Abstract
Survivors of ICU hospitalizations often experience severe and debilitating symptoms long after critical illness has resolved. Many patients experience notable psychiatric sequelae such as depression, anxiety, and posttraumatic stress disorder (PTSD) that may persist for months to years after discharge. The COVID-19 pandemic has produced large numbers of critical illness survivors, warranting deeper understanding of psychological morbidity after COVID-19 critical illness. Many patients with critical illness caused by COVID-19 experience substantial post-ICU psychological sequelae mediated by specific pathophysiologic, iatrogenic, and situational risk factors. Existing and novel interventions focused on minimizing psychiatric morbidity need to be further investigated to improve critical care survivorship after COVID-19 illness. This review proposes a framework to conceptualize three domains of risk factors (pathophysiologic, iatrogenic, and situational) associated with psychological morbidity caused by COVID-19 critical illness: (1) direct and indirect effects of the COVID-19 virus in the brain; (2) iatrogenic complications of ICU care that may disproportionately affect patients with COVID-19; and (3) social isolation that may worsen psychological morbidity. In addition, we review current interventions to minimize psychological complications after critical illness.
Key Words: ARDS, COVID-19 pandemic, postintensive care syndrome, posttraumatic stress disorder, psychological morbidity, SARS-CoV-2 virus
Over the past 20 years, cohort studies have demonstrated frequent psychological morbidity among critical illness survivors.1, 2, 3 During the COVID-19 pandemic, an unprecedented number of patients experienced critical illness over a short period of time, which further raised awareness of the psychological sequelae of critical illness.4, 5, 6 Patients with critical COVID-19 and associated ARDS may be at increased risk for psychological morbidity compared with other critically ill patients.
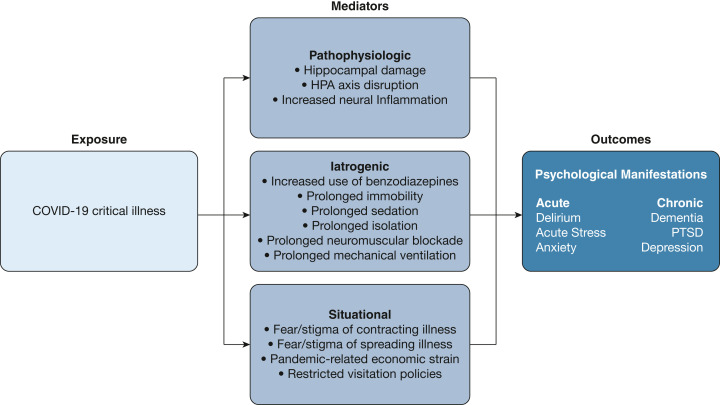
In this review, we describe acute and long-term psychological manifestations experienced by patients surviving critical illness from COVID-19, using a framework organized around three distinct risk factors for critical COVID-19-related psychological morbidity: (1) neuro-pathophysiologic changes caused by the SARS-CoV-2; (2) iatrogenic complications of ICU care that may have disproportionally affected patients with critical COVID-19; and (3) situational factors, including social isolation, which has been a characteristic feature of the pandemic (Fig 1 ). Finally, we review current therapeutic interventions for psychological sequelae of critical illness and discuss specific features of psychological morbidity after critical COVID-19 that may warrant novel therapies.
Figure 1.
Framework to conceptualize risk factors of psychological morbidity in COVID-19 critical illness. HPA = hypothalamic-pituitary-adrenal, PTSD = posttraumatic stress disorder.
Literature Search
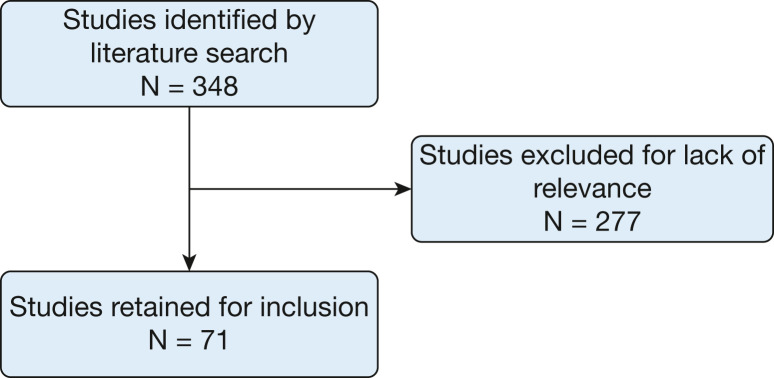
Relevant literature identified via PubMed and Ovid searches were reviewed by a single author for inclusion. The search strategy included the following descriptors: “psychiatric morbidity,” “COVID-19 critical illness,” “Post-Intensive Care Syndrome,” “Acute Respiratory Distress Syndrome,” “Depression,” “Anxiety,” and “Post-Traumatic Stress Disorder.” Titles and abstracts were screened for relevance. Relevant articles were read in full. Applicable studies of psychological morbidity in non-COVID-19 ICU survivors were also included to supplement the emerging literature in COVID-19 ICU survivors (Fig 2 ).
Figure 2.
Flowchart of literature search.
Incidence and Prevalence of Acute and Post-Acute Psychological Morbidity
Standardized measurement tools such as the Hospital Anxiety and Depression Scale and Impact of Events Scaled-Revised are frequently used to measure and quantify psychological morbidity in critically ill patients.7 In meta-analyses from the pre-COVID-19 period examining psychological outcomes of critical illness survivors using these and other tools, high rates of clinically significant anxiety (25%-42%), depression (23%-34%), and posttraumatic stress disorder (PTSD) (22%-50%) persisted as long as 1 year post-ICU stay.1, 2, 3 Cohort studies of patients surviving severe COVID-19 likewise showed a high prevalence of symptoms of anxiety (9.5%-21.4%), depression (7.1%-12.7%), and (PTSD; 4.7%-8.7%), based on the Generalized Anxiety Disorder-7, Patient Health Questionnaire-9, and PTSD Check List-5 questionnaires,8 exceeding population norms (with a prevalence ratio of 1.43 for anxiety and 1.61 for depression) at 6 months post-discharge compared with individuals without a COVID-19 diagnosis.9 Additional large cohort studies and meta-analyses are needed in patients with COVID-19 critical illness to further describe long-term prevalence and incidence of psychological morbidity.
Pathophysiology of SARS-CoV-2 and the CNS
Although SARS-CoV-2 is widely known to affect the olfactory cortex, causing anosmia via direct viral injury and local inflammation, recent studies show that the virus also has a predilection for replicating in the hippocampus, which may predispose patients to psychiatric symptoms.10, 11, 12 Brain imaging studies in patients without critical illness suggest that the hippocampus and amygdala form fear-associated memory networks, which play an important role in the development of PTSD.13 Inflammation of the hippocampus, and associated reduction in hippocampal volume, have been postulated to increase vulnerability to psychiatric disease,14, 15, 16 suggesting a mechanism by which SARS-CoV-2 infection may directly contribute to psychological symptoms.
SARS-CoV-2 also may disrupt the hypothalamic-pituitary-adrenal (HPA) axis, leading to alterations in hormone secretion.11 , 17 , 18 Although direct viral invasion of the CNS is a rare isolated cause of psychiatric morbidity, HPA axis disruption may predispose patients to suicidal ideation.11 , 17, 18, 19, 20, 21 SARS-CoV-2 enters cells via the angiotensin-converting enzyme-2 receptor,11 which is expressed in the respiratory tract, as well as the kidneys, olfactory bulb, hypothalamus, pituitary gland, and adrenal glands—predisposing these organs to SARS-CoV-2-related viral injury.19 Indeed, small cohort studies suggest there may be insufficient cortisol response and insufficient growth hormone levels in some patients up to 6 months after acute illness.18 Disruption of the HPA axis has also been implicated in other SARS viruses. For example, a 2005 study of 61 SARS survivors demonstrated that 39% of patients developed hypocortisolism secondary to HPA axis dysregulation 3 months after acute illness, with a majority recovering to normal cortisol levels within 1 year.22 It is possible that the SARS-CoV-2 virus may lead to similar long-term psychiatric consequences of HPA axis dysregulation. Additional studies are needed to better understand the magnitude and duration of suppressed cortisol levels and its nuanced effect in survivors of severe COVID-19.
Finally, neural inflammatory effects of the SARS-CoV-2 also may contribute to adverse mental health outcomes. High levels of inflammatory biomarkers specific to brain tissue such as serum 100B (s100B) and neuron-specific enolase have been correlated with development of PTSD in survivors of non-COVID-19 critical illness.14 , 23 A prospective observational study of 69 patients with mild traumatic brain injury demonstrated that increased serum s100B levels during hospitalization had a statistically significant association with PTSD symptoms at 12 months postdischarge.24 S100b has also been implicated in COVID-19 infection and is positively correlated with severity of disease.25, 26, 27 A case-control study showed that s100B levels were higher in patients with mild (n = 34) or severe COVID-19 (n = 30), as compared with 30 healthy control subjects.26 The neural inflammation caused by SARS-CoV-2 may increase the risk of detrimental psychiatric outcomes such as PTSD, as described in traumatic brain injury patients. However, the effect of s100B levels on psychological outcomes after COVID-19 has not been reported to our knowledge.
Iatrogenic Causes of ICU Delirium and Subsequent Psychiatric Sequelae
Delirium is a common complication in critically ill patients. A systematic review and meta-analysis of 27,342 patients with non-COVID-19 critical illness from 48 primary studies estimated that 31% of patients experience delirium during an ICU stay.28 Social isolation, sensory/sleep deprivation, severe medical illness, mechanical ventilation, prolonged sedation, and neuromuscular blockade (NMB) are all important risk factors for the development of delirium.29 , 30 An international cohort study of 2,088 patients from 69 ICUs demonstrated that delirium was more common and more prolonged in patients with COVID-19 ARDS vs historical control subjects with non-COVID-19 ARDS and attributed this difference, at least in part, to prolonged duration of benzodiazepine infusions in patients with COVID-19 ARDS.31 Deeper sedation and NMB are often used to mitigate ventilator desynchrony and facilitate prone positioning in patients with severe ARDS. Guidelines recommend using non-benzodiazepines sedatives (ie, propofol, dexmedetomidine) over benzodiazepines because they are associated with better patient outcomes.32 , 33 However, because of drug shortages, need for prolonged deep sedation to facilitate NMB, nursing shortages, and ICU crowding that limited 1:1 nursing care, benzodiazepine use increased during the pandemic.34 , 35 Indeed, in a multicenter cohort of 2,088 patients with COVID-19 ARDS, 56% were treated with benzodiazepines.31
NMB was also used frequently in patients with critical COVID-19.36 In a prospective observational cohort study of 1,462 ICU patients without COVID-19, NMB was one of the strongest independent risk factors for developing delirium.37 In a New York City cohort study of 258 ICU patients with COVID-19, 25% received early NMB within 48 hours of being placed on mechanical ventilation.36 , 38 Although NMB may be useful in certain scenarios, data suggest that NMB may have been used more often in COVID-19 ARDS compared with other causes of ARDS. In a single-center cohort study of patients with COVID-19 ARDS, 60% of 267 mechanically ventilated patients received NMB, whereas in the multicenter, international LUNG SAFE study, only 38% of 12,906 patients with non-COVID-19 ARDS received NMB.39 , 40
Patients with COVID-19 also experience prolonged mechanical ventilation, with a mean ventilator duration of 14.6 days compared with 5.9 days in ICU patients without COVID-19.41 , 42 As the individual risk factors of prolonged neuromuscular blockade, sedation, and mechanical ventilation compound in patients with COVID-19 ARDS, the overall risk of developing ICU delirium increases.
The presence and duration of delirium are associated with subsequent development of neuropsychiatric and neurocognitive sequelae.4 , 43 , 44 A prospective study of 381 non-ICU patients with COVID-19 demonstrated that 30% of patients developed PTSD within 4 months of acute illness and attributed in-hospital delirium as an independent risk factor.4 Another multicenter retrospective cohort study in 4,033 medical-surgical ICU patients without COVID-19 across 14 ICUs demonstrated that patients who experienced at least 1 calendar day of ICU delirium were more likely to develop clinically diagnosed neuropsychiatric disorders (Risk Ratio = 1.14) and neurocognitive disorders (Risk Ratio = 1.59) at 1 year after ICU discharge.43 Correlation between duration of delirium and likelihood of subsequent neuropsychiatric disorders was not reported and may have helped to identify a dose-dependent relationship. Additional studies are needed to assess long-term psychiatric outcomes in critical COVID-19 survivors specifically, because these patients have multiple risk factors to experience a prolonged duration of delirium.
Conversely, a preexisting diagnosis of anxiety or depression has been shown to increase the risk of developing ICU delirium. A retrospective study of 286 patients with ARDS demonstrated that in patients who experienced ICU delirium, 49.2% of patients had preexisting psychiatric conditions (OR = 1.94; P = .01).45 Subgroup analysis demonstrated that preexisting depression and anxiety were statistically significant contributing factors (OR = 1.76 and 1.88, respectively).45
Large-scale, population-based surveys demonstrated that prevalence of depression and anxiety increased during the COVID-19 pandemic.46 , 47 Fear and anxiety about contracting SARS-CoV-2 and spreading it to family members were especially prevalent during the early months of the COVID-19 pandemic.48 , 49 Prevalence of depression was also increased because of social isolation, especially in older adults.50 An international study based on online questionnaires to assess psychiatric symptoms demonstrated marked increases in anxiety, acute stress, and depression in the general worldwide population.46 Another cross-sectional study conducted in the Guangdong province of China during the early months of the pandemic found that 20% of respondents (n = 98) experienced severe depression symptoms based on Patient Health Questionnaire-9 data.47 Although selection bias and unknown pre-pandemic psychological history of respondents may limit interpretation of these data, the findings suggest that patients who develop critical COVID-19 are at increased risk for preexisting psychological diagnoses because of circumstances created by the pandemic, which may compound the risk of developing ICU delirium and subsequent post-ICU psychological sequelae.
Situational Effects of COVID-19 in the Critical Care Setting
During the pandemic, hospitals restricted visitation to inpatient areas to minimize spread of SARS-CoV-2. Limited data are available regarding the psychological effects of restricted visitation in ICU patients with COVID-19. A pre-pandemic clinical trial demonstrated that flexible vs restricted visitation policy had no effect on the incidence of ICU delirium,51 but it did not examine delirium duration or severity. However, a multicenter cohort study in ICU patients with COVID-19 found that family visitation decreased the incidence of next-day delirium by 27%, suggesting that visitation may reduce duration of delirium.31
Longer-term detrimental effects on mental health, coping mechanisms, and overall wellbeing also may be impacted by restricted visitation in patients with COVID-19. A prospective cohort study of 88 ICU patients with COVID-19 assessed in-hospital psychiatric symptoms and found that 83% and 73% experienced symptoms of anxiety and depression, respectively.52 Of 33 patients who completed the 3-month follow-up questionnaire, 73% reported a negative psychological impact of restricted visitation.52 Although the study is limited in interpretation because of self-reported data and low retention rate, it is an informative starting point to conduct additional, large cohort studies in survivors of critical COVID-19 illness.
Restricted visitation also may have an impact on patients with COVID-19 ARDS who are admitted to the ICU and monitored on maximum noninvasive ventilatory support. Not only do these patients experience significant dyspnea, but they face challenging decisions regarding mechanical ventilation and goals of care without having family members physically present. Several studies have demonstrated that dyspnea in itself is a pathophysiologic trigger for panic attacks.53 , 54 Long-term consequences of emotional distress in the peri-intubation period have not been documented in the literature and warrant additional studies. Conducting such studies with and without family presence to better understand whether restricted visitation has a compounding effect on psychological distress will be helpful.
Intubated patients with COVID-19 may face a similar challenge related to restricted visitation. Patients experience heightened levels of anxiety, agitation, and stress while mechanically ventilated.55 , 56 Patients with long-term ventilator dependence also experience feelings of helplessness and depression, because they are consciously aware of being sustained by a breathing machine.57 Family support during ventilator weaning has been correlated with ease of emotional distress, as evidenced by patient interviews.56 Pandemic-related restrictions do not allow patients with critical COVID-19 to access an important protective mechanism against acute mental health effects, which may have long-term sequelae that are currently unknown.
The use of ICU diaries in minimizing psychiatric complications has been studied and validated in the literature.1 , 58 , 59 A randomized control trial in 352 ICU patients without COVID-19 demonstrated a statistically significant reduction of PTSD symptoms 1 month post-ICU discharge in patients who were randomized to the ICU diary group.59 Data regarding the use of ICU diaries in patients with COVID-19 are lacking. In part, this may be secondary to restricted visitation, because ICU diaries are often maintained and read to patients by family members. With restricted visitation policies in place, fewer patients with critical COVID-19 may be able to psychologically benefit from sustained ICU diary use.
Current Interventions and Future Directions
Interventions to minimize psychological morbidity during and after critical illness have been studied in both the inpatient and outpatient setting, mostly in non-COVID-19 patients (Table 1 ). Implementation of the ABCDEF Bundle (Assess, prevent, and manage pain; Both spontaneous awakening and breathing trials: Choice of analgesia and sedation; Delirium assess, prevent, and manage; Early mobility and exercise; Family engagement/empowerment) has had a significant effect in reducing in-hospital delirium and subsequent psychological and cognitive impairment.60 , 61 Results of the ICU Liberation Collaborative in over 15,000 patients across 68 ICUs demonstrated that performing elements of the ABCDEF bundle resulted in a significantly reduced readmission rate and length of ICU stay, thereby minimizing iatrogenic risk factors for psychological morbidity.60 Inpatient use of ICU diaries has also improved long-term psychiatric morbidity, mainly by significantly minimizing PTSD symptoms after ICU discharge.62
Table 1.
Summary of Studies Evaluating Acute and Post-acute Psychological Morbidity
| Study Title | Study Aim | Setting | Study Population Inclusive of Patients With COVID-19 | Improvement in Psychiatric Morbidity |
|---|---|---|---|---|
| Caring for critically ill patients with the ABCDEF bundle: results of the ICU Liberation Collaborative | Assessed complete and partial utilization of ABCDEF bundle as it relates to ICU length of stay, delirium, pain, and mechanical ventilation | Inpatient | No | Yes |
| ICU diaries as a therapeutic intervention for posttraumatic stress disorder (PTSD) after critical illness (RACHEL II) | Assessed efficacy of ICU diary usage in reducing postdischarge PTSD symptoms | Inpatient | No | Yes |
| Psychological outcomes following a nurse-led preventative psychological intervention for critically ill patients (POPPI): protocol for a cluster-randomised clinical trial of a complex intervention | Evaluated clinical effectiveness of nurse-led preventative psychological interventions in reducing PTSD symptom severity and psychological morbidity | Inpatient | No | No |
| The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long-term outcomes from critical illness: a pragmatic randomised controlled trial | Compared nurse-led follow-up programs with standard follow-up in improving post-ICU quality of life | Outpatient | No | No |
| Effect of a primary care management intervention on mental health-related quality of life among survivors of sepsis: a randomized clinical trial | Compared enhanced PCP follow-up to usual PCP follow-up in improving mental-health related quality of life after ICU discharge | Outpatient | No | No |
ABCDEF = Assess, prevent, and manage pain; Both spontaneous awakening and breathing trials: Choice of analgesia and sedation; Delirium assess, prevent, and manage; Early mobility and exercise; Family engagement/empowerment; PCP = primary care physician.
A number of other interventions have been tested to mitigate psychological morbidity but have not been found beneficial. The Psychological Outcomes following a nurse-led Preventative Psychological Intervention for critically ill patients (POPPI) multicenter, cluster-randomized clinical trial of 1,353 patients treated in 24 ICUs in the United Kingdom tested a nurse-led, post-ICU preventive psychological intervention but found no difference in PTSD symptoms at 6 months after ICU discharge.63 The Sepsis Survivors Monitoring and Coordination in Outpatient Health care (SMOOTH) clinical trial of 291 ICU patients with non-COVID-19 sepsis tested an enhanced primary care follow-up intervention but found no difference in mental health outcomes compared with usual primary care physician care.64 Likewise, a Cochrane review of post-ICU clinics found insufficient evidence to determine whether these programs are effective.65 Nonetheless, post-ICU clinics are consistently liked by patients and provide an important venue for both learning about post-ICU sequalae and pilot-testing interventions. For these reasons, the 2021 Surviving Sepsis Campaign Guidelines suggest referral to post-critical illness clinics where available.66
Data regarding interventions to minimize adverse mental health effects in patients with critical COVID-19 specifically are limited. An international study conducted in 262 ICU patients with COVID-19 ARDS demonstrated a significantly lower implementation rate of the ABCDEF Bundle and ICU diaries in patients with COVID-19 compared with the reported pre-pandemic implementation rate.67 Use of the ABCDEF Bundle and ICU diaries were low in all ICU patients regardless of COVID-19 status during the pandemic, suggesting that the driving factor was resource constraints and staffing shortages.68 Additional interventions such as the creation of tailored programs to minimize ICU discomfort in patients with COVID-19 ARDS are currently being evaluated to determine effects on long-term mental health outcomes (NCT03991611).69
A potentially novel area of study could explore the outcomes of group-based therapy. Studies in psychology have demonstrated that group therapy for patients who have undergone shared trauma can substantially reduce depression, anxiety, and feelings of isolation.70 Trauma-focused cognitive behavioral therapy has been extensively studied in children and adolescents, with a known benefit of reducing psychiatric symptoms.56 Trauma-focused cognitive behavioral therapy in critically ill patients with COVID-19 both individually and in group settings may demonstrate clinical benefit as many patients share similar experiences related to the pandemic and view their ICU stay as a source of trauma.71 It will be beneficial to study these interventions in ICU patients with and without COVID-19.
Additionally, it will be important to determine whether effective postdischarge treatment can be provided in primary care settings or whether cognitive and behavioral therapy should be provided by mental health experts. Unfortunately, the current literature demonstrates that nonspecialist interventions by nurses and primary care physicians have been unsuccessful.64 , 72
Conclusion
Because critical illness has increased during the COVID-19 pandemic, there is an urgent need to expand and improve ICU survivorship care. Many patients with critical illness caused by COVID-19 experience significant psychological burden, driven by pathophysiologic, iatrogenic, and situational factors. The direct inflammatory effects of the SARS-CoV-2 virus in the brain may inherently increase patients’ risk of psychiatric sequelae. Prolonged mechanical ventilation, sedation, and paralysis caused by severe ARDS increase the risk of ICU delirium, which may subsequently increase the risk of long-term mental health effects. The added burden of restricted visitation, staffing shortages, and resource constraints during a pandemic interferes with the delivery of evidence-based interventions such as the ABCDEF bundle.
Patients with critical COVID-19 undoubtably face a complicated and psychologically challenging recovery. Although whether these complications occur at a greater incidence or with greater severity compared with other critical illness is currently unknown, the pandemic has given importance to further understanding the psychological aftermath of critical illness. The specific features of patients with COVID-19 ARDS such as prolonged mechanical ventilation and social isolation pose a challenge in generalizing data from patients with other critical illnesses. Additional studies regarding pre-ICU admission psychiatric co-morbidities, in-hospital interventions, and outpatient follow-up in ICU patients with and without COVID-19 are necessary to make conclusions that have therapeutic implications. Given the limitations of current data and the growing number of patients who will experience psychological morbidity caused by COVID-19 as a result of lasting effects of ICU stays and ongoing discovery of novel variants with unknown susceptibility to vaccines, additional research is warranted to identify unique risk factors, pathogenesis, and targeted therapies for post-ICU psychiatric sequelae.
Summary
Survivors of COVID-19 critical illness are at risk for experiencing significant psychiatric morbidity. The risk factors for developing post-ICU psychological sequelae in patients with critical COVID-19 can be conceptualized in three main domains: pathophysiological effects of the virus, iatrogenic effects of prolonged ICU hospitalizations, and situational effects of a global pandemic. Additional research is necessary to determine the severity and duration of psychological morbidity in survivors of critical COVID-19, and whether current and novel interventions will benefit this growing patient population.
Acknowledgments
Author contributions: K. S. conceptualized the topic of this manuscript, conducted the literature search with compilation of pertinent studies, and contributed substantially to writing the manuscript. M. K. G. and H. C. P. contributed substantially to determining significance of studies included in the manuscript and editing the manuscript.
Financial/nonfinancial disclosures: None declared.
References
- 1.Parker A.M., Sricharoenchai T., Raparla S., Schneck K.W., Bienvenu O.J., Needham D.M. Posttraumatic stress disorder in critical illness survivors. Crit Care Med. 2015;43(5):1121–1129. doi: 10.1097/CCM.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 2.Nikayin S., Rabiee A., Hashem M.D., et al. Anxiety symptoms in survivors of critical illness: a systematic review and meta-analysis. Gen Hosp Psychiatry. 2016;43:23–29. doi: 10.1016/j.genhosppsych.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabiee A., Nikayin S., Hashem M.D., et al. Depressive symptoms after critical illness: a systematic review and meta-analysis. Crit Care Med. 2016;44(9):1744–1753. doi: 10.1097/CCM.0000000000001811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Janiri D., Carfì A., Kotzalidis G.D., et al. Posttraumatic stress disorder in patients after severe COVID-19 infection. JAMA Psychiatry. 2021;78(5):567–569. doi: 10.1001/jamapsychiatry.2021.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park H.Y., Jung J., Park H.Y., et al. Psychological consequences of survivors of COVID-19 pneumonia 1 month after discharge. J Korean Med Sci. 2020;35(47):e409. doi: 10.3346/jkms.2020.35.e409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roth K., Upadhyay R., Paul V. The effects of COVID-19 on mental health during inpatient hospitalization. Chest. 2020;158(4):A341. [Google Scholar]
- 7.Needham D.M., Sepulveda K.A., Dinglas V.D., et al. Core outcome measures for clinical research in acute respiratory failure survivors: an international modified Delphi Consensus Study. Am J Respir Crit Care Med. 2017;196(9):1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imran J., Nasa P., Alexander L., Upadhyay S., Alanduru V. Psychological distress among survivors of moderate-to-critical COVID-19 illness: a multicentric prospective cross-sectional study. Indian J Psychiatry. 2021;63(3):285. doi: 10.4103/psychiatry.IndianJPsychiatry_1074_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnúsdóttir I., Lovik A., Unnarsdóttir A.B., et al. Acute COVID-19 severity and mental health morbidity trajectories in patient populations of six nations: an observational study. Lancet Public Health. 2022;7(5):e406–e416. doi: 10.1016/S2468-2667(22)00042-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meinhardt J., Radke J., Dittmayer C., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nature Neurosci. 2021;24(2):168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 11.Fotuhi M., Mian A., Meysami S., Raji C.A. Neurobiology of COVID-19. J Alzheimers Dis. 2020;76(1):3–19. doi: 10.3233/JAD-200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Najafloo R., Majidi J., Asghari A., et al. Mechanism of anosmia caused by symptoms of COVID-19 and emerging treatments. ACS Chem Neurosci. 2021;12(20):3795–3805. doi: 10.1021/acschemneuro.1c00477. [DOI] [PubMed] [Google Scholar]
- 13.Harnett N.G., Goodman A.M., Knight D.C. PTSD-related neuroimaging abnormalities in brain function, structure, and biochemistry. Exp Neurol. 2020;330:113331. doi: 10.1016/j.expneurol.2020.113331. [DOI] [PubMed] [Google Scholar]
- 14.Caspani G., Corbet Burcher G., Garralda M.E., et al. Inflammation and psychopathology in children following PICU admission: an exploratory study. Evid Based Ment Health. 2018;21(4):139–144. doi: 10.1136/ebmental-2018-300027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonne O., Vythilingam M., Inagaki M., et al. Reduced posterior hippocampal volume in posttraumatic stress disorder. J Clin Psychiatry. 2008;69(7):1087–1091. doi: 10.4088/jcp.v69n0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Donovan A., Chao L.L., Paulson J., et al. Altered inflammatory activity associated with reduced hippocampal volume and more severe posttraumatic stress symptoms in Gulf War veterans. Psychoneuroendocrinology. 2015;51:557–566. doi: 10.1016/j.psyneuen.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steenblock C., Todorov V., Kanczkowski W., et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the neuroendocrine stress axis. Mol Psychiatry. 2020;25(8):1611–1617. doi: 10.1038/s41380-020-0758-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Urhan E., Karaca Z., Unuvar G.K., Gundogan K., Unluhizarci K. Investigation of pituitary functions after acute coronavirus disease 2019. Endocr J. 2022;69(6):EJ21–E0531. doi: 10.1507/endocrj.EJ21-0531. [DOI] [PubMed] [Google Scholar]
- 19.Lv Q., Yang Q., Cui Y., et al. Effects of taurine on ACE, ACE2 and HSP70 expression of hypothalamic-pituitary-adrenal axis in stress-induced hypertensive rats. Adv Exp Med Biol. 2017;975(2):871–886. doi: 10.1007/978-94-024-1079-2_69. [DOI] [PubMed] [Google Scholar]
- 20.Lindqvist D., Isaksson A., Träskman-Bendz L., Brundin L. Salivary cortisol and suicidal behavior: a follow-up study. Psychoneuroendocrinology. 2008;33(8):1061–1068. doi: 10.1016/j.psyneuen.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Melhem N.M., Keilp J.G., Porta G., et al. Blunted HPA axis activity in suicide attempters compared to those at high risk for suicidal behavior. Neuropsychopharmacology. 2016;41(6):1447–1456. doi: 10.1038/npp.2015.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leow M.K.-S., Kwek D.S.-K., Ng A.W.-K., Ong K.-C., Kaw G.J.-L., Lee L.S.-U. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS) Clin Endocrinol (Oxf) 2005;63(2):197–202. doi: 10.1111/j.1365-2265.2005.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calsavara A.J., Costa P.A., Nobre V., Teixeira A.L. Prevalence and risk factors for post-traumatic stress, anxiety, and depression in sepsis survivors after ICU discharge. Brazilian J Psychiatry. 2021;43(3):269–276. doi: 10.1590/1516-4446-2020-0986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sojka P., Stålnacke B.-M., Björnstig U., Karlsson K. One-year follow-up of patients with mild traumatic brain injury: occurrence of post-traumatic stress-related symptoms at follow-up and serum levels of cortisol, S-100B and neuron-specific enolase in acute phase. Brain Injury. 2006;20(6):613–620. doi: 10.1080/02699050600676982. [DOI] [PubMed] [Google Scholar]
- 25.Perrin P., Collongues N., Baloglu S., et al. Cytokine release syndrome-associated encephalopathy in patients with COVID-19. Eur J Neurol. 2021;28(1):248–258. doi: 10.1111/ene.14491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mete E., Sabirli R., Goren T., Turkcuer I., Kurt Ö., Koseler A. Association between S100b levels and COVID-19 pneumonia: a case control study. In Vivo. 2021;35(5):2923–2928. doi: 10.21873/invivo.12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aceti A., Margarucci L.M., Scaramucci E., et al. Serum S100B protein as a marker of severity in Covid-19 patients. Sci Rep. 2020;10(1) doi: 10.1038/s41598-020-75618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krewulak K.D., Stelfox H.T., Leigh J.P., Ely E.W., Fiest K.M. Incidence and prevalence of delirium subtypes in an adult ICU: a systematic review and meta-analysis. Crit Care Med. 2018;46(12):2029–2035. doi: 10.1097/CCM.0000000000003402. [DOI] [PubMed] [Google Scholar]
- 29.Bannon L., McGaughey J., Clarke M., McAuley D.F., Blackwood B. Impact of non-pharmacological interventions on prevention and treatment of delirium in critically ill patients: protocol for a systematic review of quantitative and qualitative research. Syst Rev. 2016;5:75. doi: 10.1186/s13643-016-0254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaal I.J., Devlin J.W., Peelen L.M., Slooter A.J.C. A systematic review of risk factors for delirium in the ICU. Crit Care Med. 2015;43(1):40–47. doi: 10.1097/CCM.0000000000000625. [DOI] [PubMed] [Google Scholar]
- 31.Pun B.T., Badenes R., Heras La Calle G., et al. Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9(3):239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barr J., Fraser G.L., Puntillo K., et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 33.Devlin J.W., Skrobik Y., Gélinas C., et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 34.Shuman A.G., Fox E., Unguru Y. Preparing for COVID-19-related drug shortages. Ann Am Thorac Soc. 2020;17(8):928–931. doi: 10.1513/AnnalsATS.202004-362VP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kenes M.T., McSparron J.I., Marshall V.D., Renius K., Hyzy R.C. Propofol-associated hypertriglyceridemia in coronavirus disease 2019 versus noncoronavirus disease 2019 acute respiratory distress syndrome. Crit Care Explorations. 2020;2(12) doi: 10.1097/CCE.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobo-Valbuena B., Gordo F., Abella A., et al. Risk factors associated with the development of delirium in general ICU patients: a prospective observational study. PLOS One. 2021;16(9) doi: 10.1371/journal.pone.0255522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moss M., Huang D.T., Brower R.G., et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellani G., Laffey J.G., Pham T., et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315(8):788. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 40.Schenck E.J., Hoffman K., Goyal P., et al. Respiratory mechanics and gas exchange in COVID-19-associated respiratory failure. Ann Am Thorac Soc. 2020;17(9):1158–1161. doi: 10.1513/AnnalsATS.202005-427RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.King C.S., Sahjwani D., Brown A.W., et al. Outcomes of mechanically ventilated patients with COVID-19 associated respiratory failure. PLOS One. 2020;15(11) doi: 10.1371/journal.pone.0242651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Esteban A., Anzueto A., Frutos F., et al. Characteristics and outcomes in adult patients receiving mechanical ventilation: a 28-day international study. JAMA. 2002;287(3):345–355. doi: 10.1001/jama.287.3.345. [DOI] [PubMed] [Google Scholar]
- 43.Brown K.N., Soo A., Faris P., Patten S.B., Fiest K.M., Stelfox H.T. Association between delirium in the intensive care unit and subsequent neuropsychiatric disorders. Crit Care. 2020;24(1):476. doi: 10.1186/s13054-020-03193-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Girard T.D., Jackson J.C., Pandharipande P.P., et al. Delirium as a predictor of long-term cognitive impairment in survivors of critical illness. Crit Care Med. 2010;38(7):1513–1520. doi: 10.1097/CCM.0b013e3181e47be1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalra S.S., Jaber J., Alzghoul B.N., et al. Pre-existing psychiatric illness is associated with an increased risk of delirium in patients with acute respiratory distress syndrome. J Intensive Care Med. 2022;37(5):647–654. doi: 10.1177/08850666211019009. [DOI] [PubMed] [Google Scholar]
- 46.Shah S.M.A., Mohammad D., Qureshi M.F.H., Abbas M.Z., Aleem S. Prevalence, psychological responses and associated correlates of depression, anxiety and stress in a global population, during the coronavirus disease (COVID-19) pandemic. Commun Ment Health J. 2021;57(1):101–110. doi: 10.1007/s10597-020-00728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J., Lu H., Zeng H., et al. The differential psychological distress of populations affected by the COVID-19 pandemic. Brain Behav Immun. 2020;87:49–50. doi: 10.1016/j.bbi.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fitzpatrick K.M., Harris C., Drawve G. Fear of COVID-19 and the mental health consequences in America. Psychological Trauma: Theory, Research, Practice, and Policy. 2020;12(S1):S17–S21. doi: 10.1037/tra0000924. [DOI] [PubMed] [Google Scholar]
- 49.Bäuerle A., Teufel M., Musche V., et al. Increased generalized anxiety, depression and distress during the COVID-19 pandemic: a cross-sectional study in Germany. J Public Health (Oxf) 2020;42(4):672–678. doi: 10.1093/pubmed/fdaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sepúlveda-Loyola W., Rodríguez-Sánchez I., Pérez-Rodríguez P., et al. Impact of social isolation due to COVID-19 on health in older people: mental and physical effects and recommendations. J Nutr Health Aging. 2020;24(9):938–947. doi: 10.1007/s12603-020-1500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosa R.G., Falavigna M., Silva D.B. d.a., et al. Effect of flexible family visitation on delirium among patients in the intensive care unit. JAMA. 2019;322(3):216. doi: 10.1001/jama.2019.8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cattelan J., Castellano S., Merdji H., et al. Psychological effects of remote-only communication among reference persons of ICU patients during COVID-19 pandemic. J Intensive Care. 2021;9(1):5. doi: 10.1186/s40560-020-00520-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Benke C., Hamm A.O., Pané-Farré C.A. When dyspnea gets worse: suffocation fear and the dynamics of defensive respiratory responses to increasing interoceptive threat. Psychophysiology. 2017;54(9):1266–1283. doi: 10.1111/psyp.12881. [DOI] [PubMed] [Google Scholar]
- 54.Janssens T., Peuter S. d.e., Stans L., et al. Dyspnea perception in COPD: association between anxiety, dyspnea-related fear, and dyspnea in a pulmonary rehabilitation program. Chest. 2011;140(3):618–625. doi: 10.1378/chest.10-3257. [DOI] [PubMed] [Google Scholar]
- 55.Merchán-Tahvanainen M.E., Romero-Belmonte C., Cundín-Laguna M., Basterra-Brun P., San Miguel-Aguirre A., Regaira-Martínez E. Patients’ experience during weaning of invasive mechanical ventilation: a review of the literature. Enfermería Intensiva (English ed) 2017;28(2):64–79. doi: 10.1016/j.enfi.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Chlan L, Savik K. Patterns of anxiety in critically ill patients receiving mechanical ventilatory support. Nurs Res. 60(3 Suppl):S50-S57. [DOI] [PMC free article] [PubMed]
- 57.Jubran A., Lawm G., Kelly J., et al. Depressive disorders during weaning from prolonged mechanical ventilation. Intensive Care Med. 2010;36(5):828–835. doi: 10.1007/s00134-010-1842-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehlhorn J., Freytag A., Schmidt K., et al. Rehabilitation interventions for postintensive care syndrome. Crit Care Med. 2014;42(5):1263–1271. doi: 10.1097/CCM.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 59.Jones C., Backman C., Capuzzo M., et al. Intensive care diaries reduce new onset post traumatic stress disorder following critical illness: a randomised, controlled trial. Crit Care. 2010;14(5):R168. doi: 10.1186/cc9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pun B.T., Balas M.C., Barnes-Daly M.A., et al. Caring for critically ill patients with the ABCDEF bundle. Crit Care Med. 2019;47(1):3–14. doi: 10.1097/CCM.0000000000003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Marra A., Ely E.W., Pandharipande P.P., Patel M.B. The ABCDEF bundle in critical care. Crit Care Clin. 2017;33(2):225–243. doi: 10.1016/j.ccc.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garrouste-Orgeas M., Coquet I., Périer A., et al. Impact of an intensive care unit diary on psychological distress in patients and relatives. Crit Care Med. 2012;40(7):2033–2040. doi: 10.1097/CCM.0b013e31824e1b43. [DOI] [PubMed] [Google Scholar]
- 63.Wade D.M., Mouncey P.R., Richards-Belle A., et al. Effect of a nurse-led preventive psychological intervention on symptoms of posttraumatic stress disorder among critically ill patients: a randomized clinical trial. JAMA. 2019;321(7):665–675. doi: 10.1001/jama.2019.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schmidt K., Worrack S., Korff M. v.o.n., et al. Effect of a primary care management intervention on mental health-related quality of life among survivors of sepsis: a randomized clinical trial. JAMA. 2016;315(24):2703–2711. doi: 10.1001/jama.2016.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schofield-Robinson O.J., Lewis S.R., Smith A.F., McPeake J., Alderson P. Follow-up services for improving long-term outcomes in intensive care unit (ICU) survivors. Cochrane Database of Systematic Reviews. 2018;2018(11) doi: 10.1002/14651858.CD012701.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Evans L., Rhodes A., Alhazzani W., et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–e1143. doi: 10.1097/CCM.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 67.Liu K., Nakamura K., Katsukawa H., et al. ABCDEF bundle and supportive ICU practices for patients with coronavirus disease 2019 infection: an international point prevalence study. Crit Care Explorations. 2021;3(3) doi: 10.1097/CCE.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu K., Nakamura K., Katsukawa H., et al. Implementation of the ABCDEF bundle for critically ill icu patients during the COVID-19 pandemic: a multi-national 1-day point prevalence study. Front Med (Lausanne) 2021;8:735860. doi: 10.3389/fmed.2021.735860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.National Institutes of Health Clinical Center Reducing post-traumatic stress disorder after ICU discharge with the IPREA3 Program (PTSD-REA). NCT03991611. ClinicalTrials.gov. National Institutes of Health. 2019. https://clinicaltrials.gov/ct2/show/NCT03991611 Updated.
- 70.Deblinger E., Pollio E., Dorsey S. Applying trauma-focused cognitive-behavioral therapy in group format. Child Maltreat. 2016;21(1):59–73. doi: 10.1177/1077559515620668. [DOI] [PubMed] [Google Scholar]
- 71.Tingey J.L., Bentley J.A., Hosey M.M. COVID-19: understanding and mitigating trauma in ICU survivors. Psychol Trauma. 2020;12(S1):S100–S104. doi: 10.1037/tra0000884. [DOI] [PubMed] [Google Scholar]
- 72.Cuthbertson B.H., Rattray J., Campbell M.K., et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ. 2009;339:b3723. doi: 10.1136/bmj.b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]