Abstract
Background:
The consequences of infectious toxicity of hypomethylating agents (HMAs) on overall survival (OS) of patients diagnosed with high-risk myeloid neoplasms have not been thoroughly investigated.
Objectives:
We aimed to evaluate whether infectious events (IEs) negatively influenced the results of HMA treatment in a real-world setting.
Design:
Observational study.
Methods:
We obtained data from 412 non-selected consecutive patients from 23 Spanish hospitals who were diagnosed with high-risk myelodysplastic syndrome, chronic myelomonocytic leukemia, or acute myeloid leukemia and were treated with HMA. HMAs received after chemotherapy or stem cell transplant were excluded. All IEs were recorded. Outcomes included OS, modifications to the pre-planned treatment, incidence and characteristics of IEs, hospitalization, red blood cell transfusions, and factors associated with infection.
Results:
The rate of infection was 1.2 per patient/year. Next-cycle delay (p = 0.001) and hospitalizations (p = 0.001) were significantly influenced by IEs. Transfusion requirements during each cycle were significantly higher after infection compared with cycles without infection (coefficient = 1.55 [95% confidence interval (CI) = 1.26–1.84], p < 0.001). The median number of cycles was lower in patients experiencing any infection during the first four cycles (5 [3–8] versu 8 [5–16], p < 0.001). In the multivariable analysis, factors associated with lower OS were having any infection during the first four cycles (hazard ratio (HR) = 1.43 [95% CI = 1.09–1.88], p = 0.01), bone marrow blasts ⩾30% (HR = 2.13 [95% CI = 1.14–3.96], p = 0.01), adverse cytogenetics (HR = 1.70 [95% CI = 1.30–2.24], p < 0.001), and platelet count <50 × 109/l (HR = 1.69 [95% CI = 1.3–2.2], p < 0.001). BM blasts >20% (HR = 1.57 [95% CI = 1.19–2.01], p < 0.001) and adverse cytogenetics (HR = 1.7 [95% CI = 1.35–2.14], p < 0.001) were associated with infection, whereas hemoglobin >9 g/dl (HR = 0.65 [95% CI = 0.51–0.82], p < 0.001) and higher platelet count (HR = 0.997 [95% CI = 0.996–0.998], p = 0.016) protected from it.
Conclusion:
HMA infectious toxicity worsens OS, hinders the adherence to antineoplastic treatment and results in significant morbidity. Preventive strategies are fundamental in vulnerable patients.
Keywords: acute myeloid leukemia, hypomethylating agent, infection, myelodysplastic syndrome, treatment outcome
Introduction
The clinical efficacy of hypomethylating agents (HMAs) for the treatment of myelodysplastic syndromes (MDSs), chronic myelomonocytic leukemia (CMML), and acute myeloid leukemia (AML) in real-world practice is lower than expected from clinical trials.1,2 This discrepancy has been explained as the consequence of selection bias that hinders the inclusion of patients with comorbidities or poor performance status in these studies.
Similarly, the possibility of a higher-than-expected toxicity in unselected populations could be raised. One of the characteristic toxicities of these drugs is infection. In fact, while HMAs are considered safe drugs in this regard, infections are not uncommon. 3 The impact of infections on lowering overall survival (OS) has been addressed in small-scale studies with limited follow-up times.4,5 Infectious events (IEs) may cause mortality per se or may diminish the efficacy of antineoplastic treatment by preventing its administration according to the recommended dose and timing. In the case of HMAs, the relationship between infection, adherence to antineoplastic treatment, and survival has not been established. 6
In this context, we hypothesized that the infectious toxicity experienced by patients treated with HMA is higher than the reported in clinical trials, leading to reduced tolerance to treatment or even to an excess of mortality. In line with this hypothesis, the main objective of this multicenter study was to evaluate the impact of IEs occurring during HMA treatment on the survival of patients with high-risk MDS, CMML, or AML. For this purpose, we analyzed the incidence of IEs and their impact on adherence to antineoplastic treatment and with OS through modifications made to prescheduled protocols.
Methods
Study design and patients
This was a retrospective study from patients who were prospectively included in the Spanish Registry of myelodysplastic syndrome (RESMD).
Inclusion criteria were diagnosis of high-risk MDS, AML, or CMML-2 according to the World Health Organization (WHO) criteria and first-line treatment with azacitidine or decitabine following the European Medicines Agency–approved indications.7,8 Inclusion of low-risk MDS patients was allowed if platelet counts were <30 × 109/l, absolute neutrophil count (ANC) <0.5 × 109/l, or grade 2–3 bone marrow (BM) fibrosis was present. Similarly, patients with CMML-1 were included if transfusional dependency or thrombocytopenia <30 × 109/l was present. HMA prescriptions after intensive chemotherapy or hematopoietic stem cell transplant were excluded.
The revised International Prognostic Score System and CMML Prognostic Score System (IPSS-R and CPSS)9,10 were used in MDS and CMML, respectively. The cytogenetic category was established following Schanz, CPSS, and European Leukemia Net classifications for MDS, CMML, and AML, respectively.10 –12
Infections
All IEs were recorded.
Information on infection was extracted from detailed review of the patients’ medical records. Category of infection was defined according to the Han criteria. 13 Severity of IE was graded according to the National Cancer Institute Common Toxicity Criteria for adverse events (version 5·0). 14 In addition, those infections causing severe clinical impairment leading to treatment withdrawal were categorized as grade 4. According to the Spanish Myelodysplastic Syndromes Group (GESMD) guidelines, primary prophylaxis with fluoroquinolones (FQs), antifungals, or another antimicrobial is not advised. 15 For the purpose of this study, however, all antimicrobials administered as prophylaxis were recorded.
Outcomes
The primary outcome was OS. Secondary endpoints were modifications to the prescheduled treatment due to infection during cycles 1–4. The following events were considered modifications: dose reductions, dose delays (i.e. interval ⩾35 days between consecutive cycles), and treatment withdrawals. Investigators had to indicate if such changes were consequence of IE. Other secondary endpoints were incidence and characteristics of IEs, hospitalization, red blood cell (RBC) transfusions, and factors associated with infection.
Statistical analysis
Baseline demographics and clinical variables were summarized as median [interquartile range (IQR)] or frequency (proportion) as appropriate. The rate of infection was calculated as the total number of IEs per patient-years of follow-up. For each cycle, cumulative incidence of infection (CII) was calculated from the first day of cycle, with death as a competing event.
Within the first four and six cycles, baseline data, type of HMA, and prophylaxis with FQs were compared between cycles with and without infection using the Chi-square, Fisher’s or Wilcoxon’s tests as appropriate. The occurrence of IEs was studied using a multivariable Cox model which included those variables with a p value below 0.1 in the univariable analysis. The relationship between infection and transfusion was assessed by modeling the number of packed RBCs transfused according to infection and baseline hemoglobin level at the beginning of each cycle.
OS was calculated from the day of diagnosis and from the beginning of HMA treatment until the date of final follow-up. The probability of survival and differences between groups were estimated using the Kaplan–Meier method and the log-rank test. A landmark analysis of 6 months was performed to determine the impact of receiving less than four cycles. A multivariable Cox model was performed to evaluate variables related to survival, including all variables with p value below 0.1 in the univariable comparisons and considering only the first infection for each patient as a time-dependent covariable. In this model, hazard ratios (HRs) and 95% confidence intervals (95% CIs) were calculated.
This study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement. 16 A checklist of the STROBE statement for cohort studies is shown in Supplementary Table 1.
Results
Baseline characteristics of the study population
Four hundred and twelve patients from 21 academic and 2 non-academic centers participating in the RESMD between 21 February 2005 and 18 January 2021 were analyzed. Median age was 73 (65–78) years. According to the WHO criteria, 325 (79%) patients had MDS, 61 (15%) AML, and 26 (6%) CMML. The most frequent MDS diagnostic category was MDS with excess of blasts (263 patients, 81%). Within AML, 15 patients presented with 30% or more BM blasts. A detailed description of baseline characteristics in the global population and separated by diagnostic category is provided in Table 1 and Supplementary Tables 2 and 3.
Table 1.
Baseline patients characteristics (N = 412).
| N or median | % or range | |
|---|---|---|
| Age | 73 | 65–78 |
| Sex | ||
| Male | 239 | 58 |
| Female | 173 | 42 |
| Therapy related | 73 | 18 |
| ECOG | ||
| 0–1 | 248 | 81 |
| 2 | 59 | 19 |
| Percentage of bone marrow blasts | ||
| Global | 10 | 6–16 |
| AML | 25 | 21–30 |
| WHO diagnosis | ||
| AML | 61 | 15 |
| MDS | ||
| MDS-U | 10 | 2 |
| MDS (RS)/MDS-MD | 52 | 13 |
| MDS-EB | 263 | 64 |
| CMML | 26 | 6 |
| Cytogenetic risk | ||
| MDS | ||
| Favorable | 133 | 41 |
| Intermediate | 60 | 18 |
| Poor | 125 | 38 |
| Failure a | 7 | 2 |
| CMML | ||
| Favorable | 16 | 62 |
| Intermediate | 3 | 11 |
| Poor | 7 | 27 |
| AML | ||
| Intermediate | 39 | 64 |
| Poor | 21 | 34 |
| Failure a | 1 | 2 |
| IPSS-R risk category | ||
| Good | 6 | 2 |
| Intermediate | 74 | 23 |
| High | 238 | 73 |
| NA | 7 | 2 |
| Hemoglobin (g/dl) | 9.1 | 8.2–10.2 |
| Hb <9 g/dl | 212 | 51 |
| ANC (×109/l) | 1 | 0.5–2.3 |
| ANC <0.5 × 109/l | 116 | 28 |
| Platelets (×109/l) | 53 | 28–102 |
AML, acute myeloid leukemia; ANC, absolute neutrophil count; CMML, chronic myelomonocytic leukemia; ECOG, Eastern Cooperative Oncology Group; Hb, hemoglobin; IPSS-R, International Prognostic Score System–Revised; MDS-EB, myelodysplastic syndrome with excess of blasts; MDS-MD, myelodysplastic syndrome with multilineage dysplasia; MDS-RS, myelodysplastic syndrome with ring sideroblasts; MDS-U, myelodysplastic syndrome with unilineage dysplasia; WHO, World Health Organization.
Failure: no metaphases were obtained.
IPSS-R at the beginning of HMA treatment was low in 6 (2%) cases (Table 1). The reasons for initiating HMA treatment in these patients were platelet counts below 30 × 109/l in five patients and grade 2–3 BM fibrosis according to the WHO criteria in one patient. The median survival for these six patients was 11.9 months (0.7–20).
Twelve CMML-2 were included. In the remaining 14 CMML patients, criteria for HMA treatment were percentage of BM blasts >5% in the presence of thrombocytopenia <30 × 109/l or anemia with transfusional dependence. The median time from diagnosis to the beginning of HMA treatment was 34 days (16–92).
Treatment
Overall, 4521 cycles of HMA were delivered to 412 patients. Azacitidine was the selected HMA in 387 cases (94%), corresponding to a total of 4375 cycles. The drug was administered for 7 days in 79% of cycles (3470/4375) and for 5 days in 21% (905/4375) of cycles.
The median number of cycles was 6 (4–13), independently of HMA selected or WHO diagnosis (Supplementary Table 3). One hundred and one patients (24%) received less than four cycles of HMA, and 167 (41%) received less than six.
FQ prophylaxis was prescribed in 33% of the patients (130 MDS, 30 AML, and 8 CMML). Patients with AML, adverse cytogenetics, or lower ANC received prophylaxis more frequently at the beginning of first HMA cycle (Table 2). Regarding antifungal prophylaxis, 34 patients (8% of the whole population) received antifungal prophylaxis with fluconazole (13 patients), itraconazole (2 patients), posaconazole (13 patients), voriconazole (2 patients), and non-specified (4 patients).
Table 2.
Univariable comparisons of patients receiving or not fluoroquinolone (FQ) prophylaxis.
| FQ prophylaxis | No FQ prophylaxis | p | |
|---|---|---|---|
| Age (years) | 73 (65–80) | 73 (66–78) | 0.9 |
| Diagnostic category | |||
| MDS | 100/325 (31%) | 225/325 (69%) | 0.02 |
| AML | 30/61 (49%) | 31/61 (51%) | |
| CMML | 8/26 (31%) | 18/26 (69%) | |
| ANC* (×109/l) | 0.65 (0.37–1.4) | 1.3 (0.6–2.7) | <0.001 |
| Hemoglobin (g/dl) | 9.1 (8.2–10.2) | 9.1 (8.2–10.3) | 0.6 |
| Platelets (×109/l) | 56 (33–06) | 51 (27–101) | 0.3 |
| Cytogenetics | |||
| Adverse | 62/153 (41%) | 91/153 (59%) | 0.021 |
| Non-adverse | 76/258 (29%) | 182/258 (71%) |
AML, acute myeloid leukemia; ANC, absolute neutrophil count; CMML, chronic myelomonocytic leukemia; FQ, fluoroquinolone; MDS, myelodysplastic syndrome.
Peripheral cell counts were obtained at the beginning of the first HMA treatment. Considering ANC at the beginning of each cycle, a significant association between ANC and using FQ prophylaxis was also observed. Median ANC was 0·5 × 109/l (0.2–1.2) in those cycles in which FQs were used compared with 1.2 × 109/l (0.6–2.3) in those in which they were not used, p < 0.001.
Overall Survival
Median follow-up from diagnosis and from the beginning of HMA was 17 (9–34) and 12.5 (7–24) months, respectively. AML patients showed the lowest median OS, with 10.8 months (7.5–15.6) compared with 15.3 (14–17.6) and 20.3 (13.6–30) months in MDS and CMML patients, respectively (Supplementary Table 3).
There were 311 deaths. Infection was the main cause of death in 55 (18%) patients. Twenty-five (45%) of these deaths occurred in the first three cycles, 18 (32%) between cycles four and six, and 12 (22%) after the sixth cycle. Other causes of death were progression, hemorrhage, and toxicity secondary to subsequent treatments in 202 (64%), 8 (3%), and 46 (15%) patients, respectively.
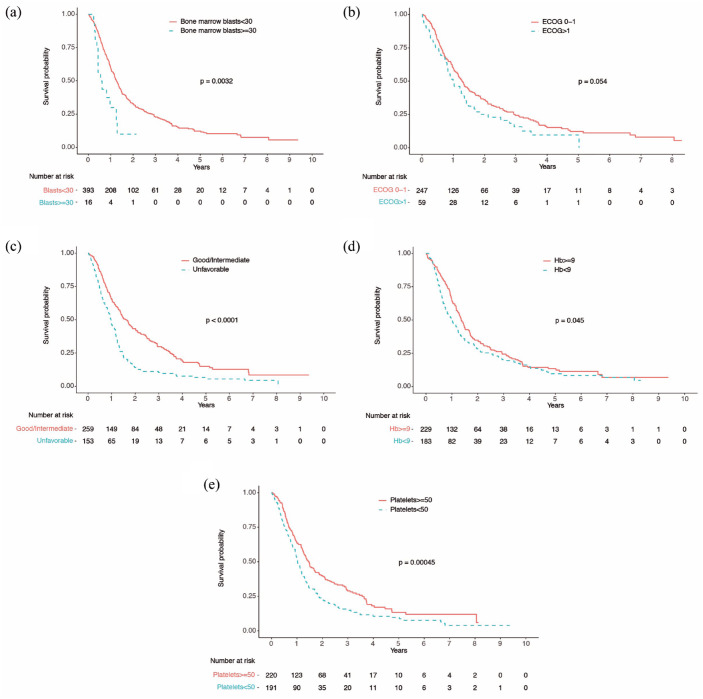
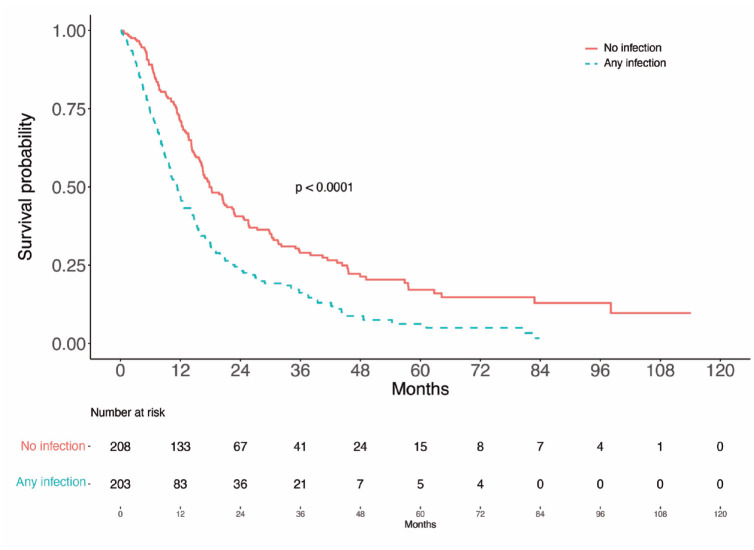
To evaluate the impact of infections on OS, we first explored in univariable analyses the association of other well-known prognostic factors with survival9,17,18 in our population (Table 3). Eastern Cooperative Oncology Group (ECOG) above one (HR = 1.3 [95% CI = 0.99–1.9], p = 0.054), more than 30% BM blasts (HR = 2.3 [95% CI = 1.34], p = 0.003), unfavorable cytogenetics (HR = 1.86 [95% CI = 1.49–2.3], p < 0.0001), hemoglobin level below 9 g/dl (HR = 1.25 [95% CI = 1.004–1.6], p = 0.045), and platelet count below 50 × 109/l (HR = 1.49 [95% CI = 1.2–1.9], p < 0.001) were associated with lower survival (Figure 1). Any infection during the first four cycles of HMA treatment was also associated with lower survival (HR = 1.7 [95% CI = 1.4–2.2], p < 0.0001; Figure 2). Age, BM blasts above 20%, ANC <0.5 or 0.8 × 109/l, or FQ prophylaxis did not significantly influence OS (Table 3 and Supplementary Figure 1).
Table 3.
Univariable and multivariable analyses for overall survival.
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Median OS (days) | p | OR [95% CI] | p | |
| Age ⩾75 <75 |
441 452 |
0.85 |
||
| ECOG 0–1 ⩾2 |
468 362 |
0.054 |
||
| Bone marrow blasts (%) <30 ⩾30 |
459 211 |
0.003 |
2.13 [1.14–3.96] | 0.01 |
| Bone marrow blasts (%) <20 ⩾20 |
468 333 |
0.17 |
||
| Cytogenetic Good or intermediate Unfavorable |
592 354 |
<0.0001 |
1.7 [1.30–2.24] | <0.001 |
| Hb ⩾9 g/dl <9 g/dl |
496 366 |
0.045 |
||
| ANC >0.8 g/l ⩽0.8 g/l >0.5 g/l ⩽0.5 g/l |
441 453 459 441 |
0.85 0.83 |
||
| Platelets ⩾50 g/l <50 g/l |
538 373 |
<0.001 |
1.69 [1.3–2.2] | <0.001 |
| Infection in first four cycles No Yes |
534 342 |
<0.0001 |
1.43 [1.09–1.88] | 0.01 |
| Fluoroquinolone prophylaxis | Global | ANC <0.5 × 109/l | AML | |
| FQ yes | 424 | 434 | 346 | |
| FQ no | 476 | 452 | 304 | |
| HR | 1.2 | 1.08 | 0.89 | |
| [95% CI] | [0.95–1.5] | [0.68–1.73] | [0.95–1.5] | |
| p | 0.7 | 0.73 | 0.13 | |
AML, acute myeloid leukemia; ANC, absolute neutrophil counts; CI, confidence interval; CMML, chronic myelomonocytic leukemia; ECOG, Eastern Cooperative Oncology Group; FQ, fluoroquinolone; Hb, hemoglobin; HR, hazard ratios; MDS, myelodysplastic syndrome; OS, overall survival; WHO, World Health Organization.
Figure 1.
Survival analysis according to (a) percentage of bone marrow blasts, (b) Eastern Cooperative Oncology Group (ECOG) performance status, (c) cytogenetic category, (d) hemoglobin level above or below 9 g/dl, and (e) platelet count above or below 50 × 109/l.
Figure 2.
Survival analysis according to occurrence of infection during the first four cycles of hypomethylating treatment. One patient without information regarding infections in the first two cycles was excluded. Univariate analysis (log-rank test) showed significant differences between patients having an infection during the first four cycles or not (p < 0.0001).
In the landmark analysis for those patients surviving 6 months or more, there were no significant differences between patients receiving more or less than four cycles of therapy (HR = 1.01 [95% CI = 0.66–1.56], p = 0.95, Supplementary Figure 2).
In the multivariable analysis considering infection as a time-dependent covariate, predictive factors for lower survival were BM blasts above 30% (HR = 2.13 [95% CI = 1.14–3.96], p = 0.01), unfavorable cytogenetics (HR = 1.70 [95% CI = 1.30–2.24], p < 0.001), platelet count below 50 × 109/l (HR = 1.69 [95% CI = 1.3–2.2], p < 0.001), and any infection during the first four cycles (HR = 1.43 [95% CI = 1.09–1.88], p = 0.01) (Table 3). Infection during the first four cycles of HMA treatment consistently increased the risk of death in all the diagnostic categories in subgroup analyses (Supplementary Table 5). Owing to the low number of patients in CMML and AML, however, it only remained statistically significant in the MDS population.
Because modifications in the management of infections derived from recommendations published in 2011 19 could have impacted the final outcome of patients, these analysis were repeated in the cohort of patients treated after that year. Out of 412 patients, 379 (92%) received their first cycle of therapy after 2011. In the multivariable Cox model, any infection during the first four cycles remained predictive for lower survival (Supplementary Table 6). Finally, the 33 months (27-NA) OS of the seven patients treated in non-academic centers was not worse compared with the OS of the whole population.
Incidence of IEs
Five hundred and twelve IEs (483 under HMA treatment and 29 after HMA were stopped) were recorded. CII followed a temporal pattern, highlighting three periods with different incidence and epidemiological features (Table 4); the early period, comprising cycles 1–3, was characterized by a CII of at least 20%; the intermediate period (cycles 4–6), in which CII decreased to 10–15%; finally, the late period (beyond the sixth cycle) was the one with the lowest CII. Within each period, the ratio of grade 4 infections relative to total number of infections ranged from 10% to 20%. These differences were accompanied by a significative rise in the median hemoglobin level and platelet count, but not in the absolute neutrophil count (ANC). CII did not change after excluding patients experiencing any dose reduction (Supplementary Table 7).
Table 4.
Total number and cumulative incidence of infection in each cycle.
| Cycle number | Treatment period | ||||||
|---|---|---|---|---|---|---|---|
| Early (cycles 1–3) | Intermediate (cycles 4–6) | Late (>sixth cycle) | |||||
| 1 | 2 | 3 | 4 | 5 | 6 | >6 | |
| Number of infectious events | 124 | 88 | 65 | 45 | 35 | 29 | 97 |
| Cumulative incidence of infection | 31 | 23 | 20 | 15 | 13 | 13 | 1–2 |
| Grade 4 infections, % a (N) | 15 (18/124) | 12 (11/88) | 17 (11/65) | 10 (12/45) | 14 (5/35) | 33 (9/29) | 12 (12/97) |
| p | |||||||
| Hb (g/dl) | 9.3 (8.3–10.3) | 10 (8.8–11.8) | <0.001 | ||||
| ANC (×109/l) | 0.9 (0.4–2.1) | 1.1 (0.5–2.2) | 0.09 | ||||
| Platelets (×109/l) | 63 (30–127) | 106 (45–181) | <0.001 | ||||
ANC, absolute neutrophil count; Hb, hemoglobin.
Lower row: peripheral blood counts at the beginning of cycle. Rate of grade 4 infections and infections leading to suspension of HMA treatment.
p indicates p value of difference in these values.
Related to number of infections in each cycle.
Collectively, documented infections were the most frequent category followed by fever of unknown origin, blood stream, viral, and fungal infections (Supplementary Table 8). Within the sites of infection, the respiratory system was the most prevalent. It must be noted that no microorganisms were identified in 141/148 (97%) infections involving the respiratory system (Supplementary Table 9).
Considering blood, urine, respiratory secretions, and tissue exudates, 109 microorganisms were identified, 102 of them being bacteria. Gram-negative bacteria were the most prevalent bacteria, with 64 isolates (28 of them Escherichia coli).
Among viral infections, Herpes virus was documented in 11 cases (2% of the total IEs). Finally, fungal infections were uncommon, with 17 infections among 483 IEs. Three patients developed five invasive fungal episodes during HMA treatment in cycles 1 (two patients) and 2 (one patient). In one patient, galactomannan antigen remained positive until death. Of note, baseline neutrophil counts at the beginning of HMA in these three patients were 0.01, 1, and 0.02 × 109/l, respectively. None of them received antifungal prophylaxis. Similarly, none of the patients who developed other type of fungal infections received prophylaxis at the beginning of cycle, except one who was receiving posaconazole and developed oral candidiasis. Additional descriptions of category of infections, sites, and identified microorganisms are provided in the Supplementary Material.
Impact of infections on healthcare resources
There were 320 hospitalizations during the study period involving 201 patients. Infections were the cause of 313 (98%) hospitalizations. Median ANC count at the time of hospitalization for infection was 0·55 × 109/l (0.11–1.7). Other reasons for hospitalization were hemorrhage (five patients), clinical deterioration and platelet refractoriness (one patient), and relapse (one patient). Median hospital stay was 9 (5–13) days.
Patients with infections during the first four cycles required more RBC transfusions within each cycle than those without them (8 [3–16] versus 5 [1–12], p = 0.002). The number of RBC transfusions was correlated with both hemoglobin (coefficient = −0.83 [95% CI = −0.91 to –0.74], p < 0.001) and infection (coefficient = 1.43 [95% CI = 1.10–1.77], p < 0.001).
Finally, 31/92 (34%) patients with any IE during the early period developed IEs in the next period compared with 28/152 (19%) patients without early IEs (p = 0.01). Furthermore, among 19 blood stream infections occurring in the intermediate period, a previous IE was identified in 14.
Impact of infections on adherence to preplanned antineoplastic treatment
When considering the first four cycles, 95/167 (57%) patients with IEs experienced a delay in the beginning of the next cycle, compared with 64/213 (39%) in patients without any infection (p = 0.001). In contrast, IEs were not correlated with dose reductions in the following cycles, because 4/167 (2%) and 6/213 (2.8%) patients with and without IEs experienced dose reductions (p = 1).
Clinical deterioration secondary to infection prompted treatment withdrawal in 15 patients (3.6%) from cycle 1 to 3. In addition, treatment withdrawal occurred in another eight patients during cycles 4 and 5.
Impact of infections on length of treatment
Median number of cycles in patients with any infection in the first four was significantly lower compared with non-infected patients (5 [3–8] versus 8 [5–16], p < 0.001). Among 101 patients receiving less than four cycles, infection was the cause of death or treatment withdrawal in 40 (40%). Cytogenetic and WHO categories, percentage of BM blasts, hemoglobin, ANC, or platelet count were not associated with receiving less than four cycles. On the opposite, 23 (39%) patients with baseline ECOG ⩾2 received less than four cycles compared with 59 (24%) patients with ECOG <2 (p = 0.02).
Infections and response to HMA treatment
The best response to HMAs was evaluated in 412 patients, 202 of them (49%) being defined as responders. During the first four cycles, IEs were most common in non-responders [119/219 (55%) versus 84/202 (42%), p = 0.002]. Karyotype, WHO diagnosis, or percentage of BM blasts were not associated with response.
Factors related to infection during the first four cycles of HMA
In univariable comparisons, ⩾20% BM blasts, unfavorable karyotype, and lower ANC were significantly associated with developing IEs, whereas higher hemoglobin and platelet count were associated with a lower rate of infection (Table 5). The use of prophylactic FQ when baseline ANC was <0.5 × 109/l was associated with a lower risk of infection [78/225 (35%) infections without FQ compared with 44/180 (24%) on FQ, p = 0.03].
Table 5.
Univariable and multivariable analyses of prognostic factors for infectious episode in the first four cycles (N = 412 patients, 308 infectious events).
| Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|
| Infection | No infection | p | OR [95% CI] | p | |
| Age (median, IQR) | 77 (69–784) | 77 (69–78) | 0.28 | ||
| Sex, N (%) | |||||
| Male | 176/835 (21) | 659/835 (79) | 0.82 | ||
| Female | 132/608 (22) | 476/608 (78) | |||
| ⩾20% BM blasts | |||||
| Yes | 65/209 (31) | 144/209 (69) | <0.001 | 1.57 a (1.19–2.01) | <0.001 |
| No | 239/1222 (20) | 983/1222 (80) | |||
| Cytogenetic risk | |||||
| Good/intermediate | 155/900 (17) | 745/900 (83) | <0.0001 | 1.7 (1.35–2.14) | 0.001 |
| Unfavorable | 146/514 (28) | 368/514 (72) | |||
| NA | 7/29 (24) | 22/29 (76) | |||
| Hemoglobin (g/dl) | 9 (8.2–9.7) | 9.5 (8.5–10.6) | <0.0001 | 0.65 (0.51–0.82) b | <0.001 |
| Platelet count (×109/l) | 53 (25–109) | 76 (34–143) | <0.0001 | 0.997 (0.996–0.998) | 0.01 |
| IPSS-R (MDS patients) | |||||
| <3.5 | 1/12 (8) | 11/12 (92) | 0.37 | ||
| 3.5–4.5 | 45/265 (17) | 220/265 (83) | |||
| >4.5 | 174/829 (21) | 655/829 (79) | |||
| NA | 6/27 (22) | 21/27 (78) | |||
| Type of HMA | |||||
| Azacitidine | 288/1352 (21) | 1064/1352 (79) | 0.98 | ||
| Decitabine | 20/91 (20) | 71/91 (106) | |||
| ANC | 0.66 (0.24–1.8) | 1.00 (0.49–2.18) | <0.0001 | 0.98 (0.95–1.01) | 0.3 |
| ANC <0.5 × 109/l | |||||
| Without FQ | 78/225 (35) | 147/225 (65) | 0.03 | 1.62 (1.12–2.34) | 0.01 |
| With FQ | 44/180 (24) | 136/180 (76) | |||
AML, acute myeloid leukemia; ANC, absolute neutrophil counts; BM, bone marrow; CMML, chronic myelomonocytic leukemia; FQ, fluoroquinolone; Hb, hemoglobin; HMA, hypomethylating agent; IPSS-R, International Prognostic Score System–Revised; MDS, myelodysplastic syndrome; WHO, World Health Organization.
Cutoff value of 20%.
Cutoff value of 9 g/dl.
When the univariable analysis was repeated in each diagnostic category, the association of infection with hemoglobin, cytogenetics, and BM blasts remained significant, with a 5% blast cutoff in MDS. Lower ANC remained significant in AML and in MDS when it was analyzed considering not receiving FQ prophylaxis. Owing to the low numbers of patients diagnosed with CMML (N = 26), no significant associations between baseline characteristics and infection were observed except with ANC (Supplementary Tables 11–13).
In a multivariable analysis performed in the whole population, BM blasts above 20% (HR = 1.57 [95% CI = 1.19–2], p < 0.001) and unfavorable cytogenetics (HR = 1.7 [95% CI = 1.35–2], p = 0.001) were associated with higher risk of infection. On the opposite, hemoglobin level >9 g/dl (HR = 0.65 [95% CI = 0.51–0.82], p < 0.001) and higher platelet count (HR = 0.997 [95% CI = 0.996–0.998], p = 0.01) protected from infection (Table 5). Inclusion of BM blasts and hemoglobin as continuous variables yielded similar results (HR = 1.02 [95% CI = 1.008–1.027], p < 0.001) and HR (0.81 [95% CI = 0.77–0.86], p < 0.001), respectively. Neither ANC nor FQ were individually associated with infection. The absence of FQ prophylaxis when ANC where below 0.5 × 109/l increased the risk of infection (HR = 1.6 [95% CI = 1.12–2.34], p = 0.01), however.
The same results were obtained when these analyses were repeated considering the first six cycles (Supplementary Table 14). Similarly, cytogenetic category remained significant after excluding the nonavailable category (data not shown).
Discussion
In this multicenter study, we have analyzed the incidence and consequences of IEs in patients undergoing treatment with HMAs. We have found that the incidence of IEs was higher than what was reported in early clinical trials20,21 and had a profoundly negative impact on outcomes.
Our data contradict early clinical trials, which suggested that HMAs were safe in terms of infectious toxicity. Indeed, the incidence of febrile neutropenia in the MDS-001 20 and AML-0013,21 trial was lower in the azacitidine arm compared with the intensive chemotherapy or low-dose cytarabine arms. The incidence of pneumonia in patients receiving azacitidine was double compared with those receiving only supportive care, however.3,20,21 Moreover, infectious toxicity was higher in patients randomized to receive decitabine than in those receiving supportive care 22 or cytarabine.23,24 This excess of toxicity was attributed to the greater length of HMA treatment. As infections occurred predominantly during the initial HMA cycles, however, 25 this increased risk of infection should not be attributed exclusively to differences in treatment exposure.
We show that IEs had a multidimensional impact that ultimately conditioned long-term outcome. The negative effects of IEs included reduction in chemotherapy dose intensity and response rate as noticed by others, 26 premature treatment withdrawal, and increased consumption of healthcare resources. The ultimate consequence of IEs was a 40% increased risk of death.
Overall, the risk for infection was closely related to the severity of the underlying disease. Low hematopoietic reserve, high disease burden in terms of BM blasts and poor prognostic cytogenetics made patients more vulnerable to infection. Thus, there was a paradox in that those patients with the greatest need of an appropriate dose intensity were also more likely to be undertreated because of their higher risk of infection.
Our results may have practical implications. First, once HMA treatment is indicated, the decision to begin with the first cycle should not be delayed, as the risk of infection increases with the severity of cytopenias. Second, FQ prophylaxis seems to be justified in severe neutropenic patients in whom it reduces the risk of bacterial infection, apparently without increasing antibiotic resistance. This information is relevant, as in the AZA-MDS-001 trial, 28% patients received prophylaxis, but information about its efficacy is not available. Moreover, in AML, where HMAs are used in combination with venetoclax and severe and prolonged neutropenia is expected, prophylaxis might be critical. Third, confronting previous reports, 27 the low incidence of invasive fungal infections found in this study do not support the use of wide-spectrum azole prophylaxis, although we cannot rule out its potential role in severe neutropenic patients. Finally, strategies aimed at preventing infection should be intensified in poor prognostic categories and during the first cycles of treatment in order to avoid cycle delays, thus maximizing the efficacy of antineoplastic treatment. 6
Several aspects ensure the reliability of our results. First, the long recruitment period and multicenter nature (23 hospitals across a country) guarantee the external validity of the study and minimize selection bias. The confirmation of the validity of classic prognostic factors9,28 and the survival rates similar to other series1,2,29 confirm the representativity of the sample. Second, we have confirmed that changes in the management of infections resulting from the implementation of recent guidelines 19 did not modify the final results. Third, all IEs that occurred throughout the treatment were reported irrespective of their severity, thus avoiding the loss of information that occurs when these patients receive assistance in centers other than in those in which HMAs were prescribed. 30 Fourth, we have excluded patients who received HMA after chemotherapy, in whom infectious toxicity is significantly higher. 31
The main limitations of the study are related to its retrospective nature and the potential bias when interpreting the consequences of infection on dose reductions and cycle delays. Dose reductions occurred in a similar rate in patients with or without pre-existing infection, however, suggesting homogeneous criteria in the management of cytopenias. Regarding the distinction between delays secondary to infection and those related to cytopenias, it must be noted that cytopenias itself exceptionally lead to hospitalization. In contrast, we have shown that infections and hospitalization were significantly associated. The need for recovery after hospitalization could have been the reason explaining the delay in the beginning of the next cycle. Another limitation of the study is the lack of molecular information, which could have helped to better categorize the risk of the underlying disease. Despite this, the accuracy of current classifications and prognostic scoring systems allowed us to identify a subgroup of patients – those with adverse cytogenetics – in whom the biology of the disease confers a higher risk of infection. Finally, although this is the largest study on infections undertaken so far, AML and CMML are under-represented, thus precluding definite conclusions about risk factors for infection other than neutropenia within these groups.
In conclusion, this study shows that infectious toxicity secondary to HMAs is common and has a profound and negative impact on the outcome of patients. This excess of toxicity contributes to explain the undertreatment and the lower than expected survival observed outside clinical trials. In order to ensure that HMAs are used in a safely and efficiently way, it is imperative to develop strategies for preventing and controlling infectious toxicity.
Supplemental Material
Supplemental material, sj-docx-1-tah-10.1177_20406207221127547 for Relevance of infections on the outcomes of patients with myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia treated with hypomethylating agents: a cohort study from the GESMD by Laura Vilorio-Marqués, Christelle Castañón Fernández, Elvira Mora, Lorena Gutiérrez, Beatriz Rey Bua, Maria José Jiménez Lorenzo, Marina Díaz Beya, Miriam Vara Pampliega, Antonieta Molero, Joaquín Sánchez-García, Marisa Calabuig, Maria Teresa Cedena, Tzu Chen-Liang, Johana Alejandra Díaz Santa, Irene Padilla, Francisca Hernández, Rosana Díez, Pedro Asensi, Blanca Xicoy, Guillermo Sanz, David Valcárcel, María Diez-Campelo and Teresa Bernal in Therapeutic Advances in Hematology
Acknowledgments
The authors are thankful to the following GESMD investigators who participated in this study:
Carlos Aguilar, Hematology Department, Hospital General Santa Bárbara Soria. Luis Benlloch, Spanish Group of Myelodysplastic Syndromes, Grupo Español de Síndromes mielodisplásicos. Nicolás Díaz Varela, Hematology Department, Hospital Universitario Santiago de Compostela. Cristián Escolano, Hematology Department, Hospital Universitario de Getafe. Ángela Gil Pérez, Hematology Department, Hospital Universitario de Guadalajara. Marta Gómez Núñez, Hematology Department, Hospital Universitario Parc Taulí Hospital. Ana Lerma Verdejo, Hematology Department, Hospital Nuestra Señora del Prado. Carolina Muñoz, Hematology Department, Hospital Infanta Leonor. M. Ángeles Pérez Sáenz, Fundación Jiménez Díaz. Andoni Urrutia, Hematology Department, Clínica Universitaria Navarra
Footnotes
ORCID iD: Teresa Bernal  https://orcid.org/0000-0002-2338-513X
https://orcid.org/0000-0002-2338-513X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Laura Vilorio-Marqués, Instituto de Investigación Sanitaria del Principado de Asturias, Oviedo, Spain.
Christelle Castañón Fernández, Instituto de Investigación Sanitaria del Principado de Asturias, Oviedo, Spain.
Elvira Mora, Hematology Department, Hospital Universitario y Politécnico La Fe, Valencia, Spain.
Lorena Gutiérrez, Hematology Department, Hospital Universitario de Canarias, La Laguna, Spain.
Beatriz Rey Bua, Hematology Department, Hospital Clínico Universitario, Salamanca, Spain.
Maria José Jiménez Lorenzo, Hematology Department, Hospital Germans Trias i Pujol, Institut Català d’Oncologia-Josep Carreras, Leukemia Research Institute, Universitat Autònoma de Barcelona, Barcelona, Spain.
Marina Díaz Beya, Hematology Department, Hospital Clínico Barcelona, Barcelona, Spain.
Miriam Vara Pampliega, Hematology Department, Hospital Universitario Cruces, Barakaldo, Spain.
Antonieta Molero, Hematology Department, Hospital Universitari Vall d’Hebrón, Barcelona, Spain.
Joaquín Sánchez-García, Hematology Department, Hospital Universitario Reina Sofía, Cordoba, Spain.
Marisa Calabuig, Hematology Department, Hospital Clínico de Valencia, Valencia, Spain.
Maria Teresa Cedena, Hematology Department, Hospital Universitario Doce de Octubre, Madrid, Spain.
Tzu Chen-Liang, Hematology Department, Hospital Universitario Morales Messeguer, Murcia, Spain.
Johana Alejandra Díaz Santa, Hematology Department, Institut Catalá de Oncología, Girona, Spain.
Irene Padilla, Hematology Department, Complejo Asistencial Universitario de León, Castilla y León, Spain.
Francisca Hernández, Hematology Department, Hospital Universitario Virgen de las Nieves, Granada, Spain.
Rosana Díez, Hematology Department, Hospital Universitario Miguel Servet, Zaragoza, Spain.
Pedro Asensi, Hematology Department, Hospital Universitario y Politécnico La Fe, Valencia, Spain.
Blanca Xicoy, Hematology Department, Hospital Germans Trias i Pujol, Institut Català d’Oncologia-Josep Carreras, Leukemia Research Institute, Universitat Autònoma de Barcelona, Barcelona, Spain.
Guillermo Sanz, Hematology Department, Hospital Universitario y Politécnico La Fe, Valencia, Spain.
David Valcárcel, Hematology Department, Hospital Universitari Vall d’Hebrón, Barcelona, Spain.
María Diez-Campelo, Hematology Department, Hospital Clínico Universitario, Salamanca, Spain.
Teresa Bernal, Hematology Department, Hospital Universitario Central de Asturias, Instituto de Investigación Sanitaria del Principado de Asturias; Instituto Universitario de Oncología del Principado de Asturias; Departamento de Medicina, Universidad de Oviedo; CIBER enfermedades respiratorias, Madrid, Spain.
Declarations
Ethics approval and consent to participate: The study was approved by the Regional Ethics Committee for Clinical Investigation of the Principado de Asturias (Oviedo, Spain, ref 204/17) and the Institutional Review Board of the Spanish Myelodysplastic Syndromes Group (GESMD). Patients were included in the registry after informed consent in accordance with the Declaration of Helsinki.
Consent for publication: Not applicable.
Author contributions: Laura Vilorio-Marqués: Data curation; Formal analysis; Investigation; Project administration; Writing – review & editing.
Christelle Castañón Fernández: Data curation; Investigation; Writing – review & editing.
Elvira Mora: Data curation; Investigation; Writing – review & editing.
Lorena Gutiérrez: Data curation; Investigation; Writing – review & editing.
Beatriz Rey Bua: Data curation; Investigation; Writing – review & editing.
Maria José Jiménez Lorenzo: Data curation; Investigation; Writing – review & editing.
Marina Díaz Beya: Data curation; Investigation; Writing – review & editing.
Miriam Vara Pampliega: Data curation; Investigation; Writing – review & editing.
Antonieta Molero: Data curation; Investigation; Writing – review & editing.
Joaquín Sánchez-García: Data curation; Investigation; Writing – review & editing.
Marisa Calabuig: Data curation; Investigation; Writing – review & editing.
Maria Teresa Cedena: Data curation; Investigation; Writing – review & editing.
Tzu Chen-Liang: Data curation; Investigation; Writing – review & editing.
Johana Alejandra Díaz Santa: Data curation; Investigation; Writing – review & editing.
Irene Padilla: Data curation; Investigation; Writing – review & editing.
Francisca Hernández: Data curation; Investigation; Writing – review & editing.
Rosana Díez: Data curation; Investigation; Writing – review & editing.
Pedro Asensi: Data curation; Investigation; Writing – review & editing.
Blanca Xicoy: Investigation; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Guillermo Sanz: Methodology; Visualization; Writing – original draft; Writing – review & editing.
David Valcárcel: Conceptualization; Methodology; Visualization; Writing – original draft; Writing – review & editing.
María Diez-Campelo: Conceptualization; Methodology; Visualization; Writing – original draft; Writing – review & editing.
Teresa Bernal: Conceptualization; Formal analysis; Investigation; Methodology; Supervision; Validation; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors declared the following potential conflict of interest with respect to the research, authorship, and/or publication of this article: T.B. has received support for attending meetings and/or travel from Jazz Pharmaceutical, honoraria from Jazz Pharmaceutical, BMS, and Pfizer. D.V. has received honoraria from Celgene/BMS, Amgen, Novartis, Jazz Pharmaceuticals, and Pfizer and support for attending meetings and/or travel from Amgen, BMS/Celgene, and Jazz. M.D.-C. reports honoraria and membership on entity’s Board of directors or advisory committees from Novartis, BMS, and Takeda. Dr G.S. has received personal fees from AbbVie, Amgen, and Astellas and has received research funding from Celgene/BMS Janssen-Cilag, Novartis, Roche, and Takeda.
Availability of data and materials: All data used in the analysis are anonymized.
References
- 1. Bernal T, Martínez-Camblor P, Sánchez-García J, et al. Effectiveness of azacitidine in unselected high-risk myelodysplastic syndromes: results from the Spanish registry. Leukemia 2015; 29: 1875–1881. [DOI] [PubMed] [Google Scholar]
- 2. Mozessohn L, Cheung MC, Fallahpour S, et al. Azacitidine in the ‘real-world’: an evaluation of 1101 higher-risk myelodysplastic syndrome/low blast count acute myeloid leukaemia patients in Ontario, Canada. Br J Haematol 2018; 181: 803–815. [DOI] [PubMed] [Google Scholar]
- 3. Seymour JF, Döhner H, Minden MD, et al. Incidence rates of treatment-emergent adverse events and related hospitalization are reduced with azacitidine compared with conventional care regimens in older patients with acute myeloid leukemia. Leuk Lymphoma 2017; 58: 1412–1423. [DOI] [PubMed] [Google Scholar]
- 4. Lorenzana N, Avila LF, Alonso S, et al. The impact of antimicrobial prophylaxis in morbidity and infections during azacitidine treatment. Ann Hematol 2017; 96: 1833–1840. [DOI] [PubMed] [Google Scholar]
- 5. Mądry K, Lis K, Biecek P, et al. Predictive model for infection risk in myelodysplastic syndromes, acute myeloid leukemia, and chronic myelomonocytic leukemia patients treated with azacitidine; azacitidine infection risk model: the Polish Adult Leukemia Group Study. Clin Lymphoma Myeloma Leuk 2019; 19: 264–274. [DOI] [PubMed] [Google Scholar]
- 6. Fenaux P, Bowen D, Gattermann N, et al. Practical use of azacitidine in higher-risk myelodysplastic syndromes: an expert panel opinion. Leuk Res 2010; 34: 1410–1416. [DOI] [PubMed] [Google Scholar]
- 7.https://www.ema.europa.eu/en/documents/product-information/vidaza-epar-product-information_es.pdf
- 8.https://www.ema.europa.eu/en/medicines/human/EPAR/dacogen
- 9. Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012; 120: 2454–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Such E, Germing U, Malcovati L, et al. Development and validation of a prognostic scoring system for patients with chronic myelomonocytic leukemia. Blood 2013; 121: 3005–3015. [DOI] [PubMed] [Google Scholar]
- 11. Schanz J, Tüchler H, Solé F, et al. New comprehensive cytogenetic scoring system for primary myelodysplastic syndromes (MDS) and oligoblastic acute myeloid leukemia after MDS derived from an international database merge. J Clin Oncol 2012; 30: 820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Döhner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129: 424–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hann I, Viscoli C, Paesmans M, et al. A comparison of outcome from febrile neutropenic episodes in children compared with adults: results from four EORTC studies. Br J Haematol 1997; 99: 580–588. [DOI] [PubMed] [Google Scholar]
- 14. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
- 15.https://www.gesmd.es/actividad-cientifica/guias-smd-y-lmmc-2/
- 16. von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61: 344–349. [DOI] [PubMed] [Google Scholar]
- 17. Itzykson R, Thépot S, Quesnel B, et al. Prognostic factors for response and overall survival in 282 patients with higher-risk myelodysplastic syndromes treated with azacitidine. Blood 2011; 117: 403–411. [DOI] [PubMed] [Google Scholar]
- 18. Ramos F, Thépot S, Pleyer L, et al. Azacitidine frontline therapy for unfit acute myeloid leukemia patients: clinical use and outcome prediction. Leuk Res 2015; 39: 296–306. [DOI] [PubMed] [Google Scholar]
- 19. Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 Update by the Infectious Diseases Society of America. Clin Infect Dis 2011; 52: 427–431. [DOI] [PubMed] [Google Scholar]
- 20. Fenaux P, Mufti GJ, Hellstrom-Lindberg E, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009; 10: 223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dombret H, Seymour JF, Butrym A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood 2015; 126: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lübbert M, Suciu S, Baila L, et al. Low-dose decitabine versus best supportive care in elderly patients with intermediate- or high-risk myelodysplastic syndrome (MDS) ineligible for intensive chemotherapy: final results of the randomized phase III study of the European Organisation for Research and Treatment of Cancer Leukemia Group and the German MDS Study Group. J Clin Oncol 2011; 29: 1987–1996. [DOI] [PubMed] [Google Scholar]
- 23. Kantarjian H, Issa JPJ, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 2006; 106: 1794–1803. [DOI] [PubMed] [Google Scholar]
- 24. Kantarjian HM, Thomas XG, Dmoszynska A, et al. Multicenter, randomized, open-label, phase III trial of decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol 2012; 30: 2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Merkel D, Filanovsky K, Gafter-Gvili A, et al. Predicting infections in high-risk patients with myelodysplastic syndrome/acute myeloid leukemia treated with azacitidine: a retrospective multicenter study. Am J Hematol 2013; 88: 130–134. [DOI] [PubMed] [Google Scholar]
- 26. Reda G, Riva M, Fattizzo B, et al. Bone marrow fibrosis and early hematological response as predictors of poor outcome in azacitidine treated high risk-patients with myelodysplastic syndromes or acute myeloid leukemia. Semin Hematol 2018; 55: 202–208. [DOI] [PubMed] [Google Scholar]
- 27. Chen TC, Wang RC, Lin YH, et al. Posaconazole for the prophylaxis of invasive aspergillosis in acute myeloid leukemia: is it still useful outside the clinical trial setting. Ther Adv Hematol 2020; 11: 2040620720965846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Malcovati L, Della Porta MG, Strupp C, et al. Impact of the degree of anemia on the outcome of patients with myelodysplastic syndrome and its integration into the WHO classification-based Prognostic Scoring System (WPSS). Haematologica 2011; 96: 1433–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pleyer L, Leisch M, Kourakli A, et al. Outcomes of patients with chronic myelomonocytic leukaemia treated with non-curative therapies: a retrospective cohort study. Lancet Haematol 2021; 8: e135–e148. [DOI] [PubMed] [Google Scholar]
- 30. Schuck A, Goette MC, Neukirchen J, et al. A retrospective study evaluating the impact of infectious complications during azacitidine treatment. Ann Hematol 2017; 96: 1097–1104. [DOI] [PubMed] [Google Scholar]
- 31. Falantes JF, Calderón C, Márquez-Malaver FJ, et al. Patterns of infection in patients with myelodysplastic syndromes and acute myeloid leukemia receiving azacitidine as salvage therapy. Clin Lymphoma Myeloma Leuk 2014; 14: 80–86. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tah-10.1177_20406207221127547 for Relevance of infections on the outcomes of patients with myelodysplastic syndromes, chronic myelomonocytic leukemia, and acute myeloid leukemia treated with hypomethylating agents: a cohort study from the GESMD by Laura Vilorio-Marqués, Christelle Castañón Fernández, Elvira Mora, Lorena Gutiérrez, Beatriz Rey Bua, Maria José Jiménez Lorenzo, Marina Díaz Beya, Miriam Vara Pampliega, Antonieta Molero, Joaquín Sánchez-García, Marisa Calabuig, Maria Teresa Cedena, Tzu Chen-Liang, Johana Alejandra Díaz Santa, Irene Padilla, Francisca Hernández, Rosana Díez, Pedro Asensi, Blanca Xicoy, Guillermo Sanz, David Valcárcel, María Diez-Campelo and Teresa Bernal in Therapeutic Advances in Hematology