Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a major genetic disorder affecting up to 12 million individuals worldwide and the fourth most common global cause for kidney replacement therapy (KRT). It is a chronic progressive condition characterized by the development and growth of cysts in the kidneys and other organs and by additional systemic manifestations (1).
Master protocols have been proposed as a pathway toward facilitating the development of safe and effective therapies, as well as making drug development more efficient (2). Approaches range from the standardization of study design elements to creating and managing a central study structure where individual researchers test their potential therapeutics. As part of the May 2021 PKD Regulatory Summit, a workshop of stakeholders aiming to establish a master protocol for ADPKD was held. This included an international group of scientists, adult and pediatric clinicians, representatives from the pharmaceutical industry, governmental health authorities (HAs), academia, patients, and their families. The attendees agreed that it was premature to produce a collaborative master protocol but agreed on the key considerations for and structural elements of a standardized trial in ADPKD. This report summarizes their scientific recommendations, including patient input, within an approach to facilitate ADPKD therapeutic developments.
Workshop Outcomes
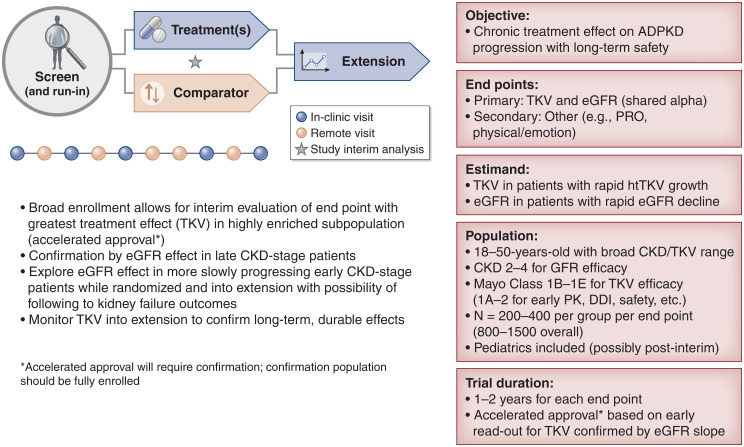
General accord was found for a common ADPKD protocol design (Figure 1). The sparsity of investigational therapies at the phase 3 stage and the lack of an infrastructure, such as is provided by the US National Cancer Institute and other global efforts, make it impractical to propose a true master protocol in the way that these have been used to develop therapies in oncology and the current viral pandemic.
Figure 1.
Autosomal dominant polycystic kidney disease (ADPKD) workshop outcome: A common ADPKD protocol design. DDI, drug-drug interaction; htTKV, height-adjusted total kidney volume; PK, pharmacokinetics; PRO, patient-reported outcome; TKV, total kidney volume.
The key objectives of an ADPKD pivotal trial are to demonstrate safe and effective slowing of ADPKD progression. Primary end points are, in part, on the basis of the stage of disease being tested as well as the mechanism of action of the treatment. Consensus was met for total kidney volume (TKV) growth and eGFR decline in adults as potential end points but should also include patient-centric concerns such as pain (3). Use of TKV (preferably using the most sensitive and reproducible techniques available [i.e., magnetic resonance imaging] or applicable to the population [i.e., ultrasound for young children]) is applicable only to interventions that might reduce the size of the kidney or rate of its growth, whereas eGFR is of universal importance as an intermediate clinical outcome. Until fully validated, it is premature to recommend available disease-specific patient-reported outcomes (PROs) for symptomatology as a key secondary end point, but PROs should be included in studies as exploratory measures and to further their validation. The meaningfulness of a PRO is expected to be supported by collateral clinician- or observer-reported outcomes. Outcome measures in the pediatric and adolescent population are also under development. Patient representatives strongly recommended assessment and collaborative refinement of freely available PROs that might demonstrate a therapeutic intervention’s effect on how patients feel and function.
Specific aspects proposed for a master protocol included estimand, population, patient number, comparator or standard of care use in the trial, and real-world evidence and PROs/clinician-reported outcomes.
Estimand
An estimand defines the specific population and statistical approaches used to reliably test a drug’s efficacy or safety. Recent international guidelines have encouraged the consideration of estimands in clinical research protocols (4). In a pivotal study, the primary estimand’s key attributes should align with the confirmatory objectives of the study. For example, TKV and eGFR change might best be evaluated in a subgroup defined by rapid progression and at low risk for early study withdrawal, whereas safety might need to be assessed in a broader population of patients with greater or lesser degrees of eGFR decline or TKV growth. The statistical analysis plan should define an estimand outlining which patients will be used for either efficacy or safety analyses. On the basis of the established relationship between TKV growth rate and future eGFR decline, Mayo classifications 1C, 1D, and 1E are proposed as the fastest progressing and most clinically relevant (5), although other clinical considerations (brought together in the PROPKD score) (6) may support classification of a rapid disease course. However, only those patients whose eGFR has reached CKD stage 3 or 4 or with historical evidence for rapid decline might demonstrate the minimally required reduction needed to reach the generally accepted outcome of a sustained and reproducible 30% eGFR decline. Patient safety might be studied across broader TKV (including Mayo classifications 2, 1A, and 1B) and CKD (including stages 1 and 2) categories, whereas the estimands focus evaluation of TKV or eGFR efficacy on more restricted, enriched subgroups. For confirmatory studies, the intercurrent event strategy is expected to be on the basis of the baseline population and a foreseen profile of treatment discontinuations and withdrawals. Measurements collected after treatment discontinuations due to reasons potentially related to treatment (e.g., treatment-related adverse events or lack of efficacy) should be included in the sensitivity analyses of the primary estimands.
Population
The age group of 18–50 years old is the group of interest as this is when rapidly progressing disease generally manifests itself. The extrapolation of outcomes from fast progressors to slower-progressing disease variants, older patients to pediatric patients, and patients with advanced stages should be considered separately and may require larger or separate trials, end points, and analysis. HAs in general have encouraged the inclusion of adolescents at phase 3 (7,8).
Patient Number
Placebo-controlled studies have used either a 1:1 or 2:1 randomization (active treatment versus placebo), with an enrollment goal adequate to satisfy the estimands (as on the basis of discussions with HAs). The actual enrollment depends on the effect size sought and safety database needed. Therefore, the “N” needed for efficacy may be smaller if enrichment designs are used.
Comparator or Standard of Care Use in the Trial
The use and development of a standard of care for background should be reviewed by the local HAs and are of particular importance in longer-term trials.
Accelerated Approval Pathways and Biomarker Use
Tolvaptan’s approvals in 2015 and 2018 by Health Canada, the European Medicines Agency (EMA) and the US Food and Drug Administration, respectively, were on the basis in the clinical studies of positive outcomes for a combination of surrogate end points (eGFR, TKV, and kidney pain) in ADPKD (9). Developers should consider using accelerated and conditional approval pathways that leverage the use of surrogate end points with a study that can be conducted in a timely fashion while generating an interpretable and confirmable result. Negotiations with HAs have set a precedent for using a reasonably likely surrogate efficacy biomarker (TKV) toward accelerated approval after an interim analysis, with full approval contingent on the success of an accepted clinical end point (e.g., 30% decline in eGFR by the conclusion of the trial) (3). HAs expressed the importance of taking measures to ensure the completion of the full study cohort, despite patients potentially having access to the drug commercially during the confirmatory portion of the ADPKD study. Studies should be fully enrolled at the time of the accelerated approval and have measures in place to address any missing patient data due to dropouts. Although TKV has not been validated in the pediatric population as a prognostic surrogate, children with increased TKV experience early hypertension and an accelerated rate of kidney growth (10).
Use of Real-World Evidence and Patient-Reported Outcomes/Clinician-Reported Outcomes
The incorporation of real-world evidence and PROs/clinician-reported outcomes as novel study end points that capture how the therapeutic intervention affects how patients feel or function as well as health-related quality of life was encouraged for inclusion. Use of the Autosomal Dominant Polycystic Kidney Disease Pain and Discomfort Scale was suggested (11).
Discussion
A workshop of key stakeholders in ADPKD development, including HA and patient input, discussed the potential design of a phase 3 master protocol in ADPKD.The workshop participants agreed that a master protocol approach as used in oncology may be less practical for ADPKD due to the relative lack of specific infrastructure funding (e.g., via the US National Institutes of Health or public-private consortium–based grants) that supports the majority of costs of running these trials, as well as the relatively smaller number of innovator companies in this area of development. Therefore, the workshop focused on near-term and longer-term goals for the ADPKD research community to consider. Even if a master protocol is not feasible by a consortium, the standardization of the key elements to support a common approach to a trial could still serve to facilitate and potentially accelerate the development of therapies. Patient representatives highlighted the importance of designing trials that are accessible to patients to enter and remain in across the 2+-year study duration that ADPKD trials require. Leveraging technology to permit remote patient visits and monitoring to ease the burden or need for on-site visits and assessments was related.
The outcome of the workshop was that further discussion and progress are needed to ultimately attain a phase 3 master protocol in ADPKD. Therefore, for this workshop, common study elements for a phase 3 ADPKD study were agreed to. This study template could be leveraged for use by drug developers for their own development programs and might further encourage investment and industry interest by lowering the risk and barrier to entry by providing clarity on how ADPKD studies can be conducted. Patient-level benefits in addition to the expected regulatory or clinical outcomes should be included in future studies.
Disclosures
F.S. Czerwiec is an employee of Sparrow Pharmaceuticals, Inc.; was an employee of Goldfinch Bio, Inc. at the time of the workshop; reports an investment portfolio that includes stock or stock options of several pharmaceutical and health care companies, including Goldfinch Bio, Inc., Merck, Novartis, Otsuka Holding Company, and Sparrow Pharmaceuticals, Inc.; reports research funding from Goldfinch Bio, Inc. and Sparrow Pharmaceuticals, Inc.; reports advisory or leadership roles for the Polycystic Kidney Disease Outcomes Consortium as the Industry Co-Director and Sparrow Pharmaceuticals, Inc. as the Chief Medical Officer; and reports speakers bureau for Goldfinch Bio, Inc. and Sparrow Pharmaceuticals, Inc. C. Ostroff is owner of Mill Street Consulting LLC, a practice providing regulatory affairs advice to drug developers; was an employee of Goldfinch Bio, Inc. at the time of the workshop and is a stockholder of Goldfinch Bio, Inc.; was an employee and stockholder of Janssen Pharmaceuticals, a JNJ company, within the past 3 years; reports stock options in Protagonist Therapeutics; reports honoraria from Gerson Lehrman Group, Inc.; and reports consultancy agreements with Mill Street Consulting LLC, Protagonist Therapeutics, and PureTech Health. R.D. Perrone reports consultancy agreements with Caraway, Navitor, Otsuka, Palladiobio, Reata, and Sanofi-Genzyme; research funding from Kadmon, Palladiobio, Reata, and Sanofi; honoraria from Otsuka, Reata, and Sanofi-Genzyme; advisory or leadership roles for Otsuka, Palladiobio, and Sanofi-Genzyme; speakers bureau for Haymarket Media; and other interests or relationships with the Critical Path Institute, the PKD Foundation, and UpToDate.
Funding
Funding for the 2021 PKD Regulatory Summit was provided by Otsuka Pharmaceutical and the PKD Foundation. Critical Path Institute is supported by the Food and Drug Administration (FDA) of the US Department of Health and Human Services (HHS) and is 54.2% funded by FDA/HHS, totaling $13,239,950, and 45.8% funded by nongovernment source(s), totaling $11,196,634.
Acknowledgments
We acknowledge the following participants of the workshop: Frank Czerwiec (Goldfinch Bio; now with Sparrow Pharmaceuticals), Aliza Thompson (the US Food and Drug Administration [FDA]), Romaldas Maciulaitis (EMA), Gregory Mainolfi (patient speaker), Peter Cory Fiduccia (patient speaker), Karl Cremer (Regulus Therapeutics), Kevin Fowler (patient advocate), Ken Gruchalla (Health Canada), Tess Harris (PKD charity), Neil Shusterman (Palladio Bio), Liron Walsh (Goldfinch Bio; now with Intellia Therapeutics), Alan Yu (the University of Kansas), Irina Barash (The Rogosin Institute), Arlene Chapman (the University of Chicago), Fouad Chebib (the Mayo Clinic), Michel Chonchol (the University of Colorado), Christian Hanna (the Mayo Clinic), Marie Hogan (the Mayo Clinic), Lesley Inker (Tufts Medical Center, Tufts University School of Medicine), Pravin Jadhav (Otsuka Pharmaceuticals), Max Liebau (the University of Cologne), Djalila Mekahli (Universitair Ziekenhuis Leuven), Colin Meyer (Reata Pharmaceuticals), Craig Ostroff (Goldfinch Bio; now with Mill Street Consulting), York Pei (the University of Toronto), Ronald Perrone (Tufts Medical Center, Tufts University School of Medicine), and Vicente Torres (the Mayo Clinic).
The contents are those of the author(s) and do not necessarily represent the official views of nor an endorsement by FDA/US Department of Health and Human Services or the US Government.
The content of this article reflects the personal experience and views of the author(s) and should not be considered medical advice or recommendations. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or CJASN. Responsibility for the information and views expressed herein lies entirely with the author(s).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
F.S. Czerwiec and R.D. Perrone conceptualized the study; R.D. Perrone provided supervision; C. Ostroff wrote the original draft; and F.S. Czerwiec, C. Ostroff, and R.D. Perrone reviewed and edited the manuscript.
References
- 1.Kidney Disease Improving Global Outcomes Consortium : Proposed Scope of Work for KDIGO Clinical Practice Guideline for the Evaluation, Management and Treatment of ADPKD, 2021. Available at: https://kdigo.org/wp-content/uploads/2021/01/ADPKD-Guideline-Scope-of-Work-for-Public-Review.pdf. Accessed April 28, 2022
- 2.US Food and Drug Administration : Master Protocols: Efficient Clinical Trial Design Strategies to Expedite Development of Oncology Drugs and Biologics Guidance for Industry, 2022. Available at: https:///www.fda.gov/regulatory-information/search-fda-guidance-documents/master-protocols-efficient-clinical-trial-design-strategies-expedite-development-oncology-drugs-and. Accessed April 28, 2022
- 3.Smith KA, Thompson AM, Baron DA, Broadbent ST, Lundstrom GH, Perrone RD: Addressing the need for clinical trial end points in autosomal dominant polycystic kidney disease: A report from the Polycystic Kidney Disease Outcomes Consortium (PKDOC). Am J Kidney Dis 73: 533–541, 2019. 10.1053/j.ajkd.2018.11.001 [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration : E9(R1) Statistical Principles for Clinical Trials: Addendum: Estimands and Sensitivity Analysis in Clinical Trials, 2021. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/e9r1-statistical-principles-clinical-trials-addendum-estimands-and-sensitivity-analysis-clinical. Accessed April 28, 2022
- 5.Irazabal MV, Rangel LJ, Bergstralh EJ, Osborn SL, Harmon AJ, Sundsbak JL, Bae KT, Chapman AB, Grantham JJ, Mrug M, Hogan MC, El-Zoghby ZM, Harris PC, Erickson BJ, King BF, Torres VE; CRISP Investigators : Imaging classification of autosomal dominant polycystic kidney disease: A simple model for selecting patients for clinical trials. J Am Soc Nephrol 26: 160–172, 2015. 10.1681/ASN.2013101138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cornec-Le Gall E, Audrézet MP, Rousseau A, Hourmant M, Renaudineau E, Charasse C, Morin MP, Moal MC, Dantal J, Wehbe B, Perrichot R, Frouget T, Vigneau C, Potier J, Jousset P, Guillodo MP, Siohan P, Terki N, Sawadogo T, Legrand D, Menoyo-Calonge V, Benarbia S, Besnier D, Longuet H, Férec C, Le Meur Y: The PROPKD score: A new algorithm to predict renal survival in autosomal dominant polycystic kidney disease. J Am Soc Nephrol 27: 942–951, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Medicines Agency : Extrapolation of Efficacy and Safety in Paediatric Medicine Development, 2018. Available at: https://www.ema.europa.eu/en/extrapolation-efficacy-safety-paediatric-medicine-development. Accessed April 28, 2022
- 8.Sun H, Temeck JW, Chambers W, Perkins G, Bonnel R, Murphy D: Extrapolation of efficacy in pediatric drug development and evidence-based medicine: Progress and lessons learned. Ther Innov Regul Sci 2017: 1–7, 2017. 10.1177/2168479017725558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Perrone RD, Koch G, Ouyang J, McQuade RD, Blais JD, Czerwiec FS, Sergeyeva O; REPRISE Trial Investigators : Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 377: 1930–1942, 2017. 10.1056/NEJMoa1710030 [DOI] [PubMed] [Google Scholar]
- 10.Cadnapaphornchai MA: Clinical trials in pediatric autosomal dominant polycystic kidney disease. Front Pediatr 5: 53, 2017. 10.3389/fped.2017.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oberdhan D, Cole JC, Krasa HB, Cheng R, Czerwiec FS, Hays RD, Chapman AB, Perrone RD: Development of the autosomal dominant polycystic kidney disease impact scale: A new health-related quality-of-life instrument. Am J Kidney Dis 71: 225–235, 2018 [DOI] [PubMed] [Google Scholar]