Abstract
Haemophilus influenzae has an absolute requirement for NAD (factor V) because it lacks almost all the biosynthetic enzymes necessary for the de novo synthesis of that cofactor. Factor V can be provided as either nicotinamide adenosine dinucleotide (NAD), nicotinamide mononucleotide (NMN), or nicotinamide riboside (NR) in vitro, but little is known about the source or the mechanism of uptake of these substrates in vivo. As shown by us earlier, at least two gene products are involved in the uptake of NAD, the outer membrane lipoprotein e (P4), which has phosphatase activity and is encoded by hel, and a periplasmic NAD nucleotidase, encoded by nadN. It has also been observed that the latter gene product is essential for H. influenzae growth on media supplemented with NAD. In this report, we describe the functions and substrates of these two proteins as they act together in an NAD utilization pathway. Data are provided which indicate that NadN harbors not only NAD pyrophosphatase but also NMN 5′-nucleotidase activity. The e (P4) protein is also shown to have NMN 5′-nucleotidase activity, recognizing NMN as a substrate and releasing NR as its product. Insertion mutants of nadN or deletion and site-directed mutants of hel had attenuated growth and a reduced uptake phenotype when NMN served as substrate. A hel and nadN double mutant was only able to grow in the presence of NR, whereas no uptake of NMN was observed.
Haemophilus influenzae, a gram-negative facultative anaerobic bacterium, is responsible for significant morbidity and mortality in young children (9, 35). In order to cultivate H. influenzae, complex medium is required, and if it is not blood based, it must contain two growth factors: nicotinamide adenine dinucleotide (NAD) and hemin (6). Early biochemical investigations established that nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) can substitute for NAD, whereas nicotinamide, niacin, or other nicotine-based intermediates of the Preiss-Handler pathway cannot (10, 20, 31). The NAD dependency of H. influenzae was confirmed by the absence of the genes encoding the enzymes necessary for the de novo biosynthesis of NAD (8). Accumulation of nicotinamide nucleotides derived from NAD or NR has been demonstrated in H. influenzae and Haemophilus parainfluenzae (4, 11). For H. parainfluenzae the Km for transport is about 0.55 μM for NAD and 0.14 μM for NR, while the Vmax for NR is about four times that of NAD (4). This implies that NR is the substrate for an as-yet-unidentified inner membrane transporter, a proposal that is supported by the observation that NAD cannot be taken up into the cytosolic compartment as an intact molecule. Limited NAD salvage capacity resides within the H. influenzae cytosol, which can be demonstrated if cell extracts are incubated with NR or NMN, indicating the presence of an NMN adenylyl transferase or an NAD pyrophosphorylase activity (5, 16).
In other bacteria, NAD is degraded into NMN or NR prior to uptake. In Salmonella enterica serovar Typhimurium, an inner membrane-associated NAD pyrophosphatase is present with its activity expressed in the periplasm (7). The encoding gene is pnuE (22), and PnuE is needed to utilize NAD and to produce NMN. NMN is transported across the cytoplasmic membrane via two independent routes. One system resembles that of an active transport, consisting of two gene products encoded by pnuC and nadR. PnuC was characterized as an integral membrane protein essential for transport (37), and NadR was characterized in several ways. It is a repressor protein involved in feedback regulation coupled with de novo biosynthesis of NAD (14, 24), and it participates in NAD uptake mechanisms acting on PnuC (37), but it is nonessential (38). Recently, it was shown that in Escherichia coli NadR itself has NAD pyrophosphorylase activity (25). In addition, a second NAD transport route exists, in which a membrane-bound NMN glycohydrolase, presumably an inner membrane protein facing the periplasm, effects the release of nicotinamide, which is then assumed to diffuse across the inner membrane (for a review, see reference 23).
Recently, our investigators presented data showing that two gene products appear to be involved in the NAD utilization pathway of H. influenzae (26). The genes were identified as the hel-encoding outer membrane lipoprotein e (P4) and a periplasm-encoded gene product termed NadN. Knockout mutations of both genes resulted in growth-deficient phenotypes which were dependent on the NAD concentrations provided in the growth medium. The enzymatic activities of both proteins were partially characterized. It was shown by Reidl, Reilly, and colleagues (26–28) that e (P4) is an acid phosphatase, while NadN-enriched protein fractions have NAD pyrophosphatase activity. NadN is thought to be identical to a 64-kDa periplasmic NAD pyrophosphatase described earlier (16). In nontypeable H. influenzae (NTHi), nadN was also identified but was named nucA. The protein product of nucA possessed a 5′-nucleotidase activity that acted on 5′-phosphorylated nucleosides, e.g., adenosine monophosphate (AMP) (36). Green and coworkers showed that e (P4) and NadN proteins are antigenically highly conserved among both typeable H. influenzae and NTHi isolates (12, 13, 36), perhaps indicating the physiological importance of these proteins (36).
In this report, we define the functions of NadN and e (P4) in the NAD uptake pathway of H. influenzae. We present data demonstrating that NadN is identical to NucA and that this protein has the ability to act as an NAD pyrophosphatase as well as an NMN 5′-nucleotidase. Furthermore, we provide data indicating that e (P4) also acts as an NMN 5′-nucleotidase, and deletion or point mutants in hel affect the growth of H. influenzae and the uptake of NAD and NMN. Finally, a nadN hel double mutant was constructed and shown to be unable to utilize NAD or NMN and only able to survive when NR was provided in the growth medium.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
All plasmid constructs were cloned in E. coli strains XL-1 and LE392 (New England Biolabs, Frankfurt, Germany). Reference strain H. influenzae Rd KW20 was obtained from A. Wright (Tufts University, Boston, Mass.), and strain R906 (3) was from A. Smith (University of Missouri, Columbia). These strains were used for the construction of mutants in hel and nadN (Table 1). H. influenzae was grown at 37°C under aerobic conditions on 3.8% brain heart infusion (BHI) agar (Difco Laboratories, Detroit, Mich.) supplemented with NAD (15 to 30 μM) and hemin-chloride (20 μg/ml) (Sigma, Deisenhofen, Germany). H. influenzae mutants REI1010 (nadN::cat), REI1012 (Δhel::kan), GK02 (helD86L), and GK03 (helD84A) were grown on BHI agar supplemented with NMN (30 μM). The double mutant GK04 (nadN::cat Δhel::kan) was grown on NR (30 μM). Antibiotics were used for H. influenzae and E. coli as follows: chloramphenicol (Cm), 2 and 30 μg/ml, respectively; kanamycin (Kan), 10 and 50 μg/ml; ampicillin (Amp), 6 and 100 μg/ml (Sigma). Plasmids used are listed in Table 1. Plasmids were isolated according to the Qiagen kit protocol (Qiagen, Hilden, Germany).
TABLE 1.
Relevant strains and plasmids used in this study
| Strain or plasmid | Phenotype or genotypea | Reference or source |
|---|---|---|
| Strains | ||
| H. influenzae Rd KW20 | Genome determined | 8 |
| R906 | NTHi | 3 |
| REI1010 | Strain Rd (nadN::cat) | 26 |
| REI1012 | Strain Rd (Δhel::kan) Kanr | This study |
| GK02 | Strain Rd (helD86L), with exchange of D to L on aa position 86; Cmr | This study |
| GK03 | Strain Rd (helD84A), with exchange of D to A on aa position 84, Cmr | This study |
| GK04 | Strain Rd (nadN::cat Δhel::kan) Cmr Kanr | This study |
| Plasmids | ||
| pACYC177 | Ampr Kanr | New England Biolabs |
| pACYC184 | Cmr Tetr | New England Biolabs |
| phel1 | EcoRI fragment of ATCC GhiGU90 into pBKS | 28 |
| pGK01 | pACYC184, Cmrhel+ (cat in SwaI site) | This study |
| pSEhel | pACYC184, Cmrhel+ | This study |
| pSEΔhel | pACYC184, Cmr Δhel | This study |
| pSEΔhelkan | pACYC184, Kanr/Cmr Δhel | This study |
| pSE2 | pACYC177, Kanr HI0205+nadN::cat | 26 |
aa, amino acid.
Construction of H. influenzae mutant strains REI1012 (Δhel::kan), GK02 (helD86L), GK03 (helD84A), and GK04 (nadN::cat Δhel::kan).
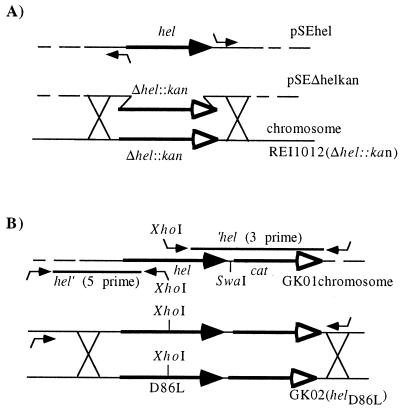
All primers used for cloning and constructions were synthesized by MWG-Biotech, Ebersberg, Germany. For construction of strain REI1012, a chromosomal DNA fragment encoding hel (HI0693) along with adjacent gene sequences was generated by PCR from strain Rd chromosomal DNA with primers hel-5′ and helEcoRV-3′ (Table 2). PCR amplification was carried out with the Thermoprime PCR kit (Advanced Biotechnologies, Hamburg, Germany), using the protocol of Mullis and Faloona (21). Primer helEcoRV-3′ contained an EcoRV site for further subcloning. After PCR amplification, a 3.1-kbp DNA fragment containing bp 736123 to 739333 of the Rd genome (8) was purified, digested with EcoRV and BamHI (a BamHI site is located at bp 736160 on the chromosome, 37 bp in from the 5′ end of the PCR fragment), and ligated into BamHI- and EcoRV-digested pACYC184 (30). The resulting construct was named pSEhel (Fig. 1A). The hel gene was deleted in plasmid pSEhel by inverse PCR using primers designed to contain flanking HpaI sites, ΔP4HpaI-5′ and ΔP4HpaI-3′ (Fig. 1A; Table 2). The resulting 6.35-kbp amplicon was digested with HpaI and then religated to obtain plasmid pSEΔhel. An aminoglycoside 3′ phosphotransferase gene (kan), derived from pACYC177 (29), was isolated as a HincII and StuI fragment and ligated into the HpaI site of pSEΔhel, resulting in plasmid pSEΔhelkan (Fig. 1A). This plasmid served as the template for the amplification of the Δhel::kan region by PCR with primers helScaI-5′ and a pACYC184-specific primer, BamHI-3′ (Table 2). The resulting 3.5-kbp DNA fragment was transformed into H. influenzae Rd according to the method of Tomb et al. (33). Kanr transformants were selected on BHI agar containing hemin (20 μg/ml), NMN (30 μM), and kanamycin (10 μg/ml), and the resultant strain was designated REI1012 (Δhel::kan). Strain construction was verified by PCR and Southern (data not shown) and Western blot analyses (Fig. 2B).
TABLE 2.
Primers used for strain constructions
| Primer | Sequence (5′ to 3′) |
|---|---|
| hel-5′ | TTGAGCTCTTGTTCCAGCTTTCACAGGCT |
| helEcoRV-3′ | TCGATATCACAAATGCGCTATTCTGACGGG |
| ΔP4Hpal-5′ | CCGTTAACTTGAGGGGCTAAGCTCAGTT |
| ΔP4Hpal-3′ | GGGTTAACGGGTATAGTAAGTCTTTCTG |
| BamHI-3′ | TAAGGGGATGCGTCCGGCGTA |
| helBamHI5′ | GGATTCATATGCACGCGGT |
| P4XhoI3′ | AGTCTCGAGTAAATCAGCCACAAGCGCTTT |
| P4XhoI5′ | TTACTCGAGACTATGTTAGACAACAGCCCT |
| P4cat3′ | TGGTTTCATCCAACGACGACAACG |
| D84A | GCGGTTGTGGCTGCTTTAGATGAAACTATGTTAG |
| HI0206-L′ | TAATCCCTTACCAATAGGAG |
| HI0206-R′ | ACGCGCTATTTTTAGCCTAT |
FIG. 1.
Construction of H. influenzae mutants. (A) REI1012 (Δhel::kan); (B) GK02 (helD86L). Restriction enzymes were XhoI, SwaI, SalI, and KpnI. DNA crossover constructions are indicated by crossing, encoding regions are indicated as solid arrows, and genes encoding antibiotic resistance are indicated as arrows with open arrowheads; for details see the text.
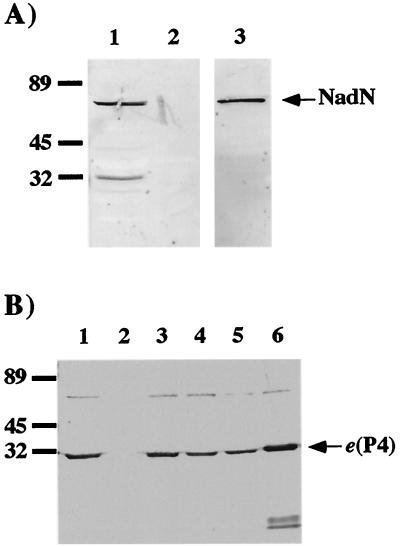
FIG. 2.
Western blot analysis of NadN and e (P4) of H. influenzae Rd. (A) Detection of NadN in periplasmic extracts. Protein sizes (in kilodaltons) are as indicated. Lane 1, Rd extract; lane 2, extract of REI1010 (nadN::cat); lane 3, purified NadN. (B) Detection of e (P4) in OMP extracts. Lane 1, Rd extract; lane 2, extract of REI1012 (Δhel::kan); lane 3, extract of REI1012 (Δhel::kan) complemented with e (P4) on plasmid pJRP4; lane 4, GK02 (helD86L) extract; lane 5, GK03 (helD84A) extract; lane 6, purified e (P4). Samples were adjusted to a protein concentration of 20 μg/ml and subjected to SDS–12% PAGE.
A D86L point mutation was constructed in hel, resulting in mutant strain GK02. A purified cat gene, encoding chloramphenicol acetyltransferase derived from pACYC184 (18, 30), was digested with SnaBI and FspI and ligated into the SwaI site 7 bp downstream of the hel stop codon in pSEhel. The resulting plasmid, pGK01, was digested with EcoRV and SnaBI, releasing a 2.5-kbp DNA fragment encoding the cat gene and 393 bp of the hel gene. This was transformed into H. influenzae Rd with Cmr transformants selected on BHI agar containing hemin (20 μg/ml), NMN (30 μM), and chloramphenicol (2 μg/ml). The resulting mutant strain (GK01) encoded cat downstream from the 3′ end of hel and in the same orientation (Fig. 1B). Two PCR products were generated from the hel gene of strain GK01, each encoding an XhoI restriction site at either the 5′ or the 3′ end. Normally, no XhoI site can be found within hel; however, by changing the sequence motif GATGAA at bp 256 to 261 to an XhoI site, CTCGAG, the aspartate at position 86 is replaced with leucine. The hel 5′ PCR product (1,550 bp) (Fig. 1B), containing the promoter region and the N terminus of hel with a 3′ XhoI site, was generated with primers helBamHI5′ and P4XhoI3′ (Table 2). The hel 3′ PCR product (1,800 bp; Fig. 1B) was generated with primers P4XhoI5′ and P4cat3′ and started at the 5′ XhoI site, contained the C terminus of hel, and ended downstream of the cat cassette (Table 2). Both hel-5′ and hel-3′ were digested with XhoI and ligated, and the ligation product was PCR amplified with helBamHI5′ and P4cat3′. The amplicon was purified and transformed into REI1012 (Fig. 1B). Cmr transformants were selected on BHI agar containing hemin (20 μg/ml), NMN (30 μM), and chloramphenicol (2 μg/ml) and replicated to BHI agar containing kanamycin (10 μg/ml). Cmr and Kans colonies were isolated and purified, and the resultant strain, GK02, was verified by PCR and DNA sequence analysis (data not shown). This D86L hel point mutation (strain GK02) was characterized by Western blot analysis (Fig. 2B) and phosphatase assays (see Fig. 4).
FIG. 4.
Enzymatic characterization of e (P4). (A) TLC of [14C]NMN (10 nCi; 0.1 μM) incubated with purified e (P4) (lane 1) and outer membrane extracts of strains Rd (lane 2), REI1012 (lane 3), and REI1012 (lane 4) complemented with pJRP4. (B) Activity of e (P4) phosphatase point mutants, GK02 and GK03. [14C]NMN (10 nCi; 0.1 μM) was incubated with Rd (lane 1), GK02 (lane 2), and GK03 extracts (lane 3).
A D84A point mutation was differently constructed in hel, resulting in mutant strain GK03. Wild-type hel cloned into the EcoRI site of pBluescript and designated phel1 (27) was used as the substrate for PCR mutagenesis. Site-directed mutations were generated using the QuickChange site-directed mutagenesis kit (Stratagene, Amsterdam, The Netherlands) according to the manufacturer's instructions. The primer D84A, containing the degenerate nucleotide (indicated by underlining, Table 2), and the reverse complement primer were used for PCR amplification of plasmid phel1. PCR-mutated phel1 was restricted with DpnI to remove nonmutated phel1, prior to transformation into E. coli DH5α. Transformants selected on Luria-Bertani agar containing ampicillin were assayed for phosphomonoesterase activity as described earlier (28). E. coli clones lacking phosphomonoesterase activity were isolated and mutated phel1 was recovered (Wizard Plus Miniprep kit; Promega). A 1.3-kb HincII fragment containing the cat gene was cloned into a SwaI site 3′ to the hel open reading frame in mutant phel1 plasmids. cat-containing phel1 plasmid, designated phel1cat, was transformed into DH5α and selected on Luria-Bertani agar containing ampicillin and chloramphenicol. Subsequently, the mutant hel gene associated with cat was excised from mutant phel1cat with EcoRI and transformed into H. influenzae strain R906. Transformants were selected on chocolate agar containing chloramphenicol. Selected transformants were analyzed for phosphomonoesterase activity and for the presence of e (P4) by Western blot analysis (data not shown). Subsequent transformation of chromosomal DNA from strain R906, containing the hel point mutation (D84A) and cat gene, into strain REI1012 (Δhel::kan) resulted in a Kans Cmr Rd helD84A mutant, GK03. This strain was also tested for phosphomonoesterase activity (see Fig. 4B) and the presence of the e (P4) antigen by Western blot analysis (Fig. 2B).
Plasmid pSE2, containing nadN::cat (26), was used for the construction of GK04 (nadN::cat Δhel::kan) in strain Rd. A 2.8-kbp nadN::cat DNA fragment was PCR amplified with primers HI0206-L′ and HI0206-R′ (Table 2) and was transformed into H. influenzae strain Rd REI1012. Cmr colonies of strain GK04 were isolated and purified on BHI agar containing hemin (20 μg/ml), NR (30 μM), and chloramphenicol (2 μg/ml). Isolated clones were verified by PCR and Southern and Western blot analyses (data not shown).
Nicotinamide nucleotide reagents.
β-NAD, β-NMN, and AMP were obtained from Sigma. NR was prepared by incubating β-NAD with shrimp alkaline phosphatase in shrimp phosphatase buffer according to the manufacturer's instructions (Amersham Pharmacia Biotech, Freiburg, Germany). Carbonyl-[14C]NAD was obtained from Amersham, and [14C]NMN was prepared from carbonyl-[14C]NAD by treatment with snake venom nucleotide pyrophosphatase (Sigma). [14C]NR was prepared by incubating carbonyl-[14C]NAD, snake venom nucleotide pyrophosphatase, and alkaline phosphatase in alkaline phosphatase buffer for 1 h at 37°C. Enzymes were inactivated by adding 5% trichloroacetic acid, followed by 10-min incubation on ice. Subsequently, the supernatants were recovered after centrifugation (5 min, 16,000 × g) and neutralized to pH 7 with 6 N NaOH.
Isolation of OMP and periplasmic protein extracts.
Outer membrane proteins (OMPs) were prepared according to a modified (26) protocol of Carlone et al. (2). Samples were kept in HEPES (10 mM) and glycerol (5%) and were stored at −20°C. For the preparation of periplasmic extracts, overnight cultures (5 ml) of strain Rd or REI1010 were harvested by centrifugation and washed once with Tris-HCl (50 mM, pH 8.5) at 4°C. The pellets were resuspended in 200 μl of Tris-HCl (50 mM, pH 8.5) containing polymyxin B (2 mg/ml) and incubated for 10 min on ice. Cells were then centrifuged (20,000 × g, 4°C, 10 min) and the supernatant was recovered. These extracts are referred to as periplasmic extracts (15).
Purification of recombinant NadN (NucA) and e (P4) protein.
Recombinant NadN (NucA), originally derived from nontypeable H. influenzae (NTHi) was purified from E. coli INVF′(pPX691) as previously described (36). The purified recombinant protein was dialyzed into phosphate-buffered saline (pH 7.2) and stored frozen at −20°C. The recombinant e (P4) protein used for this study was isolated as described earlier (28).
Protein analysis.
Protein concentrations of OMP extracts, periplasmic extracts, and purified recombinant proteins were determined according to the method of Bradford (1), using the Bio-Rad protein assay kit (Bio-Rad, München, Germany). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (19). After SDS-PAGE, proteins were detected by Western blot analysis, according to the methods of Towbin et al. (34), with monoclonal antibodies (MAbs) directed against e (P4) and NadN, respectively, as described earlier (12, 36).
Characterization and quantification of NAD pyrophoshatase and 5′-nucleotidase activities.
Purified NadN (NucA) (0.12 mg of protein/ml) was incubated with 0.1 μM specific radioactive labeled substrate in shrimp alkaline phosphatase buffer for 1.5 h at 37°C. The enzymatic activity of periplasmic extracts was determined by incubating 1 mg of extract/ml with sample buffer (50 mM MgCl2, pH 8.5) and 0.01 to 0.1 μM concentrations of radioactive 14C-labeled substrate for 1.5 h at 37°C. Reactions were terminated by adding 5% trichloroacetic acid, followed by 10-min incubation on ice. Subsequently, the supernatants were recovered after centrifugation (5 min, 10,000 rpm) and NaOH (6 N) was added to neutralize the samples to pH 7. Aliquots of 2 μl were subsequently analyzed by thin-layer chromatography (TLC). Purified e (P4) protein was incubated with [14C]NAD, [14C]NMN, or [14C]NR (a 0.1 μM concentration of each) in HEPES buffer (10 mM, pH 6) for 1.5 h at 37°C. OMP extracts were incubated with sample buffer (50 mM MgCl2, pH 6.0) and [14C]NAD, [14C]NMN, or [14C]NR (each at a 0.1 μM concentration) for 1.5 h at 37°C. Samples were then treated as described for NadN (see above).
Activity of NadN against NMN, NAD, and AMP and of e (P4) against NMN was determined by measuring the release of inorganic phosphate from these substrates as previously described (27, 36). The NadN Kms for AMP, NAD, and NMN were determined by using a Lineweaver-Burk double-reciprocal plot based on individual substrate saturation curves. The e (P4) Km for NMN was determined by using the computer-based method of Brooks (1a).
TLC.
Radioactively labeled samples were separated by TLC in a solvent system consisting of 1 M ammonium acetate (pH 5) and ethanol (70:30) (17) using Cellulose F plates (Merck, Darmstadt, Germany). After separation, the plates were dried and exposed to radiation-sensitive film (Eastman Kodak Co., Rochester, N.Y.). Spots were identified by comparison with reference samples of [14C]NAD, [14C]NMN, and [14C]NR.
Uptake studies.
H. influenzae strains from overnight cultures were inoculated in BHI (3.8%) medium supplemented with NR (30 μM) and hemin chloride (20 μg/ml) and grown to an optical density at 490 nm (OD490) of ≈1. After centrifugation (4,000 × g, 5 min) the pellets were resuspended in BHI (3.8%) medium without supplements to an OD490 of ≈2. Aliquots of 3 ml were incubated on a heating block to 37°C, and radioactively labeled substrates (1 μM concentration) were added; 500-μl samples were removed after 1, 3, 5, 7, and 9 min. The samples were filtered through ME 25 filters (0.45-μm pore size; Schleicher & Schuell, Dassel, Germany) which had been soaked in 0.1 M NaCl. The filters were washed with 5 ml of NaCl (0.1 M) and placed in vials containing 5 ml of scintillation liquid (Emulsifier-Safe; Packard, Dreieich, Germany). Radioactivity was measured with an SL 6000SC scintillation counter (Beckman, München, Germany). Results are expressed as the percent total cellular accumulation of 14C-labeled metabolite, compared to 100% of the 14C-labeled substrate provided in the assay. Results (means) are shown together with the standard deviation from at least three independent experiments.
RESULTS
Characterization of enzymatic activities of NadN.
Recently, two new gene loci have been described in typeable H. influenzae and NTHi, nadN and nucA, respectively (26, 36). The nadN gene product, NadN, was shown to possess NAD pyrophosphatase activity localized to periplasmic extracts (26). Independently, NucA was purified from an NTHi strain and shown to be a 5′-nucleotidase (36). By comparison of the nucleotide sequences, it was apparent that both proteins were encoded by the same gene.
To demonstrate further that NadN and NucA were the same, periplasmic extracts were isolated from strains Rd and REI1010 and Western blot analysis was performed with a NucA-specific MAb (36). The MAb recognized NadN as a protein of approximately 64 kDa (Fig. 2A). Additional weak bands of about 30 kDa observed only in the Rd extract may have arisen from NadN degradation by copurified proteolytic enzymes in the periplasmic extracts.
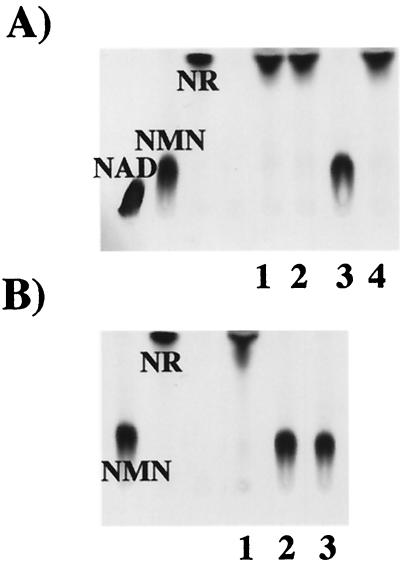
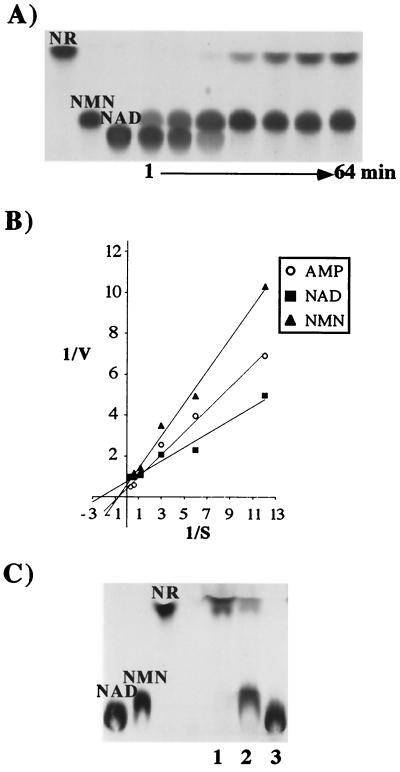
In enzymatic characterization experiments, purified NucA exhibited not just 5′-nucleotidase but also NAD pyrophosphatase activity. Purified NucA sequentially released NMN and then NR from NAD (Fig. 3A). Purified NucA was further assayed to compare the enzyme activity of NAD with alternative substrates, NMN and AMP. Lineweaver-Burk double-reciprocal plots were used to determine Km values for each substrate (Fig. 3B). NAD, NMN, and AMP exhibited Km values of 0.43, 1.26, and 1.09 mM, respectively.
FIG. 3.
Enzymatic characterization of NadN. (A) Kinetic analysis of NAD processing by NadN. [14C]NAD (0.1 mM) was incubated with 0.012 mg of NadN/ml. Sampling was done at the indicated time points. Lanes 1 to 7 correspond to reaction times of 1, 2, 4, 8, 16, 32, and 64 min. (B) Determination of Km values of purified NadN for the substrates NAD, NMN, and AMP; V was determined as change in OD660 over 20 min with 0.16 μg of protein. Reaction mixtures were prepared as described earlier (36). (C) TLC of [14C]NAD used as substrate for Rd periplasmic extracts. [14C]NAD (10 nCi; 0.1 mM) was incubated with purified NadN (lane 1), periplasmic extracts of H. influenzae Rd (lane 2), and REI1010 (lane 3).
The ability of periplasmic extracts of strains Rd and REI1010 to metabolize [14C]NAD to [14C]NMN and [14C]NR was determined by TLC. The periplasmic extract of Rd hydrolyzed [14C]NAD to [14C]NMN and [14C]NR (Fig. 3C, lane 2), whereas the extract from the nadN mutant had no activity against [14C]NAD (Fig. 3C, lane 3).
Characterization of e (P4).
The OMP e (P4) is an acid phosphomonoesterase (26, 27) involved in NAD utilization. To determine a substrate specificity for e (P4), purified e (P4) and OMP preparations from Rd and REI1012 were incubated with 14C-labeled NAD, NMN, and NR. As shown by TLC (Fig. 4A), NMN was dephosphorylated to NR by purified e (P4) and by Rd, but not REI1012, OMP fractions. The absent 5′-nucleotidase activity in the REI1012 OMPs was complemented by hel on plasmid pJRP4. Neither the purified e (P4) nor Rd OMP fractions possessed the ability to use NAD or NR as substrates (data not shown). Results from kinetic characterization of e (P4)-mediated hydrolysis suggest that initial velocity is linearly proportional to enzyme concentration and that the concentration of released inorganic phosphate is directly proportional to the time of incubation for the first 30 min of the reaction at 37°C. The estimated Km and Vmax of the e (P4)-mediated hydrolysis of NMN was 0.318 mM and 0.033 mmoles of Pi released/min/mg of e (P4), respectively. Assays were performed in which substrate concentration [S] were varied from 0.03 to 14.1 times the Km and V varied from 0.01 to 1.01 times the Vmax (data not shown).
Growth phenotypes of H. influenzae Rd, REI1012 (Δhel::kan), and GK04 (nadN::cat Δhel::kan).
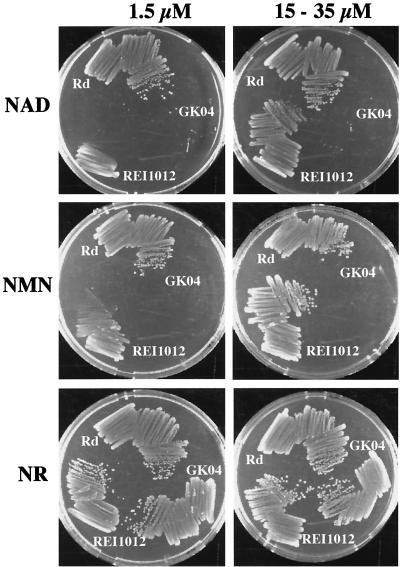
We previously demonstrated that growth of a hel transposon mutant, hel::TnI0d-bla, was dependent on the concentration of NAD provided in the growth medium (26). To confirm the role of e (P4) in the utilization of NAD, a hel-deficient mutant (REI1012) was constructed. The growth of Rd and REI1012 on BHI agar plates supplemented with hemin with various concentrations of NAD, NMN, and NR was tested (Fig. 5).
FIG. 5.
Growth of mutant H. influenzae Rd strains. The strains Rd, REI1012 (Δhel::kan), and GK04 (nadN::cat Δhel::kan) were grown on BHI agar plates supplemented with hemin (20 μg/ml) and different nicotinamide nucleotide concentrations and sources. Growth phenotype is shown with 1.5 and 35 μM NAD, with 1.5 and 15 μM NMN, and with 1.5 and 15 μM NR, as indicated.
Compared with Rd, REI1012 had significantly reduced growth with limiting concentrations of NAD (1.5 μM) or NMN (1.5 μM) but had similar growth with high concentrations of NAD (35 μM) and NMN (15 μM) (Fig. 5). However, REI1012 was able to grow as well as strain Rd even with NR, and even when the concentrations were limiting (Fig. 5).
To investigate whether e (P4) and NadN are the sole proteins that are able to process NMN to NR, a double mutant, GK04, was constructed. GK04 was unable to grow on BHI agar containing either NAD or NMN at 1.5 or 35 μM (Fig. 5). However, in the presence of NR, GK04 grew as well as strain Rd, even at the low NR concentration (1.5 μM) (Fig. 5).
Uptake of 14C-labeled nicotinamide nucleotides.
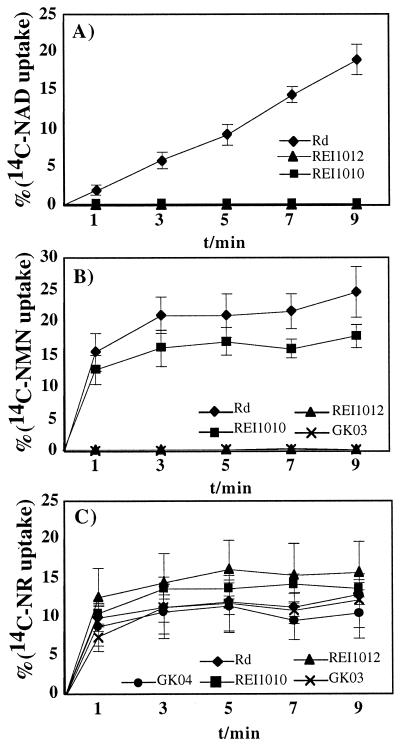
The uptakes of 14C-labeled NAD, NMN, and NR by Rd and the various mutants were compared. Rd incorporated about 20% of the available 14C-labeled NAD and NMN within 9 min. REI1010 and REI1012 were unable to take up [14C]NAD (Fig. 6A). Accumulation of [14C]NMN was not observed for REI1012 and decreased uptake was detected for REI1010 (Fig. 6B). Rd, REI1010, REI1012, and GK04 all showed similar accumulations of [14C]NR, at approximately 11 to 15% (Fig. 6C).
FIG. 6.
Uptake of labeled nicotinamide nucleotide substrates by H. influenzae. Strains used were H. influenzae Rd, REI1010 (nadN::cat), REI1012 (Δhel::kan), GK03 (helD84A), and GK04 (nadN::cat Δhel::kan). Each point represents the mean value with standard deviation obtained from at least three independent measurements. Uptake is given as the percentage of the initial 1 μM substrate concentration. (A) Uptake of [14C]NAD by Rd, REI1010, and REI1012; (B) uptake of [14C]NMN by Rd, REI1010, REI1012, and GK03; (C) uptake of [14C]NR by Rd, REI1010, REI1012, GK03, and GK04.
Characterization of GK02 (helD86L) and GK03 (helD84A).
The surface location of e (P4), originally described by Green et al. (12), led to the question of whether e (P4) acts only as a phosphomonoesterase or possesses other functions necessary for nicotinamide nucleotide utilization, e.g., involvement in substrate binding or transport. To test for other functions, two phosphatase-negative e (P4) point mutants, GK02 and GK03, were constructed. These point mutants are predicted to lack acid phosphatase activity, as the mutations modify two of the four conserved aspartates in group C phosphatases (32). The expression of the mutated e (P4) was verified by Western blot analysis (Fig. 2B, lanes 4 and 5). The expression of helD86L was decreased about 30-fold compared to that of helD84A and wild-type hel, perhaps due to instability, decreased expression, or insufficient translocation of the mutated protein. The protein concentrations of these samples were adjusted to yield equivalent concentrations in Western blotting and enzymatic assays.
To verify the loss of the phosphomonoesterase activity of the mutated e (P4) proteins, OMP preparations of Rd, GK02, and GK03 were incubated with NMN. As shown by TLC, the membrane fractions of GK02 and GK03 were not able to dephosphorylate NMN (Fig. 4B, lanes 2 and 3). In contrast, the Rd membrane extracts readily hydrolyzed NMN (Fig. 4B, lane 1). OMP preparations of GK02 and GK03 were also unable to hydrolyze pNPP (data not shown). GK02 and GK03 showed attenuated growth on NAD and NMN, comparable with that of REI1012 (Fig. 5), but all strains grew well if NR was the nicotinamide nucleotide source (data not shown).
To confirm the observed phenotypes with the acid phosphatase mutants, we performed uptake studies with [14C]NMN and [14C]NR, comparing Rd with REI1012 and GK03. There was no measurable uptake of 14C-labeled NAD or NMN by GK03, as was seen with REI1012 (Fig. 6A and B). Rd, REI1012, and GK03 all incorporated 12 to 15% of the available [14C]NR (Fig. 6C). We conclude that the phosphatase-negative mutants behave exactly like strain REI1012 in [14C]NAD and [14C]NMN uptake, in their growth, and in their ability to dephosphorylate NMN.
DISCUSSION
Recently, we described two gene products, e (P4) and NadN, both involved in the utilization of NAD (26). Individually constructed knockout mutations of both genes gave rise to mutants with growth deficiencies on media containing various concentrations of NAD. We demonstrated that the nadN mutant was unable to grow on NAD-supplemented media and had no detectable NAD pyrophosphatase activity (26). Subsequently, a gene in NTHi was characterized and called nucA. NucA was purified to homogeneity (36), and its activity was defined as a 5′-nucleotidase acting on phosphorylated nucleosides, especially monophosphate nucleosides (AMP, CMP, GMP, UMP, and TMP) (36). The NucA amino acid sequence matched that of the open reading frame HI0206 (NadN) of strain Rd with nearly complete identity (36); thus, NucA in NTHi and NadN in Rd are encoded by the same gene. Therefore we have renamed nucA as nadN, for NAD nucleotidase in H. influenzae.
In this study we showed that both NAD and NMN are substrates for purified NadN. NadN was shown to exhibit NAD nucleotidase activity with hydrolysis of NAD to NMN and dephosphorylation of NMN to NR. Consequently, loss of NadN function causes a complete inability to utilize NAD. Furthermore, the nadN mutant does not take up as much NR derived from NMN as Rd. However, no difference in accumulation of NR was observed. Earlier, it was postulated that NR might serve as the only nicotinamide nucleotide substrate that is able to cross the cytoplasmic membrane by active transport (4). For nadN mutants to be able to accumulate NR derived from NMN, another enzyme must also have NMN 5′-nucleotidase activity. A potential enzyme was the outer membrane lipoprotein e (P4), which was recently identified as an acid phosphatase encoded by hel (26, 27). We had already demonstrated that dephosphorylation of pNPP by e (P4) is strongly inhibited by NMN (26), indicating that NMN might serve as an e (P4) substrate. Furthermore, a hel knockout mutant had reduced growth if NAD concentrations were low. Therefore, we reasoned that e (P4) could be involved in some step in the processing and utilization of NAD.
To test whether NMN, as an intermediate in NAD uptake, is a substrate for e (P4), TLC analyses were performed to identify NMN-specific phosphatase activity. The substrates used were 14C-labeled NAD, NMN, and NR. Purified e (P4) protein and e (P4)-containing OMP fractions were able to catalyze the dephosphorylation of NMN and, subsequently, release of NR was detected. No activity was observed if NAD or NR was added as a substrate (data not shown). A second approach was to quantify the enzyme kinetics, and we determined the Michaelis constant of e (P4) for NMN. A third approach addressed the uptake of NAD, NMN, and NR and the ability to use these substrates for growth by comparing the wild type and the hel mutant. The uptake analyses indicated that the Δhel strain had lost the ability to utilize NR from NAD and NMN (substrate concentrations used corresponded to limiting concentrations in growth media) but was able to take up NR. Consequently, the Δhel strain could not grow with limiting concentrations of NAD and NMN, but it grew well with NR. The phosphatase activity of e (P4) on NMN appears to be important for NMN utilization and hence indirectly also for the utilization of NAD. Therefore, NAD must first be hydrolyzed to NMN, as the only substrate that e (P4) can utilize appears to be NMN. To address whether the hel mutant phenotype is solely explained by loss of phosphatase activity, site-directed point mutations in hel were constructed by replacing amino acid D residues at position 84 or 86 with A and L, respectively. The growth, substrate uptake, and NMN dephosphorylation of these mutants correlated exactly with that of the Δhel mutant. Therefore, we conclude that only the phosphatase activity of e (P4) is involved in NMN utilization.
In conclusion, e (P4) and NadN are both able to hydrolyze NMN. Mutants of nadN or hel are able to grow on high concentrations of NMN (26), but under limiting concentrations the uptake of substrate and growth abilities are reduced. A distinct difference between the nadN and hel mutants can be described as follows: in transport assays a hel deletion mutant is unable to accumulate NR derived from NMN; however, in the nadN mutant the accumulation is only reduced but not completely abrogated because of the remaining e (P4) function. Consistent with that observation, the Km for NMN of e (P4) is lower (0.318 mM) than that of NadN (1.26 mM). Both results may indicate that e (P4) rather than NadN is relevant for production of NR from NMN. As e (P4) is an outer membrane-located lipoprotein (12), one can speculate that NMN might be a relevant and available nicotinamide nucleotide source in vivo and is mainly recognized by e (P4) as a substrate.
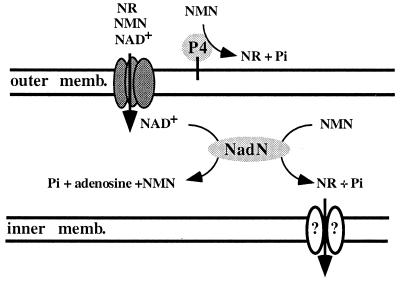
Since NadN also possesses NMN phosphatase activity, a double mutant of hel and nadN was characterized to exclude the possibility that any other enzyme of H. influenzae participates in the NAD pathway. A double mutant was indeed unable to grow on NMN, even at high concentrations, and was only able to grow with and take up the substrate NR. This result suggests strongly that NR is the final and only substrate which is eventually utilized by H. influenzae, as shown in the model (Fig. 7). Based on our deductions from these data, we emphasize that no other enzymatic activity contributes to the release of NR, from either NMN or NAD, and that NR indeed represents the minimal nicotinamide nucleotide requirement and acts as the substrate for an as-yet-unidentified transport system. Both e (P4) and NadN are immunodominant, conserved antigens, and both are ubiquitously expressed by typeable and NTHi strains (12, 13, 27, 36). Therefore, it seems predictable that the combined enzyme action of both gene products is needed by the organism to support its parasitic lifestyle and to obtain a broader nicotinamide nucleotide substrate spectrum. We finally conclude that both enzymes, e (P4) and NadN, act together in the NAD uptake pathway and are both needed for an efficient processing of NAD and NMN to generate NR.
FIG. 7.
Model for nicotinamide nucleotide utilization in H. influenzae. The outer membrane contains e (P4) and a putative diffusion porin, the periplasmic compartment contains NadN, and the inner membrane contains a putative transport complex. The enzymatic activities of the characterized proteins e (P4) and NadN, which are illustrated (for details, see text), lead to the uptake of NR.
ACKNOWLEDGMENTS
We thank J. Blaß for technical assistance and W. Boos for helpful discussions and support.
This work was funded by the BMBF (grant 01KI8906), the DFG (grant Re 1561/1-1), and the National Institutes of Health (grants AI44002 and AI07276).
REFERENCES
- 1.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 1a.Brooks S P J. A simple computer program with statistical tests for the analysis of enzyme kinetics. BioTechniques. 1992;13:901–911. [PubMed] [Google Scholar]
- 2.Carlone A C Y, Myrtle L T, Rumschlag H S, Sottner F O. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J Clin Microbiol. 1986;24:330–332. doi: 10.1128/jcm.24.3.330-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Catlin B W, Bendler J W, Goodgal S H. The type b capsulation locus of Haemophilus influenzae: map location and size. J Gen Microbiol. 1972;70:411–422. doi: 10.1099/00221287-70-3-411. [DOI] [PubMed] [Google Scholar]
- 4.Cynamon M H, Sorg T B, Patapow A. Utilization and metabolism of NAD by Haemophilus parainfluenzae. J Gen Microbiol. 1988;134:2789–2799. doi: 10.1099/00221287-134-10-2789. [DOI] [PubMed] [Google Scholar]
- 5.Denicola-Seoane A, Anderson B M. Studies of NAD kinase and NMN:ATP adenylyltransferase in Haemophilus influenzae. J Gen Microbiol. 1990;136:425–430. doi: 10.1099/00221287-136-3-425. [DOI] [PubMed] [Google Scholar]
- 6.Evans N M, Smith D D, Wicken A J. Hemin and nicotinamide adenine dinucleotide requirements of Haemophilus influenzae. J Med Microbiol. 1974;7:359–365. doi: 10.1099/00222615-7-3-359. [DOI] [PubMed] [Google Scholar]
- 7.Falconer D F, Spector M P, Foster W. Membrane association of NAD pyrophosphatase in Salmonella typhimurium. Curr Microbiol. 1984;10:237–242. [Google Scholar]
- 8.Fleischmann R D, Adams M D, White O, Clayton R A, Kirkness E F, Kerlavage A R, Bult C J, Tomb J F, Dougherty B A, Merrick J M, McKenney K, Sutton G, FitzHugh W, Fields C, Gocayne J D, Scott J, Shirley R, Liu L I, Glodek A, Kelley J M, Weidman J F, Phillips C A, Spriggs T, Hedblom E, Cotton M D, Utterback T R, Hanna M C, Nguyen D T, Saudek D M, Brandon R C, Fine L D, Frichman J L, Fuhrmann J L, Geoghagen N S M, Gnehm C L, McDonald L A, Small K V, Fraser C M, Smith H O, Venter J C. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 9.Funkhouser A, Steinhoff M C, Ward J. Haemophilus influenzae disease and immunization in developing countries. Rev Infect Dis. 1991;13:542–554. doi: 10.1093/clinids/13.supplement_6.s542. [DOI] [PubMed] [Google Scholar]
- 10.Gingrich W, Schlenk F. Codehydrogenase I and other pyridinium compounds as V factor for Haemophilus influenzae and Haemophilus parainfluenzae. J Bacteriol. 1944;47:535–550. doi: 10.1128/jb.47.6.535-550.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Godek C P, Cynamon M H. In vitro evaluation of nicotinamide riboside analogs against Haemophilus influenzae. Antimicrob Agents Chemother. 1990;34:1473–1479. doi: 10.1128/aac.34.8.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green B A, Farley J E, Quinn-Dey T, Deich R A, Zlotnick G W. The e (P4) outer membrane protein of Haemophilus influenzae: biologic activity of anti-e serum and cloning and sequencing of the structural gene. Infect Immun. 1991;59:3191–3198. doi: 10.1128/iai.59.9.3191-3198.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green B A, Vazquez M E, Zlotnick G W, Quigley-Reape G, Swarts J D, Green I, Cowell J L, Bluestone C D, Doyle W J. Evaluation of mixtures of purified Haemophilus influenzae outer membrane proteins in protection against challenge with nontypeable H. influenzae in the chinchilla otitis media model. Infect Immun. 1993;61:1950–1957. doi: 10.1128/iai.61.5.1950-1957.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holley E A, Spector M P, Foster J W. Regulation of NAD biosynthesis in Salmonella typhimurium: expression of nad-lac gene fusions and identification of a nad regulatory locus. J Gen Microbiol. 1985;131:2759–2770. doi: 10.1099/00221287-131-10-2759. [DOI] [PubMed] [Google Scholar]
- 15.Hovde C J, Calderwood S B, Mekalanos J J, Collier R J. Evidence that glutamic acid 167 is an active site residue of shiga-like toxin I. Proc Natl Acad Sci USA. 1988;85:2568–2572. doi: 10.1073/pnas.85.8.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kahn D W, Anderson B M. Characterization of Haemophilus influenzae nucleotide pyrophosphatase. J Biol Chem. 1986;261:6016–6025. [PubMed] [Google Scholar]
- 17.Kasarov L B, Moat A G. Convenient method for enzymatic synthesis of 14C-nicotinamide riboside. Anal Biochem. 1972;46:181–186. doi: 10.1016/0003-2697(72)90410-1. [DOI] [PubMed] [Google Scholar]
- 18.Kraiß A, Schlör S, Reidl J. In vivo transposon mutagenesis in Haemophilus influenzae. Appl Environ Microbiol. 1998;64:4697–4702. doi: 10.1128/aem.64.12.4697-4702.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Leder I G, Handler P. Synthesis of nicotinamide mononucleotide by human erythrocytes in vitro. J Biol Chem. 1951;189:889–899. [PubMed] [Google Scholar]
- 21.Mullis K B, Faloona F. Specific synthesis of DNA in vitro via a polymerase chain reaction. Methods Enzymol. 1987;155:335–340. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 22.Park U E, Roth J R, Olivera B M. Salmonella typhimurium mutants lacking NAD pyrophosphatase. J Bacteriol. 1988;170:3725–3730. doi: 10.1128/jb.170.8.3725-3730.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Penfound T, Foster J W. Biosynthesis and recycling of NAD. In: Neidhard F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1996. pp. 721–730. [Google Scholar]
- 24.Penfound T, Foster J W. NAD-dependent DNA binding activity of the bifunctional NadR regulator of Salmonella typhimurium. J Bacteriol. 1999;181:648–655. doi: 10.1128/jb.181.2.648-655.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raffaelli N, Lorenzi P L, Emanuelli M, Amici A, Ruggieri S, Magni G. The Escherichia coli NadR regulator is endowed with nicotinamide mononucleotide adenylytransferase activity. J Bacteriol. 1999;181:5509–5511. doi: 10.1128/jb.181.17.5509-5511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reidl J, Schlör S, Kraiss A, Schmidt-Brauns J, Kemmer G, Soleva E. NADP and NAD utilization in Haemophilus influenzae. Mol Microbiol. 2000;35:1573–1581. doi: 10.1046/j.1365-2958.2000.01829.x. [DOI] [PubMed] [Google Scholar]
- 27.Reilly T J, Chance D L, Smith A L. Outer membrane lipoprotein e (P4) of Haemophilus influenzae is a novel phosphomonoesterase. J Bacteriol. 1999;181:6797–6800. doi: 10.1128/jb.181.21.6797-6805.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reilly T J, Smith A L. Purification and characterization of a recombinant Haemophilus influenzae outer membrane phosphomonoesterase e (P4) Protein Expr Purif. 1999;17:401–409. doi: 10.1006/prep.1999.1157. [DOI] [PubMed] [Google Scholar]
- 29.Rose R E. The nucleotide sequence of pACYC177. Nucleic Acids Res. 1988;16:356. doi: 10.1093/nar/16.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose R E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988;16:355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shifrine M, Biberstein E L. A growth factor for Haemophilus species secreted by a Pseudomonad. Nature. 1960;187:623. [Google Scholar]
- 32.Thaller M C, Schippa S, Rossolini G M. Conserved sequence motifs among bacterial, eukaryotic, and archaeal phosphatases that define a new phosphohydrolase superfamily. Protein Sci. 1998;7:1647–1652. doi: 10.1002/pro.5560070722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomb J F, Barcak G J, Chandler M S, Redfield R J, Smith H O. Transposon mutagenesis, characterization, and cloning of transformation genes of Haemophilus influenzae Rd. J Bacteriol. 1989;171:3796–3802. doi: 10.1128/jb.171.7.3796-3802.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Turk D C. The pathogenicity of Haemophilus influenzae. J Med Microbiol. 1984;18:1–16. doi: 10.1099/00222615-18-1-1. [DOI] [PubMed] [Google Scholar]
- 36.Zagursky R J, Ooi P, Jones K F, Fiske M J, Smith R P, Green B A. Identification of a Haemophilus influenzae 5′-nucleotidase protein: cloning of the nucA gene and immunogenicity and characterization of the NucA protein. Infect Immun. 2000;68:2525–2534. doi: 10.1128/iai.68.5.2525-2534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhu N, Olivera M, Roth J R. Genetic characterization of the pnuC gene, which encodes a component of the nicotinamide mononucleotide transport system in Salmonella typhimurium. J Bacteriol. 1989;171:4402–4409. doi: 10.1128/jb.171.8.4402-4409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu N, Roth J R. The nadI region of Salmonella typhimurium encodes a bifunctional regulatory protein. J Bacteriol. 1991;173:1302–1310. doi: 10.1128/jb.173.3.1302-1310.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]