All a low protein diet does is to shrink the patient down to the size of his kidneys.
F. Parsons
The Guidelines
In 2020, the Kidney Disease Quality Outcomes Initiative (KDOQI) guidelines on nutrition in CKD patients stated: for “Protein Restriction, CKD Patients Not on Dialysis and Without Diabetes. In adults with CKD (stages) 3–5 who are metabolically stable, we recommend, under close supervision, protein restriction with or without keto acid analogs, to reduce risk for end stage kidney disease (ESKD)/death (1A) and improve quality of life (2C)” (1).
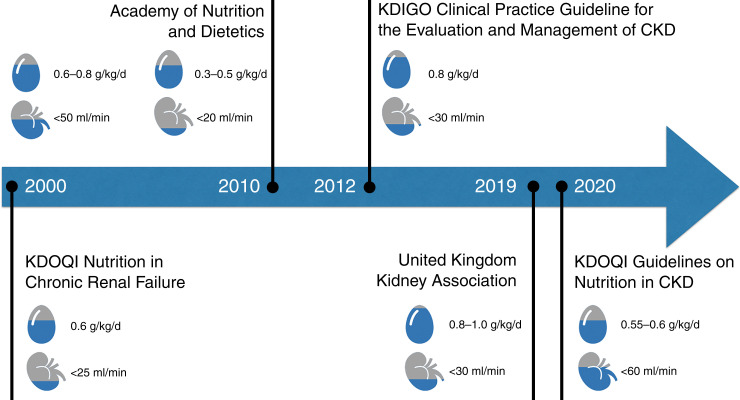
The guideline went on to define a low-protein diet (LPD) as 0.55–0.60 g of dietary protein per kilogram of body weight (g/kg) and very low protein intake as 0.28–0.43 g/kg. This was similar, although stronger, than the guideline published in 2010 by the Academy of Nutrition and Dietetics, which recommended 0.6–0.8 g/kg for patients with a GFR of <50 ml/min per 1.73 m2 (2). In contrast, the Kidney Disease Improving Global Outcomes (KDIGO) 2012 CKD guideline recommended protein restriction with advanced CKD “suggest lowering protein intake to 0.8 g/kg/day in adults with diabetes (2C) or without diabetes (2B) and GFR of 30 ml/min/1.73 m2 (GFR categories G4–G5), with appropriate education” (3). Of note, the recommended daily allowance of protein in the United States is 0.8 g/kg per day, so there was not much restriction in this guideline (4). Similarly, in 2019, the UK Kidney Association recommended a normal protein intake (i.e., no restriction) of 0.8–1 g/kg per day for patients with CKD stages 4 and 5. See Figure 1 for a summary of this differing and somewhat conflicting guidance.
Figure 1.
Summary of various guidelines on nutrition in CKD patients.
Asking individuals with CKD to change their diet substantially and reduce protein intake is a big ask and has the potential to force patients to change cultural norms. It can even separate people from communal meals and experiences, potentially reducing their quality of life. In the case of CKD stage 3, according to Turin et al., the lifetime risk of kidney failure for a middle-aged person is 8% for men and 3% for women (5). Thus, patients will need to make and maintain this dietary change for decades to see a small benefit and, for the ≥90% of people who will never reach dialysis, no benefit at all. We believe that the dietary recommendations from KDOQI are creeping to higher GFRs without adequate evidence of benefit.
The Science
Whether high-protein intake causes or accelerates preexisting kidney disease is a long-standing debate in nephrology. The origin comes from the 1920s when researchers found that amino acid infusions increased GFR, proteinuria, and glomerular sclerosis on biopsy in animal models (6,7). The presumed mechanism for the increased GFR is afferent arteriolar vasodilation from nitric oxide (8,9). It is interesting that this presumed mechanism of afferent vasoconstriction in response to low-protein diets aligns with one of the primary glomerular effects of sodium-glucose co-transporter 2 inhibitors (10).
Several randomized controlled trials have tackled this question with mixed results, summarized in Table 1. The KDOQI guidelines rely heavily on a single trial from 1991 where Locatelli et al. enrolled 456 adult patients who were randomized to either a LPD (0.6 g/kg body weight daily; n=226) or a “normal” controlled-protein diet (NPD; 1 g/kg body weight daily; n=230) and were stratified into three groups by baseline plasma creatinine concentrations (group A: 1.5–2.5 mg/dl; group B: 2.5–5 mg/dl; group C: 5–7 mg/dl) (11). Notably, the investigators specifically avoided the use of angiotensin-converting enzyme inhibitors in all patients. The overall difference between the dietary groups in cumulative renal survival (27 LPD, 42 NPD) did not reach traditional levels of significance with a P value of 0.06, and the authors relied on splicing to suggest a benefit in certain subgroups without adjusting for multiple comparisons. Notably, 14% of patients withdrew because they could not tolerate the LPD. Also of note, the loss of kidney function was greater in the LPD groups as was the rise in serum creatinine (NS).
Table 1.
The major protein restriction randomized controlled trials
| Trial | Population | Planned Intervention | Achieved Protein Intake | ESKD Outcome | Change in GFR or Creatinine Clearance | Adherence/Tolerance of Low-Protein Diet |
|---|---|---|---|---|---|---|
| Rosman et al. 1989 (13) | 228 patients with CrCl 10–60 ml/min | 118 patients were randomly assigned to a LPD group (0.4 or 0.6 g/kg per day); 110 patients were assigned to a control group | Not provided | Dialysis or transplant 6 in LPD group versus 11 in control group | Significant decline in control group versus LPD group based on reciprocal of serum creatinine analysis | Subjective acceptance of LPD was rated “bad” by one third of patients at 3 and 6 months |
| Locatelli et al. 1991 (11) | 456 patients with diabetes CKD | NPD (1 g/kg per day) versus LPD (0.6 g/kg per day), follow-up for 2 years | Dietary protein intake higher than required in LPD: 21% (interview) to 40% (24 hour urine urea calculation) | Doubling in serum creatinine or ESKD development, 27 in LPD group compared with 42 in NPD group (P=0.06) | Change in creatinine 0.029 μmol/L per month in NPD group versus 0.036 μmol/L per month in LPD group | 64 participants withdrew (“lack of cooperation” for 58, “intolerance of low protein food” for 6) |
| Klahr et al. (MDRD) 1994 (16) | Study 1: 585 patients with GFR 25–55 ml/min per 1.73 m2 Study 2: 255 patients with GFR 13–24 ml/min per 1.73 m2 |
LPD (0.58 g/kg per day) versus NPD (1.3 g/kg per day) Very LPD (0.28 ml/kg per day) versus LPD (0.58 g/kg per day) Follow-up 18–45 months |
Mean 1.1 g/kg per day (1–1.3) versus mean 0.7 g/kg per day (0.6–0.8) Mean 0.5 g/kg per day (0.4–0.6) versus mean 0.7 g/kg per day (0.6–0.8) |
The relative risk of ESKD or death was 0.93 (95% CI, 0.65 to 1.33) for the patients assigned to the very LPD compared with those assigned to the LPD | No difference in GFR decline | Differences in protein intake between the dietary groups were achieved by the fourth month of follow-up and remained relatively constant throughout the follow-up period |
| Hansen et al. 2002 (19) | 82 patients with type 2 diabetes and progressive diabetic nephropathy (prestudy GFR decline of 7.1 ml/min per 1.73 m2 per year) | NPD versus LPD (0.6 g/kg per day) based on dietitian advice every 3 months | LPD group achieved mean 0.89 g/kg per day versus prescribed 0.6 g/kg per day | 2 Dialysis or transplant need in 4 in NPD group versus 2 in LPD group | GFR decline was 3.9 ml/min per 1.73 m2 per year in the NPD group and 3.8 ml/min per 1.73 m2 in the LPD group (P=0.87) | Tolerance or quality of life not reported |
| Cianciaruso et al. 2009 (14) | 423 patients with CKD stages 4–5 | LPD (0.55 g/kg per day) versus MPD (0.8 g/kg per day) Follow-up 32 months |
Average protein intakes were 0.73±0.04 g/kg per day for the LPD group and 0.9±0.06 g/kg/d for the MPD | Effects of LPD on death, ESKD, or the composite outcome of both were 1.01 (95% CI, 0.57 to 1.79), 0.96 (95% CI, 0.62 to 1.48), and 0.98 (95% CI, 0.68 to 1.42), respectively | No difference between the two groups | 3 (0.7%) patients met the criteria for protein-caloric malnutrition |
CrCl, creatinine clearance; LPD, low-protein diet; NPD, normal protein diet; CI, confidence interval; MPD, moderate-protein diet.
Before Locatelli et al., in the 1980s, Rosman et al. (12) randomized 248 patients to a LPD (0.4–0.6 g/kg per day) versus usual care. Although the authors report that a LPD was only helpful in patients with primary glomerulonephritis, this was not a prespecified analysis, and clear numbers about event counts across subgroups are not reported. Overall, there was no difference in ESKD (6 versus 11), and self-reported acceptance was “bad” in a third of patients. In the longer-term follow-up, the authors tempered their original conclusions further, stating “after four years of follow-up, we are only moderately optimistic about DPR [dietary protein restriction] as a general measure for the management of the progression of chronic renal insufficiency” (13).
Another 4-year randomized controlled trial from 2002 compared the effects of a LPD (0.6 g/kg per day) with a NPD in 82 patients with type 1 diabetes and diabetic nephropathy, with a mean decline in GFR of 7.1 ml/min per 1.73 m2 per year in the year before enrollment. Against the planned 0.6 g/kg per day, the achieved protein intake in the LPD group was 0.89 (0.83–0.95) g/kg per day, again highlighting the low adherence. The rate of GFR decline was 3.9 ml/min per 1.73 m2 per year in the NPD group and 3.8 ml/min per 1.73 m2 per year in the LPD group (P=0.87). Although ESKD or death occurred in 27% of patients on a NPD compared with 10% on a LPD (P=0.04), this difference was largely driven by a difference in death between the groups, with seven deaths in the NPD group and only two deaths in the LPD group, and was significant within the first year of follow-up. The causes of death were heart failure (4) and myocardial infarction (5), which is likely the effect of chance and not the LPD.
In 2009, Cianciaruso et al. (14) randomized 423 patients with CKD stages 4 and 5 to either a LPD (0.55 g/kg per day) or a NPD (0.8 g/kg per day). After a median follow-up of 32 months, the LPD had no effect on any of the outcomes, protein-caloric malnutrition, dialysis, death, or the composite outcome of dialysis and death. In the KDOQI discussion, the guideline authors attribute the negative result to “a relatively small sample size,” despite having the second highest sample size among the five trials discussed so far.
In summary, the trials used to justify the KDOQI guideline do not support that a LPD lowers the risk of ESKD or slows the progression of kidney disease unless one relies on isolated subgroups and ignores the totality of the evidence. These diets are also poorly tolerated, and adherence falls short of the goal, even in these trial settings.
Evidence Synthesis
The other incongruity is the fact that the KDOQI guideline states that low protein is able to delay dialysis while simultaneously saying that it is unable to reduce the progression of GFR. As stated in the guideline, “Results from all the studies indicated that an LPD (0.55–0.6 g/kg body weight) had no significant effect on GFR compared with the control group (0.8 g/kg protein).” The same year in which the 2020 KDIGO guidelines were published, a Cochrane systematic review was published examining the same question (15). In regard to initiating dialysis, they reported no effect (six studies, 1814 participants; relative risk 1.05, 95% confidence interval, 0.73 to 1.53). Similarly, they reported no signal for rate of loss of GFR. However, the meta-analysis did find that a very LPD was likely to prevent dialysis compared with a LPD, although again there was no effect on GFR, and no attempt was made to reconcile these apparently contradictory findings. The inability of a LPD to prevent loss of GFR was most famously demonstrated in the Modification of Diet in Renal Disease study (16). The Modification of Diet in Renal Disease also had the advantage of not using serum creatinine or and creatinine clearance to determine GFR but rather used iothalamate clearance to insulate measurement from changes in muscle mass that may occur with uremia and dietary changes. The inability to slow loss of GFR while possibly being able to prevent the initiation of dialysis suggests that a LPD may prevent some symptoms of uremia and hence delay doctors from pulling the trigger to initiate dialysis. If this turns out to be the explanation for the apparent contradiction, then there would be no advantage to starting a LPD early in CKD when there are no symptoms of uremia and that it should be reserved for advanced CKD where patients are near dialysis.
Another possible explanation for the conflicting signals is that we are looking at the wrong aspect of dietary protein. It may not be the quantity of protein but rather the quality of protein. Not all proteins produce the same amount of acid that needs to be neutralized. Animal protein, specifically red meat, tends to be higher in methionine and cysteine, both of which generate sulfuric acid in their catabolism. Lew et al. used the Singapore Chinese Health Study to look at total protein and the types of protein in more than 63,000 people and examined the risk of ESKD with 15.5 years of follow-up (17). Although total protein was related to the risk of ESKD, it was not dose related. However, there was a strong dose-dependent relationship with red meat intake and increased risk of ESKD. This wasn’t seen with other protein sources (poultry, fish, eggs, or dairy products). In an associated editorial, Wesson and Goraya speculated that the cause of this may be increased metabolic acidosis associated with red meat (18).
One last point that must be kept in mind when evaluating these data was that the majority of the studies were done in a pre-renin-angiotensin system blockade era. Now, we have those drugs, as well as flozins and mineralocorticoid antagonists, which are not just effective but much less complicated to implement. Thus, dietary protein restriction, which has an imperfect evidence base, will also likely have a much smaller benefit (if any) when added to these foundational therapies.
Conclusion
Good food and dietary variety are some of the great joys of life. The data supporting a LPD were largely collected before widespread adoption of renin-angiotensin system blockade and entirely before the addition of sodium-glucose co-transporter 2 inhibitors in the management of CKD. We believe that given the commitment required of patients, dietary restrictions should only be made when there is clear, conclusive, coherent, and consistent evidence. As we describe, this is not true in any respect. The current KDOQI guideline, with an evidence grade of 1A, overstates the evidence, and we advise practitioners only to implement dietary changes after shared decision making and a critical review of the evidence.
Disclosures
S. Hiremath reports research funding (research salary support) from the Department of Medicine, University of Ottawa; and an advisory or leadership role for the American Journal of Kidney Disease, Canadian Journal of Cardiology, and American Journal of Hypertension (editorial board) and NephJC (board of directors; not for profit educational entity; unpaid volunteer position). J. Topf reports consultancy for Davita; ownership interest in Davita; research funding from Natera; an advisory or leadership role for the American Journal of Kidney Disease, American Society of Nephrology, AstraZeneca, Bayer, CJASN, Cara Therapeutics, and Triceda; and being the author of Precious Bodily Fluids blog, editor of NephMadness, co-creator of NephJC, Author of Nephrology Secrets, and president of NephJC. The remaining author has nothing to disclose.
Funding
None.
Acknowledgments
The content of this article reflects the personal experience and views of the authors and should not be considered medical advice or recommendation. The content does not reflect the views or opinions of the American Society of Nephrology (ASN) or Kidney360. Responsibility for the information and views expressed herein lies entirely with the authors.
Author Contributions
All authors wrote the original draft of the manuscript.
References
- 1.Ikizler TA, Burrowes JD, Byham-Gray LD, Campbell KL, Carrero J-J, Chan W, Fouque D, Friedman AN, Ghaddar S, Goldstein-Fuchs DJ, Kaysen GA, Kopple JD, Teta D, Yee-Moon Wang A, Cuppari L: KDOQI Clinical Practice Guideline for Nutrition in CKD: 2020 update [published correction appears in Am J Kidney Dis 77: 308, 2021 10.1053/j.ajkd.2020.11.004]. Am J Kidney Dis 76: S1–S107, 2020. [DOI] [PubMed] [Google Scholar]
- 2.Academy of Nutrition and Dietetics : 2010 Chronic Kidney Disease (CKD) Evidence-based Nutrition Practice Guideline. Available at: https://www.andeal.org/topic.cfm?menu=5303&cat=3927. Accessed June 1, 2022
- 3.Eknoyan G, Lameire N, Eckardt K, Kasiske B, Wheeler D, Levin A, Stevens PE, Bilous RW, Lamb EJ, Coresh J: KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int 3: 5–14, 2013 [Google Scholar]
- 4.Rodriguez NR, Miller SL: Effective translation of current dietary guidance: Understanding and communicating the concepts of minimal and optimal levels of dietary protein. Am J Clin Nutr 101: 1353S–1358S, 2015. 10.3945/ajcn.114.084095 [DOI] [PubMed] [Google Scholar]
- 5.Turin TC, Tonelli M, Manns BJ, Ahmed SB, Ravani P, James M, Hemmelgarn BR: Lifetime risk of ESRD. J Am Soc Nephrol 23: 1569–1578, 2012. 10.1681/ASN.2012020164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hostetter TH, Meyer TW, Rennke HG, Brenner BM: Chronic effects of dietary protein in the rat with intact and reduced renal mass. Kidney Int 30: 509–517, 1986. 10.1038/ki.1986.215 [DOI] [PubMed] [Google Scholar]
- 7.Ko G-J, Rhee CM, Kalantar-Zadeh K, Joshi S: The effects of high-protein diets on kidney health and longevity. J Am Soc Nephrol 31: 1667–1679, 2020. 10.1681/ASN.2020010028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sällström J, Carlström M, Olerud J, Fredholm BB, Kouzmine M, Sandler S, Persson AEG: High-protein-induced glomerular hyperfiltration is independent of the tubuloglomerular feedback mechanism and nitric oxide synthases. Am J Physiol Regul Integr Comp Physiol 299: R1263–R1268, 2010. 10.1152/ajpregu.00649.2009 [DOI] [PubMed] [Google Scholar]
- 9.Wei J, Zhang J, Jiang S, Wang L, Persson AEG, Liu R: High-protein diet-induced glomerular hyperfiltration is dependent on neuronal nitric oxide synthase β in the macula densa via tubuloglomerular feedback response. Hypertension 74: 864–871, 2019. 10.1161/HYPERTENSIONAHA.119.13077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherney DZI, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, Fagan NM, Woerle HJ, Johansen OE, Broedl UC, von Eynatten M: Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 129: 587–597, 2014. 10.1161/CIRCULATIONAHA.113.005081 [DOI] [PubMed] [Google Scholar]
- 11.Locatelli F, Alberti D, Graziani G, Buccianti G, Redaelli B, Giangrande A; Northern Italian Cooperative Study Group : Prospective, randomized, multicentre trial of effect of protein restriction on progression of chronic renal insufficiency. Lancet 337: 1299–1304, 1991. 10.1016/0140-6736(91)92977-A [DOI] [PubMed] [Google Scholar]
- 12.Rosman JB, ter Wee PM, Meijer S, Piers-Becht TP, Sluiter WJ, Donker AJ: Prospective randomized trial of early dietary protein restriction in chronic renal failure. Lancet 2: 1291–1296, 1984. 10.1016/S0140-6736(84)90818-3 [DOI] [PubMed] [Google Scholar]
- 13.Rosman JB, Langer K, Brandl M, Piers-Becht TP, van der Hem GK, ter Wee PM, Donker AJ: Protein-restricted diets in chronic renal failure: A four year follow-up shows limited indications. Kidney Int Suppl 27: S96–S102, 1989 [PubMed] [Google Scholar]
- 14.Cianciaruso B, Pota A, Bellizzi V, Di Giuseppe D, Di Micco L, Minutolo R, Pisani A, Sabbatini M, Ravani P: Effect of a low- versus moderate-protein diet on progression of CKD: Follow-up of a randomized controlled trial. Am J Kidney Dis 54: 1052–1061, 2009. 10.1053/j.ajkd.2009.07.021 [DOI] [PubMed] [Google Scholar]
- 15.Hahn D, Hodson EM, Fouque D: Low protein diets for non-diabetic adults with chronic kidney disease. Cochrane Database Syst Rev 10: CD001892, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klahr S, Levey AS, Beck GJ, Caggiula AW, Hunsicker L, Kusek JW, Striker G; Modification of Diet in Renal Disease Study Group : The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease. N Engl J Med 330: 877–884, 1994. 10.1056/NEJM199403313301301 [DOI] [PubMed] [Google Scholar]
- 17.Lew QJ, Jafar TH, Koh HWL, Jin A, Chow KY, Yuan J-M, Koh W-P: Red meat intake and risk of ESRD. J Am Soc Nephrol 28: 304–312, 2017. 10.1681/ASN.2016030248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goraya N, Wesson DE: Is dietary red meat kidney toxic? J Am Soc Nephrol 28: 5–7, 2017. 10.1681/ASN.2016060664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hansen HP, Tauber-Lassen E, Jensen BR, Parving H-H: Effect of dietary protein restriction on prognosis in patients with diabetic nephropathy. Kidney Int 62: 220–228, 2002. 10.1046/j.1523-1755.2002.00421.x [DOI] [PubMed] [Google Scholar]