Abstract
Although it is often stated that only vaccination would be able to contain or protect the population against a catastrophic smallpox outbreak, the acyclic nucleoside phosphonate analog cidofovir offers a valuable alternative for the therapy and short-term pre- and post-exposure prophylaxis, not only of smallpox but also of other poxvirus infections and DNA viruses. Cidofovir has proven effective against vaccinia, cowpox and monkeypox in various animal model infections. In cell culture, cidofovir has demonstrated activity against variola virus, the etiological agent of smallpox, and in patients it has shown marked efficacy against molluscum contagiosum and orf, two poxvirus infections. Cidofovir is available as an aqueous solution for intravenous administration and could be reformulated for topical (cream or gel), intranasal (aerosol) or peroral (as a lipid prodrug) use, should the need arise.
Keywords: Cidofovir, variola virus, poxviruses, smallpox, bioterrorism, vaccinia virus
In the abeyance of a widely available and effective smallpox virus vaccine, cidofovir is a valuable alternative to be considered in the treatment and pre- and post-exposure prophylaxis of poxvirus infections.
The discontinuation of vaccination against smallpox, as a result of global eradication of the infection since 1978, has rendered most of the world's population vulnerable to severe or fatal smallpox infection in the event that illicitly preserved stocks of variola virus, the etiological agent of smallpox, were to be employed as a biological weapon in a bioterrorism scenario 1., 2.. Facing the threat of a potentially deadly poxvirus infection, whether as a result of variola virus or any other poxvirus, it is generally stated that no treatment is available. In a recent feature article on biodefense, D.A. Henderson was quoted as having said that he is very doubtful that an antiviral drug would prove effective against symptomatic smallpox or would be preferable to a vaccine during the window of time between infection and disease [3].
Cidofovir
Here, I would like to counteract the viewpoint of Henderson, and provide circumstantial evidence for an effective therapeutic strategy that could be used in the treatment and short-term (pre- and post-exposure) prophylaxis of infections with poxviruses in general and variola virus in particular. The compound concerned is cidofovir {(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC)}, an acyclic nucleoside phosphonate (Fig. 1 ). Cidofovir has been formally licensed (since 1996) for the treatment of cytomegalovirus (CMV) retinitis in AIDS patients. The compound is available as an aqueous solution of 375 mg per 5 ml (Vistide®) and is intended for intravenous infusion at a dose of (maximally) 5 mg kg−1 in the treatment of CMV retinitis [once weekly for the first two weeks (induction therapy), and thereafter once every other week (maintenance therapy)].
Fig. 1.
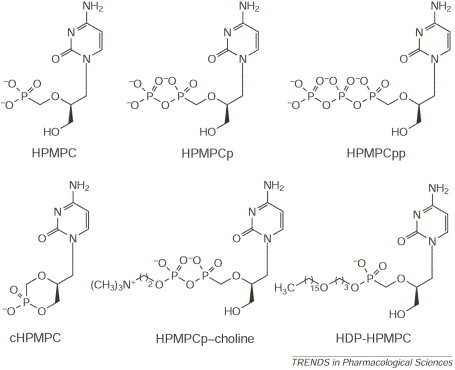
Cidofovir {(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine; HPMPC} and its derivatives HPMPCp (monophosphate), HPMPCpp (diphosphate), HPMPCp–choline, cyclic HPMPC (cHPMPC) and hexadecyloxypropyl-HPMPC (HDP-HPMPC).
Activity spectrum
Cidofovir is not only active against CMV but also against other herpes viruses [e.g. herpes simplex virus (HSV) and varicella-zoster virus (VZV), including thymidine kinase (TK)-deficient HSV and VZV strains, Epstein-Barr virus (EBV) and human herpes viruses type 6, 7 and 8] [4], in addition to polyoma-, papilloma-, adeno- and poxviruses [5]. Among poxviruses, vaccinia, cowpox, molluscum contagiosum, orf (ecthyma infectiosum), monkeypox, camelpox and variola (smallpox) have been found to be sensitive to the compound. In a comparative study of the susceptibility of different poxviruses to cidofovir, the lowest IC50 of the drug (1.5 μg ml−1) (i.e. the most potent activity) was found against variola virus (J.W. Huggins, unpublished data cited in [6]).
Experimental poxvirus infections
In 1993 we were the first to report the successful use of cidofovir in the prevention and therapy of a lethal vaccinia virus infection in immunosuppressed [severe combined immune deficiency (SCID)] mice [7], and these data have been corroborated in more recent studies 8., 9.. Furthermore, cidofovir was able to protect mice against a lethal intranasal infection with cowpox 10., 11. and, even when given intranasally as an aerosol, cidofovir proved effective in the prevention and therapy of intranasal cowpox virus infection in mice [12]. Even if administered as a single dose (10, 20 or 40 mg kg−1) 24 h after intranasal challenge with cowpox virus, cidofovir conferred up to 100% protection against mortality [12]. That aerosolized cidofovir would be efficacious in both pre- and post-exposure prophylaxis of an aerosolized cowpox virus infection has, again, been confirmed in a recent study [13]. In this study, aerosol doses of 0.5–5.0 mg kg−1 cidofovir were invariably more effective than subcutaneous doses of 25–100 mg kg−1, as monitored by a series of parameters (body and lung weight, lung virus titers, lung pathology and survival). Thus, the efficacy of cidofovir has been unequivocally demonstrated in several animal models for poxvirus infections (Table 1 ).
Table 1.
Mouse model poxvirus infections in which efficacy of cidofovir has been demonstrated
| Virus | Route of virus infection | Route of drug administration | Refs |
|---|---|---|---|
| Vaccinia | Intravenous | Subcutaneous | [7] |
| Vaccinia | Intranasal | Subcutaneous or intraperitoneal | 8., 9. |
| Cowpox | Intranasal | Subcutaneous | 10., 11. |
| Cowpox | Intranasal (aerosolized) | Intranasal (aerosolized) | 12., 13. |
| Cowpox | Aerosolized | Oral HDP-HPMPCa | [17] |
Abbreviation: HDP–HPMPC, hexadecyloxypropyl-(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)-cytosine (cidofovir).
Cidofovir has also been shown to be efficacious in monkeys, against an experimental infection with monkeypox virus, supposedly a poxvirus that, although less virulent and less contagious than variola virus, might have the potential for rapid spread in populous areas and could also be used as a bioterrorist weapon. Monkeys exposed to large quantities (as much as 1000 LD50) of aerosolized monkeypox could be rescued from this overwhelming viral challenge, provided treatment with cidofovir was started within a few days of viral exposure (J.W. Huggins, unpublished data cited in [10]).
Mechanism of action
Once it has been taken up by the cells (by endocytosis), cidofovir (HPMPC) is converted to its monophosphate (HPMPCp) and diphosphate (HPMPCpp), which then acts, in competition with dCTP (deoxycytidine triphosphate), at the DNA polymerase level with viral DNA synthesis [14]. Also formed intracellularly is the HPMPCp–choline adduct (Fig. 1), which serves as a reservoir for the slow intracellular release of cidofovir, and thus secures its long-lasting antiviral action.
Lipid prodrug
Cidofovir penetrates only poorly and slowly into cells and its bioavailability via the oral route is negligible. In attempts to circumvent these problems, several prodrugs of cidofovir have been constructed such as its cyclic form (cHPMPC) and the alkyloxyalkyl (1-O-hexadecyloxypropyl) ester (HDP-HPMPC) (Fig. 1). Linking cidofovir onto a lipid tail such as HDP enhanced the in vitro potency against several poxviruses, including variola virus, by 1–3 orders of magnitude 15., 16.. HDP-HPMPC also showed markedly increased oral bioavailability (93% compared with 0.6% for cidofovir itself) and provided 100% protection against aerosolized cowpox virus infection in mice when administered orally at 5, 10 or 20 mg kg−1, once daily for 5 days [17]. This might make it feasible to use cidofovir for the oral treatment of smallpox and all other virus infections that are sensitive to the drug.
Human use against poxviruses
In humans, cidofovir has so far been used in the treatment of only two varieties of poxvirus infections, namely molluscum contagiosum {by both the parenteral (intravenous) and local route 18., 19., 20., 21., 22.}, and orf (ecthyma infectiosum) (by topical application [23]). In these cases, cidofovir proved highly effective in curbing the infection and the associated symptoms; in the case of orf, the (monstruous) ecthyma lesion completely disappeared following topical application of cidofovir [23].
Illustrated in Fig. 2 is a patient presenting with smallpox. Obviously, the prophylactic and/or therapeutic effectiveness of cidofovir against smallpox has not been, and could not be, proven for smallpox because the disease was officially declared eradicated in 1980 (and cidofovir was first described in 1987 [24]). However, there is compelling evidence to assume that cidofovir should be efficacious in the therapy and short-term prophylaxis of smallpox. For example, cidofovir has proven effective, whether administered intravenously, subcutaneously, topically, intranasally (aerosolized) or perorally (as a lipid prodrug), in several animal models of experimental infections caused by poxviruses that in vitro are even less sensitive to the compound than variola virus. Furthermore, cidofovir has already been used with success in humans, against a wide variety of infections caused not only by poxviruses, but also by herpesviruses and papillomaviruses.
Fig. 2.

A patient suffering from smallpox.
It should be pointed out that cidofovir is approved, and available, only for parenteral (i.e. intravenous) administration, which might limit its practical use in a massive outbreak of smallpox. However, repeated observations have pointed to the efficacy of cidofovir, when applied topically, in the treatment of pox-, herpes- and papillomavirus infections. In addition, the results obtained in experimental animal model infections with aerosolized cidofovir [13] and its oral lipid (HDP) prodrug [17] clearly indicate that the drug could be useful when administered by different delivery routes.
Concluding remarks
Cidofovir offers an important alternative to vaccination for short-term prophylaxis against smallpox should the smallpox vaccine (vaccinia) not be available. In addition, cidofovir represents a supplement to vaccination, should the vaccine still allow breakthrough of the variola virus infection, or should vaccination by itself lead to severe complications, as might happen in immunosuppressed patients [25].
References
- 1.Henderson D.A. Bioterrorism as a public health threat. Emerg. Infect. Dis. 1998;4:488–492. doi: 10.3201/eid0403.980340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Toole T. Smallpox: an attack scenario. Emerg. Infect. Dis. 1999;5:540–546. doi: 10.3201/eid0504.990416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen J., Marshall E. Vaccines for biodefense: a system in distress. Science. 2001;294:498–501. doi: 10.1126/science.294.5542.498. [DOI] [PubMed] [Google Scholar]
- 4.De Clercq E. In search of a selective antiviral chemotherapy. Clin. Microbiol. Rev. 1997;10:674–693. doi: 10.1128/cmr.10.4.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Clercq E. Vaccinia virus inhibitors as paradigm for the chemotherapy of poxvirus infections. Clin. Microbiol. Rev. 2001;14:382–397. doi: 10.1128/CMR.14.2.382-397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Safrin S., et al. Clinical uses of cidofovir. Rev. Med. Virol. 1997;7:145–156. doi: 10.1002/(sici)1099-1654(199709)7:3<145::aid-rmv196>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 7.Neyts J., De Clercq E. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxy-propyl)cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SCID) mice. J. Med. Virol. 1993;41:242–246. doi: 10.1002/jmv.1890410312. [DOI] [PubMed] [Google Scholar]
- 8.Smee D.F., et al. Treatment of lethal vaccinia virus respiratory infections in mice with cidofovir. Antiviral Chem. Chemother. 2001;12:71–76. doi: 10.1177/095632020101200105. [DOI] [PubMed] [Google Scholar]
- 9.Smee D.F., et al. Effects of cidofovir on the pathogenesis of a lethal vaccinia virus respiratory infection in mice. Antiviral Res. 2001;52:55–62. doi: 10.1016/s0166-3542(01)00159-0. [DOI] [PubMed] [Google Scholar]
- 10.Bray M., et al. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J. Infect. Dis. 2000;181:10–19. doi: 10.1086/315190. [DOI] [PubMed] [Google Scholar]
- 11.Smee D.F., et al. Treatment of cowpox virus respiratory infections in mice with ribavirin as a single agent or followed sequentially by cidofovir. Antiviral Chem. Chemother. 2000;11:303–309. doi: 10.1177/095632020001100406. [DOI] [PubMed] [Google Scholar]
- 12.Smee D.F., et al. Intranasal treatment of cowpox virus respiratory infections in mice with cidofovir. Antiviral Res. 2000;47:171–177. doi: 10.1016/s0166-3542(00)00105-4. [DOI] [PubMed] [Google Scholar]
- 13.Bray M., et al. Treatment of aerosolized cowpox virus infection in mice with aerosolized cidofovir. Antiviral Res. 2002;54:129–142. doi: 10.1016/S0166-3542(01)00220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Clercq E. Cidofovir in the treatment of poxvirus infections. Antiviral Res. 2002;55:1–13. doi: 10.1016/S0166-3542(02)00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kern E.R., et al. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 2002;46:991–995. doi: 10.1128/AAC.46.4.991-995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huggins, J.W. et al. (2002) Orally active ether lipid prodrugs of cidofovir for the treatment of smallpox. In Abstracts of the Fifteenth International Conference on Antiviral Research, Antiviral Res. 53, A67 (No. 104)
- 17.Winegarden, K.L. et al. (2002) Oral pharmacokinetics and preliminary toxicology of 1-O-hexadecyloxypropyl-cidofovir in mice. In Abstracts of the Fifteenth International Conference on Antiviral Research, Antiviral Res. 53, A67 (No. 105)
- 18.Meadows K.P., et al. Resolution of recalcitrant molluscum contagiosum virus lesions in human immunodeficiency virus-infected patients treated with cidofovir. Arch. Dermatol. 1997;133:987–990. [PubMed] [Google Scholar]
- 19.Davies E.G., et al. Topical cidofovir for severe molluscum contagiosum. Lancet. 1999;353:2042. doi: 10.1016/s0140-6736(99)01782-1. [DOI] [PubMed] [Google Scholar]
- 20.Zabawski E.J., Cockerell C.J. Topical cidofovir for molluscum contagiosum in children. Pediatr. Dermatol. 1999;16:414–415. [PubMed] [Google Scholar]
- 21.Ibarra V., et al. Efficacy of cidofovir in the treatment of recalcitrant molluscum contagiosum in an AIDS patient. Acta Derm. Venereol. 2000;80:315–316. doi: 10.1080/000155500750012333. [DOI] [PubMed] [Google Scholar]
- 22.Toro J.R., et al. Topical cidofovir. A novel treatment for recalcitrant molluscum contagiosum in children infected with human immunodeficiency virus 1. Arch. Dermatol. 2000;136:983–985. doi: 10.1001/archderm.136.8.983. [DOI] [PubMed] [Google Scholar]
- 23.Geerinck K., et al. A case of human orf in an immunocompromised patient treated successfully with cidofovir cream. J. Med. Virol. 2001;64:543–549. doi: 10.1002/jmv.1084. [DOI] [PubMed] [Google Scholar]
- 24.De Clercq E., et al. Antiviral activity of phosphonylmethoxyalkyl derivatives of purine and pyrimidines. Antiviral Res. 1987;8:261–272. doi: 10.1016/s0166-3542(87)80004-9. [DOI] [PubMed] [Google Scholar]
- 25.Redfield R.R., et al. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. New Engl. J. Med. 1987;316:673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]


