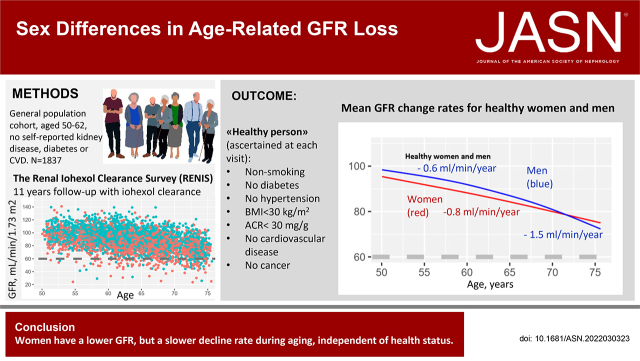
Significance Statement
Although more women than men have CKD, more men develop kidney failure. Sex or gender disparities in health status or access to health care, or sex-specific rates of kidney function decline, may explain sex or gender disparities in CKD epidemiology. In this study of a general northern European population, baseline kidney function (GFR measured by plasma iohexol clearance) was lower in middle-aged women than in men, whereas rate of decline in kidney function during aging was steeper among men. Sex disparities in comorbidity or CKD risk factors did not explain the sex differences in kidney function decline rates. This study suggests that sex differences in kidney function and kidney function decline rates may, in part, explain sex and gender disparities in the epidemiology of CKD.
Keywords: chronic kidney disease, clinical epidemiology, gender difference, glomerular filtration rate, glomerular hyperfiltration, kidney failure, microalbuminuria, renal function decline, risk factors, health status
Visual Abstract
Abstract
Background
CKD is more prevalent in women, but more men receive kidney replacement therapy for kidney failure. This apparent contradiction is not well understood.
Methods
We investigated sex differences in the loss of kidney function and whether any sex disparities could be explained by comorbidity or CKD risk factors. In the Renal Iohexol Clearance Survey (RENIS) in northern Europe, we recruited 1837 persons (53% women, aged 50–62 years) representative of the general population and without self-reported diabetes, CKD, or cardiovascular disease. Participants’ GFR was measured by plasma iohexol clearance in 2007–2009 (n=1627), 2013–2015 (n=1324), and 2018–2020 (n=1384). At each study visit, healthy persons were defined as having no major chronic diseases or risk factors for CKD. We used generalized additive mixed models to assess age- and sex-specific GFR decline rates.
Results
Women had a lower GFR than men at baseline (mean [SD], 90.0 [14.0] versus 98.0 [13.7] ml/min per 1.73 m2; P<0.001). The mean GFR change rate was −0.96 (95% confidence interval [CI], −0.88 to −1.04) ml/min per 1.73 m2 per year in women and −1.20 (95% confidence interval [CI], −1.12 to −1.28) in men. Although the relationship between age and GFR was very close to linear in women, it was curvilinear in men, with steeper GFR slopes at older ages (nonlinear effect; P<0.001). Healthy persons had a slower GFR decline, but health status did not explain the sex difference in the GFR decline.
Conclusion
Among middle-aged and elderly individuals in the general population, decline in the mean GFR in women was slower than in men, independent of health status.
CKD is projected to become the fifth leading cause of years of life lost in 2040.1 In most countries, more women than men develop CKD stage G3, which is defined as a reduced GFR, whereas more men start RRT.2,3 This apparent contradiction is poorly understood, but proposed explanations include gender disparities in access to health care and RRT, biologic differences between women and men leading to different GFR decline rates, bias in creatinine-based formulas to estimate the GFR, and overestimation of the CKD prevalence in women.3 In addition, sex and gender disparities in health status could cause differences in GFR loss.3 For example, women have a lower prevalence of myocardial infarction and a longer life expectancy than men.4 However, although cross-sectional population studies have found a higher mean GFR in healthy than in unhealthy persons,5 it is unknown whether good health is associated with preserved GFR during aging at the individual level, and whether this can explain the sex difference in CKD prevalence.3,5,6
Population-based longitudinal studies with repeated assessments of GFR in the same individuals are necessary to investigate the associations between sex, health status, and age-related GFR decline. The few existing studies on GFR change rates were not population based, did not investigate the association with health status, or used equations to calculate the eGFR on the basis of endogenous substances.6–13 These eGFR equations are biased by non–GFR-related factors, such as muscle mass, affecting men and women differently, particularly during aging.14–16 Measurements of GFR by an exogenous filtration marker, e.g., iohexol, avoid these methodologic problems.17
Accordingly, we investigated age- and sex-specific GFR decline rates in the Renal Iohexol Clearance Survey (RENIS), which is the only general population cohort with repeated measurements of GFR.18 The aim of the study was to report a reference range for age-related GFR decline in the general population and to investigate possible sex disparities in GFR decline rates by health status.
Methods
Study Sample
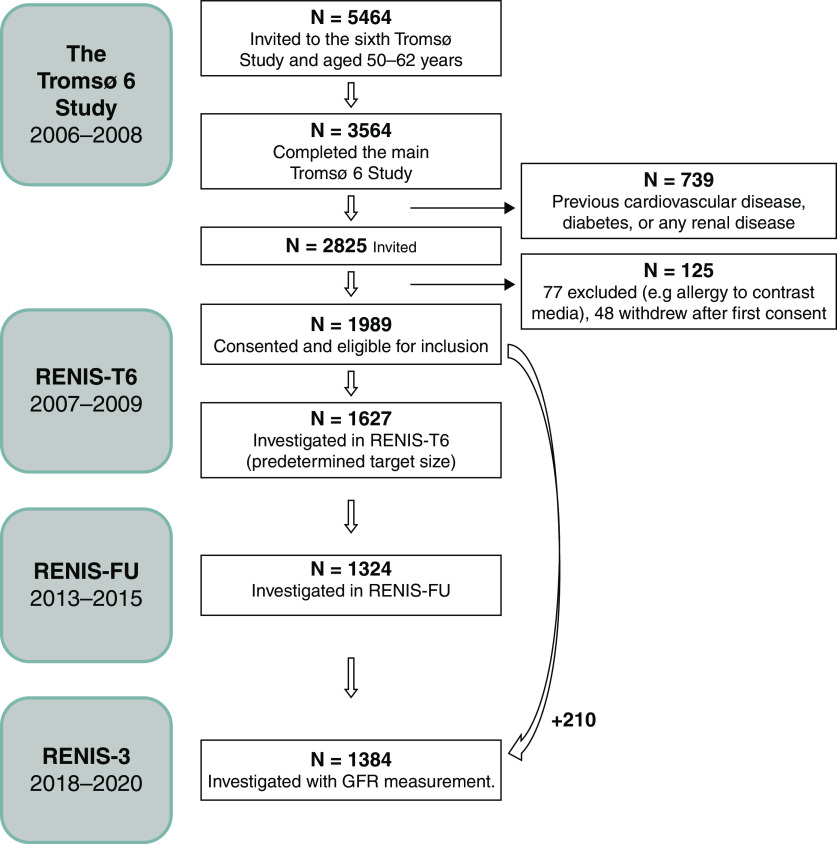
The RENIS in Tromsø 6 (RENIS-T6) study was a substudy of the sixth Tromsø population-based health survey in the municipality of Tromsø in northern Norway.19 The vast majority of the participants were White subjects because the Tromsø population has relatively few immigrants. A 40% random sample of individuals aged 50–59 years and all individuals aged 60–62 years (5464 total subjects) in Tromsø were invited to participate in the sixth Tromsø study. Of these, 3564 (65%) individuals completed the main part of the Tromsø 6, and those without self-reported diabetes, cardiovascular disease (CVD), or kidney disease were invited to participate in the RENIS-T6 (2007–2009) (Figure 1). A total of 2114 (75%) people consented to participate; 1989 were eligible for inclusion, and 1627 were included until the predetermined study size of the RENIS-T6 was obtained. The characteristics of the cohort were comparable to those of the total group of eligible recruits (n=2825; Supplemental Table 1).
Figure 1.
Flow diagram of the study subjects in RENIS. Inclusion of subjects in the Renal Iohexol Clearance Survey (RENIS). Participants were recruited from the sixth wave of the Tromsø Study.
Among those assessed at baseline, 1324 (83%) had follow-up GFR data in the RENIS-Follow-Up (RENIS-FU) (2013–2015) and 1174 (72%) had follow-up GFR data in the RENIS-3 (2018–2020). To counteract the tendency for internal selection bias in longitudinal cohort studies, in the RENIS-3, we also invited 353 persons who were eligible for inclusion in the RENIS-T6 but were not investigated. Because subjects were invited to participate in the RENIS-T6 in a random order until the inclusion target was met, this group (n=353) represented a random sample of all eligible persons. A total of 210 of these 353 persons were included, resulting in a total of 1384 participants with GFR measurement data in the RENIS-3 (Figure 1). The numbers of participants in RENIS with at least one, two, or three GFR measurements were 1837, 1410, and 1088 persons, respectively (Supplemental Figure 1). A random sample of 88 participants underwent two GFR measurements in the RENIS-FU to estimate day-to-day variability in the GFR. These measurements were also included in the analyses. Power calculations relevant for this study are given in the Supplemental Appendix.
The research protocol was approved by the Norwegian Data Inspectorate and the Regional Ethics Committee of Northern Norway (2016/2320/REK nord). All subjects provided informed written consent.
Data Collection and Definition of Variables
All measurements at each visit were performed in the morning between 8 and 10 am at the Clinical Research Unit, University Hospital of North Norway. Height and body weight were measured, and body mass index (BMI) was calculated. BP was measured three times, with 1-minute intervals, using an automated device (model UA-799; A&D). Fasting serum samples were drawn for standard laboratory measurements. Three samples of first-void morning spot urine were collected on consecutive days at each visit. The urinary albumin and creatinine concentrations were measured in fresh urine, and the albumin-creatinine ratio (ACR) in milligrams per millimole was calculated for each urine specimen.20 The median ACR value was used in the analyses.
Outcome of Interest
The outcome of interest for this investigation was the decline rate of the GFR. The GFR was measured by single-sample plasma iohexol clearance at all visits, as previously described in detail.21 In brief, 5 ml iohexol (300 mgI/ml) was injected through a Teflon catheter placed in an antecubital vein. Serum iohexol was measured at the optimal time point for each person on the basis of their eGFR, and the GFR was calculated with the formulas described by Jacobsson.21 Serum iohexol was measured using high-performance liquid chromatography in the RENIS-T6 and RENIS-FU and using liquid chromatography–mass spectrometry in the RENIS-3. The measurements in the RENIS-T6 and RENIS-3 were calibrated to the RENIS-FU measurements by reanalysis of frozen samples, as described in the Supplemental Appendix. The intraindividual (day-to-day) coefficient of variation in the GFR measurement was 4.2%, as previously reported.18
Covariates
Data regarding comorbidities, smoking habits, medication use, and hospital admissions were obtained through questionnaires at each visit. The use of lipid-lowering medications, antidiabetic drugs, cardiac glycosides (digoxin/digitoxin), or antihypertensive medications was registered as a dichotomous variable.
Tobacco smoking was categorized as current, previous, or never. Hypertension was defined as a systolic BP of ≥140 mm Hg, diastolic BP of ≥90 mm Hg, or the use of antihypertensive medication. Diabetes was defined as self-reported diabetes, use of antidiabetic medication, or measured hemoglobin A1c of ≥6.5% (≥48 mmol/mol) or fasting glucose of ≥7 mmol/L.
For the 210 participants in RENIS-3 who did not take part in the RENIS baseline investigation, we substituted baseline variables with the same variables registered in the main part of the Tromsø 6 that were collected a median of 3.8 (interquartile range, 2.4–4.7) months before RENIS-T6. In the total study population (n=1837), there were 11 missing values for hemoglobin A1c, one for diabetes, five for smoking, and six for albuminuria.
We measured serum creatinine and cystatin C as previously described.21 External quality control of both assays was provided by Equalis (www.equalis.se). The eGFR was calculated from creatinine (eGFRcrea), cystatin C (eGFRcys), and both (eGFRcreacys) using the Chronic Kidney Disease Epidemiology Collaboration equation.22
We defined health status as a time-dependent dichotomous variable that was ascertained concurrently with GFR measurements. A healthy person was defined as a nonsmoking person with no diabetes or hypertension; a BMI of <30 kg/m2; an ACR of <3.4 mg/mmol (30 mg/g); and without self-reported previous myocardial infarction, angina pectoris, coronary revascularization procedure, stroke, cancer, or use of lipid-lowering medication or cardiac glycosides.5 Information about persons who died was obtained from the Norwegian Cause of Death Registry.
Statistical Analyses
Differences in baseline characteristics between women and men were calculated using two-sample t tests or two-sample tests of proportions.
The associations between the GFR (ml/min per 1.73 m2) as the dependent variable and age, sex, and time-dependent health status as independent variables were explored in linear mixed models with a random intercept and slope and an unstructured covariance matrix. Because cross-sectional age differences in the GFR (between persons) and longitudinal GFR changes (within-person change) in this cohort study converged into a common trajectory (see Supplemental Appendix), we used chronologic age as the time variable and adjusted the analyses for sex-specific baseline age.23 A negative sign for the time coefficient indicates a decline in the GFR. The effects of sex and health status on the rate of change in the GFR were assessed by including two-way interaction terms between the variables in question and the time variable. We used generalized additive mixed models (GAMMs) to investigate a possible sex-specific nonlinear relationship between GFR and age.24 All study participants (n=1837) were included in the linear mixed model and GAMM analyses because mixed models allow for missing observations at one or more points in time.25,26 The Akaike information criterion (AIC) was used to compare the fit of the different models.27 On the basis of the GAMM with the best fit, the GFR change rate was calculated as the numeric time derivative of the GFR as a function of sex, age, and health status. The estimated best linear unbiased predictions of the random slope for each person were taken to represent the interindividual distribution of the GFR change rates and used to obtain percentiles of the change rates.
We constructed smoothed histograms of the distributions of the predicted GFR change rates separately for men and women by health status. This was performed by computing kernel density estimates of the variables using the geom density procedure in the ggplot2 package in R. Because the GFR decline curves in the best-fitting GAMM were approximately linear, and for the purpose of creating histograms, we calculated the mean predicted GFR decline rate for each person by subtracting the baseline predicted GFR from the predicted GFR at the last follow-up and dividing by the corresponding observation time for persons with at least two GFR measurements (n=1410).
Statistical analyses were performed using the mgcv and mgcViz packages in R version 4.1.0 (May 18, 2021; https://www.r-project.org/) and STATA version 16 (College Station, TX).24,28 Statistical significance was set at P<0.05.
Results
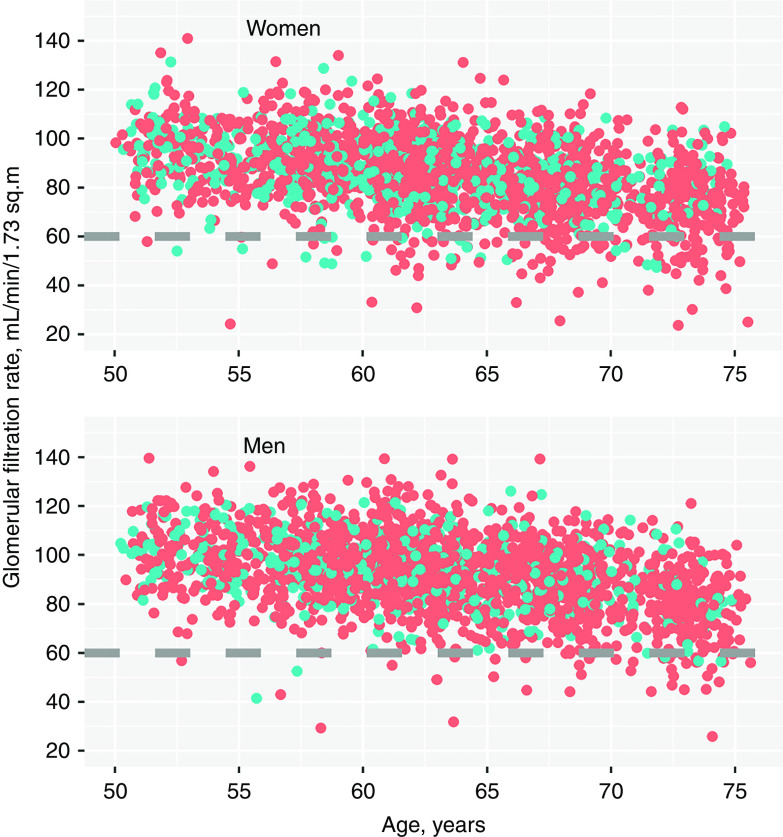
The study population consisted of 1837 persons with 4423 GFR measurements. The baseline characteristics are shown in Table 1. The mean (SD) baseline age was 58 (3.8) years, and 53% of the participants were women. The mean (SD) GFR at baseline was 90.0 (14.0) ml/min per 1.73 m2 in women and 98.0 (13.7) ml/min per 1.73 m2 in men. The median (interquartile range) follow-up time for those with GFR measurements at baseline (n=1627) was 10.7 (6.3–11.3) years. Thirty-two women (4%) and 50 men (6%) died during follow-up, which was ascertained on February 1, 2020. The proportions of healthy persons at the three RENIS visits were 26%, 27%, and 22%, respectively, and the proportions were higher in women than in men (Table 2). Healthy women (n=242) had, on average, an 8.3 ml/min per 1.73 m2 lower GFR at baseline than healthy men (n=179; P<0.001). A scatterplot showing the association between all GFR measurements and age in women and men separately is presented in Figure 2. A total of 127 of 2281 GFR measurements (5.6%) in women and 48 of 2142 GFR measurements (2.2%) in men were <60 ml/min per 1.73 m2 (the CKD stage 3 cutoff) at any time point.
Table 1.
Baseline characteristics of the RENIS cohort
| Baseline Characteristics | All (n=1837) | Women (n=974) | Men (n=863) |
|---|---|---|---|
| Age (yr), mean (SD) | 58.2 (3.8) | 58.1 (3.9) | 58.3 (3.8) |
| BMI (kg/m2), mean (SD) | 27.2 (4.0) | 26.7 (4.4) | 27.8 (3.4) |
| Obese (BMI>30 kg/m2), n (%) | 401 (22) | 187 (19) | 214 (25) |
| Systolic BP (mm Hg), mean (SD) | 129.9 (17.9) | 125.8 (17.6) | 134.4 (17.1) |
| Diastolic BP (mm Hg), mean (SD) | 82.7 (10.2) | 79.7 (9.8) | 86.0 (9.5) |
| Use of antihypertensive medication, n (%) | 361 (20) | 176 (18) | 185 (21) |
| Hypertension, n (%) | 762 (41) | 327 (34) | 435 (50) |
| Hemoglobin A1c (mmol/mol), mean (SD) | 37.2 (4.0) | 37.1 (3.9) | 37.4 (4.0) |
| Diabetes, n (%)a | 38 (2) | 16 (2) | 21 (2) |
| Current smoking, n (%) | 384 (21) | 216 (22) | 168 (20) |
| Total cholesterol (mmol/L), mean (SD) | 5.68 (0.95) | 5.75 (0.96) | 5.60 (0.94) |
| Lipid-lowering medication, n (%) | 121 (7) | 73 (7) | 48 (6) |
| Albuminuria (ACR>3.4 mg/mmol), n (%)b | 24 (1) | 11 (1) | 13 (1.5) |
| mGFR (ml/min per 1.73 m2), mean (SD)c | 93.9 (14.4) | 90.0 (14.0) | 98.0 (13.7) |
Estimates are given as the mean (SD) or number (percent). There were 11 missing values hemoblobin A1C, one for diabetes, five for current smoking, and six for albuminuria.
Numbers are undiagnosed diabetes on the basis of fasting glucose or hemoglobin A1c. Those with self-reported diabetes were excluded at baseline.
ACR>30 mg/g.
GFR measured using single-sample iohexol clearance. GFR was not measured at baseline for the additional 210 persons included in RENIS-3 who did not attend RENIS-T6.
Table 2.
Proportion of healthy women and men at baseline and follow-up
| Health Status | RENIS-T6 (2007–2009) | RENIS-FU (2013–2015) | RENIS-3 (2018–2020) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All (n=1627) | Women (n=826) | Men (n=801) | P Valuea | All (n=1324) | Women (n=667) | Men (n=657) | P Valuea | All (n=1384) | Women (n=744) | Men (n=640) | P Valuea | |
| Healthy, n (%)b | 421 (26) | 242 (30) | 179 (22) | 0.001 | 360 (27) | 197 (30) | 163 (25) | 0.05 | 299 (22) | 176 (24) | 123 (19) | <0.05 |
Chi-squared test for difference between women and men.
Healthy, defined as a nonsmoking person without diabetes, hypertension, myocardial infarction, angina pectoris, coronary revascularization procedures, stroke, cancer, and use of lipid-lowering medication or digoxin, and with a BMI of <30 kg/m2 and urinary ACR of <3.4 mg/mmol (30 mg/g).
Figure 2.
The association between GFR and age for women and men. Blue dots are healthy subjects and red dots are subjects defined as not healthy (prevalent comorbidity or CKD risk factors). Age was used as the time variable (baseline age plus follow-up time).
GFR Decline
There was a statistically significant interaction between sex and GFR change rates in the linear mixed model (P<0.001; model 1, Table 3). Men had a 25% steeper mean GFR decline than women (1.20 [95% confidence interval (CI), 1.12 to 1.28] versus 0.96 [95% CI, 0.88 to 1.04] ml/min per 1.73 m2 per year; model 1, Table 3). We introduced the dichotomous variable of health status into the model to see if it would modify the relationship between sex, age, and GFR. Persons defined as “not healthy” had a more rapid GFR decline of 0.28 (95% CI, 0.15 to 0.40) ml/min per 1.73 m2 per year than healthy persons (P<0.001; model 2, Table 3). The sex effect on the GFR change rate was very similar to that in the model without health status. There was no effect modification between sex and health status on GFR decline (P=0.34), indicating that health status had the same association with the GFR change rate in men and women.
Table 3.
Associations between sex, health status, and GFR change rates in linear mixed models
| Characteristics | Model 1 | Model 2 | ||
|---|---|---|---|---|
| Change in GFR, ml/min per 1.73 m2 per Year (95% Confidence Interval) | P Value | Change in GFR, ml/min per 1.73 m2 per Year (95% Confidence Interval) | P Value | |
| Women | −0.96 (−0.88 to −1.04) | <0.001 | −1.04 (−1.12 to −0.95) | <0.001 |
| Men | −1.20 (−1.12 to −1.28) | <0.001a | −1.26 (−1.18 to −1.35) | <0.001 |
| Healthyb | 0.28 (0.15 to 0.40) | <0.001 | ||
| Difference between men and women | −0.24 (−0.12 to −0.35) | <0.001 | −0.23 (−0.11 to −0.34) | <0.001 |
Both models were adjusted for age at baseline, with separate terms for women and men.
P<6 × 10−5 for effect modification by sex.
Healthy, defined at each visit as no CVD, cancer, diabetes, hypertension, smoking, lipid-lowering medication or digoxin, and a BMI of <30 kg/m2 and urinary ACR of <3.4 mg/mmol (30 mg/g).
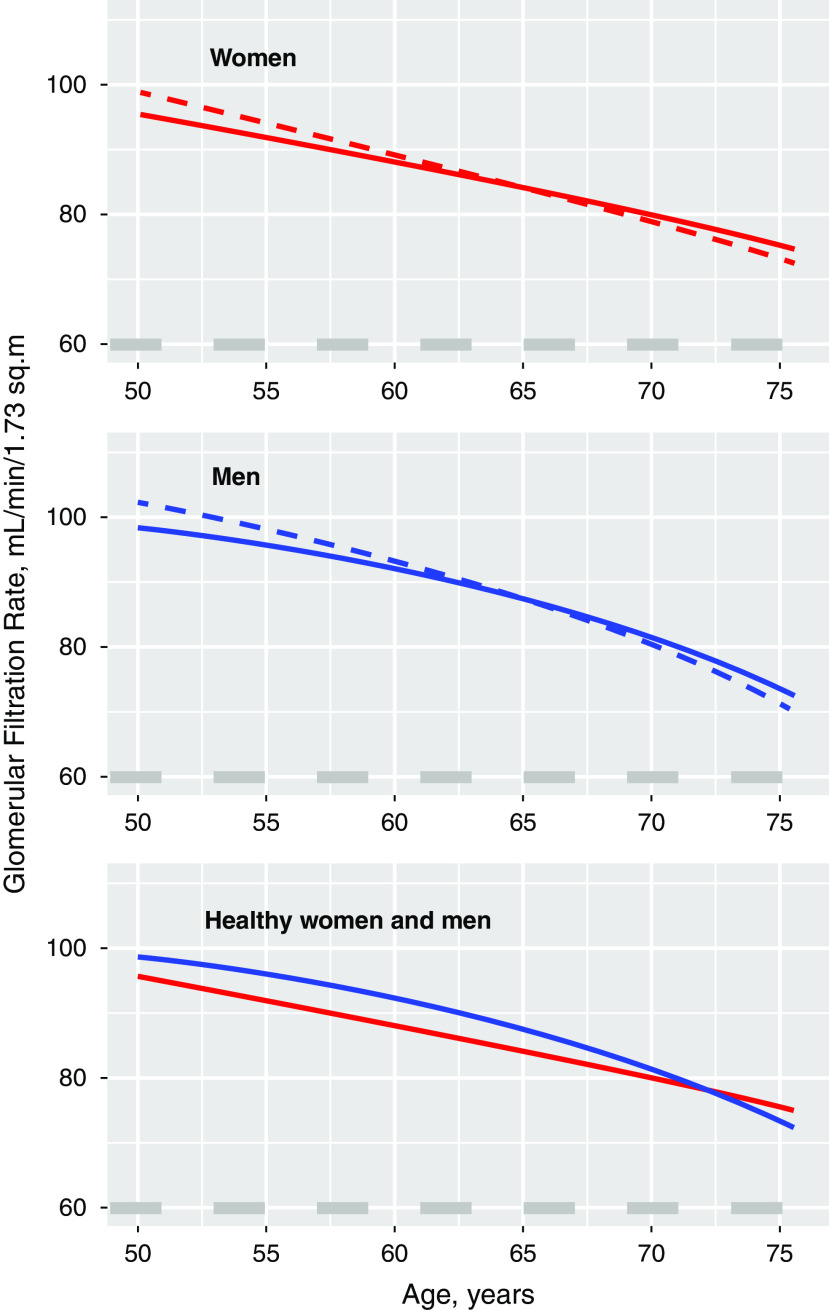
We then included sex-specific nonlinear terms for GFR change rates in this model and found that the fit of the GAMM was improved, as indicated by a substantial decrease in the Akaike information criterion relative to a model with only linear effects (from 33948 to 33929; model 3, Supplemental Table 2). Whereas the GFR-age relationship in women was very close to linear, the relationship in men was curvilinear, with a steeper GFR decline at older ages, as illustrated in Figure 3. The effects of sex and health status on the GFR change rate were similar in a model that added adjustments for BMI, fasting glucose, and systolic BP as continuous variables (model 4, Supplemental Table 3). Persons defined as “unhealthy” had a higher GFR by 3.5 (95% CI, 1.7 to 5.2) ml/min per 1.73 m2 at 50 years of age and a steeper GFR decline by 0.24 ml/min per 1.73 m2 per year (Figure 3, Supplemental Tables 2 and 3). We also obtained similar results when we used the absolute GFR in milliliters per minute as the dependent variable and added body weight and height as independent variables to adjust for body size (Supplemental Table 4).
Figure 3.
Mean GFR decline with age for women and men by health status (“healthy” in solid and “not healthy” in dashed). The lower panel depicts the mean GFR decline with age for healthy women versus healthy men. Calculated using a GAMM (model 3, Supplemental Table 2).
In analyses using the eGFRcrea and eGFRcreacys instead of the measured GFR (mGFR), there was no effect of sex on eGFR decline rates. For eGFRcys, the effect of sex was statistically significant, although smaller than with mGFR. For all eGFR equations, the association between health status and eGFR decline rates was weaker than that of the mGFR (Supplemental Table 5).
Age-Specific GFR Decline Rates in Healthy Women and Men
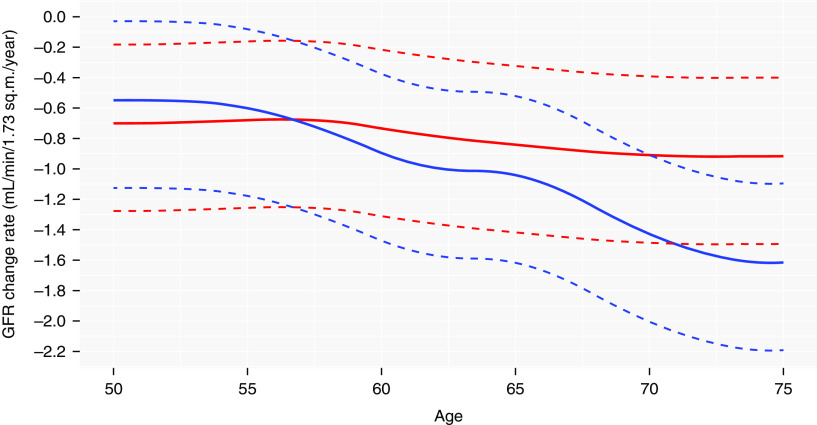
The GFR change rates in healthy women and men as a function of age were calculated by obtaining the numeric time derivative of the GAMM in Supplemental Table 2. The mean and 2.5th and 97.5th percentiles of the distributions of the GFR change rates are shown in Figure 4 and tabulated in Table 4. The maximum 97.5th percentile was less than −0.20 ml/min per 1.73 m2 for all subjects except men between 50 and 54 years, for whom it was −0.04 ml/min per 1.73 m2 (Figure 4), demonstrating very few persons had a stable GFR. Smoothed histograms of the distribution of individual mean predicted GFR decline rates for healthy and unhealthy women and men (n=1410 with at least two GFRs) using iohexol clearance compared with eGFRcrea, eGFRcys, and eGFRcreacys, are shown in Figure 5. Whereas the location and shape of the histograms for mGFR and eGFRcrea were similar, the mode of the histogram for eGFRcys was more negative and its spread greater.
Figure 4.
Sex-specific GFR change rates for healthy women and men as a function of age with 95% reference intervals. The solid lines represent the GFR change rates for women (red) and men (blue). The dashed lines represent the 95% reference intervals estimated from the best linear unbiased predictions of the random slopes of the GAMM in Supplemental Table 2.
Table 4.
Age-specific annual GFR change rates for healthy women and men
| Age Group, yr | Women | Men | ||||
|---|---|---|---|---|---|---|
| Mean | Percentiles | Mean | Percentiles | |||
| 2.5th | 97.5th | 2.5th | 97.5th | |||
| 50–54 | −0.72 | −1.29 | −0.20 | −0.58 | −1.15 | −0.06 |
| 55–59 | −0.72 | −1.30 | −0.20 | −0.73 | −1.30 | −0.21 |
| 60–64 | −0.79 | −1.36 | −0.27 | −0.98 | −1.55 | −0.46 |
| 65–69 | −0.86 | −1.43 | −0.34 | −1.22 | −1.79 | −0.70 |
| 70–75 | −0.88 | −1.46 | −0.37 | −1.52 | −2.09 | −1.00 |
The values are means (ml/min per 1.73 m2 per year) for each 5-year interval. The 95% reference intervals were estimated from the best linear unbiased predictions of the random slopes of the generalized additive model in Supplemental Table 2.
Figure 5.
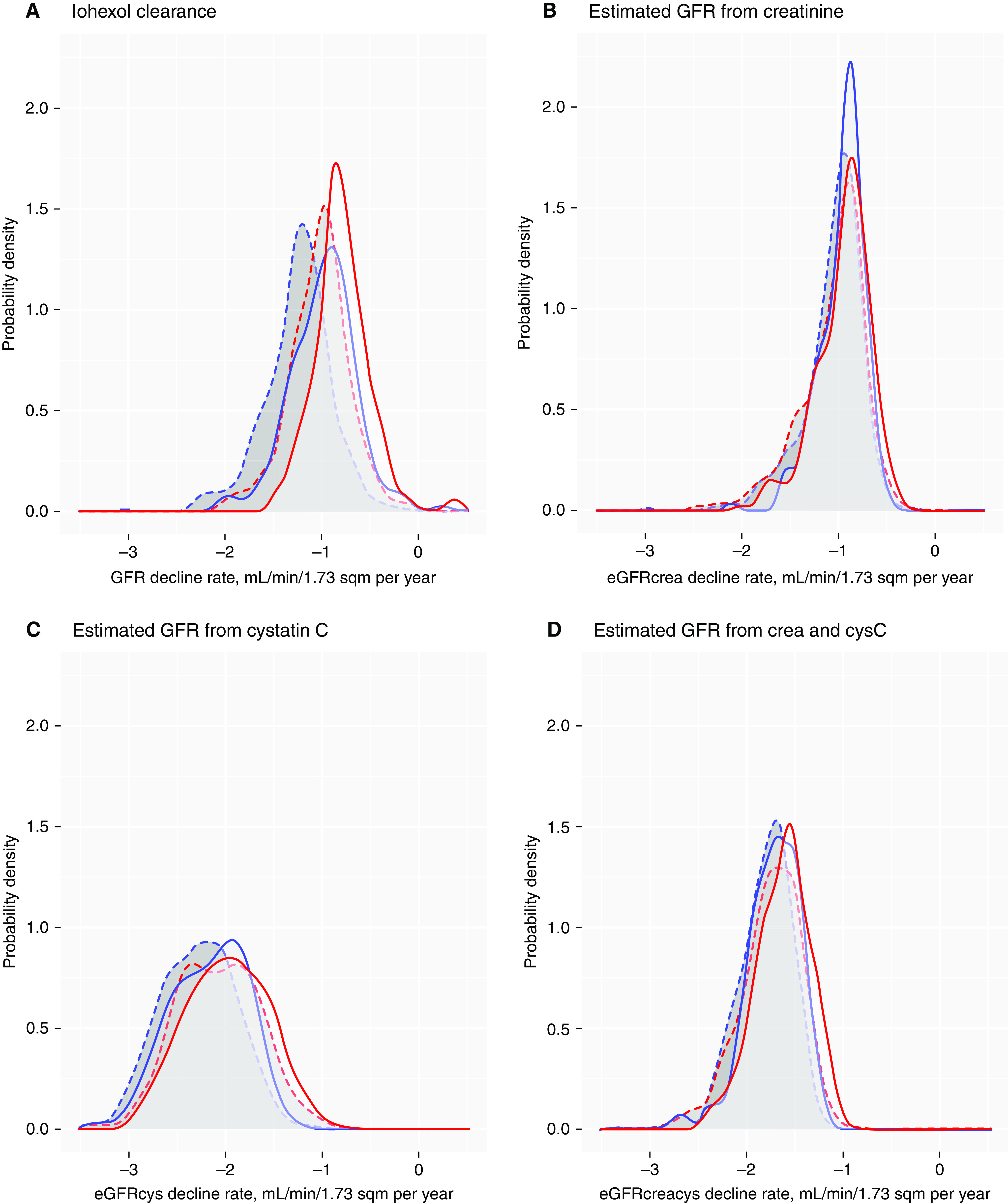
Smoothed histograms of the distribution of individual predicted mean GFR decline rates. GFR change rates were measured using (A) iohexol clearance and estimated from (B) creatinine, (C) cystatin C, and (D) creatinine and cystatin C for healthy (solid) and unhealthy (dashed) women (red) and men (blue), for participants with at least one follow-up (n=1410). Predicted by the GAMM in model 3, Supplemental Table 2 for mGFR, and Supplemental Table 5 for eGFR.
Discussion
In the general population without major chronic diseases or risk factors for CKD, we found women had a slower mean GFR decline rate than men. More women than men were defined as healthy, but this did not explain the difference in the GFR decline rate.
Previous population studies that investigated sex differences in kidney function decline, as assessed by eGFR, yielded mixed results. Some studies reported steeper eGFR declines in men,8–10,29 a few reported no sex differences,9,13 and two studies found steeper eGFR declines in women after adjustment for CKD risk factors.11,30 Because these studies included patients with CVD, diabetes, or CKD; used the creatinine-based eGFR; or were based on annual health checkup data, the results may not reflect GFR decline rates in the healthy general population. We obtained different results using the eGFRcrea or eGFRcys compared with the mGFR, suggesting non–GFR-related factors influence eGFR decline rates in men and women differently.
CKD stage 3 (GFR of 30–60 ml/min per 1.73 m2) accounts for a large proportion of the total CKD population, with a significantly higher prevalence in women than in men, ranging from 10% to 100% higher in women in different countries.3 Overdiagnosis of CKD stage 3a (GFR of 45–60 ml/min per 1.73 m2) by eGFR has been suggested as an explanation.3,31,32 We found that healthy, middle-aged women had an 8.3 ml/min per 1.73 m2 lower GFR than healthy men at baseline, indicating true sex differences in kidney function. As a consequence, more healthy women in these age groups had CKD stage 3a (GFR of 45–60 ml/min per 1.73 m2; Figure 2). However, because women had a slower GFR decline, women maintained a higher mean GFR than men at ages >72 years (Figure 3). If the steeper GFR decline in men continues at lower GFR levels in both healthy and diseased individuals, as suggested by others,29,33,34 it may contribute to the higher prevalence rates of stage 5 CKD (GFR of <15 ml/min per 1.73 m2), dialysis, and kidney transplantation in men.3 It may also contribute to lower life expectancy in men than in women because rapid GFR loss (>3 ml/min per 1.73 m2 per year) and an GFR of <45 ml/min per 1.73 m2 are both independent risk factors for all-cause mortality in older people from the general population.35,36
The sex differences described above and the GFR loss observed even in healthy persons have relevance for the ongoing discussion about whether the CKD definition should be age and sex adjusted.31,37 If normality is determined on the basis of the distribution of GFR values in healthy persons, our findings support age- and sex-specific cutoff values for defining CKD. For example, a 70-year-old healthy woman with a GFR of 59 ml/min per 1.73 m2 and no albuminuria is currently labeled with CKD stage 3a, although her GFR is within the 95% age- and sex-specific reference range in European populations.5,31 A CKD diagnosis may cause anxiety and referral to a specialist health care center, but, according to our study, her risk of accelerated GFR loss is low and the risk of ESKD has been found to be minimal.37,38 Conversely, a GFR of 65 ml/min per 1.73 m2 in a man <50 years does not fulfill the CKD criteria, although his GFR is clearly abnormal,5 and his lifetime risk of progression to CKD stage 4 and 5 may be significant. However, the association between GFR levels and the risk of morbidity and mortality should also be considered.37 For people >65 years, the relative risk is small, if any, until the GFR has fallen <45 ml/min per 1.73 m2.35,37,39 Studies on mGFR that include sex-specific morbidity or mortality end points are needed to decide whether the CKD definition should be adjusted for age and sex.
Differences in nitric oxide metabolism and oxidative stress between women and men, and the influence of sex hormones, have been proposed as explanations for the sex difference in GFR.3,40 The female participants in our study were 50–62 years old at baseline, and the sex difference in GFR decline rates was more prominent at older ages, making the influence of female sex hormones unlikely. Although the majority of experimental studies support deleterious renal effects of testosterone, a delayed progression of CKD in hypogonadal men treated with low-dose testosterone has been reported.41,42 Whether a gradual loss of testosterone in men during aging may influence the GFR decline rate and the risk of CKD is unknown.40–45
We found that persons classified as unhealthy had a higher GFR at a younger age and a steeper GFR decline. A possible explanation may be the increased prevalence of an abnormally elevated GFR, i.e., hyperfiltration, associated with some of the conditions included in our definition of “unhealthy.” Hyperfiltration leads to podocyte stress, glomerulosclerosis, and progression of CKD. In the general population, it is associated with diabetes, obesity, prediabetes, hypertension, and subsequent GFR loss and may also be a risk factor for cardiovascular events and all-cause mortality.46–51 However, adjustment for health status, BMI, BP, and fasting glucose did not influence the effect modification by sex on GFR decline rates, which makes hyperfiltration a less likely explanation for sex differences.
Previous longitudinal studies indicated the GFR is preserved or increases with age in a significant proportion of healthy persons.6–13,52 These studies used the eGFR or creatinine clearance rate; some were limited by short follow-up times, and some calculated the GFR change rate as the difference between GFR measurements divided by time. The variation in the GFR change rates calculated by this method includes both interindividual variation and random measurement error, resulting in a wide distribution and a higher proportion of change rates greater than zero, i.e., a preserved or increased GFR. The generalized additive linear mixed model used in this study estimated interindividual variation and random error separately and found very few persons with a preserved GFR during aging (Figures 4 and 5).
Although we did not observe sex differences in eGFR decline rates using creatinine, we found the mean and distribution of eGFRcrea decline rates were more comparable with the mean and distribution of mGFR than eGFRcys (Figure 5). The annual mean decline rates were approximately −1.0 ml/min per 1.73 m2 for women and men using eGFRcrea, and for eGFRcys, they were −2.2 and −2.3 ml/min per 1.73 m2 for women and men, respectively. Because the quality of the cystatin C assay has been under continuous external quality control, influence from non–GFR-related factors seems the most likely explanation for the discrepancy.
The strength of this study was the repeated GFR measurements in a well-described cohort representative of the general population without preexisting diabetes or CVD. The participation rate was fair, and the day-to-day variation in GFR measurements was low.
There are also some limitations. Study participants were of European ancestry, limiting the generalizability. The average GFR levels for men and women in this study were higher than those reported in some other general population studies. However, we aimed to study age-related GFR decline in healthy people, and GFR levels were comparable to studies in healthy kidney donors.53,54 Although relatively few participants were lost to follow-up, we cannot exclude bias due to a higher dropout rate among those with poor health or the retention of healthy persons. Bias due to competing risk of death was unlikely because only 5% of participants died during follow-up. We chose a stringent definition of “healthy,” but we cannot exclude that some mechanisms contributing to GFR decline in this category should be classified as pathologic rather than age related. For example, the long-term effect of glucose and BP levels within the normal range on GFR variations is poorly defined. Any such pathologic mechanism would need to have sex-specific effects in healthy persons to change our conclusion of differences in GFR decline rates between the sexes. Because we did not find any interaction between sex and health status on GFR decline, this possibility seems less likely.
In conclusion, we found men had a steeper GFR decline rate than women in a representative sample of the general population aged between 50 and 75 years and without diabetes, CKD, or CVD at baseline. Good health did not explain the sex difference in the decline rate.
Disclosures
T.G. Jenssen reports having consultancy agreements with AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, and Novo Nordisk; receiving honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, and Novo Nordisk; and serving in an advisory or leadership role as medical advisor for the Norwegian Association of Diabetes. T. Melsom reports receiving honoraria from Novo Nordisk Norway AS and for providing a lecture at a local meeting. M.D. Solbu reports receiving honoraria from AstraZeneca; and having other interests in, or relationships with, European Renal Association–European Dialysis and Transplant Association, International Society of Nephrology, Norwegian Society of Hypertension, and Norwegian Society of Nephrology. V. Stefansson reports receiving research funding from Boehringer Ingelheim (via a grant to the RENIS-FU study); having ownership interest in Biosergen, Komplett, NordNet, Norsk Hydro, Norwegian, Storebrand, Telenor, and Volvo; and having other interests in, or relationships with, the Norwegian Medical Association and UiT The Arctic University of Norway. The RENIS-FU was also supported by an unrestricted grant from Boehringer Ingelheim, not related to this work. All remaining authors have nothing to disclose.
Funding
The RENIS studies were funded by Northern Norway Regional Health Authority and the University Hospital of North Norway grants HNF1422-18 and SFP 1100-13.
Supplementary Material
Acknowledgments
We thank the staff at the Clinical Research Unit, University Hospital of North Norway, for their assistance in planning the study, performing the procedures, and collecting the data according to Good Clinical Practice standards, particularly Britt-Ann Winther Eilertesen and Annika Gustafsson. We thank all participants for their participation in the study. We also thank the following members of the Metabolic and Renal Research laboratory: Gro Bolstad and Dmitri Svistounov.
RENIS was funded by the University Hospital of North Norway and Northern Norway Regional Health Authority.
The funding source had no role in the design and conduct of the study.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Author Contributions
I.T. Enoksen, B.O. Eriksen, O.M. Fuskevåg, T.G. Jenssen, U.D. Mathisen, J.V. Norvik, M.D. Solbu, and V. Stefansson reviewed and edited the manuscript; I.T. Enoksen, B.O. Eriksen, O.M. Fuskevåg, U.D. Mathisen, T. Melsom, J.V. Norvik, and V. Stefansson were responsible for data curation; I.T. Enoksen, B.O. Eriksen, U.D. Mathisen, T. Melsom, J.V. Norvik, and V. Stefansson were responsible for investigation; B.O. Eriksen provided supervision and was responsible for visualization; B.O. Eriksen, O.M. Fuskevåg, and T. Melsom were responsible for methodology; B.O. Eriksen, T.G. Jenssen, T. Melsom, and M.D. Solbu were responsible for funding acquisition; B.O. Eriksen and T. Melsom conceptualized the study and were responsible for formal analysis, project administration, and resources; and T. Melsom wrote the original draft.
Data Sharing Statement
The data underlying this article cannot be shared publicly because this was not included in the research permission due to ethical considerations and the privacy of individuals who participated in the study. The data can be shared on request as part of a research collaboration. Please contact the corresponding author, T. Melsom (toralf.melsom@unn.no), or the last author, B.O. Eriksen (bjorn.odvar.eriksen@unn.no).
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2022030323/-/DCSupplemental.
Supplemental Appendix. Supplemental methods.
Supplemental Table 1. Characteristics of all persons invited to the Renal Iohexol Clearance Survey (RENIS) and of persons actually included in each of its three waves as registered in the main part of the sixth Tromsø study (before RENIS baseline).
Supplemental Table 2. The relationship between age, sex, health status, and GFR in the generalized additive mixed model.
Supplemental Table 3. The relationship between age, sex, health status, and GFR in a generalized additive mixed models.
Supplemental Table 4. The relationship between age, sex, health status and absolute GFR in ml/min in generalized additive mixed models.
Supplemental Table 5. The relationship between age, sex, health status and eGFR in generalized additive mixed models.
Supplemental Figure 1. The total study population with at least one GFR measurement in the Renal Iohexol Clearance Survey (RENIS).
References
- 1.Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, et al. : Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 392: 2052–2090, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nelson RG, Grams ME, Ballew SH, Sang Y, Azizi F, Chadban SJ, et al. : CKD Prognosis Consortium: Development of risk prediction equations for incident chronic kidney disease. JAMA 322: 2104–2114, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ: Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 14: 151–164, 2018 [DOI] [PubMed] [Google Scholar]
- 4.Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. : Lifetime risks of cardiovascular disease. N Engl J Med 366: 321–329, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eriksen BO, Palsson R, Ebert N, Melsom T, van der Giet M, Gudnason V, et al. : GFR in healthy aging: An individual participant data meta-analysis of iohexol clearance in European population-based cohorts. J Am Soc Nephrol 31: 1602–1615, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muntner P: Longitudinal measurements of renal function. Semin Nephrol 29: 650–657, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Lindeman RD, Tobin J, Shock NW: Longitudinal studies on the rate of decline in renal function with age. J Am Geriatr Soc 33: 278–285, 1985 [DOI] [PubMed] [Google Scholar]
- 8.Toyama T, Kitagawa K, Oshima M, Kitajima S, Hara A, Iwata Y, et al. : Age differences in the relationships between risk factors and loss of kidney function: A general population cohort study. BMC Nephrol 21: 477, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai E, Horio M, Yamagata K, Iseki K, Hara S, Ura N, et al. : Slower decline of glomerular filtration rate in the Japanese general population: A longitudinal 10-year follow-up study. Hypertens Res 31: 433–441, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Halbesma N, Brantsma AH, Bakker SJ, Jansen DF, Stolk RP, De Zeeuw D, et al. : PREVEND study group: Gender differences in predictors of the decline of renal function in the general population. Kidney Int 74: 505–512, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Kronborg J, Solbu M, Njølstad I, Toft I, Eriksen BO, Jenssen T: Predictors of change in estimated GFR: A population-based 7-year follow-up from the Tromso study. Nephrol Dial Transplant 23: 2818–2826, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Hemmelgarn BR, Zhang J, Manns BJ, Tonelli M, Larsen E, Ghali WA, et al. : Progression of kidney dysfunction in the community-dwelling elderly. Kidney Int 69: 2155–2161, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Cohen E, Nardi Y, Krause I, Goldberg E, Milo G, Garty M, et al. : A longitudinal assessment of the natural rate of decline in renal function with age. J Nephrol 27: 635–641, 2014 [DOI] [PubMed] [Google Scholar]
- 14.van Rijn MHC, Metzger M, Flamant M, Houillier P, Haymann J-P, van den Brand JAJG, et al. : Performance of creatinine-based equations for estimating glomerular filtration rate changes over time. Nephrol Dial Transplant 35: 819–827, 2020 [DOI] [PubMed] [Google Scholar]
- 15.Rule AD, Bailey KR, Lieske JC, Peyser PA, Turner ST: Estimating the glomerular filtration rate from serum creatinine is better than from cystatin C for evaluating risk factors associated with chronic kidney disease. Kidney Int 83: 1169–1176, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathisen UD, Melsom T, Ingebretsen OC, Jenssen T, Njølstad I, Solbu MD, et al. : Estimated GFR associates with cardiovascular risk factors independently of measured GFR. J Am Soc Nephrol 22: 927–937, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens LA, Levey AS: Measured GFR as a confirmatory test for estimated GFR. J Am Soc Nephrol 20: 2305–2313, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Eriksen BO, Stefansson VTN, Jenssen TG, Mathisen UD, Schei J, Solbu MD, et al. : High ambulatory arterial stiffness index is an independent risk factor for rapid age-related glomerular filtration rate decline in the general middle-aged population. Hypertension 69: 651–659, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Jacobsen BK, Eggen AE, Mathiesen EB, Wilsgaard T, Njølstad I: Cohort profile: The Tromso study. Int J Epidemiol 41: 961–967, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solbu MD, Kronborg J, Eriksen BO, Jenssen TG, Toft I: Cardiovascular risk-factors predict progression of urinary albumin-excretion in a general, non-diabetic population: A gender-specific follow-up study. Atherosclerosis 201: 398–406, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Eriksen BO, Mathisen UD, Melsom T, Ingebretsen OC, Jenssen TG, Njølstad I, et al. : Cystatin C is not a better estimator of GFR than plasma creatinine in the general population. Kidney Int 78: 1305–1311, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. : CKD-EPI Investigators: Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sliwinski M, Hoffman L, Hofer SM: Evaluating convergence of within-person change and between-person age differences in age-heterogeneous longitudinal studies. Res Hum Dev 7: 45–60, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wood SN: Generalized Additive Models. An Introduction with R, 2nd Ed., Boca Raton, FL, CRC Press, 2017 [Google Scholar]
- 25.Leffondre K, Boucquemont J, Tripepi G, Stel VS, Heinze G, Dunkler D: Analysis of risk factors associated with renal function trajectory over time: A comparison of different statistical approaches. Nephrol Dial Transplant 30: 1237–1243, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Twisk J, de Boer M, de Vente W, Heymans M: Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J Clin Epidemiol 66: 1022–1028, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Burnham K: Model selection and multimodel inference: A practical information-theoretic approach, New York, Springer, 2002 [Google Scholar]
- 28.Fasiolo M, Nedellec R, Goude Y, Wood SN: Scalable visualization methods for modern generalized additive models. J Comput Graph Stat 29: 78–86, 2020 [Google Scholar]
- 29.van der Burgh AC, Rizopoulos D, Ikram MA, Hoorn EJ, Chaker L: Determinants of the evolution of kidney function with age. Kidney Int Rep 6: 3054–3063, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baba M, Shimbo T, Horio M, Ando M, Yasuda Y, Komatsu Y, et al. : Longitudinal study of the decline in renal function in healthy subjects. PLoS One 10: e0129036, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wetzels JF, Kiemeney LA, Swinkels DW, Willems HL, den Heijer M: Age- and gender-specific reference values of estimated GFR in Caucasians: The Nijmegen Biomedical Study. Kidney Int 72: 632–637, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Inker LA, Levey AS, Tighiouart H, Shafi T, Eckfeldt JH, Johnson C, et al. : Performance of glomerular filtration rate estimating equations in a community-based sample of Blacks and Whites: The multiethnic study of atherosclerosis. Nephrol Dial Transplant 33: 417–425, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eriksen BO, Ingebretsen OC: The progression of chronic kidney disease: A 10-year population-based study of the effects of gender and age. Kidney Int 69: 375–382, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Neugarten J, Acharya A, Silbiger SR: Effect of gender on the progression of nondiabetic renal disease: A meta-analysis. J Am Soc Nephrol 11: 319–329, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Hallan SIMK, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, et al. : Chronic Kidney Disease Prognosis Consortium: Age and association of kidney measures with mortality and end-stage renal disease. JAMA 308: 2349–2360, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, et al. : Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delanaye P, Jager KJ, Bökenkamp A, Christensson A, Dubourg L, Eriksen BO, et al. : CKD: A call for an age-adapted definition. J Am Soc Nephrol 30: 1785–1805, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tangri N, Stevens LA, Griffith J, Tighiouart H, Djurdjev O, Naimark D, et al. : A predictive model for progression of chronic kidney disease to kidney failure. JAMA 305: 1553–1559, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Liu P, Quinn RR, Lam NN, Elliott MJ, Xu Y, James MT, et al. : Accounting for age in the definition of chronic kidney disease. JAMA Intern Med 181: 1359–1366, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baylis C: Sexual dimorphism of the aging kidney: Role of nitric oxide deficiency. Physiology (Bethesda) 23: 142–150, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Alwani M, Al-Zoubi RM, Al-Qudimat A, Yassin A, Aboumarzouk O, Al-Rumaihi K, et al. : The impact of long-term testosterone therapy (TTh) in renal function (RF) among hypogonadal men: An observational cohort study. Ann Med Surg (Lond) 69: 102748, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharma R, Oni O, Wiegmann P, Sharma M, Garcia-Touza M, Goel A, et al. : Testosterone replacement therapy (TRT) is associated with delayed progression of chronic kidney disease: A retrospective analysis of testosterone normalization in US veterans. Ann Nephrol 5: 51–59, 2020 [Google Scholar]
- 43.Zhao JV, Schooling CM: Sex-specific associations of sex hormone binding globulin with CKD and kidney function: A univariable and multivariable mendelian randomization study in the UK Biobank. J Am Soc Nephrol 32: 686–694, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kurita N, Horie S, Yamazaki S, Otani K, Sekiguchi M, Onishi Y, et al. : Low testosterone levels and reduced kidney function in Japanese adult men: The Locomotive Syndrome and Health Outcome in Aizu Cohort Study. J Am Med Dir Assoc 17: 371.e1–371.e6, 2016 [DOI] [PubMed] [Google Scholar]
- 45.Zhao JV, Schooling CM: The role of testosterone in chronic kidney disease and kidney function in men and women: a bi-directional Mendelian randomization study in the UK Biobank. BMC Med 18: 122, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tonneijck L, Muskiet MH, Smits MM, van Bommel EJ, Heerspink HJ, van Raalte DH, et al. : Glomerular hyperfiltration in diabetes: Mechanisms, clinical significance, and treatment. J Am Soc Nephrol 28: 1023–1039, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Melsom T, Mathisen UD, Ingebretsen OC, Jenssen TG, Njolstad I, Solbu MD, et al. : Impaired fasting glucose is associated with renal hyperfiltration in the general population. Diabetes Care 34: 1546–1551, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S: Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol Dial Transplant 27: 1821–1825, 2012 [DOI] [PubMed] [Google Scholar]
- 49.Melsom T, Nair V, Schei J, Mariani L, Stefansson VTN, Harder JL, et al. : Correlation between baseline GFR and subsequent change in GFR in Norwegian adults without diabetes and in Pima Indians. Am J Kidney Dis 73: 777–785, 2019 [DOI] [PubMed] [Google Scholar]
- 50.Park M, Yoon E, Lim YH, Kim H, Choi J, Yoon HJ: Renal hyperfiltration as a novel marker of all-cause mortality. J Am Soc Nephrol 26: 1426–1433, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dupuis ME, Nadeau-Fredette AC, Madore F, Agharazii M, Goupil R: Association of glomerular hyperfiltration and cardiovascular risk in middle-aged healthy individuals. JAMA Netw Open 3: e202377, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jiang S, Sun X, Gu H, Chen Y, Xi C, Qiao X, et al. : Age-related change in kidney function, its influencing factors, and association with asymptomatic carotid atherosclerosis in healthy individuals—a 5-year follow-up study. Maturitas 73: 230–238, 2012 [DOI] [PubMed] [Google Scholar]
- 53.Gaillard F, Courbebaisse M, Kamar N, Rostaing L, Del Bello A, Girerd S, et al. : The age-calibrated measured glomerular filtration rate improves living kidney donation selection process. Kidney Int 94: 616–624, 2018 [DOI] [PubMed] [Google Scholar]
- 54.Pottel H, Delanaye P, Weekers L, Selistre L, Goffin K, Gheysens O, et al. : Age-dependent reference intervals for estimated and measured glomerular filtration rate. Clin Kidney J 10: 545–551, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.