Abstract
As of August 5, 2022, >26,000 cases of monkeypox have been diagnosed worldwide and the steep increase of cases has spurred renewed concern about the risk for another viral pandemic. In this narrative review, we address etiology, epidemiology and virology of monkeypox, describing routes of transmission and modes of spread. We also describe the current clinical presentation of monkeypox, focusing on circumstances where the disease should be suspected, and the methods to diagnose it. Finally, we briefly describe available treatments and strategies for active immune prophylaxis.
Keywords: Monkeypox, Viral spread, Routes of transmission, Clinical presentation, Antiviral treatment, Prophylaxis
1. What is Monkeypox?
Monkeypox (MPX) is a viral infectious disease characterized by symptoms that are similar to, but less severe than those of smallpox. It is caused by the monkeypox virus (MPXV) [1].
2. Is Monkeypox a new infection?
Monkeypox isn't at all a ‘novel’ infection. The first description of MPX dates back to the 1958, when a new virus, belonging to the Poxviridae family, was detected in captive monkeys at the State Serum Institute in Copenhagen [2]. After the initial discovery, several cases among different animal species were reported [3], [4], [5].
On September 1, 1970, a 9-month-old child, who had not been previously vaccinated for smallpox, and who was suspected of having the disease, was admitted to the Basankusu Hospital, Democratic Republic of Congo. Different specimens from the patient were sent to the WHO Smallpox Reference Center in Moskov, where a virus almost identical to MPXV was isolated. Only limited clinical information regarding this child illness was made available, with the exception of the presence of a skin eruption resembling that of smallpox [6]. In 1970, several MPX cases were diagnosed in Liberia and Sierra Leone. Subsequent serological examination of monkeys in the Congo region showed that these animals had had contact with an infectious agent belonging to the Poxviruses. This observation led to hypothesise that transmission from these animals to humans had occurred.
Early epidemiological analyses showed a low grade of contagiousness to men, and no human-to-human transmission among households was reported [7]. Between 1970 and 1979, 47 human MPX cases were diagnosed in different countries of Africa [8]. An analysis from the Centers for Disease Control and Prevention showed significant epidemiological changes during the years 1970–1997: between 1970 and 79 primary cases, i.e. cases stemming from animal-to-human transmission, were 91% (43/47) with a fatality rate as high as 17%; between 1981 and 86, primary cases decreased to 72% (243/338) and fatality rate to 10%; very few cases were reported thereafter (due to surveillance failure), and in 1996–97 primary cases were only 22% (92/419) with a fatality rate of <5% [9].
MPX was not reported outside Africa until 2003. In June 2003, 47 cases of MPX were reported in Illinois, Indiana and Wisconsin [10,11]. Traceback investigation identified an international shipment of about 800 small mammals from Ghana to Texas as the probable source of MPXV introduction in the USA [12]. In recent years, travel-associated cases of MPX related to a Nigeria outbreak were reported in Israel [13], UK [14,15] and Singapore [16]. Between January and September 2020, 4594 suspected cases were reported from Democratic Republic of Congo [17], while the second most affected country was Nigeria [18].
3. What is the current epidemiologic scenario?
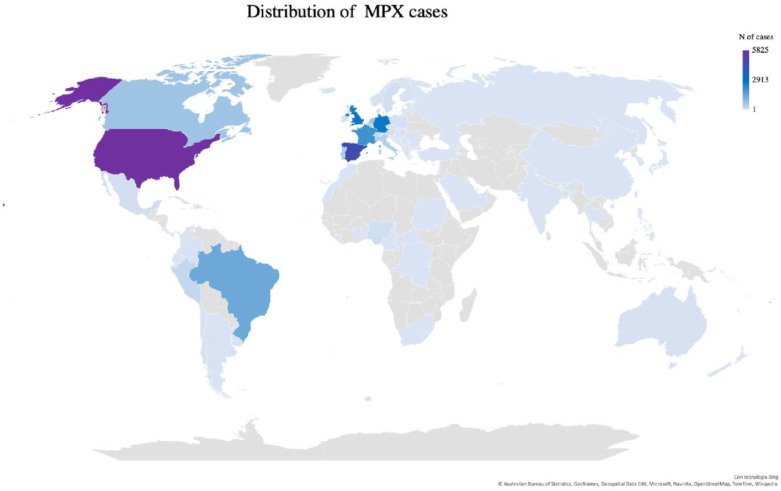
MPX received ‘renewed’ attention in May 2022, when an unprecedented outbreak of MPXV infection was reported in Europe, and beyond. At variance from the past, newly infected patients did not report any travel history to endemic regions or close contact with MPX confirmed or suspected cases, highlighting the emergence of a new area of endemic spread [19]. After the initial report, new cases were detected in different countries. On July 25, the WHO published an External Situation Report declaring this outbreak as a ‘public health emergency of international concern’ [20]. As of August 5, 2022, 26017 confirmed MPX cases were diagnosed, with 9 reported deaths (mortality <0.0005%). Cases were listed in >75 countries belonging to all six WHO regions (Fig. 1 ).
Fig. 1.
Distribution of Monkeypox cases from January 1, 2022 through August 3, 2022. Prevalence map constructed based on WHO data [https://worldhealthorg.shinyapps.io/mpx_global/, Accessed 6 August 2022].
Current epidemiological data show an overwhelming predominance of affected males (>99%) and young adult (median age 36 years, interquartile range 31–43). Among cases with reported sexual orientation, 98% (5470/5561) self-identified as being gay, bisexual, or men having sex with men (MSM), and as many as 41% (1873/4614) of cases with reported HIV status were indeed living with HIV [20].
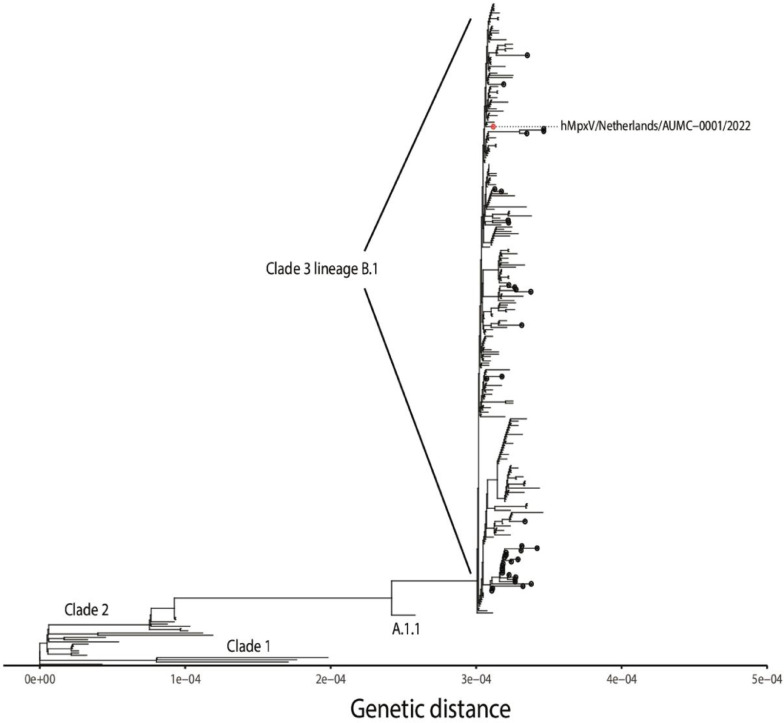
Genome analysis of current outbreak cases reveals a distinct monophyletic lineage of the current human MPXV. This lineage (B.1, belonging to clade 3) is characterized by heightened mutational signature comparing with its ancestors. This feature could explain the higher transmission rates observed and the related growing number of cases [21]. Phylogenetic analysis and the other MPXV clades are shown in Fig. 2 [22].
Fig. 2.
Phylogenetic analysis of the currently spreading MPXV and the other MPXV clades. Reprinted under CC-BY license from Tutu van Furth et al. [22].
4. What characterises the MPX causative agent and how does it spread?
MPXV is a 197 kb linear DNA genome Orthopoxvirus, member of the Poxviridae family, which includes smallpox, cowpox and vaccinia viruses. MPXV is 200–250 nm in size with virions enveloped into a lipoprotein outer membrane. A recognised cause of zoonosis endemic in West Africa and the Congo Basin [23], MPXV may spread via animal-to-human or human-to-human transmission.
Before the current outbreak, major risk factors for human-to-human transmission were identified in sharing the same bedroom or bed or household, and drinking or eating from the same dish. In contrast, behaviors implying increased exposure to animals were related to a higher risk of animal-to-human transmission [24]. Transmission occurs through direct skin/mucous membranes contact or inhalation of contaminated aerosols. Upon entry, MPXV infection starts via dermis or respiratory epithelium invasion, followed by lymphatic and then hematogenous spread [25]. In light of the high prevalence in MSM groups during the current outbreak, a sexual transmission also appears obvious. Indeed, analysis of samples obtained from infected patients showed high viral loads in saliva, rectal swab, semen, urine and fecal samples [26]. A preliminary study about transmissibility showed an R0 >1, with the highest figure of 1.60 for the Spanish study group. However, it should be kept in mind that in this early phase of outbreak R0 estimation has many limitations [27]. Miura et al estimated mean incubation period as 8.5 days [28].
5. Clinical presentation of monkeypox in the current outbreak
Before the current outbreak, symptoms of MPX included an initial febrile prodrome (1–4 days) followed by appearance of characteristic, deep-seated, well circumscribed skin lesions, typically showing a centrifugal distribution. Lesions on one area of the body were often synchronous in their development and in rash progression (macule → papule → vesicle → pustule → crust) to desquamation, which occurred 14–24 days after rash onset. Lymphadenopathy was seen in many MPX patients [29].
Since the first report of the ongoing (2022) outbreak, a copious amount of case descriptions were published. We summarized in Table 1 the chief epidemiological and clinical features from the three major studies published by July 25, 2022 [30], [31], [32].
Table 1.
Current clinical features of MPX as described in 3 recently-published, large case series.
| Reference | Number of patients | Country | Age range | Sex (%) | Sexual behaviour, (%) | HIV status, (%) | Concomitant STI, (%) | Systemic symptoms, (%) | Distribution of lesion | Lymphadenopathy, (%) | Complications, (%) | Source of MPXV DNA detection (%) | Antiviral treatment, (%) | Hospitalization, (%) | Mortality, (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [30] | 508 | Spain | 20-69 | M 503 (99) | MSM 397 (78) | 225 (44) | N/A | Fever (63) Asthenia (47) Myalgia (36) Odynophagia (28) Proctitis (16) |
Genital and perineal 71% Legs and arms 44% Face 39% Chest abdomen 31% Palms and soles 24% |
311 (61) | Parapharyngeal abscess, mouth ulcers, bacterial superinfection (1.3) |
Vesicular lesion (100) |
0 (0) | 19 (3.7) | 0 (0) |
| [31] | 27* | Portugal | 22-51 | M 27 (100) | MSM 18 (95) | 14 (52) | N/A | Fever (48) Exanthema (52) |
Genital ulcers 22% | 14 (52) | 0 (0) | Vesicular lesion (100) |
0 (0) | 3 (11) | 0 (0) |
| [32] | 528 | 16 countries | 18 (68%) |
M 527 (99) | MSM 509 (96) Heterosexual 9 (2) Bisexual 10 (2) |
218 (41) | 109 (29) | Fever 330 (62) Pharyngitis 113 (21) Proctitis or anorectal pain 75 (14) Rash 500 (95) |
Anogenital area 383 (73%) Trunk or limbs 292 (55%) Face 134 (25%) Palms and soles 51 (20%) |
295 (56) | Epiglottitis 1 (< 1) Myocarditis 2 (< 1) |
Skin or anogenital lesion (97) Semen (29) |
25 (5) | 70 (13) | 0 (0) |
MSM: men having sex with men; HIV, human immunodeficiency virus; STI, sexually transmitted infection; MPXV, monkeypox virus.
in this study the total number of MKPV cases was 96 but data were available only for 27 patients
The WHO reported data from 9099 patients [20]. The clinical presentation of some MPX cases associated with this outbreak was atypical, and many cases were not presenting with the clinical picture and timing of signs and symptoms previously described. Any rash was described in 90% of cases (8216/9099) and genital rash in 37% (3426/9099); fever was present in 48% of cases (4429/9099) but no specific information was available about its timing. Other major signs and symptoms were: lymphadenopathy (32.8%), fatigue (24%), headache (21.7%) and oral lesions (11%). Asymptomatic cases were 780 (8.6%) [20].
These data clearly indicate the current disease is different from prior outbreaks. There is no longer a clear distinction between prodromal symptoms and the appearance of skin lesions, and systemic symptoms are described in only about half of cases. Skin lesions are often asynchronous. In addition, the new evidence of genital and perineal lesions pinpoints the route of transmission. In this regard, it is interesting to analyse the case of two MSM patients having both anal and oro-anal sex [33]. Twenty-four hours after sexual intercourse, the receptive partner developed perioral white spots and painful perianal blistering lesions. Forty-eight hours later, the insertive partner had symptoms of perioral papules which blistered and ulcerated, with subsequent papules on the mons pubis and penis which evolved into painful ulcers. Neither individual reported prodromal symptoms; rather, skin eruptions were followed by systemic manifestations of lymphadenopathy, fever, headache and diarrhoea [33].
A macroscopic and microscopic description of skin lesions was reported by Maronese et al. [34]. Skin lesions were represented by multiple vesicular-pustular lesions with marked umbilication and brownish central crusting and perilesional erythema at dermoscopy. Histology revealed a central area of full-thickness epidermal necrosis with adjacent acanthosis and keratinocyte degeneration. Full-thickness inflammatory infiltrate and cytopathic changes on vascular walls were found in the underlying dermis [34].
6. When to suspect and how to diagnose monkeypox
According to the ECDC, a confirmed case is a person with a laboratory-confirmed MPX infection: this implies a positive result on MPXV-specific PCR assay or an orthopoxvirus-specific PCR positive result plus confirmation of the detected virus as MPXV by nucleotide sequence determination. A probable case is defined as either A) a person with an unexplained rash on any part of the body AND one or more additional symptom(s) of MPXV infection (fever higher >38.5 °C, headache, backache, fatigue, localized or generalized lymphadenopathy) PLUS one of the following: 1) positive laboratory test result for orthopoxviral infection; 2) epidemiological link to a confirmed or probable case of MPX in the 21 days before symptom onset; 3) travel to MPXV endemic countries in the 21 days before symptom onset; 4) multiple or anonymous sexual partners in the 21 days before symptom onset; 5) man having sex with men, or B) a person with an unexplained generalized or localized maculopapular or vesiculopustular rash with centrifugal spread, with lesions showing umbilication or scabbing, lymphadenopathy and one or more additional MPX-compatible symptoms [35].
At present, there is no role for serology in the diagnosis of MPX.
7. Is there any treatment for monkeypox?
In the attempt to avert a potential bioterrorism threat, a few drugs were recently developed in the US to treat smallpox infection. These antiviral agents are also active against MPXV. The FDA approved tecovirimat in 2018 and oral brincidofovir in 2021. Another active antiviral, already available since decades, is cidofovir, both in topical and intravenous formulations. Tecovirimat was also approved by the EMA in 2021 [36]. It acts inhibiting the viral protein p27, thereby preventing viral egress from infected cells. In contrast, brincidofovir and cidofovir block viral DNA polymerase [37]. Two clinical trials had actually been planned before the current outbreak to evaluate tecovirimat in MPXV infected patients [38]. NIOCH-14 is an analogue of tecovirimat that showed efficacy in a small number of patients.
Recommended tecovirimat dose for adult patients is 600 mg twice daily for 14 days, given either orally or intra-venously. Co-administration with repaglinide may cause hypoglycaemia. Interference of tecovirimat with cytochrome P450 enzymes translates into numerous interactions with antiviral agents, including anti-retroviral agents for the treatment of HIV-infection. The intra-venous formulation should not be administered to patients with severe renal impairment. [39]
Due to limited supply of MPX-specific antivirals, these drugs should be considered solely for those patients at risk for severe disease (or those who present or develop severe MPX, as recommended by WHO. Pregnant women, children, immune compromised subjects should be considered to be at higher risk for complications [40].
Vaccina immune globulin (VIG) is a recently FDA-approved medicinal product showing efficacy in complications resulting from smallpox vaccination [41]. It could have a role against MPXV but no clinical data are presently available on the use of VIG in MPXV-infected patients.
In light of current clinical, morbidity and mortality data, the goals of MPX management are essentially represented by supportive care, pain management and treatment of uncommon complications such as bacterial superinfections.
8. Who should be prioritized for prophylaxis
The progressive weaning of herd immunity against smallpox due to withdrawal of the relevant vaccine campaign is considered one of the main causes of the growing MPX incidence over the past decades [42]. Interestingly, median age of patients infected during the current MPX outbreak is about 10 years lower than that of latest smallpox vaccinees.
Both pre- and post-exposure MPXV prophylaxis may be prescribed. Available vaccines include MVA-BN (Bavarian Nordic) and ACAM 2000. The former is fully market authorized for MPX prophylaxis in the US and Canada, while it is licensed for smallpox and authorized under exceptional circumstances in Europe. The latter is approved in the US for smallpox and as emergency drug under investigation by FDA for MPXV post-exposure prophylaxis. MVA-BN is a 3rd generation, attenuated, non-replicating orthopoxvirus; ACAM 2000 is a 2nd generation live vaccinia virus [39,43].
Post-exposure prophylaxis is recommended for high- and medium-risk contacts. These are respectively defined as: 1) Direct exposure of skin or mucous membranes to skin or respiratory secretions of a person with confirmed, probable or suspected MPX, their body fluids or potentially infectious materials if not wearing appropriate personal protection equipment; 2) No direct contact but close proximity in the same room or indoor physical space with a symptomatic MPX patient, if not wearing appropriate protection.
Pre-exposure prophylaxis is indicated only for health-care workers at risk of exposure, research laboratory personnel, clinical laboratory personnel performing diagnostic testing for orthopoxviruses, and designated response team members at risk for occupational exposure to MPX [43].
Some authorities have suggested studying optimal strategies for pre-exposure prophylaxis in subgroups with a high prevalence of infection, such as MSM and patients living with HIV [44].
9. Take home message
MPX is spreading fast in previously non-endemic countries, and now represents a reason for concern. Mutations in MPXV have likely increased infectivity and favored human-to-human transmission, which is mostly confined to MSM as a sexually transmitted infection. Clinical presentation appears to have changed, and translates into subtle morbidity and exceedingly low mortality. For severe cases, active antivirals are available. Vaccine prophylaxis should be considered in at risk groups.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
EDM reports Consulting fees and Advisory Board membership for Roche, Genentech, Pfizer, Angelini and Advanz pharma, research support to his Institution from Pfizer, Merck, Angelini, Advanz pharma and Infectopharm, and Honoraria/Travel expenses support from Pfizer, Merck, Angelini, Advanz pharma, Infectopharm, Menarini, Shionogi, Biotest, and Abbvie. FP and RG have no conflicts of interest.
References
- 1.WHO. Monkeypox. 19/05/2022 available at https://www.who.int/news-room/fact-sheets/detail/monkeypox [Accessed 5 August 2022].
- 2.Magnus P.von, Andersen E.K., Petersen K.B., Birch-Andersen A. Acta Path Microbiol Scand. 1959;46:156–176. [Google Scholar]
- 3.Prier JE, Sauer RM, Malsberger RG, Sillaman JM. Studies on a pox disease of monkeys. II. Isolation of the etiologic agent. Am J Vet Res. 1960;21:381–384. MayPMID: 14434871. [PubMed] [Google Scholar]
- 4.McConnell S.J., Herman Y.F., Mattson D.E., Erickson L. Monkey pox disease in irradiated cynomolgus monkeys. Nature (Lond.) 1962;195:1128–1129. [Google Scholar]
- 5.Gispen R, Verlinde JD, Zwart P. Histopathological and virological studies on monkeypox. Arch Gesamte Virusforsch. 1967;21(2):205–216. doi: 10.1007/BF01241445. PMID: 4298424. [DOI] [PubMed] [Google Scholar]
- 6.Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46(5):593–597. PMID: 4340218; PMCID: PMC2480792. [PMC free article] [PubMed] [Google Scholar]
- 7.Marennikova SS, Seluhina EM, Mal'ceva NN, Cimiskjan KL, Macevic GR. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Health Org. 1972;46:599–611. [PMC free article] [PubMed] [Google Scholar]
- 8.Breman JG, Kalisa-Ruti, Steniowski MV, Zanotto E, Gromyko AI, Arita I. Human monkeypox, 1970-79. Bull World Health Org. 1980;58(2):165–182. PMID: 6249508; PMCID: PMC2395797. [PMC free article] [PubMed] [Google Scholar]
- 9.Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4(1):15–25. doi: 10.1016/s1473-3099(03)00856-9. JanErratum in: Lancet Infect Dis. 2004 Apr;4(4):251. PMID: 14720564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC) Multistate outbreak of monkeypox–Illinois, Indiana, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(23):537–540. Jun 13PMID: 12803191. [PubMed] [Google Scholar]
- 11.Reynolds MG, Yorita KL, Kuehnert MJ, Davidson WB, Huhn GD, Holman RC, Damon IK. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194(6):773–780. doi: 10.1086/505880. Sep 15Epub 2006 Aug 8. PMID: 16941343. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Update: multistate outbreak of monkeypox–Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(26):616–618. Jul 4PMID: 12844080. [PubMed] [Google Scholar]
- 13.Erez N, Achdout H, Milrot E, Schwartz Y, Wiener-Well Y, Paran N, et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25(5):980–983. doi: 10.3201/eid2505.190076. PMID: 30848724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaughan A, Aarons E, Astbury J, Balasegaram S, Beadsworth M, Beck CR, Chand M, O'Connor C, Dunning J, Ghebrehewet S, Harper N, Howlett-Shipley R, Ihekweazu C, Jacobs M, Kaindama L, Katwa P, Khoo S, Lamb L, Mawdsley S, Morgan D, Palmer R, Phin N, Russell K, Said B, Simpson A, Vivancos R, Wade M, Walsh A, Wilburn J. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23(38) doi: 10.2807/1560-7917.ES.2018.23.38.1800509. SepPMID: 30255836; PMCID: PMC6157091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, Osborne JC, Rampling T, Beadsworth MB, Duncan CJ, Dunning J, Fletcher TE, Hunter ER, Jacobs M, Khoo SH, Newsholme W, Porter D, Porter RJ, Ratcliffe L, Schmid ML, Semple MG, Tunbridge AJ, Wingfield T, Price NM, NHS England High Consequence Infectious Diseases (Airborne) Network Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;S1473-3099(22):00226–00228. doi: 10.1016/S1473-3099(22)00228-6. May 24Epub ahead of print. Erratum in: Lancet Infect Dis. 2022 Jul;22(7):e177. Erratum in: Lancet Infect Dis. 2022 Jul;22(7):e177. PMID: 35623380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yong SEF, Ng OT, Ho ZJM, Mak TM, Marimuthu K, Vasoo S, Yeo TW, Ng YK, Cui L, Ferdous Z, Chia PY, Aw BJW, Manauis CM, Low CKK, Chan G, Peh X, Lim PL, Chow LPA, Chan M, Lee VJM, Lin RTP, Heng MKD, Leo YS. Imported Monkeypox, Singapore. Emerg Infect Dis. 2020;26(8):1826–1830. doi: 10.3201/eid2608.191387. AugEpub 2020 Apr 27. PMID: 32338590; PMCID: PMC7392406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Health Organization. Regional Office for Africa. 2020. Weekly bulletin on outbreak and other emergencies: week 41: 05–11 October 2020. Available from: https://apps.who.int/iris/handle/10665/336026 [Accessed 5 August 2022].
- 18.Nigeria Centre for Disease Control. Nigeria monkeypox monthly situation report. December 2019. Available from: https://reliefweb.int/sites/reliefweb.int/files/resources/5a1a9820f21136842ba43f186b8d09e7.pdf [Accessed 5 August 2022].
- 19.Vivancos R, Anderson C, Blomquist P, Balasegaram S, Bell A, Bishop L, Brown CS, Chow Y, Edeghere O, Florence I, Logan S, Manley P, Crowe W, McAuley A, Shankar AG, Mora-Peris B, Paranthaman K, Prochazka M, Ryan C, Simons D, Vipond R, Byers C, Watkins NA, UKHSA Monkeypox Incident Management team. Welfare W, Whittaker E, Dewsnap C, Wilson A, Young Y, Chand M, Riley S, Hopkins S, Monkeypox Incident Management Team Community transmission of monkeypox in the United Kingdom, April to May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200422. JunErratum in: Euro Surveill. 2022 Jun;27(23): PMID: 35656834; PMCID: PMC9164677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO. Multi-country outbreak of monkeypox, External situation report #2 - 25 July 2022 available at https://www.who.int/publications/m/item/multi-country-outbreak-of-monkeypox-external-situation-report-2-25-july-2022 [Accessed 5 August 2022].
- 21.Luna N, Ramírez AL, Muñoz M, Ballesteros N, Patiño LH, Castañeda SA, Bonilla-Aldana DK, Paniz-Mondolfi A, Ramírez JD. Phylogenomic analysis of the monkeypox virus (MPXV) 2022 outbreak: Emergence of a novel viral lineage? Travel Med Infect Dis. 2022;49 doi: 10.1016/j.tmaid.2022.102402. Jul 13Epub ahead of print. PMID: 35840078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tutu van Furth AM, van der Kuip M, van Els AL, Fievez LC, van Rijckevorsel GG, van den Ouden A, Jonges M, Welkers MR. Paediatric monkeypox patient with unknown source of infection, the Netherlands, June 2022. Euro Surveill. 2022;27(29) doi: 10.2807/1560-7917.ES.2022.27.29.2200552. JulPMID: 35866435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Subramaniam G, Karuppanan K. Human monkeypox outbreak in 2022. J Med Virol. 2022 doi: 10.1002/jmv.27894. May 30Epub ahead of print. PMID: 35637363. [DOI] [PubMed] [Google Scholar]
- 24.Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, Steffen R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2) doi: 10.1371/journal.pntd.0010141. Feb 11PMID: 35148313; PMCID: PMC8870502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saied AA. Should not airborne transmission be ignored in the 2022 monkeypox outbreak? Int J Surg. 2022;104 doi: 10.1016/j.ijsu.2022.106762. Jul 5Epub ahead of print. PMID: 35798203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peiró-Mestres A, Fuertes I, Camprubí-Ferrer D, Marcos MÁ, Vilella A, Navarro M, Rodriguez-Elena L, Riera J, Català A, Martínez MJ, Blanco JL, Hospital Clinic de Barcelona Monkeypox Study Group Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022;27(28) doi: 10.2807/1560-7917.ES.2022.27.28.2200503. JulPMID: 35837964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwok KO, Wei WI, Tang A, Shan Wong SY, Tang JW. Estimation of local transmissibility in the early phase of monkeypox epidemic in 2022. Clin Microbiol Infect. 2022;S1198-743X(22):00340–00348. doi: 10.1016/j.cmi.2022.06.025. Jul 8Epub ahead of print. PMID: 35817231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCollum AM, Nakazawa Y, Ndongala GM, Pukuta E, Karhemere S, Lushima RS, Ilunga BK, Kabamba J, Wilkins K, Gao J, Li Y, Emerson G, Damon IK, Carroll DS, Reynolds MG, Malekani J, Tamfum JJ. Human monkeypox in the Kivus, a conflict region of the Democratic Republic of the Congo. Am J Trop Med Hyg. 2015;93(4):718–721. doi: 10.4269/ajtmh.15-0095. OctEpub 2015 Aug 17. PMID: 26283752; PMCID: PMC4596588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miura F, van Ewijk CE, Backer JA, Xiridou M, Franz E, Op de Coul E, Brandwagt D, van Cleef B, van Rijckevorsel G, Swaan C, van den Hof S, Wallinga J. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27(24) doi: 10.2807/1560-7917.ES.2022.27.24.2200448. JunPMID: 35713026; PMCID: PMC9205160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iñigo Martínez J, Gil Montalbán E, Jiménez Bueno S, Martín Martínez F, Nieto Juliá A, Sánchez Díaz J, García Marín N, Córdoba Deorador E, Nunziata Forte A, Alonso García M, Humanes Navarro AM, Montero Morales L, Domínguez Rodríguez MJ, Carbajo Ariza M, Díaz García LM, Mata Pariente N, Rumayor Zarzuelo M, Velasco Rodríguez MJ, Aragón Peña A, Rodríguez Baena E, Miguel Benito Á, Pérez Meixeira A, Ordobás Gavín M, Lopaz Pérez MÁ, Arce Arnáez A. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Euro Surveill. 2022;27(27) doi: 10.2807/1560-7917.ES.2022.27.27.2200471. JulPMID: 35801519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perez Duque M, Ribeiro S, Martins JV, Casaca P, Leite PP, Tavares M, Mansinho K, Duque LM, Fernandes C, Cordeiro R, Borrego MJ, Pelerito A, de Carvalho IL, Núncio S, Manageiro V, Minetti C, Machado J, Haussig JM, Croci R, Spiteri G, Casal AS, Mendes D, Souto T, Pocinho S, Fernandes T, Firme A, Vasconcelos P, Freitas G. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Euro Surveill. 2022;27(22) doi: 10.2807/1560-7917.ES.2022.27.22.2200424. JunPMID: 35656830; PMCID: PMC9164676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thornhill JP, Barkati S, Walmsley S, Rockstroh J, Antinori A, Harrison LB, Palich R, Nori A, Reeves I, Habibi MS, Apea V, Boesecke C, Vandekerckhove L, Yakubovsky M, Sendagorta E, Blanco JL, Florence E, Moschese D, Maltez FM, Goorhuis A, Pourcher V, Migaud P, Noe S, Pintado C, Maggi F, Hansen AE, Hoffmann C, Lezama JI, Mussini C, Cattelan A, Makofane K, Tan D, Nozza S, Nemeth J, Klein MB, Orkin CM, SHARE-net Clinical Group Monkeypox virus infection in humans across 16 Countries - April-June 2022. N Engl J Med. 2022 doi: 10.1056/NEJMoa2207323. Jul 21Epub ahead of print. PMID: 35866746. [DOI] [PubMed] [Google Scholar]
- 33.Heskin J, Belfield A, Milne C, Brown N, Walters Y, Scott C, Bracchi M, Moore LS, Mughal N, Rampling T, Winston A, Nelson M, Duncan S, Jones R, Price DA, Mora-Peris B. Transmission of monkeypox virus through sexual contact - A novel route of infection. J Infect. 2022;S0163-4453(22):00335–00338. doi: 10.1016/j.jinf.2022.05.028. Jun 1Epub ahead of print. PMID: 35659548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maronese CA, Beretta A, Avallone G, Boggio FL, Marletta DA, Murgia G, Cusini M, Gori A, Carrera CG, Di Benedetto A, Ramoni S, Marzano AV. Clinical, dermoscopic and histopathological findings in localized human monkeypox: a case from northern Italy. Br J Dermatol. 2022 doi: 10.1111/bjd.21773. Jul 13Epub ahead of print. PMID: 35822390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.European Centre for Disease Prevention and Control/WHO Regional Office for Europe. Monkeypox, joint epidemiological overview, 27 July, 2022. Available at https://cdn.who.int/media/docs/librariesprovider2/monkeypox/monkeypox_euro_ecdc_final_jointreport_2022-07-27_datecorrected.pdf?sfvrsn=45ba08a7_1&download=true [Accessed 5 August 2022].
- 36.Tecovirimat SIGA European medicines agency; 2021 available at https://www.ema.europa.eu/en/medicines/human/EPAR/tecovirimat-siga [Accessed 5 August 2022].
- 37.Grosenbach DW, Honeychurch K, Rose EA, Chinsangaram J, Frimm A, Maiti B, Lovejoy C, Meara I, Long P, Hruby DE. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379(1):44–53. doi: 10.1056/NEJMoa1705688. Jul 5PMID: 29972742; PMCID: PMC6086581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harris E. What to know about monkeypox. JAMA. 2022;327(23):2278–2279. doi: 10.1001/jama.2022.9499. Jun 21PMID: 35622356. [DOI] [PubMed] [Google Scholar]
- 39.Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Prevention and Treatment of Monkeypox. Drugs. 2022:1–7. doi: 10.1007/s40265-022-01742-y. Jun 28Epub ahead of print. PMID: 35763248; PMCID: PMC9244487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO. Clinical management and infection prevention and control for monkeypox: Interim rapid response guidance, 10 June 2022 available at https://www.who.int/publications/i/item/WHO-MPX-Clinical-and-IPC-2022.1 [Accessed 5 August 2022].
- 41.Vaccinia immune globulin intravenous (Human) https://www.fda.gov/vaccines-blood-biologics/approved-blood-products/vaccinia-immune-globulin-intravenous-human [Accessed 5 August 2022].
- 42.Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, Steffen R. The changing epidemiology of human monkeypox-A potential threat? A systematic review. PLoS Negl Trop Dis. 2022;16(2) doi: 10.1371/journal.pntd.0010141. Feb 11PMID: 35148313; PMCID: PMC8870502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.WHO. Vaccines and immunization for monkeypox: interim guidance, 14 June 2022. WHO reference number: WHO/MPX/Immunization/2022.1 available at https://www.who.int/publications/i/item/who-mpx-immunization-2022.1 [Accessed 5 August 2022].
- 44.Petersen E, Zumla A, Hui DS, Blumberg L, Valdoleiros SR, Amao L, Ntoumi F, Asogun D, Simonsen L, Haider N, Traore T, Kapata N, Dar O, Nachega J, Abbara A, Al Balushi A, Kock R, Maeurer M, Lee SS, Lucey DR, Ippolito G, Koopmans M. Vaccination for monkeypox prevention in persons with high-risk sexual behaviours to control on-going outbreak of monkeypox virus clade 3. Int J Infect Dis. 2022;S1201-9712(22):00372–00378. doi: 10.1016/j.ijid.2022.06.047. Jul 1Epub ahead of print. PMID: 35788415. [DOI] [PMC free article] [PubMed] [Google Scholar]