Abstract
Background
Human monkeypox (MPX) is a neglected zoonotic disease caused by the MPX virus a double-stranded DNA virus which belongs to the Poxviridae family genus Orthopoxvirus. It is endemic in the rural rainforests of Central and Western Africa where it is responsible of human sporadic cases and outbreaks since 1970. Outside Africa MPXV caused an outbreak in 2003 in the United States linked to importation of infected rodents from Ghana and a few travel-related cases in the USA, United Kingdom, Israel and Singapore. Actually, a worldwide outbreak with more than 1200 confirmed cases mainly concentrated among men who have sex with men is ongoing.
Case report
We present the case of an Italian man living in Portugal that was diagnosed with MPX at our clinic in Milan, Italy. Monkeypox virus infection was confirmed by a specific homemade Real-Time PCR. Samples obtained from different sites (pharynx, skin lesions, anal ulcer, seminal fluid) turned all positive with different viral load.
Conclusions
Our report illustrates the challenge of a disease that seems to present in a different way from classic description with possible human-to-human transmission through sexual contact.
Keywords: Monkeypox, Outbreak, HIV, Italy, Diagnosis
1. Introduction
Monkeypox (MPX) is a neglected zoonosis endemic in the tropical rain forests of Central and West Africa [1]. Monkeypox virus (MPXV) was first identified in 1958 in Cynomolgus monkeys in Denmark but nonhuman primates are considered “incidental” hosts in the same way of human beings with rodents and other small mammals actually considered (although unproven) the natural hosts [2]. The first human case was reported in 1970 in a 9-month child admitted for suspected smallpox to the Basankusu hospital in the Democratic Republic of Congo (DRC) [3]. Soon after six cases of human infection with MPXV were identified in one adult and five unvaccinated children in Liberia, Sierra Leone and Nigeria with isolation of an identical virus [4,5]. Since then several outbreaks of human monkeypox have been regularly reported in Equatorial Africa especially in DRC and Nigeria [[6], [7], [8], [9]]. However, in recent years an increasing number of suspected and confirmed cases has been reported with over 19,000 cases in the period 2000–2019 according to a systematic review of the literature [10] and 15,600 cases in 2021–2022 according to the WHO Bulletin [11,12]. Outside Africa a large multistate outbreak with 71 confirmed and suspected cases was described in 2003 in the USA linked to importation of infected animals from Ghana [13]. Sporadic cases associated with travels to Africa have been observed in the United Kingdom (UK), USA, Singapore and Israel [[14], [15], [16], [17], [18]]. However, starting from 7, May 2022, nearly contemporary cases of human MPX apparently not linked with travel to Africa were reported from 13 countries in Europe (UK, Portugal, Spain, Italy, France, Germany, Belgium, Austria, Denmark, Slovenia, Sweden and Czech Republic), Canada, USA, Australia, Israel [19].
We present the case of a patient diagnosed with human MPX infection in Milan, Italy on May 24. We also made a review of monkeypox diagnosed in Africa and outside Africa with a discussion of many of the challenges faced by clinicians in non-endemic countries who provide care for patients with MPX.
2. Patient case presentation
A 33-year old Italian man attended our clinic on May 24th at Luigi Sacco Hospital in Milan, Italy to investigate a perianal lesion that appeared a few days before. He was living in Lisbon (Portugal) since January 2022 and returned to Italy on May 18th. His past medical history was notable for HIV infection diagnosed in 2016 that was under treatment with dolutegravir/rilpivirine 50/25 mg with good viro-immunological response (CD4+ lymphocyte 771 cell/μL, HIV-RNA < 20 cp/mL). He was fully vaccinated against COVID-19 (two doses of Comirnaty and one dose of Moderna mRNA vaccines). In the previous six months he had travelled only once for a week in January 2022 to Madrid. On May 15th he complained of asthenia, malaise, anorexia with the appearance of two small papular lesions on both elbows and an ulcerated perianal lesion; 3 days later (May 18th) a new lesion appeared on the right cheek associated with symptoms involving the upper respiratory tract (faryngodynia and sneezing). Before flying to Italy he underwent a nasopharyngeal swab for SARS-CoV-2 that resulted negative. On May 21 he complained of the appearance of fever (38.5 °C) with spontaneous resolution in one day. On May 24th he presented to our ward complaining of pain in the perianal region. He reported a receptive anal intercourse on May 8th with a casual partner in Lisbon. On physical examination he appeared in good clinical condition with the following parameters: blood pressure 100/55 mm Hg; pulse rate 105/min; oxygen saturation 95% on ambient air. Mild oropharyngeal hyperemia and several scattered skin lesions with different stage of evolution were observed on the face, both elbows, the trunk, the buttock and the right foot (total number of lesions <10) (Fig. 1 A–F). Bilateral inguinal lymphadenopathy was confirmed by means of ultrasound. Blood test were all within normal limits with the exception of moderate thrombocytopenia (146,000/μL) and mild increase of C-reactive protein (30 mg/L). He underwent a rectal swab for research of sexually transmitted agents (herpes simplex virus, Chlamydia trachomatis and Neisseria gonorrhoeae) using a real-time PCR that gave negative results. Swabs taken from the oropharynx, anus, the perianal ulcerated lesion, a foot vesicle and plasma at the time of hospital admission turned positive for non-human Orthopoxvirus (as depicted in Fig. 2 and Table 1 ) with subsequent confirmation of MPXV infection. The specimens from skin lesion and oropharyngeal swab were collected by mean of Universal Transport Medium swabs (UTM-RT®; COPAN Diagnostics, Italy). Sterile screw cap containers were used to collect urine and seminal fluid while blood samples were collected in the BD Vacutainer® K2EDTA tube and Thrombin tube to obtain plasma and serum respectively. The DNA was extracted from 200 μL of sample and eluted in 100 μL using the automated ELITe InGenius® system.
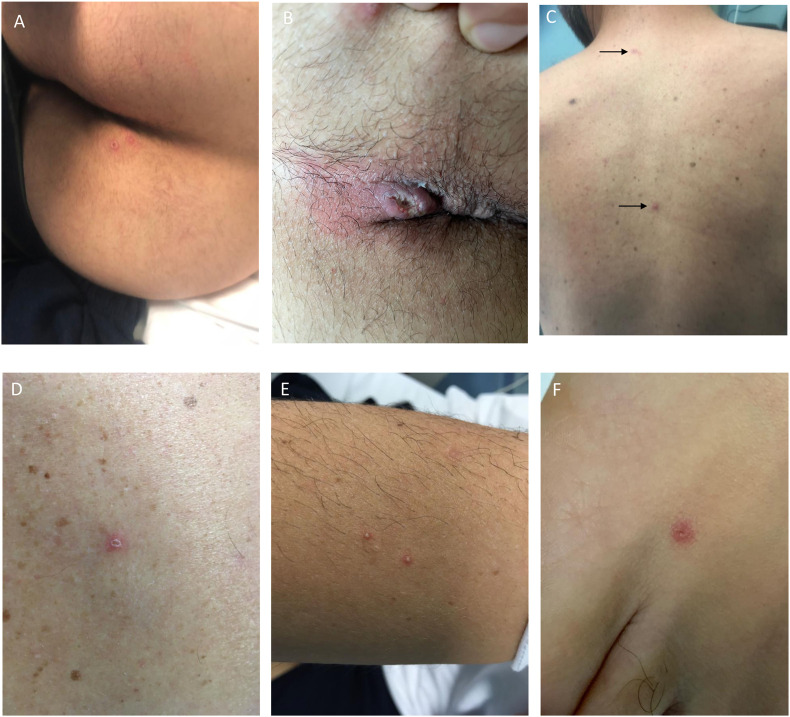
Fig. 1.
A–E. Skin lesions with different appearance obtained at time of diagnosis of MPX. A, buttock. B, perianal ulcerated lesion. C, trunk. D, one of the trunk lesions at major magnification. E, two vesicula/pustular lesion of the arm. F, one foot lesion. Arrows indicate two lesions of the trunk.
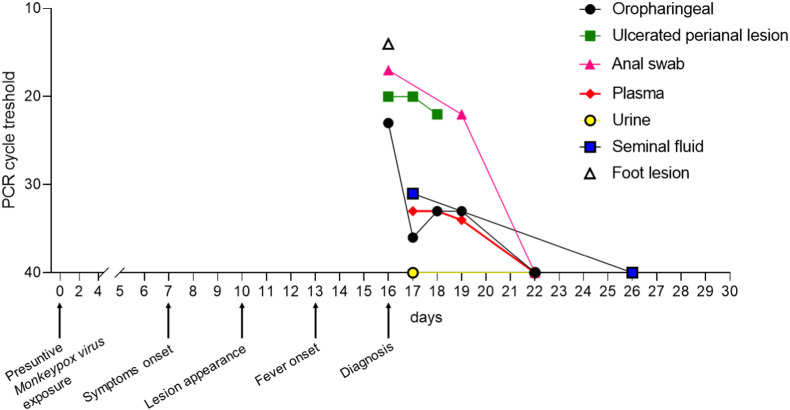
Fig. 2.
Clinical and virological timeline of the patient affected by monkeypox. Real-Time PCR cycle threshold value indicates the number of PCR cycle required to a positive result. The value is inversely proportional to the viral DNA. Ct value of 40 cycle is the PCR negative cut-off.
Table 1.
Timeline of Polymerase Chain Reaction (PCR) for monkeypox virus and virus isolation.
| Days from symptoms onset | Oropharyngeal swab |
Ulcerated perianal lesion |
Anal swab |
Foot lesion |
Plasma |
Seminal fluid |
Urine |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR cycle threshold | Viral Isolation | PCR cycle threshold | Viral Isolation | PCR cycle threshold | Viral Isolation | PCR cycle threshold | Viral Isolation | PCR cycle threshold | Viral Isolation | PCR cycle threshold | Viral Isolation | PCR cycle threshold | Cell culture | |
| 9 | 23 | Positive | 20 | Positive | 17 | Positive | 14 | Positive | / | n.a. | / | n.a. | / | |
| 10 | 36 | Negative | 20 | Positive | n.a. | / | n.a. | / | 33 | Negative | 31 | Negative | Negative | / |
| 11 | 33 | Negative | 22 | Positive | n.a. | / | n.a. | / | 33 | Negative | n.a. | / | n.a. | / |
| 12 | 33 | Negative | n.a. | / | 22 | Positive | n.a. | / | 34 | Negative | n.a. | / | n.a. | / |
| 15 | Negative | / | n.a. | / | Negative | / | n.a. | / | Negative | / | n.a. | / | Negative | / |
| 19 | 35 | a.o. | n.a. | / | Negative | / | n.a. | / | Negative | / | n.a. | / | Negative | / |
List of abbreviations: n.a, not available; a.o, analysis ongoing; Polymerase Chain Reaction, PCR.
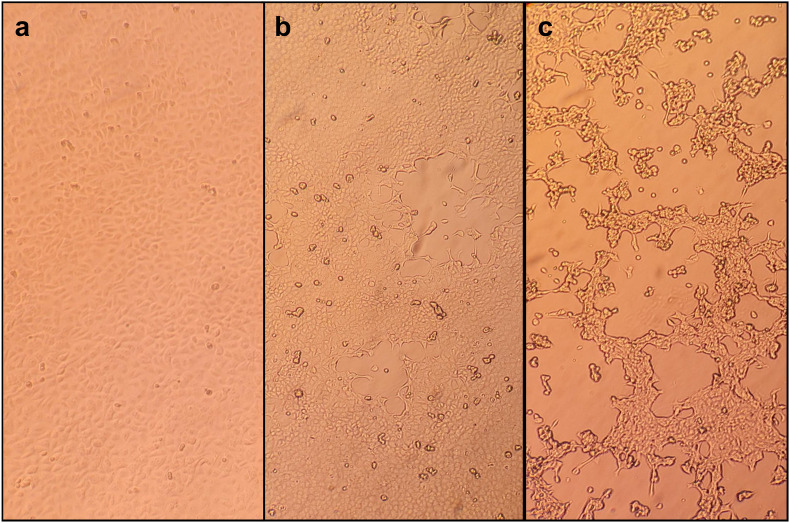
Real-time Polymerase Chain Reaction (PCR) [RealStar Orthopoxvirus PCR Kit 1.0 – altona DIAGNOSTICS) targeting variola virus and non-variola Orthopoxvirus species (Cowpox virus, Monkeypox virus, Raccoonpox virus, Camelpox virus, Vaccinia virus] was used as Orthopoxvirus screening PCR. Monkeypox virus infection was confirmed by a specific homemade Real-Time PCR performed on Orthopoxvirus screening PCR positive samples as previously described [20]. Sampling was repeated on a daily basis to evaluate viral load variation in terms of Cycle threshold (Ct) values over time, viral shedding and virus clearance collecting also plasma, urine and seminal fluid (Fig. 2). All the positive samples were used to attempt viral isolation. In brief, a 200 μL aliquot of each sample was plated in duplicate in 24-wells plates containing 80%–90% confluent Vero E6 cells with 800 μL of Dulbecco's Modified Eagle Medium with l-glutamine (Gibco™ ThermoFisher Scientif) supplemented with 2% of heat-inactivated fetal bovine serum (Gibco™ ThermoFisher Scientific) and 1% Penicillin-Streptomycin [5000 U mL−1] (Pen-Strep, Gibco™ ThermoFisher Scientific). Plates was incubated at 37 °C and 5% CO2 atmospheric pressure and checked every 24 h. Cytopathic effect was observed in Vero E6 cells showing typical monolayer separation and cell rounding (Fig. 3 ).
Fig. 3.
Isolation of Monkeypox virus in Vero E6 cells from skin lesions and oropharyngeal swab. a: mock-infected Vero E6 cells; b: Vero E6 cells at 3 days post-infection; c: Vero E6 cells at 6 days post-infection.
The patient was isolated in a negative-pressure room and remained well throughout admission until discharge. He was examined daily for new skin lesions and was discharged to home on day 7 of hospitalization. The patient was instructed to remain isolated in his residence until all skin lesions recovered. During follow-up the RT-PCR turned negative on all examined specimens except the last oropharyngeal swab which showed a Ct of 35.
2.1. Search strategy and selection criteria
We undertook a search of PubMed from Jan 1, 1970 to June 3, 2022, with no language restriction using the following search terms: “Monkeypox”, “Monkeypox virus”, “Imported monkeypox”, “Monkeypox and Africa”, “Monkeypox and travel”, “Monkeypox and Europe”, “Monkeypox outbreak”, “Monkeypox reservoir”, “Monkeypox and treatment”. We selected key references and seminal papers, review articles, patient reports. We also reviewed publications from international organizations such as the WHO, ECDC and CDC. We completed manual search with references from selected reports.
3. Review and discussion
3.1. Monkeypox virus
MPXV was first identified in 1959 in Copenhagen (Denmark) in captive cynomolgus monkeys (Macaca fascicularis) shipped from Singapore and showing a pox-like disease [2]. Subsequently the causal agent named MPXV has been recovered in outbreaks of illness involving monkeys or apes in the USA, The Netherland and France [4,21]. However, MPX is considered a misnomer because monkeys are not the natural reservoir of the virus with small rodents considered although not proved the probable animal reservoir. Till now the virus has been isolated in wild animals only twice: in 1985 in DRC in a rope squirrel (Funisciurus anerythrus) and in 2012 in a sooty mangabey (Cercocebus atys) in Ivory Coast [22,23]. The virus belongs to the Poxviridae family genus Orthopoxvirus with a double-stranded DNA genome with approximately 200,000 base pairs. On electron microscopy observation the virus shows a brick-like shape measuring 200–400 nm. Genomic sequencing of MPXV isolates from cases diagnosed in Western and Central Africa and during the 2003 USA outbreak showed the existence of two clades: the West Africa clade and the Central Africa (Congo basin) clade [[24], [25], [26]]. Difference in pathogenicity has been observed with a milder disease and a better outcome associated with the West Africa clade in comparison with the Congo basin clade [10,25]. D14L encoding VCP-MPXV an ortholog VACV complement binding protein has been proposed as a candidate virulence gene [25]. Interestingly the first published genome sequence of MPXV associated with the actual multi-country outbreak indicates that the virus belongs to the West African clade [27,28]. MPXV is considered endemic in ten countries of the tropical rain forests of Africa (DRC, Republic of Congo, Nigeria, Central African Republic, Cameroon, Liberia, Sierra Leone, Ivory Coast, South Sudan) [1,9,10]. Humans can be infected from either a direct contact or a bite/scratch with an infected animal (i.e. primary zoonotic transmission) or by human-to-human transmission [[29], [30], [31]]. Activities such as hunting or butchering of bushmeat are considered as risk factors for primary zoonotic acquisition of MPXV [7,10,32]. Transmission of MPXV occurs through salivary or respiratory droplets, direct contact with skin lesions and with fomites. Among previous imported cases in UK both household and nosocomial infections were observed [14]. Secondary attack rate reported in the literature is extremely variable and seems to be influenced by several variables such as the geographic area involved by the outbreak, the period of study, a history of previous vaccination against smallpox and the degree of contact [7,[33], [34], [35], [36]]. In the first survey of human MPX cases diagnosed in West and Central Africa (mainly in DRC) during the first decade of ‘70s, Breman et al. reported an overall secondary attack rate of 3.3% but it increased to 7.7% among very close family members [33]. A subsequent study conducted in DRC assessing more than two thousand people with close contacts with monkeypox patients showed an overall 3% probability to become infected from a human source [34]. However, the attack rate increased to 7.5% among unvaccinated persons, to 9.3% among unvaccinated household contact and to 11.7% among household contact in the 0–4 year age group [34]. Independently from history of smallpox vaccination, household contacts had a significantly higher attack rate than for other contacts being four times higher for unvaccinated and seven times higher for vaccinated [35]. Another study conducted in DRC during a 1996–1997 outbreak reported a household secondary attack rate of 8.3% [7]. More recently the median attack rate in an outbreak in DRC was reported to be 50% [36]. Sexual transmission of MPXV has never been demonstrated but it is hypothesized based on previous reports of vaccinia transmission through sexual intercourses and the high rate of genital lesions (68%) observed among monkeypox cases in Nigeria [[37], [38], [39], [40]]. The incubation period of human monkeypox is not well characterized although it is generally reported to be in the range of 10–14 days [41]. Experts joining at a WHO meeting in 1984 indicated in cases of human-to-human transmission an interval between contacts and onset of rash ranging between 7 and 23 days [42]. More recently during an outbreak of monkeypox in Nigeria the reported exposure to another patient before the onset of the disease was 7–21 days in 73% of subjects for whom this information was available [7]. Finally, in a study using either cases described in the literature with well defined incubation period and their own well characterized cases Nolen and coworkers provided indication of a median incubation period of 9 days with 75% of patients having an incubation period of 5–12 days [36]. A significant increase of the number of individual outbreak reports in Africa has been observed since 1970 [9,10,43]. Particularly in the DRC a 20-fold increase was observed between 1981-1986 and 2006–2007 with an average annual cumulative incidence of 5.53 per 10.000 [43]. Interestingly, the median age of presentation increased from 4 years (in the 70s) to the actual 21 years (2010–2019) [10]. Several possible explanations for the increasing number of monkeypox outbreaks have been proposed: enhanced surveillance system with improved reporting of cases [10,44]; deforestation with increasing exposure to animal reservoir harboring monkeypox virus thus providing more opportunities for zoonotic transmission [[45], [46], [47]]; declining vaccine coverage against smallpox with decreased herd immunity against poxviruses [[30], [43], [46], [48]]; genetic evolution of MPXV with increased capacity of human-to-human transmission [49]. Old calculation of the basic reproduction number using data collected in the DRC in 1980–1984 resulted in a R0 of 0.81 with a net reproduction number (Rnet) of 0.3 and an upper limit of 1.0 thus not excluding the possibility of MPXV persistence in the human population [30]. Another study again performed in DRC with data collected in 2006–2007 calculated an Rnet of 0.6 [49]. However, researchers from Pasteur using the data of 85% vaccine protection (induced by smallpox vaccine) calculated an R0 for monkeypox in DRC of 2.13 (uncertainty bounds 1.46–2.67) [50]. A new proposed model consisting of eight mutually exclusive compartments five of which regarding the human population (exposed and isolated humans, exposed population, infected humans and recovered humans) and three the rodent population (exposed, susceptible and infected rodents) provided evidence that isolation of infected humans helps to reduce disease transmission [51].
3.2. Monkeypox in West and Central Africa
Since the first recognized case of human monkeypox in the DRC on the wake of smallpox eradication MPXV has been responsible of regular outbreaks especially in rural communities of central and western Africa with possible expanding frequency and geographic extension in the last decade [1,3,[6], [7], [8], [9], [10]]. However, the disease is mostly neglected and our knowledge about transmission, clinical manifestations and outcome are largely derived from a few old studies [30,33,52,53] and even in recent studies important information are lacking [7,8,40,54,55] (Table 2 ). The appearance of rash is considered a distinctive feature of human MPX present in all the patients [7,8,33,40,[53], [54], [55]] although in some studies its presence was inferred only retrospectively from the residual skin scars [7,54]. The rash is reported to be similar to that of smallpox with monomorphic lesions and centrifugal distribution thus fairly different from that observed in chickenpox [56]. However, such description is based on the classic studies conducted by Jezek and coworkers during the 80s [52,53] and a more recent study reported a pleomorphic characteristic of the rash in nearly 38% of patients being even more frequent among people living with HIV infection [40]. Moreover, it should be highlighted that cases identified as probable MPX might be indeed cases of chickenpox [54,57]. In the outbreak of Kasai Oriental in 1996–1997 testing of skin lesion vesicles in nineteen patients identified MPXV in nine cases and varicella zoster virus (VZV) in four cases [54]. The investigation of seven outbreaks observed in DRC in 2001 showed that two were caused only by VZV and in another two both MPXV and VZV were involved [57]. However, also the opposite can be true with cases diagnosed as chickenpox that are instead MPX [58]. In a recent study conducted in DRC 12.1% of suspected MPX cases were confirmed MPX/chickenpox coinfections and such patients presented significant higher lesion counts than patients with chickenpox [59]. The rash of MPX is frequently localized to the face (75–97%) and the trunk (72–92%) with palms and soles generally not spared [8,40,53]. Involvement of genital area was reported in an old study in less than 30% of affected patients [53] but in more recent studies nearly 68% of subjects had such localization [[8], [40]]. The skin lesions usually progress through macules, papules, vesicles and pustules before umbilicating and crusting [52]. The number of skin lesions exceeds one hundred (an indirect sign of severity) in 49–66% of patients [30,40,53,55] with more than one thousand lesions in 17.5% of cases [40]. Secondary bacterial infection of skin has been recorded in 18.4–54% of cases [40,52]. Hypo-hyperpigmented scars were reported to be still present 1–4 years following the acute disease [33,52] but in a recent study skin scars were no longer visible after 8 weeks of follow-up [40]. Jezek et al. reported that illness started with fever in most patients with 5% of patients developing fever and rash on the same day [52]. Two recent studies reported fever preceding the rash only in 34.3% and 57% of patients, respectively [8,40]. Lymphadenopathy has been considered a distinctive clinical feature of MPX in the differential diagnosis with either smallpox and chickenpox [52,56] with possible generalized, cervical, submandibular, axillary and inguinal involvement [33,40,52]. However, it has been reported in 38–87% in different case series [7,8,33,40,54,55]. A case fatality rate (CFR) ranging from 0 to 17% has been reported with a calculated pooled estimate in a recent meta-analysis of 8.7% [10]. However, a significant difference emerged according to the different MPXV clades involved with a CFR of 10.6% for Central African clade and 4.6% for West African clade [10].
Table 2.
Clinical characteristics of human monkeypox infection in West and Central Africa.
| Author [reference] | Breman [33] | Jezek [53] | Hutin [7] | CDC [54] | Formenty [55] | Yinka-Ogunleye [8] | Ogoina [40] |
|---|---|---|---|---|---|---|---|
| Country (ies) | DRC, Liberia, Sierra leone, Nigeria, Ivory Coast | DRC (ex Zaire) | DRC (ex Zaire) | DRC (ex Zaire) | Sudan | Nigeria | Nigeria |
| Year | 1970–1979 | 1981–1986 | 1996–1997 | 1996–1997 | 2005–2006 | 2017–2018 | 2017–2018 |
| N° of cases/Confirmed/Probable cases | 47/37/10 | 338/NR/NR | 88/7/81 | 344/NR/NR | 19/10/9 | 122/118/4 | 40/NR/NR |
| Male gender (%) | 24 (51.1) | 182 (53.8) | 50 (56.8) | NR | 9 (47.4) | 84 (69) | 31 (77.5) |
| Age, median (range) | 4y (7 mo-40y) | 4.4y (3mo-69y) | 10y (1mo-62y) | NR | NR (8mo-32y) | 29 (2d-50y) | 32y (28d-54y) |
| Previous smallpox vaccination | 4 (8.5%) | 43 (13%) | 13/84 (15.5) | 39 (11.3) | NR | NR | NR |
| Rash (%) | 47 (100%) | 338 (100) | 82/82 (100) | 344 (100) | 19 (100) | 122 (100) | 40 (100) |
| Rash involvement | NR | NR | |||||
| *Face | 256 (75.7%) | 3/11 (27.3) | 68/71 (96) | 34/35 (97.5) | |||
| * Trunk | NR | 8/11 (72.7) | 56/70 (80) | 32/35 (92.5) | |||
| *Palms | 206 (60.9%) | 5/7 (71.4) | 3/11 (27.3) | 48/70 (69) | 19/35 (55) | ||
| * Soles of feet | 196 (58%) | 5/7 (71.4) | 3/11 (27.3) | 42/66 (64) | 17/35 (50) | ||
| *Genitalia | 88 (26%) | NR | 44/65 (68) | 24/35 (67.5) | |||
| Rash characteristic^ | NR | NR | NR | NR | NR | ||
| * Monomorphic | 233/295 (79) | 25 (62.5) | |||||
| * Pleomorphic | 62/295 (21) | 15 (37.5) | |||||
| Diffusion, N° (%) | NR | NR | NR | NR | NR | NR | |
| *Centrifugal | 246/295 (83.4) | ||||||
| *Centripetal | 13/295 (4.4) | ||||||
| *Indefinite | 36/295 (12.2) | ||||||
| N° skin lesions (%) | NR | NR | NR | ||||
| *< 25 | 6 (12.7) | 22 (6.5) | - | ||||
| *26-99 | 18 (38.3) | 55 (16.3) | 3 (50) | 16 (40) | |||
| *> 100 | 23 (48.9) | 218 (66.5) | 3 (50) | 24 (60)§ | |||
| Fever | NR | NR | NR | NR | 16 (84.2) | 81/92 (88) | 36 (90) |
| Lymphadenopathy, N° (%) | 18 (38.3) | 164 (48.5) | 47/85 (55.3) | 237 (69) | 15 (79) | 45/65 (69) | 35 (87.5) |
| Case fatality rate (%) | 8 (17%) | 33 (9.7) | 3/81 (3.7) | 5 (1.5) | 0 (0) | 7 (5.7)* | 5 (12.5) |
List of abbreviations: DRC, Democratic Republic of Congo; NR, not reported; mo, months; y, year; d, days; * four patients had AIDS; § 17 patients had 101-1000 lesions, 7 patients >1000 lesions; ^ monomorphic indicates similar size and appearance, pleomorphic indicates different sizes and appearance.
3.3. Monkeypox cases in non-endemic areas (2003–2021)
Up to 2021, 48 confirmed MPX cases have been reported outside the endemic African regions [[13], [14], [15], [16], [17], [18],31,60,61] and epidemiology, clinical characteristics and outcome are summarized in Table 3 . Most of the patients belong to a large multistate monkeypox outbreak occurred in 2003 in the USA following the importation from Ghana of infected African rodents causing direct human infections or indirect infections by means of an intermediate animal host (i.e. prairie dogs) [31,62]. All the cases involved in the 2003 US outbreak had direct contact with infected exotic or wild mammalian pets, whereas human-to-human transmission had never been confirmed. Females were slightly prevalent (53.2%) and 23.4% of patients were <18 years of age [31]. Vaccination against smallpox was reported in approximately one out of three patients of the initial outbreak and none of this received the vaccine shot after 1972 [13]. The median incubation period was 12 days which was shorter for patients with a complex exposure (bite or scratch sufficient to provoke a break in the skin) when compared to those with a noninvasive exposure (9 vs 13 days, respectively) [31]. The clinical course was usually characterized, in patients with a noninvasive exposure, by a short prodromic phase (2–3 days) with fever, chills, lymphadenopathy, headache, sore throat, myalgias, and gastrointestinal symptoms, followed by the onset of the rash [31,62,63]. On the contrary, for patients with a complex exposure the rash usually preceded the acme of febrile illness [31]. Lymphadenopathy was frequently reported (71% of 34 confirmed cases) [63]. The rash presented with the centrifugal distribution in only 48% of cases and the involvement of palms in 28% and soles was observed only 9%, respectively [63]. Only 5 patients (12.5%) experienced >100 skin lesions [31]. In addition, healing with prominent hemorrhagic crusts was distinctive of the US cases and the resolution of lesions occurred after sloughing of these crusts usually without significant scares. Fourteen patients were hospitalized (only 9 for longer than 48 h) and five met the criteria for severe disease. All patients fully recovered without any specific antiviral treatment although it is worth mentioning that one 6-year-old child developed encephalitis with seizures and required mechanical ventilation for 48 h [31,63].
Table 3.
Epidemiology, characteristics, clinical presentation and outcome of patients with monxeypox infection in non-endemic countries.
| Country/Year/Ref | N° | Sex | Age | Exposure risk | Type of exposure* | Incubation | Respiratory symptoms | GI symptoms | Systemic illness | Number of lesions | Fever | Specific Treatment | Duration | Adverse events | Post exposure vaccination | Hospitalized | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| USA/2003/13,31 | 47 (37 confirmed and 10 probable) | 25 (53.2%) F 22 (46.8%)M |
11 (23.4%) <18 years | Prairie dogs exposed to imported animals from Ghana | Noninvasive 30 (63.8%) Complex 17 (36.2%) |
9–13 days | 39 (83%) pronounced 8 (17%) mild |
14 (29.8%) | Pronounced 30 (72.3%) Mild 17 (26.7%) |
</ = 25 22 (46.8%) 26-100 13 (27.7%) 101-249 2 (4%) >/ = 250 3 (6.4%) |
31 (66%) | None | NA | NA | None | 14 (29.8%) | Recovered |
| UK/2018/60 | 2 | 2 M | Age range 30–40 years | Travel from Nigeria, bush meat possible for one case | Noninvasive | Unknown | NR | NR | Mild in both patients | Unknown, vesiculopustular rash present in both cases, lymphadenopathy | Yes | BCV 200 mg orally | One dose in one case and 2 dose in onother | Increase in liver enzyme in both patients (ALT peak 331 IU/L and 550 IU/L) | None | Yes | Recovered |
| Israel/2018/15 | 1 | M | 38 years | Rodent carcasses in Nigeria | Noninvasive | 12 days | NR | NR | Mild | Unknown, vesiculopustular rash present, lymphadenopathy | Yes | NR | NR | NR | NR | Yes | Recovered |
| Singapore 2019/17 | 1 | M | 38 years | Travel to Nigeria and attended a wedding and eat bushmeat | Noninvasive | 9 days | NR | NR | Mild | Unknown, vesiculopustular rash present, lymphadenopathy | Yes | None | NA | NA | None | Yes | Recovered |
| UK/2021/61 | 3 | 2 M 1 F |
18 months and age range 30–40 years | Travel to Nigeria (index case), family cluster | Noninvasive | Index case unknow Secondary cases 19 and 14 days |
NR | NR | Mild 3 | Unknown, vesiculopustular rash, lymphadenopathy in one adult case and in the baby | None | TCV 600 mg twice orally in one case | 2 weeks | None | None | Yes | Recovered |
| USA/2021/16 | 1 | M | Middle aged | Travel to Nigeria, large social gathering | Noninvasive | Unknown | Yes | Yes | Mild | Unknown, vesiculopustular rash | Yes | TCV | Not specified | NR | None | Yes | Recovered |
| USA/2021/18 | 1 | M | 28 years | Travel to Nigeria | Noninvasive | Unknown | NR | NR | Mild | Unknown, vesiculopustular rash present, lymphadenopathy | Yes | None | NA | NA | None | Yes | Recovered |
| UK/2018/14 | 1 | F | Age range 30–40 | Secondary exposure during healthcare in UK | Noninvasive | 18 days | NR | NR | Mild | 32 lesions | No | BCV 200 mg orally |
2 dose | Increase in liver enzyme (ALT peak 127 IU/L), nausea and abdominal discomfort | Modified vaccina Ankara 6 days post-exposure | Yes | Recovered |
List of abbreviations: BCV, brincidofovir; NR, not reported; NA, not available; TCV, tecovirimat; GI, gastrointestinal.
Since 2018, eleven additional cases of human MPX have been reported in non-endemic countries: seven in UK [14,60,61], two in the USA [16,18], one each in Israel [15] and in Singapore [17]. All but one patient aged <2 years were middle-aged adults (9 males and 2 females). All the cases had an epidemiological link with Nigeria (a travel to the country or the exposure to MPX cases imported from Nigeria). Notably, in three cases diagnosed in the UK human to human transmission was proven for the first time outside endemic areas [14,61]. In the first two cases it was in a family cluster [61] and in the third case the exposure was related to the health care assistance of a patient with MPX [14]. Reported exposures among travel-related cases were attending social gathering with bushmeat consumption [17,60] and contact with two rodent carcasses [15]. Lymphadenopathy was a common finding and the clinical presentation, and the distribution of the vesiculo-pustular rash (present in all cases) appeared to be similar to that described in the noninvasive exposed patients of the 2003 US outbreak [31]. Notably three patients experienced low mood (with also emotional lability in one case) [14] and ulcerated inguinal lesions with a protracted healing were reported in two cases [14]. All patients were hospitalized experiencing a mild illness and fully recovered. Three patients were treated with brincidofovir (all experiencing a significant increase in liver enzyme) [14,60], two with tecovirimat [14,16] and one patient received post- exposure modified vaccina Ankara [14].
3.4. Monkeypox laboratory diagnosis
The swab of a lesion exudate or crust specimens is considered the best sample to obtain a rapid and definite diagnosis of MPX. In particular, the direct recognition of the viral DNA by means of real-time PCR allow the rapid discrimination between smallpox and other poxvirus [20,[64], [65], [66], [67], [68], [69]]. The nucleic acid of the virus could be also retrieved in blood, urine, upper respiratory tract and seminal fluid [14,69]. In addition, the GeneXpert assay has been demonstrated to perform well with both crust and vesicle samples providing an additional diagnostic platform that may expand and expedite MPX detection capabilities [70]. As an additional molecular tool useful for epidemiological purpose high-throughput shotgun metagenomics should be used to reconstruct the genome sequences and to inform about the evolutionary trajectory of MPX outbreak by means of phylogenomic data [71]. Viral isolation from a clinical specimen, electron microscopy, and immunohistochemistry are techniques that require advanced technical skills and training, and should be reserved to reference laboratories with appropriate expertise and skills [56]. Serological examination is useful for epidemiological purpose although limited by cross-reactivity to a variety of Orthopoxviruses. Consequently, the presence of anti-Orthopoxvirus IgG does not allow to reach a definitive diagnosis since a previous exposure to Orthopoxviruses or smallpox vaccine could cause a false positive result. The presence of anti-Orthopoxvirus IgM could be helpful, also in individuals with prior vaccination, in case of a recent exposure to MPX although again limited by the absence of intra Orthopoxvirus specificity [72].
3.5. Monkeypox treatment
Currently there are no treatments proven to be effective in clinical trial against human MPX; nevertheless, two orally bioavailable drugs, brincidofovir and tecovirimat, approved in the USA for the treatment of smallpox in preparation for a potential bioterrorism event [73,74], have been demonstrated to be effective against orthopoxviruses (including MPX) in animal models [[75], [76], [77], [78]].
Tecovirimat is a small synthetic molecule that inhibits the production of extracellular viruses by interacting with the F13L gene product, which encodes a phospholipase involved in the formation of a protein complex that catalyzes the envelopment of intracellular mature virus particles [79]. The drug has been proven to be tolerated following oral administration at a single oral dose of 400 mg or 600 mg per day during 14 days in healthy volunteers in a phase I clinical trial [80,81]. On May 18, 2022 the FDA also approved an intravenous formulation of tecovirimat to treat smallpox as an option for those who are unable to swallow the oral capsule [82].
Brincidofovir (hexadecyloxypropyl-cidofovir), is a lipid conjugate of cidofovir with a broad antiviral activity against several DNA viruses [83]. Cidofovir after conversion to cidofovir diphosphate by intracellular kinases when incorporated in a growing DNA strand results in slower incorporation of the next nucleotide before a complete block of DNA synthesis with a subsequent impairment of genome encapsidation and virion assembly [84,85]. The advantage of brincidofovir when compared to cidofovir is an increased cellular uptake and better conversion to the active form by intracellular enzymes [86]. This if on the one hand results in at least 25-fold higher efficacy than that cidofovir against vaccinia strains on the other it exerts a higher cellular toxicity [87]. Brincidofovir takes the advantage of oral administration and the absence of nephrotoxicity, although in a phase I study mild gastrointestinal adverse effects and transient elevation of transaminases were reported and confirmed in phase II and III trials performed in immunocompromised adults and children [88,89].
The clinical experience with both these compounds in the treatment of Orthopoxvirus infections is extremely limited. A press release in 2021 announced an expanded access programme for tecovirimat is in progress in the Central African Republic, although no data is published to date [90]. There are three reports of compassionate use of tecovirimat for complicated vaccinia [[91], [92], [93], [94]], without significant safety signals identified. Few anecdotal cases reported the use of these compounds in patients with MPX in non-endemic countries. Three patients with a diagnosis of human MPX were treated in the UK with brincidofovir (all experiencing a significant increase in liver enzyme [14,60] and two with tecovirimat one in the UK [14] and the other in the USA [16]. However, no definitive conclusions can be drawn about the efficacy of both drugs in the treatment of MPX.
4. Ongoing Monkeypox virus outbreak (April–June 2022) and discussion
As of June 8, 1200 confirmed cases of human MPX have been reported worldwide (29 countries) with cases observed in seventeen European countries (including UK and Switzerland), the Americas (USA, Canada, Mexico, Argentina), Middle East (Israel, United Arab Emirates), Asia (Thailand) and Australia [95]. This is the largest ever reported multi-country outbreak of human MPX outside Africa [96]. As far as UK, three distinct incidents have been identified: 1) one isolated imported case from Nigeria; 2) a separate household cluster (3 subjects with two confirmed cases) without a link with travel to endemic countries; 3) 82 confirmed cases not linked to the first two incidents [97]. For the latter, available information indicates that all subjects were males (79), 66 (83%) reported sex with men (MSM), the median age was 38 years and 18 reported foreign travel to multiple countries outside Africa [97]. Portugal reported 96 confirmed MPX cases up to May, 27 with detailed information for 27 cases [98]. All were males with a median age of 33 years and resided mainly (92.6%) in the Lisbon and Tangus Valley but four reported travelling abroad [98]. Eighteen subjects identified themselves as MSM, fourteen were people living with HIV. An exanthema with inguinal lymphadenopathy was reported in fourteen patients but such information was missing for the remaining patients. Six presented genital ulcers and four anal ulcers [98]. Genital and anal lesions were also reported at the beginning of the illness in four MSM diagnosed in Rome (Italy) [69], two MSM who had sexual intercourse with each other in UK [99], one MSM diagnosed in Australia who had insertive anal intercourse with four casual male partners in Europe [100], one MSM diagnosed in Czech Republic who had unprotected sexual intercourses in Gran Canaria (Spain) [101] and our patient who reported a single anal receptive intercourse in Portugal seven days before the appearance of lesions. Also in the CDC report from USA, 94% of confirmed cases (16/17) identified themselves as MSM with 35% complaining of perianal lesions and 24% on genitals [102]. All such cases raised the possibility that MPXV can be transmitted by close contact during sexual intercourse as previously reported for vaccinia virus [[36], [37], [38]]. Interestingly in our case we were able to demonstrate MPXV DNA in the seminal fluid with a Ct value of 31 which is similar to the results obtained by our colleagues in Rome who found positivity in the seminal fluid of 3 patients with a Ct ranging from 27 to 30 [69]. However, we were not able to culture MPXV from this specimen and actually it is not demonstrated a direct sexual transmission although the lesions localized on genitals suggests close physical contact during sexual intercourse as the route of acquisition. Different from the study by Adler and coworkers (reporting the UK experience on eight patients diagnosed between 2018 and 2021) [14] who were not able to confirm positive PCR results with viral culture assays, we showed positive culture from four sites with Ct ranging from 14 to 23 demonstrating shedding of viable virus.
In conclusion, we observed in our patient a mild disease with a low number of skin lesions localized also to the genital area and with an asynchronous pattern evolution which is consistent with the clinical presentation reported so far in few reports [69,97] regarding the actual worldwide outbreak of MPX.
5. Conclusion
This case report highlights the difficulties encountered by clinicians facing with patients with MPXV infection during the ongoing outbreak outside Africa. Diagnostic methods to confirm the diagnosis are sensitive but generally available only in few national reference laboratories. Differential diagnosis should consider several diseases including secondary syphilis, disseminated gonorrhea, herpes simplex, lymphogranuloma venereum, molluscum contagiosus, disseminated cryptococcosis. Our case as well as other well described cases in the literature seems to present with few skin lesions sometimes localized to genital and anal area with an asynchronous pattern and with inguinal lymphadenopathy that is different from classical description of the illness. Contact tracing is not an easy task since many patients engaged sex with multiple anonymous partners. Awareness among health-care providers in non-endemic countries need to be strengthened.
Contributors
DM drafted the manuscript and tables, made the laboratory diagnosis and participated in the isolation of the virus. AR cared for the patient. MC participated in the laboratory diagnosis and isolation of the virus. DM cared for the patient and sampled all the specimens. MC participated in the laboratory diagnosis and isolation of the virus. LM cared for the patient. AG drafted the manuscript and did the scientific literature search. GB cared for the patient. MRG participated in the laboratory diagnosis and isolation of the virus. GR drafted the manuscript. SA drafted the manuscript and tables, did the scientific literature search and cared for the patient. All the authors revised the drafted manuscript and approved the final version.
Declaration of interests
We declare no competing interests.
Ethical statement
The patient provided written informed consent to publish this manuscript and for material sampling.
Acknowledgments
We would like to thank Dr Fabrizio Carletti of the National Institute for Infectious Diseases “Lazzaro Spallanzani” (IRCCS), Rome, Italy for providing reagents for specific RT-PCR for Monkeypox virus.
References
- [1.Durski K.N., McCollum A.M., Nakazawa Y., Petersen B.W., Reynolds M.G., Briand S., Djingarey M.H., Olson V., Damon I.K., Khalakdina A. Emergence of monkeypox in West Africa and central Africa, 1970-2017. Wkly Epidemiol Rec. 2018;11:125–132. [Google Scholar]
- 2.Von Magnus P., Andersen E.A., Petersen K.B., Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46:156–176. [Google Scholar]
- 3.Ladnyj I.D., Ziegler P., Kima E. A human infection caused by monkeypox virus in Basakunsu Territory, Democratic Republic of Congo. Bull World Health Organ. 1972;46:593–597. [PMC free article] [PubMed] [Google Scholar]
- 4.Lourie B., Bingham P.G., Evans H.H., Foster S.O., Nakano J.H., Hermann K.L. Human infection with monkeypox virus: laboratory investigation of six cases in West Africa. Bull World Health Organ. 1972;46:633–639. [PMC free article] [PubMed] [Google Scholar]
- 5.Marennikova S.S., Seluhina E.M., Mal’ceva N.N., Cimiskian K.L., Macevic G.R. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Health Organ. 1972;46:599–611. [PMC free article] [PubMed] [Google Scholar]
- 6.Heymann D.L., Szczeniowski M., Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54:693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 7.Hutin Y.J.F., Williams J., Malfait P., Pebody R., Loparev V.N., Ropp S.L., et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996-1997. Emerg Infect Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yinka-Ogunleye A., Aruna O., Dalhat M., Ogoina D., McCallum A., Disu Y., et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beer E.M., Rao V.B. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13 doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., baer L.R., et al. The changing epidemiology of human monkeypox- A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16 doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization Regional office for Africa. Weekly Bulletin on Outbreak and other Emergencies : Met Week. January 2021;4:18–24. [Google Scholar]
- 12.World Health Organization Regional office for Africa. Weekly Bulletin on Outbreak and other Emergencies : Met Week. January 2022;4:17–23. [Google Scholar]
- 13.Centers for Disease Control and Prevention Update: multistate outbreak of monkeypox- Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(27):642–646. [PubMed] [Google Scholar]
- 14.Adler H., Gould S., Hine P., Snell L.B., Wong W., Houlihan C.F., et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00228-6. published online May 24, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erez N., Achoudot H., Milrot E., Schwartz Y., Wiener-Well Y., Paran N., et al. Diagnosis of imported monkeypox. Israel, 2018. Emerg Infect Dis. 2019;25:980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao A.K., Schulte J., Chen T.-H., Hughes C.M., Davidson W., Neff J.M., et al. Monkeypox in a traveller returning from Nigeria- Dallas. Texas, July 2021. MMWR (Morb Mortal Wkly Rep) 2022;71(14):509–516. doi: 10.15585/mmwr.mm7114a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yong S.E.F., Ng O.T., Ho Z.J.M., Mak T.M., Marimuthu K., Vasoo S., et al. Imported monkeypox. Singapore. Emerg Infect Dis. 2020;26:1826–1830. doi: 10.3201/eid2608.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Costello V., Sowash M., Gaur A., Cardis M., Pasieka H., Wortmann G., Ramdeen S. Imported monkeypox from international traveller, Maryland, USA. Emerg Infect Dis. 2022;28(5):1002–1005. doi: 10.3201/eid2805.220292. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.European Centre for Disease Prevention and Control . ECDC; Stockholm: May 2022. Monkeypox multi-country outbreak- 23. [Google Scholar]
- 20.Li Y., Zhao H., Wilkins K., Hughes C., Damon I.K. Real-time PCR assays for the specific detection of monkeypox virus West African and Congo Basin strain DNA. J Virol Methods. 2010;169(1):223–227. doi: 10.1016/j.jviromet.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arita I., Henderson D.A. Smallpox and monkeypox in non-human primates. Bull World Health Organ. 1968;39:277–283. [PMC free article] [PubMed] [Google Scholar]
- 22.Khodakevich L., Jezek Z., Kinzanzka K. Isolation of monkeypox virus from wild squirrel infected in nature. Lancet. 1986;1:98–99. doi: 10.1016/S0140-6736(86)90748-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Radonic A., Metzger S., Dabrowski P.W., Couacy-Hymann E., Schuenadel L., Kurth A., et al. Fatal monkeypox in wild-living sooty mangabey. Cote d’Ivoire, 2021. Emerg Infect Dis. 2014;20:1009–1011. doi: 10.3201/eid2006.131329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Likos A.M., Sammons S.A., Olson V.A., Frace A.M., Li Y., Olsen-Rasmussen M., et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 25.Chen N., Li G., Liszewski M.K., Atkinson J.P., Jahrling P.B., Feng Z., et al. Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology. 2005;340:46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berthet N., Descorps-Declere S., Besombes C., Curaudeau M., Meyong A.A.K., Selekon B., et al. Genomic history of human monkey pox infections in the Central African Republic between 2001 and 2018. Sci Rep. 2021;11:13085. doi: 10.1038/s41598-021-92315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isidro J., Borges V., Pinto M., Ferreira R., Sobral D., Nunes A., et al. First draft genome sequence of Monkeypox virus associated with the suspected multi-country outbreak. May 2022. https://virological.org confirmed case in Portugal.
- 28.Martinez-Puchol S., Coello A., Bordoy A.E., Soler L., Panisello D., Gonzalez-Gomez S., et al. Spanish draft genome sequence of Monkeypox virus related to multi-country outbreak. May 2022. https://vitological.org
- 29.Mutombo M., Arita I., Jezek Z. Human monkeypox transmitted by a chimpanzee in a tropical rain-forest area of Zaire. Lancet. 1983;1(8327):735–737. doi: 10.1016/S0140-6736(83)92027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fine P.E., Jezek Z., Grab B., Dixon H. The transmission potential of monkeypox virus in human population. Int J Epidemiol. 1988;17:643–650. doi: 10.1093/ije/17.3.643. [DOI] [PubMed] [Google Scholar]
- 31.Reynolds M.G., Yorita K.L., Kuehnert M.J., Davidson W.B., Huhn G.D., Holman R.C., Damon I.K. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773–780. doi: 10.1086/505880. [DOI] [PubMed] [Google Scholar]
- 32.Quiner C.A., Moses C., Monroe B.P., Nakazawa Y., Doty J.B., Hughes C.M., et al. Presumptive risk factors for monkeypox in rural communities in the Democratic Republic of the Congo. PLoS One. 2017;12(2) doi: 10.1371/journal.pone.0168664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Breman J.G., Ruti K., Steniowski M.V., Zanotto E., Gromyko A.I., Arita I. Human monkeypox, 1970-79. Bull World Health Organ. 1980;58:165–182. [PMC free article] [PubMed] [Google Scholar]
- 34.Jezek Z., Grab B., Szczeniowski M.V., Paluku K.M., Mutombo M. Human monkeypox: secondary attack rates. Bull World Health Organ. 1988;66:465–470. [PMC free article] [PubMed] [Google Scholar]
- 35.Jezek Z., Marennikova S.S., Mutumbo M., Nakano J.H., Paluku K.M., Szczeniowski M. Human monkeypox: a study of 2,510 contacts of 214 patients. J Infect Dis. 1986;154:551–555. doi: 10.1093/infdis/154.4.551. [DOI] [PubMed] [Google Scholar]
- 36.Nolen L.D., Osadebe L., Katomba J., Likofata J., Mukadi D., Monroe B., et al. Extended human-to-human transmission during a monkeypox outbreak in the Democratic Republic of Congo. Emerg Infect Dis. 2016;22:1014–1021. doi: 10.3201/eid2206.150579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.CDC Secondary and tertiary transmission of vaccinia virus after sexual contact with a smallpox vaccine-San Diego, California. MMWR Morb Mortal Wkly Rep. 2013;62:145–147. [PMC free article] [PubMed] [Google Scholar]
- 38.CDC Vulvar vaccinia infection after sexual contact with a military smallpox vaccine-Alaska, 2006. MMWR (Morb Mortal Wkly Rep) 2007;56:417–419. [PubMed] [Google Scholar]
- 39.CDC Vaccinia virus infection after sexual contact with a military smallpox vaccine-Washington, 2010. MMWR (Morb Mortal Wkly Rep) 2010;59:773–775. [PubMed] [Google Scholar]
- 40.Ogoina D., Iroezindu M., James H.I., Oladokun R., Yinka-Ogunleye A., Wakama P., et al. Clinical course and outcome of human Monkeypox in Nigeria. Clin Infect Dis. 2020;71:e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 41.Di Giulio D.B., Eckburg P.B. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15–25. doi: 10.1016/S1473-3099(03)00856-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anonymous The current status of human monkeypox: memorandum from a WHO meeting. Bull World Health Organ. 1984;62:702–713. [PMC free article] [PubMed] [Google Scholar]
- 43.Rimoin A.W., Mulembakani P.M., Johnston S.C., Lloyd Smith J.O., Kisalu N.K., et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hoff N.A., Doshi R.H., Colwell B., Kebela-Lllunga B., Mukadi P., Mossoko M., et al. Evolution of a disease surveillance system: an increase in reporting of human monkeypox disease in Democratic Republic of the Congo. 2001-2013. Int J Trop Dis Health. 2017;25(2) doi: 10.9734/IJTDH/2017/35885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khodakevich L., Jezek Z., Messenger D. Monkeypox virus: ecology and public health significance. Bull World Health Organ. 1988;66:747–752. [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen P.Y., Ajisegiri W.S., Costantino V., Chughtai A.A., MacIntyre C.R. Reemergence of human monkey-pox and declining population immunity in the context of urbanization. Nigeria , 2017-2020. Emerg Infect Dis. 2021:27. doi: 10.3201/eid2704.203569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson K., Heymann D., Brown C.S., Edmunds W.J., Elsgaard J., Fine P., et al. Human monkeypox- after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077–5081. doi: 10.1016/j.vaccine.2020.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McMullen C.L., Mulembakani P., Hoff N.A., Doshi R.H., Mukadi P., Shongo R., et al. Human monkeypox transmission dynamics thirty years after smallpox eradication in the Sankuru district, Democratic Republic of Congo. Am J Trop Med Hyg. 2015;93(Suppl.4):341. [Google Scholar]
- 49.Kugelman J.R., Johnston S.C., Mulembakani P.M., Kisalu N., Lee M.S., Koroleva G., et al. Genomic variability of monkeypox virus among humans, Democratic Republic of Congo. Emerg Infect Dis. 2017;20:232–239. doi: 10.3201/eid2002.130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grant R., Nguyen L.B.L., Breban R. Modelling human-to-human transmission of monkeypox. Bull World Health Organ. 2020;98:638–640. doi: 10.2471/BLT.19.242347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peter O.J., Kumar S., Kumari N., Oguntolu F.A., Oshinubi K., Musa R. Transmission dynamics of Monkeypox virus: a mathematicl modelling approach. Model Earth Syst Environ. 2021:1–12. doi: 10.1007/s40808-021-01313-2. Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jezek Z., Szczeniowski M., Paluku K.M., Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156:293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 53.Jezek Z., Grab B., Szczeniowski M., Paluku K.M., Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66:459–464. [PMC free article] [PubMed] [Google Scholar]
- 54.CDC Human monkeypox- Kasai oriental, democratic republic of Congo, february 1996-october 1997. MMWR (Morb Mortal Wkly Rep) 1997;46:1168–1171. [PubMed] [Google Scholar]
- 55.Formenty P., Muntasir M.O., Damon I., Chowdhary V., Opoka M.L., Monimart C., et al. Human monkeypox outbreak caused by novel virus belonging to Congo Basin Clade, Sudan, 2005. Emerg Infect Dis. 2010;16:1539–1545. doi: 10.3201/eid1610.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCollum A.M., Damon I.K. Human monkeypox. Clin Infect Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 57.Meyer H., Perrichot M., Stemmler M., Emmerich P., Schmitz H., Varaine F., et al. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J Clin Microbiol. 2002;40:2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jezek Z., Szczeniowski M., Paluku K.M., Mutombo M., Grab B. Human monkeypox : confusion with chickenpox. Acta Trop. 1988;45:297–307. [PubMed] [Google Scholar]
- 59.Hughes C.M., Liu L., Davidson W.B., Radford K., Wilkins K., Monroe B., et al. A tale of two viruses: coinfections of Monkeypox and Varicella Zoster virus in the Democratic Republic of Congo. Am J Trop Med Hyg. 2020;104:604–611. doi: 10.4269/ajtmh.20-0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vaughan A., Aarons E., Astbury J., Balasegaram S., Beadsworth M., Beck C.R., et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hobson G., Adamson J., Adler H., et al. Family cluster of three cases of monkeypox imported from Nigeria to the UK, May 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.32.2100745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reed K.D., Melski J.W., Graham M.B., Regnery R.L., Sotir M.J., Wegner M.V., et al. The detection of monkeypox in humans in the western hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 63.Huhn G.D., Bauer A.M., Yorita K., Graham M.B., Sejvar J., Likos A., et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41:1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 64.Kulesh D.A., Loveless B.M., Norwood D., Garrison J., Whitehouse C.A., Hartmann C., et al. Monkeypox virus detection in rodents using real-time 3′-minor groove binder TaqMan assays on the Roche LightCycler. Lab Invest. 2004;84:1200–1208. doi: 10.1038/labinvest.3700143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Olson V.A., Laue T., Laker M.T., Baskin I.V., Drosten C., Shchelkunov S.N., et al. Real-time PCR system for detection of orthopoxviruses and simultaneous identification of smallpox virus. J Clin Microbiol. 2004;42:1940–1946. doi: 10.1128/JCM.42.5.1940-1946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li Y., Olson V.A., Laue T., Laker M.T., Damon I.K. Detection of monkeypox virus with real-time PCR assays. J Clin Virol. 2006;36:194–203. doi: 10.1016/j.jcv.2006.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shchelkunov S.N., Shcherbakov D.N., Maksyutov R.A., Gavrilova E.V. Species-specific identification of variola, monkeypox, cowpox, and vaccinia viruses by multiplex real-time PCR assay. J Virol Methods. 2011;175:163–169. doi: 10.1016/j.jviromet.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shchelkunov S.N., Gavrilova E.V., Babkin I.V. Multiplex PCR detection and species differentiation of orthopoxviruses pathogenic to humans. Mol Cell Probes. 2005;19:1–8. doi: 10.1016/j.mcp.2004.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Antinori A., Mazzotta V., Vita S., Carletti Tacconi D., Lapini L.E., et al. Epidemiological, clinical and virological characteristics of four cases on monkeypox support transmission through sexual contact. Italy, may 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200421. pii=2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li D., Wilkins K., McCollum A.M., Osadebe L., Kabamba J., Nguete B., et al. Evaluation of the GeneXpert for human monkeypox diagnosis. Am J Trop Med Hyg. 2017;96(2):405–410. doi: 10.4269/ajtmh.16-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gomes J.P., Isidro J., Borges V., et al. Multi-country outbreak of monkeypox virus: phylogenomic characterization and signs of microevolution. May 2022. PREPRINT (Version 1) available at: Research Square. 31. [DOI] [PMC free article] [PubMed]
- 72.Karem K.L., Reynolds M., Braden Z., et al. Characterization of acute phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12:867–872. doi: 10.1128/CDLI.12.7.867-872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grosenbach D.W., Honeychurch K., Rose E.A., Chinsangaram J., Frimm A., Maiti B., Lovejoy C., Meara I., Long P., Hruby D.E. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379(1):44–53. doi: 10.1056/NEJMoa1705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chittick G., Morrison M., Brundage T., Nichols W.G. Short-term clinical safety profile of brincidofovir: a favorable benefit-risk proposition in the treatment of smallpox. Antivir Res. 2017 Jul;143:269–277. doi: 10.1016/j.antiviral.2017.01.009. https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/smallpox-preparedness-and-response-updates-fda [DOI] [PubMed] [Google Scholar]
- 75.Yang G., Pevear D.C., Davies M.H., Collett M.C., Bailey T., Rippen S., et al. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus Challenge. J Virol. 2005;79(20):13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Russo A.T., Grosenbach D.W., Brasel T.L., Baker R.O., Cawthon A.G., Reynolds C., et al. Effects of treatment delay on efficacy of tecovirimat following lethal aerosol monkeypox virus challenge in cynomolgus macaques. J Infect Dis. 2018;218(9):1490–1499. doi: 10.1093/infdis/jiy326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Delaune D., Iseni F. Drug development against smallpox: present and future. Antimicrob Agents Chemother. 2020;64(4) doi: 10.1128/AAC.01683-19. e01683–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hutson C.L., Kondas A.V., Mauldin M.R., Doty J.B., Grossi I.M., Morgan C.N., et al. Pharmacokinetics and efficacy of a potential smallpox therapeutic, brincidofovir, in a lethal monkeypox virus animal model [published correction appears in mSphere. mSphere. 2021;6(1) doi: 10.1128/mSphere.00927-20. 2021 Feb 17;6(1):] e00927–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jordan R., Leeds J.M., Tyavanagimatt S., Hruby D.E. Development of ST-246 for treatment of poxvirus infections. Viruses. 2010;2:2409–2435. doi: 10.3390/v2112409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jordan R., Tien D., Bolken T.C., Jones K.F., Tyavanagimatt S.R., Strasser J., Frimm A., Corrado M.L., Strome P.G., Hruby D.E. Single-dose safety and pharmacokinetics of ST-246, a novel orthopoxvirus egress inhibitor. Antimicrob Agents Chemother. 2008;52:1721–1727. doi: 10.1128/AAC.01303-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chinsangaram J., Honeychurch K.M., Tyavanagimatt S.R., Leeds J.M., Bolken T.C., Jones K.F., Jordan R., Marbury T., Ruckle J., Mee-Lee D., Ross E., Lichtenstein I., Pickens M., Corrado M., Clarke J.M., Frimm A.M., Hruby D.E. Safety and pharmacokinetics of the anti-orthopoxvirus compound ST-246 following a single daily oral dose for 14 days in human volunteers. Antimicrob Agents Chemother. 2012;56:4900–4905. doi: 10.1128/AAC.00904-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/smallpox-preparedness-and-response-updates-fda
- 83.De Clercq E., Holy A., Rosenberg I., Sakuma T., Balzarini J., Maudgal P.C. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986;323:464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 84.Magee W.C., Hostetler K.Y., Evans D.H. Mechanism of inhibition of vaccinia virus DNA polymerase by cidofovir diphosphate. Antimicrob Agents Chemother. 2005;49:3153–3162. doi: 10.1128/AAC.49.8.3153-3162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jesus D.M., Costa L.T., Goncalves D.L., Achete C.A., Attias M., Moussatche N., Damaso C.R. Cidofovir inhibits genome encapsidation and affects morphogenesis during the replication of vaccinia virus. J Virol. 2009;83:11477–11490. doi: 10.1128/JVI.01061-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hostetler K.Y. Synthesis and early development of hexadecyloxypropylcidofovir: an oral antipoxvirus nucleoside phosphonate. Viruses. 2010;2:2213–2225. doi: 10.3390/v2102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kern E.R., Hartline C., Harden E., Keith K., Rodriguez N., Beadle J.R., Hostetler K.Y. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob Agents Chemother. 2002;46:991–995. doi: 10.1128/AAC.46.4.991-995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marty F.M., Winston D.J., Chemaly R.F., Mullane K.M., Shore T.B., Papanicolou G.A., et al. A randomized, double-blind, placebo-controlled phase 3 trial of oral brincidofovir for cytomegalovirus prophylaxis in allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2019;25(2):369–381. doi: 10.1016/j.bbmt.2018.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grimley M.S., Chemaly R.F., Englund J.A., Kustzberg J., Chittick G., Brundage T.M., et al. Brincidofovir for asymptomatic adenovirus viremia in pediatric and adult allogeneic hematopoietic cell transplant recipients: a randomized placebo-controlled phase II trial. Biol Blood Marrow Transplant. 2017;23(3):512–521. doi: 10.1016/j.bbmt.2016.12.621. [DOI] [PubMed] [Google Scholar]
- 90.SIGA announces collaboration with oxford university to support expanded access protocol for use of tpoxx (tecovirimat) to treat monkeypox in Central African Republic. https://www.globenewswire.com/news-release/2021/07/29/2270930/9738/en/SIGA-Announces-Collaboration-with-Oxford-University-to-Support-Expanded-Access-Protocol-for-Use-of-TPOXX-Tecovirimat-To-Treat-Monkeypox-in-Central-African-Republic.html
- 91.Vora S., Damon I., Fulginiti V., Weber S.G., Kahama M., Stein S.L., et al. Severe eczema vaccinatum in a household contact of a smallpox vaccinee. Clin Infect Dis. 2008;46(10):1555–1561. doi: 10.1086/587668. [DOI] [PubMed] [Google Scholar]
- 92.Lindholm D.A., Fisher R.D., Montgomery J.R., Davidson W., Yu P.A., Yu Y.C., et al. Preemptive tecovirimat use in an active duty service member who presented with acute myeloid leukemia after smallpox vaccination. Clin Infect Dis. 2019;69(12):2205–2207. doi: 10.1093/cid/ciz286. [DOI] [PubMed] [Google Scholar]
- 93.Kiernan M., Koutroumanos N. Orbital cowpox. N Engl J Med. 2021;384(23):2241. doi: 10.1056/NEJMicm2033620. [DOI] [PubMed] [Google Scholar]
- 94.Wendt R., Tittelbach J., Schrick L., Kellner N., Kalbitz S., Ruehe B., et al. Generalized cowpox virus infection in an immunosuppressed patient. Int J Infect Dis. 2021;106:276–278. doi: 10.1016/j.ijid.2021.03.076. [DOI] [PubMed] [Google Scholar]
- 95.Cdc. 2022 Monkeypox and Orthopoxvirus outbreak global map. CDC.gov accessed on June 9, 2022.
- 96.Leon-Figueroa D.A., Bonilla-Aldana K., Pachar M., Romani L., Saldana-Cumpa H.M., Anchay-Zuloeta C., et al. The never-ending global emergence of viral zoonoses after COVID-19? The rising concern of monkeypox in Europe, North America and beyond. Travel Med Infect Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vivancos R., Anderson C., Blomquist P., Balasegaram Bell A., Bishop L., et al. Community transmission of monkeypox in the United Kingdom. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200422. April to May 2022. pii=2200422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perez Duque M., Ribeiro S., Martins J.V., Casaca P., Leite P.P., Tavares M., et al. Ongoing monkeypox virus outbreak, Portugal. Euro Surveill. 2022;(22):27. doi: 10.2807/1560-7917.ES.2022.27.22.2200424. 29 April to 23 May 2022. pii=2200424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Heskin J., Belfield A., Milne C., Brown N., Walters Y., Scott C., et al. Transmission of monkeypox virus through sexual contact- A novel route of infection. J Infect. 2022 doi: 10.1016/j.jinf.2022.05.028. pii:S0163-4453(22) 00335–00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hammerschlag Y., MacLeod G., Papadakis G., Sanchez A.A., Druce J., Taiaroa G., et al. Monkeypox infection presenting as genital rash, Australia, May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200411. pii=2200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bizova B., Vesely D., Trojanek M., Rob F. Coinfection with syphilis and monkeypox in HIV positive man in Prague, Czech Republic. Travel Med Infect Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Minhaj F.S., Ogale Y.P., Whitehill F., Schultz J., Foote M., Davidson W., et al. Monkeypox outbreak- nine States, May 2022. MMWR (Morb Mortal Wkly Rep) 2022;71:764–769. doi: 10.15585/mmwr.mm7123e1. [DOI] [PMC free article] [PubMed] [Google Scholar]