Abstract
Background:
Social well-being of patients with inflammatory bowel disease (IBD) is garnering increased attention; however, the impact of social isolation remained poorly understood.
Objectives:
We investigated the joint association of social isolation and IBD with premature deaths to articulate the profound impact of social isolation in IBD prognosis.
Design:
Longitudinal cohort study.
Methods:
We leveraged data of 486,014 participants from UK Biobank (including 5791 with IBD), the mean follow-up was 11.84 years. Diagnoses of IBD and its subtypes of Crohn’s disease (CD) and ulcerative colitis were confirmed with the combination of self-reporting, primary care, and hospital admission data. Social isolation was measured by the frequency of meeting family/friends, leisure and social activity, and communal/solitary living. Mortality was ascertained through data linkage with national death registries. Multivariable Cox regression models were conducted to estimate hazard ratio (HR) and 95% confidence interval (CI).
Results:
Comparing non-isolated non-IBD population, the HRs of mortality in patients with IBD who were socially isolated or not were 2.06 (95% CI: 1.69, 2.51) and 1.33 (95% CI: 1.21, 1.45), respectively. The excess risk of death was observed in socially isolated patients with IBD (HR = 1.69, 95% CI: 1.36, 2.11), particularly among patients with CD (HR = 2.06, 95% CI: 1.48, 2.87) than their non-isolated counterparts. Data from subgroup and sensitivity analyses were consistent with those from the primary analysis.
Conclusion:
Socially isolated patients with IBD especially CD increases the risk of premature death. Preventing social isolation might be a promising approach to improve IBD prognosis.
Plain language summary
Social isolation as a risk factor to excess mortality in patients with IBD: findings from a longitudinal cohort study
Social isolation is prevalent in individuals with inflammatory bowel disease (IBD); however, its potential health impact on IBD prognosis has not been quantitatively well examined. In this study, we explored the association between social isolation and subsequent death, with the focus on patients with IBD.
We leveraged data of 486,014 participants (including 5791 with IBD) from UK Biobank. We measured social isolation by the frequency of meeting family/friends, leisure and social activity, and communal/solitary living. We ascertained patients with IBD and mortality by self-report data and data linkage with primary care, hospital, and national death registry. Participants were followed up for a mean of 11.84 years.
Comparing non-isolated non-IBD population, we found that patients with IBD who were deemed as socially isolated or not were associated with a 2.06-fold (1.69–2.51) and 1.33-fold (1.21–1.45) risk of death, respectively. Furthermore, we revealed that socially isolated patients with IBD and subtype Crohn’s disease (CD) had 69% (36–111%) and 106% (48–187%) increased risk of premature death compared with their non-isolated counterparts, respectively.
Social isolation merits attention in IBD care and management. Patients with IBD, especially CD, are more likely to be affected when socially isolated. Targeted social support strategies ought to be devised to improve IBD prognosis.
Keywords: Crohn’s disease, inflammatory bowel disease, mortality, social isolation, ulcerative colitis
Introduction
Inflammatory bowel disease (IBD) encompasses Crohn’s disease (CD) and ulcerative colitis (UC), and its symptoms can persist over a patient’s lifetime due to no cure for this disease yet. 1 Patients with IBD are known to have distressing and severe physical symptoms such as abdominal pain, diarrhea, fecal urgency, weight loss, and rectal bleeding. These can cause emotional and social stress that persists even during remission periods, contributing to the deterioration of the quality of life.2,3
Social isolation refers to an absence of social interactions, social support structures, and engagement with wider community activities. 4 There has been an increasing concern regarding the health impact that is attributable to social isolation in patients with IBD, especially in the current COVID-19 pandemic. 5 According to a recent qualitative meta-synthesis, social isolation in patients with IBD was identified as one of the main obstacles to leading a normal life. 6 However, so far, evidence has been largely limited to qualitative studies. While such qualitative studies captured that patients with IBD might be particularly more prone to experience social isolation due to disease-related stress, 7 negative perception of social support, 8 lack of public awareness, and surrounding stigma, 9 they failed to recognize the effects of social isolation on IBD prognosis. A few previous quantitative studies relevant to the topic were based on a small sample size, cross-sectional design, or were limited by exclusive focus on one side of social isolation yet it is a multidimensional concept,3,5,10–13 which might not generate robust evidence to adequately address the problem. Nevertheless, patients with IBD always have protracted course of conditions, placing them with demanding care throughout lifetime. 14 Thus, as part of IBD care, identifying the impact of social isolation is rather essential.
In this study, to better understand social isolation of patients with IBD, and help devise targeted interventions to improve the prognosis, we prospectively examined the joint association of social isolation and IBD with premature death using a large cohort, UK Biobank, to articulate the severe impact caused by social isolation to patients with IBD.
Methods
Data source and study population
The study followed STROBE reporting guidelines for cohort studies. 15 Data were obtained from the UK Biobank Cohort Study (https://www.ukbiobank.ac.uk/). UK Biobank participants were recruited between 2007 and 2010 from 22 assessment centers across the UK; over half a million individuals aged 40–69 years participated in this study. Extensive health-related information was collected through self-report questionnaires, medical records, and a range of physical and laboratory measures. Ethical approval for UK Biobank was obtained from the North West Multicentre Research Ethics Committee (REC reference: 16/NW/0274). 16
To investigate our research questions, 486,041 participants including 5791 individuals who were diagnosed with either CD or UC were documented after excluding those who failed to complete the social isolation assessment (N = 8828), ambiguous diagnosis of IBD (N = 18), those who diagnosed with IBD during the follow-up to ensure the stability of the state (N = 2885), and those who died within 1 year (N = 923) following baseline to reduce reverse causation (Figure 1).
Figure 1.
Flowchart of the study.
IBD, inflammatory bowel disease.
Ascertainment of IBD
In line with previous studies17–19, we ascertained diagnoses of IBD and its subtypes based on the combination of self-reporting, the primary care, and hospital admission data with International Classification of Disease (ICD) codes; namely, ICD-10 (K50 and K51) and ICD-9 (555 and 556). In case of revised diagnoses among patients with multiple diagnostic records, we used the most recent of those for IBD subtype confirmation. For those who were not classified into specific IBD subtypes, we included them in the analysis of overall IBD despite not being in the subtype analyses.
Assessment of social isolation
Social isolation was assessed by three questions used in previous UK Biobank studies,20–22 which were also similar to other investigated UK cohorts,23,24 as follows: (1) ‘How often do you visit friends or family or have them visit you’, (2) ‘Which of the following (leisure/social activities) do you engage in once a week or more’, and (3) ‘Including yourself, how many people are living together in your household?’ The answers to each question were dichotomized as follows: 1 point for friends and family visits occurring fewer times than once a month, 1 point for no participation in leisure/social activities, and 1 point for living alone. The total score for social isolation ranged from 0 to 3, and those who scored 2 or 3 points were defined as socially isolated individuals (Supplemental Table S1).
Ascertainment of mortality
UK Biobank obtains mortality records by linking them to national death registries. We ascertained the participants’ date of death (from any cause) during the follow-up period from baseline to 23 March 2021.
Assessment of covariates
In primary analyses, to reduce possible confounding, a broad range of covariates including sociodemographic, anthropometric, and lifestyle factors were considered. Information was collected via self-reported questionnaires or during visits to the clinic. Detailed covariates included age, sex, race (white or non-white), education (with or without university/college degree), Townsend deprivation index (TDI, grouped by tertiles and with lower values indicating higher socioeconomic statuses), body mass index (BMI), alcohol consumption (current drinker or not), smoking status (never smoker or not), and physical activity (assessed by the International Physical Activity questionnaire and categorized as low intensity, moderate intensity, and high intensity).
For further adjustment, psychological variables were included. To be specific, symptoms of depression were assessed using the validated two-item Patient Health Questionnaire-2 at baseline, which comprised the following questions: ‘Over the past 2 weeks, how often have you felt down, depressed, or hopeless?’ and ‘Over the past 2 weeks, how often have you had little interest or pleasure in doing things?’ Answers were scored on a four-point scale (not at all = 0, several days = 1, more than half the days = 2, and nearly every day = 3) with scores of 3 or greater classified as symptoms of depression 25 ; loneliness, which was measured using two questions that were similar to revised UCLA loneliness scale 26 : ‘Do you often feel lonely’ (no = 0, yes = 1) and ‘How often are you able to confide in someone close to you?’ (almost daily to approximately once a month=0; once every few months to never or almost never = 1). Scores of 2 denoted loneliness. 20 In addition, to account for potential confounding caused by health status, also the severity or activity of IBD, we furtherly incorporated Charlson Comorbidity Index (CCI), which was constructed by summing weighted ICD-10 codes of 17 comorbidities, with weights ranging from 1 to 6 depending on disease severity and risk of mortality 27 ; history of cardiovascular disease and metabolic diseases (diabetes, hypertension, coronary heart disease, stroke), which were identified from hospital, primary care, and self-report data28,29; serum C-reactive protein (CRP) level (mg/L, an acute-phase reactant whose level correlates well with disease activity in IBD 30 ; measured by an immunoturbidimetric high sensitivity analysis on a Beckman Coulter AU5800 31 ), and history of bowel resection surgery at baseline (Office of Population Censuses and Surveys was used to identify participants with any kind of intestinal surgical history). To address surveillance bias, we furtherly included seeing a psychiatrist or primary care doctor for nerves, anxiety, tension, or depression (for more details, see Supplemental Methods 1).
Various imputation approaches were used to compensate for missing values on covariates before performing the subsequent multivariate analyses; we used the median to fill continuous variables and used the largest group (missing rate <3%) or the missing indicator method by setting the missing value of each variable as the missing group (⩾3%) to impute categorical variables (Supplemental Table S2). Results after imputation are displayed in Supplemental Table S3.
Statistical analyses
Baseline characteristics are expressed as mean ± standard deviation (SD) for continuous variables and counts (percentages) for categorical variables, by IBD status. The F-test or the χ2 test was used for group comparisons as applicable. To assess the reproducibility of social isolation in repeated measurement, we further calculated intraclass correlation (ICC = 0.60), confirming the optimal reproducibility.
Person-time was computed for each participant from baseline to the date of death from any cause, that of loss to follow-up, or that of the endpoint of the study (23 March 2021), whichever came first. We first plotted Kaplan–Meier curves with the numbers at risk at each time interval reported; differences were evaluated using log-rank tests. Next, after confirming the association of IBD and social isolation with the risk of mortality, respectively (Supplemental Table S4), multivariable Cox regression models were fitted followed by verifying the proportional hazards assumption through Schoenfeld residuals (the minimum p > 0.09). The non-isolated participants who were free of IBD was used as referent category within each stratum of IBD and subtypes. Subsequently, the multiplicative interaction was tested by comparing the models with and without the interaction term. The additive interaction was estimated by the relative excess risk due to interaction (RERI). 32 We also performed the subgroup analyses by each covariate to test the effect modification. Then, we ran the Cox regression models to compare the excess risk of death between the binary categories of social isolation status among patients with IBD and its subtypes, respectively.
Two models were applied to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) by adjusting for covariates as follows: minimally adjusted model, adjusted for age, age squared, and sex; fully adjusted model, further adjusted for race, education, TDI, BMI, alcohol consumption, smoking status, and physical activity.
A series of sensitivity analyses were conducted to test the robustness of the results. First, considering that mortality in patients with social isolation might be influenced by associated confounders, we further adjusted for loneliness and symptoms of depression as covariates. 20 Meanwhile, to examine whether participants’ health status, conditions related to IBD activity or severity, and doctor visits affected results, we additionally adjusted for CCI, CRP, history of bowel resection surgery, and counseling of psychiatrist or primary care doctor visits at baseline. In addition, since cardiovascular and metabolic diseases are well-established risk factors for premature death, and are prevalent in participants, we excluded those who were diagnosed with such disease.28,29 Second, to furtherly minimize possible bias caused by reverse association, we excluded individuals who died within the first 2 and 3 years after recruitment. Third, given that there are up to 18.71% missing values in physical activity, we used the multiple imputation method by chained equations, with five imputed databases generated. Meanwhile, we also included handgrip strength (lower than 0.58%) as a proxy variable for further results verification. Fourth, we test the potential influence across different disease location according to the currently accepted Montreal classification for IBD subtypes. To be specific, using ICD codes, we identified CD location as small bowel disease or terminal ileitis (L1), colon (L2), and ileocecal CD or location not defined (L3/LX), and UC extent as ulcerative proctitis (E1), left-sided ulcerative proctitis (E2), extensive ulcerative proctitis (E3), and extent not defined (EX). 33 Lastly, we also performed the stratified Cox regression model by categorizing patients with IBD according to duration of time since the disease onset (diagnosis until assessment >20 years or ⩽20 years).
All statistical analyses were performed using the R software (version 4.0.5), and statistical significance was set at a two-sided p value of less than 0.05.
Results
Table 1 presents the baseline characteristics of the study population stratified by IBD and social isolation categories in detail. Among 486,014 eligible participants [whose mean age was 56.5 (SD: 8.1) and among whom 54.5% were women], we identified 5791 individuals with IBD. Social isolation represented 9.2% (N = 44,041) of the total non-IBD population and 10.4% (N = 600) of patients with IBD. Specifically, these socially isolated patients with IBD comprised 229 of 1897 with CD and 354 of the 3759 with UC.
Table 1.
Baseline characteristic of participants as IBD status and social isolation.
| Overall (N = 486,014) | Non-IBD (N = 480,223) | IBD (N = 5791) | p Value a | |||
|---|---|---|---|---|---|---|
| Non-isolated (N = 436,182) | Isolated (N = 44,041) | Non-isolated (N = 5191) | Isolated (N = 600) | |||
| Age at recruitment, years, mean (SD) | 56.5 (8.1) | 56.5 (8.1) | 56.6 (7.8) | 57.2 (7.9) | 57.7 (7.7) | <0.001 |
| Female, n (%) | 265,020 (54.5) | 239,218 (54.8) | 22,743 (51.6) | 2769 (53.3) | 290 (48.3) | <0.001 |
| White, n (%) | 459,440 (94.8) | 413,200 (95.0) | 40,674 (92.9) | 4990 (96.4) | 576 (96.2) | <0.001 |
| With university/college degree | 158,744 (32.9) | 144,026 (33.3) | 13,076 (30.1) | 1486 (28.8) | 156 (26.2) | <0.001 |
| TDI, mean (SD) | −1.3 (3.1) | −1.5 (3.0) | 0.1 (3.5) | −1.4 (3.0) | −0.1 (3.4) | <0.001 |
| BMI, kg/m2, mean (SD) | 27.4 (4.8) | 27.4 (4.7) | 27.9 (5.4) | 27.1 (4.6) | 27.5 (5.2) | <0.001 |
| Current drinker, n (%) | 447,318 (92.1) | 404,161 (92.7) | 37,954 (86.4) | 4708 (90.8) | 495 (82.6) | <0.001 |
| Never smoker, n (%) | 265,768 (54.9) | 241,812 (55.6) | 21,206 (48.4) | 2494 (48.2) | 256 (43.0) | <0.001 |
| Physical activity b , n (%) | <0.001 | |||||
| Low | 74,491 (18.9) | 64,296 (18.1) | 9195 (26.6) | 862 (20.7) | 138 (30.0) | |
| Moderate | 161,234 (40.8) | 145,044 (40.7) | 14,334 (41.5) | 1667 (40.1) | 189 (41.1) | |
| High | 159,360 (40.3) | 146,610 (41.2) | 10,986 (31.8) | 1631 (39.2) | 133 (28.9) | |
| Missing | 90,929 (18.7) | 80,232 (18.4) | 9526 (21.6) | 1031 (19.9) | 140 (23.3) | |
| Depression symptoms, n(%) | 26,573 (5.8) | 21,182 (5.2) | 4969 (12.3) | 336 (6.9) | 86 (15.4) | <0.001 |
| Loneliness, n(%) | 22,152 (4.8) | 16,489 (4.0) | 5364 (12.9) | 224 (4.5) | 75 (12.8) | <0.001 |
| CCI, mean (SD) | 0.3 (0.9) | 0.3 (0.9) | 0.4 (1.0) | 0.4 (1.1) | 0.6 (1.3) | <0.001 |
| CRP, mean (SD) | 2.6 (4.3) | 2.5 (4.2) | 3.0 (4.9) | 3.6 (5.6) | 4.6 (7.5) | <0.001 |
| Bowel resection, n(%) | 11,418 (2.3) | 9481 (2.2) | 1070 (2.4) | 758 (14.6) | 109 (18.2) | <0.001 |
| Left-hand grip strength (Kg) (mean (SD)) | 29.6 (11.3) | 29.7 (11.3) | 28.8 (11.4) | 28.9 (11.5) | 28.3 (11.2) | <0.001 |
| Right hand grip strength (kg) (mean (SD)) | 31.7 (11.3) | 31.8 (11.3) | 31.0 (11.3) | 31.1 (11.4) | 30.3 (11.1) | <0.001 |
| Seen a psychiatrist for nerves, anxiety, tension, or depression (%) | 55,466 (11.5) | 47,054 (10.8) | 7648 (17.5) | 633 (12.3) | 131 (21.9) | <0.001 |
| Seen primary care doctor for nerves, anxiety, tension, or depression (%) | 163,990 (34.0) | 143,906 (33.2) | 18,010 (41.3) | 1814 (35.2) | 260 (43.6) | <0.001 |
p values were calculated by F-tests (continuous variables) or Chi-square tests (categorical variables).
Physical activity was measured by IPAQ.
BMI, body mass index; CCI, Charlson Comorbidity Index; CRP, C-reactive protein; IBD, inflammatory bowel disease; IPAQ, International Physical Activity Questionnaire; SD, standard deviation; TDI, Townsend deprivation index.
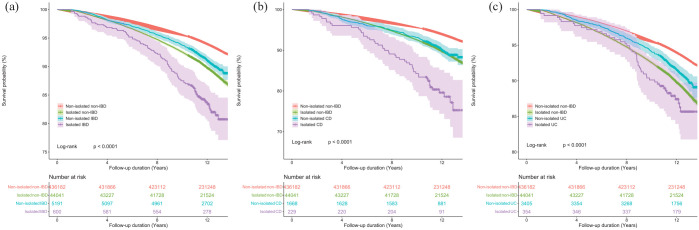
There were 32,390 incident cases of all-cause mortality documented during a mean follow-up of 11.84 (median: 12.06, interquartile range: 11.34, 12.76) years, representing 5756,146 person-years (Table 2). Kaplan–Meier survival curves (Figure 2) stratified by social isolation and IBD subtype showed significant differences between all four groups (all p < 0.05), with clear patterns of decreased survival among patients with IBD who were socially isolated.
Table 2.
The association of social isolation – IBD status with the risk of all-cause mortality.
| Death (N) | Person-years | Minimally adjusted model a | Fully adjusted model b | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |||
| Non-isolated non-IBD | 27,210 | 5,176,011 | Ref. | Ref. | ||
| Isolated non-IBD | 4603 | 512,294 | 1.75 (1.70, 1.81) | <0.001 | 1.45 (1.40, 1.50) | <0.001 |
| Non-isolated IBD | 476 | 61,024 | 1.41 (1.28, 1.54) | <0.001 | 1.33 (1.21, 1.45) | <0.001 |
| Non-isolated CD | 168 | 19,520 | 1.64 (1.41, 1.91) | <0.001 | 1.49 (1.28, 1.74) | <0.001 |
| Non-isolated UC | 298 | 40,125 | 1.30 (1.16, 1.45) | <0.001 | 1.24 (1.11, 1.39) | <0.001 |
| Isolated IBD | 101 | 6817 | 2.62 (2.16, 3.19) | <0.001 | 2.06 (1.69, 2.51) | <0.001 |
| Isolated CD | 48 | 2541 | 3.58 (2.70, 4.76) | <0.001 | 2.75 (2.07, 3.64) | <0.001 |
| Isolated UC | 46 | 4100 | 1.90 (1.42, 2.53) | <0.001 | 1.52 (1.14, 2.03) | 0.005 |
Minimally adjusted model adjusted for age, age-square, sex.
Fully adjusted model adjusted for age, age-square, sex, race, education, TDI, BMI, alcohol consumption status, smoking status, and physical activity.
BMI, body mass index; CD, Crohn’s disease; CI, confidence interval; HR, hazard ratios; IBD, inflammatory bowel disease; TDI, Townsend deprivation index; UC, ulcerative colitis.
Figure 2.
Kaplan–Meier curves of all-cause mortality for (a) IBD patients, (b) CD patients, (c) UC patients.
Proportional hazard assumption verified using Schoenfeld residual test: social isolation – IBD: p = 0.16; social isolation – CD: p = 0.10; social isolation – UC: p = 0.09.
CD, Crohn’s disease; IBD, inflammatory bowel disease; UC, ulcerative colitis.
According to the multivariate Cox models (Table 2), compared with non-socially isolated individuals without IBD diagnoses, all three other groups, that is, isolated non-IBD (HR = 1.45, 95% CI: 1.40, 1.50), non-socially isolated patients with IBD (HR = 1.33, 95% CI: 1.21, 1.45), and socially isolated patients with IBD (HR = 2.06, 95% CI: 1.69, 2.51) had elevated risks of mortality after fully adjusting for covariates. Likewise, the similar trend remained when stratifying patients with CD and UC; socially isolated patients with CD and UC showed a 2.75-fold (95% CI: 2.07, 3.64) and 1.52-fold (95% CI: 1.14, 2.03) increased risk, respectively. As shown in Supplemental Tables S5, S6, and S7, significant elevated risk of all-cause mortality was consistently found among patients with IBD and subtypes who were younger than 60 years old at recruitment, female, with higher socioeconomic deprivation, with lower BMI, and had a history of smoking (p for interactions < 0.05). However, both multiplicative interaction (p = 0.38) and multiplicative interaction [RERI = −0.27 (−0.64, 0.03), p = 0.11] of IBD and social isolation were not significant.
In Table 3, results of the association between social isolation and premature death were presented stratified by IBD and subtypes; patients who were not deemed as socially isolated were set as the referent group. Excess risks of mortality were found in IBD (HR = 1.69, 95% CI: 1.36, 2.11), especially in CD group (HR = 2.06, 95% CI: 1.48, 2.87). However, for UC subtype, a marginally (but not significant) increased risk of mortality was observed (HR = 1.30, 95% CI: 0.95, 1.79) after the fully adjustment.
Table 3.
The association of social isolation with the risk of all-cause mortality stratified by IBD status.
| Death (N) | Person-years | Minimally adjusted model a | Fully adjusted model b | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |||
| IBD | ||||||
| Non-isolated | 476 | 61,024 | Ref. | Ref. | ||
| Isolated | 101 | 6817 | 1.77 (1.43, 2.20) | <0.001 | 1.69 (1.36, 2.11) | <0.001 |
| CD | ||||||
| Non-isolated | 168 | 19,520 | Ref. | Ref. | ||
| Isolated | 48 | 2541 | 2.28 (1.66, 3.15) | <0.001 | 2.06 (1.48, 2.87) | <0.001 |
| UC | ||||||
| Non-isolated | 298 | 40,125 | Ref. | Ref. | ||
| Isolated | 46 | 4100 | 1.33 (0.97, 1.81) | 0.077 | 1.30 (0.95, 1.79) | 0.106 |
Minimally adjusted model adjusted for age, age-square, sex.
Fully adjusted model adjusted for age, age-square, sex, race, education, TDI, BMI, alcohol consumption status, smoking status, and physical activity.
BMI, body mass index; CD, Crohn’s disease; CI, confidence interval; HR, hazard ratio; IBD, inflammatory bowel disease; TDI, Townsend deprivation index; UC, ulcerative colitis.
On sensitivity analyses that additionally adjusted for symptoms of depression, loneliness, CRP level, bowel resection surgery, and doctor counselling for nerves, anxiety, tension, or depression, findings were consistent with the results of the primary analysis with no notable change in effect of estimators (Supplemental Tables S8 and S9). Likewise, when excluding patients who died within the second-, and third-year post-recruitment (Supplemental Table S10), and applying the multiple imputation methods (Supplemental Table S11) or replacing physical activity with hand grip strength (Supplemental Table S9) to deal with the relatively high missing rate, results were in agreement with the findings of our primary analysis. However, the elevated risk of death diminished among isolated patients with UC after adjusting for CCI, and excluding participants with cardiovascular or metabolic diseases. Significant increased HRs were specifically observed in ileocecal or location not defined CD patients (HR = 2.09, 95% CI: 1.40, 3.12) (Supplemental Table S12), and UC patients with ulcerative proctitis (HR = 2.09, 95% CI: 1.40, 3.12) (Supplemental Table S13). However, since the sample size in each disease location stratum was small, the results need to be interpreted cautiously. The association was attenuated for patients who were diagnosed with IBD no more than 20 years (HR = 1.36, 95% CI: 1.00, 1.84), but their counterparts showed a significantly higher risk of premature death (HR = 2.14, 95% CI: 1.52, 3.03) (Supplemental Table S14).
Discussion
To our knowledge, ours is the first study to quantify the relationship between social isolation and mortality, with the focus on patients with IBD. With a large sample size, adequate follow-up period, and rigorous statistical analyses, we were able to produce reliable data on the relationship between IBD and social isolation, and mortality. Moreover, we attempted to address the possible influence of social isolation by adjusting covariates related to IBD activity, severity, location, duration, and history of surgery, which might shed light on IBD prognosis and management improvement.
Results of our study consistently indicated that social isolation compounded the risk of premature death in patients with IBD than their non-isolated counterparts after adjusting for known covariates. Besides, the magnitude of this phenomenon we observed when restricted patients with IBD (HR = 1.69) especially its subtype CD (HR = 2.06) appeared higher than the reported pooled result in the general population (OR = 1.29), 34 as well as patients with other chronic diseases reported to date, including cardiovascular disease (HR = 1.16), 35 stroke (HR = 1.32), and acute myocardial infarction (HR = 1.25), 21 emphasizing the greater impact of social isolation on patients with IBD.
Interestingly, our study found that social isolation plays a crucial role in the prognosis of IBD, and the effect appears to be more severe among patients who suffered from CD than UC. Since population-based evidence consistently reported higher mortality rate in patients with CD than UC,36–38 our findings might expand our knowledge on explaining the discrepant prognosis of IBD subtypes. Although a lack of previous studies assessing the impact of social isolation on patients with IBD and its subtypes precludes making comparisons, our findings complement those of Kappelman et al. 39 , whose study of 10,634 patients with IBD revealed that patients with CD reported poorer health and well-being measures across all domains than did those with UC. 39 Furthermore, a recent study conducted by Nass et al. 5 also endorses our findings. It reported that social isolation during the COVID-19 pandemic lockdown coincided with more frequent flare-ups in patients with CD than in those with UC. Our study indicated the plausibility of the notion that social isolation leads to greater symptomatic and overall dysfunction in patients with CD, which, in turn, worsens long-term outcomes and reduces lifespans. 5
In addition, the different measures of social isolation impact on patients with CD and those with UC may relate to the non-similarity in these diseases’ symptoms. While UC and CD both cause abdominal pain, chronic diarrhea, bowel urgency, and might need for a stoma (which may curtail social life),6,40 inflammation is limited to the colonic mucosa in patients with UC but can affect any segment of the gastrointestinal tract (leading to transmural lesions with complications such as abscesses and fistulae) in those with CD. 2 Postoperative complications and malnutrition are also more common in patients with CD, as this disease can affect any part of the gastrointestinal tract and is more likely than UC to lead to a direct malabsorptive effect. 41 Thus, once socially isolated, patients might lack prompt medical help or sufficient care, thereby altering their lifestyles in adverse ways such as developing poor dietary habits. 35 These are likely to exacerbate the course of disease and resulted in a much more adverse outcome in patients with CD than in those with UC.
It is necessary to formulate public health policies and social support strategies that address or mitigate social isolation within the population with IBD, in particular, for female, with higher socioeconomic deprivation, lower BMI, had the history of smoking, and experience longer course of disease as noted in our sub-analyses. Meanwhile, more research is needed to elucidate the relevant triggers as well as the biological and psychological pathways that link social isolation to IBD prognosis. To that end, targeted suggestions include (1) physicians and caregivers need to pay attention to life experiences and psychological symptoms beyond the physical symptoms of patients with IBD. 42 Integrating psychological support into IBD care is warranted. 43 Notably, despite patients with CD might more likely to withdraw from work and socialize less often due to more severe fatigue44,45 and perceived burden 46 reported than in those with UC, more attention need to be paid on them to mitigate the long-term effects; (2) support groups for patients with IBD should be encouraged to help increase social interaction and improve coping strategies3,47; and (3) both patients and the public should be educated with relevant information to bridge the knowledge gap, improve understanding, and reduce the stigma associated with IBD. 6
Although we performed the rigorous statistical study with a series of sensitivity analyses, our findings still need to be interpreted with caution in light of several limitations. First, although we used the same assessment of social isolation as in previous UK-based studies, evaluating loneliness and depression depends on responses to a limited number of questions; therefore, other dimensions related to these factors may be missed. Future studies can incorporate specific social isolation measures such as the Berkman–Syme Social Network Index to improve the reliability of the data. 48 Second, while we use ICC to confirm the optimal reproducibility of social isolation assessment, the assessment data were obtained only at baseline, which limited the examination of directionality and changes in the relationship between IBD and social isolation. Thus, additional studies with repeated measurements are required to validate our results. Third, although we included a wide range of identifiable covariates, others may still exist (which is also the case for previous studies). For example, given that we used a population-based database, although we incorporated key variables acting as a proxy for IBD severity and activity in analysis, we could not access more precise disease-specific data like disease treatment, which may have influenced the results over time. Meanwhile, although we made efforts to minimize the reverse causality, as with other observational studies, it is also a factor worth noting, since disease course and other variables may affect the patients’ social connections. Finally, as with other studies conducted using UK Biobank, the dataset was dominated by participants of Caucasian, which may limit generalizability.
Conclusions
Patients with IBD, especially CD, are more likely to be affected when socially isolated, associating with a higher risk of early death in patients with CD. Therefore, social isolation merits attention, and targeted social support strategies and interventions ought to be devised. In addition, the differing consequences of social isolation between patients with CD and those with UC, as well as the underlying mechanisms thereof, remain to be examined in future studies.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848221127474 for Associations between inflammatory bowel disease, social isolation, and mortality: evidence from a longitudinal cohort study by Jie Chen, Jiawei Geng, Jiayi Wang, Zhenhua Wu, Tian Fu, Yuhao Sun, Xuejie Chen, Xiaoyan Wang and Therese Hesketh in Therapeutic Advances in Gastroenterology
Acknowledgments
This project was conducted using the UK Biobank Resource under Application 73595. We thank all participants in the UK Biobank. We also thank Wiley Editing Services for providing writing assistance.
Footnotes
ORCID iD: Jie Chen  https://orcid.org/0000-0002-4029-4192
https://orcid.org/0000-0002-4029-4192
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jie Chen, Department of Gastroenterology, The Third Xiangya Hospital of Central South University, Changsha, China; Center for Global Health, Zhejiang University, Hangzhou, China.
Jiawei Geng, Center for Global Health, Zhejiang University, Hangzhou, China.
Jiayi Wang, Department of Gastroenterology, The Third Xiangya Hospital of Central South University, Changsha, China.
Zhenhua Wu, Zhejiang Provincial Hospital of Chinese Medicine, Hangzhou, China.
Tian Fu, Department of Gastroenterology, The Third Xiangya Hospital of Central South University, Changsha, China.
Yuhao Sun, Center for Global Health, Zhejiang University, Hangzhou, China.
Xuejie Chen, Department of Gastroenterology, The Third Xiangya Hospital of Central South University, Changsha, China.
Xiaoyan Wang, Department of Gastroenterology, The Third Xiangya Hospital, Central South University, 138 Tongzipo Road, Changsha, Hunan 410013, P.R. China.
Therese Hesketh, Center for Global Health, Zhejiang University, Hangzhou, China; Institute for Global Health, University College London, UK.
Declarations
Ethics approval and consent to participate: The study used data from the UK Biobank. Ethical approval of UK Biobank was obtained from the North West Multicentre Research Ethics Committee (REC reference: 16/NW/0274). Every participants provided written informed consent.
Consent for publication: Not applicable.
Author contribution(s): Jie Chen: Conceptualization; Data curation; Investigation; Methodology; Writing – review & editing.
Jiawei Geng: Investigation; Methodology; Writing – original draft.
Jiayi Wang: Formal analysis; Methodology; Writing – original draft.
Zhenhua Wu: Writing – review & editing.
Tian Fu: Methodology; Writing – review & editing.
Yuhao Sun: Formal analysis; Methodology.
Xuejie Chen: Methodology.
Xiaoyan Wang: Conceptualization; Funding acquisition; Writing – review & editing.
Therese Hesketh: Writing – review & editing
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project is supported by National Natural Science Foundation of China [grant number, 81970494] and Key Project of Research and Development Plan of Hunan Province [grant number, 2019SK2041].
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: The data underlying this article were from UK Biobank and are available to researchers, at https://www.ukbiobank.ac.uk/.
References
- 1. Alatab S, Sepanlou SG, Ikuta K, et al. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol 2020; 5: 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farrell D, McCarthy G, Savage E. Self-reported symptom burden in individuals with inflammatory bowel disease. J Crohns Colitis 2016; 10: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kubesch A, Boulahrout P, Filmann N, et al. Real-world data about emotional stress, disability and need for social care in a German IBD patient cohort. PloS one 2020; 15: e0227309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Escalante E, Golden RL, Mason DJ. Social isolation and loneliness: imperatives for health care in a post-COVID world. JAMA 2021; 325: 520–521. [DOI] [PubMed] [Google Scholar]
- 5. Nass BY, Dibbets P, Markus CR. Impact of the COVID-19 pandemic on inflammatory bowel disease: the role of emotional stress and social isolation. Stress Health 2022; 38: 222–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Byron C, Cornally N, Burton A, et al. Challenges of living with and managing inflammatory bowel disease: a meta-synthesis of patients’ experiences. J Clin Nurs 2020; 29: 305–319. [DOI] [PubMed] [Google Scholar]
- 7. Larsson K, Lööf L, Nordin K. Stress, coping and support needs of patients with ulcerative colitis or Crohn’s disease: a qualitative descriptive study. J Clin Nurs 2017; 26: 648–657. [DOI] [PubMed] [Google Scholar]
- 8. Palant A, Himmel W. Are there also negative effects of social support? A qualitative study of patients with inflammatory bowel disease. BMJ Open 2019; 9: e022642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fourie S, Jackson D, Aveyard H. Living with inflammatory bowel disease: a review of qualitative research studies. Int J Nurs Stud 2018; 87: 149–156. [DOI] [PubMed] [Google Scholar]
- 10. Qualter P, Rouncefield-Swales A, Bray L, et al. Depression, anxiety, and loneliness among adolescents and young adults with IBD in the UK: the role of disease severity, age of onset, and embarrassment of the condition. Qual Life Res 2021; 30: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Slonim-Nevo V, Sarid O, Friger M, et al. Effect of social support on psychological distress and disease activity in inflammatory bowel disease patients. Inflamm Bowel Dis 2018; 24: 1389–1400. [DOI] [PubMed] [Google Scholar]
- 12. LaVana G-H, Jeff B, Megan R, et al. Social isolation, loneliness, and caring for IBD patients. Am J Gastroenterol 2021; 116: S373. [Google Scholar]
- 13. Damas OM, Kuftinec G, Khakoo NS, et al. Social barriers influence inflammatory bowel disease (IBD) outcomes and disproportionally affect Hispanics and non-Hispanic blacks with IBD. Therap Adv in Gastroenterol 2022; 15: 17562848221079162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fiorino G, Allocca M, Chaparro M, et al. ‘Quality of Care’ standards in inflammatory bowel disease: a systematic review. J Crohns Colitis 2019; 13: 127–137. [DOI] [PubMed] [Google Scholar]
- 15. Von EE, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014; 12: 1495–1499. [DOI] [PubMed] [Google Scholar]
- 16. Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med 2015; 12: e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Z, Chen L, Qian ZM, et al. Residential green and blue space associated with lower risk of adult-onset inflammatory bowel disease: findings from a large prospective cohort study. Environ Int 2022; 160: 107084. [DOI] [PubMed] [Google Scholar]
- 18. Meyers TJ, Weiner AB, Graff RE, et al. Association between inflammatory bowel disease and prostate cancer: a large-scale, prospective, population-based study. Int J Cancer 2020; 147: 2735–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alayo QA, Deepak P. S933 risk factors for cardiovascular diseases in participants with inflammatory bowel diseases: analysis of the United Kingdom biobank. Am J Gastroenterol 2021; 116: S443. [Google Scholar]
- 20. Elovainio M, Hakulinen C, Pulkki-Råback L, et al. Contribution of risk factors to excess mortality in isolated and lonely individuals: an analysis of data from the UK Biobank cohort study. Lancet Public Health 2017; 2: e260–e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hakulinen C, Pulkki-Råback L, Virtanen M, et al. Social isolation and loneliness as risk factors for myocardial infarction, stroke and mortality: UK Biobank cohort study of 479 054 men and women. Heart 2018; 104: 1536–1542. [DOI] [PubMed] [Google Scholar]
- 22. Allen SF, Gilbody S, Atkin K, et al. The associations between loneliness, social exclusion and pain in the general population: a N = 502,528 cross-sectional UK Biobank study. J Psychiatr Res 2020; 130: 68–74. [DOI] [PubMed] [Google Scholar]
- 23. Hakulinen C, Pulkki-Råback L, Jokela M, et al. Structural and functional aspects of social support as predictors of mental and physical health trajectories: whitehall II cohort study. J Epidemiol Community Health 2016; 70: 710–715. [DOI] [PubMed] [Google Scholar]
- 24. Valtorta NK, Kanaan M, Gilbody S, et al. Loneliness, social isolation and risk of cardiovascular disease in the English longitudinal study of ageing. Eur J Prev Cardiol 2018; 25: 1387–1396. [DOI] [PubMed] [Google Scholar]
- 25. Harshfield EL, Pennells L, Schwartz JE, et al. Association between depressive symptoms and incident cardiovascular diseases. JAMA 2020; 324: 2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hughes ME, Waite LJ, Hawkley LC, et al. A short scale for measuring loneliness in large surveys: results from two population-based studies. Res Aging 2004; 26: 655–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mak JKL, Kuja-Halkola R, Wang Y, et al. Frailty and comorbidity in predicting community COVID-19 mortality in the UK Biobank: the effect of sampling. J Am Geriatr Soc 2021; 69: 1128–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fan M, Sun D, Zhou T, et al. Sleep patterns, genetic susceptibility, and incident cardiovascular disease: a prospective study of 385 292 UK biobank participants. Eur Heart J 2020; 41: 1182–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the uk biobank study. JAMA Cardiol 2018; 3: 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen P, Zhou G, Lin J, et al. Serum biomarkers for inflammatory bowel disease. Front Med 2020; 7: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. UK Biobank. Biomarker assay quality procedures: approaches used to minimise systematic and random errors (and the wider epidemiological implications). 1.2 ed. UK Biobank. https://biobank.ndph.ox.ac.uk/showcase/ukb/docs/biomarker_issues.pdf (accessed 11 August 2021). [Google Scholar]
- 32. Knol MJ, VanderWeele TJ. Recommendations for presenting analyses of effect modification and interaction. Int J Epidemiol 2012; 41: 514–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shrestha S, Olén O, Eriksson C, et al. The use of ICD codes to identify IBD subtypes and phenotypes of the Montreal classification in the Swedish national patient register. Scand J Gastroenterol 2020; 55: 430–435. [DOI] [PubMed] [Google Scholar]
- 34. Holt-Lunstad J, Smith TB, Baker M, et al. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci 2015; 10: 227–237. [DOI] [PubMed] [Google Scholar]
- 35. Yu B, Steptoe A, Chen L-J, et al. Social isolation, loneliness, and all-cause mortality in patients with cardiovascular disease: a 10-year follow-up study. Psychosom Med 2020; 82: 208–214. [DOI] [PubMed] [Google Scholar]
- 36. Lin WC, Weng MT, Tung CC, et al. Trends and risk factors of mortality analysis in patients with inflammatory bowel disease: a Taiwanese nationwide population-based study. J Transl Med 2019; 17: 414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Card T, Hubbard R, Logan RF. Mortality in inflammatory bowel disease: a population-based cohort study. Gastroenterology 2003; 125: 1583–1590. [DOI] [PubMed] [Google Scholar]
- 38. Manninen P, Karvonen AL, Huhtala H, et al. Mortality in ulcerative colitis and Crohn’s disease. A population-based study in Finland. J Crohns Colitis 2012; 6: 524–528. [DOI] [PubMed] [Google Scholar]
- 39. Kappelman MD, Long MD, Martin C, et al. Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clin Gastroenterol Hepatol 2014; 12: 1315–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Rooy EC, Toner BB, Maunder RG, et al. Concerns of patients with inflammatory bowel disease: results from a clinical population. Am J Gastroenterol 2001; 96: 1816–1821. [DOI] [PubMed] [Google Scholar]
- 41. De Simone B, Davies J, Chouillard E, et al. WSES-AAST guidelines: management of inflammatory bowel disease in the emergency setting. World J Emerg Surg 2021; 16: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kamp KJ, West P, Holmstrom A, et al. Systematic review of social support on psychological symptoms and self-management behaviors among adults with inflammatory bowel disease. J Nurs Scholarsh 2019; 51: 380–389. [DOI] [PubMed] [Google Scholar]
- 43. Keefer L, Bedell A, Norton C, et al. How should pain, fatigue and emotional wellness be incorporated into treatment goals for optimal management of IBD? Gastroenterology 2022; 162: 1439–1451. [DOI] [PubMed] [Google Scholar]
- 44. Borren NZ, van der Woude CJ, Ananthakrishnan AN. Fatigue in IBD: epidemiology, pathophysiology and management. Nat Rev Gastroenterol Hepatol 2019; 16: 247–259. [DOI] [PubMed] [Google Scholar]
- 45. McGing JJ, Radford SJ, Francis ST, et al. The aetiology of fatigue in inflammatory bowel disease and potential therapeutic management strategies. Aliment Pharmacol Ther 2021; 54: 368–387. [DOI] [PubMed] [Google Scholar]
- 46. Le Berre C, Ananthakrishnan AN, Danese S, et al. Ulcerative colitis and crohn’s disease have similar burden and goals for treatment. Clin Gastroenterol Hepatol 2020; 18: 14–23. [DOI] [PubMed] [Google Scholar]
- 47. Volpato E, Bosio C, Previtali E, et al. The evolution of IBD perceived engagement and care needs across the life-cycle: a scoping review. BMC Gastroenterol 2021; 21: 293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine year follow-up study of alameda county residents. Am J Epidemiol 1979; 109: 186–204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848221127474 for Associations between inflammatory bowel disease, social isolation, and mortality: evidence from a longitudinal cohort study by Jie Chen, Jiawei Geng, Jiayi Wang, Zhenhua Wu, Tian Fu, Yuhao Sun, Xuejie Chen, Xiaoyan Wang and Therese Hesketh in Therapeutic Advances in Gastroenterology