Abstract
Orthopoxviruses, including smallpox, monkeypox and molluscipox, pose risks to human health through bioterrorist acts or natural transmission. There is no approved therapy for orthopoxvirus infections; however, cidofovir (CDV) has been approved as an investigational new drug for emergency treatment of adverse effects following smallpox vaccination. For evaluation of new therapies directed against orthopoxvirus infections, we have utilized immunocompetent, hairless mice (SKH-1) inoculated by a cutaneous route with cowpox virus (CV) or vaccinia virus (VV). Mice subsequently developed skin lesions and virus was recovered from the site of inoculation and quantified. Skin biopsies were evaluated microscopically, revealing brick-like eosinophilic, intracytoplasmic inclusion bodies characteristic of orthopoxvirus infection. SKH-1 mice fully recovered from either CV or VV infection. Immunodeficient Athymic or Rhino mice inoculated with CV or VV had more lesions and severe disease than SKH-1 mice. CV-infected SKH-1 mice were treated either with systemic or topical CDV. Although some protection was achieved with systemic treatment, 5% topical CDV was most effective at reducing virus titers in skin, lung, kidney, and spleen. These models may provide a means for evaluating efficacy of new therapies directed against orthopoxvirus diseases and further confirm the topical activity of CDV against cutaneous infections.
Keywords: Orthopoxvirus, Cutaneous, Mice, Cidofovir, Antiviral
1. Introduction
The intentional release of smallpox by bioterrorists is considered to be a small but significant threat (Fulginiti et al., 2003a) and vaccination was offered to health care workers and first responders during 2002–2003 in order to increase national preparedness. Adverse events to vaccination of immunosuppressed individuals include progressive vaccinia which can be life threatening. Treatment options include administration of vaccinia immune globulin (VIG) or intravenous cidofovir (CDV) which has recently been given investigational new drug approval for use in complications to smallpox vaccination (Fulginiti et al., 2003b). Currently there are few models to evaluate the effectiveness of topical or parenteral therapies for progressive vaccinia or other cutaneous orthopoxvirus infections.
The number of cases of human molluscum contagiosum, caused by molluscipox virus, has also increased, especially in the HIV-infected population (Epstein, 1992). The disease prevalence in HIV positive patients may be as high as 5–18% and the severity of lesions is related inversely to CD4 T-lymphocyte counts (Kauffman and Yoon, 2001). Transmission occurs through direct contact, but large quantities of virus can be recovered from a single lesion and autoinoculation can occur (Fields et al., 1996). Treatments for this disease have been largely unsuccessful. A report of successful topical CDV therapy was published in which two HIV-infected children exhibiting mollusca lesions were treated topically with CDV for 2 months and showed significant improvement without any adverse effects (Toro et al., 2000).
Seventy-one human cases of monkeypox, another orthopoxvirus disease, were documented during the recent US outbreak (CDC, 2003b). The majority of people purchased or had contact with prairie dogs which had been exposed to imported monkeypox-infected giant Gambian rats (CDC, 2003a, CDC, 2003b; Enserink, 2003, Maskalyk, 2003). Considering the trace back data in this outbreak, the rapid dissemination of imported infected animals within the US raised awareness concerning the potential for future foreign animal disease outbreaks and helped to prompt permanent changes in importation and quarantine policies. Thirty people were vaccinated as a result of this outbreak, but no requests for VIG were made. Widespread vaccination is impractical for control of these orthopoxvirus infections due to the increasing numbers of immunodeficient patients affected by infectious disease processes such as HIV, pregnancy, or immunosuppressive therapy for organ transplantation, etc.
Worldwide cases of monkeypox have also increased in recent decades with previous outbreaks in 1996–1997 of up to 88 clinical cases in the Republic of Congo with a 3.7% case-fatality rate (Hutin et al., 2001) and an outbreak in 2001 also in the Republic of Congo with a 33% case-fatality rate (Meyer et al., 2002). It is also known that human-to-human transmission of monkeypox occurs by aerosol route and approximately 30% of cases occur in this way (Hutin et al., 2001, Melnick, 1988). While investigational intravenous administration of CDV may be effective in treating this disease, CDV infusions would require hospital admission and monitoring each patient for renal toxicity. It may be that development of topical preparations of CDV, or other antiviral agents, could provide some benefit for treatment of cutaneous lesions.
Topical treatment of acute adenoviral keratoconjunctivitis with cidofovir or cidofovir plus cyclosporine has also been shown to be beneficial and without adverse effect (Gordon et al., 1994, Hillenkamp et al., 2001). Topical CDV treatment of acyclovir-resistant herpes simplex virus in HIV-infected patients proved efficacious without significant side effects (Lalezari et al., 1997). Animal studies in rabbits, however, have shown increases in CDV absorption through abraded skin with bioavailabilities up to 41% (Cundy et al., 1997). There is one report of CDV-induced renal failure in a bone marrow recipient who was treated topically for genital condyloma with 1% CDV for 5 days and 4% for 12 days. The patient had prior renal insufficiency but renal recovery occurred after CDV treatment was terminated (Bienvenu et al., 2002).
Development of appropriate small animal models, adequately simulating human disease, is essential for further progress in evaluation of antiviral chemotherapies for orthopoxvirus infections. There are relatively few animal models available for evaluation of antiviral compounds against these infections, particularly for cutaneous disease. The systemic murine models of orthopoxvirus disease, using cowpox virus (CV) or vaccinia virus (VV) given intravenously, intranasally or by aerosol routes, are currently the only small animal models available for screening new compounds for antiviral activity (Boyle et al., 1967, Bray et al., 2000, Buller, 1985, De Clercq and De Somer, 1968, De Clercq et al., 1976, De Clercq et al., 1989, Martinez et al., 2000, Mims, 1968, Neyts and De Clercq, 1993, Neyts and De Clercq, 2001, Quenelle et al., 2003, Quenelle et al., 2004; Smee et al., 2000a, Smee et al., 2000b). For evaluation of topical preparations we have utilized immunocompetent hairless or immunocompromised mice infected with VV and CV by applying the virus onto abraded skin.
2. Materials and methods
2.1. Mice
Female SKH-1 mice, 4–6 weeks of age, were obtained from Charles River Laboratories (Raleigh, NC). SKH-1 mice are hairless and have normal immunity. Female Athymic, immunodeficient (Crl:NU/NU-BR, Charles River Laboratories) and Rhino mice (RHJ/LeJ hr/+, Jackson Laboratories, Bar Harbor, ME), which are immunocompromised due to an impaired antibody response to T-dependent antigens, were used as models for immunocompromised hosts. Mice were group-housed in microisolator cages and utilized at a quantity of 10–15 mice per treatment group. Mice were obtained, housed, utilized and euthanized according to policies of USDA and Association for Assessment and Accreditation for Laboratory Animal Care. All animal procedures were approved by University of Alabama at Birmingham, Institutional Animal Care and Use Committee prior to initiation of studies.
2.2. Virus
Cowpox virus, strain Brighton, was kindly provided by John W. Huggins (Department of Viral Therapeutics, Virology Division, U.S. Army Medical Research Institute of Infectious Disease, Frederick, MD). Vaccinia virus, strain WR, was obtained from American Type Culture Collection, Manassas, VA.
2.3. Antiviral compounds
Cidofovir (Gilead Pharmaceuticals, Foster City, CA) was weighed and dissolved in sterile saline to yield the desired dosages within a 0.1 ml volume. It was administered i.p. once daily or three times weekly, depending on the experimental protocol. A 5% CDV topical formulation was prepared in 1.5% carbopol gel (Noveon Inc., Cleveland, OH, 980NF). This was applied to the orofacial area three times daily for 7 days beginning 24 h post-viral inoculation using approximately 0.03 ml of gel per mouse per treatment.
2.4. Virus quantitation
To determine the effect of treatment on viral replication on the orofacial area, swabs of the area were obtained every 48 h through day 15 post-infection. Swabs were placed in tubes containing 2.0 ml of media and frozen at −70 °C until titrated for CV or VV. When all samples had been collected they were thawed, diluted serially 10-fold with an autodilutor machine, and viral titers determined in Vero cells using a microtiter cytopathic effect (CPE) assay. Wells were seeded with 105 cells per well and inoculated in triplicate 24 h later. The plates were read under a microscope 5 days later and the presence/absence of viral CPE in each well were recorded.
Tissue samples were collected aseptically and prepared as 10% (w/v) homogenates in minimal essential medium (MEM) with 10% fetal bovine serum (FBS) and standard antibiotics and stored at −70 °C until assayed for virus. Samples were thawed and assayed on Vero cells using an agarose overlay plaque assay to determine VV titers. Briefly, samples of organ homogenates were diluted serially and a 0.2 ml volume placed in duplicate into each of 12-wells of Vero cell monolayers and incubated for 1 h. An overlay solution containing 2× MEM and 1% SeaKem®, ME agarose in equal volumes (FMC BioProducts, Rockland, ME) was added to each well and the cultures incubated for 3 days. Cultures were stained with Neutral Red (Gibco, Grand Island, NY) for approximately 6 h prior to enumeration of viral plaques.
2.5. Experimental infections
For all studies, mice were quarantined for at least 3 days after arrival. SKH-1 mice were routinely placed on antibiotic therapy (sulfamethoxazole-trimethoprim) provided in the drinking water just prior to viral inoculation. On the day of infection, mice were anesthetized (20 mg/ml ketamine per 0.6 mg/ml xylazine) with 0.1 ml anesthetic per 25 g mouse given i.p. Mice were individually identified by implantation of electronic microchips (Stoelting, Wood Dale, IL). Skin over the snout area was abraded using a variable speed Dremmel Tool (Racine, WI) with a no. 193 bit at 4500 rpm. The procedure was performed so that superficial abrasions were created without bleeding. Then 4×104 to 4×106 pfu/ml of CV or 5×103 to 5×105 pfu/ml of VV was applied to the abraded area for 10 s using a Dacron swab saturated with virus solution. For efficacy studies, an inoculum of 4×106 or 1×106 pfu/ml was selected for CV or VV, respectively. To quantify local viral replication, the site of inoculation on the snout was sampled at intervals after viral inoculation using a moistened Dacron swab. Swabs were placed into tubes containing 2 ml of media and frozen at −70 °C until assayed on Vero cells using a CPE assay to determine virus titers. Clinical signs and skin lesions were scored and recorded daily. Lesions that occurred on the orofacial site of inoculation were scored from 0 to 3 based on the percentage of orofacial area affected. Likewise, lesions which occurred over the body were scored from 0 to 1.5 based on the percentage of the body affected. Finally, the pock-like vesicular lesions were scored from 0 to 1.5 based on the regions of the body affected.
Histopathology was performed on pock-like vesicular lesions of SKH-1 mice that were inoculated cutaneously with CV. Skin biopsies were obtained from anesthetized mice and fixed in 10% neutral-buffered formalin. Samples were processed and stained with hematoxylin-eosin.
To determine the extent of systemic viral replication, samples of liver, lung, spleen, kidney, or orofacial skin were obtained at days 1, 3, 5, 7, and 10 post-viral inoculation. Samples were weighed and homogenized as a 10% (w/v) solution in MEM with 10% FBS. Virus titers were determined using a plaque assay in Vero cells, as described previously (Kern et al., 2002).
2.6. Statistical evaluations
Mortality rates were analyzed by two-tailed Fisher’s exact test and mean day of death, and virus titers in tissues using Mann–Whitney U rank sum test. Peak lesion scores, peak virus titers, areas under virus titer–day and lesion score–day curves were compared using the Mann–Whitney U rank sum test. A P-value of 0.05 or less was considered significant.
3. Results
3.1. Cutaneous infections of SKH-1 mice with CV or VV
SKH-1 mice readily became infected with either VV or CV when virus was applied to abraded skin on the orofacial region. All SKH-1 mice developed orofacial skin lesions when inoculated with either CV at 4×106 to 4×104 pfu/ml or VV at 5×105 to 5×103 pfu/ml with area under the curve (AUC) values of 37–44. Vesicular lesions developed in CV- and VV-inoculated mice in a dose-dependent manner (Table 1 ). No mortality was observed in mice infected with VV or CV. Skin lesions that developed in these mice were categorized into three types and scored daily for severity. The first type of lesion occurred at the site of virus inoculation, the snout area, beginning on days 3 and 4 post-viral inoculation. The severity of these lesions peaked on days 9 and 10 and continued through day 20 (Fig. 1A ). The second type of skin lesion developed over the body beginning on day 4 and severity peaked on days 10–14 (Fig. 1B). This particular rash was follicular in distribution and no virus was isolated from this generalized follicular skin rash. A third type of skin lesion consisted of distinct vesicular “pock-type” lesions appearing on all skin surfaces (Fig. 1C) by day 6 post-inoculation and severity peaked on days 10–14 and continued through day 21. Biopsies of these vesicular lesions were obtained on day 12 from CV-inoculated mice for histopathology. Sections stained with hematoxylin-eosin showed large intracytoplasmic, eosinophilic brick-shaped inclusion bodies (Fig. 1D), indicative of orthopoxvirus infection. In addition, lesion scores and AUC values were analyzed statistically for orofacial and vesicular lesions.
Table 1.
Virus titers and vesicular lesion development in a cutaneous cowpox or vaccinia virus infection in SKH-1 mice
| Virusa (pfu/ml) | No. of virus positive/no. of inoculated | Virus titer–day AUC | Mean peak virus titer | No. of mice with lesions/no. of inoculated | Lesion day AUC | Mean peak lesion score |
|---|---|---|---|---|---|---|
| Cowpox | ||||||
| 4 × 106 | 10/10 | 46.1 | 5.2 | 8/10 | 3.8 | 0.8 |
| 4 × 105 | 10/10 | 32.0 | 4.0 | 7/10 | 4.3 | 0.7 |
| 4 × 104 | 10/10 | 8.4 | 2.2 | 6/10 | 1.7 | 0.6 |
| Vaccinia | ||||||
| 5 × 105 | 10/10 | 31.9 | 4.9 | 8/10 | 1.8 | 0.6 |
| 5 × 104 | 10/10 | 35.7 | 4.9 | 9/10 | 5.3 | 0.8 |
| 5 × 103 | 10/10 | 14.9 | 2.8 | 6/10 | 1.0 | 0.5 |
Virus was delivered using a virus-soaked Dacron swab applied to the snout for approximately 10s in 0.02ml doses.
Fig. 1.
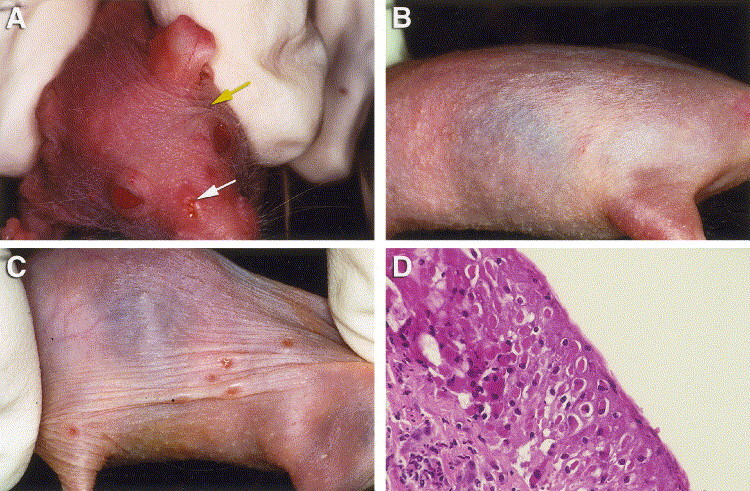
(A) Orofacial area of SKH-1 mouse. White arrow indicates site of inoculation. Yellow arrow indicates general location of facial skin lesions. (B) Rash-like lesions on infected SKH-1 mouse. (C) Vesicular lesions on lateral thorax. (D) Histopathology of biopsy of vesicular lesion from CV-infected SKH-1 mouse 6 days post-viral inoculation.
Swab samples from the orofacial area were assayed for virus quantitation. Both CV and VV were isolated from samples of the orofacial areas (Table 1), and all samples were positive for CV or VV at all virus concentrations used. Generally, mean peak virus titers and virus–titer day AUC values were highest in mice inoculated with the highest inoculum and lowest in mice inoculated with the lowest inoculum. Virus was first detected beginning 1 day after infection and persisted through 15 days post-infection.
3.2. Cutaneous infection of immunocompromised mice with CV or VV
Neither orofacial nor the rash-like body lesions seen in the SKH-1 mice occurred in the immunodeficient Athymic or Rhino mice (data not shown). The vesicular lesions, however, were more numerous, severe and prolonged in appearance in both Athymic and Rhino mice than the SKH-1 mice. The AUC values were higher in both strains of immunodeficient mice compared to the SKH-1 mice (Table 2 ). Samples for virus quantitation from the orofacial site of inoculation indicated that CV or VV replicated well in orofacial areas from each strain of mice. Virus was recovered from 1 day post-viral inoculation through day 15, indicating prolonged viral replication in the skin of all mice inoculated with CV or VV. Since Athymic mice had a 100% mortality rate to CV or VV and CV also produced mortality in Rhino mice, treatment studies were not performed using these immunocompromised animals.
Table 2.
Virus titers and vesicular lesion development in a cutaneous cowpox or vaccinia virus infection in Athymic, Rhino, or SKH-1 mice
| Virusa (pfu/ml) | No. of virus positive/no. of inoculated | Virus titer–day AUC | Mean peak virus titer | No. of mice with lesions/no. of inoculated | Lesion day AUC | Mean peak lesion score |
|---|---|---|---|---|---|---|
| Cowpox (4 × 105) | ||||||
| Athymic | 10/10 | 81.3 | 8.1 | 10/10 | 16.1 | 1.5 |
| Rhino | 8/10 | 88.7 | 8.2 | 10/10 | 20.8 | 1.5 |
| SKH-1 | 10/10 | 55.7 | 6.0 | 9/10 | 5.1 | 0.9 |
| Vaccinia (5 × 104) | ||||||
| Athymic | 10/10 | 90.2 | 8.0 | 10/10 | 13.3 | 1.5 |
| Rhino | 10/10 | 64.5 | 7.4 | 10/10 | 7.2 | 1.5 |
| SKH-1 | 10/10 | 52.5 | 7.0 | 8/10 | 5.0 | 1.4 |
Virus was delivered using a virus-soaked Dacron swab applied to the snout for approximately 10s in approximately 0.02ml doses.
3.3. Efficacy of cidofovir against CV or VV infections in SKH-1 mice
SKH-1 mice were inoculated with CV and treated i.p. with 50, 25, 20, 6.7, or 2.2 mg of CDV/kg beginning 24 h post-inoculation. Only mice treated with 50 mg/kg had significantly reduced lesion–day AUC and mean peak lesion scores for vesicular lesions when compared to untreated controls (Table 3 ). CDV at 25 mg/kg also slightly reduced virus-titer day AUC and mean peak virus titers in the orofacial swab samples (Table 3). No toxicity was observed at the highest dosage given.
Table 3.
Effect of i.p. treatment with CDVa on virus titers and vesicular lesion development in SKH-1 mice inoculated cutaneously with cowpox virus
| Treatment | No. of virus positive/no. of inoculated | Virus titer–day AUC | P-value | Mean peak virus titer | P-value | No. of mice with lesions/no. of inoculated | Lesion–day AUC | P-value | Mean Peak lesion score | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Experiment no. 1 | ||||||||||
| Untreated | 15/15 | 47.6 | – | 5.4 ± 1.33 | – | 7/9 | 7.9 | – | 0.9 ± 0.6 | – |
| Vehicle | 15/15 | 57.6 | – | 6.3 ± 1.06 | – | 11/15 | 5.0 | – | 0.8 ± 0.5 | – |
| CDV 20 mg/kg | 15/15 | 53.8 | NSb | 5.9 ± 1.24 | NS | 11/15 | 3.5 | NS | 0.6 ± 0.46 | NS |
| CDV 6.7 mg/kg | 15/15 | 48.8 | NS | 5.4 ± 1.41 | NS | 11/15 | 6.8 | NS | 0.8 ± 0.63 | NS |
| CDV 2.2 mg/kg | 15/15 | 45.4 | NS | 5.1 ± 1.42 | NS | 11/15 | 5.8 | NS | 0.8 ± 0.59 | NS |
| Experiment no. 2 | ||||||||||
| Untreated | 9/9 | 56.7 | – | 6.3 ± 1.25 | – | 7/9 | 7.9 | – | 0.9 ± 0.6 | – |
| Vehicle | 9/9 | 57.3 | – | 6.4 ± 1.24 | – | 6/9 | 2.7 | – | 0.5 ± 0.5 | – |
| CDV 50 mg/kg | 10/10 | 56.6 | NS | 6.0 ± 1.54 | NS | 7/10 | 4.4 | 0.02 | 0.6 ± 0.44 | 0.05 |
| CDV 25 mg/kg | 10/10 | 41.5 | 0.07 | 4.8 ± 1.70 | 0.09 | 8/10 | 4.1 | NS | 0.7 ± 0.48 | NS |
CDV was prepared in sterile saline and administered i.p. in 0.1ml volume beginning +24h after viral inoculation, three times weekly for 1 week.
NS: not statistically different from placebo-treated group.
When SKH-1 mice infected with VV were treated with 50 mg/kg of CDV i.p. or topically with a 5% CDV preparation, both treatment regimens significantly reduced the lesion–day AUC and mean peak scores of the vesicular lesions (P<0.01, Table 4 ). Also, topical 5% CDV treatment reduced facial lesions, but increased severity of body lesions (data not shown). Virus titers from orofacial swabs indicated that only topical CDV treatment significantly reduced both virus titer–day AUC and mean peak virus titers (Table 4). There was, however, some toxicity associated with topical administration of 5% CDV which was most pronounced in infected animals which had weight loss and 20% mortality. Weight loss was also observed in uninfected animals.
Table 4.
Effect of parenteral or topical CDV on virus titers and vesicular lesion development in a cutaneous vaccinia virus infection in SKH-1 mice
| Treatment | No. of virus positive/no. of inoculated | Virus titer–day AUC | P-value | Mean peak virus titer | P-value | No. of mice with lesions/no. of inoculated | Lesion–day AUC | P-value | Mean peak lesion score | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Vehiclea | 15/15 | 54.0 | – | 6.5 ± 0.48 | – | 8/15 | 3.1 | – | 1.0 | – |
| CDV 50 mg/kga | 15/15 | 45.7 | NSb | 6.4 ± 0.46 | NS | 0/15 | 0.0 | <0.01 | 0.0 | <0.01 |
| Vehicle-carbopolc | 15/15 | 49.5 | – | 6.7 ± 0.40 | – | 6/15 | 4.0 | – | 0.8 | – |
| CDV 5%c | 14/15 | 9.3 | 0.001 | 2.7 ± 0.99 | 0.001 | 1/15 | 0.2 | <0.01 | 0.0 | <0.01 |
Treatment was initiated 24h post-inoculation once a day i.p. for 7 days at 0.1ml per dose.
NS: not statistically significant when compared to appropriate control or placebo-treated group.
Treatment was initiated 24h post-inoculation three times a day topically for 7 days at 0.03ml per dose.
Virus titers obtained from organ samples indicated that topical 5% CDV was the most effective treatment in the cutaneous VV infection. Skin samples from the site of virus inoculation had a minimum of 2 log10 reduction in virus titers after treatment began and was sustained through the course of the study (Fig. 2 ). In addition, in mice treated with topical 5% CDV there was no virus detected in lung, kidney, or spleen.
Fig. 2.
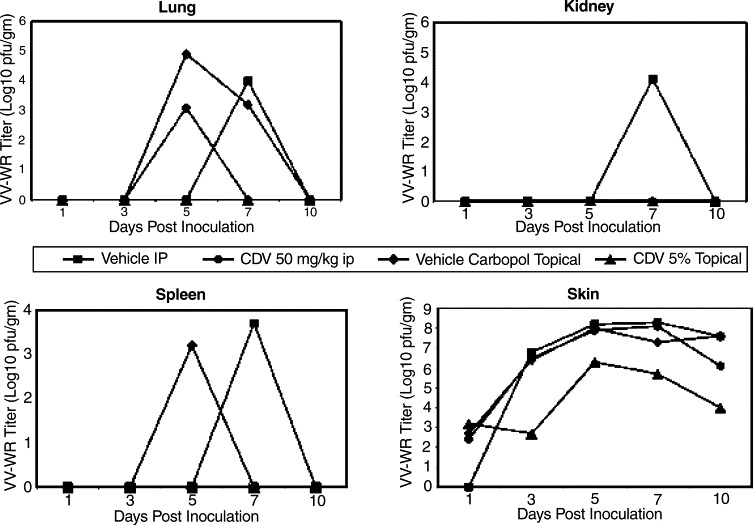
Alteration of VV–WR virus titers in organs of SKH-1 mice treated topically three times daily with 5% CDV or i.p. once daily with 50 mg/kg CDV beginning at 24 h after infection and continued through day 7.
4. Discussion
SKH-1 mice-infected cutaneously with CV or VV provide excellent, easily reproducible models for human orthopoxvirus disease and provide a means for evaluation of new antiviral compounds. These mice exhibit self-limiting disease, including typical vesicular dermatological lesions, and then recover fully from infection. Virus is shed from the site of inoculation in untreated mice for as long as 15 days post-viral inoculation.
The pathogenesis of these orthopoxviruses in the SKH-1 model was documented by viral quantitation from tissue homogenates obtained from untreated or treated mice over the initial course of disease. Viral replication occurred first in the orofacial skin on day 1 post-infection and later in systemic organs, including lung, spleen and kidney with viral replication beginning by day 5, just prior to appearance of vesicular skin lesions on day 6. These lesions occurred in skin covering all parts of the body including ears, nose, thorax, abdomen, digits, neck, and tail and developed approximately 6 days post-viral inoculation. This CV or VV pathogenesis is quite similar to the well described mousepox disease caused by ectromelia virus infection in mice (Fenner, 1948). In mousepox, virus invades the skin, replicates, enters the bloodstream as a primary viremia, replicates in organs such as liver and spleen by day 3, enters the blood for a secondary viremia on day 5, and then goes to the skin for focal infection and multiplication on day 6. The resultant ectromelia virus papules also contain numerous intracytoplasmic type A inclusions.
In our models using CV or VV, several different types of skin lesions were associated with infection. The most significant lesion was the vesicular “pock-type” lesion that was demonstrated to contain characteristic pox-like inclusion bodies. Both strains of immunodeficient mice also had more numerous, severe, persistent, and extensive vesicles and significant mortality which again supports the concept that the vesicular “pock-type” lesion is the most significant, virally-induced dermatological pathology. Other facial or body type lesions in CV and VV dermal infections of SKH-1 did not contain virus and are likely to be associated with an immunological response to the orthopoxvirus infections. Athymic mice and rhino mice did not exhibit the facial or body lesions which would support the concept of immune-mediated processes for those lesions.
CDV efficacy in these infections presents an interesting phenomenon. The i.p. administration of CDV at various dosages did not generally alter these infections. It is possible that systemically administered CDV does not reach adequate levels in the skin to alter viral replication, as quantities of virus detected by swabs of the facial region were generally unchanged by systemic administration. Although the numbers of animals that developed the pock-like vesicular lesions were reduced, it is possible that parenteral CDV reduced levels of virus during the primary or secondary viremic stages but not sufficient to prevent subsequent disease. The topical application of CDV, however, appeared to halt the replication at the site of infection and prevented the primary or secondary viremia from occurring, as documented by lack of virus in homogenates of the orofacial skin at the site of viral inoculation and the complete lack of vesicular lesions on day 6.
There was significant systemic absorption of CDV following topical application as evidenced by the degree of toxicity observed and inhibition of virus replication in lung, kidney, and spleen. Some toxicity, indicated by weight loss, was seen in uninfected mice that were treated three times daily. The infected mice, however, maintained some degree of dermal abrasion due to the repeated trauma of drug application and vigorous swabbing for virus quantitation. This atypical skin structure might allow for greater absorption of CDV into the systemic circulation. The infected mice treated topically with CDV also had significant weight loss, dehydration, and 20% mortality. The dehydration of the skin during the infection with topical CDV treatment made the body lesions appear worse. Further investigations of topical CDV may be quite useful to pursue as a potential benefit to patients with dermal lesions due to a variety of poxvirus infections, including progressive vaccinia, monkeypox, and molluscum contagiosum.
Smee and coworkers have utilized cutaneous VV infections in SKH-1 mice immunosuppressed with cyclophosphomide and reported that topical application of CDV was superior to parenteral CDV (Smee et al., 2003). Neyts and coworkers evaluated topical and systemic CDV in a cutaneous VV model and also found that topical CDV treatment initiated up to 24 h post-viral inoculation was protective against disseminated disease (Neyts et al., 2003). These two observations are in agreement with our results which clearly indicated that a topical 5% CDV preparation was more effective in the treatment of cutaneous CV or VV lesions than a high dose of CDV delivered parenterally.
These models provide a means for determining efficacy of new chemotherapeutic agents, vaccine candidates or immunomodulatory agents for cutaneous orthopoxvirus infections. Both systemic and topical applications of potential antiviral agents can be evaluated in these animal model systems to identify local versus systemic effects of treatment.
Acknowledgments
This work was supported by Public Health Services, contract no. NO1-AI-15439, from NIAID, NIH, Bethesda, MD. Excellent technical assistance by Ms. Kathy Keith is appreciated.
References
- Bienvenu B, Martinez F, Devergie A, Rybojad M, Rivet J, Bellenger P, Morel P, Gluckman E, Lebbe C. Topical use of cidofovir induced acute renal failure. Transplantation. 2002;73:661–662. doi: 10.1097/00007890-200202270-00033. [DOI] [PubMed] [Google Scholar]
- Boyle J.J, Haff R.F, Stewart R.C. Evaluation of antiviral compounds by suppression of tail lesions in vaccinia-infected mice. Antimicrob. Agents Chemother. 1967;6:536–539. [PubMed] [Google Scholar]
- Bray M, Martinez M, Smee D.F, Kefauver D, Thompson E, Huggins J.W. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J. Infect. Dis. 2000;181:10–19. doi: 10.1086/315190. [DOI] [PubMed] [Google Scholar]
- Buller R.M.L. The BALB/c mouse as a model to study orthopoxviruses. Curr. Top. Microbiol. Immunol. 1985;122:148–153. doi: 10.1007/978-3-642-70740-7_22. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, 2003a. Multistate outbreak of monkeypox—Illinois, Indiana, and Wisconsin, 2003. MMWR Morb. Mortal. Wkly. Rep. 52, 537–540. [PubMed]
- Centers for Disease Control and Prevention, 2003b. Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morbid. Mortal. Wkly. Rep. 52, 616–618. [PubMed]
- Cundy K.C, Lynch G, Lee W.A. Bioavailability and metabolism of cidofovir following topical administration to rabbits. Antivir. Res. 1997;35:113–122. doi: 10.1016/s0166-3542(97)00022-3. [DOI] [PubMed] [Google Scholar]
- De Clercq E, De Somer P. Effect of interferon, polyacrylic acid, and polymethacrylic acid on tail lesions in mice infected with vaccinia virus. Appl. Microbiol. 1968;16:1314–1319. doi: 10.1128/am.16.9.1314-1319.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E, Luczak M, Shugar D, Torrence P.F, Waters J.A, Witkop B. Effect of cytosine arabinoside, iododeoxyuridine, ethyldeoxyuridine, thiocyanatodeoxyuridine, and ribavirin on tail lesion formation in mice infected with vaccinia virus. Proc. Soc. Exp. Biol. Med. 1976;151:487–490. doi: 10.3181/00379727-151-39241. [DOI] [PubMed] [Google Scholar]
- De Clercq E, Holý A, Rosenberg I. Efficacy of phosphonylmethoxyalkyl derivatives of adenine in experimental herpes simplex virus and vaccinia virus infections in vivo. Antimicrob. Agents Chemother. 1989;33:185–191. doi: 10.1128/aac.33.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enserink M. Infectious diseases. US monkeypox outbreak traced to Wisconsin pet dealer. Science. 2003;300:1639. doi: 10.1126/science.300.5626.1639a. [DOI] [PubMed] [Google Scholar]
- Epstein W.L. Molluscum contagiosum. Semin. Dermatology. 1992;11:184–189. [PubMed] [Google Scholar]
- Fenner F. The pathogenesis of acute exanthems. An interpretation based on experimental investigations with mouse-pox (infectious ectromelia of mice) Lancet. 1948;2:915–920. doi: 10.1016/s0140-6736(48)91599-2. [DOI] [PubMed] [Google Scholar]
- Fields, B.N., Knipe, D.M., Howley, P.M. (Eds.), 1996. Fields Virology, vol. 2. Poxviruses, pp. 2693–2696.
- Fulginiti V.A, Papier A, Lane J.M, Neff J.M, Henderson D.A. Smallpox vaccination: a review. Part I. Background, vaccination technique, normal vaccination and revaccination, and expected normal reactions. Clin. Infect. Dis. 2003;37:241–250. doi: 10.1086/375824. [DOI] [PubMed] [Google Scholar]
- Fulginiti V.A, Papier A, Lane J.M, Neff J.M, Henderson D.A. Smallpox vaccination: a review. Part II. Adverse events. Clin. Infect. Dis. 2003;37:251–271. doi: 10.1086/375825. [DOI] [PubMed] [Google Scholar]
- Gordon Y.J, Romanowski E, Araullo-Cruz T. Topical HPMPC inhibits adenovirus type 5 in the New Zealand rabbit ocular replication model. Invest. Ophthalmol. Vis. Sci. 1994;35:4135–4143. [PubMed] [Google Scholar]
- Hillenkamp J, Reinhard T, Ross R.S, Bohringer D, Cartsburg O, Roggendorf M, De Clercq E, Godehardt E, Sundmacher R. Topical treatment of acute adenoviral keratoconjunctivitis with 0.2% cidofovir and 1% cyclosporine. Arch. Ophthalmol. 2001;119:1487–1491. doi: 10.1001/archopht.119.10.1487. [DOI] [PubMed] [Google Scholar]
- Hutin Y.J.F, Williams R.J, Malfait P, Pebody R, Loparev V.N, Ropp S.L, Rodriguez M, Knight J.C, Tshioko F.K, Khan A.S, Szczeniowski M.V, Esposito J.J. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauffman, C.L., Yoon, S.W., 2001. Molluscum contagiosum, eMedicine http://www.emedicine.com/derm/topic270.htm.
- Kern E.R, Hartline C, Harden E, Keith K, Rodriguez N, Beadle J.R, Hostetler K.Y. Enhanced inhibition of orthopoxvirus replication in vitro by alkoxyalkyl esters of cidofovir and cyclic cidofovir. Antimicrob. Agents Chemother. 2002;46:991–995. doi: 10.1128/AAC.46.4.991-995.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalezari J, Schacker T, Feinberg J, Gathe J, Lee S, Cheung T, Kramer F, Kessler H, Corey L, Drew W.L, Boggs J, McGuire B, Jaffe H.S, Safrin S. A randomized, double-blind, placebo-controlled trial of cidofovir gel for the treatment of acyclovir-unresponsive mucocutaneous herpes simplex virus infection in patients with AIDS. J. Infect. Dis. 1997;176:892–898. doi: 10.1086/516542. [DOI] [PubMed] [Google Scholar]
- Martinez M.J, Bray M.P, Huggins J.W. A mouse model of aerosol-transmitted orthopoxviral disease. Arch. Pathol. Lab. Med. 2000;124:362–377. doi: 10.5858/2000-124-0362-AMMOAT. [DOI] [PubMed] [Google Scholar]
- Maskalyk J. Monkeypox outbreak among pet owners. Can. Med. Assoc. J. 2003;169:44–45. [PMC free article] [PubMed] [Google Scholar]
- Melnick, J.L. (Ed.), 1988. Human Monkeypox, Monographs in Virology, S. Karger AG.
- Meyer H, Perrichot M, Stemmler M, Emmerich P, Schmitz H, Varaine F, Shungu R, Tshioko F, Formenty P. Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002;40:2919–2921. doi: 10.1128/JCM.40.8.2919-2921.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mims C.A. The response of mice to the intravenous injection of cowpox virus. Br. J. Exp. Pathol. 1968;49:24–32. [PMC free article] [PubMed] [Google Scholar]
- Neyts J, De Clercq E. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl) cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SCID) mice. J. Med. Virol. 1993;41:242–246. doi: 10.1002/jmv.1890410312. [DOI] [PubMed] [Google Scholar]
- Neyts J, De Clercq E. Efficacy of 2-amino-7-(1,3-dihydroxy-2-propoxymethyl) purine for treatment of vaccinia virus (orthopoxvirus) infections in mice. Antimicrob. Agents Chemother. 2001;45:84–87. doi: 10.1128/AAC.45.1.84-87.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyts, J., Leyssen, P., Verbeken, E., De Clercq, E., 2003. Effect of cidofovir on disseminated/progressive vaccinia in mice. In: Abstract LB-7 of 16th International Conference on Antiviral Research, Savannah, GA, 27 April–1 May.
- Quenelle D.C, Collins D.J, Kern E.R. Efficacy of multiple- or single-dose cidofovir against vaccinia and cowpox virus infections in mice. Antimicrob. Agents Chemother. 2003;47:3275–3280. doi: 10.1128/AAC.47.10.3275-3280.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenelle D.C, Collins D.J, Wan W.B, Beadle J.R, Hostetler K.Y, Kern E.R. Oral treatment of cowpox and vaccinia virus infections in mice with ether lipid esters of cidofovir. Antimicrob. Agents Chemother. 2004;48:404–412. doi: 10.1128/AAC.48.2.404-412.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee D.F, Bailey K.W, Sidwell R.W. Treatment of cowpox virus respiratory infections in mice with ribavirin as a single agent or followed sequentially by cidofovir. Antivir. Chem. Chemother. 2000;11:303–309. doi: 10.1177/095632020001100406. [DOI] [PubMed] [Google Scholar]
- Smee D.F, Bailey K.W, Wong M.H, Sidwell R.W. Intranasal treatment of cowpox virus respiratory infections in mice with cidofovir. Antivir. Res. 2000;47:171–177. doi: 10.1016/s0166-3542(00)00105-4. [DOI] [PubMed] [Google Scholar]
- Smee, D.F., Bailey, K.W., Sidwell, R.W., 2003. Vaccinia skin lesions in immunosuppressed hairless mice can be treated topically but not parenterally with cidofovir. In: Abstract 129 of 16th International Conference on Antiviral Research, Savannah, GA, 27 April–1 May, 2003. Antivir. Res. 57, A79.
- Toro J.R, Wood L.V, Patel N.K, Turner M.L. Topical cidofovir: a novel treatment for recalcitrant molluscum contagiosum in children infected with human immunodeficiency virus 1. Arch. Dermatol. 2000;136:983–985. doi: 10.1001/archderm.136.8.983. [DOI] [PubMed] [Google Scholar]


