Abstract
Objective
To investigate different parameters derived from the quantity and quality of perinephric fat, and to compare their effectiveness in predicting the malignant pathology of renal tumours.
Methods
Data from patients diagnosed with renal tumour between April 2014 and December 2020 were retrospectively reviewed, and patients were categorized into malignant or benign tumour groups. Fat parameters, including perinephric fat volume (PFV), perinephric fat area (PFA), perinephric fat thickness (PFT), and Mayo adhesive probability (MAP) score were measured using abdominal computed tomography scans. Between-group differences were assessed by analysis of variance and χ2-test. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the performance of perinephric fat parameters in diagnosing malignancy.
Results
A total of 109 patients were included. MAP score, PFV, PFA, and PFT were significantly increased in the malignant versus benign tumour group, and after correction for body mass index (BMI), the indexed PFV/BMI, PFA/BMI, and PFT/BMI values remained significantly higher in the malignant tumour group. All parameters showed fair predictivity of malignancy, with comparable area under the curve values in the ROC curve.
Conclusion
An increased amount of perinephric fat is predictive of malignant pathology for renal tumours. The predictive accuracy for each perinephric fat parameter remained fair after correcting for BMI.
Keywords: Renal tumour, perinephric fat, prediction of malignancy, Mayo adhesive probability, body mass index, computed tomography
Introduction
The incidental discovery of renal masses has risen due to the increasing accessibility and popularity of abdominal image modalities, with a reported 13–27% of abdominal examinations revealing renal masses. 1 Although most are clinically insignificant, small, simple cysts, and about 15% of surgically resected suspicious malignant lesions are proven to be benign, 2 there remains increased concern regarding asymptomatic malignant tumours in clinical practice. In fact, the incidence of renal cell carcinoma (RCC) has been on an upward trend worldwide, in parallel with the increasing use of axial imaging. 3
A renal tumour biopsy is sometimes performed for definite histology when the malignant potential of a tumour remains uncertain in imaging studies. Due to the invasive nature of this procedure, there may be inevitable associated complications, and it yields a maximum 22.6% non-diagnostic rate. 4 As such, the use of advanced imaging techniques has been investigated for the noninvasive characterization of renal tumours. 3
Obesity has been linked to the incidence of multiple malignant conditions, with a higher body mass index (BMI) found to be associated with the incidence of RCC.5,6 Additional parameters regarding visceral fat have been studied to further understand the interactions between adipose tissue and the kidney. The Mayo Adhesive Probability (MAP) score is a 0 to 6 scoring system composed of perinephric fat thickness (PFT) and fat stranding quality.7,8 Although the MAP score was originally developed for preoperative prediction of partial nephrectomy, it has also been associated with malignant renal histology. However, there are reports that pure quantitative measurement of perinephric fat area (PFA) fails to predict the malignant pathology of renal tumours.7,8
Compared with one-dimensional fat thickness or two-dimensional PFA, three-dimensional perinephric fat volume (PFV) may more accurately describe the amount of perinephric adipose tissue and its relationship with renal tumours. An increased PFV has been shown to influence the complexity of robotic-assisted partial nephrectomy with a significantly increased operation time, while MAP score was not a significant predictor. 9
The aim of the present study was to focus on the relationship between PFV and RCC, which to the best of our knowledge, has not been previously investigated, and to compare other parameters derived from the quantity and quality of perinephric fat, between benign and malignant tumours. Through these results, a further study aim was to evaluate the ability of perinephric fat to predict the malignant pathology of renal tumours.
Patients and methods
Study population
In this retrospective observational study, the medical records of all patients who had received a clinical or pathology diagnosis of renal tumour, either benign or malignant, from Mackay Memorial Hospital, Taipei, between April 2014 and December 2020, were reviewed. Patients without available abdominal computed tomography (CT) scan images, who had undergone haemodialysis, with history of any abdominal operation, with history of other malignancy, and/or with history of acute pyelonephritis of the same kidney were excluded. Patients having a solitary renal tumour with complete clinical and radiological data were enrolled, including those who underwent either partial or radical nephrectomy and patients who did not receive surgery but displayed a typical presentation of renal angiomyolipoma in a CT scan (<−10 HU). The clinical diagnosis of angiomyolipoma was unanimously agreed by two experienced radiologists (DCL and WMH). All participant data were deidentified for the study, and patients were grouped according to benign or malignant renal tumour for analyses.
The study protocol was reviewed and approved by the Institutional Review Board at Mackay Memorial Hospital, approval number 20MMHIS020e. Due to the retrospective study design and deidentification of patient data, the requirement of informed consent from study participants was waived by the same committee. The reporting of this study conforms to STROBE guidelines. 10
CT scan and measurement of subcutaneous and visceral adipose tissue
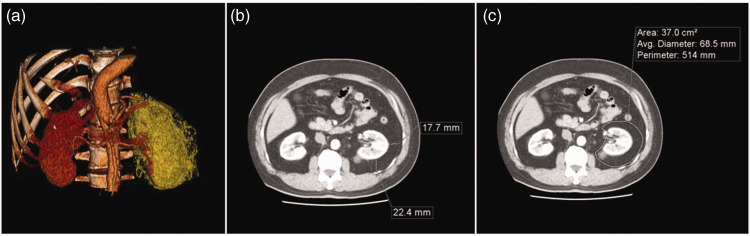
All CT studies were performed using a 128-slice (Somatom definition AS; Siemens Healthcare, Forchheim, Germany) or a 256-slice (Somatom definition Flash; Siemens Healthcare) multidetector CT scanner. The settings for all examinations were identical and used the same scanning parameters, including: scan acquisition within a single breath-hold and obtained with a detector width of 128 × 0.6 or 256 × 0.6 mm, a tube voltage of 120 kVp, automatic exposure control for tube current, 0.5 s gantry rotation time, and 1.5 mm reconstructed slice thickness. The scan coverage was from the top of the hemi-diaphragm to the pelvic symphysis. All images were acquired with the patient in a supine position and at full inspiration status. The adipose tissue measure was quantified using a dedicated workstation (Aquarius iNtuition, version 4.4; TeraRecon, San Mateo, CA, USA). The region of interest was manually traced and the fat tissue was defined as pixels within a window of –190 to –30 HU and a window centre of –110 HU. 11 PFV was measured according to the visible boundaries of Gerota’s fascia on the CT images. The adrenal glands, kidneys, and renal hilar structures were subtracted from the surrounding perinephric fat to obtain the calculated PFV (Figure 1). The axial image slice at the level of the renal vein was selected for measuring PFA and PFT. The distance between the posterior, lateral wall of the kidney and Gerota’s fascia, and the area between the kidney and Gerota’s fascia were measured. To measure the visceral fat area (VFA), and the subcutaneous fat area (SFA), axial view CT images at the L4–L5 level were selected. VFA and SFA were automatically quantified by the software to calculate the adipose tissue in the peritoneal cavity and the subcutaneous region. Subcutaneous fat thickness was measured as the largest distance between the skin and the outer limit of the muscular wall of the abdomen at the level of the umbilicus.
Figure 1.
Representative multidetector computed tomography images demonstrating perinephric fat measures, including volume, thickness, and area. The perinephric fat volume was defined as the fat inside the boundaries of Gerota’s fascia, as shown in (a) a 3D reconstruction (orange-coloured regions indicate visceral fat tissue). Axial view CT was used to measure (b) perinephric fat thickness and (c) area.
The RENAL nephrometry score, based on five anatomical features of renal tumour, including (R)adius (maximal diameter), (E)xophytic/endophytic properties, (N)earness of tumour deepest portion to the collecting system, (A)nterior (a)/posterior (p) descriptor and the (L)ocation relative to the polar line, was calculated for each patient. Of the five components, four are scored on a 1, 2 or 3-point scale with the 5th indicating the anterior or posterior location of the mass relative to the coronal plane of the kidney. 12
Statistical analyses
Continuous variables are presented as mean ± SD and categorical data are presented as numbers and percentages. All statistical analyses were performed using IBM SPSS Statistics software, version 22 (IBM Corp. Armonk, NY, USA). Between-group differences were assessed using analysis of variance and χ2-test. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the performance of perinephric fat parameters in diagnosing renal tumour malignancy. The cut-off value was calculated by the area under the ROC curve (AUC) with optimal sensitivity and specificity. A P-value <0.05 was considered to indicate statistical significance.
Results
A total of 109 patients with a solitary renal tumour and complete clinical and radiological data were included (benign tumour group, n = 54; malignant tumour group, n = 55). Of those who underwent either partial or radical nephrectomy, 55 were diagnosed with RCC and 28 were found to have a benign renal tumour by pathological examination. The remaining 26 patients did not receive surgery, but displayed a typical presentation of renal angiomyolipoma in a CT scan (<−10 HU). The patients’ demographic and pathological characteristics are summarised in Table 1. Most benign tumours were clinically or pathologically diagnosed as angiomyolipoma (47 cases [87%]), while oncocytoma was the second most common benign pathology (5 cases [9.3%]). There were no statistically significant between-group differences in patient age, or history of diabetes mellitus, hypertension or coronary artery disease.
Table 1.
Demographic and pathological characteristics of 109 patients with renal tumour.
|
Study group |
||||
|---|---|---|---|---|
| Parameter | Total (n = 109) | Benign (n = 54) | Malignant (n = 55) | Statistical significance |
| Age, years | 55.4 ± 13.6 | 56.2 ± 13.9 | 54.6 ± 13.4 | NS |
| Sex, male/female | 53/56 | 11/43 | 42/13 | P <0.01 |
| BMI, kg/m2 | 25.5 ± 4.2 | 24.3 ± 3.9 | 26.4 ± 4.1 | P = 0.01 |
| Tumour size, cm | 3.99 ± 2.39 | 4.56 ± 2.98 | 3.44 ± 1.46 | P = 0.01 |
| Medical history | ||||
| DM | 27 (24) | 12 (22) | 15 (27) | NS |
| Hypertension | 49 (45) | 25 (46) | 24 (43) | NS |
| CAD | 10 (9) | 6 (11) | 4 (7) | NS |
| Smoking | 20 (18) | 4 (7) | 16 (29) | P <0.01 |
| Pathology type | ||||
| Angiomyolipoma | Clear cell RCC | |||
| 47 (87) | 42 (76.4) | |||
| Oncocytoma | Papillary RCC | |||
| 5 (9.3) | 9 (16.4) | |||
| Others | Chromophobe RCC | |||
| 2 (3.7) | 3 (5.4) | |||
| Xp11 translocation | ||||
| 1 (1.8) | ||||
| T Stage | ||||
| T1a | 39 (70.9) | |||
| T1b | 15 (27.3) | |||
| T2a | 1 (1.8) | |||
Data presented as mean ± SD or n (%) prevalence.
BMI, body mass index; DM, diabetes mellitus; CAD, coronary artery disease; RCC, renal cell carcinoma.
NS, no statistically significant between-group difference (P >0.05; analysis of variance or χ2-test).
Comparison of perinephric fat properties between the malignant and benign tumour groups (Table 2) revealed a significantly increased MAP score, PFV, PFA, and PFT in the malignant group compared with the benign group. The VFA and SFA values were also significantly increased in patients with malignant renal tumour. After correction for BMI, the indexed PFV/BMI, PFA/BMI, and PFT/BMI values remained significantly higher in the malignant tumour group (P <0.05). There was no statistically significant between-group difference in RENAL scores.
Table 2.
Comparison of perinephric fat properties in 109 patients with renal tumour.
|
Study group |
|||
|---|---|---|---|
| Parameter | Benign (n = 54) | Malignant (n = 55) | Statistical significance |
| PFV, cm3 | 114.1 ± 106.2 | 222.7 ± 174.2 | P <0.01 |
| PFA, cm2 | 13.5 ± 9.4 | 26.8 ± 20.2 | P <0.01 |
| PFT, cm | 9.1 ± 6.1 | 18.4 ± 12.3 | P <0.01 |
| VFA, cm2 | 108.5 ± 59.7 | 142.1 ± 81.0 | P = 0.015 |
| SFA, cm2 | 174.9 ± 71.9 | 206.0 ± 89.6 | P = 0.048 |
| RENAL score | 7.8 ± 2.0 | 7.7 ± 1.6 | NS |
| MAP score | 1.5 ± 1.3 | 2.1 ± 1.6 | P = 0.037 |
| PFV/BMI | 4.3 ± 2.7 | 8.2 ± 5.9 | P <0.01 |
| PFA/BMI | 0.55 ± 0.30 | 0.98 ± 0.65 | P <0.01 |
| PFT/BMI | 0.39 ± 0.22 | 0.69 ± 0.42 | P <0.01 |
Data presented as mean ± SD.
PFV, perinephric fat volume; PFA, perinephric fat area; PFT, perinephric fat thickness; VFA, visceral fat area; SFA, subcutaneous fat area; MAP, Mayo adhesive probability; BMI, body mass index.
RENAL score, nephrometry score based on tumour radius (maximal diameter), exophytic/endophytic properties, nearness of tumour deepest portion to the collecting system, anterior (a)/posterior (p) descriptor and location relative to the polar line.
NS, no statistically significant between-group difference (P >0.05; analysis of variance).
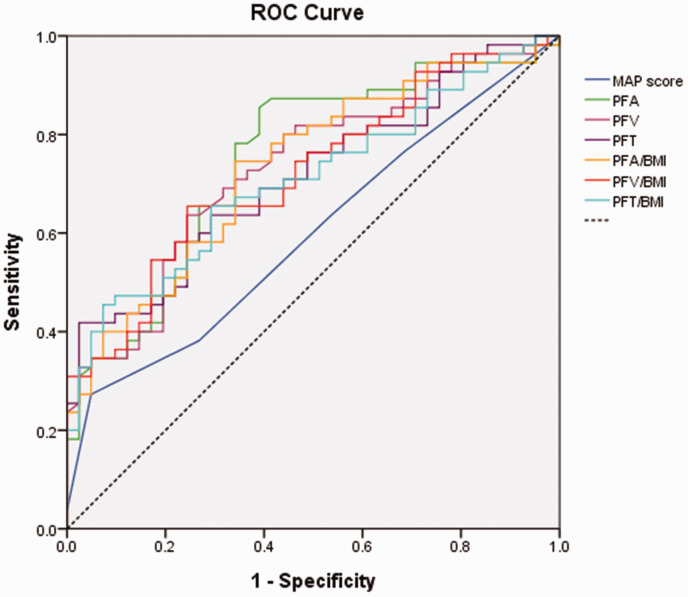
The ROC curve for perinephric fat parameters is presented in Figure 2. The AUC was 0.598 for MAP score, 0.733 for PFV, 0.748 for PFA, and 0.714 for PFT. The adjusted AUC after correction for BMI was 0.723 for PFV/BMI, 0.734 for PFA/BMI, and 0.709 for PFT/BMI. The optimal cut-off points, sensitivities, and specificities are summarised in Table 3. PFA displayed the highest sensitivity and PFT displayed the highest specificity.
Figure 2.
Receiver operating characteristic curves comparing parameters for measuring perinephric fat. The area under the curve (AUC) was 0.598 for Mayo adhesive probability (MAP) score, 0.733 for perinephric fat volume (PFV), 0.748 for perinephric fat area (PFA), and 0.714 for perinephric fat thickness (PFT). After correction for body mass index (BMI), the AUC was 0.723 for PFV/BMI, 0.734 for PFA/BMI, and 0.709 for PFT/BMI.
Table 3.
Receiver operating characteristic (ROC) curve analysis of the ability of perinephric fat properties to predict malignancy in 109 patients with renal tumour.
| Parameter | Cut-off value | Sensitivity | Specificity |
|---|---|---|---|
| PFV, cm3 | 127.5 | 0.636 | 0.756 |
| PFA, cm2 | 11.6 | 0.855 | 0.61 |
| PFT, cm | 20.05 | 0.418 | 0.976 |
| PFV/BMI | 5.01 | 0.655 | 0.756 |
| PFA/BMI | 0.574 | 0.745 | 0.659 |
| PFT/BMI | 0.702 | 0.455 | 0.927 |
PFV, perinephric fat volume; PFA, perinephric fat area; PFT, perinephric fat thickness; BMI, body mass index.
Discussion
For patients diagnosed with a renal tumour by CT scan, it is feasible to evaluate features in the vicinity of the tumour and to conduct noninvasive measurements of fat with modern computer software. The information acquired from CT scans, including the existence of local invasion, the presence of enlarged collateral vessels, the amount and pattern of perinephric fat, and the amount of visceral fat, may provide a clinically significant estimate of RCC prognosis.7,13–17 An increased amount of visceral fat is indicated to be associated with poor oncologic outcomes, larger tumour size, and higher Furhman’s grade of RCC.18,19 Additionally, an increase in fat thickness is shown to be a predictor of clear cell RCC (ccRCC) pathology, a risk factor of poor progression-free survival, and a predictor of tumour outward growth pattern.20–22 Interestingly, an increased amount of perinephric adipose tissue has also been found to be associated with poor outcomes for colorectal and ovarian cancer.23,24 However, to date, most available literature on perinephric fat addresses its effect on surgical difficulty and perioperative outcomes in partial nephrectomy.7,9,25–27
Based on abundant evidence, the correlation between adiposity and malignancy is well-established, particularly in organs with close anatomical proximity to adipose tissue depots, such as the prostate and breast.28,29 Thus, it is reasonable to apply this concept to renal tumours, which are also embedded in rich adipose tissue, and to develop a noninvasive tool that may help predict malignant histology and prevent complications from unnecessary invasive procedures.
Bernstein et al. 8 concluded that, instead of PFA, fat adhesiveness is predictive of malignant histology. However, the quantitative influence of adjacent adipose tissue may not be fully elucidated, as studies regarding epicardial fat and coronary artery disease have suggested.30,31 An increased VFA has been demonstrated to be a potential parameter for distinguishing ccRCC from renal angiomyolipoma with minimal fat. 32 In the present study, the parameters for measuring perinephric fat, including MAP score, PFT, PFA, PFV, and their BMI-corrected values, were all demonstrated to be significantly increased in patients with malignant renal tumour versus those with a benign tumour. The major outcome of the study, according to ROC curve analysis, was that perinephric fat measurements may potentially serve as a predictor of renal tumour malignancy. According to an academic points system, all perinephric fat parameters were categorized as fair accuracy, with an AUC between 0.7 to 0.8 both before and after being corrected for BMI. Among these parameters, PFA displayed the highest sensitivity and PFT had the highest specificity.
Investigation into the role of adipose tissue in tumorigenesis and metastasis is an emerging field of study. Hypertrophied adipose tissue depots in obese individuals are in a state of chronic low-grade inflammation, with macrophages and inflammatory cells comprising up to half of the adipose tissue cellularity compared with just 5–10% in lean subjects.31,33 Excessive reactive oxygen species, proinflammatory cytokines, and adipokines secreted by adipose tissue, are considered to be tumour promotors. 34 Angiogenesis and alterations in the tumour microenvironment, promoted by regions of hypoxia caused by visceral adipose tissue, are hypothesized to be associated with the growth of RCC, and transcriptomic analyses have revealed a significant upregulation of hypoxia- and angiogenesis-related genes in obese patients with ccRCC compared with patients of normal weight. 35 The tumorigenesis role of adipose tissue in RCC may also vary between histological subtypes and different types of genetic mutation. An Italian research group demonstrated that, in comparison with control group, both ccRCC and non-clear cell RCC (nccRCC) groups have significantly increased VFA and VFA/SFA, yet the statistically significant increased SFA was only observed in the ccRCC group.36,37 An earlier Chinese study reported a significantly greater VFA in patients with ccRCC versus those with nccRCC by an average of 25 cm2. 38 Even amongst the same ccRCC subtype, a significant difference in VFA has been measured between von Hippel–Lindau (VHL) gene mutation and lysine (K)-specific demethylase 5C (KDM5C) gene mutation groups. 39
The abovementioned hormonal and immune phenomena regarding adipose tissue were also observed in excessive perinephric fat with increased levels of tumour necrosis factor-α, and interleukin-6, as well as increased numbers of macrophages. 40 Previous molecular studies have reported the overexpression of uncoupling protein-1 and the under expression of homeobox protein 8 and 9 in the perinephric adipose tissue of patients with RCC. 41 However, to date, the comprehensive molecular basis of perinephric fat involvement in carcinogenesis and tumour development remains unclear.
The results of the present study may be limited by its retrospective nature, small cohort size, lack of hormonal status, and use of single-centre data. In addition, tumours of different pathology subtypes and stages were included in the same group, and this heterogeneous composition may lead to a within-group variation in adipose tissue amount.38,42 A proportion of patients with angiomyolipoma were diagnosed based on radiological examination, and the lack of pathology proof may have led to a bias. However, only lipid-rich angiomyolipomas (<−10 HU) are considered benign and left unoperated at Mackay Memorial Hospital in Taipei, and the radiological examination applied a region of interest (ROI) density measurement, which is reported to have a specificity of 100% with a threshold of <−10 HU for diagnosing angiomyolipoma. 43 Thus, we believe that the potential bias has been reduced to the lowest possible level. Different male to female ratios between the two groups should also be highlighted, as it is suggested that males have a greater amount of perinephric fat compared with females.7,8,44 Although small sample size was a limitation, an increased perinephric fat volume was not observed in male patients with benign renal tumours in the present study. Further investigations on a larger scale may be required to verify the present results. Of note, inter-interpreter variation in radiological diagnosis and measurement is an additional potential bias in studies that use such manually derived variables of perinephric fat.
In conclusion, an increased amount of perinephric fat is predictive of malignant pathology for renal tumours, regardless of whether it is measured in one, two, or three-dimensions, and the predictive accuracy for each perinephric fat parameter remained fair after correction for BMI. Further investigation is required to evaluate the diagnostic value of non-invasive radiological measurements of perinephric fat, as well as the underlying mechanism of adipose-tumour interaction.
Author contributions: YHC wrote the draft manuscript; JPT, DCL and WMH measured the CT scan-derived adipose tissue parameters; DCL performed the statistical analyses; MC supervised manuscript preparation and revision; and all authors read and approved the final manuscript.
Declaration of conflicting interest: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Data accessibility
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
ORCID iD
Yi-Hsuan Chen https://orcid.org/0000-0002-4362-7001
References
- 1.Gill IS, Aron M, Gervais DA, et al. Clinical practice. Small renal mass. N Engl J Med 2010; 362: 624–634. [DOI] [PubMed] [Google Scholar]
- 2.Corcoran AT, Russo P, Lowrance WT, et al. A review of contemporary data on surgically resected renal masses–benign or malignant? Urology 2013; 81: 707–713. [DOI] [PubMed] [Google Scholar]
- 3.Sanchez A, Feldman AS, Hakimi AA. Current management of small renal masses, including patient selection, renal tumor biopsy, active surveillance, and thermal ablation. J Clin Oncol 2018; 36: 3591–3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richard PO, Jewett MA, Bhatt JR, et al. Renal tumor biopsy for small renal masses: a single-center 13-year experience. Eur Urol 2015; 68: 1007–1013. [DOI] [PubMed] [Google Scholar]
- 5.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371: 569–578. [DOI] [PubMed] [Google Scholar]
- 6.Flaherty KT, Fuchs CS, Colditz GA, et al. A prospective study of body mass index, hypertension, and smoking and the risk of renal cell carcinoma (United States). Cancer Causes Control 2005; 16: 1099–1106. [DOI] [PubMed] [Google Scholar]
- 7.Kocher NJ, Kunchala S, Reynolds C, et al. Adherent perinephric fat at minimally invasive partial nephrectomy is associated with adverse peri-operative outcomes and malignant renal histology. BJU Int 2016; 117: 636–641. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein AP, Fram EB, Sankin A, et al. A comparison of perinephric fat surface area and Mayo Adhesive Probability score in predicting malignancy in T1 renal masses. Urol Oncol 2018; 36: 499.e17–499.e22. [DOI] [PubMed] [Google Scholar]
- 9.Motoyama D, Matsushita Y, Watanabe H, et al. Significant impact of three-dimensional volumetry of perinephric fat on the console time during robot-assisted partial nephrectomy. BMC Urol 2019; 19: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Von Elm E, Altman DG, Egger M, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007; 335: 806–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Irving BA, Weltman JY, Brock DW, et al. NIH ImageJ and Slice-O-Matic computed tomography imaging software to quantify soft tissue. Obesity (Silver Spring) 2007; 15: 370–376. [DOI] [PubMed] [Google Scholar]
- 12.Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol 2009; 182: 844–853. [DOI] [PubMed] [Google Scholar]
- 13.Zhai T, Zhang B, Qu Z, et al. Elevated visceral obesity quantified by CT is associated with adverse postoperative outcome of laparoscopic radical nephrectomy for renal clear cell carcinoma patients. Int Urol Nephrol 2018; 50: 845–850. [DOI] [PubMed] [Google Scholar]
- 14.Nguyen GK, Mellnick VM, Yim AK, et al. Synergy of sex differences in visceral fat measured with CT and tumor metabolism helps predict overall survival in patients with renal cell carcinoma. Radiology 2018; 287: 884–892. [DOI] [PubMed] [Google Scholar]
- 15.Thiel DD, Davidiuk AJ, Meschia C, et al. Mayo adhesive probability score is associated with localized renal cell carcinoma progression-free survival. Urology 2016; 89: 54–60. [DOI] [PubMed] [Google Scholar]
- 16.Kutluhan MA, Unal S, Eren S, et al. Predictive features of pre-operative computed tomography and magnetic resonance imaging for advanced disease in renal cell carcinoma. Arch Ital Urol Androl 2022; 94: 1–6. [DOI] [PubMed] [Google Scholar]
- 17.Bradley AJ, MacDonald L, Whiteside S, et al. Accuracy of preoperative CT T staging of renal cell carcinoma: which features predict advanced stage? Clin Radiol 2015; 70: 822–829. [DOI] [PubMed] [Google Scholar]
- 18.Park YH, Lee JK, Kim KM, et al. Visceral obesity in predicting oncologic outcomes of localized renal cell carcinoma. J Urol 2014; 192: 1043–1049. [DOI] [PubMed] [Google Scholar]
- 19.Otunctemur A, Dursun M, Ozer K, et al. Renal cell carcinoma and visceral adipose index: a new risk parameter. Int Braz J Urol 2016; 42: 955–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okhunov Z, Mues AC, Kline M, et al. Evaluation of perirenal fat as a predictor of cT 1a renal cortical neoplasm histopathology and surgical outcomes. J Endourol 2012; 26: 911–916. [DOI] [PubMed] [Google Scholar]
- 21.Huang H, Chen S, Li W, et al. High perirenal fat thickness predicts a poor progression-free survival in patients with localized clear cell renal cell carcinoma. Urol Oncol 2018; 36: 157.e1–157.e6. [DOI] [PubMed] [Google Scholar]
- 22.Kashiwagi E, Imada K, Abe T, et al. Thickness of perirenal fat predicts the growth pattern of renal cell carcinoma. Kidney Cancer 2020; 4: 41–48. [Google Scholar]
- 23.Jung M, Volonté F, Buchs NC, et al. Perirenal fat surface area as a risk factor for morbidity after elective colorectal surgery. Dis Colon Rectum 2014; 57: 201–209. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Coletta AM, Allen PK, et al . Perirenal adiposity is associated with lower progression-free survival from ovarian cancer. Int J Gynecol Cancer 2018; 28: 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin L, Rouviere O, Bezza R, et al. Mayo adhesive probability score is an independent computed tomography scan predictor of adherent perinephric fat in open partial nephrectomy. Urology 2017; 103: 124–128. [DOI] [PubMed] [Google Scholar]
- 26.Ishiyama R, Kondo T, Takagi T, et al. Impact of the Mayo adhesive probability score on the complexity of robot-assisted partial nephrectomy. J Endourol 2018; 32: 928–933. [DOI] [PubMed] [Google Scholar]
- 27.Jin D, Zhang J, Zhang Y, et al. A combination of the Mayo adhesive probability score and the RENAL score to predict intraoperative complications in small renal masses. Urol Int 2020; 104: 142–147. [DOI] [PubMed] [Google Scholar]
- 28.Avgerinos KI, Spyrou N, Mantzoros CS, et al. Obesity and cancer risk: emerging biological mechanisms and perspectives. Metabolism 2019; 92: 121–135. [DOI] [PubMed] [Google Scholar]
- 29.Cozzo AJ, Fuller AM, Makowski L. Contribution of adipose tissue to development of cancer. Compr Physiol 2017; 8: 237–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ueno K, Anzai T, Jinzaki M, et al. Increased epicardial fat volume quantified by 64-multidetector computed tomography is associated with coronary atherosclerosis and totally occlusive lesions. Circ J 2009; 73: 1927–1933. [DOI] [PubMed] [Google Scholar]
- 31.Venuraju SM, Lahiri A, Jeevarethinam A, et al. Association of epicardial fat volume with the extent of coronary atherosclerosis and cardiovascular adverse events in asymptomatic patients with diabetes. Angiology 2021; 72: 442–450. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Bao J, Zhang W, et al. The potential of visceral adipose tissue in distinguishing clear cell renal cell carcinoma from renal angiomyolipoma with minimal fat. Cancer Manag Res 2021; 13: 8907–8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003; 112: 1796–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieman KM, Romero IL, Van Houten B, et al. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta 2013; 1831: 1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanchez A, Furberg H, Kuo F, et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol 2020; 21: 283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greco F, Quarta LG, Grasso RF, et al. Increased visceral adipose tissue in clear cell renal cell carcinoma with and without peritumoral collateral vessels. Br J Radiol 2020; 93: 20200334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greco F, Mallio CA, Grippo R, et al. Increased visceral adipose tissue in male patients with non-clear cell renal cell carcinoma. Radiol Med 2020; 125: 538–543. [DOI] [PubMed] [Google Scholar]
- 38.Wang HK, Song XS, Cheng Y, et al. Visceral fat accumulation is associated with different pathological subtypes of renal cell carcinoma (RCC): a multicentre study in China. BJU Int 2014; 114: 496–502. [DOI] [PubMed] [Google Scholar]
- 39.Greco F, Mallio CA. Relationship between visceral adipose tissue and genetic mutations (VHL and KDM5C) in clear cell renal cell carcinoma. Radiol Med 2021; 126: 645–651. [DOI] [PubMed] [Google Scholar]
- 40.Favre G, Grangeon-Chapon C, Raffaelli C, et al. Perirenal fat thickness measured with computed tomography is a reliable estimate of perirenal fat mass. PLoS One 2017; 12: e0175561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grigoras A, Balan RA, Caruntu ID, et al. Perirenal adipose tissue–Current knowledge and future opportunities. J Clin Med 2021; 10: 1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greco F, Quarta LG, Carnevale A, et al. Subcutaneous adipose tissue reduction in patients with clear cell renal cell carcinoma and peritumoral collateral vessels: a retrospective observational study. Applied Sciences 2021; 11: 6076. [Google Scholar]
- 43.Simpson E, Patel U. Diagnosis of angiomyolipoma using computed tomography-region of interest < or =-10 HU or 4 adjacent pixels < or =-10 HU are recommended as the diagnostic thresholds. Clin Radiol 2006; 61: 410–416. [DOI] [PubMed] [Google Scholar]
- 44.Macleod LC, Hsi RS, Gore JL, et al. Perinephric fat thickness is an independent predictor of operative complexity during robot-assisted partial nephrectomy. J Endourol 2014; 28: 587–591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.