fphys.2019.00733) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu S, Lu Q, Wang Q, Ding Y, Ma Z, Mao X, Huang K, Xie Z, Zou M-H. 2017. Binding of FUN14 domain containing 1 with inositol 1,4,5-trisphosphate receptor in mitochondria-associated endoplasmic reticulum membranes maintains mitochondrial dynamics and function in hearts in vivo. Circulation 136, 2248-2266. ( 10.1161/CIRCULATIONAHA.117.030235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kapoor N, Tran A, Kang J, Zhang R, Philipson KD, Goldhaber JI. 2015. Regulation of calcium clock-mediated pacemaking by inositol-1,4,5-trisphosphate receptors in mouse sinoatrial nodal cells. J. Physiol. 593, 2649-2663. ( 10.1113/JP270082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hirose M, Stuyvers B, Dun W, ter Keurs H, Boyden PA. 2008. Wide long lasting perinuclear Ca2+ release events generated by an interaction between ryanodine and IP3 receptors in canine Purkinje cells. J. Mol. Cell. Cardiol. 45, 176-184. ( 10.1016/j.yjmcc.2008.05.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yaniv Y, Lakatta EG, Maltsev VA. 2015. From two competing oscillators to one coupled-clock pacemaker cell system. Front. Physiol. 6, 28. ( 10.3389/fphys.2015.00028) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tsutsui K, et al. 2018. A coupled-clock system drives the automaticity of human sinoatrial nodal pacemaker cells. Sci. Signal. 11, eaap7608. ( 10.1126/SCISIGNAL.AAP7608) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ju Y-K, Liu J, Lee BH, Lai D, Woodcock EA, Lei M, Cannell MB, Allen DG. 2011. Distribution and functional role of inositol 1,4,5-trisphosphate receptors in mouse sinoatrial node. Circ. Res. 109, 848-857. ( 10.1161/CIRCRESAHA.111.243824) [DOI] [PubMed] [Google Scholar]
- 134.Gorza L, Schiaffino S, Volpe P. 1993. Inositol 1,4,5-trisphosphate receptor in heart: evidence for its concentration in Purkinje myocytes of the conduction system. J. Cell Biol. 121, 345-353. ( 10.1083/jcb.121.2.345) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boyden PA, Dun W, Barbhaiya C, ter Keurs HEDJ. 2004. 2APB- and JTV519(K201)-sensitive micro Ca2+ waves in arrhythmogenic Purkinje cells that survive in infarcted canine heart. Heart Rhythm 1, 218-226. ( 10.1016/J.HRTHM.2004.03.068) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stuyvers BD, Dun W, Matkovich S, Sorrentino V, Boyden PA, Ter Keurs HEDJ. 2005. Ca2+ sparks and waves in canine Purkinje cells: a triple layered system of Ca2+ activation. Circ. Res. 97, 35-43. ( 10.1161/01.RES.0000173375.26489.FE) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Uchiyama T, Yoshikawa F, Hishida A, Furuichi T, Mikoshiba K. 2002. A novel recombinant hyperaffinity inositol 1,4,5-trisphosphate (IP3) absorbent traps IP3, resulting in specific inhibition of IP3-mediated calcium signaling. J. Biol. Chem. 277, 8106-8113. ( 10.1074/jbc.M108337200) [DOI] [PubMed] [Google Scholar]
- 138.Saleem H, Tovey SC, Molinski TF, Taylor CW. 2014. Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br. J. Pharmacol. 171, 3298-3312. ( 10.1111/BPH.12685) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jaimovich E, Mattei C, Liberona JL, Cardenas C, Estrada M, Barbier J, Debitus C, Laurent D, Molgó J. 2005. Xestospongin B, a competitive inhibitor of IP3-mediated Ca2+ signalling in cultured rat myotubes, isolated myonuclei, and neuroblastoma (NG108-15) cells. FEBS Lett. 579, 2051-2057. ( 10.1016/J.FEBSLET.2005.02.053) [DOI] [PubMed] [Google Scholar]
- 140.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 1997. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 122, 498-505. ( 10.1093/OXFORDJOURNALS.JBCHEM.A021780) [DOI] [PubMed] [Google Scholar]
- 141.Bultynck G, Sienaert I, Parys JB, Callewaert G, De Smedt H, Boens N, Dehaen W, Missiaen L. et al. 2003. Pharmacology of inositol trisphosphate receptors. Pflugers Arch. 445, 629-642. ( 10.1007/S00424-002-0971-1) [DOI] [PubMed] [Google Scholar]
- 142.De Smet P, Parys JB, Callewaert G, Weidema AF, Hill E, De Smedt H, Erneux C, Sorrentino V, Missiaen L. 1999. Xestospongin C is an equally potent inhibitor of the inositol 1,4,5-trisphosphate receptor and the endoplasmic-reticulum Ca2+ pumps. Cell Calcium 26, 9-13. ( 10.1054/ceca.1999.0047) [DOI] [PubMed] [Google Scholar]
- 143.Ta TA, Feng W, Molinski TF, Pessah IN. 2006. Hydroxylated xestospongins block inositol-1,4,5-trisphosphate-induced Ca2+ release and sensitize Ca2+-induced Ca2+ release mediated by ryanodine receptors. Mol. Pharmacol. 69, 532-538. ( 10.1124/MOL.105.019125) [DOI] [PubMed] [Google Scholar]
- 144.Peppiatt CM, Collins TJ, Mackenzie L, Conway SJ, Holmes AB, Bootman MD, Berridge MJ, Seo JT, Roderick HL. 2003. 2-aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell Calcium 34, 97-108. ( 10.1016/S0143-4160(03)00026-5) [DOI] [PubMed] [Google Scholar]
- 145.Haase H, Hebel S, Engelhardt G, Rink L. 2009. Zinc ions cause the thimerosal-induced signal of fluorescent calcium probes in lymphocytes. Cell Calcium 45, 185-191. ( 10.1016/J.CECA.2008.09.003) [DOI] [PubMed] [Google Scholar]
- 146.Bello D, Aslam T, Bultynck G, Slawin AMZ, Roderick HL, Bootman MD, Conway SJ. 2007. Synthesis and biological action of novel 4-position-modified derivatives of D-myo-inositol 1,4,5-trisphosphate. J. Org Chem. 72, 5647-5659. ( 10.1021/jo070611a) [DOI] [PubMed] [Google Scholar]
- 147.Shipton ML, Riley AM, Rossi AM, Brearley CA, Taylor CW, Potter BVL. 2020. Both d- and l-glucose polyphosphates mimic d- myo-inositol 1,4,5-trisphosphate: new synthetic agonists and partial agonists at the Ins(1,4,5)P3 receptor. J. Med. Chem. 63, 5442-5457. ( 10.1021/ACS.JMEDCHEM.0C00215) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We have analysed previously published data. The data are freely available in the previous publication.