Abstract
Background:
Kidney disease is a major public health issue arising from loss of glomerular podocyte function, and there are considerable sex differences in its prognosis. Evidence suggests a renoprotective effect of estrogen and soy diet-derived phytoestrogens, although the molecular basis for this is poorly understood.
Objective:
Here, we aim to assess sex differences in expression of key proteins associated with podocyte survival and determine the effects of dietary soy on glomerular and podocyte signaling.
Methods:
Male and female FVB mice were fed control, low (1%), and high (20%) doses of isolated soy protein (ISP) in utero and until 100 days of age. Spot urine was collected to measure proteinuria and isolated glomeruli were used to quantify activated and total levels of nephrin, Akt, and ERK1/2. To investigate protective effects of specific soy phytoestrogens, cultured podocytes were treated with or without daidzein and subject to control or high glucose as a model of podocyte injury.
Results:
Nephrin and Akt were elevated at baseline in glomeruli from females compared to males. Both sexes that were fed 1% and 20% ISP displayed robust increases in total glomerular Akt compared to controls, and these effects were more prominent in females. A similar trend at both doses in both sexes was observed with activated Akt and total nephrin. Notably, males exclusively showed increased phosphorylation of nephrin and extracellular signal-regulated kinase (ERK) at the 1% ISP dose; however, no overt changes in urinary albumin excretion or podocin levels were observed, suggesting that the soy diets did not impair podocyte function. Finally, in cultured male and female podocytes, daidzein treatment suppressed high glucose-induced ERK activation.
Conclusions:
Together, our findings reveal a putative mechanism to explain the protective influence of sex on kidney disease progression, and they provide further evidence to support a beneficial role for dietary soy in preserving glomerular function.
Keywords: sex differences, soy, podocyte, cell survival, glomerular function, nephrin
Abrégé
Contexte:
L’insuffisance rénale est un problème majeur de santé publique résultant d’une perte de fonction des podocytes glomérulaires, et son pronostic diffère selon le sexe. Bien que le fondement moléculaire en soit mal compris, des données suggèrent que les œstrogènes et des phytoestrogènes dérivés du soja alimentaire auraient un effet néphroprotecteur.
Objectifs:
Évaluer les différences selon le sexe dans l’expression des protéines clés associées à la survie des podocytes, et déterminer les effets du soja alimentaire sur la signalisation glomérulaire et les podocytaire.
Méthodologie:
Des souris FVB mâles et femelles ont reçu un régime alimentaire témoin ou un regime à faible dose (1 %) ou à dose élevée (20 %) de protéines de soja isolées (PSI) in utero et jusqu’à l’âge de 100 jours. Des échantillons aléatoires d’urine ont été recueillis pour mesurer la protéinurie et des glomérules isolés ont été utilisés pour quantifier les niveaux activés et totaux de néphrine, d’Akt et d’ERK1/2. Pour évaluer l’effet protecteur de certains phytoestrogènes du soja, des podocytes cultivés ont été traités avec ou sans daidzéine et soumis à une dose témoin ou à une dose élevée de glucose comme modèle de lésion podocytaire.
Résultats:
Les taux initiaux de néphrine et d’Akt étaient plus élevés dans les glomérules des souris femelles. Les souris mâles et femelles nourries avec des doses de 1 % et de 20 % de PSI ont montré des augmentations significatives de l’Akt glomérulaire totale par rapport aux témoins, et ces effets étaient plus importants chez les femelles. Une tendance semblable a été observée chez les deux sexes et pour les deux doses en ce qui concerne l’Akt activée et la néphrine totale. Seuls les mâles ont montré une augmentation de la phosphorylation de la néphrine et de l’ERK à 1 % de PSI; aucun changement manifeste n’a cependant été observé dans l’excrétion urinaire d’albumine ou dans le taux de podocine, ce qui suggère que le soja alimentaire n’a pas altéré la fonction des podocytes. Dans les podocytes cultivés, tant mâles que femelles, le traitement à la daidzéine a inhibé l’activation de l’ERK induite par une forte dose de glucose.
Conclusion:
Ensemble, nos résultats révèlent un mécanisme putatif pouvant expliquer l’effet protecteur du sexe du patient sur la progression de l’insuffisance rénale. Ces résultats fournissent des preuves supplémentaires soutenant l’hypothèse d’un rôle bénéfique du soja alimentaire dans la préservation de la fonction glomérulaire.
Introduction
Podocytes are specialized epithelial cells which comprise the outer layer of the glomerular filtration barrier. 1 As a primary target of injury in many forms of kidney disease, podocytes are lost over time, such that the kidneys are no longer able to properly filter the blood, leading to an accumulation of metabolic waste products and loss of essential proteins such as albumin into the urine (proteinuria). 2 Presently, dialysis and transplantation are the only forms of treatment for advanced kidney disease, and these approaches are associated with significant morbidity, mortality, and health care costs.3,4 As a consequence, there is intense interest in identifying alternative strategies such as dietary alterations to maintain podocyte function and slow the progression of disease.
Epidemiological and animal-based studies have demonstrated that kidney disease progression is worse in males than females and that men are more likely to experience end-stage renal disease and death.5-7 Mechanisms to explain these sex-related disparities are poorly understood. Differences in sex hormones in addition to sex-specific cellular responses have been proposed to play a role in these divergent outcomes.5-7 In this regard, estrogens are associated with preservation of the filtration barrier through their effects on podocytes.8-10 Interestingly, phytoestrogens such as the isoflavones, genistein, and daidzein found in soy protein may also exert renoprotective effects. Several studies have shown that consumption of a soy protein–rich diet can improve glomerular hyperfiltration as well as reduce proteinuria and blood cholesterol levels in humans with diabetes-induced kidney disease.11-14 The effects of isoflavones and other flavonoids on kidney function have also been examined in mouse and rat models of diabetes, where their administration is associated with reduced kidney damage and podocyte injury. 15 Despite evidence to support the efficacy of plant-based protein in slowing the progression of glomerular disease and reducing comorbidities such as cardiovascular disease,16,17 the molecular pathways that are targeted by phytoestrogens in podocytes have yet to be completely defined.
Here, we investigate sex differences in expression of key cell signaling proteins associated with podocyte survival (nephrin, Akt, and ERK1/2) and determine the effects of soy phytoestrogens on their expression. Nephrin is a key molecular component of the glomerular filtration barrier, and loss of nephrin is a hallmark of glomerular injury leading to proteinuria.18-22 Tyrosine phosphorylation of the nephrin intracellular region can activate Akt kinase.23,24 Among the Akt paralogs, Akt2 is central to podocyte survival, and loss of Akt activation is associated with human chronic kidney disease progression. 25 Intriguingly, sex differences in Akt expression have been reported in several biological settings.26-28 By contrast, sustained activation of extracellular signal-regulated kinase (ERK) induces podocyte apoptosis.29,30 Previous studies in other systems have shown that soy or its constituents can alter these survival signals. For instance, soy protein supplementation in fructose-fed male rats increases nephrin mRNA expression and preserves kidney architecture, 31 genistein increases Akt activation in neonatal mouse hearts, 32 and phytoestrogens have cell-specific effects on ERK1/2 signaling.33-37 In this report, we demonstrate that a soy-based diet increases expression and phosphorylation of Akt and nephrin in both male and female mice. This effect is more prominent in female mice, which we further show possess higher baseline levels of both proteins. In male mice only, soy diet induces marked activation of ERK1/2, along with low-level albuminuria following exposure to supraphysiological levels of isolated soy protein (ISP). Finally, using cultured male and female human podocytes, we show that daidzein can suppress activation of ERK1/2 phosphorylation in a podocyte injury model. Altogether, our studies uncover sex differences in baseline and soy-fed expression levels of critical podocyte signaling pathways which may have implications for dissimilar disease progression in males versus females.
Materials and Methods
Animal Care and Study Approval
Animals were housed at the University of Guelph Central Animal Facility and maintained using guidelines established by the Canadian Council of Animal Care. This study was approved by the Animal Care Committee at the University of Guelph (AUPs 3291, 3123, and 3394).
Animal Husbandry and Diets
FVB mice were obtained from an established breeding colony 38 and fed diets containing 20% casein protein (for controls), 20% ISP, 5% ISP/15% casein, or 1% ISP/19% casein (Harlan Laboratories, Madison, WI). The ISP used for these diets contained approximately 1660 ppm of isoflavones. The isoflavone content is made up of 463 ppm daidzin, 95 ppm daidzein, 933 ppm genistin, 101 ppm genistein, 57 ppm glycetin, and 9 ppm glycetein, all in the aglycone form. Experimental diet conditions began in utero, up until sacrifice at 100 days of age, as described previously. 38 Animals were sacrificed by CO2 asphyxiation.
Glomerular Isolation
Glomeruli were isolated as described previously. 39 Kidneys were extracted and longitudinally cut to separate medulla from cortex. The medulla was discarded and cortex samples were processed individually (such that one mouse represents one biological replicate). Cortices were minced using a #10 scalpel blade, incubated in 5 mL Dulbecco’s phosphate-buffered saline (D-PBS) solution (Wisent, St-Bruno, QC) containing 1 μg/mL collagenase for 30 minutes at 37°C with shaking at 30 rpm and then passed through a 100 μm sterile cell strainer with 40 mL cold D-PBS to enrich glomeruli. The flow-through was pelleted at 1000 rpm for 1 minute followed by removal of the supernatant. To lyse red blood cells, 5 to 10 mL of sterile Ack lysis buffer (0.15 M NH4Cl, 7 mM KCO3, 0.1 mM ethylenediaminetetraacetic acid [EDTA]) was added to the pellet, inverted 5 to 7 times at room temperature, and immediately spun down at 1000 rpm for 1 minute. The supernatant was removed and the glomeruli were lysed by re-suspending the pellet in 500 to 600 μL of lysis buffer, as described below.
Tissue Lysis
Whole kidney, brain, and glomeruli were lysed in Phospholipase C (PLC) lysis buffer (50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] pH 7.5, 10% Glycerol, 1 mM ethylene glycol tetraacetic acid (EGTA), 10 mM NaPPi, 1.5 mM MgCl2, 150 mM NaCl, 100 mM NaF, 1% Triton X-100) containing protease inhibitors (0.1 mg/mL aprotinin, 0.1 mg/mL leupeptin), along with 1 mM sodium orthovanadate and 1 mM phenylmethanesulfonyl fluoride (PMSF) (PLC+). Lysates were sonicated using Vibra-Cell (Sonics & Materials Inc., Newtown, CT) for 5 to 10 seconds followed by incubation on ice for 10 minutes and then centrifuged at 12 000× g for 12 minutes to pellet cell debris. Lysates were then subject to bicinchoninic acid (BCA) assay to quantify protein concentration (Pierce, Waltham, MA). Whole cell lysates were prepared by combining equal amounts of total protein with 5× sodium dodecyl sulfate (SDS) loading buffer (20% SDS, 0.3125 M Tris Base, 50% glycerol, 20% β-mercaptoethanol) and boiled at 100°C for 5 minutes.
Cell Culture and Lysis
Immortalized human podocyte cells (HPCs; AB line, 40 from a 3-year-old male patient, and LY line, 41 from a 6-year-old female patient) were provided by Dr Moin Saleem and cultured as previously described.40,41 Short tandem repeat (STR) analyses were performed on HPCs for cell line authentication and confirmation of sex (performed at The Centre for Applied Genomics, Toronto, ON; Supplemental Table 1). Mouse podocyte cells (MPCs) were generated and cultured according to standardized protocols.42,43 AB line HPCs and MPCs were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 200 U/mL penicillin, and 200 µg/mL streptomycin (Invitrogen, Burlington, ON) and allowed to proliferate at 33°C with 5% CO2. The LY line HPCs were cultured in these same conditions with the addition of 1% insulin, transferrin, and selenium (ITS, Sigma, St. Louis, MO). Proliferating MPCs were further supplemented with 10 U/mL interferon-gamma (PeproTech, Cranbury, NJ). Once grown to 70% confluence, the cells were transferred to 37°C to promote growth arrest and differentiation for 10 to 14 days. One week prior to treatment, cells were switched to media containing 2% FBS and 5.5 mM glucose (Sigma). Cells were serum-starved 16 to 24 hours before treatment, and combinations of 10 μM daidzein (Sigma), 20 to 25 mM glucose, and 1 μM angiotensin II (Ang II; Sigma) were used for 24 hours. Where noted, mannitol (Sigma) and dimethyl sulfoxide (DMSO) were used as osmotic and solvent controls. For lysis, media was removed and cells were washed twice with phosphate-buffered saline (PBS). Cells were scraped off dishes using PLC+ lysis buffer, and cells were sonicated, incubated, and centrifuged as with tissue lysis.
Antibodies
Commercial antibodies used were as follows: rabbit anti-Akt (C67E7, 4691, Cell Signaling Technology, Danvers, MA), rabbit anti-pAkt S473 (D9E, 4060, Cell Signaling Technology), rabbit anti-Akt1 (C73H10, 2938, Cell Signaling Technology), rabbit anti-Akt2 (5B5, 2964, Cell Signaling Technology), rabbit anti-Akt3 (4059, Cell Signaling Technology), rabbit anti-ERK1/2 (137F5, 4695, Cell Signaling Technology), rabbit anti-pERK1/2 T202/Y204 (D13.14.4E, 4370, Cell Signaling Technology), rabbit anti-p38 (9212, Cell Signaling Technology), rabbit anti-p-p38 T180/Y182 (D3F9, 4511, Cell Signaling Technology), rabbit anti-podocin (PO372, Sigma), mouse anti-β-actin (AC15, A1978, Sigma), rabbit anti-cadherin (ab16505, Abcam, Cambridge, UK), and rabbit anti-ERβ (H-150, SC-8974, Santa Cruz). Rabbit anti-nephrin was described previously. 44 Phospho-specific anti-nephrin antibodies were generated and validated previously. 45 All primary antibodies were used at 1:1000 except for podocin and β-actin, which were used at 1:5000. Secondary horseradish peroxidase-conjugated goat anti-mouse (170-6516, BioRad, Hercules, CA) and goat anti-rabbit (170-6515, BioRad) antibodies were used at 1:5000 for immunoblot detection.
Immunoblotting
Total protein (20 to 30 μg) was loaded on 10% SDS polyacrylamide gels and electrophoresed for 20 minutes at 120 V before increasing the voltage to 180 V for 1 hour. Proteins were transferred onto polyvinylidene fluoride (PVDF) membranes (Roche, Basel, Switzerland) via wet transfer at 100 V for 50 minutes. Membranes were blocked for 1 hour in 5% skim milk in 1× Tris-buffered saline with Tween (TBST; 0.15 M NaCl, 0.02 M Tris Base, 0.005% Tween-20) for non-phosphorylated proteins and in 5% bovine serum albumin (BSA; Roche) in TBST for phosphorylated proteins. Membranes were briefly washed for 1 minute in MilliQ water and then TBST, before being incubated at 4°C overnight with diluted primary antibodies. The following day, membranes were washed 5 times for 5 minutes with TBST and incubated with diluted secondary antibodies for 1 hour. Membranes were then washed again with TBST 5 times for 5 minutes each. Signals were detected using chemiluminescent 1:1 ECL Western Blot Substrate (Pierce) for 2 minutes and then developed using either a Konica Minolta Automatic Film Developer SRX-101A with X-ray blue film (VWR, Radnor, PA) or ChemiDoc XRS+ (BioRad). To probe loading controls, membranes were stripped with 20 mL of stripping buffer (200 mM glycine, 0.1% SDS, 1% Tween-20, pH 2.2) for 30 minutes and washed briefly with TBST before blocking with 5% BSA or milk/TBST for 15 minutes. Densitometry on all images was performed using Fiji software, with biological replicates normalized to the sum of each separately ran gel 46 and individual lanes normalized to loading controls β-actin or cadherin.
Proteinuria Assessment
Urine was collected at the time of mouse sacrifice. Urine samples were spun down quickly, and the pellet was discarded. Albumin-creatinine ratios were measured by enzyme-linked immunosorbent assay (ELISA; Albuwell M from Ethos Biosciences, Newtown Square, PA) as per manufacturer’s instruction. Equal volumes of urine and 2× SDS loading buffer were added and samples were boiled at 100°C for 5 minutes. In all, 4 μL of each sample was resolved on a 10% gel with BSA (2.5 μg) as a positive control. Gels were stained with Coomassie brilliant blue R (ThermoFisher Scientific, Waltham, MA) for 30 minutes and destained with 45% methanol: 10% glacial acetic acid for 1 hour. Gels were imaged on a ChemiDoc XRS+ (BioRad).
Statistical Analysis
Comparisons between sex or dietary conditions were completed using Student’s t test. Comparisons between cell culture treatment groups were completed using a one-way analysis of variance (ANOVA) with a post-hoc Sidak’s test. Statistical significance was considered when P < .05. All figures and statistical analyses were generated using Prism 9 by GraphPad Software (San Diego, CA).
Results
Female Mice Show Higher Baseline Expression of Akt and Nephrin in Glomeruli Relative to Male Mice
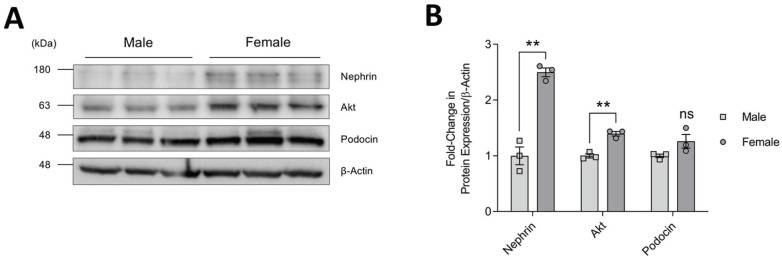
The sex distinct progression of kidney disease prompted us to first investigate whether differences in expression of key podocyte survival proteins might exist in male and female mice. To this end, we compared the levels of nephrin and Akt in glomerular isolates prepared from kidneys of littermate male and female FVB mice at 100 days of age. Akt2 is the predominant paralog in mouse glomeruli (Supplemental Figure 1), consistent with previous reports showing that Akt2 localizes to podocytes while Akt1 is enriched in surrounding tubules. 25 Western blotting followed by densitometric analysis revealed that total Akt protein levels were significantly higher in glomeruli isolated from female mice and that an even greater difference was observed with nephrin (Figure 1A and 1B). Of note, expression levels of podocin, a podocyte marker protein which binds to nephrin, were comparable between males and females, indicating that the changes were not due to differences in podocyte number. Together, these findings suggest sex differences in baseline expression of Akt and nephrin in mouse podocytes in vivo.
Figure 1.
Baseline expression profile of nephrin and Akt in glomeruli of male and female mice. (A) Immunoblots of glomerular whole cell lysates prepared from individual male and female control mice (n = 3 of each shown, 2 litters matched, 100 days of age). β-actin was used as a loading control and podocin was used to show comparable podocyte number. (B) Densitometry of immunoblot in (A), normalized to β-actin and male mice.
Note. Data are presented as mean ± standard error of the mean. Statistical significance compared to male mice was determined using Student’s t test and is marked by asterisks. ns = no significance.
**P < .01.
Soy Protein Diets Induce Sex Distinct Changes in Glomerular Akt, Nephrin, and ERK1/2 in Mice
We next investigated the effects of soy supplementation on these key podocyte signaling pathways in male and female FVB mice. In line with previous reports, 47 glomerular isolates from male and female mice show similar expression levels of ERβ (Supplemental Figure 2). This estrogen receptor (ER) is preferentially bound by isoflavones, 48 highlighting the potential for a comparable response in both sexes. Animals were fed a diet containing low (1%), intermediate (5%), or high (20%) levels of ISP or casein control (20%) throughout their lifetime. The 1% ISP diet falls within the normal range of soy intake (20-141 g/day) in Asian populations while the 20% ISP diet is 15-fold higher and considered supraphysiological. 49 Mice were maintained on the diet up until sacrifice at 100 days of age, at which point urine was collected and kidneys/glomeruli were isolated. Analysis of urine by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) demonstrated that none of the diets led to overt proteinuria in either sex (Figure 2A and Supplemental Figure 3). Measurement of albumin-creatinine ratio (ACR) showed a slight increase in the observed ACR in male mice on the 20% ISP diet compared to male casein diet controls; however, values from all diets are within the range reported for healthy male FVB mice of a similar age (Figure 2B and Supplemental Figure 3). 50
Figure 2.
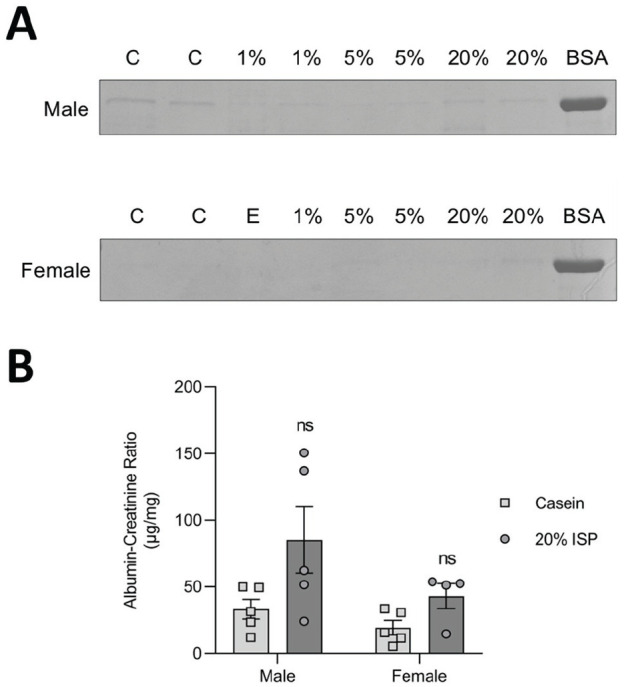
Urinary albumin assessment following consumption of variable amounts of ISP. (A) Coomassie stain of spot urine collections electrophoresed using SDS-PAGE. Albumin bands are shown and compared with 2.5 μg of BSA. “C” indicates casein-fed controls; “E” indicates empty well; and 1%, 5%, and 20% indicate mice fed diets with the specified percentage of ISP in total chow. (B) ACR of spot urine collections displayed in (A). n = 4 to 5 animals per diet.
Note. ACR = albumin-creatinine ratio; ISP = isolated soy protein; BSA = bovine serum albumin; ns = no significance; SDS-PAGE = sodium dodecyl sulfate polyacrylamide gel electrophoresis.
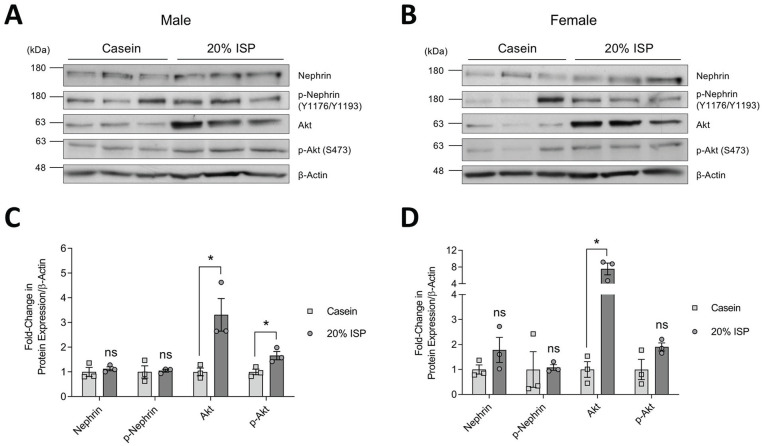
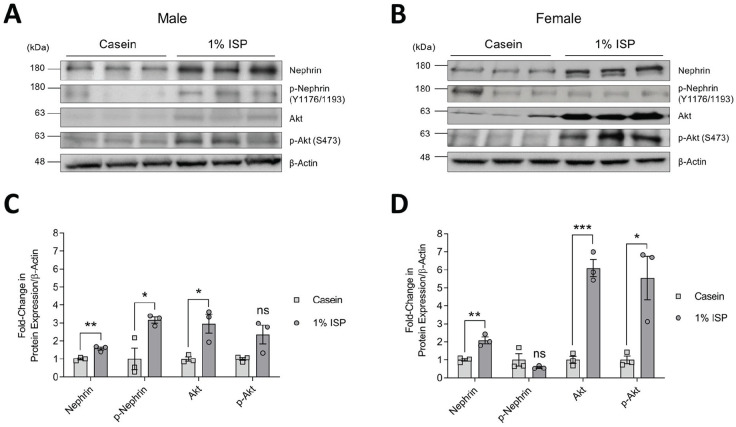
Western blotting of glomerular lysates followed by densitometric analysis showed that when soy was the sole source of protein in the diet (20% ISP), both sexes had significant increases in total Akt protein levels, as well as enhanced Akt activation (significant only in males), as indicated by phosphorylation of Ser473 (Figure 3A-3D). The increase in total Akt was approximately 2-fold higher in females than males. No significant differences were seen with total or phosphorylated nephrin (Figure 3A-3D). Similarly, on diets containing normal range 1% ISP, both male and female mice showed significant increases in Akt expression and activation, and females again displayed a more robust response with approximately 2-fold higher levels of total and activated Akt compared to males (Figure 4A-4D). Notably, these increases correlated with elevated total nephrin in both sexes as well as phosphorylation of nephrin in males (Figure 4A-4D).
Figure 3.
Total and phosphorylated nephrin and Akt expression in mice fed diet of 20% ISP. (A & B) Immunoblots of glomerular whole cell lysates from individual male (A) and female (B) mice fed casein or ISP as an exclusive source of protein (n = 3 shown). β-actin was used as a loading control. (C & D) Densitometry of immunoblots in A and B (C & D, respectively), normalized to casein controls.
Note. Data are presented as mean ± standard error of the mean. Statistical significance compared to casein control mice was determined using Student’s t test and is marked by an asterisk. ISP = isolated soy protein; ns = no significance.
*P < .05.
Figure 4.
Total and phosphorylated nephrin and Akt expression in mice fed diet of 1% ISP. (A & B) Immunoblots of glomerular whole cell lysates from individual male (A) and female (B) mice fed casein or casein supplemented with 1% ISP (n = 3 shown). β-actin was used as a loading control. (C & D) Densitometry of immunoblots in A and B (C & D, respectively), normalized to casein controls.
Note. Data are presented as mean ± standard error of the mean. Statistical significance compared to casein control mice was determined using Student’s t test and is marked by asterisks. ISP = isolated soy protein; ns = no significance.
*P < .05. **P < .01. ***P < .001.
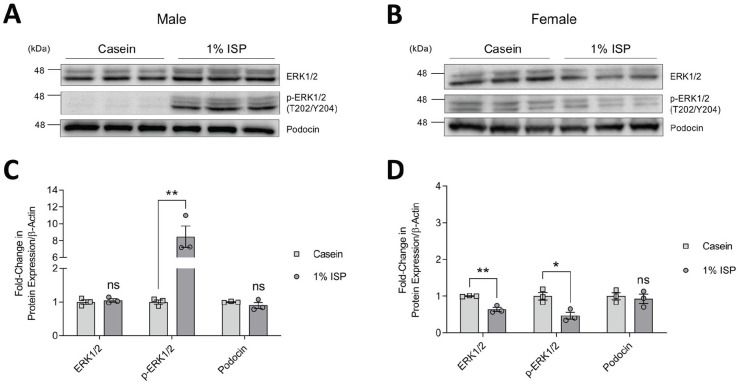
In parallel, as a readout of potential podocyte injury,29,30 we also investigated ERK1/2 levels in glomeruli and found that male mice fed 1% ISP showed a significant 8-fold increase in ERK1/2 activation compared to casein-fed controls (Figure 5A and 5C). This effect was not seen in soy-fed females, which instead had slightly lower total and activated ERK1/2 compared to casein-fed controls (Figure 5B and 5D). We verified that podocin levels were unchanged in both male and female mice fed 1% ISP diet compared to casein controls, suggesting that the soy diet did not impact podocyte number (Figure 5A-5D).
Figure 5.
Total and phosphorylated ERK expression in mice fed diet of 1% ISP. (A & B) Immunoblots of glomerular whole cell lysates from individual male (A) and female (B) mice fed casein or casein supplemented with 1% ISP (n = 3 shown). (C & D) Densitometry of immunoblots in A and B (C & D, respectively), normalized to casein controls.
Note. Podocin was used to show comparable podocyte number. β-actin was used as a loading control (shown in Figure 4). Statistical significance compared to casein control mice was determined using Student’s t test and is marked by asterisks. ISP = isolated soy protein; ns = no significance; ERK = extracellular signal-regulated kinase.
*P < .05. **P < .01.
Taken together, these results demonstrate that soy supplementation increases expression and activation of nephrin and Akt in glomeruli of both male and female mice and that activation of ERK1/2 occurs exclusively in glomeruli of male mice.
Daidzein Suppresses ERK1/2 Activation Following High Glucose Injury in Cultured Podocytes
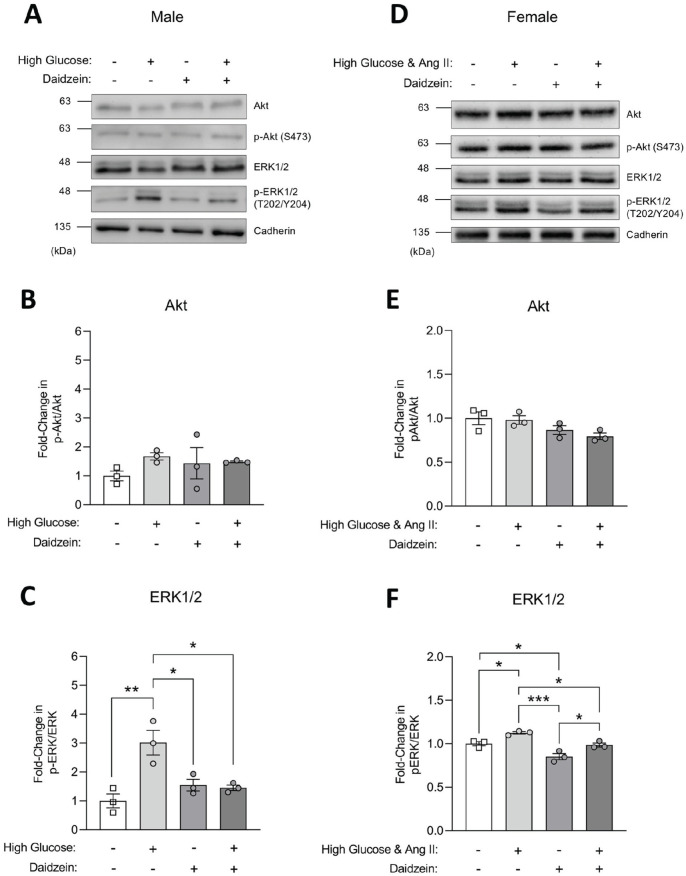
Given the effects of ISP on augmenting cell signaling pathways associated with podocyte survival and apoptosis in vivo, we were interested in exploring potential renoprotective effects of soy-derived phytoestrogens in a podocyte injury model. Using a simplified in vitro setting of cultured HPC lines derived from male or female human donors,40,41 cells were treated with daidzein in the presence of 5.5 or 25 mM glucose and/or Ang II to induce podocyte injury.51,52 In male-derived cells, Western blotting of HPC lysates followed by densitometric analysis revealed that cells cultured in high glucose had increased activation of ERK1/2 compared to untreated cells, consistent with previous reports,52-54 and that daidzein treatment could significantly suppress this increase (Figure 6A and 6C). However, in contrast with ISP exposure in vivo, daidzein alone did not induce changes in expression or activation of ERK1/2 or Akt in male podocytes in vitro, and no changes in activated or total Akt were seen in high glucose-treated cells (Figure 6A-6C). In female-derived cells, which required inclusion of angiotensin in the injury model to increase ERK1/2 activation, 51 treatment with daidzein similarly suppressed the effects of high glucose (Figure 6A and 6C). A comparable overall effect was seen with p38 activation, which is another mitogen-activated protein kinase (MAPK) pathway associated with podocyte injury (Supplemental Figure 4). 55 Interestingly, treatment with daidzein alone decreased ERK1/2 signaling in female podocytes, paralleling the changes seen in soy-fed female mice in vivo. As observed in male cells, female cells exposed to daidzein did not show the changes in Akt that occurred with the ISP diets in vivo (Figure 6A and 6C). This observation may be due to species difference between mice and human podocytes, the inability of antibody reagents to distinguish between human Akt1 and Akt2, or the difference in protein expression between glomeruli and cultured podocytes (Supplemental Figure 1). Altogether, these findings demonstrate that isoflavones can inhibit signaling pathways associated with podocyte injury in both male- and female-derived cell lines.
Figure 6.
Total and phosphorylated Akt and ERK1/2 expression in HPCs. (A-C) Differentiated male HPCs were treated with high glucose (20-25 mM), daidzein (10 μM), or high glucose with daidzein for 24 hours. Differentiated HPCs with 0.1% DMSO were used as controls. (A) Immunoblots of total and phospho Akt and ERK1/2 from male HPC lysates with cadherin shown as a loading control. (B & C) Densitometry from Akt (B) and ERK1/2 (C) immunoblots from 3 separate biological replicates (n = 3) normalized to control cells. (D-F) Differentiated female HPCs were treated with a combination of high glucose (25 mM)/1 µM angiotensin II (Ang II), daidzein (10 µM), or high glucose/Ang II and daidzein for 24 hours. Differentiated HPCs with 19.5 mM mannitol and 0.1% DMSO were used as controls. (D) Immunoblots of total and phospho Akt and ERK1/2 from female HPC lysates with cadherin shown as a loading control. (E & F) Densitometry from Akt (E) and ERK1/2 (F) immunoblots from 3 separate biological replicates (n = 3) normalized to control cells.
Note. All data are presented as mean ± standard error of the mean. Statistical significance compared to control cells was determined using one-way ANOVA with post-hoc Sidak’s test. Significance is marked by asterisks. HPCs = human podocyte cells; ANOVA = analysis of variance; ERK = extracellular signal-regulated kinase; DMSO = dimethyl sulfoxide.
*P < .05. **P < .01. ***P < .001.
Discussion
In this study, we reveal that signaling pathways controlling podocyte survival are differentially regulated in males and females and that they can be augmented with soy diet. Notably, we found significant glomerular sex differences in baseline expression of nephrin as well as Akt, which we showed likely corresponds to the Akt2 paralog. Glomeruli isolated from female mice showed higher baseline levels of both proteins compared to glomeruli isolated from male mice. Intriguingly, sex differences in nephrin have been reported previously in humans, with greater nephrin mRNA traces in female blood, as well as type 2 diabetic rats, with greater nephrin protein in female kidney cortices.56,57 For Akt, similar sex disparities in basal expression have also been identified in the heart and uterus.26-28 Such differences may reflect alterations in Akt translation or stability, perhaps due to the activity of Akt regulators such as mTORC2 and Pin1 in podocytes. 58 Alternately, the increase in total nephrin and Akt may correlate with higher levels of ERα observed in female kidney cortices. 59 Non-nuclear actions of estrogen include modulation of intracellular signaling pathways including Akt, wherein 17β-estradiol can induce Akt expression and/or phosphorylation.26,32,60 Moreover, a positive feedback loop may exist, as ERα can be activated by Akt.61,62 In podocytes, ERα itself has shown to be protective against apoptosis, 9 presumably through its effect on Akt signaling, 63 and reduced nephrin expression is found in ERα knockout mice. 8 Taken together, higher baseline levels of key survival factors in female glomeruli may confer intrinsic sex-specific resistance to cell death.
Consistent with the influence of endogenous estrogen, mice fed a diet containing soy-derived phytoestrogens showed increased nephrin and Akt expression compared with mice fed a casein diet. In both low- and high-level ISP diets, the most pronounced effects were on total Akt, with females showing 2-fold higher levels than males. We also noted activation of Akt in both males and females. These findings suggest that the renoprotective effect of soy protein might be due in part to an increase in the available pool of Akt to facilitate survival signaling. The increase in nephrin expression was only observed with low-level ISP diet, and though it was elevated in both males and females, only males showed a parallel increase in nephrin phosphorylation. Tyrosine phosphorylation of nephrin is required to maintain podocyte function 39 ; yet, it also leads to AP-1 and p38 stress signal activation and can induce nephrin internalization from the slit diaphragm.64-66 In line with this, only males showed an increase in activated ERK1/2, and this was paralleled by a mild elevation in ACR with supraphysiological levels of ISP diet. Although our study was limited by low animal numbers and should be repeated to confidently characterize the trend, our results suggest a potential negative effect exacerbated by higher doses.
Sustained ERK activation is correlated with podocyte apoptosis;29,30 yet, its activation by estradiol has also been reported to protect podocytes from injury. 8 In vivo, we cannot rule out the possibility that the increase in ERK1/2 is occurring within other glomerular cell types. We, therefore, specifically examined cultured podocytes and found that daidzein alone had no effect on ERK in males, though it did decrease ERK1/2 signaling in female cells, in line with our findings in female soy-fed mice. Moreover, daidzein suppressed high glucose-induced ERK1/2 activation in both male and female cells. These results support previous studies where genistein mitigated high glucose injury to cultured podocytes and reduced activated ERK1/2 in diabetic kidneys.67,68 To further probe the effects of daidzein on apoptosis, we also monitored p38 activation and performed lactate dehydrogenase (LDH) and terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling (TUNEL) assays. While female HPCs demonstrated only a minimal increase in p38 activation following a combined injury of high glucose and angiotensin II, daidzein still showed a small degree of protection in this setting. However, our injury model did not lead to notable changes in apoptosis (data not shown), and consistent with the weak effect on p38 activation, it is likely that a more robust injury will be required to induce a sufficient level of apoptosis for these assays. Moreover, we did not detect changes in Akt activation in daidzein-treated male or female cells, unlike our findings in vivo. Given the differences in expression of the Akt paralogs in podocytes grown in culture compared to within intact glomeruli, the in vitro setting may not be a suitable model system to study Akt signaling. Altogether, it remains unclear whether the observed changes to nephrin and ERK in male mice may reflect an early stage of injury or an uncharacterized beneficial state for podocytes. Beyond the scope of this study, future investigation testing soy diets in mouse models of diabetic kidney disease will be important to determine whether a similar mechanism of renoprotection occurs in vivo.
Soy contains 2 major phytoestrogens, genistein and daidzein, which can be further metabolized into the isoflavones equol, 5-hydroxy-equol, and O-desmethylangolensin.48,69,70 These compounds mostly target ERβ but can target ERα at higher concentrations. 48 This dose-dependent effect on ER activity is one possible reason why we observed differences in nephrin and Akt activity and expression in mice fed 1% ISP compared to mice fed 20% ISP. Nevertheless, the primary target of these phytoestrogens, ERβ, is similarly expressed in male and female glomeruli, implying an equivalent potential to respond to dietary soy. In line with our results, ERβ has been previously shown to mediate cardiac Akt activation and anti-apoptotic signaling. 71 However, ER-independent signaling pathways for soy constituents likely exist as well, as genistein provokes cellular changes in triple-negative breast cancer cells that lack ER expression.72,73 Currently, there is no accepted mechanism of action for genistein, daidzein, or equols, as treatments of various cell lines with these isoflavones have resulted in myriad signaling effects. 74 As a novel function for soy isoflavones, we show that daidzein can limit high glucose-induced ERK1/2 activation in cultured podocytes. Nevertheless, the complete influence of soy on glomerular signaling in vivo likely involves additional isoflavones like genistein and equols as well as indirect systemic effects (eg, lowering cholesterol, improved insulin response, and immune modulation).75-77
Conclusions
Diet is a significant risk factor in the progression of kidney disease; yet, dietary intervention is a relatively underused management approach. 78 While the benefits of dietary protein restriction remain controversial, 79 changing dietary protein quality appears to have more favorable effects. 16 Substituting plant-sourced for animal-sourced protein may reduce oxidative stress, metabolic acidosis, and production of nephrotoxic gut metabolites. 80 Our study now suggests that the kidney-protective effect of plant-derived phytoestrogens may arise in parallel through enhancement of a survival signaling axis in podocytes. These findings, along with baseline sex differences in nephrin and Akt expression, contribute to our understanding of the disparity in kidney disease outcomes between men and women, and they provide further evidence to support the beneficial effects of soy consumption.
Supplemental Material
Supplemental material, sj-pdf-1-cjk-10.1177_20543581221121636 for Sex Differences in Glomerular Protein Expression and Effects of Soy-Based Diet on Podocyte Signaling by Afreeda Mahesaniya, Casey R. Williamson, Ava Keyvani Chahi, Claire E. Martin, Alexander E. Mitro, Peihua Lu, Laura A. New, Katrina L. Watson, Roger A. Moorehead and Nina Jones in Canadian Journal of Kidney Health and Disease
Acknowledgments
The authors thank Dr Moin Saleem (Bristol, UK) for gifting cell lines, Dr Glen Pyle (Guelph, ON) for providing estrogen receptor antibodies, Robert Roe (Soy 20/20, Guelph, ON) for helpful discussions, and Jing Zhang for training and data collections from the Advanced Analysis Center Genomics Facility at the University of Guelph.
Footnotes
Ethics Approval and Consent to Participate: Standards for animal housing were maintained using the guidelines established by the Canadian Council of Animal Care. All experimental procedures involving mice were previously approved by the Animal Care Committee at the University of Guelph (AUPs 3291, 3123, and 3394).
Consent for Publication: All authors provided consent for the publication of this manuscript.
Availability of Data and Materials: All data used for the preparation of this manuscript is available upon request.
Author Contributions: A.M., C.R.W., R.A.M., and N.J. conceived and designed research; A.M., C.R.W., A.K.C., and P.L. performed experiments; A.M., C.R.W., A.K.C., L.A.N., and N.J. analyzed data and interpreted results of experiments; C.E.M., A.E.M., and K.L.W. provided assistance with experiments; A.M., C.R.W., and N.J. prepared figures; and C.R.W., L.A.N., and N.J. prepared the manuscript; all authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA)–University of Guelph Partnership Program (grant number 2013-1538, to N.J.); the Canadian Cancer Society (CCS) Research Institute (grant number 702774, to R.A.M.); and the Canadian Institutes of Health Research (CIHR) (grant number OCP-137736, to R.A.M.).
ORCID iDs: Claire E. Martin  https://orcid.org/0000-0001-8346-414X
https://orcid.org/0000-0001-8346-414X
Nina Jones  https://orcid.org/0000-0003-2786-2404
https://orcid.org/0000-0003-2786-2404
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Pavenstädt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253-307. [DOI] [PubMed] [Google Scholar]
- 2. Scott RP, Quaggin SE. Review series: the cell biology of renal filtration. J Cell Biol. 2015;209:199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Foley RN, Collins AJ. The growing economic burden of diabetic kidney disease. Curr Diab Rep. 2009;9(6):460-465. [DOI] [PubMed] [Google Scholar]
- 4. Manns B, McKenzie SQ, Au F, Gignac PM, Geller LI, Canadians Seeking Solutions and Innovations to Overcome Chronic Kidney Disease Network. The financial impact of advanced kidney disease on Canada Pension Plan and private disability insurance costs. Can J Kidney Health Dis. 2017;4:2054358117703986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carrero JJ. Gender differences in chronic kidney disease: underpinnings and therapeutic implications. Kidney Blood Press Res. 2010;33(5):383-392. [DOI] [PubMed] [Google Scholar]
- 6. Yanes LL, Sartori-Valinotti JC, Reckelhoff JF. Sex steroids and renal disease: lessons from animal studies. Hypertension. 2008;51(4):976-981. [DOI] [PubMed] [Google Scholar]
- 7. Ricardo AC, Yang W, Sha D, et al. Sex-related disparities in CKD progression. J Am Soc Nephrol. 2019;30(1):137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doublier S, Lupia E, Catanuto P, et al. Testosterone and 17β-estradiol have opposite effects on podocyte apoptosis that precedes glomerulosclerosis in female estrogen receptor knockout mice. Kidney Int. 2011;79(4):404-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kummer S, Jeruschke S, Wegerich LV, et al. Estrogen receptor alpha expression in podocytes mediates protection against apoptosis in-vitro and in-vivo. PLoS ONE. 2011;6(11):e27457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Catanuto P, Doublier S, Lupia E, et al. 17 beta-estradiol and tamoxifen upregulate estrogen receptor beta expression and control podocyte signaling pathways in a model of type 2 diabetes. Kidney Int. 2009;75(11):1194-1201. [DOI] [PubMed] [Google Scholar]
- 11. Stephenson TJ, Setchell KD, Kendall CW, Jenkins DJ, Anderson JW, Fanti P. Effect of soy protein-rich diet on renal function in young adults with insulin-dependent diabetes mellitus. Clin Nephrol. 2005;64(1):1-11. [DOI] [PubMed] [Google Scholar]
- 12. Teixeira SR, Tappenden KA, Carson L, et al. Isolated soy protein consumption reduces urinary albumin excretion and improves the serum lipid profile in men with type 2 diabetes mellitus and nephropathy. J Nutr. 2004;134(8):1874-1880. [DOI] [PubMed] [Google Scholar]
- 13. Zhang J, Liu J, Su J, Tian F. The effects of soy protein on chronic kidney disease: a meta-analysis of randomized controlled trials. Eur J Clin Nutr. 2014;68(9):987-993. [DOI] [PubMed] [Google Scholar]
- 14. Jing Z, Wei-Jie Y. Effects of soy protein containing isoflavones in patients with chronic kidney disease: a systematic review and meta-analysis. Clin Nutr. 2016;35(1):117-124. [DOI] [PubMed] [Google Scholar]
- 15. Vargas F, Romecín P, García-Guillén AI, et al. Flavonoids in kidney health and disease. Front Physiol. 2018;9:394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Garneata L, Stancu A, Dragomir D, Stefan G, Mircescu G. Ketoanalogue-supplemented vegetarian very low-protein diet and CKD progression. J Am Soc Nephrol. 2016;27(7):2164-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lichtenstein AH. Soy protein, isoflavones and cardiovascular disease risk. J Nutr. 1998;128:1589-1592. [DOI] [PubMed] [Google Scholar]
- 18. Kestilä M, Lenkkeri U, Männikkö M, et al. Positionally cloned gene for a novel glomerular protein–nephrin–is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1(4):575-582. [DOI] [PubMed] [Google Scholar]
- 19. Cooper ME, Mundel P, Boner G. Role of nephrin in renal disease including diabetic nephropathy. Semin Nephrol. 2002;22(5):393-398. [DOI] [PubMed] [Google Scholar]
- 20. Yuan H, Takeuchi E, Taylor GA, McLaughlin M, Brown D, Salant DJ. Nephrin dissociates from actin, and its expression is reduced in early experimental membranous nephropathy. J Am Soc Nephrol. 2002;13(4):946-956. [DOI] [PubMed] [Google Scholar]
- 21. Verma R, Venkatareddy M, Kalinowski A, et al. Nephrin is necessary for podocyte recovery following injury in an adult mature glomerulus. PLoS ONE. 2018;13(6):e0198013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin CE, Jones N. Nephrin signaling in the podocyte: an updated view of signal regulation at the slit diaphragm and beyond. Front Endocrinol (Lausanne). 2018;9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu J, Sun N, Aoudjit L, et al. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73(5):556-566. [DOI] [PubMed] [Google Scholar]
- 24. Huber TB, Hartleben B, Kim J, et al. Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol. 2003;23(14):4917-4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Canaud G, Bienaimé F, Viau A, et al. AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med. 2013;19(10):1288-1296. [DOI] [PubMed] [Google Scholar]
- 26. Dery M-C, Leblanc V, Shooner C, et al. Regulation of Akt expression and phosphorylation by 17β-estradiol in the rat uterus during estrous cycle. Reprod Biol Endocrinol. 2003;1:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bell JR, Porrello ER, Huggins CE, Harrap SB, Delbridge LM. The intrinsic resistance of female hearts to an ischemic insult is abrogated in primary cardiac hypertrophy. Am J Physiol Heart Circ Physiol. 2008;294(4):H1514-H1522. [DOI] [PubMed] [Google Scholar]
- 28. Reichelt ME, Mellor KM, Curl CL, Stapleton D, Delbridge LM. Myocardial glycophagy — a specific glycogen handling response to metabolic stress is accentuated in the female heart. J Mol Cell Cardiol. 2013;65:67-75. [DOI] [PubMed] [Google Scholar]
- 29. Das R, Xu S, Nguyen TT, et al. Transforming growth factor β1-induced apoptosis in podocytes via the extracellular signal-regulated kinase-mammalian target of rapamycin complex 1-NADPH oxidase 4 axis. J Biol Chem. 2015;290:30830-30842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu S, Ding J, Fan Q, Zhang H. The activation of extracellular signal-regulated kinase is responsible for podocyte injury. Mol Biol Rep. 2010;37(5):2477-2484. [DOI] [PubMed] [Google Scholar]
- 31. Palanisamy N, Anuradha CV. Soy protein preserves basement membrane integrity through a synergistic effect on nephrin, matrix metalloproteinase and vascular endothelial growth factor. Am J Nephrol. 2011;34(6):529-533. [DOI] [PubMed] [Google Scholar]
- 32. Camper-Kirby D, Welch S, Walker A, et al. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circ Res. 2001;88:1020-1027. [DOI] [PubMed] [Google Scholar]
- 33. Jeng YJ, Kochukov MY, Watson CS. Membrane estrogen receptor-α-mediated nongenomic actions of phytoestrogens in GH3/B6/F10pituitary tumor cells. J Mol Signal. 2009;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Maggiolini M, Vivacqua A, Fasanella G, et al. The G protein-coupled receptor GPR30 mediates c-fos up-regulation by 17beta-estradiol and phytoestrogens in breast cancer cells. J Biol Chem. 2004;279:27008-27016. [DOI] [PubMed] [Google Scholar]
- 35. Liao Q-C, Li Y-L, Qin Y-F, et al. Inhibition of adipocyte differentiation by phytoestrogen genistein through a potential downregulation of extracellular signal-regulated kinases 1/2 activity. J Cell Biochem. 2008;104:1853-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yanagihara N, Toyohira Y, Shinohara Y. Insights into the pharmacological potential of estrogens and phytoestrogens on catecholamine signaling. Ann N Y Acad Sci. 2008;1129:96-104. [DOI] [PubMed] [Google Scholar]
- 37. Frey RS, Singletary KW. Genistein activates p38 mitogen-activated protein kinase, inactivates ERK1/ERK2 and decreases Cdc25C expression in immortalized human mammary epithelial cells. J Nutr. 2003;133(1):226-231. [DOI] [PubMed] [Google Scholar]
- 38. Watson KL, Sauerzopf K, Moorehead RA. Isolated soy protein promotes mammary tumor development induced by the Type I insulin-like growth factor receptor in transgenic mice. Nutr Cancer. 2021;73:1340-1349. [DOI] [PubMed] [Google Scholar]
- 39. New LA, Martin CE, Scott RP, et al. Nephrin tyrosine phosphorylation is required to stabilize and restore podocyte foot process architecture. J Am Soc Nephrol. 2016;27(8):2422-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saleem MA, O’Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13(3):630-638. [DOI] [PubMed] [Google Scholar]
- 41. Haley KE, Kronenberg NM, Liehm P, et al. Podocyte injury elicits loss and recovery of cellular forces. Sci Adv. 2018;4(6):eaap8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Martin CE, New LA, Phippen NJ, et al. Multivalent nephrin-Nck interactions define a threshold for clustering and tyrosine-dependent nephrin endocytosis. J Cell Sci. 2020;133:jcs236877. [DOI] [PubMed] [Google Scholar]
- 43. Shankland SJ, Pippin JW, Reiser J, Mundel P. Podocytes in culture: past, present, and future. Kidney Int. 2007;72(1):26-36. [DOI] [PubMed] [Google Scholar]
- 44. Li H, Lemay S, Aoudjit L, Kawachi H, Takano T. Src-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol. 2004;15(12):3006-3015. [DOI] [PubMed] [Google Scholar]
- 45. Jones N, New LA, Fortino MA, et al. Nck proteins maintain the adult glomerular filtration barrier. J Am Soc Nephrol. 2009;20(7):1533-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Degasperi A, Birtwistle MR, Volinsky N, Rauch J, Kolch W, Kholodenko BN. Evaluating strategies to normalise biological replicates of western blot data. PLoS ONE. 2014;9(1):e87293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lindenmeyer MT, Eichinger F, Sen K, et al. Systematic analysis of a novel human renal glomerulus-enriched gene expression dataset. PLoS ONE. 2010;5:e11545. doi: 10.1371/JOURNAL.PONE.0011545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jiang Y, Gong P, Madak-Erdogan Z, et al. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 2013;27(11):4406-4418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Watson KL, Stalker L, Jones RA, et al. High levels of dietary soy decrease mammary tumor latency and increase incidence in MTB-IGFIR transgenic mice. BMC Cancer. 2015;15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chang JH, Paik SY, Mao L, et al. Diabetic kidney disease in FVB/NJ Akita mice: temporal pattern of kidney injury and urinary nephrin excretion. PLoS ONE. 2012;7(4):e33942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Che G, Gao H, Hu Q, Xie H, Zhang Y. Angiotensin II promotes podocyte injury by activating Arf6-Erk1/2-Nox4 signaling pathway. PLoS ONE. 2020;15(3):e0229747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoshi S, Nomoto K, Kuromitsu J, et al. High glucose induced VEGF expression via PKC and ERK in glomerular podocytes. Biochem Biophys Res Commun. 2002;290:177-184. [DOI] [PubMed] [Google Scholar]
- 53. Sakai N, Wada T, Furuichi K, et al. Involvement of extracellular signal-regulated kinase and p38 in human diabetic nephropathy. Am J Kidney Dis. 2005;45(1):54-65. [DOI] [PubMed] [Google Scholar]
- 54. Okada T, Wada J, Hida K, et al. Thiazolidinediones ameliorate diabetic nephropathy via cell cycle-dependent mechanisms. Diabetes. 2006;55(6):1666-1677. [DOI] [PubMed] [Google Scholar]
- 55. Cardoso VG, Gonçalves GL, Costa-Pessoa JM, et al. Angiotensin II-induced podocyte apoptosis is mediated by endoplasmic reticulum stress/PKC-δ/p38 MAPK pathway activation and trough increased Na + /H + exchanger isoform 1 activity. BMC Nephrol. 2018;19:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Orlandi E, Butt A, Goldsmith D, Swaminathan R. Factors affecting circulating mRNA for nephrin. Clin Chem. 2005;51(10):1982-1983. [DOI] [PubMed] [Google Scholar]
- 57. Spires DR, Palygin O, Levchenko V, et al. Sexual dimorphism in the progression of type 2 diabetic kidney disease in T2DN rats. Physiol Genomics. 2021;53:223-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liao Y, Hung MC. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19-42. [PMC free article] [PubMed] [Google Scholar]
- 59. Rogers JL, Mitchell AR, Maric C, Sandberg K, Myers A, Mulroney SE. Effect of sex hormones on renal estrogen and angiotensin type 1 receptors in female and male rats. Am J Physiol Regul Integr Comp Physiol. 2007;292(2):R794-R799. [DOI] [PubMed] [Google Scholar]
- 60. Knowlton AA, Lee AR. Estrogen and the cardiovascular system. Pharmacol Ther. 2012;135:54-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Campbell RA, Bhat-Nakshatri P, Patel NM, et al. Phosphatidylinositol 3-Kinase/AKT-mediated activation of estrogen receptor α. J Biol Chem. 2001;276:9817-9824. [DOI] [PubMed] [Google Scholar]
- 62. Bratton MR, Duong BN, Elliott S, et al. Regulation of ERα-mediated transcription of Bcl-2 by PI3K-AKT crosstalk: implications for breast cancer cell survival. Int J Oncol. 2010;37:541-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Catanuto P, Fornoni A, Pereira-Simon S, et al. In vivo 17β-estradiol treatment contributes to podocyte actin stabilization in female db/db Mice. Endocrinology. 2012;153(12):5888-5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martin CE, Petersen KA, Aoudjit L, et al. ShcA adaptor protein promotes nephrin endocytosis and is upregulated in proteinuric nephropathies. J Am Soc Nephrol. 2018;29(1):92-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huber TB, Köttgen M, Schilling B, et al. Interaction with podocin facilitates nephrin signaling. J Biol Chem. 2001;276:41543-41546. [DOI] [PubMed] [Google Scholar]
- 66. Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T. Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J Am Soc Nephrol. 2009;20(12):2534-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Elmarakby AA, Ibrahim AS, Faulkner J, Mozaffari MS, Liou GI, Abdelsayed R. Tyrosine kinase inhibitor, genistein, reduces renal inflammation and injury in streptozotocin-induced diabetic mice. Vascul Pharmacol. 2011;55(5-6):149-156. [DOI] [PubMed] [Google Scholar]
- 68. Wang Y, Li Y, Zhang T, et al. Genistein and Myd88 activate autophagy in high glucose-induced renal podocytes in vitro. Med Sci Monit. 2018;24:4823-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Matthies A, Loh G, Blaut M, Braune A. Daidzein and genistein are converted to equol and 5-hydroxy-equol by human intestinal Slackia isoflavoniconvertens in gnotobiotic rats. J Nutr. 2012;142(1):40-46. [DOI] [PubMed] [Google Scholar]
- 70. Atkinson C, Frankenfeld CL, Lampe JW. Gut bacterial metabolism of the soy isoflavone daidzein: exploring the relevance to human health. Exp Biol Med (Maywood). 2005;230(3):155-170. [DOI] [PubMed] [Google Scholar]
- 71. Wang M, Wang Y, Weil B, et al. Estrogen receptor β mediates increased activation of PI3K/Akt signaling and improved myocardial function in female hearts following acute ischemia. Am J Physiol Regul Integr Comp Physiol. 2009;296:R972-R978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pan H, Zhou W, He W, et al. Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-κB activity via the Notch-1 pathway. Int J Mol Med. 2012;30(2):337-343. [DOI] [PubMed] [Google Scholar]
- 73. Li Z, Li J, Mo B, et al. Genistein induces cell apoptosis in MDA-MB-231 breast cancer cells via the mitogen-activated protein kinase pathway. Toxicol Vitro. 2008;22(7):1749-1753. [DOI] [PubMed] [Google Scholar]
- 74. Sirotkin AV, Harrath AH. Phytoestrogens and their effects. Eur J Pharmacol. 2014;741:230-236. [DOI] [PubMed] [Google Scholar]
- 75. Borradaile NM, de Dreu LE, Wilcox LJ, et al. Soya phytoestrogens, genistein and daidzein, decrease apolipoprotein B secretion from HepG2 cells through multiple mechanisms. Biochem J. 2002;366:531-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wagner JD, Zhang L, Shadoan MK, et al. Effects of soy protein and isoflavones on insulin resistance and adiponectin in male monkeys. Metabolism. 2008;57(7, suppl 1):S24-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yellayi S, Naaz A, Szewczykowski MA, et al. The phytoestrogen genistein induces thymic and immune changes: a human health concern? Proc Natl Acad Sci U S A. 2002;99:7616-7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Goraya N, Wesson DE. Dietary protein as kidney protection: quality or quantity? J Am Soc Nephrol. 2016;27:1877-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Klahr S, Levey AS, Beck GJ, et al. The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med. 1994;330:877-884. [DOI] [PubMed] [Google Scholar]
- 80. Goraya N, Wesson DE. Novel dietary and pharmacologic approaches for acid-base modulation to preserve kidney function and manage uremia. Curr Opin Nephrol Hypertens. 2020;29(1):39-48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cjk-10.1177_20543581221121636 for Sex Differences in Glomerular Protein Expression and Effects of Soy-Based Diet on Podocyte Signaling by Afreeda Mahesaniya, Casey R. Williamson, Ava Keyvani Chahi, Claire E. Martin, Alexander E. Mitro, Peihua Lu, Laura A. New, Katrina L. Watson, Roger A. Moorehead and Nina Jones in Canadian Journal of Kidney Health and Disease