Abstract
Neuromuscular blocking agents (NMBA) are a controversial therapeutic option in the approach to the critically ill patient. They are not innocuous, and the available evidence does not support their routine use in the intensive care unit. If necessary, monitoring protocols should be established to avoid residual relaxation, adverse effects, and associated complications. This narrative review discusses the current indications for the use of NMBA and the different tools for monitoring blockade in the intensive care unit. However, expanding the use of NMBA in critical settings merits the development of prospective studies.
Keywords: Neuromuscular blocking agent, muscle weakness, intensive care unit, train of four, post-tetanic count, review
Introduction
Neuromuscular blocking agents (NMBA) are drugs that affect the motor endplate of striated muscles 1 by blocking the transmission of nerve impulses at the neuromuscular junction. Normally, to cause contraction at the neuromotor endplate, acetylcholine is released from the presynaptic motor nerve ending, spreads across the synaptic cleft, and binds to ligand-gated nicotinic acetylcholine receptors on the postsynaptic motor endplate. If the threshold potential is reached, the action potential diffuses over the surface of the skeletal muscle cells, giving rise to contraction. 1
NMBA are widely used in controlled surgical settings. When neuromuscular function is assessed by measuring the muscle response to stimulation of a peripheral nerve, one of the most commonly used methods is the train of four (TOF). 2 NMBA have variable utility in the intensive care unit (ICU), and the most common reason for using them is rapid sequence intubation because of their short-term effects. 3 Long-term use of NMBA has been recommended in patients with refractory hypoxemia, especially when associated with acute respiratory distress syndrome (ARDS), and NMBA have been widely used in the current COVID-19 pandemic, 4 in patients with status asthmaticus, and in patients with elevated intra-abdominal pressure (IAP). Some controversial indications include post-arrest hypothermia, severe head injury (concussive brain injury), tremor control, correction of patient–ventilator asynchrony, and elevated intracranial pressure (ICP), among other scenarios. 3
Despite their many benefits, NMBA are associated with several potential complications. It is imperative that staff have accurate knowledge regarding monitoring of neuromuscular blockade (NMB) in the ICU based on both pathophysiological concepts and data obtained from clinical studies.
This narrative review was developed after conducting a search for primary studies, systematic reviews, and meta-analyses in the main scientific databases Google Scholar, Embase, Scopus, and Medline using the MeSH terms “neuromuscular blocking agents,” “intensive care unit,” “train of four,” and “post-tetanic count,” which were linked with the Boolean AND connector for each of the components of the PICO question (Problem, Intervention, Comparator, Results). Duplicate articles were excluded, and those that met the inclusion criteria, including the keywords in the title or abstract, were selected. Finally, to select relevant articles for this review, a consensus was reached among all the authors to unify and review the database. We herein provide an overview of the current indications for the use of NMBA in the ICU and available options for monitoring these drugs according to the most recent literature.
Physiological and pharmacological basis of NMBA
Neuromuscular transmission (NMT) comprises three fundamental components: the neuron, the neurotransmitter, and the muscle fiber. Initially, the motor units comprise lower motor neurons and their associated muscle, with a single nerve capable of innervating up to 100 individual muscle fibers. The neurotransmitter acetylcholine is accumulated in vesicles in presynaptic neurons; once the action potential is generated, it is released into the synaptic cleft through depolarization mediated by sodium influx.4–6
Generally, more acetylcholine molecules are released than are necessary to activate the nicotinic receptors and guarantee transmission of the action potential because of the ability of acetylcholinesterase to rapidly hydrolyze acetylcholine, given its high affinity. 6 About 4 million molecules of acetylcholine are released into the synaptic cleft to exert their effect on about half a million postsynaptic nicotinic receptors. These receptors have a conformation of several subunits (two types α, β, δ, and γ) that require the binding of at least two molecules of acetylcholine to generate a conformational change in the receptor and allow massive entry of sodium and potassium, which produces membrane depolarization and generates muscle contraction.3,7 Only the motor endplate of skeletal muscles is affected (smooth muscle and cardiac muscle are not affected). 1 Finally, the action of acetylcholine is rapidly terminated by the enzyme acetylcholinesterase. 3
NMBA are classified according to their mechanism of inducing NMB: depolarizing agents, including succinylcholine, and non-depolarizing agents, which are further classified as aminosteroids (such as rocuronium, vecuronium, and pancuronium) and benzylisoquinolines (including cisatracurium and atracurium).1–3 The characteristics of the main NMBA are summarized in Table 1.
Table 1.
Pharmacokinetic and pharmacodynamic characteristics of NMBA commonly used in the ICU.
| Type | Agent | SD 95/intubation dose (mg/kg) | Start time (minutes) | Infusion dose (µg/kg/minute) | Duration of action (minutes) | Elimination | Adverse effects |
|---|---|---|---|---|---|---|---|
| Depolarizing | Succinylcholine | 0.5–0.6/1–1.2 | <1 | NF | 10–12 | Metabolized by plasma cholinesteraseNo active metabolite | Transient increase in potassium, malignant hyperthermia |
| Non-depolarizing: Aminosteroids | Rocuronium | 0.3/0.6 (1.2 for fast sequence induction) | 1.5–3 (1 for rapid induction dose) | 5–12 | 20–70 | Eliminated by liver (90%) and kidneys (10%)No active metabolite | Tachycardia |
| Non-depolarizing: Aminosteroids | Pancuronium | 0.07/0.1 | 3–5 | 0.8–1.7 | 20–40 | Eliminated by liver (15%) and kidneys (85%)Active metabolite =3-OH-pancuronium, which accumulates in renal failure | Tachycardia, hypotension, flushing |
| Non-depolarizing: Aminosteroids | Vecuronium | 0.05/0.08–0.1 | 3–5 | 0.8–1.7 | 20–40 | Eliminated by liver (60%) and kidneys (40%)Active metabolite =3-desacetyl-vecuronium, which accumulates in renal failure | Bradycardia |
| Non-depolarizing: Benzylisoquinolines | Cisatracurium | 0.05–0.07/0.15 | 4–7 | 1–3 | 35–50 | Hofmann elimination. No active metabolite | Generally well tolerated |
| Non-depolarizing: Benzylisoquinolines | Atracurium | 0.4/0.4–0.5 | 3–5 | 5–20 | 20–35 | Plasma esterase and Hofmann elimination | Generally well tolerated |
NMBA, neuromuscular blocking agents; ICU, intensive care unit; SD 95%, amount of NMBA needed to reduce the height of contractions by 95%; NF, not feasible.
Source: self-made. Adapted from Reference 3.
Depolarizing NMB
Succinylcholine is a nicotinic receptor agonist that allows ion-gated channels to open and remain open in the presence of succinylcholine. 8 From a clinical point of view, depolarization allows the observation of fasciculations in the early phases that later give way to paralysis. The rapid effect and 3- to 5-minute duration of succinylcholine make it useful in short procedures, such as orotracheal intubation (OTI). 8 Potential adverse effects of succinylcholine include hyperkalemia, anaphylaxis, bronchospasm, cardiac arrhythmia, disorders up to asystole, and the eventual possibility of triggering malignant hyperthermia. 9
Non-depolarizing NMB
Non-depolarizing NMB drugs (NDNMB) are competitive antagonists of nicotinic receptors, bind to the receptor for a longer period, and prevent acetylcholine from binding to the receptor, resulting in NMB. 8 There are two classes of NDNMB: benzylisoquinolines, which are metabolized at physiological pH through Hoffmann elimination (an organ-independent mechanism), 10 and aminosteroids, which undergo hepatic metabolism and renal elimination to varying degrees depending on the drug. 11
According to the indication, drugs must be selected in the ICU because they must be compatible with critical conditions and comorbidities to avoid the greatest number of undesirable effects. 8 Thus, rocuronium is commonly used as an alternative to succinylcholine for OTI because of its rapid onset and intermediate duration of action without the high risk of generating hyperkalemia or malignant hyperthermia. Atracurium or cisatracurium may be the preferred agent for continuous infusion because their metabolism is not related to renal or hepatic function. Additionally, unlike succinylcholine, NDNMB infusions can be safely used in critically ill patients with conditions that cause receptor proliferation without the concern for sustained membrane permeability or acute hyperkalemia. 3
Notably, caution is needed in some patients, such as those with myasthenia gravis (who are especially sensitive to the effects of NMBA) and those with burns (who are resistant to the effects of NMB because of the proliferation/upregulation of nicotinic receptors in the sarcolemma). 8
Use of NMBA in the ICU
The most common indications for the administration of NMBA are to facilitate OTI and mechanical ventilation, especially in patients with ARDS; to ensure ICP control; and to ablate muscle spasms associated with tetanus and decreased oxygen consumption. 12 The use of NMBA has also been proposed for control of IAP and induction of therapeutic hypothermia. 3 However, the most relevant factors to consider when choosing specific NMBA in the ICU are clinical experience with the drug, the duration and mechanism of action, and patient-specific factors.8,12
1. OTI
The most commonly used NMBA are succinylcholine and rocuronium, with rocuronium being particularly preferred for rapid sequence intubation. 10 NMBA have precise indications for the sake of improving intubation conditions and plans, fewer attempts and complications related to the procedure have been described. 3
Commonly used NMBA for rapid sequence intubation management are succinylcholine and rocuronium, the latter of which is the drug of choice. Rocuronium may be administered if succinylcholine is contraindicated. However, once the patient’s muscles are relaxed, difficulties in performing intubation and ventilation may progress to a life-threatening situation. 13 To reduce this risk, sugammadex is also available. Sugammadex is a novel cyclodextrin (a selective relaxant binding drug) that is used to reverse NMB after administration of aminosteroid NDNMB such as vecuronium or rocuronium. 14 Its action is immediate and produces recovery from deep NMB in a shorter time than when succinylcholine is used in rapid sequence doses; notably, however, the use of sugammadex does not supplant adequate airway management. 14 Sugammadex in the ICU, although not widely used, could be an important tool in reversal of the muscle relaxation caused by NMBA. 15 It may also be useful in enhanced recovery protocols after surgery and in postoperative residual NMB. 16
2. ARDS
NMBA can facilitate protective ventilation of the lungs, prevent the start of spontaneous respiratory efforts, decrease the work of breathing, reduce oxygen consumption, and abolish resting muscle tone; 3 they can also increase chest wall compliance, reduce patient–ventilator asynchrony, facilitate lung recruitment, and reduce the inflammatory response of ARDS. 17 The ACURASYS study was one of the first to show that pharmacological intervention with cisatracurium in patients with moderate to severe ARDS was able to achieve a significant decrease in mortality. 18
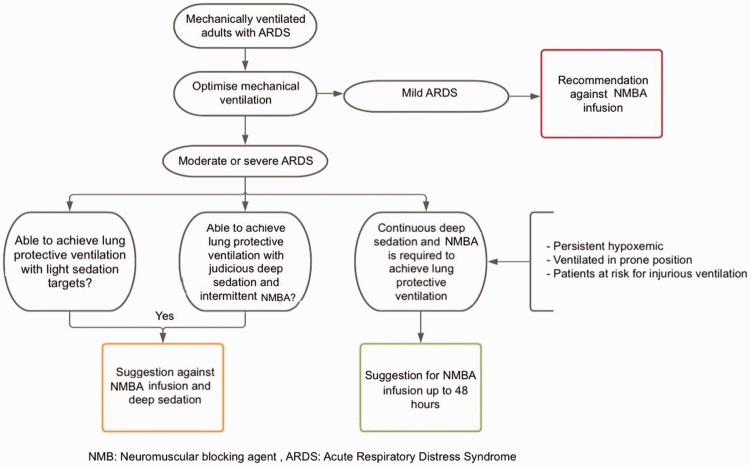
Recently published studies show controversial evidence. In the Re-evaluation of Systemic Early Neuromuscular Blockade (ROSE) study, the use of cisatracurium infusion for 48 hours was compared with its intermittent administration in patients with ARDS scenario, and no significant differences in mortality were found (group difference in mortality reduction, −0.3; 95% confidence interval, −6.4 to 5.9; p = 0.93). 19 The evidence derived from NMBA use in the COVID-19 pandemic is reflected in the work carried out in a Korean ICU, in which the driving pressure and use of NMBA were evaluated in patients with ARDS due to COVID-19. 20 The study showed that the short-term use of NMB (<6 days) facilitated lung-protective ventilation and was independently associated with decreased mortality (adjusted odds ratio, 0.27; 95% confidence interval, 0.09–0.81; p = 0.02). 20 However, the guidelines support NMBA use and recommend using them in continuous intravenous infusion early during ARDS for patients with a PaO2/FiO2 of <150. 8 These strategies are recommended by various ARDS guidelines, such as the Surviving Sepsis Campaign’s COVID-19 guide, which recommends using NMB in continuous infusion for up to 48 hours because of its benefits in refractory hypoxemia. 21 This proposal has been used in many patients during the current pandemic17,18 (Figure 1).
Figure 1.
Algorithm for the use of neuromuscular blocking agents in ARDS. Source: self-made.
ARDS, acute respiratory distress syndrome; NMBA, neuromuscular blocking agent.
3. Status asthmaticus
Among patients with status asthmaticus, those who require mechanical ventilation may require NMBA to facilitate synchronization with the ventilator, avoid excessive hyperinflation, increase airway pressure, and reduce the activity of the respiratory muscles. 22 Administration of NMBA in controlled boluses instead of continuous infusions is recommended because intermittent doses allow serial evaluation and reduce the risk of developing myopathies due to prolonged paralysis. 23 Additional benefits include reducing oxygen consumption and carbon dioxide production. 3
4. Elevated ICP
NMBA facilitate mechanical ventilation strategies that reduce ICP. 3 They promote carbon dioxide clearance, reduce positive end-expiratory pressure, decrease metabolic output, and limit ICP surges after stimuli such as tracheal suctioning, coughing, movement, agitation, and postural changes. 3 Despite the lack of scientific evidence, the use of NMBA in these patients is part of the routine management once the need for surgical intervention has been ruled out. 22
5. Elevated IAP
Intra-abdominal hypertension, defined as sustained IAP of >12 mmHg, is present in almost one-third of ICU patients and can result in abdominal compartment syndrome. 24 It can develop from reduced compliance of the abdominal wall due to fluid overload/increased vascular permeability, abdominal closure, or pain and can predispose the patient to multiple organ failure (especially kidney failure) and death. 24 By reducing abdominal muscle tone, NMBA not only reduce IAP but can also give clinicians time to monitor and manage patients with positioning changes, nasogastric/rectal decompression, diuresis, and therapeutic paracentesis. 3
6. Therapeutic hypothermia after cardiac arrest
People who experience out-of-hospital cardiac arrest attain better scores in the brain performance category during their hospital stay and are more likely to survive to hospital discharge when their core body temperature is reduced to the range of 32°C to 34°C for 12 to 24 hours. 25 Shivering due to therapeutic hypothermia leads to heat production, an increased metabolic rate, swelling, increased ICP, decreased oxygen levels in brain tissue, and muscle pain. 3 NMBA are commonly given during therapeutic hypothermia to more quickly achieve the target temperature and to control shivering. 26
A multicenter study evaluated 136 patients who, after having been successfully resuscitated after ventricular fibrillation, underwent therapeutic hypothermia of 32°C to 34°C for 24 hours versus normothermia. 25 The hypothermia group showed more favorable neurological outcomes and lower mortality than the normothermia group. 25 The HYPERION study, in which 584 patients who had been successfully resuscitated after non-shockable cardiac arrest were randomized to therapeutic hypothermia and normothermia groups, also showed a benefit in terms of an improved neurological status at 90 days. 27
Less favorable evidence exists for patients with traumatic brain injury. In one study that examined the efficacy of hypothermia in reducing ICP, morbidity, and mortality at 6 months after severe TBI, successful ICP reduction was achieved but with a higher mortality rate and worse functional outcome. 28
Adverse effects of muscle relaxants in the ICU
Most of the effects of NMBA that occur outside the neuromuscular junction are cardiac in nature and are due to histamine release and ganglionic or muscarinic stimulation manifested by vagolytic actions, ganglionic blockade, or sympathetic stimulation. 8 Complications of NMBA are related to immobility, including eschar formation, keratitis, and corneal abrasions secondary to loss of lid closure reflexes. 12 Paralysis is associated with pooling and stasis of blood, which can increase the risk of venous thromboembolism. ICU-acquired weakness is another potential complication of NMBA use. 29
Another concern surrounding the administration of NMBA is the potential for patient awareness if not adequately sedated. Because NMBA do not have amnesic or analgesic properties, it is imperative that patients receive adequate sedation and analgesia before receiving them. Without sedoanalgesia, significant distress and increases in the blood pressure and heart rate can result. 10
Adverse events of NMBA are multifactorial in nature and include muscle atrophy, inflammation, and immobility as well as drug interactions with corticosteroids and aminoglycosides. 12 Absence of deep tendon reflexes, loss of distal sensory mechanisms, and inability to wean from the ventilator result in a prolonged hospital stay and increased mortality.12,29
Monitoring and level of NMB
A continuous infusion of NMBA seems to be more effective that intermittent boluses in terms of recovery. 30 However, titration of the level of NMB is essential to achieve the therapeutic goal and prevent complications. 29 Physicians who prescribe NMBA should closely monitor the degree of relaxation and ensure adequate sedation. 21 The use of deep sedation is recommended in critically ill patients receiving therapy with neuromuscular relaxants, which cannot be monitored with routine clinical parameters such as the Richmond Agitation Sedation Scale. 31
The use of NMT monitoring is based on an understanding of the different muscle sensitivities to NMB. 2 Several techniques can be used to monitor neuromuscular function: clinical evaluation, objective quantitative methods, or stimulation patterns.2,32 Monitoring facial muscles can prove challenging because direct muscle stimulation easily occurs; however, the corrugator supercilii muscle reportedly responds to NMBA similarly to the diaphragm and laryngeal muscles, while the orbicularis oculi can respond similarly to the extremities. 33 Experts’ consensuses discourage monitoring facial muscles because there is a more than five-fold higher risk of residual paralysis when monitoring is performed at the eye muscles than at the hand muscles. 33
Quantitative monitoring methods use devices that stimulate the peripheral nerve while also recording, quantifying, and numerically displaying the evoked responses patterns.2,32 Many monitoring techniques have been described. Acceleromyography is the most widely used technique; it measures the acceleration of a muscle, usually the adductor pollicis, in response to neurostimulation. 32 Similarly, kinemyography measures the electrical signal generated by the bending of a piezoelectric sensor placed between the thumb and index after neurostimulation. 2 Electromyography does not rely on an unrestricted freely moving thumb because it measures muscle action potentials across the neuromuscular unit and has the advantage of working in ICU patients who may have wrist cuffs to prevent accidental removal of invasive catheters.2,32 All these devices allow automated measurements at a user-defined time interval and can perform various patterns of neurostimulation; they include TOF, single contraction, double burst, and post-tetanic potentiation catheters.2,32
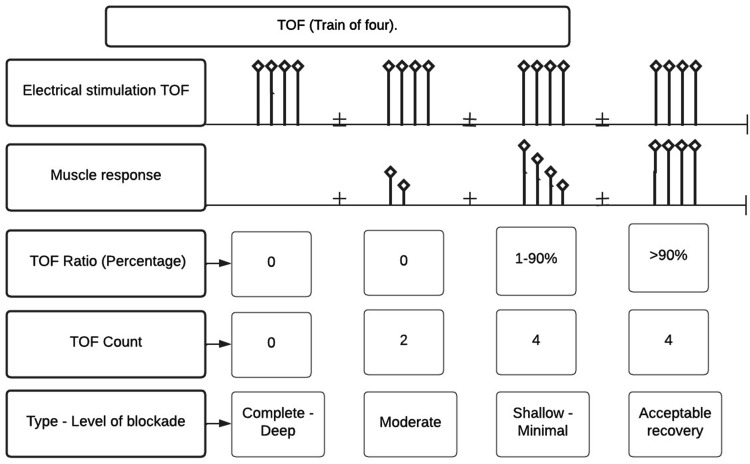
The most commonly used method of NMT monitoring is evaluation of NMT through peripheral nerve stimulation by TOF. 34 This method ensures an appropriate level of blockade and decreases the total dose of NMBA administered. 34 However, because it is a qualitative type of monitoring, it still has several limitations and should be complemented with clinical evaluation. 12 TOF consists of the administration of four supramaximal stimuli for 0.5 s (2 Hz), and each set (train) of four is repeated each time the evaluation of neuromuscular relaxation is needed. 2 The repetitive stimulus produced by the four contractions in a row produces a decrease in the muscular response and constitutes the basis of the evaluation in such a way that the ratio of the amplitude of the fourth contraction to the first provides the TOF value. When there is no effect of any neuromuscular relaxant, the TOF value is equal to 1 (100%), and in conditions of partial NDNMBD, the TOF value is inversely proportional to the level of blockade 35 (Figure 2).
Figure 2.
TOF response patterns according to the level of neuromuscular blockade. In complete or deep blockade, there is an absence of TOF. In moderate blockade, there is a TOF count of 1 to 3 but there is no TOF ratio percentage. In the shallow or minimal (recovery) phase, there is a TOF count of 4 and a TOF ratio of at least 1%. Source: self-made.
TOF, train of four.
Although TOF is the most widely used method for evaluating blockade, it is impossible to adequately classify the level of blockade under conditions of deep and intense NMB. In such patients, we can use the post-tetanic count (PTC); this is obtained by evaluating the neuromuscular contraction at 1 Hz beginning 3 s after a tetanic stimulation (stimulus at 50–200 Hz for 5 s), which will appear before the first TOF stimulus. 35 A patient is considered fit for extubation when they have a TOF value of ≥90%. 36
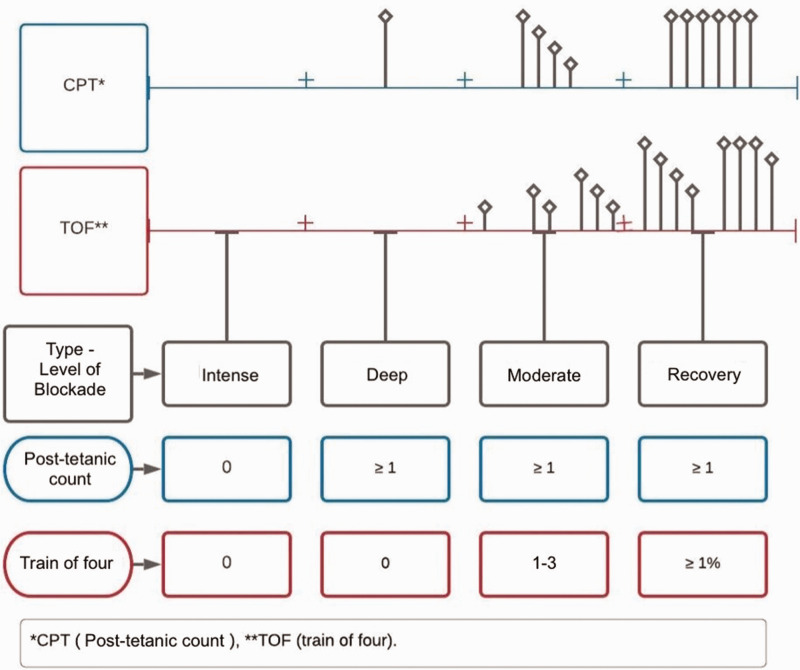
It is important to clarify that in the presence of intense NMB, there is no TOF response or PTC response; in deep blockade, however, although there is no response to TOF, there is at least one response to PTC. During moderate NMB, there is no TOF ratio; however, there is a response from train 1 to 3 and there is a response to PTC. In the recovery phase, there is a TOF ratio that varies from 1% to 100%. The monitoring of neuromuscular relaxation through peripheral nerve stimulation is performing using various patterns such as single contraction, tetanic and post-tetanic contraction, and TOF, of which the last two are the most commonly used to determine the level of NMB in patients 2 (Figure 3).
Figure 3.
Different degrees of non-depolarizing neuromuscular blockade according to the type of stimulus. When there is complete blockade, there is absolutely no response. When the degree of blockade is deep, there is at least one PTC response but no TOF response. In moderate neuromuscular blockade, there is a response in the PTC, and the first responses of the TOF appear (from 1 to 3) but the TOF percentage (T1/T4) does not yet appear. In the shallow or minimal (recovery) phase, the four TOF responses appear and a percentage of the relationship between the first stimulus and the fourth (T1/T4) appears. Source: self-made.
TOF, train of four; CPT, post-tetanic count.
The ACURACYS study showed that a protocol based on TOF allowed for a reduction in the consumption of cisatracurium without affecting the quality of NMB. 17 The study used a protocol that began with a conventional starting dose and then increased the cisatracurium infusion when at least one muscular TOF response was obtained. 17 Another publication compared the effect of fixed doses of cisatracurium versus a titrated infusion strategy based on TOF monitoring to evaluate the ventilatory and clinical outcomes in 167 patients with ARDS. The authors reported that fixed doses were associated with similar ventilatory and clinical outcomes compared with the TOF titration strategy. Nevertheless, fixed doses were associated with a three-fold increase in the administered dose. 37
By contrast, a study of 38 patients with ARDS showed that the addition of TOF to NMT monitoring did not change mortality in the ICU or the days with mechanical ventilation, but it did increase the consumption of atracurium compared with clinical evaluation. 38 Additionally, a small retrospective study provided information on the doses of cisatracurium needed in patients with ARDS to reduce the time needed to reach target TOF. 39 However, the methodology of the study limited the extrapolation of its conclusions, and further research is required.
Although NMT monitoring is not free of biases and inaccuracies, and although well-designed prospective trials on such monitoring are lacking, its use is an element that we believe should be essential to effectively titrate the dose of neuromuscular relaxants to reduce the risk of myopathy or alterations of the myoneural endplate associated with these drugs, especially in the context of the ICU. 12
We therefore consider it fundamental to use NMT monitoring not only to titrate doses but also as part of the checklist prior to scheduled extubation to limit the possibility of failed extubation associated with neuromuscular weakness. 12
Conclusion
NMBA are not routinely used in the ICU; they are mainly used in well-documented precise scenarios. However, the current pandemic and the tsunami of ARDS cases has forced the massive use of mechanical ventilation and the use of neuromuscular relaxants for several days. This has generated, among other things, a concern about the doses used, adverse effects, and ability to adequately evaluate the effect of these drugs with a monitoring tool. For this reason, interest has reappeared in the use of TOF and PTC to evaluate and quantify peripheral stimuli to establish and quantify the goals, dose, and duration of use of these drugs. Given the limited literature in this regard, clinical trials are needed to evaluate this type of regimen to reduce unwanted effects of NMBA.
Author contributions: CDC, JRB: Conceptualization; Writing – original draft, review & editing. AAH: Writing – original draft, review & editing; Visualization. TRY: Writing – original draft, review & editing; Visualization. MCM: Writing – review & editing. JDRB: Writing – review & editing. DBN: Writing – original draft, review & editing; Visualization. MCAC: Writing – review & editing.
Declaration of conflicting interests: The authors declare that there are no conflicts of interest.
Funding: This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Consent for publication
No written consent was obtained from the patients because no patient-identifiable data are included in this narrative review.
Ethics approval
This is a review of previously published articles. No humans or animals were involved; therefore, no ethics approval was required.
ORCID iD
María Cristina Martínez-Ávila https://orcid.org/0000-0002-1542-0249
References
- 1.Fierro MA, Bartz RR. Management of sedation and paralysis. Clin Chest Med 2016; 37: 723–739. doi:10.1016/j.ccm.2016.07.012 [DOI] [PubMed] [Google Scholar]
- 2.Duţu M, Ivaşcu R, Tudorache O, et al. Neuromuscular monitoring: an update. Rom J Anesth Intensive Care. 2018; 25: 55–60. doi:10.21454/rjaic.7518.251.nrm [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.deBacker J, Hart N, Fan E. Neuromuscular blockade in the 21st century management of the critically ill patient. Chest. 2017; 151: 697–706. doi:10.1016/j.chest.2016.10.040 [DOI] [PubMed] [Google Scholar]
- 4.Hraiech S, Yoshida T, Annane D, et al. Myorelaxants in ARDS patients. Intensive Care Med. 2020; 46: 2357–2372. doi:10.1007/s00134-020-06297-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fagerlund MJ, Eriksson LI. Current concepts in neuromuscular transmission. Br J Anaesth. 2009; 103: 108–114. doi:10.1093/bja/aep150 [DOI] [PubMed] [Google Scholar]
- 6.Johnson M, Dabrowski M, Gurley DA, et al. Activation and inhibition of human muscular and neuronal nicotinic acetylcholine receptors by succinylcholine. Anesthesiology. 2006; 104: 724–733. doi:10.1097/00000542-200604000-00017 [DOI] [PubMed] [Google Scholar]
- 7.Fagerlund MJ, Dabrowski M, Eriksson LI. Pharmacological characteristics of the inhibition of nondepolarizing neuromuscular blocking agents at human adult muscle nicotinic acetylcholine receptor. Anesthesiology. 2009; 110: 1244–1252. doi:10.1097/ALN.0b013e31819fade3 [DOI] [PubMed] [Google Scholar]
- 8.Murray MJ, DeBlock H, Erstad B, et al. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2016; 44: 2079–2103. doi:10.1097/CCM.0000000000002027 [DOI] [PubMed] [Google Scholar]
- 9.Zoremba N, Schälte G, Bruells C, et al. [Update on muscle relaxation: what comes after succinylcholine, rocuronium and sugammadex?]. Anaesthesist. 2017; 66: 353–359. doi:10.1007/s00101-017-0289-1 [DOI] [PubMed] [Google Scholar]
- 10.Mefford B, Donaldson JC, Bissell BD. To block or not: updates in neuromuscular blockade in acute respiratory distress syndrome. Ann Pharmacother. 2020; 54: 899–906. doi:10.1177/1060028020910132 [DOI] [PubMed] [Google Scholar]
- 11.Grawe ES, Bennett S, Hurford WE. Early paralysis for the management of ARDS. Respir Care. 2016; 61: 830–838. doi:10.4187/respcare.04734 [DOI] [PubMed] [Google Scholar]
- 12.Renew JR, Ratzlaff R, Hernandez-Torres V, et al. Neuromuscular blockade management in the critically ill patient. J Intensive Care. 2020; 8: 37. doi:10.1186/s40560-020-00455-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosier JM, Sakles JC, Law JA, et al. Tracheal intubation in the critically ill. Where we came from and where we should go. Am J Respir Crit Care Med. 2020; 201: 775–788. doi: 10.1164/rccm.201908-1636CI. [DOI] [PubMed] [Google Scholar]
- 14.Miller RD. Sugammadex: an opportunity to change the practice of anesthesiology? Anesth Analg. 2007; 104: 477–478. doi: 10.1213/01.ane.0000255645.64583.e8. [DOI] [PubMed] [Google Scholar]
- 15.Carron M, Baratto F, Pettenuzzo T, et al. Sugammadex as rescue therapy for residual neuromuscular blockade in the intensive care unit. Can J Anaesth. 2016; 63: 1384–1385. doi: 10.1007/s12630-016-0736-5. Epub 2016 Sep 19. PMID: 27646527. [DOI] [PubMed] [Google Scholar]
- 16.Abrishami A, Ho J, Wong J, et al. Sugammadex, a selective reversal medication for preventing postoperative residual neuromuscular blockade. Cochrane Database Syst Rev. 2009; (4): CD007362. doi: 10.1002/14651858.CD007362.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Ammar MA, Sacha GL, Welch SC, et al. Sedation, analgesia, and paralysis in COVID-19 patients in the setting of drug shortages. J Intensive Care Med. 2021; 36: 157–174. doi:10.1177/0885066620951426 [DOI] [PubMed] [Google Scholar]
- 18.Papazian L, Forel JM, Gacouin A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010; 363: 1107–1116. doi:10.1056/NEJMoa1005372 [DOI] [PubMed] [Google Scholar]
- 19.National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss M, Huang DT, Brower RG, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019; 380: 1997–2008. doi:10.1056/NEJMOA1901686/SUPPL_FILE/NEJMOA1901686_DATA-SHARING.PDF [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee BY, Lee SI, Baek MS, et al. Lower driving pressure and neuromuscular blocker use are associated with decreased mortality in patients with COVID-19 ARDS. Respir Care. 2022; 67: 216–226. doi:10.4187/RESPCARE.09577 [DOI] [PubMed] [Google Scholar]
- 21.Alhazzani W, Belley-Cote E, Møller MH, et al. Neuromuscular blockade in patients with ARDS: a rapid practice guideline. Intensive Care Med. 2020; 46: 1977–1986. doi:10.1007/s00134-020-06227-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sandiumenge A, Anglés R, Martínez-Melgar JL, et al. [Use of neuromuscular blocking agents in critically ill patients.] Med Intensiva. 2008; 32(Spec No. 1): 69–76. [Article in Spanish] [PubMed] [Google Scholar]
- 23.Dos Santos Rocha A, Südy R, Peták F, et al. Physiologically variable ventilation in a rabbit model of asthma exacerbation. Br J Anaesth. 2020; 125: 1107–1116. doi:10.1016/J.BJA.2020.08.059/ATTACHMENT/3431DDEB-C4B1-4B3B-8CE2-CA07CEEC768F/MMC1.DOCX [DOI] [PubMed] [Google Scholar]
- 24.Kühn A, Fuchs C, Hahnenkamp K. [Intra-abdominal pressure measurement]. Dtsch Med Wochenschr. 2021; 146:1211–1217. doi:10.1055/a-1287-5112 [DOI] [PubMed] [Google Scholar]
- 25.Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurological outcome after cardiac arrest. N Engl J Med. 2002; 346: 549–556. doi:10.1056/NEJMoa012689 [DOI] [PubMed] [Google Scholar]
- 26.Riker RR, Gagnon DJ, May T, et al. Analgesia, sedation, and neuromuscular blockade during targeted temperature management after cardiac arrest. Best Pract Res Clin Anaesthesiol. 2015; 29: 435–450. doi:10.1016/j.bpa.2015.09.006 [DOI] [PubMed] [Google Scholar]
- 27.Lascarrou JB, Meziani F, le Gouge A, et al. Therapeutic hypothermia after nonshockable cardiac arrest: the HYPERION multicenter, randomized, controlled, assessor-blinded, superiority trial. Scand J Trauma Resusc Emerg Med. 2015; 23: 26. doi:10.1186/s13049-015-0103-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews PJD, Sinclair HL, Rodriguez A, et al. Therapeutic hypothermia to reduce intracranial pressure after traumatic brain injury: the Eurotherm3235 RCT. Health Technol Assess. 2018; 22: 1–134. doi:10.3310/hta22450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deem S, Lee CM, Curtis JR. Acquired neuromuscular disorders in the intensive care unit. Am J Respir Crit Care Med. 2003; 168: 735–739. doi:10.1164/rccm.200302-191UP [DOI] [PubMed] [Google Scholar]
- 30.de Lemos JM, Carr RR, Shalansky KF, et al. Paralysis in the critically ill: intermittent bolus pancuronium compared with continuous infusion. Crit Care Med. 1999; 27: 2648–2655. doi:10.1097/00003246-199912000-00007 [DOI] [PubMed] [Google Scholar]
- 31.Devlin JW, Skrobik Y, Gelinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018; 46: e825–e873. doi:10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 32.Naguib M, Brull SJ, Johnson KB. Conceptual and technical insights into the basis of neuromuscular monitoring. Anaesthesia. 2017; 72 Suppl 1: 16–37. doi: 10.1111/anae.13738. [DOI] [PubMed] [Google Scholar]
- 33.Chaves-Cardona H, Hernandez-Torres V, Kiley S, et al. Neuromuscular blockade management in patients with COVID-19. Korean J Anesthesiol. 2021; 74: 285–292. doi: 10.4097/kja.21106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Foster JG, Kish SK, Keenan CH. A national survey of critical care nurses’ practices related to administration of neuromuscular blocking agents. Am J Crit Care. 2001; 10: 139–145. doi:10.4037/ajcc2001.10.3.139 [PubMed] [Google Scholar]
- 35.Forkin KT, Nemergut EC. Miller’s Anesthesia, 8th Edition. Anesthesiology. 2016; 124: 977–978. doi:10.1097/ALN.0000000000001020 [Google Scholar]
- 36.Schepens T, Janssens K, Maes S, et al. Respiratory muscle activity after spontaneous, neostigmine- or sugammadex-enhanced recovery of neuromuscular blockade: a double blind prospective randomized controlled trial. BMC Anesthesiol. 2019; 19: 187. doi:10.1186/s12871-019-0863-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson Bastin ML, Smith RR, Bissell BD, et al. Comparison of fixed dose versus train-of-four titration of cisatracurium in acute respiratory distress syndrome. J Crit Care. 2021; 65: 86–90. doi:10.1016/j.jcrc.2021.05.011 [DOI] [PubMed] [Google Scholar]
- 38.Rezaiguia-Delclaux S, Laverdure F, Genty T, et al. Neuromuscular blockade monitoring in acute respiratory distress syndrome: randomized controlled trial of clinical assessment alone or with peripheral nerve stimulation. Anesth Analg 2021; 132: 1051–1059. doi:10.1213/ANE.0000000000005174 [DOI] [PubMed] [Google Scholar]
- 39.Merkel A, Massey K, Bellamy C, et al. Predictors of cisatracurium continuous infusion dose in acute respiratory distress syndrome. J Pharm Pract. 2021; 34: 600–605. doi:10.1177/0897190019888103 [DOI] [PubMed] [Google Scholar]