Abstract
Background
The incidence of sepsis can be estimated between 250 and 500 cases/100.000 people per year and is responsible for up to 6% of total hospital admissions. Identified as one of the most relevant global health problems, sepsis is the condition that generates the highest costs in the healthcare system. Important changes in the management of septic patients have been included in recent years; however, there is no information about how changes in the management of sepsis-associated organ failure have contributed to reduce mortality.
Methods
A retrospective analysis was conducted from hospital discharge records from the Minimum Basic Data Set Acute-Care Hospitals (CMBD-HA in Catalan language) for the Catalan Health System (CatSalut). CMBD-HA is a mandatory population-based register of admissions to all public and private acute-care hospitals in Catalonia. Sepsis was defined by the presence of infection and at least one organ dysfunction. Patients hospitalized with sepsis were detected, according ICD-9-CM (since 2005 to 2017) and ICD-10-CM (2018 and 2019) codes used to identify acute organ dysfunction and infectious processes.
Results
Of 11.916.974 discharges from all acute-care hospitals during the study period (2005–2019), 296.554 had sepsis (2.49%). The mean annual sepsis incidence in the population was 264.1 per 100.000 inhabitants/year, and it increased every year, going from 144.5 in 2005 to 410.1 in 2019. Multiorgan failure was present in 21.9% and bacteremia in 26.3% of cases. Renal was the most frequent organ failure (56.8%), followed by cardiovascular (24.2%). Hospital mortality during the study period was 19.5%, but decreases continuously from 25.7% in 2005 to 17.9% in 2019 (p < 0.0001). The most important reduction in mortality was observed in cases with cardiovascular failure (from 47.3% in 2005 to 31.2% in 2019) (p < 0.0001). In the same way, mean mortality related to renal and respiratory failure in sepsis was decreased in last years (p < 0.0001).
Conclusions
The incidence of sepsis has been increasing in recent years in our country. However, hospital mortality has been significantly reduced. In septic patients, all organ failures except liver have shown a statistically significant reduction on associated mortality, with cardiovascular failure as the most relevant.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-022-04176-w.
Keywords: Sepsis, Sepsis syndrome, Septic shock, Sepsis mortality, Epidemiologic methods, Sepsis epidemiology, Multiple organ dysfunction syndrome, Sequential organ failure assessment score, Sepsis-related organic failure
Background
Sepsis is defined as an organ dysfunction secondary to a dysregulated immune response to an infectious process [1]. Incidence can be estimated between 250 and 500 cases/100.000 people per year [2–7] and is responsible for up to 6% of total hospital admissions [4]; up to 50% can require ICU admission [4]. Incidence of community acquired sepsis that requires admission to ICU has been estimated to be 50 cases/100.000 inhabitants per year [8].
Identified as one of the most relevant global health problems, sepsis is the condition that generates the highest costs in the US healthcare system [5] and is responsible for practically half of hospital deaths [6], even above myocardial infarction and stroke [7]. Improvements in knowledge of the epidemiological behavior of sepsis could be useful for a better approach and management of this pathology.
Although the absolute values obtained from epidemiological studies may be biased by different selection procedures, they allow the monitoring of incidence and mortality trends in large patient populations.
Important changes in the management of septic patients have been included in recent years. Strategies to increase awareness in healthcare workers [9, 10], emphasis on the achievement of early empiric effective antibiotic treatment [9–11], simplification of the hemodynamic management (early goal directed therapy [12] versus last evidence [13–15]), changes on respiratory failure management (use of prone positioning [16], protective ventilation [17, 18], ECMO [19, 20]), or renal failure management [21] presented some differences during the last years. However, there is no information about how changes in the management of sepsis-associated organ failure have contributed to mortality reduction during these years.
The objective of our study is to observe trends in mortality in septic patients according to the different organ failure in the population of Catalonia over a period of 15 years.
Material and methods
Data sources
A retrospective analysis was conducted from hospital discharge records from the Minimum Basic Data Set Acute-Care Hospitals (CMBD-HA in Catalan language) for the Catalan Health System (CatSalut). The study was approved by the Mataró’s Hospital and Maresme Health Consortium Ethical Review Board with a waiver of informed consent in 21th of March in 2018 (code CEIC_20/18), and all study’s procedures were followed in accordance with the Helsinki Declaration of 1975.
CMBD-HA is a mandatory population-based register of admissions to all public and private acute-care hospitals in Catalonia that enables evaluation and optimization of resource use, provides support, improves healthcare planning and facilitates procurement management and payments. All the codes are provided directly by the patient's treating physicians and subsequently verified by the technical secretariat of each health center. To ensure data quality, the CMBD-HA input data are systematically validated internally in CatSalut with an automated data validation system that checks data consistency and identifies potential errors or inconsistencies between variables. Furthermore, as this information is used for provider payment purposes, external audits are regularly performed to ensure the quality and reliability of the data. These external audits are performed whenever a suspicious deviation is detected or, if none is detected, every 3 or 5 years. The data set contains demographic and clinical data for patient care episodes, including age, sex, length of stay (days), one primary diagnosis, up to fourteen secondary diagnoses, one primary procedure, up to nineteen secondary procedures and status on discharge (alive, dead, or transferred to another hospital). Official data from the register of insured persons maintained by CatSalut were used to estimate crude and specific hospitalization rates (universal coverage for 7.570.430 inhabitants in 2019).
Patients
Sepsis was defined by the presence of infection and at least one organ dysfunction. In Catalonia, the diagnostic coding system changed in January 2018 from ICD-9-CM to ICD-10-CM. In this way, ICD-9-CM codes were used until December 31, 2017, and ICD-10-CM were used subsequently.
Using ICD-9-CM and ICD-10-CM codes, all hospitalized patients with and infection and an organ dysfunction were detected following the Angus methodology [22], over a 15-year period (2005–2019). All ICD-9-CM diagnostic codes used for detection of infection-related organ dysfunction have been provided in the Additional file 1, and refer to acute dysfunction of any organ as a result of sepsis, since each of them is associated with the diagnosis of infection or sepsis. All of these used codes were translated into the new coding system (CID-10-CM) as of 2018.
To avoid overlaps, we excluded patients who were transferred from one acute-care hospital to another during the same sepsis episode; 18.992 admissions were excluded from non-residents in Catalonia.
Coding
Diagnoses and procedures were coded using the ICD-9-CM until the end of 2017 and ICD-10-CM for 2018–2019. ICD-9-CM codes to identify patients with sepsis were updated in 2000 to the following: 995.91 (sepsis), 995.92 (severe sepsis) and 785.52 (septic shock). ICD-10-CM codes to identify patients with sepsis were equated to ICD-9-CM codes and were: R652 (severe sepsis), R6520 (severe sepsis without septic shock) and R6521 (severe sepsis with septic shock).
Although information was not available regarding the unit or department where patients were treated (intensive care unit (ICU), internal medicine unit, etc.), we indirectly deduced ICU admission from procedures typically used in intensive care management (Additional file 1). The Charlson comorbidity index with its 17 comorbid disease categories [23] was used to assess the presence of underlying comorbidities. The ICD-9-CM codes used to identify acute organ dysfunction and infectious processes are listed in Supplementary Material. With ICD-10-CM codification system, all used codes to identify acute organ dysfunction were equated to ICD-10-CM system.
Statistical analysis
The hospitalization rate was defined as the yearly number of admissions per 100.000 population. Crude overall and specific hospitalization rates by age and sex were calculated. Continuous variables and discrete variables were compared using analysis of variance and the Chi-square test, respectively. Multivariate logistic regression, adjusted for other significant variables, was used to analyze hospital mortality risk by year of admission for the study population and for the ICU and non-ICU patient groups; variables were entered one by one and retained when their significance was < 0.10 and were clinically plausible. For the regression analysis, each of the clinical attributes included (comorbidities, acute organ failure and infection) was treated as binary (dummy) variables indicating the presence or absence of these conditions; a single patient could therefore account for more than one attribute. The area under the receiver operating characteristic curve (AUROC) was used to evaluate how well the multivariate logistic regression model discriminated between patients with severe sepsis who were discharged alive versus those who died in hospital [24]. Data analysis was performed using SPSS 18.0 software (SPSS Inc, Chicago, IL, USA).
Results
Of 11.916.974 discharges from all acute-care hospitals during the study period (2005–2019), 296.554 had sepsis (2.49%). Demographic characteristics and comorbidities for patients with sepsis are shown in Table 1.
Table 1.
Profile of patients with severe sepsis in Catalonia
| Total N = 296,554 | Alive N = 238,775 | Dead N = 57,779 | |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Sex (males) | 166,808 (56.2%) | 133,428 (55.9%) | 33,380 (57.8%) |
| Mean (SD) | Mean (SD) | Mean (SD) | |
|---|---|---|---|
| Charlson index | 5.51 (2.74) | 5.37 (2.72) | 6.08 (2.74) |
| Mean age | 72.9 (18.1) | 72.3 (18.7) | 75.2 (15.3) |
| Length of hospital stay (days) | 15.3 (24.2) | 15.5 (24.8) | 14.5 (21.6) |
| Age (years) | |||
| < 15 | 6430 (2.17%) | 5905 (2.47%) | 525 (0.91%) |
| 15–44 | 15,072 (5.08%) | 13,332 (5.58%) | 1740 (3.01%) |
| 45–64 | 47,027 (15.9%) | 38,020 (15.9%) | 9007 (15.6%) |
| 65–74 | 53,585 (18.1%) | 42,924 (18.0%) | 10,661 (18.5%) |
| 75–84 | 94,322 (31.8%) | 75,577 (31.7%) | 18,745 (32.4%) |
| > 84 | 80,118 (27.0%) | 63,017 (26.4%) | 17,101 (29.6%) |
| Comorbidities | |||
| Myocardial infarction | 12,377 (4.17%) | 9481 (3.97%) | 2896 (5.01%) |
| Congestive heart failure | 65,456 (22.1%) | 50,537 (21.2%) | 14,919 (25.8%) |
| Peripheral vascular disease | 16,134 (5.44%) | 12,452 (5.21%) | 3682 (6.37%) |
| Cerebrovascular disease | 22,538 (7.60%) | 17,568 (7.36%) | 4970 (8.60%) |
| Dementia | 23,510 (7.93%) | 18,791 (7.87%) | 4719 (8.17%) |
| COPD | 74,240 (25.0%) | 60,892 (25.5%) | 13,348 (23.1%) |
| Rheumatic diseases | 6952 (2.34%) | 5801 (2.43%) | 1151 (1.99%) |
| Peptic ulcer disease | 3512 (1.18%) | 2666 (1.12%) | 846 (1.46%) |
| Liver disease: mild | 29,962 (10.1%) | 22,517 (9.43%) | 7445 (12.9%) |
| Uncomplicated diabetes | 64,848 (21.9%) | 54,717 (22.9%) | 10,131 (17.5%) |
| Complicated diabetes | 14,909 (5.03%) | 12,689 (5.31%) | 2220 (3.84%) |
| Hemiplegia/paraplegia | 4896 (1.65%) | 3966 (1.66%) | 930 (1.61%) |
| Chronic kidney disease | 83,949 (28.3%) | 69,525 (29.1%) | 14,424 (25.0%) |
| Cancer | 41,129 (13.9%) | 29,039 (12.2%) | 12,090 (20.9%) |
| Liver disease: moderate–severe | 9121 (3.08%) | 6291 (2.63%) | 2830 (4.90%) |
| Metastasis | 15,473 (5.22%) | 9952 (4.17%) | 5521 (9.56%) |
| AIDS/HIV infection | 2781 (0.94%) | 2113 (0.88%) | 668 (1.16%) |
| ICU admissions* | 70,578 (23.8%) | 46,354 (19.4%) | 24,224 (41.9%) |
| Sepsis origins | |||
| Urinary | 113,565 (38.3%) | 99,236 (41.6%) | 14,329 (24.8%) |
| Respiratory | 96,940 (32.7%) | 76,410 (32.0%) | 20,530 (35.5%) |
| Abdominal | 31,518 (10.6%) | 24,001 (10.1%) | 7517 (13.0%) |
| Skin and soft tissues | 13,465 (4.54%) | 10,985 (4.60%) | 2480 (4.29%) |
| Endocarditis | 3789 (1.28%) | 2712 (1.14%) | 1077 (1.86%) |
| Device related | 22,063 (7.44%) | 17,968 (7.53%) | 4095 (7.09%) |
| CNS | 2457 (0.83%) | 1849 (0.77%) | 608 (1.05%) |
| Others | 9537 (3.22%) | 8175 (3.42%) | 1362 (2.36%) |
| Unspecific | 40,578 (13.7%) | 27,231 (11.4%) | 13,347 (23.1%) |
| Bacteremia | 84,191 (28.4%) | 56,291 (23.6%) | 27,900 (48.3%) |
| Total N = 296,554 | Alive | Dead | |
| Organ Dysfunction | |||
| Kidney | 168,603 (56.9%) | 135,059 (56.6%) | 33,544 (58.1%) |
| Lung | 45,016 (15.2%) | 28,734 (12.0%) | 16,282 (28.2%) |
| CNS | 61,377 (20.7%) | 53,209 (22.3%) | 8168 (14.1%) |
| Hematologic | 30,615 (10.3%) | 24,547 (10.3%) | 6068 (10.5%) |
| Cardiovascular | 71,929 (24.3%) | 46,306 (19.4%) | 25,623 (44.3%) |
| Liver | 3956 (1.33%) | 1819 (0.76%) | 2137 (3.70%) |
| Number of organ failures | |||
| 1 | 231,622 (78.1%) | 197,135 (82.6%) | 34,487 (59.7%) |
| 2 | 48,898 (16.5%) | 33,865 (14.2%) | 15,033 (26.0%) |
| 3 or more | 16,034 (5.41%) | 7775 (3.26%) | 8259 (14.3%) |
Data are presented as mean and standard deviation or %
AIDS: acquired immune deficiency syndrome, CNS: central nervous system, COPD: chronic obstructive pulmonary disease, HIV: human immunodeficiency virus, ICU: intensive care unit
ICU admissions* are estimated from invasive procedures related to organ failure management
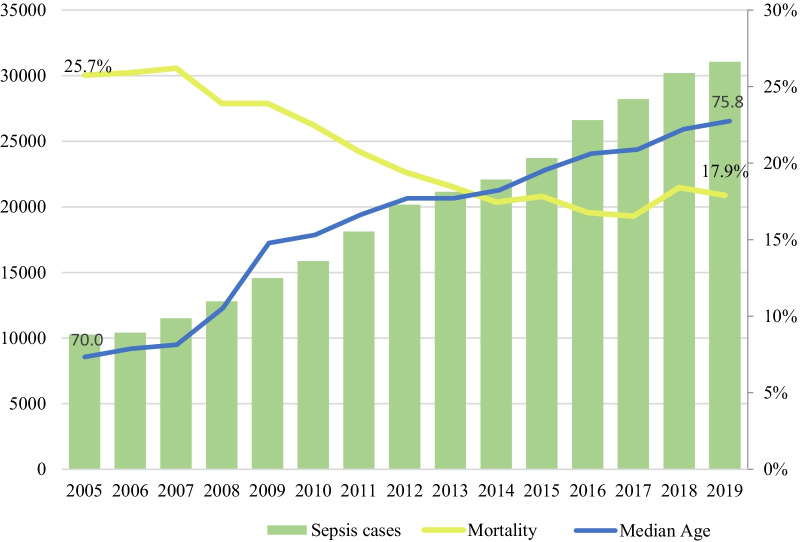
The annual sepsis incidence in the population was 264.1 per 100.000 inhabitants/year, and it increased every year, going from 144.5 in 2005 to 410.1 in 2019.
Sepsis was significantly associated with age (76.9% of the cases occurred in patients older than 65 years) and sex (56.2 of cases occurred in men) (p < 0.001).
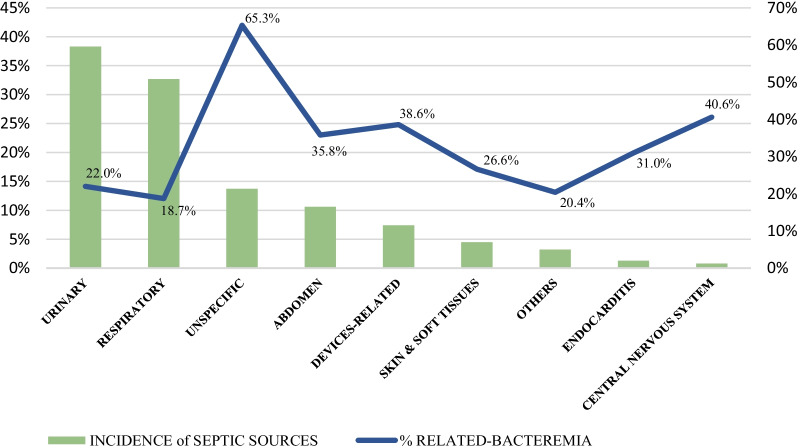
The most frequent origins of sepsis were urinary and respiratory tract infections, accounting for 38.3% and 32.7% of cases, respectively, followed by sepsis of unknown origin (13.7%). The central nervous system was the least frequent origin of sepsis (0.8%) (Fig. 1).
Fig. 1.
Incidence of different septic sources and its related-bacteremia
More than a quarter of cases (26.3%) presented bacteremia. Infections with most presence of bacteremia were those related to unknown origin (65.3%), followed by central nervous system (40.6%), external devices (38, 6%) and abdominal sources (35.8%). The source with the lowest presence of bacteremia was the respiratory tract (18.7%) (Fig. 1).
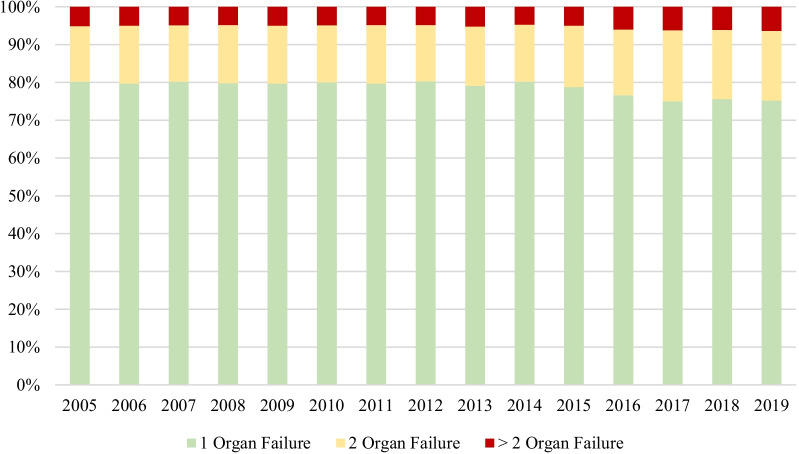
The majority of patients (78.1%) presented single organ failure at the time of sepsis diagnosis and slightly more than 5% had failure of 3 or more organs in this moment (Table 1, Fig. 2).
Fig. 2.
Incidence of 1, 2 or ≥ 2 organ failures in sepsis during study period (2005 – 2019)
Global length of hospital stay was 15.3 (± 24.2) days, and it was shorter in those patients who died (14.5 (± 21.6) vs 15.5 (± 24.8). Length of hospital stay has been decreasing from 19.5 (± 25.4) days in 2005 to 14.9 (± 21.4) days in 2019 (p < 0.0001). Hospital mortality during the study period was 19.5% but decreases continuously from 25.7% in 2005 to 17.9% in 2019 (p < 0.0001) (Fig. 3). Hospital mortality was higher in older patients (p < 0.05) and in those cases that presented some comorbidity included in the Charlson index assessment (p < 0.0001) (Table 1).
Fig. 3.
Incidence, mortality and median age of Sepsis in Catalonia during study period (2005–2019)
Organ failure-associated mortality
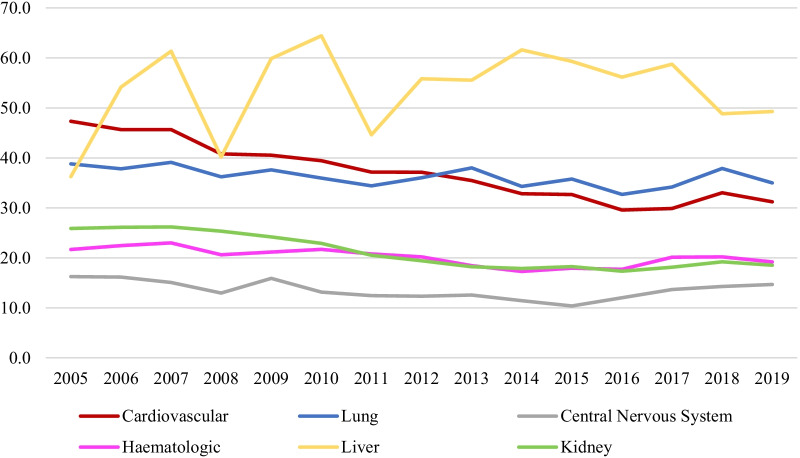
Renal was the most frequent organ failure (56.9%), followed by cardiovascular (24.3%), central nervous system (20.7%) and respiratory failure (15.2%). Hepatic failure was the less frequent organ failure present in septic patients (1.3%) (Table 1).
Sepsis-related cardiovascular failure mortality was 35,6% during the study period. However, a reduction in this rate was observed in recent years (from 47.3% in 2005 to 31.2% in 2019) (p < 0.0001) (Fig. 4). In the same way, mean mortality related to renal failure in sepsis was 19.9%, but a reduction in this mortality was observed in last years (from 25.9% in 2005 to 18.5% in 2019) (p < 0.0001) (Fig. 4). In relation to respiratory failure, mortality also declined in last period from 38.8% in 2005 to 35% in 2019 (p < 0.0001), as well as in central nervous system failure (from 16.3% in 2005 to 14.7% in 2019, p < 0.0001) and hematological failure (from 21.7% in 2005 to 19.2% in 2019, p < 0.0001) (Fig. 4).
Fig.4.
Evolution of mortality (%) in sepsis according to organic dysfunction
Although hepatic failure is the least frequent organic failure in sepsis, mortality in these patients was high (52.0%) and has been increasing over the years (from 36.3% in 2005 to 49.3% in 2019, p < 0.0001) (Fig. 4). These differences were confirmed in the multivariate analysis adjusted for all significant variables (age group, sex, comorbidities, organ dysfunction, infection source and presence of bacteremia) (Table 2).
Table 2.
Univariate and multivariate analyses of organ failure associated in-hospital mortality by year of admission in Catalonia (2005–2017)
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Year | n | Mortality | p | OR | IC95% | p |
| Respiratory failure | ||||||
| 2005 | 2662 | 38,8 | 1 | – | ||
| 2006 | 2459 | 37,8 | 0,8,555,715 | 0.755–0.970 | 0.0146 | |
| 2007 | 2643 | 39,2 | 0,89,399,059 | 0.791–1.011 | 0.0731 | |
| 2008 | 2799 | 36,2 | 0,77,898,524 | 0.690–0.880 | < 0.0001 | |
| 2009 | 2996 | 37,6 | 0,75,801,748 | 0.673–0.854 | < 0.0001 | |
| 2010 | 2816 | 36,0 | 0,69,558,657 | 0.616–0.786 | < 0.0001 | |
| 2011 | 2802 | 34,4 | 0,60,252,572 | 0.533–0.681 | < 0.0001 | |
| 2012 | 2776 | 36,0 | 0,58,824,668 | 0.520–0.665 | < 0.0001 | |
| 2013 | 2891 | 38,0 | 0,61,456,548 | 0.545–0.693 | < 0.0001 | |
| 2014 | 2642 | 34,3 | 0,51,394,317 | 0.453–0.583 | < 0.0001 | |
| 2015 | 2745 | 35,8 | 0,52,879,873 | 0.468–0.598 | < 0.0001 | |
| 2016 | 3380 | 32,7 | 0,46,018,306 | 0.408–0.518 | < 0.0001 | |
| 2017 | 3849 | 34,3 | 0,48,323,359 | 0.431–0.542 | < 0.0001 | |
| 2018 | 3742 | 37,9 | < 0.0001 | 0,56,156,227 | 0.501–0.630 | < 0.0001 |
| 2019 | 3814 | 35,0 | 0,4,733,619 | 0.422–0.531 | < 0.0001 | |
| AUC ROC | ||||||
| 0.762 (0.758 – 0.767) | ||||||
| Cardiovascular failure | ||||||
| 2005 | 2491 | 47,3 | 1 | – | ||
| 2006 | 2858 | 45,7 | 0,69,393,529 | 0.615–0.783 | < 0.0001 | |
| 2007 | 3225 | 45,7 | 0,69,297,304 | 0.616–0.779 | < 0.0001 | |
| 2008 | 3568 | 40,8 | 0,55,888,678 | 0.498–0.627 | < 0.0001 | |
| 2009 | 3945 | 40,5 | 0,54,844,547 | 0.490–0.614 | < 0.0001 | |
| 2010 | 4081 | 39,4 | 0,48,303,417 | 0.431–0.541 | < 0.0001 | |
| 2011 | 4487 | 37,1 | 0,44,050,755 | 0.394–0.493 | < 0.0001 | |
| 2012 | 4520 | 37,1 | 0,40,863,827 | 0.365–0.457 | < 0.0001 | |
| 2013 | 4774 | 35,5 | 0,37,430,685 | 0.335–0.418 | < 0.0001 | |
| 2014 | 4802 | 32,8 | 0,33,977,245 | 0.304–0.380 | < 0.0001 | |
| 2015 | 5256 | 32,7 | 0,32,401,597 | 0.290–0.362 | < 0.0001 | |
| 2016 | 6389 | 29,6 | 0,27,741,318 | 0.249–0.309 | < 0.0001 | |
| 2017 | 5791 | 29,9 | 0,25,132,744 | 0.225–0.281 | < 0.0001 | |
| 2018 | 7504 | 33,0 | < 0.0001 | 0,32,937,548 | 0.297–0.366 | < 0.0001 |
| 2019 | 8238 | 31,2 | 0,3,003,618 | 0.271–0.333 | < 0.0001 | |
| AUC ROC | ||||||
| 0.764 (0.761 – 0.768) | ||||||
| CNS failure | ||||||
| 2005 | 1809 | 16,2 | 1 | – | ||
| 2006 | 1676 | 16,2 | 1,003,113,881 | 0.827–1.217 | 0.9749 | |
| 2007 | 2036 | 15,1 | 0,984,914,164 | 0.818–1.186 | 0.8727 | |
| 2008 | 2392 | 12,9 | 0,783,057,153 | 0.651–0.941 | 0.0092 | |
| 2009 | 2648 | 15,9 | 0,983,827,452 | 0.826–1.171 | 0.8547 | |
| 2010 | 2865 | 13,1 | 0,821,556,085 | 0.689–0.980 | 0.0292 | |
| 2011 | 3197 | 12,4 | 0,727,698,472 | 0.611–0.866 | 0.0003 | |
| 2012 | 3860 | 12,3 | 0,676,469,996 | 0.571–0.801 | < 0.0001 | |
| 2013 | 3855 | 12,5 | 0,703,124,481 | 0.594–0.832 | < 0.0001 | |
| 2014 | 4237 | 11,4 | 0,65,610,759 | 0.555–0.776 | < 0.0001 | |
| 2015 | 4704 | 10,4 | 0,55,191,349 | 0.467–0.652 | < 0.0001 | |
| 2016 | 6238 | 12,0 | 0,569,267,239 | 0.486–0.667 | < 0.0001 | |
| 2017 | 7081 | 13,7 | 0,624,865,732 | 0.536–0.729 | < 0.0001 | |
| 2018 | 7542 | 14,2 | < 0.0001 | 0,673,377,407 | 0.578–0.784 | < 0.0001 |
| 2019 | 7237 | 14,7 | 0,64,039,723 | 0.549–0.746 | < 0.0001 | |
| AUC ROC | ||||||
| 0.761 (0.755 – 0.766) | ||||||
| Hematological failure | ||||||
| 2005 | 1089 | 21,7 | 1 | – | ||
| 2006 | 1122 | 22,4 | 1,02,282,062 | 0.808–1.295 | 0.8513 | |
| 2007 | 1218 | 23,0 | 1,03,249,027 | 0.819–1.301 | 0.7866 | |
| 2008 | 1304 | 20,6 | 0,85,500,995 | 0.679–1.077 | 0.1829 | |
| 2009 | 1578 | 21,2 | 0,80,145,653 | 0.643–0.999 | 0.0492 | |
| 2010 | 1705 | 21,7 | 0,81,385,536 | 0.655–1.011 | 0.0625 | |
| 2011 | 1814 | 20,8 | 0,7,338,104 | 0.592–0.910 | 0.0048 | |
| 2012 | 1931 | 20,2 | 0,67,176,921 | 0.542–0.832 | 0.0003 | |
| 2013 | 2141 | 18,4 | 0,56,482,716 | 0.457–0.698 | < 0.0001 | |
| 2014 | 2060 | 17,3 | 0,52,934,679 | 0.426–0.657 | < 0.0001 | |
| 2015 | 2239 | 18,0 | 0,56,450,526 | 0.457–0.697 | < 0.0001 | |
| 2016 | 2822 | 17,7 | 0,4,413,267 | 0.360–0.542 | < 0.0001 | |
| 2017 | 2883 | 20,3 | 0,45,095,202 | 0.368–0.552 | < 0.0001 | |
| 2018 | 3327 | 20,2 | < 0.0001 | 0,529,861 | 0.435–0.646 | < 0.0001 |
| 2019 | 3382 | 19,2 | 0,47,667,204 | 0.391–0.581 | < 0.0001 | |
| AUC ROC | ||||||
| 0.835 (0.829 – 0.840) | ||||||
| Liver failure | ||||||
| 2005 | 80 | 36,2 | 1 | – | ||
| 2006 | 96 | 54,2 | 1,83,497,874 | 0.892–3.776 | 0.0992 | |
| 2007 | 119 | 61,3 | 1,96,503,495 | 0.995–3.882 | 0.0518 | |
| 2008 | 107 | 40,2 | 0,85,993,432 | 0.430–1.721 | 0.6698 | |
| 2009 | 147 | 59,9 | 2,07,901,315 | 1.072–4.032 | 0.0304 | |
| 2010 | 149 | 64,4 | 1,93,607,915 | 0.998–3.754 | 0.0505 | |
| 2011 | 186 | 44,6 | 0,95,603,365 | 0.506–1.805 | 0.8898 | |
| 2012 | 240 | 55,8 | 1,35,968,041 | 0.736–2.513 | 0.3269 | |
| 2013 | 252 | 55,6 | 1,31,101,581 | 0.712–2.415 | 0.3848 | |
| 2014 | 250 | 61,6 | 1,31,593,221 | 0.712–2.434 | 0.3815 | |
| 2015 | 371 | 59,3 | 1,22,325,659 | 0.678–2.208 | 0.5035 | |
| 2016 | 365 | 56,2 | 0,97,385,455 | 0.540–1.756 | 0.9298 | |
| 2017 | 382 | 58,9 | 0,96,491,164 | 0.534–1.744 | 0.9059 | |
| 2018 | 514 | 48,8 | < 0.0001 | 0,72,797,746 | 0.400–1.326 | 0.2992 |
| 2019 | 698 | 49,3 | 0,68,868,416 | 0.383–1.239 | 0.2134 | |
| AUC ROC | ||||||
| 0.801 (0.788 – 0.815) | ||||||
| Kidney failure | ||||||
| 2005 | 4780 | 25,9 | 1 | – | ||
| 2006 | 4937 | 26,1 | 0,940,454,002 | 0.850–1.040 | 0.2332 | |
| 2007 | 5195 | 26,2 | 0,923,138,453 | 0.836–1.020 | 0.1160 | |
| 2008 | 5930 | 25,3 | 0,873,826,895 | 0.793–0.963 | 0.0065 | |
| 2009 | 7090 | 24,2 | 0,830,444,748 | 0.756–0.912 | 0.0001 | |
| 2010 | 8326 | 22,9 | 0,758,868,867 | 0.692–0.832 | < 0.0001 | |
| 2011 | 10,341 | 20,5 | 0,659,592,374 | 0.603–0.721 | < 0.0001 | |
| 2012 | 12,025 | 19,4 | 0,609,565,863 | 0.558–0.665 | < 0.0001 | |
| 2013 | 12,980 | 18,2 | 0,53,201,268 | 0.488–0.580 | < 0.0001 | |
| 2014 | 13,721 | 17,9 | 0,535,202,205 | 0.491–0.584 | < 0.0001 | |
| 2015 | 14,896 | 18,2 | 0,531,784,828 | 0.488–0.579 | < 0.0001 | |
| 2016 | 15,659 | 17,3 | 0,456,185,713 | 0.419–0.497 | < 0.0001 | |
| 2017 | 17,538 | 18,1 | 0,465,317,937 | 0.428–0.506 | < 0.0001 | |
| 2018 | 17,311 | 19,2 | < 0.0001 | 0,511,196,703 | 0.470–0.556 | < 0.0001 |
| 2019 | 17,874 | 18,5 | 0,45,881,633 | 0.422–0.499 | < 0.0001 | |
| AUC ROC | ||||||
| 0.783 (0.780 – 0.786) | ||||||
Data are presented as number of death or %. The multivariate analysis is adjusted by sex, age group, comorbidities, ICU admission, emergency admission, organ dysfunction, number of organ failures, sepsis origin and bacteremia
CI confidence interval, ICU intensive care unit, OR odds ratio, NS non-significant
Discussion
This observational study shows that the association of organ failure with mortality has changed over time depending on the affected organ. To our knowledge, there are no epidemiological studies that have analyzed the evolution of the behavior of mortality associated with the different organ failures in septic patients.
Protocols to increase the detection of sepsis [25], better antimicrobial stewardship [26] and initiate early source control [27] have led to an improvement in the vital prognosis of patients with multiorgan failure. For this reason, an improvement in survival of all organ failures analyzed separately would be expected. Nevertheless, our study shows that this impact is not homogeneous. Although the nature of our study does not allow us to establish causal relationships, we suggest that the differences in the evolution of mortality associated with each organ failure could be related to an improvement in the care of some of them (cardiovascular failure) compared to those without specific treatment (liver failure). However, studies designed for this purpose should be developed to confirm this hypothesis.
In our opinion, the most relevant result in our analysis is the reduction in mortality in the cardiovascular failure group. The continuous reduction observed, from 47.3 to 31.2% of mortality, supposes about a one third relative reduction in mortality. Evolution in management protocols has greatly simplified the initial management of septic shock. Current protocols advocate for a lower positive fluid balance [28, 29] and an early use of norepinephrine [30, 31], which allows an earlier recovery of tissue perfusion [32, 33].
We do not believe that the reduction in mortality in renal failure could be due to an improvement in extrarenal clearance techniques, the lack of consensus on which is the best modality or the moment of initiation of the technique may hinder a greater impact [34]. However, the close relationship between the improvement in tissue perfusion and renal function is well known, which could explain the parallelism between improved cardiovascular and renal failure survival.
There is also a reduction in mortality in respiratory failure, although not so marked. Although non-invasive techniques (high-flow nasal cannulas, non-invasive mechanical ventilation) [35, 36] have failed to significantly impact the general prognosis of patients with sepsis, in some subpopulations they do appear to be useful. The incorporation of recruitment maneuvers (prone position, PEEP,) and the use of extracorporeal techniques can also explain this better prognosis.
Improvement in each organ failure mortality rates results in a reduction in global mortality on septic patients. The general trend observed in our study is also present in other observational studies, both in the epidemiological characteristics of the patients and in the origin and impact of infections [3, 4, 22, 37–40]. Liver failure, however, presents an opposite trend.
Sepsis is not considered in epidemiological studies as a major cause of acute liver failure [41]. However, when it appears, it defines a scenario of high mortality. Liver failure in sepsis does not have specific treatment or organ support measures. The use of extracorporeal techniques in sepsis for liver support is still anecdotic and cannot be considered a standardized technique [42]. Macrophage activation-like syndrome (MALS) in septic patients causes hepatic dysfunction and hematological alterations and, when present, significantly increases mortality in these patients [43]. MALS, which does not respond to standard sepsis treatment, could explain the high mortality of septic patients with liver failure and the lack of prognostic improvement that septic patients with hematological dysfunction have experienced over the years.
Knowing the dimensions of sepsis at the population level is essential for a rational use of economic and health resources. The incidence of sepsis increases year after year, and mortality has been decreasing in parallel. Our data are consistent with other epidemiological studies both in Europe [3, 39, 40] and in other settings [4, 22, 37, 38] and also with clinical data from population studies in our territory [8]. The increase in incidence is attributed to a better control of other pathologies, increase in life expectancy and increase in patient’s age [44] (Fig. 3), though it should be noted that an increase in diagnostic coding in recent years could have contributed to the progressive increase in the incidence of sepsis.
As mentioned above, population-based epidemiological studies such as ours do not allow causality to be established, but the improvement in the results could be attributed to the improvement in the knowledge of the physiopathology of sepsis and the improvement and standardization of treatments, related to the implementation of action plans to improve septic patient care. Since the beginning of 2015, the Interhospital Sepsis Code (CSI) has existed in Catalonia. The CSI is a territorial strategy that involves all the CatSalut acute hospitals and defines an emergency care plan for patients with sepsis; its objective is to speed up detection and coordinate care between hospitals of different complexity at the territorial level throughout the country [45].
Our study has been carried out in the population of Catalonia. Although other studies have shown results similar to ours [3, 4, 22, 37–40], this marks a limitation that must be considered since the results may not be extrapolated to other countries with different socioeconomic levels or dissimilar health systems. Admission to the ICU was deduced by procedure coding and, although in our environment the procedures related to critical or semi-critical patients are performed mostly in intensive care units, this does not happen in 100% of cases. For this reason, the percentage of patients admitted to the ICU could be overestimated as a result of selection bias.
The most relevant limitation of this study is that it is a retrospective epidemiological study based on hospital discharge data. Although we have used a validated methodology for the case definition, there is a risk of bias related to inconsistency in the definition of the processes and in the coding. Despite that the clinical diagnostic criteria for sepsis were modified and updated during the study period [46] (a fact that may limit homogeneity when identifying cases), infection and organ failure definitions remained unchanged, so inclusion criteria are unmodified. On the other hand, codification system was changed in Catalonia in the beginning of 2018 from ICD-9-CM to ICD-10-CM. This change could be the responsible for the slight increase in mortality during 2018 and 2019 compared to previous years although this does not affect the organ failure associated trend.
We would like also comment that we excluded the pandemic period from the analysis. It is unknown how could affect sepsis prognosis the impact of changes in hospital’s structures, the effect of limited human and structural resources to attend critically ill patients or the impact of social restrictions during the pandemic. In our opinion, it requires an specific and very interesting analysis.
Conclusions
The incidence of sepsis has been increasing in recent years in our setting. However, hospital mortality has been significantly reduced. In septic patients, all organ failures except liver have shown a statistically significant reduction on associated mortality, with cardiovascular failure as the most relevant. Early source control and the simplification of algorithms to recover tissue perfusion could explain these results. On the contrary, mortality associated with liver failure in sepsis is very high and has not changed, a fact that could be explained by the lack of specific treatment for the failure of this particular organ.
Supplementary Information
Additional file 1. ICD‑9‑MC codes used to identify infectious process, site of infection, acute organ dysfunction and procedures used in intensive care unit.
Acknowledgements
Not Applicable.
Author contributions
CL-C contributes to conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; supervision; validation; visualization; writing original draft; writing review and editing. JCY contributes to conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing review and editing. EV and MC contributes to formal analysis; software; supervision. JMªS, CF, CR, AR, JCRR, JT contribute to supervision. EE contributes to conceptualization; data curation; investigation; methodology; project administration; resources; supervision; validation; visualization; writing review and editing. All authors read and approved the final manuscript.
Funding
The study is part of a project that has received a grant from the "Fundació Marató TV3", entitled: "Sepsis Training, Analysis and Feedback (STAF) strategy for the implementation of Sepsis Code" (Id Num: 201836_10).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Mataró’s Hospital and Maresme Health Consortium Ethical Review Board with a waiver of informed consent in 21th of March in 2018 (code CEIC_20/18), and all study’s procedures were followed in accordance with the Helsinki Declaration of 1975.
Consent for publication
Not Applicable.
Competing interests
All the signing authors state that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Carolina Lorencio Cárdenas, Email: carol_lorencio@hotmail.com.
Juan Carlos Yébenes, Email: jyebenes@csdm.cat.
Emili Vela, Email: evela@catsalut.cat.
Montserrat Clèries, Email: mcleries@catsalut.cat.
Josep Mª Sirvent, Email: jsirvent.girona.ics@gencat.cat.
Cristina Fuster-Bertolín, Email: cristinafusterbertolin@gmail.com.
Clara Reina, Email: creinaag@csdm.cat.
Alejandro Rodríguez, Email: ahr1161@yahoo.es.
Juan Carlos Ruiz-Rodríguez, Email: jcruiz@vhebron.net.
Josep Trenado, Email: jtrenado@mutuaterrassa.es.
Elisabeth Esteban Torné, Email: elisabeth.esteban@sjd.es.
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fleischmann C, Scherag A, Adhikari NK, et al. Assessment of global incidence and mortality of hospital-treated sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–72. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 3.Yébenes JC, Ruiz-Rodríguez JC, Ferrer R, et al. Epidemiology of sepsis in catalonia: analysis of incidence and outcomes in a European setting. Ann Intensive Care. 2017;7(1):19. doi: 10.1186/s13613-017-0241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee C, Dantes R, Epstein L, et al. Incidence and trends of sepsis in US hospitals using clinical vs claim data, 2009–2014. JAMA. 2017;318(13):1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rudd K, Delaney A, Finfer S. Counting sepsis, an imprecise but improving science. JAMA. 2017;318(13):1228–1229. doi: 10.1001/jama.2017.13697. [DOI] [PubMed] [Google Scholar]
- 6.Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312(1):90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 7.Hall MJ, Levant S, DeFrances CJ. Trends inpatient hospital deaths: national hospital discharge survey, 2000–2010. NCHS Data Brief. 2013;118:1–8. [PubMed] [Google Scholar]
- 8.Almirall J, Güell E, Capdevila JA, et al. Epidemiology of community-acquired severe sepsis. Population Based Study Med Clin (Barc) 2016;147(4):139–143. doi: 10.1016/j.medcli.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Ferrer R, Martínez ML, Gomà G, et al. ABISS-edusepsis Study group Improved empirical antibiotic treatment of sepsis after an educational intervention: the ABISS-edusepsis study. Crit Care. 2018;22(1):167. doi: 10.1186/s13054-018-2091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esteban E, Belda S, García-Soler P, et al. A multifaceted educational intervention shortened time to antibiotic administration in children with severe sepsis and septic shock: ABISS edusepsis pediatric study. Intensive Care Med. 2017;43(12):1916–1918. doi: 10.1007/s00134-017-4721-4. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34(6):1589–1596. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 12.Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345(19):1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 13.Yealy DM, Kellum JA, Huang DT, et al. N Engl J Med. 2014;370:1683–1693. doi: 10.1056/NEJMoa1401602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peake P, et al. Goal-directed resuscitation for patients with early septic shock. New Engl J Med. 2014;371(16):1496–1506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 15.Mouncey PR, Osborn TM, Power GS, et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015;372(14):1301–1311. doi: 10.1056/NEJMoa1500896. [DOI] [PubMed] [Google Scholar]
- 16.Guérin C, Beuret P, Constantin JM, et al. A prospective international observational prevalence study on prone positioning of ARDS patients: the APRONET (ARDS Prone Position Network) study. Intensive Care Med. 2018;44(1):22–37. doi: 10.1007/s00134-017-4996-5. [DOI] [PubMed] [Google Scholar]
- 17.Needham DM, Colantuoni E, Mendez-Tellez PA, et al. Lung protective mechanical ventilation and two year survival in patients with acute lung injury: prospective cohort study. BMJ. 2012;5(344):e2124. doi: 10.1136/bmj.e2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amato MB, Barbas CS, Medeiros DM, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 19.Bréchot N, Luyt CE, Schmidt M, et al. Venoarterial extracorporeal membrane oxygenation support for refractory cardiovascular dysfunction during severe bacterial septic shock. Crit Care Med. 2013;41(7):1616–1626. doi: 10.1097/CCM.0b013e31828a2370. [DOI] [PubMed] [Google Scholar]
- 20.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 21.Suarez D, Ferrer R, Artigas A, et al. Cost-effective-ness of the surviving sepsis campaign protocol for severe sepsis: a pro-spective nation-wide study in Spain. Intensive Care Med. 2011;37:444–452. doi: 10.1007/s00134-010-2102-3. [DOI] [PubMed] [Google Scholar]
- 22.Angus DC, Linde-Swirble T, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 24.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 25.Schorr C, Odden A, Evans L, et al. Implementation of a multi-center performance improvement program for early detection and treatment of severe sepsis in general medical–surgical wards. J Hosp Med. 2016;11(S1):S32–S39. doi: 10.1002/jhm.2656. [DOI] [PubMed] [Google Scholar]
- 26.Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235–2244. doi: 10.1056/NEJMoa1703058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez ML, Ferrer R, Torrents E, et al. Impact of source control in patients with severe sepsis and septic shock*. Crit Care Med. 2017;45(1):11–19. doi: 10.1097/CCM.0000000000002011. [DOI] [PubMed] [Google Scholar]
- 28.Marik PE, Linde-Zwirble WT, Bittner EA, et al. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med. 2017;43(5):625–632. doi: 10.1007/s00134-016-4675-y. [DOI] [PubMed] [Google Scholar]
- 29.Sirvent JM, Ferri C, Baró A, Murcia C, Lorencio C. Fluid balance in sepsis and septic shock as a determining factor of mortality. Am J Emerg Med. 2015;33(2):186–189. doi: 10.1016/j.ajem.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 30.Beck V, Chateau D, Bryson GL, et al. Timing of vasopressor initiation and mortality in septic shock: a cohort study. Crit Care. 2014;18(3):R97. doi: 10.1186/cc13868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Y, Li H, Zhang D. Timing of norepinephrine initiation in patients with septic shock: a systematic review and meta-analysis. Crit Care. 2020;24(1):488. doi: 10.1186/s13054-020-03204-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coopersmith CM, De Backer D, Deutschman C, et al. Surviving sepsis campaign: research priorities for sepsis and septic shock. Intensive Care Med. 2018;44(9):1400–1426. doi: 10.1007/s00134-018-5175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Evans L, Rhodes A, Alhazzan W, et al. Surviving Sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Crit Care Med. 2021;49(11):e1063–e1143. doi: 10.1097/CCM.0000000000005337. [DOI] [PubMed] [Google Scholar]
- 34.Li P, Qu LP, Shen B, et al. High-dose versus low-dose haemofiltration for the treatment of critically ill patients with acute kidney injury: an updated systematic review and meta-analysis. BMJ Open. 2017;7(10):e014171. doi: 10.1136/bmjopen-2016-014171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.David-João PG, Guedes MH, Réa-Neto Á, et al. Noninvasive ventilation in acute hypoxemic respiratory failure: a systematic review and meta-analysis. J Crit Care. 2019;49:84–91. doi: 10.1016/j.jcrc.2018.10.012. [DOI] [PubMed] [Google Scholar]
- 36.Schnell D, Timsit JF, Darmon M, et al. Noninvasive mechanical ventilation in acute respiratory failure: trends in use and outcomes. Intensive Care Med. 2014;40(4):582–591. doi: 10.1007/s00134-014-3222-y. [DOI] [PubMed] [Google Scholar]
- 37.Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality related to severe sepsis and septic shock among critically ill patients in Australia and New Zealand, 2000–2012. JAMA. 2014;311(13):1308–16. doi: 10.1001/jama.2014.2637. [DOI] [PubMed] [Google Scholar]
- 38.Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the United States: an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018;46(12):1889. doi: 10.1097/CCM.0000000000003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adrie C, Alberti C, Chaix-Couturier C, et al. Epidemiology and economic evaluation of severe sepsis in France: age, severity, infection site, and place of acquision (community, hospital, or intensive care unit) as determinants of workload and cost. J Crit Care. 2005;20(1):46–58. doi: 10.1016/j.jcrc.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 40.Iñigo J, Sendra JM, Díaz R, et al. Epidemiology and costs of severe sepsis in Madrid. A hospital discharge study. Med Intensiva. 2006;30(5):197–203. doi: 10.1016/S0210-5691(06)74507-7. [DOI] [PubMed] [Google Scholar]
- 41.Ostapowicz G, Fontana RJ, Schiødt FV, et al. Acute liver failure study group. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137(12):947–54. doi: 10.7326/0003-4819-137-12-200212170-00007. [DOI] [PubMed] [Google Scholar]
- 42.Lee KC, Baker LA, Stanzani G, Alibhai H, Chang YM, Palacios CJ, Leckie PJ, Giordano P, Priestnall SL, Antoine DJ, Jenkins RE. Extracorporeal liver assist device to exchange albumin and remove endotoxin in acute liver failure: Results of a pivotal pre-clinical study. J Hepatol. 2015;63(3):634–42. doi: 10.1016/j.jhep.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karakike E, Giamarellos-Bourboulis EJ. Macrophage activation-like syndrome: a distinct entity leading to early death in Sepsis. Front Immunol. 2019;31(10):55. doi: 10.3389/fimmu.2019.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Padro T, Smotherman C, Gautam S, et al. Admission characteristics predictive of in-hospital death from hospital-acquired sepsis: a comparison to community-acquired sepsis. J Crit Care. 2019;51:145–148. doi: 10.1016/j.jcrc.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yébenes JC, Lorencio C, Esteban E, et al. Interhospital Sepsis Code in Catalonia (Spain): Territorial model for initial care of patients with sepsis. Med Intensiva. 2020;44(1):36–45. doi: 10.1016/j.medin.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315(8):762. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. ICD‑9‑MC codes used to identify infectious process, site of infection, acute organ dysfunction and procedures used in intensive care unit.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.