Abstract
Very rarely, vasoactive intestinal peptide-related diarrhea (VIP-D) is observed in patients with high-risk neuroblastoma (HR-NB) where the associated fluid and electrolyte abnormalities can pose a major clinical challenge for administering the required aggressive multimodality treatment. Two patients with HR-NB developed VIP-D during induction and were found to have a somatic BRAF V600E mutation. Serum VIP levels and diarrhea promptly resolved in both patients after initiating treatment with BRAF and MEK inhibitors. This illustrates an association of VIP-D with BRAF V600E mutations and demonstrates a therapeutic strategy in the specific context of VIP-D and BRAF V600E mutations in HR-NB patients. The addition of BRAF and MEK inhibitors allows continued conventional tumor-directed treatment by decreasing the severity of symptoms caused by this life-threatening complication.
Keywords: MYCN-amplified neuroblastoma, vasoactive intestinal peptide, paraneoplastic syndrome, BRAF V600E mutation
INTRODUCTION
Neuroblastoma (NB) is the most common extracranial solid tumor in children.[1] Up to 3% of patients present with a paraneoplastic syndrome characterized by excessive production of vasoactive intestinal peptide (VIP), which causes secretory diarrhea (VIP-D).[1,2] These patients typically have low-risk, localized disease, and resection is curative of the neoplasm and of the VIP-D.[1,2] With high-risk neuroblastoma (HR-NB), VIP-D is exceedingly rare and emerges during induction chemotherapy, suggesting that VIP is secreted by treatment-induced differentiated NB.[3,4] Two patients with HR-NB developed chemotherapy-related VIP-D that was successfully treated with oral tyrosine kinase inhibitors (TKIs) targeting the BRAF V600E mutation in their tumors complementing conventional treatment for their HR-NB.
METHODS
After obtaining IRB approval, medical records on all patients with NB seen at Memorial Sloan Kettering Cancer Center (MSKCC) between 1998–2020 with VIP-D and/or somatic BRAF mutations identified by a DataLine search were reviewed. Of the seven patients with VIP-D, three had somatic BRAF V600E mutations including the two discussed in the case reports below. Four patients with VIP-D at presentation did not have BRAF mutations. An additional six patients had BRAF mutations without elevated VIP levels or VIP-D (Table 1).
TABLE 1.
Patients with BRAF-mutated neuroblastoma or VIP-D without BRAF mutation treated at Memorial Sloan Kettering Cancer Center from 2002–2019.
| Patient | Age at Diagnosis of NB | Clinical Presentation | Stage | Mutations | Diarrhea | Highest VIP Level with Diarrhea |
|---|---|---|---|---|---|---|
| 1 | 19 months old | Abdominal primary w/ metastases in liver, lung, bone, and bone marrow | 4 |
Initial: BRAF p.V600E, MYCN Amplification Relapse: ALK p.F1174L, MYCN Amplification |
After chemotherapy | >3200pg/mL |
| 2 | 11 months old | Adrenal mass w/ metastases in liver and pleura | 4 | BRAF p.V600E, MYC-N amplification | After chemotherapy | 1416pg/mL |
| 3 | 4 months old | Adrenal mass w/ metastases in liver, soft tissue, and bone | 4 |
Biopsy: MYCN Amplification Resection: ALK p.R1275Q, BRAF p.V600E, MYCN Amplification |
None | N/A |
| 4 | 11 months old | Abdominal primary w/ extension to spinal canal | 3 | BRAF p.V600E | None | N/A |
| 5 | 17 months old | Abdominal primary | 2 | BRAF p.V600E | Before chemotherapy | 486pg/mL |
| 6 | 20 months old | Left neck mass | 2 | BRAF p.L597R | None | N/A |
| 7 | 9 years old | Abdominal primary w/ metastases to bone and bone marrow | 4 |
Initial: ALK p.R1275Q, MYCN Amplification, ATRX p.R250 Relapse: ALK p.R1275Q, BRAF p.G466E, ATRX p.R250 |
None | N/A |
| 8 | 11 years old | Adrenal primary w/ metastases in liver and bone | 4 | BRAF p.G469A, ATRX p.R1739Hfs*8 | None | N/A |
| 9 | 13 years old | Adrenal primary w/ metastases in liver, bone, and bone marrow | 4 | BRAF F595L | None | N/A |
| 10 | 21 months old | Abdominal primary w/ metastases in liver, bone marrow, bones | 4 | None | Before chemotherapy | >400 pg/mL |
| 11 | 21 months old | Abdominal primary | 3 | None | Before chemotherapy | 370 pg/mL |
| 12 | 2 years old | Abdominal primary w/ metastases in soft tissue of neck | 4 | None | Before chemotherapy | 142 pg/mL |
| 13 | 2 years old | Abdominal primary w/ metastases in soft tissue, pleura, bone marrow | 4 | None | Before chemotherapy | 184 pg/mL |
CASE REPORTS
Patient 1 (Fig. 1A) was diagnosed at age 19 months with MYCN-amplified HR-NB with an abdominal primary tumor and metastases in liver, lung, bone, and bone marrow. After three cycles of induction chemotherapy,[5] she acutely developed VIP-D with serum VIP levels >3200pg/mL (normal <400pg/mL). She produced 3–5 liters/day of diarrhea refractory to chemotherapy, steroids, and octreotide therapy. Identification of BRAF V600E in resected post-chemotherapy tumor [6] prompted treatment with dabrafenib, a TKI specific for this mutation – and VIP-D resolved within 72 hours. However, six weeks later, she relapsed in the brain with a BRAF-wild type metastasis. Dabrafenib was discontinued; diarrhea recurred and VIP levels rose to 879pg/mL. VIP-D improved with resumption of dabrafenib in combination with the MEK inhibitor, trametinib, but NB rapidly progressed leading to death 13 months post-diagnosis. Serial whole genome sequencing of pre- and post-treatment adrenal tumor, brain metastasis, and liver and lung lesions obtained at autopsy documented disease evolution leading to subclones with distinct driver mutations (Fig. 2). BRAF V600E was noted only in post-treatment adrenal tumor, while brain, lung, and liver metastases had two ALK mutations, F1174L and F1245I without the BRAF mutation.
FIGURE 1.
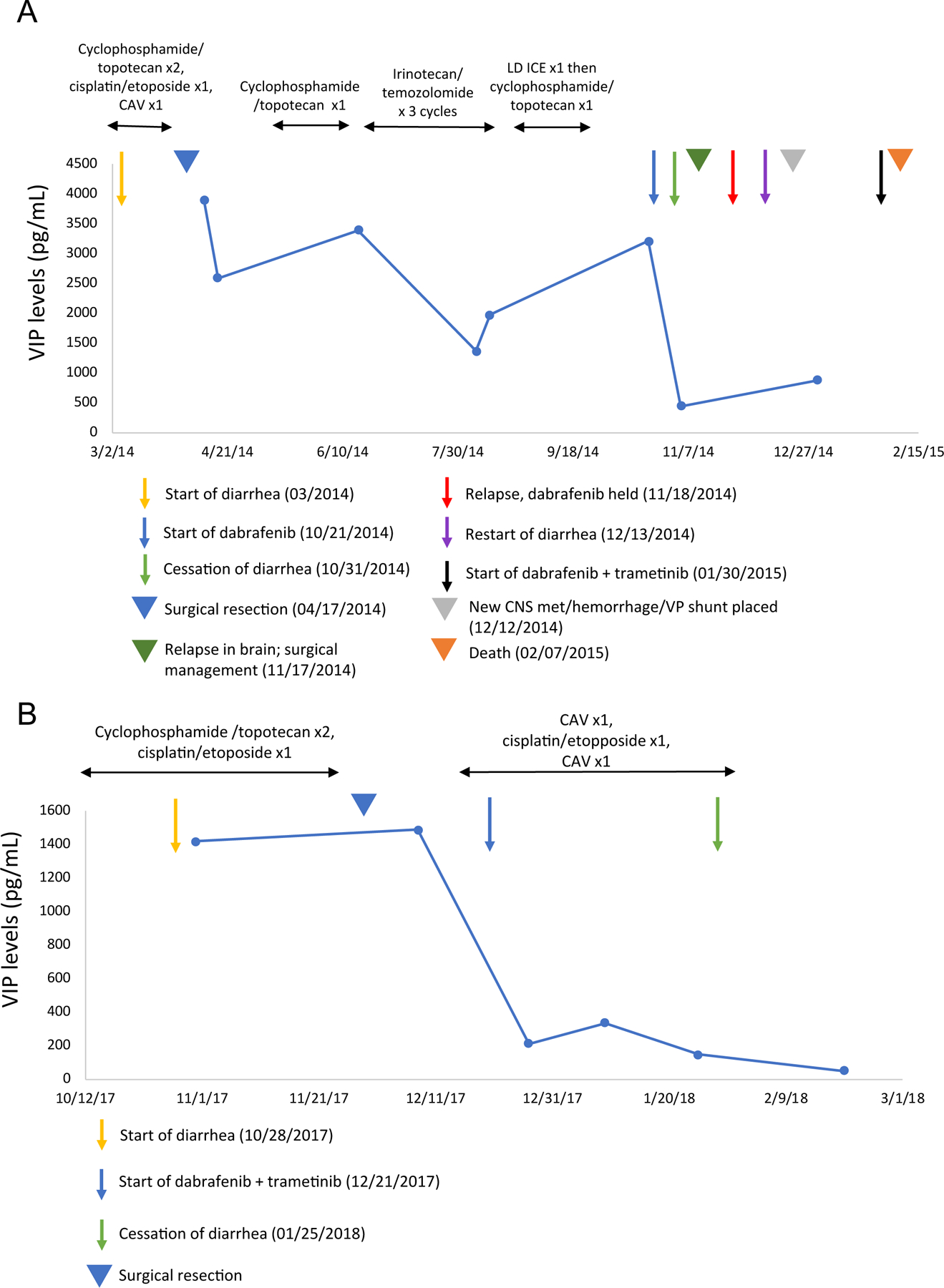
Timeline of treatment, presence of diarrhea, and VIP levels in patients 1 and 2.
A. Patient 1: Dabrafenib treatment was started seven months after the onset of VIP-D with rapid resolution of diarrhea and prompt reduction of serum VIP level. Upon relapse two weeks later, dabrafenib was held, VIP-D resumed within one month, and combination therapy with dabrafenib and trametinib was started one month later. Although the diarrhea promptly improved again, disease rapidly progressed leading to death 13 months post-diagnosis.
B. Patient 2: Combination therapy with dabrafenib and trametinib was started two months after the onset of VIP-D with rapid resolution of diarrhea and prompt reduction of serum VIP level.
CAV: cyclophosphamide, doxorubicin, vincristine; LD ICE: low dose ifosfamide, carboplatin, etoposide
FIGURE 2.

Sub-clonal evolution of tumor in patient 1 through the course of diagnosis and treatment.
Serial whole genome sequencing (83–107X) of the pre-treatment and post-treatment primary adrenal tumor as well as metastases from CNS, liver, and bilateral lungs indicated that there was branching evolution leading to subclones with distinct driver mutations confined to different tumor sites. The BRAF V600E mutation was found in the post-treatment adrenal tumor only, while CNS tumors and lung/liver tumors had two different ALK mutations, F1174L and F1245I, respectively.
Patient 2 (Fig. 1B) was 11 months old at diagnosis. She had a MYCN-amplified adrenal mass and metastases in liver and pleura. After two cycles of induction,[5] she developed VIP-D. Diarrhea persisted despite tumor resection and octreotide therapy. When BRAF V600E was identified in liver metastasis,[7] therapy was started with two TKIs, dabrafenib and trametinib. Within one week, diarrhea resolved and serum VIP levels decreased from 1416pg/mL to 209pg/mL (normal). Post-induction, she received chemoimmunotherapy with irinotecan, temozolomide, anti-GD2 antibody dinutuximab, and granulocyte-macrophage colony-stimulating factor (GM-CSF) [8] with a complete response. TKIs were discontinued after six months without recurrence of VIP-D. Further treatment included anti-GD2 antibody naxitamab+GM-CSF [9] followed by anti-NB vaccine.[10] Sequencing of post-treatment disease [6] revealed BRAF V600E in a lymph node but not in adrenal tumor which instead had ALK F1174L. She remains progression-free 40 months post-diagnosis.
Based on these two patients, we retrospectively reviewed our experience with patients with NB who had VIP-D and/or somatic BRAF mutations (Table 1). Of seven additional patients with NB and BRAF mutations, four had metastatic disease, three had BRAF V600E, and only one patient had VIP-D. The latter was the presenting symptom of localized BRAF V600E mutation-positive disease, and the diarrhea resolved promptly after surgical resection, following the typical clinical scenario for NB and VIP-D. The sole patient similar to the two subjects of the current report, with MYCN-amplified metastatic HR-NB and BRAF V600E mutation detected not at diagnosis, but at 2nd-look surgery, never developed VIP-D and is a long-term event-free survivor. The other five patients with BRAF mutations, including one BRAF V600E, did not have VIP-D. Four additional patients with NB and VIP-D had diarrhea present at diagnosis and did not have BRAF mutations. This brief retrospective analysis demonstrates that BRAF V600E mutations are not always associated with VIP-D or HR-NB.
DISCUSSION
BRAF mutations lead to increased cell proliferation, migration, and angiogenesis. Their role in selected adult malignancies is well documented.[11,12] Recent reports note an association with pediatric neoplasms including gliomas and Langerhans cell histiocytosis,[13,14] but not with NB or VIP-D. The onset of VIP-D in patients with HR-NB during induction has been described anecdotally,[3] but to our knowledge, ours is the first report on underlying genomic aberrations.
Although a clear mechanistic association between VIP and BRAF in NB has not yet been elucidated, in vitro studies in other cell lines have identified potential relationships between the two: in HT29 colonic adenocarcinoma cells, VIP stimulated cell proliferation and induced a signaling pathway involving RAS, RAP1, BRAF, and ERK.[15] In LNCaP prostate cancer cells, VIP induced neuroendocrine differentiation by activating RAS, PKA, ERK1/2, and PI3K pathways, and VIP-stimulated ERK1/2 phosphorylation was completely abolished by a MEK inhibitor.[16] In contrast, in T cells VIP exposure directly inhibited RAS kinase activity and impaired the activation of RAF1 and its binding to RAS.[17] Therefore, it appears that VIP effects on the RAS-RAF-MEK-ERK signaling cascade are cell-type specific.
BRAF inhibition in our patients rapidly reversed the massive VIP-D which complicated administration of their strongly myelosuppressive induction. The inhibitors had negligible toxicity and were compatible with conventional NB-directed chemotherapy. These clinical findings were expected, based on recent phase I/II pediatric clinical trials which confirmed the safety and tolerability of dabrafenib [18,19] and trametinib [20,21] in BRAF V600E mutation-positive tumors. The transient response of patient 1 to monotherapy informed management for patient 2. When the BRAF mutation was detected, both dabrafenib and trametinib were started, with elimination of VIP-D and no excess toxicity during induction. Eventually these TKIs were discontinued; their role in contributing to the child’s favorable outcome remains speculative.
Tumor heterogeneity and clonal evolution of NB in patients undergoing therapy was vividly demonstrated in our patients. In patient 1, serial whole genome sequencing showed ongoing tumor evolution leading to subclones with distinct driver mutations (Figure 2). The BRAF V600E mutation found in post-treatment adrenal tumor was absent in brain, lung, and liver metastases; these had ALK mutations (F1174L and F1245I) not previously detected. These findings document tumor heterogeneity and clonal evolution. In contrast, patient 2 had the BRAF V600E mutation initially detected in liver biopsy; it persisted post-chemotherapy in a metastatic lymph node but was absent in adrenal tumor, which instead had ALK F1174L mutation. We hypothesize that chemotherapy provided selective pressure for the BRAF-mutated clone. In both patients, BRAF and ALK mutations were mutually exclusive per site.
CONCLUSIONS
In summary, we describe an effective strategy for the management of VIP-D in patients with HR-NB. Our experience highlights the need to perform tumor sequencing in all patients with HR-NB, especially those who develop VIP-D because timely treatment with concurrent BRAF and MEK inhibitors can facilitate ongoing tumor-directed treatment of HR-NB and probably contribute to a favorable outcome. Although our patients tolerated the TKI combination well, current early-phase studies (clinicaltrials.gov NCT02684058, NCT02124772, NCT03919071) will formally confirm the safety profile of this combination in children with BRAF-mutated tumors.
ACKNOWLEDGEMENTS
We acknowledge support of the NCI Cancer Center Support Grant P30 CA008748. We thank Joseph Olechnowicz for editorial assistance.
ABBREVIATION KEY
- NB
Neuroblastoma
- VIP
Vasoactive intestinal peptide
- VIP-D
Vasoactive intestinal peptide-related diarrhea
- HR-NB
High-risk neuroblastoma
- TKI
Tyrosine kinase inhibitor
- MSKCC
Memorial Sloan Kettering Cancer Center
- GM-CSF
Granulocyte-macrophage colony-stimulating factor
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest relevant to this manuscript.
Accepted as a poster to ASPHO 2020 but ultimately not presented due to COVID-19. Abstract published: Shahid, S., Kushner, B., Modak, S., Basu, E., Rubin, E., Roberts, S. (2020). MYCN-amplified neuroblastoma with vasoactive intestinal peptide syndrome and BRAF V600E mutation. 2020 ASPHO Conference Paper and Poster Index. Pediatr Blood Cancer, 67: e28321. https://doi.org/10.1002/pbc.28321.
REFERENCES
- 1.Matthay KK, Maris JM, Schleiermacher G, et al. Neuroblastoma. Nature Reviews Disease Primers 2016:2(1):16078. [DOI] [PubMed] [Google Scholar]
- 2.Han W, Wang HM. Refractory diarrhea: A paraneoplastic syndrome of neuroblastoma. World J Gastroenterol 2015:21(25):7929–7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourdeaut F, de Carli E, Timsit S, et al. VIP hypersecretion as primary or secondary syndrome in neuroblastoma: A retrospective study by the Société Française des Cancers de l’Enfant (SFCE) 2009:52(5):585–590. [DOI] [PubMed] [Google Scholar]
- 4.Czkwianianc E, Zalewska-Szewczyk B, Kobos J, et al. Uncommon reasons of the digestive tract-related paraneoplastic syndromes in children with neuroblastic tumors: three case reports. Contemp Oncol (Pozn) 2018:22(1):42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JR, Kreissman SG, London WB, et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma: A Randomized Clinical Trial. JAMA 2019:322(8):746–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn 2015:17(3):251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodhouse R, Li M, Hughes J, et al. Clinical and analytical validation of FoundationOne Liquid CDx, a novel 324-Gene cfDNA-based comprehensive genomic profiling assay for cancers of solid tumor origin. PLoS One 2020:15(9):e0237802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mody R, Yu AL, Naranjo A, et al. Irinotecan, Temozolomide, and Dinutuximab With GM-CSF in Children With Refractory or Relapsed Neuroblastoma: A Report From the Children’s Oncology Group. J Clin Oncol 2020:38(19):2160–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kushner BH, Cheung IY, Modak S, et al. Humanized 3F8 Anti-GD2 Monoclonal Antibody Dosing With Granulocyte-Macrophage Colony-Stimulating Factor in Patients With Resistant Neuroblastoma: A Phase 1 Clinical Trial. JAMA Oncology 2018:4(12):1729–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kushner BH, Cheung IY, Modak S, et al. Phase I trial of a bivalent gangliosides vaccine in combination with β-glucan for high-risk neuroblastoma in second or later remission. Clin Cancer Res 2014:20(5):1375–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holderfield M, Deuker MM, McCormick F, et al. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer 2014:14(7):455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bond CE, Whitehall VLJ. How the BRAF V600E Mutation Defines a Distinct Subgroup of Colorectal Cancer: Molecular and Clinical Implications. Gastroenterol Res Pract 2018:2018:9250757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieran MW. Targeting BRAF in pediatric brain tumors. Am Soc Clin Oncol Educ Book 2014:e436–440. [DOI] [PubMed]
- 14.Kieran MW, Geoerger B, Dunkel IJ, et al. A Phase I and Pharmacokinetic Study of Oral Dabrafenib in Children and Adolescent Patients with Recurrent or Refractory BRAF V600 Mutation-Positive Solid Tumors. Clin Cancer Res 2019:25(24):7294–7302. [DOI] [PubMed] [Google Scholar]
- 15.Alleaume C, Eychène A, Caigneaux E, et al. Vasoactive intestinal peptide stimulates proliferation in HT29 human colonic adenocarcinoma cells: concomitant activation of Ras/Rap1-B-Raf-ERK signalling pathway. Neuropeptides 2003:37(2):98–104. [DOI] [PubMed] [Google Scholar]
- 16.Gutiérrez-Cañas I, Juarranz MG, Collado B, et al. Vasoactive intestinal peptide induces neuroendocrine differentiation in the LNCaP prostate cancer cell line through PKA, ERK, and PI3K. Prostate 2005:63(1):44–55. [DOI] [PubMed] [Google Scholar]
- 17.Anderson P, Gonzalez-Rey E. Vasoactive intestinal peptide induces cell cycle arrest and regulatory functions in human T cells at multiple levels. Mol Cell Biol 2010:30(10):2537–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kieran MW, Geoerger B, Dunkel IJ, et al. A Phase I and Pharmacokinetic Study of Oral Dabrafenib in Children and Adolescent Patients with Recurrent or Refractory BRAF V600 Mutation–Positive Solid Tumors. Clinical Cancer Research 2019:25(24):7294–7302. [DOI] [PubMed] [Google Scholar]
- 19.Hargrave DR, Bouffet E, Tabori U, et al. Efficacy and Safety of Dabrafenib in Pediatric Patients with BRAF V600 Mutation–Positive Relapsed or Refractory Low-Grade Glioma: Results from a Phase I/IIa Study. Clinical Cancer Research 2019:25(24):7303–7311. [DOI] [PubMed] [Google Scholar]
- 20.Perreault S, Larouche V, Tabori U, et al. A phase 2 study of trametinib for patients with pediatric glioma or plexiform neurofibroma with refractory tumor and activation of the MAPK/ERK pathway: TRAM-01. BMC Cancer 2019:19(1):1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kondyli M, Larouche V, Saint-Martin C, et al. Trametinib for progressive pediatric low-grade gliomas. J Neurooncol 2018:140(2):435–444. [DOI] [PubMed] [Google Scholar]


