Abstract
Background
The beneficial role of gut microbiota and bacterial metabolites, including short-chain fatty acids (SCFAs), is well recognized, although the available literature around their role in colorectal cancer (CRC) has been inconsistent.
Methods
We performed a systematic review and meta-analysis to examine the associations of fecal SCFA concentrations to the incidence and risk of CRC. Data extraction through Medline, Embase, and Web of Science was carried out from database conception to June 29, 2022. Predefined inclusion/exclusion criteria led to the selection of 17 case-control and six cross-sectional studies for quality assessment and analyses. Studies were categorized for CRC risk or incidence, and RevMan 5.4 was used to perform the meta-analyses. Standardized mean differences (SMD) with 95% confidence intervals (CI) were calculated using a random-effects model. Studies lacking quantitation were included in qualitative analyses.
Results
Combined analysis of acetic, propionic, and butyric acid revealed significantly lower concentrations of these SCFAs in individuals with a high-risk of CRC (SMD = 2.02, 95% CI 0.31 to 3.74, P = 0.02). Additionally, CRC incidence was higher in individuals with lower levels of SCFAs (SMD = 0.45, 95% CI 0.19 to 0.72, P = 0.0009), compared to healthy individuals. Qualitative analyses identified 70.4% of studies reporting significantly lower concentrations of fecal acetic, propionic, butyric acid, or total SCFAs in those at higher risk of CRC, while 66.7% reported significantly lower concentrations of fecal acetic and butyric acid in CRC patients compared to healthy controls.
Conclusions
Overall, lower fecal concentrations of the three major SCFAs are associated with higher risk of CRC and incidence of CRC.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02529-4.
Keywords: Colorectal cancer, Adenoma, Short-chain fatty acid, Incidence, Risk, Meta-analysis
Background
According to the Global Cancer Incidence, Mortality and Prevalence (GLOBOCAN) 2020 report, colorectal cancer (CRC) is the third most commonly diagnosed cancer (10% of all diagnosed cancers) and the second (9.4%) leading cause of cancer-related death [1]. It has been estimated that the overall risk of CRC in all age groups will increase 60% worldwide by 2030, leading to more than 1.1 million deaths and 2.2 million new cases [2]. Colorectal cancer develops from precursor lesions collectively known as colorectal adenomas (CRA), in the form of adenomatous polyps or to a lesser extent (10–20%) serrated polyps [3, 4]. It is a heterogeneous disease and environmental factors have a potential impact on the development of CRC, among which diet is a risk factor [4–6]. According to several meta-analyses, high consumption of processed and unprocessed meat is related to high CRC risk [7, 8], and high fiber intake is suggested as a protective factor against CRC progression and incidence [9–11].
The effect of diet on colonic health is partly mediated through alteration of gut microbiota composition, diversity, and metabolism [6, 12]. Gut microbiota constitutes the largest community of commensal microorganisms in the body, which mainly resides in the lower small intestine and colon [6, 12, 13]. The gut microbiota-derived metabolites are in constant crosstalk with colonocytes, and short-chain fatty acids (SCFAs) make up a large group of these metabolites [6, 12, 13].
Short-chain fatty acids are small molecules generated via the fermentation of dietary fibers by gut microbiota. Acetic, propionic, and butyric acid constitutes the majority of colonic SCFA content [14, 15] and the beneficial anti-inflammatory and anti-carcinogenic effects of dietary fibers on colonocytes are mediated through these SCFA molecules [16, 17]. Among the three major SCFA molecules, butyric acid is also considered as one of the main energy sources for colonocytes [12, 14, 17]. Therefore, alteration in SCFA levels could impact the colonic health and predisposition of colonocytes to aberrant proliferation and tumor formation [15, 16].
Several studies have assessed fecal SCFA concentration in patients with colorectal carcinoma or adenoma [18–34]. However, due to variable results, the conclusive evaluation of SCFA profiles from CRC patients versus healthy subjects is lacking. In addition, other studies have compared SCFA concentration within healthy individuals from various countries and ethnic groups with the highest and lowest prevalence of CRC; although with inconsistent results [35–40].
Therefore, systematic analyses designed to better understand the link between SCFA concentration in CRC risk and incidence is highly desired. We divided our analyses on the available evidence into two broad categories: (1) CRC-risk and (2) incidence. We aimed to systematically analyze the results of all primary observational human studies, which measured fecal SCFA levels in “at-risk” individuals or in CRC patients. In the CRC risk category, the focus was on at-risk individuals, which was further sub-divided into two groups based on (1a) studies that analyzed clinical data (presence of colorectal adenomas) or (1b) those that assigned CRC risk based on non-clinical evaluation of study participants (ethnic background or location). The CRC incidence category included studies that compared fecal SCFA levels in individuals with clinically diagnosed CRC and healthy individuals. Our results underline the potential association of the three major SCFA molecules (acetic, propionic, and butyric acid) with CRC risk and incidence.
Methods
We used Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guideline [41, 42] to systematically search and extract data from primary human studies with SCFA measurement in CRC risk or incidence.
Database search
The Medline, Embase, and Web of Science database search was performed for articles involving human subjects that are in English from database conception until June 29, 2022. The details of the search keywords and strategies utilized in Ovid and Web of Science are available in the Additional file 1: Supplementary methods.
Eligibility criteria
All the records, including abstracts, were imported to EndNote X9 (Clarivate Analytics, Toronto, Canada). Duplicate records were first removed. The records were then filtered using EndNote’s built-in search tool for the following criteria: (i) searching for concentration*, level*, quanti*, measure*, assess*, evaluat*, estimat*, calculat*, mmol, and μmol as the inclusion criteria to capture studies which reported the SCFA measurement based on these terms, and (ii) searching for mouse, mice, murine, rats, conference, ethyl acetate (EtOAc), and phorbol as the exclusion criteria to exclude rodent studies, conference proceedings, and studies that have stated the use of any unrelated chemicals (such as EtOAc and 12-O-Tetradecanoylphorbol-13-acetate) - the asterisk symbol (*) applied was to include all the variations of the search terms. The abstracts of the remaining records were then screened to exclude reviews, methodology, human studies not related to SCFAs in CRC or CRA, and non-human studies (i.e., in vitro or other non-rodent animal studies), to identify the human studies on SCFA measurement in CRC or CRA. The full text of the remaining (n = 57) records were then screened to include only the observational studies which have measured fecal SCFA concentration. A final set of 23 observational studies qualified for further data extraction, quality assessment, and statistical analyses.
Data extraction and quality assessments
The data and additional details available for analysis (such as study subjects and SCFA levels) from the finalized primary studies were extracted and added to an Excel worksheet. The Newcastle-Ottawa Scale (NOS) [43] was used as a standard tool for quality assessment of 17 case-control studies in the selection, comparability, and exposure categories, to provide a score range between 0 and 9 (≤ 6, 7–8, and 9 indicate high, medium, and low risk of bias, respectively) [42]. Evaluation of six cross-sectional studies was performed using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist tool [44], as recommended [45].
Statistical analyses
Review Manager (RevMan) software version 5.4 (Cochrane, Copenhagen, Denmark) was used to analyze the quantitative fecal SCFA concentration data, which were available in 10 of the final 23 observational studies (9 of 17 case-control, plus 1 of 6 cross-sectional studies). The fecal concentration of acetic, propionic, or butyric acid was considered as the subgroups. Before data entry, SEM or 95% CI upper and lower bound values were converted to SD. Due to variation in the reported SCFA concentration units between different papers, standardized mean difference (SMD) was selected as a measure of effect size for each study. The statistical heterogeneity among studies was calculated using χ2 and I2 tests and a P-value of 0.05 was considered significant [46]. To normalize the use of different SCFA measurement methods, a random-effects model was applied to analyze the pooled effect size and P-value for each SCFA molecule in each subgroup. One overall effect size and P-value of combined acetic, propionic, and butyric acid were also calculated. In all analyses, the effect size was reported with 95% confidence intervals, and the P-value < 0.05 was considered significant. Furthermore, the fixed-effect model was also applied in the case of non-significant heterogeneity of I2 < 50 [46]. All the data conversions, as well as qualitative and quantitative analyses, were validated by the second team member and confirmed by the senior authors.
One study [34] reported values that could not be converted to mean and SD for the meta-analysis and was therefore only included in our qualitative analysis. Another study [35] reported the numeric values of butyric acid concentration and other SCFA molecules in graphs and hence was included in both quantitative (for butyric acid) and qualitative data (for acetic acid, propionic acid and total SCFA). Therefore, in addition to studies in which the fecal SCFA concentration was presented using graphs (with no reported actual values), 14 of 23 studies were considered as qualitative studies (8 of 17 case-control, plus all 6 cross-sectional studies—including Ocvirk et al. 2020). The outcome of analyses from these qualitative studies was plotted as stacked bar charts, using Microsoft Excel (ver. 2016; Microsoft Corporation, Redmond, WA, USA).
Results
Study selection and quality assessment
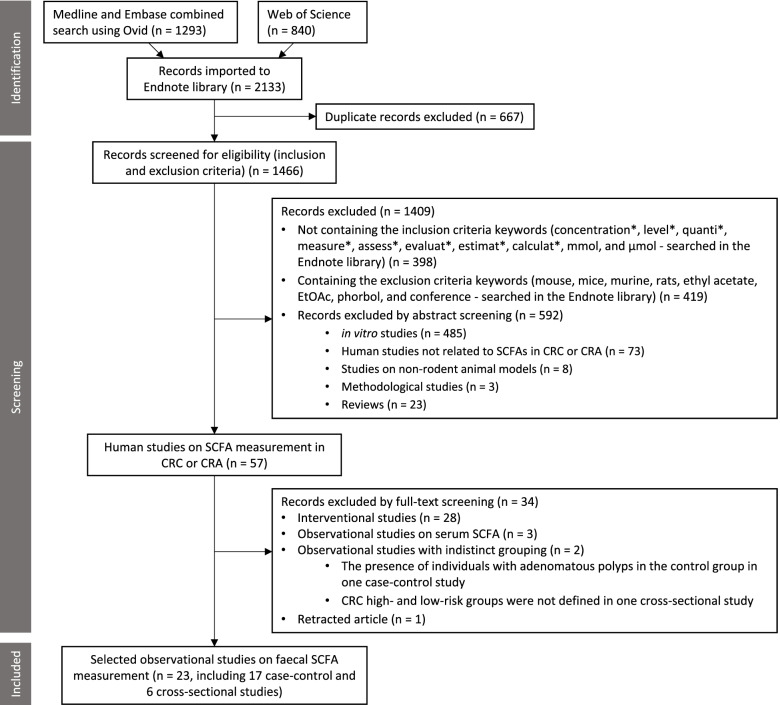
The workflow on the identification and stepwise selection of the observational studies is presented in Fig. 1. Initially, a total of 2133 English language records obtained from searching through the three databases (Medline, Embase, and Web of Science) were imported to EndNote along with their abstracts. After removing duplicate records, the titles and abstracts of the remaining 1466 records were filtered and screened for eligibility as detailed in the Methods section. In total, 1409 records were excluded, of which most were in vitro studies. From the remaining 57 human studies, 34 studies were excluded. Of these 28 were interventional studies, three were observational studies on serum SCFA [47–49], two studies had indistinct grouping (one case-control study with the presence of individuals with adenomatous polyps in the healthy control group [50] and one cross-sectional study with no clear definition of CRC high- and low-risk group [51]), and one retracted observational study [52].
Fig. 1.
The PRISMA flowchart shows the selection process of the systematic review. The abstracts of all the studies were imported into Endnote from the indicated databases. SCFA, short-chain fatty acid; CRC, colorectal cancer; CRA, colorectal adenoma
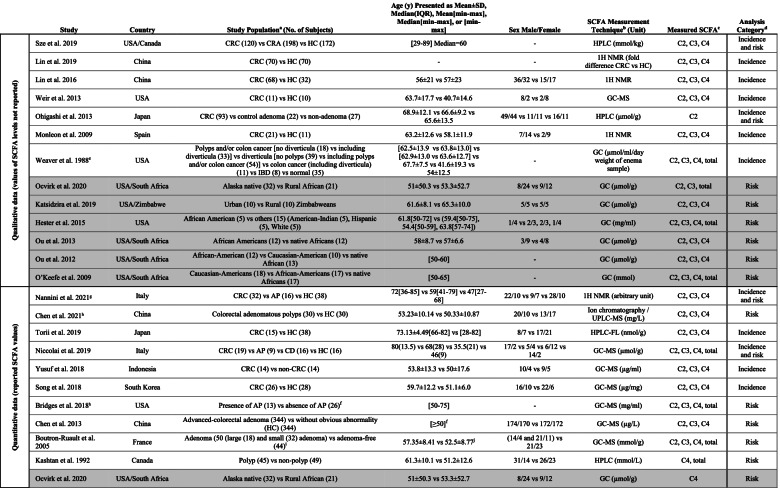
Finally, 17 case-control and 6 cross-sectional studies were selected for data extraction and analysis. Table 1 summarizes the characteristics of these observational studies. The results of quality assessment using NOS and JBI tools on case-control and cross-sectional studies are provided in Additional file 1: Tables S1 and S2, respectively.
Table 1.
Characteristics of the selected studies. Cross-sectional studies are highlighted in gray, and case-control studies are not highlighted
aCRC colorectal cancer, AP adenomatous polyposis, CD celiac disease, CRA colorectal adenoma, HC healthy controls, IBD inflammatory bowel disease. bGC-MS gas chromatography-mass spectrometry, HPLC high-performance liquid chromatography, FL fluorescence, 1H NMR 1H nuclear magnetic resonance spectroscopy, UPLC-MS ultra-performance liquid chromatography-tandem mass spectrometry, GLC gas-liquid chromatography. cC2 acetic acid, C3 propionic acid, C4 butyric acid. dRefer to the text for the definition of CRC risk and incidence category. eValues in this paper were measured on enema samples, not feces. Therefore, they used in qualitative analysis. fMore details are provided in the article. gRemoved from quantitative analysis as the reported SCFA values could not be converted to mean and SD. hRemoved from meta-analysis due to insufficient data on SCFA measurement method. iSCFAs were measured in only a subset of these subjects (n = 25 large/small adenoma and n = 23 adenoma-free). jCombined values of males and females
Stratifications based on CRC risk or incidence
Studies listed in Table 1 are presented based on the type of data provided (qualitative or quantitative) and CRC risk and/or incidence. Among the 17 case-control studies (not highlighted in Table 1), 8 studies comparing CRC cases and healthy control subjects were allocated to the CRC incidence category, 5 studies comparing individuals with CRA and healthy controls assigned to the CRC risk category, and the remaining 4 studies were included in both incidence and risk categories since they compared CRC patients, CRA individuals, and healthy subjects. All 6 cross-sectional studies (highlighted gray in Table 1) comparing populations with high- versus low-risk of CRC were allocated to the risk category. Therefore, the CRC incidence and risk category included 12 and 15 studies, respectively (Table 1). For each study, the details of the measured SCFA and CRC risk and/or incidence grouping are provided in Additional file 1: Table S3. Some studies reported total SCFA concentration in addition to the individual (acetate, propionate, and butyrate) SCFAs.
The primary studies analyzed in this systematic review were performed in various countries and ethnic groups. Age was matched in some of the studies [18, 24, 28, 38–40], although the male-to-female ratio was not similar between the study groups in most studies (Table 1). The SCFA concentrations were measured using different techniques, such as gas chromatography, liquid chromatography, gas-liquid chromatography and 1H nuclear magnetic resonance spectroscopy.
Data analyses
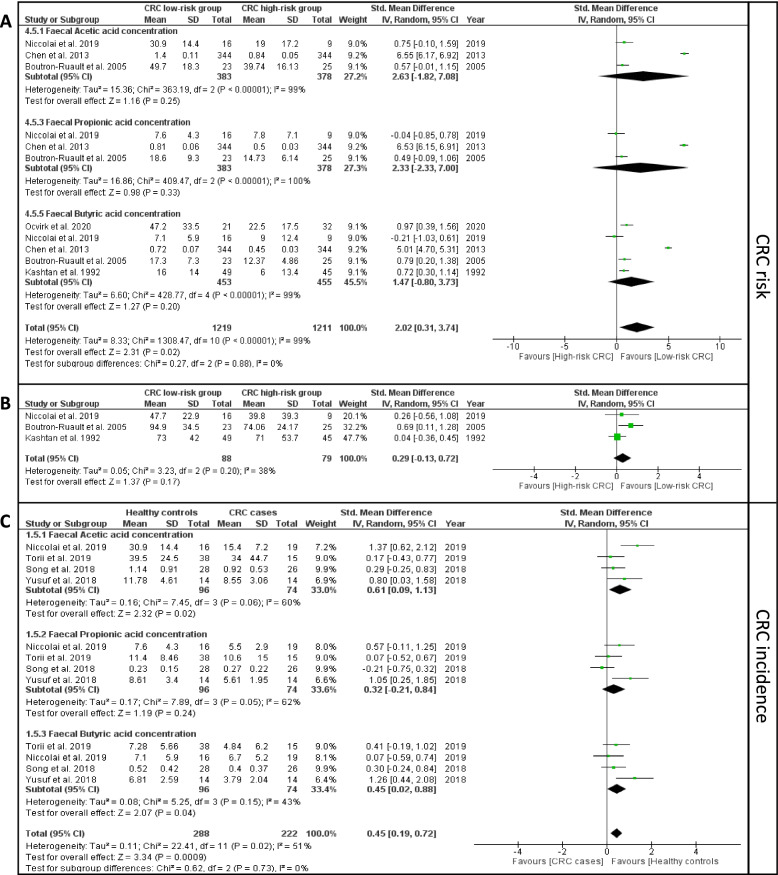
The meta-analysis of the quantitative data extracted from the 10 selected studies [18–22, 26, 28, 30, 33, 35] are presented in Fig. 2. In the risk category (Fig. 2A. B), two studies [19, 20] were excluded from the meta-analysis due to the lack of sufficient details of the methods used for SCFA measurement from stool samples. In CRC risk meta-analysis, the effect size of each of the three SCFAs was not statistically significant; however, their combined effect size was significantly higher in low risk compared to high-risk CRC (SMD = 2.02, 95% CI 0.31 to 3.74, P = 0.02, Fig. 2A). The effect size of total SCFA concentration was not statistically significant in the low- vs high-risk group (Fig. 2B).
Fig. 2.
Forest plots representing the meta-analyses of the fecal concentrations of A acetic, propionic, and butyric acid in CRC risk category; B total SCFA in CRC risk category; and C acetic, propionic, and butyric acid in CRC incidence category. Note that in B, the total SCFA indicates the collection of all the SCFA molecules—not only acetic, propionic, and butyric acid
In the CRC incidence analysis (Fig. 2C), the fecal concentrations of acetic acid (SMD = 0.61, 95% CI 0.09 to 1.13, P = 0.02) and butyric acid (SMD = 0.45, 95% CI 0.02 to 0.88, P = 0.04) were significantly higher in the healthy control compared to CRC cases. In addition, the combined effect size of acetic, propionic, and butyric acid remained significant between CRC cases and healthy controls (SMD = 0.45, 95% CI 0.19 to 0.72, P = 0.0009, Fig. 2C).
Furthermore, the I2 heterogeneity index was in the “moderate” range (30 to 60%) [46] for the meta-analysis of total SCFA concentration in CRC risk (Fig. 2B) and butyric acid in CRC incidence (Fig. 2C) category. Therefore, we performed another meta-analysis using the fixed-effect model on the same data instead of the random-effect model presented in Table 2. This resulted in a more pronounced difference in butyric acid concentration between CRC cases and healthy controls (SMD = 0.42, 95% CI 0.1 to 0.74, P = 0.009). The results of the fixed-effect model meta-analyses are presented in Additional file 1: Fig. S1 and S2, respectively, and the findings of all quantitative meta-analyses are summarized in Table 2.
Table 2.
Summary of the outcomes of each meta-analysis. Significant P values of the effect size are in bold
| Measured SCFA | Number of Studies | Heterogeneity (I2 %, P value) |
Statistical Model | Effect size (SMD [95% CI], P value) | |
|---|---|---|---|---|---|
| CRC risk | Acetic acid | 3 | 99, < 0.00001 | Random effect | 2.63 [− 1.82 to 7.08], 0.25 |
| Propionic acid | 3 | 99, < 0.00001 | Random effect | 2.33 [− 2.33 to 7.00], 0.33 | |
| Butyric acid | 5 | 99, < 0.00001 | Random effect | 1.47 [− 0.80 to 3.73], 0.2 | |
| Combineda | 11 | 99, < 0.00001 | Random effect | 2.02 [0.31 to 3.74], 0.02 | |
| Total SCFA | 3 | 38, 0.2 | Random effect | 0.29 [− 0.13 to 0.72], 0.17 | |
| Total SCFA | 3 | 38, 0.2 | Fixed effect | 0.25 [− 0.05 to 0.56], 0.11 | |
| CRC incidence | Acetic acid | 4 | 60, 0.06 | Random effect | 0.61 [0.09 to 1.13], 0.02 |
| Propionic acid | 4 | 62, 0.05 | Random effect | 0.32 [− 0.21 to 0.84], 0.24 | |
| Butyric acid | 4 | 43, 0.15 | Random effect | 0.45 [0.02 to 0.88], 0.04 | |
| Combineda | 12 | 51, 0.02 | Random effect | 0.45 [0.19 to 0.72], 0.0009 | |
| Butyric acid | 4 | 43, 0.15 | Fixed effect | 0.42 [0.1 to 0.74], 0.009 |
aCombined effect size of acetic, propionic, and butyric acid. Note that the total SCFA indicates the collection of all the SCFA molecules—not only acetic, propionic, and butyric acid
Qualitative analysis was carried out on the studies which reported lower, higher or no changes to the concentration of SCFAs between high-risk CRC (for risk category) or CRC case (for incidence) and low-risk or control, respectively [23–25, 27, 29, 31, 32, 34–40] (Additional file 1: Fig. S3). In the risk category, more studies (70.4%) reported significantly lower concentrations of fecal acetic, propionic, and butyric acid as well as total SCFA in individuals at high risk of CRC. In the incidence category, more studies (66.7%) reported significantly lower concentrations of fecal acetic and butyric acid in CRC patients compared to healthy controls. However, the number of studies reporting no significant difference in the propionic acid was the highest in the incidence category. Overall, our qualitative analysis (Additional file 1: Fig. S3) corroborates with the meta-analysis results (Fig. 2).
Discussion
For more than three decades, in vitro, animal, and human studies have identified numerous potentially beneficial anti-inflammatory and anti-carcinogenic roles of SCFA molecules in gut health and colonic diseases [6, 14–17, 53]. In addition, several meta-analyses (Additional file 1: Table S4) have assessed the role of colonic microbiota [54], non-digestible carbohydrates [55] and dietary fiber in colorectal carcinoma [11, 56] or adenoma [9, 10] as well as the alteration of SCFAs in irritable bowel syndrome (IBS) [57], or inflammatory bowel disease (IBD) [58].
This systematic review and meta-analysis were conducted on 23 studies to better determine the potential association between fecal SCFA concentration and CRC risk and incidence. The combined mean difference of acetic, propionic, and butyric acid in the CRC risk category analysis revealed a significantly lower concentration of these SCFAs in individuals at risk of developing CRC compared to healthy subjects, indicating a potential association between these three major SCFA molecules and CRC development. This finding was further confirmed in the CRC incidence category analysis where the fecal SCFA levels in CRC patients were significantly lower compared to those in healthy subjects.
Our findings in CRC risk and incidence were consistent with the observations reported in other meta-analyses, which focused on the association between dietary fiber intake and the risk of colorectal adenoma [9, 10], and carcinoma [11]. These systematic reviews suggested a protective effect of dietary fiber intake against CRA and CRC [9–11]. Since SCFAs are produced by gut-microbiota via the fermentation of dietary fibers [14–17], our meta-analysis of SCFA concentrations in CRC further confirms earlier observations and underlines the importance of dietary fibers/SCFAs in the risk and progression of CRC.
Another meta-analysis, which assessed the effect of non-digestible carbohydrate [resistance starch (RS)] or inulin supplementation on the risk of colorectal neoplasia, did not find significant increase in fecal total SCFA or butyric acid concentration and excretion before and after the intervention [55]. Many studies which investigated the effect of RS on healthy subjects or individuals with sporadic CRC or adenoma had a period of ≤ 4-week of intervention. A few studies reported 7- and 8-week intervention on adenoma or healthy individuals and the remaining studies were conducted on individuals with inherited CRC syndromes after > 2-year intervention [55]. The duration of intervention was longest (> 2 years) for studies involving hereditary CRC cases with reported germ-line mutations, which may have outweighed the effect of RS supplementation, while interventions involving sporadic cases or healthy subjects had much shorter periods of RS intervention (< 8 weeks) [55]. In our meta-analysis, we also did not observe a significant difference in total fecal SCFAs in the CRC risk category. This could be due to other SCFA molecules such as valeric, iso-butyric, and iso-valeric acid being included in total SCFA measurements; the latter two are the branched SCFAs mainly produced via fermentation of branched amino acids in the colon and not from non-digestible carbohydrates [59, 60].
Another systematic review on the food-microorganism-SCFA axis, without any meta-analysis, concluded that most evidence demonstrated higher SCFA levels in individuals at risk of CRC compared to healthy individuals [61], which contrasts with findings in our systematic review which showed lower fecal SCFA concentration in at-risk individuals (Fig. 2). In comparison to our systematic review, their search strategy restricted their analysis to only 8 of the final 23 studies that we analyzed [19, 27, 31, 32, 37–40]. Therefore, their conclusion was based on a smaller subset of the primary studies available and was also not supported by a meta-analysis.
Both the quantitative and qualitative analyses of CRC risk identified comparable findings of significantly lower concentration of acetic and butyric acid in the high- versus low-risk CRC group. For the CRC incidence category, the quantitative meta-analysis of butyric acid was consistent with observations identified in most of the articles from the qualitative analysis, supporting the evidence of lower concentration of three SCFAs in CRC cases compared to healthy controls. The meta-analysis of propionic acid was not significantly different between cases and controls. Similarly, most of the studies (5 of 7) reported no significant difference in fecal propionic acid concentration between CRC and healthy control in the qualitative analysis. The meta-analysis on IBS revealed a significantly higher concentration of fecal propionic acid in these patients in comparison to healthy controls [57]. Therefore, further studies comparing SCFA profiles among multiple gut diseases could shed more light on the importance of these molecules in the development of varied medical conditions.
To our knowledge, this systematic review is the first to provide a comprehensive search and data collection on observational studies linking SCFA molecules with the CRC risk and incidence. A limitation of our analysis is the heterogeneity of the studies evaluated in this systematic review, which is very difficult to control for. One such factor was the age group assessed for CRC incidence and risk. The mean age of the group in the studies was greater than 50 years and fecal SCFA concentration was not measured in younger populations to provide a comparison with low-risk, young age individuals. Although CRC is most often diagnosed in individuals > 50 years, the incidence for early-onset CRC (EOCRC) in adults aged 20–49 years has increased over the past decade in the USA, Australia, and Europe [62–65]. It would be of interest in the future to study different age group populations for CRC risk and incidence. Family history [66], diet, and lifestyle [67] are known factors contributing to CRC incidence. Only a few studies assessed in this systematic review provided information on the dietary difference between groups [19, 21, 35, 36, 40]. There was also no information about the type of polyps (conventional vs serrated) in individuals with CRA.
One common limitation is related to the nature of observational studies. Case-control studies are inherently prone to recall bias and appropriate matching of case and control groups [68]. Similarly, the results of the cross-sectional studies could be affected by bidirectional relationship [69] and confounding factors. We have assessed the effect of these inherent limitations on our analysis by undertaking appropriate quality checks such as the “comparability” category of NOS quality assessment (Additional file 1: Table S1) and the JBI tool (Additional file 1: Table S2) for all the studies included in our systematic review and meta-analysis.
Another limitation is the diversity in sample handling/storage workflows and the methodologies used to measure fecal SCFA across the studies (Table 1). We did not have enough studies to perform separate meta-analyses to understand the effect of each of these variables on the SCFA concentrations. While these factors could influence our interpretation, the levels of SCFAs were lower in high-risk as well as incident CRC cases, irrespective of the method used to measure SCFAs. Since these are well-known and established techniques for measuring SCFA concentrations, it appears that the standardized sample handling workflows and analytical methods had little impact on differences across study groups, so long as optimized procedures for SCFA assessment were followed. This systematic review did not include non-English records. To our knowledge, no longitudinal studies have reported fecal SCFA measurements at different time points during CRC progression, nonetheless, the 23 studies assessed in this systematic review and meta-analysis provide a comparison between CRC risk/incidence and respective controls from various countries and ethnic groups.
In addition to the SCFAs assessed in this systematic review, other metabolites such as bile acids were also measured in six of the selected studies [20, 30, 32, 35, 36, 39]. Among the bile acids investigated, a significantly higher fecal concentration of deoxycholic acid in the CRC high- versus low-risk group was reported in three studies [35, 36, 39]. Dietary fiber and fat promote the production of SCFA and bile acid molecules in the gut, respectively, and the latter is associated with gastrointestinal carcinogenesis [70–72]. Measurement of fecal SCFAs and other gut metabolites (such as bile acids) in longitudinal studies comparing individuals with colorectal adenoma/risk and healthy subjects could strengthen their association with CRC progression.
This study supports further exploration into fecal concentration of SCFAs: acetic, propionic, and butyric acids, as biomarkers for CRC risk. Among the current CRC screening methods, colonoscopy is the gold standard [73]; however, being invasive, it presents some procedural risk [74]. The guaiac fecal occult blood test (gFOBT) and fecal immunochemical test (FIT) are other, in practice, non-invasive stool-based methods for CRC screening, which however require improvement, in particular for detection of CRA or early-stage colonic carcinogenesis [74–76]. Fecal SCFA could be considered as a potential non-invasive biomarker to be measured in combination with or as an alternative to the commonly used non-invasive and current CRC screening methods [74, 77], to improve specificity and sensitivity of current screening, as well as for potential early detection of CRA.
Conclusions
Gut microbiota dysbiosis and changes in their metabolites have been the focus of epidemiological studies aimed at uncovering associations with colonic inflammation and carcinogenesis. In line with the protective role of fecal SCFAs against the development of gut diseases [15, 16], and the protective effect of dietary fibers against CRC risk and/or incidence [9–11], we determined that the combined fecal concentration of the three major SCFA molecules was significantly lower not only in CRC patients compared to healthy controls, but also in high-risk CRC individuals. Gut SCFA concentrations are inversely associated with CRC-risk as well as CRC-incidence and could be biomarkers for predicting CRC-progression, as well as a drug target (in future intervention studies) aimed to retard or prevent CRC progression.
Registration
The study is registered in PROSPERO database (registration code: CRD42021256123).
Supplementary Information
Acknowledgements
The authors acknowledge Dr. Ho Pham for his assistance and the infrastructure support through the School of Medicine, Western Sydney University. The authors also acknowledge Mrs. Ameneh Najdi for cross-checking the data entry in the analyses.
Abbreviations
- CRC
Colorectal cancer
- CRA
Colorectal adenoma
- SCFA
Short-chain fatty acid
- GLOBOCAN
Global Cancer Incidence, Mortality and Prevalence
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- NOS
Newcastle-Ottawa Scale
- JBI
Joanna Briggs Institute
- RevMan
Review Manager
- SMD
Standardized mean difference
- RS
Resistance starch
- IBS
Irritable bowel syndrome
- IBD
Inflammatory bowel disease
- EOCRC
Early-onset colorectal cancer
- gFOBT
Guaiac fecal occult blood test
- FIT
Fecal immunochemical test
Authors’ contributions
Conceptualization: AAH; original search and analysis: EA; validation: WKMW; writing—first draft: EA; writing—review and editing: WKMW, MVJ, KS, AAH; writing—finalization: KS and AAH. Revisions: approved by all authors. KS and AAH are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final manuscript.
Funding
EA acknowledges PhD scholarship from the WSU, WKMW is partly supported through the Leona M. and Harry B. Helmsley Charitable Trust (Grant 2018PG-T1D009 to AAH) in collaboration with the JDRF Australian Type 1 Diabetes Clinical Research Network funding (Grant 3-SRA-2019-694-M-B to AAH). AAH is supported by grants from Juvenile Diabetes Research Foundation (JDRF) Australia T1D Clinical Research Network (JDRF/4-CDA2016-228-MB) and Visiting Professorships (2016-18 and 2019-22) from the Danish Diabetes Academy, funded by the Novo Nordisk Foundation, grant number NNF17SA0031406. The authors acknowledge all the infrastructure and publication support through the Western Sydney University, School of Medicine, and library facilities at WSU, MacArthur, NSW.
Availability of data and materials
All the analyses are available within an institutional repository and can be provided upon request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable; this manuscript does not include any details, images, or videos relating to an individual person.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kevin J. Spring, Email: k.spring@westernsydney.edu.au
Anandwardhan A. Hardikar, Email: a.hardikar@westernsydney.edu.au
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen LH, Goel A, Chung DC. Pathways of colorectal carcinogenesis. Gastroenterology. 2020;158(2):291–302. doi: 10.1053/j.gastro.2019.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467–1480. doi: 10.1016/S0140-6736(19)32319-0. [DOI] [PubMed] [Google Scholar]
- 5.Mármol I, Sánchez-De-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez YM. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017;18(1):197. doi: 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong SH, Yu J. Gut microbiota in colorectal cancer: mechanisms of action and clinical applications. Nat Rev Gastroenterol Hepatol. 2019;16(11):690–704. doi: 10.1038/s41575-019-0209-8. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Z, Feng Q, Yin Z, Shuang J, Bai B, Yu P, et al. Red and processed meat consumption and colorectal cancer risk: a systematic review and meta-analysis. Oncotarget. 2017;8(47):83306–83314. doi: 10.18632/oncotarget.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan DS, Lau R, Aune D, Vieira R, Greenwood DC, Kampman E, et al. Red and processed meat and colorectal cancer incidence: meta-analysis of prospective studies. PLoS One. 2011;6(6):e20456. doi: 10.1371/journal.pone.0020456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nucci D, Fatigoni C, Salvatori T, Nardi M, Realdon S, Gianfredi V. Association between dietary fibre intake and colorectal adenoma: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(8):4168. [DOI] [PMC free article] [PubMed]
- 10.Oh H, Kim H, Lee DH, Lee A, Giovannucci EL, Kang SS, et al. Different dietary fibre sources and risks of colorectal cancer and adenoma: a dose-response meta-analysis of prospective studies. Br J Nutr. 2019;122(6):605–615. doi: 10.1017/S0007114519001454. [DOI] [PubMed] [Google Scholar]
- 11.Gianfredi V, Salvatori T, Villarini M, Moretti M, Nucci D, Realdon S. Is dietary fibre truly protective against colon cancer? A systematic review and meta-analysis. Int J Food Sci Nutr. 2018;69(8):904–915. doi: 10.1080/09637486.2018.1446917. [DOI] [PubMed] [Google Scholar]
- 12.O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13(12):691–706. doi: 10.1038/nrgastro.2016.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J, Yu J. The association of diet, gut microbiota and colorectal cancer: what we eat may imply what we get. Protein Cell. 2018;9(5):474–487. doi: 10.1007/s13238-018-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander C, Swanson KS, Fahey GC, Jr, Garleb KA. Perspective: Physiologic importance of short-chain fatty acids from nondigestible carbohydrate fermentation. Adv Nutr. 2019;10(4):576–589. doi: 10.1093/advances/nmz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parada Venegas D, De la Fuente MK, Landskron G, González MJ, Quera R, Dijkstra G, et al. Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front Immunol. 2019;10(277):1-16. [DOI] [PMC free article] [PubMed]
- 16.Liu P, Wang Y, Yang G, Zhang Q, Meng L, Xin Y, et al. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol Res. 2021;165:105420. doi: 10.1016/j.phrs.2021.105420. [DOI] [PubMed] [Google Scholar]
- 17.van der Beek CM, Dejong CHC, Troost FJ, Masclee AAM, Lenaerts K. Role of short-chain fatty acids in colonic inflammation, carcinogenesis, and mucosal protection and healing. Nutr Rev. 2017;75(4):286–305. doi: 10.1093/nutrit/nuw067. [DOI] [PubMed] [Google Scholar]
- 18.Boutron-Ruault MC, Marteau P, Lavergne-Slove A, Myara A, Gerhardt MF, Franchisseur C, et al. Effects of a 3-mo consumption of short-chain fructo-oligosaccharides on parameters of colorectal carcinogenesis in patients with or without small or large colorectal adenomas. Nutr Cancer. 2005;53(2):160–168. doi: 10.1207/s15327914nc5302_5. [DOI] [PubMed] [Google Scholar]
- 19.Bridges KM, Diaz FJ, Wang Z, Ahmed I, Sullivan DK, Umar S, et al. Relating stool microbial metabolite levels, inflammatory markers and dietary behaviors to screening colonoscopy findings in a racially/ethnically diverse patient population. Genes. 2018;9(3):119. [DOI] [PMC free article] [PubMed]
- 20.Chen CY, Niu M, Pan JX, Du N, Liu SM, Li HQ, et al. Bacteroides, butyric acid and t10,c12-CLA changes in colorectal adenomatous polyp patients. Gut Pathogens. 2021;13(1):9. doi: 10.1186/s13099-021-00404-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen HM, Yu YN, Wang JL, Lin YW, Kong X, Yang CQ, et al. Decreased dietary fiber intake and structural alteration of gut microbiota in patients with advanced colorectal adenoma. Am J Clin Nutr. 2013;97(5):1044–1052. doi: 10.3945/ajcn.112.046607. [DOI] [PubMed] [Google Scholar]
- 22.Kashtan H, Stern HS, Jenkins DJA, Jenkins AL, Thompson LU, Hay K, et al. Colonic fermentation and markers of colorectal-cancer risk. Am J Clin Nutr. 1992;55(3):723–728. doi: 10.1093/ajcn/55.3.723. [DOI] [PubMed] [Google Scholar]
- 23.Lin Y, Ma C, Liu C, Wang Z, Yang J, Liu X, et al. NMR-based fecal metabolomics fingerprinting as predictors of earlier diagnosis in patients with colorectal cancer. Oncotarget. 2016;7(20):29454–29464. doi: 10.18632/oncotarget.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Ma CC, Bezabeh T, Wang ZN, Liang JH, Huang Y, et al. H-1 NMR-based metabolomics reveal overlapping discriminatory metabolites and metabolic pathway disturbances between colorectal tumor tissues and fecal samples. Int J Cancer. 2019;145(6):1679–1689. doi: 10.1002/ijc.32190. [DOI] [PubMed] [Google Scholar]
- 25.Monleon D, Morales JM, Barrasa A, Lopez JA, Vazquez C, Celda B. Metabolite profiling of fecal water extracts from human colorectal cancer. NMR Biomed. 2009;22(3):342–348. doi: 10.1002/nbm.1345. [DOI] [PubMed] [Google Scholar]
- 26.Niccolai E, Baldi S, Ricci F, Russo E, Nannini G, Menicatti M, et al. Evaluation and comparison of short chain fatty acids composition in gut diseases. World J Gastroenterol. 2019;25(36):5543–5558. doi: 10.3748/wjg.v25.i36.5543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohigashi S, Sudo K, Kobayashi D, Takahashi O, Takahashi T, Asahara T, et al. Changes of the intestinal microbiota, short chain fatty acids, and fecal pH in patients with colorectal cancer. Dig Dis Sci. 2013;58(6):1717–1726. doi: 10.1007/s10620-012-2526-4. [DOI] [PubMed] [Google Scholar]
- 28.Song EM, Byeon JS, Lee SM, Yoo HJ, Kim SJ, Lee SH, et al. Fecal fatty acid profiling as a potential new screening biomarker in patients with colorectal cancer. Dig Dis Sci. 2018;63(5):1229–1236. doi: 10.1007/s10620-018-4982-y. [DOI] [PubMed] [Google Scholar]
- 29.Sze MA, Topcuoglu BD, Lesniak NA, Ruffin MT, Schloss PD. Fecal short-chain fatty acids are not predictive of colonic tumor status and cannot be predicted based on bacterial community structure. mBio. 2019;10(4):e01454-19. [DOI] [PMC free article] [PubMed]
- 30.Torii T, Kanemitsu K, Hagiwara A. Simultaneous assay of fecal short-chain fatty and bile acids and ratio of total bile acids to butyrate in colon cancer. Chromatography. 2019;40(2):49–57. doi: 10.15583/jpchrom.2018.022. [DOI] [Google Scholar]
- 31.Weaver GA, Krause JA, Miller TL, Wolin MJ. Short chain fatty acid distributions of enema samples from a sigmoidoscopy population: an association of high acetate and low butyrate ratios with adenomatous polyps and colon cancer. Gut. 1988;29(11):1539–1543. doi: 10.1136/gut.29.11.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weir TL, Manter DK, Sheflin AM, Barnett BA, Heuberger AL, Ryan EP. Stool microbiome and metabolome differences between colorectal cancer patients and healthy adults. PLoS One. 2013;8(8):e70803. [DOI] [PMC free article] [PubMed]
- 33.Yusuf F, Adewiah S, Fatchiyah F. The level short chain fatty acids and HSP 70 in colorectal cancer and non-colorectal cancer. Acta Informatica Medica. 2018;26(3):160–163. doi: 10.5455/aim.2018.26.160-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nannini G, Meoni G, Tenori L, Ringressi MN, Taddei A, Niccolai E, et al. Fecal metabolomic profiles: a comparative study of patients with colorectal cancer vs adenomatous polyps. World J Gastroenterol. 2021;27(38):6430–6441. doi: 10.3748/wjg.v27.i38.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ocvirk S, Posma JM, Li JV, Koller KR, Day GM, Flanagan CA, et al. A prospective cohort analysis of gut microbial co-metabolism in Alaska Native and rural African people at high and low risk of colorectal cancer. Am J Clin Nutr. 2020;111(2):406–419. doi: 10.1093/ajcn/nqz301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katsidzira L, Ocvirk S, Wilson A, Li J, Mahachi CB, Soni D, et al. Differences in fecal gut microbiota, short-chain fatty acids and bile acids link colorectal cancer risk to dietary changes associated with urbanization among Zimbabweans. Nutr Cancer. 2019;71(8):1313–1324. doi: 10.1080/01635581.2019.1602659. [DOI] [PubMed] [Google Scholar]
- 37.Hester CM, Jala VR, Langille MGI, Umar S, Greiner KA, Haribabu B. Fecal microbes, short chain fatty acids, and colorectal cancer across racial/ethnic groups. World J Gastroenterol. 2015;21(9):2759–2769. doi: 10.3748/wjg.v21.i9.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ou J, Carbonero F, Zoetendal EG, DeLany JP, Wang M, Newton K, et al. Diet, microbiota, and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am J Clin Nutr. 2013;98(1):111–120. doi: 10.3945/ajcn.112.056689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ou J, DeLany JP, Zhang M, Sharma S, O'Keefe SJD. Association between low colonic short-chain fatty acids and high bile acids in high colon cancer risk populations. Nutr Cancer. 2012;64(1):34–40. doi: 10.1080/01635581.2012.630164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Keefe SJD, Ou J, Aufreiter S, O'Connor D, Sharma S, Sepulveda J, et al. Products of the colonic microbiota mediate the effects of diet on colon cancer risk. J Nutr. 2009;139(11):2044–2048. doi: 10.3945/jn.109.104380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muka T, Glisic M, Milic J, Verhoog S, Bohlius J, Bramer W, et al. A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. 2020;35(1):49–60. doi: 10.1007/s10654-019-00576-5. [DOI] [PubMed] [Google Scholar]
- 43.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2011. [Google Scholar]
- 44.Moola S, Munn Z, Tufanaru C, Aromataris E, Sears K, Sfetcu R, et al. JBI manual for evidence synthesis [Internet]. JBI. 2020. Systematic reviews of etiology and risk. [Google Scholar]
- 45.Ma LL, Wang YY, Yang ZH, Huang D, Weng H, Zeng XT. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil Med Res. 2020;7(1):7. doi: 10.1186/s40779-020-00238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions [Internet]. Version 6.2. 2021. Analysing data and undertaking meta-analyses. 2021. [Google Scholar]
- 47.Baldi S, Menicatti M, Nannini G, Niccolai E, Russo E, Ricci F, et al. Free fatty acids signature in human intestinal disorders: Significant association between butyric acid and celiac disease. Nutrients. 2021;13(3):1–14. doi: 10.3390/nu13030742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bartolucci G, Pallecchi M, Menicatti M, Moracci L, Pucciarelli S, Agostini M, et al. A method for assessing plasma free fatty acids from C2 to C18 and its application for the early detection of colorectal cancer. J Pharm Biomed Anal. 2022;215:114762. doi: 10.1016/j.jpba.2022.114762. [DOI] [PubMed] [Google Scholar]
- 49.Genua F, Mirković B, Mullee A, Levy M, Gallagher WM, Vodicka P, et al. Association of circulating short chain fatty acid levels with colorectal adenomas and colorectal cancer. Clin Nutr ESPEN. 2021;46:297–304. doi: 10.1016/j.clnesp.2021.09.740. [DOI] [PubMed] [Google Scholar]
- 50.Amiot A, Dona AC, Wijeyesekera A, Tournigand C, Baumgaertner I, Lebaleur Y, et al. H-1 NMR spectroscopy of fecal extracts enables detection of advanced colorectal neoplasia. J Proteome Res. 2015;14(9):3871–3881. doi: 10.1021/acs.jproteome.5b00277. [DOI] [PubMed] [Google Scholar]
- 51.Segal I, Hassan H, Walker ARP, Becker P, Braganza J. Fecal short chain fatty acids in South African urban Africans and whites. Dis Colon Rectum. 1995;38(7):732–734. doi: 10.1007/BF02048031. [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Wang J, Rao B, Deng LI. Gut flora profiling and fecal metabolite composition of colorectal cancer patients and healthy individuals. Exper Therapeutic Med. 2017;13(6):2848–2854. doi: 10.3892/etm.2017.4367. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 53.Song M, Chan AT, Sun J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology. 2020;158(2):322–340. doi: 10.1053/j.gastro.2019.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Borges-Canha M, Portela-Cidade JP, Dinis-Ribeiro M, Leite-Moreira AF, Pimentel-Nunes P. Role of colonic microbiota in colorectal carcinogenesis: a systematic review. Rev Esp Enferm Dig. 2015;107(11):659–671. doi: 10.17235/reed.2015.3830/2015. [DOI] [PubMed] [Google Scholar]
- 55.Rao M, Gao C, Hou J, Gu J, Law BYK, Xu Y. Non-digestible carbohydrate and the risk of colorectal neoplasia: a systematic review. Nutr Cancer. 2021;73(1):31–44. doi: 10.1080/01635581.2020.1742360. [DOI] [PubMed] [Google Scholar]
- 56.Ma Y, Hu M, Zhou L, Ling S, Li Y, Kong B, et al. Dietary fiber intake and risks of proximal and distal colon cancers: a meta-analysis. Medicine (Baltimore) 2018;97(36):e11678. doi: 10.1097/MD.0000000000011678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun Q, Jia Q, Song L, Duan L. Alterations in fecal short-chain fatty acids in patients with irritable bowel syndrome: a systematic review and meta-analysis. Medicine (Baltimore) 2019;98(7):e14513. doi: 10.1097/MD.0000000000014513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhuang X, Li T, Li M, Huang S, Qiu Y, Feng R, et al. Systematic review and meta-analysis: short-chain fatty acid characterization in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2019;25(11):1751–1763. doi: 10.1093/ibd/izz188. [DOI] [PubMed] [Google Scholar]
- 59.Rios-Covian D, González S, Nogacka AM, Arboleya S, Salazar N, Gueimonde M, et al. An overview on fecal branched short-chain fatty acids along human life and as related with body mass index: associated dietary and anthropometric factors. Front Microbiol. 2020;11:973. doi: 10.3389/fmicb.2020.00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gill PA, van Zelm MC, Muir JG, Gibson PR. Review article: short chain fatty acids as potential therapeutic agents in human gastrointestinal and inflammatory disorders. Aliment Pharmacol Ther. 2018;48(1):15–34. doi: 10.1111/apt.14689. [DOI] [PubMed] [Google Scholar]
- 61.Shuwen H, Miao D, Quan Q, Wei W, Zhongshan Z, Chun Z, et al. Protective effect of the “food-microorganism-SCFAs” axis on colorectal cancer: from basic research to practical application. J Cancer Res Clin Oncol. 2019;145(9):2169–2197. doi: 10.1007/s00432-019-02997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akimoto N, Ugai T, Zhong R, Hamada T, Fujiyoshi K, Giannakis M, et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol. 2021;18(4):230–243. doi: 10.1038/s41571-020-00445-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burnett-Hartman AN, Lee JK, Demb J, Gupta S. An update on the epidemiology, molecular characterization, diagnosis, and screening strategies for early-onset colorectal cancer. Gastroenterology. 2021;160(4):1041–1049. doi: 10.1053/j.gastro.2020.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Saad El Din K, Loree JM, Sayre EC, Gill S, Brown CJ, Dau H, et al. Trends in the epidemiology of young-onset colorectal cancer: a worldwide systematic review. BMC Cancer. 2020;20(1):288. doi: 10.1186/s12885-020-06766-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut. 2019;68(10):1820–1826. doi: 10.1136/gutjnl-2018-317592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kastrinos F, Samadder NJ, Burt RW. Use of family history and genetic testing to determine risk of colorectal cancer. Gastroenterology. 2020;158(2):389–403. doi: 10.1053/j.gastro.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 67.Baena R, Salinas P. Diet and colorectal cancer. Maturitas. 2015;80(3):258–264. doi: 10.1016/j.maturitas.2014.12.017. [DOI] [PubMed] [Google Scholar]
- 68.Setia MS. Methodology series module 2: case-control studies. Indian J Dermatol. 2016;61(2):146–151. doi: 10.4103/0019-5154.177773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Setia MS. Methodology series module 3: cross-sectional studies. Indian J Dermatol. 2016;61(3):261–264. doi: 10.4103/0019-5154.182410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ocvirk S, Wilson AS, Appolonia CN, Thomas TK, O'Keefe SJD. Fiber, fat, and colorectal cancer: new insight into modifiable dietary risk factors. Curr Gastroenterol Rep. 2019;21(11):62. doi: 10.1007/s11894-019-0725-2. [DOI] [PubMed] [Google Scholar]
- 71.Nguyen TT, Ung TT, Kim NH, Jung YD. Role of bile acids in colon carcinogenesis. World J Clin Cases. 2018;6(13):577–588. doi: 10.12998/wjcc.v6.i13.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15(2):111–128. doi: 10.1038/nrgastro.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for colorectal cancer: US Preventive Services Task Force Recommendation Statement. Jama. 2021;325(19):1965–1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 74.Ferrari A, Neefs I, Hoeck S, Peeters M, Van Hal G. Towards novel non-invasive colorectal cancer screening methods: a comprehensive review. Cancers (Basel). 2021;13(8):1820. [DOI] [PMC free article] [PubMed]
- 75.Jodal HC, Helsingen LM, Anderson JC, Lytvyn L, Vandvik PO, Emilsson L. Colorectal cancer screening with faecal testing, sigmoidoscopy or colonoscopy: a systematic review and network meta-analysis. BMJ Open. 2019;9(10):e032773. doi: 10.1136/bmjopen-2019-032773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Imperiale TF, Gruber RN, Stump TE, Emmett TW, Monahan PO. Performance characteristics of fecal immunochemical tests for colorectal cancer and advanced adenomatous polyps: a systematic review and meta-analysis. Ann Intern Med. 2019;170(5):319–329. doi: 10.7326/M18-2390. [DOI] [PubMed] [Google Scholar]
- 77.Anghel SA, Ionita-Mindrican CB, Luca I, Pop AL. Promising epigenetic biomarkers for the early detection of colorectal cancer: a systematic review. Cancers (Basel). 2021;13(19):4965. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the analyses are available within an institutional repository and can be provided upon request.