Key Points
KidneyIntelX, a bioprognostic test for assessing risk of CKD progression, risk stratified individuals for kidney, heart failure, and death outcomes in the Canagliflozin Cardiovascular Assessment Study.
Individuals scored as high risk seemed to derive more of benefit from treatment with canagliflozin versus placebo.
These findings may serve to increase adoption of underutilized therapies for cardiorenal risk reduction in patients with diabetic kidney disease.
Keywords: clinical nephrology, chronic renal disease, diabetes, diabetes mellitus, diabetic nephropathy
Diabetic kidney disease (DKD) is the commonest cause of CKD (1). In addition to progression to kidney failure, patients with DKD are also at risk for worsening of heart function and hospitalizations for heart failure (HHF) (2). Sodium-glucose cotransporter 2 inhibitors (SGLT2i) have beneficial effects on both DKD progression and heart failure (3,4). KidneyIntelX is a bioprognostic test validated for assessing risk of progression of prevalent DKD and is currently utilized in clinical practice for this indication (5,6). SGLT2i are underutilized in the United States, despite robust evidence and guideline recommendations (7). Risk stratification for clinically relevant outcomes, including DKD progression and HHF, can prioritize patients for intensive management and identify those with most to gain from SGLT2i treatment. Due to shared pathophysiology of DKD and heart failure, we hypothesized that KidneyIntelX would also risk stratify patients with prevalent DKD for a clinically relevant kidney outcome, HHF, and all-cause mortality.
The Canagliflozin Cardiovascular Assessment Study (CANVAS) trial enrolled 4330 participants from 24 countries (8). Participants were randomly assigned using a central Web-based response system in a 1:1:1 ratio for treatment with canagliflozin 100 mg, canagliflozin 300 mg, or a matching placebo. Participants assigned to treatment with canagliflozin or the placebo were followed for a median of 6.1 years. KidneyIntelX was evaluated in the subgroup of the CANVAS population that met the criteria for prevalent DKD (eGFR ≥30–59.9 ml/min per 1.73 m2 [G3a and G3b] or those with an eGFR ≥60 ml/min per 1.73 m2 with a urine albumin-creatinine ratio [uACR] ≥30 mg/g) at the time of enrollment with existing biobanked blood samples (6). Thus, of the 4330 participants in the CANVAS trial, 1396 had prevalent DKD, and of those, 1278 had available blood samples for KidneyIntelX ascertainment and analysis. We have previously demonstrated that KidneyIntelX robustly stratified patients for risk of kidney disease progression in this subgroup of the CANVAS trial population (6). In this subsequent post hoc analysis, we assessed the association of KidneyIntelX at baseline with the time-to-event composite end point of 57% decline in eGFR or adjudicated ESKD, HHF, or death. We measured soluble TNF receptors (sTNFR) 1 and 2, and kidney injury molecule-1 (KIM-1) via proprietary assays (9), and calculated KidneyIntelX scores using the existing validated algorithm (5,6). The model was not recalibrated, reweighted, or retrained for this new composite outcome. We divided the patient population into high- (score >85), intermediate- (score 50–85), and low-risk (score 5–45) strata using the clinical risk score cutoffs for KidneyIntelX (5,6). We calculated adjusted hazard ratios (aHR) with 95% confidence intervals (CI) for high- versus low-risk strata for the composite outcome after adjusting for age, sex, race, randomization arm, baseline cardiovascular disease, and baseline measures of hemoglobin A1C, BP, low-density lipoprotein cholesterol, body mass index, baseline eGFR, and baseline uACR.
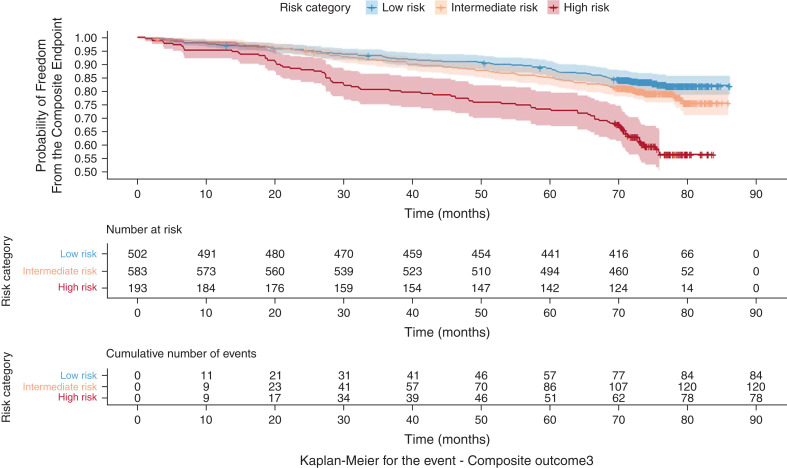
Among the 1278 CANVAS participants in this post hoc analysis, the mean age was 64 years, 32% were women, the mean baseline eGFR was 65 ml/min per 1.73 m2, the median uACR was 56 mg/g, 498 (40%) had an eGFR< 60 ml/min per 1.73 m2, and 209 (16%) had heart failure at baseline. During a mean of 5.6 years follow-up, 282 (22%) experienced the composite outcome, 41 (3%) developed a 57% decline in eGFR or ESKD, 78 (6%) were hospitalized for heart failure, and 209 (16%) died. The proportion with events was 17%, 21%, and 40% for low-, intermediate-, and high-risk strata, respectively. The aHR for the composite outcome was 2.1 (95% CI, 1.4 to 3) in the high- versus low-risk group, and it was 1.4 (95% CI, 1.02 to 1.9) in the intermediate- versus low-risk group. Additionally, we conducted a sensitivity analysis using a sustained 40% decline in eGFR rather than a doubling in serum creatinine (57% decline in eGFR) in the composite outcome. The aHR for this composite outcome was 2.2 (95% CI, 1.3 to 2.8) in the high- versus low-risk group, and it was 1.5 (95% CI, 1.1 to 2) in the intermediate- versus low-risk group (Table 1). Figure 1 shows the time to the composite events stratified by KidneyIntelX risk categories (low, intermediate, and high), and Table 2 shows the aHRs for high versus low risk in each component of the composite outcome. The risk for the composite event was reduced by 22%–24% across all risk strata in participants randomized to canagliflozin versus placebo, with absolute risk reductions of 11% in the high-risk stratum, 6% in the intermediate-risk stratum, and 4% in the low-risk stratum (P<0.01 for high versus low risk).
Table 1.
Associations with different composite outcomes for KidneyIntelX high versus low risk strata after successive adjustment for risk factors
| Adjusted HR for High versus Low Risk (95% Confidence Interval) | Adjusted HR for Intermediate versus Low Risk (95% Confidence Interval) | |
|---|---|---|
| Time-to-event composite end point of 57% decline in eGFR or adjudicated ESKD, HHF, or death | ||
| Model 1 | 3.04 (2.23 to 4.16) | 1.36 (1.03 to 1.81) |
| Model 2 | 2.67 (1.9 to 3.75) | 1.35 (1.01 to 1.82) |
| Model 3 | 2.10 (1.42 to 2.97) | 1.40 (1.02 to 1.86) |
| Time-to-event composite end point of sustained 40% decline in eGFR or adjudicated ESKD, HHF, or death | ||
| Model 1 | 3.25 (2.31 to 4.3) | 1.47 (1.13 to 1.96) |
| Model 2 | 2.91 (1.97 to 3.85) | 1.46 (1.1 to 1.97) |
| Model 3 | 2.16 (1.3 to 2.75) | 1.47 (1.08 to 1.94) |
Model 1 adjusted for age, sex, and randomization arm; model 2=model 1+baseline cardiovascular disease, hemoglobin A1C, systolic and diastolic BPs, low-density lipoprotein, and body mass index; model 3=model 2+baseline eGFR and baseline uACR. HR, hazard ratio; CI, confidence interval; HHF, hospitalizations for heart failure; uACR, urine albumin-creatinine ratio.
Figure 1.
Kaplan–Meier curves for the composite event by KidneyIntelX risk strata. The composite end point consisted of time to first occurrence of 57% decline in eGFR or adjudicated ESKD, hospitalization for heart failure, or death.
Table 2.
Adjusted hazard ratios for individual components of the composite clinical outcome
| Component | n/N | Adjusted Hazard Ratio for High versus Low Risk (95% Confidence Interval) |
|---|---|---|
| Composite outcome | 282/1278 | 2.1 (1.4–2.9) |
| Kidney outcome | 41/1278 | 20.7 (4.6–93.3) |
| Hospitalizations for heart failure | 78/1278 | 1.9 (1.0–3.9) |
| Death | 209/1278 | 1.3 (0.9–2.2) |
Adjusted for age, sex, randomization arm, baseline cardiovascular disease, hemoglobin A1C, systolic and diastolic BPs, low-density lipoprotein, body mass index, eGFR, and urine albumin-creatinine ratio.
Although KidneyIntelX has been validated for an outcome of DKD progression, the results from this subsequent post hoc analysis from CANVAS demonstrated that KidneyIntelX robustly stratified patients for a composite end point consisting of clinically relevant outcomes. KidneyIntelX combines two inflammatory markers (sTNFR1 & 2), one tubule injury marker (KIM-1), ratios of the markers, and seven clinical variables to create an individualized risk score using random forests that allows for complex nonlinear interaction modeling between biomarkers and clinical variables. In prior analyses from CANVAS, each of the three biomarkers, sTNFR1, sTNFR2, and KIM-1, were associated with HHF after adjustment only for demographics and randomized treatment, but the point estimates were attenuated to null after full covariate adjustment (9). KidneyIntelX is a commercially available test that is in use clinically at various health systems in the United States and is CLIA-certified as a laboratory developed test in all 50 states. Real-world deployment of new risk stratification tests (including biomarkers) necessitates a comprehensible message and integration into clinical workflow to drive clinician behavior and overcome therapeutic inertia. This could be potentially done through a composite risk score, such as the KidneyIntelX bioprognostic test, which combines both the biomarkers and clinical features. Because the SGLT2 inhibitors, including canagliflozin which was studied in the CANVAS population, have not only beneficial effects on kidney outcomes but also robust effects on heart failure hospitalizations (10), this study has clinical implications. Indeed, although the relative risks for the composite outcome for canagliflozin versus placebo were similar across the three strata of KidneyIntelX risk, the absolute risk reductions achieved with canagliflozin compared with placebo were greatest in the high-risk KidneyIntelX stratum, thereby potentially allowing its use to identify patients most likely to benefit from treatment. Limitations of this post hoc analysis include the lack of an independent external validation cohort, the use of an algorithm not specifically trained for the broad clinical composite assessed herein, and, although we adjusted for 11 clinical covariates, potential for residual confounding.
In conclusion, we demonstrated that KidneyIntelX, a composite risk score trained and validated for a kidney-specific outcome, provided risk stratification for a triple composite end point that included not only the kidney-specific outcome of progression, but also clinically relevant outcomes of hospitalizations for heart failure and all-cause mortality, even after adjusting for several other risk factors for these outcomes. These findings suggest that KidneyIntelX may have utility as a clinical trial enrichment tool for therapies to ameliorate cardiorenal risk and provides further impetus to increase adoption of underutilized guideline-recommended therapies to reduce risk of kidney disease progression, HHF, and death in clinical practice.
Disclosures
S.G. Coca reports consultancy for Axon Therapies, Bayer, Boehringer Ingelheim, Nuwellis, Renalytix, Reprieve Cardiovascular, Takeda, Vifor, and 3ive; ownership interest in pulseData and Renalytix; research funding from ProKidney, Renalytix, Renal Research Institute, and XORTX; patents or royalties from Renalytix; being a scientific co-founder of Renalytix and having equity and royalties and being a consultant and member of the scientific advisory board; an advisory or leadership role with Renalytix and Reprieve Cardiovascular; and being an associate editor for Kidney360 and on the editorial boards of JASN, CJASN, and Kidney International. F. Fleming reports being the chief technology officer and co-founder of Renalytix; being an employee of Renalytix; ownership interest in Renalytix and Verici Dx; and having an advisory or leadership role with Renalytix. M. Hansen reports being an employee of Janssen Research & Development, and ownership interest in Johnson & Johnson. H.L. Heerspink reports being an employee of University Medical Center Groningen; ongoing consultancy agreements with AstraZeneca, Bayer, Boehringer Ingelheim, CSL Behring, Chinook, Dimerix, Eli-Lilly, Gilead, GoldFinch, Janssen, Merck, NovoNordisk, and Travere Pharmaceuticals; research funding from AstraZeneca, Janssen research support (grant funding directed to employer), and NovoNordisk; honoraria from AstraZeneca (lecture fees); and participating in a speakers’ bureau for AstraZeneca. K. Mahaffey reports consultancy for Amgen, Applied Therapeutics, AstraZeneca, Bayer, CSL Behring, Elsevier, Fribrogen, Invo, Johnson & Johnson, Lexicon, Myokardia, Novartis, Novo Nordisk, Otsuka, Phasebio, Portola, Precordior, Quidel, Sanofi, and Theravance; research funding from the American Heart Assocation, Apple, Inc., Bayer, California Institute Regenerative Medicine, Eidos, Ferring, Gilead, Google (Verily), Idorsia, Johnson & Johnson, Luitpold, PAC-12, Precordior, and Sanifit; and honoraria from Amgen, Anthos, Applied Therapeutics, AstraZeneca, Bayer, CSL Behring, Elsevier, Inova, Intermountain Health, Johnson & Johnson, Medscae, Mount Sinai, Mundi Pharma, Myokardia, Novartis, Novo Nordisk, Otsuka, Portola, Sanofi, SmartMedics, and Theravance. G.N. Nadkarni reports consultancy for Daiichi Sankyo, GLG consulting, Qiming Capital, Reata, Renalytix, Siemens Healthineers, and Variant Bio; ownership interest in Data2Wisdom, LLC, Doximity, Nexus iConnect, Pensieve Health, Renalytix, and Verici; research funding from Renalytix; honoraria from Daiichi Sankyo; patents or royalties from Renalytix; an advisory or leadership role with Renalytix; participating in a speakers’ bureau with Daiichi Sankyo; and being a scientific co-founder of Renalytix and having equity and royalties and being a consultant and member of the scientific advisory board. B. Neal reports consultancy for Janssen Research & Development, LLC; research funding from the Australian National Health and Medical Research Council Principal Research Fellowship and Janssen; honoraria from Janssen Research & Development, LLC (paid to the institution); and serving on advisory boards and/or involvement in continuing medical education (CME) programs for Janssen, with any consultancy, honoraria, or travel support paid to his institution. D. Takale reports being an employee of Persistent. Y. Yavin reports being an employer of Janssen, and ownership interest in Bristol-Myers Squibb and Johnson & Johnson.
Funding
None.
Footnotes
See related editorial, “The Next Frontier: Biomarkers and Artificial Intelligence Predicting Cardiorenal Outcomes in Diabetic Kidney Disease,” on pages 1480–1483.
Author Contributions
S.G. Coca was responsible for visualization; S.G. Coca, F. Fleming, M.K. Hansen, H.J.L. Heerspink, K.W. Mahaffey, G.N. Nadkarni, B. Neal, D. Takale, and Y. Yavin reviewed and edited the manuscript; S.G. Coca, F. Fleming, M.K. Hansen, H.J.L. Heerspink, G.N. Nadkarni, and B. Neal were responsible for the methodology; S.G. Coca, F. Fleming, M.K. Hansen, K.W. Mahaffey, G.N. Nadkarni, B. Neal, and Y. Yavin were responsible for the investigation; S.G. Coca, F. Fleming, M.K. Hansen, K.W. Mahaffey, D. Takale, and Y. Yavin were responsible for validation; S.G. Coca, F. Fleming, H.J.L. Heerspink, and G.N. Nadkarni were responsible for conceptualization; S.G. Coca, F. Fleming, G.N. Nadkarni, and D. Takale were responsible for formal analysis; S.G. Coca, H.J.L. Heerspink, and G.N. Nadkarni were responsible for supervision; S.G. Coca and G.N. Nadkarni wrote the original draft of the manuscript; F. Fleming, M.K. Hansen, H.J.L. Heerspink, K.W. Mahaffey, B. Neal, D. Takale, and Y. Yavin were responsible for resources; M.K. Hansen, K.W. Mahaffey, and Y. Yavin were responsible for funding acquisition; H.J.L. Heerspink and D. Takale were responsible for data curation; and G.N. Nadkarni, D. Takale, and Y. Yavin were responsible for project administration.
References
- 1.Centers for Disease Control and Prevention : Chronic Kidney Disease in the United States. Available at: https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html. Accessed May 5, 2022.
- 2.Oshima M, Shimizu M, Yamanouchi M, Toyama T, Hara A, Furuichi K, Wada T: Trajectories of kidney function in diabetes: A clinicopathological update. Nat Rev Nephrol 17: 740–750, 2021. 10.1038/s41581-021-00462-y [DOI] [PubMed] [Google Scholar]
- 3.Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, Fulcher G, Blais J, Li Q, Jardine MJ, Perkovic V, Wheeler DC: Relative and absolute risk reductions in cardiovascular and kidney outcomes with canagliflozin across KDIGO risk categories: Findings from the CANVAS Program. Am J Kidney Dis 77: 23–34.e1, 2021. 10.1053/j.ajkd.2020.06.018 [DOI] [PubMed] [Google Scholar]
- 4.Neuen BL, Young T, Heerspink HJL, Neal B, Perkovic V, Billot L, Mahaffey KW, Charytan DM, Wheeler DC, Arnott C, Bompoint S, Levin A, Jardine MJ: SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: A systematic review and meta-analysis. Lancet Diabetes Endocrinol 7: 845–854, 2019. 10.1016/S2213-8587(19)30256-6 [DOI] [PubMed] [Google Scholar]
- 5.Chan L, Nadkarni GN, Fleming F, McCullough JR, Connolly P, Mosoyan G, El Salem F, Kattan MW, Vassalotti JA, Murphy B, Donovan MJ, Coca SG, Damrauer SM: Derivation and validation of a machine learning risk score using biomarker and electronic patient data to predict progression of diabetic kidney disease. Diabetologia 64: 1504–1515, 2021. 10.1007/s00125-021-05444-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam D, Nadkarni GN, Mosoyan G, Neal B, Mahaffey KW, Rosenthal N, Hansen MK, Heerspink HJL, Fleming F, Coca SG: Clinical utility of KidneyIntelX in early stages of diabetic kidney disease in the CANVAS trial. Am J Nephrol 53: 21–31, 2022. 10.1159/000519920 [DOI] [PubMed] [Google Scholar]
- 7.Eberly LA, Yang L, Eneanya ND, Essien U, Julien H, Nathan AS, Khatana SAM, Dayoub EJ, Fanaroff AC, Giri J, Groeneveld PW, Adusumalli S: Association of race/ethnicity, gender, and socioeconomic status with sodium-glucose cotransporter 2 inhibitor use among patients with diabetes in the US. JAMA Netw Open 4: e216139, 2021. 10.1001/jamanetworkopen.2021.6139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, Shaw W, Law G, Desai M, Matthews DR; CANVAS Program Collaborative Group : Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 377: 644–657, 2017. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 9.Sen T, Li J, Neuen BL, Neal B, Arnott C, Parikh CR, Coca SG, Perkovic V, Mahaffey KW, Yavin Y, Rosenthal N, Hansen MK, Heerspink HJL: Effects of the SGLT2 inhibitor canagliflozin on plasma biomarkers TNFR-1, TNFR-2 and KIM-1 in the CANVAS trial. Diabetologia 64: 2147–2158, 2021. 10.1007/s00125-021-05512-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qiu M, Ding L, Zhou H: Effects of SGLT2 inhibitors on cardiovascular and renal outcomes in type 2 diabetes: A meta-analysis with trial sequential analysis. Medicine (Baltimore) 100: e25121, 2021. 10.1097/MD.0000000000025121 [DOI] [PMC free article] [PubMed] [Google Scholar]