Abstract
Lrp (leucine-responsive regulatory protein) plays a global regulatory role in Escherichia coli, affecting expression of dozens of operons. Numerous lrp-related genes have been identified in different bacteria and archaea, including asnC, an E. coli gene that was the first reported member of this family. Pairwise comparisons of amino acid sequences of the corresponding proteins shows an average sequence identity of only 29% for the vast majority of comparisons. By contrast, Lrp-related proteins from enteric bacteria show more than 97% amino acid identity. Is the global regulatory role associated with E. coli Lrp limited to enteric bacteria? To probe this question we investigated LrfB, an Lrp-related protein from Haemophilus influenzae that shares 75% sequence identity with E. coli Lrp (highest sequence identity among 42 sequences compared). A strain of H. influenzae having an lrfB null allele grew at the wild-type growth rate but with a filamentous morphology. A comparison of two-dimensional (2D) electrophoretic patterns of proteins from parent and mutant strains showed only two differences (comparable studies with lrp+ and lrp E. coli strains by others showed 20 differences). The abundance of LrfB in H. influenzae, estimated by Western blotting experiments, was about 130 dimers per cell (compared to 3,000 dimers per E. coli cell). LrfB expressed in E. coli replaced Lrp as a repressor of the lrp gene but acted only to a limited extent as an activator of the ilvIH operon. Thus, although LrfB resembles Lrp sufficiently to perform some of its functions, its low abundance is consonant with a more local role in regulating but a few genes, a view consistent with the results of the 2D electrophoretic analysis. We speculate that an Lrp having a global regulatory role evolved to help enteric bacteria adapt to their ecological niches and that it is unlikely that Lrp-related proteins in other organisms have a broad regulatory function.
Lrp (leucine-responsive regulatory protein) is a global regulator of metabolism in Escherichia coli, affecting expression of at least 30 operons (7, 29). Targets of Lrp action include operons involved in amino acid biosynthesis and degradation, nitrogen metabolism, one-carbon metabolism, and transport. In addition, Lrp affects a sizeable number of operons that encode fimbrial systems. Lrp activates expression of some operons and represses expression of others, and in some instances the effect of Lrp is modulated by high exogenous levels of leucine and/or alanine. The moderately high abundance of Lrp in E. coli, about 3,000 dimers per cell (40), is consistent with Lrp playing a global regulatory role.
Here we consider the distribution and function of proteins having sequence similarity to Lrp, herein called Lrp-related proteins. The first such protein described, AsnC, is an activator of asnA, a gene in E. coli involved in asparagine biosynthesis (13, 21). Lrp-related proteins are sometimes referred to as the Lrp/AsnC family of proteins, and this may be an apt name because the two may represent paradigms for global versus local regulatory roles. asnC controls its own expression and that of adjacent asnA, the two being transcribed divergently (13, 21). A number of other lrp-related genes are organized similarly with respect to their target genes, including bkdR in Pseudomonas putida (25), putR in Agrobacterium tumefaciens (8), putR in Rhodobacter capsulatus (19), and mdeR in P. putida (18). Other Lrp-related genes are organized differently with respect to target genes; for example, azlB is the first gene in an operon encoding proteins involved in branched-chain amino acid transport in Bacillus subtilis (4). There is no direct evidence that AsnC, BkdR, PutR, MdeR, or AzlB controls only a limited number of genes, but we take this as a working hypothesis. Madhusudhan et al. have shown that the abundance of BkdR is 25 to 40 tetramers per cell, and this supports the idea that BkdR is more a local than a global regulator in P. putida (24).
Lrp from E. coli has limited amino acid sequence identity with most other Lrp-related proteins, in the range of about 29% (see below). On the other hand, E. coli Lrp shares high amino acid sequence identity (between 97 and 100%) with a group of what are almost certainly orthologous proteins from some other enteric bacteria (17). This high sequence identity is not simply a consequence of these organisms being relatively closely related, because the corresponding nucleotide sequences differ for the most part by more than 10% and greater amino acid sequence variation is expected in the absence of evolutionary constraints (17). Friedberg et al. speculated that the high degree of sequence conservation might be related to the fact that Lrp with a monomer size of only 164 amino acids must have amino acid sequences that allow binding to DNA, transcription activation, and regulation by leucine. In addition, they noted potential evolutionary constraints upon a regulatory protein that affects expression of many different operons (17).
Given the considerations above, we focused on an Lrp-related protein from Haemophilus influenzae that we called LrfB (HI1596). LrfB has 75% amino acid identity with Lrp, the highest value among a group of 42 Lrp-related proteins that we surveyed, excluding those from enteric organisms. We entertained three scenarios with respect to a local versus global regulatory function for LrfB: it plays a global regulatory role analogous to that in enteric organisms; it plays a global regulatory role, but perhaps a more modest one that is appropriate for an organism having a genome size about 40% the size of that of E. coli; or it has a local regulatory role, more like that postulated for other Lrp-related proteins. The latter two possibilities seem equally consonant with the amino acid identity value of 75%. We report here the results of studies of LrfB from H. influenzae that we performed to investigate these scenarios.
MATERIALS AND METHODS
Bacterial strains, media, and growth conditions.
The E. coli strains used in this study are listed in Table 1. E. coli cells were grown in L broth (LB) or SSA minimal salts (6) supplemented with 0.2% glucose, 50 μg each of proline, isoleucine, and valine per ml, and 10 μg of thiamine per ml (sSSA medium). Ampicillin was added at 50 and 100 μg/ml for plasmid-containing strains grown in minimal and LB media, respectively. Cultures were grown at 37°C with shaking.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Origin and relevant characteristics | Reference or source |
|---|---|---|
| E. coli | ||
| CV975 | P90C ilvIH::Mu dI1734 | 34 |
| CV1008 | P90C ilvIH::Mu dI1734 lrp-35::Tn10 | 34 |
| CV1445 | P90C lrp-35::Tn10 λ243 (lrp-lacZ) | 22 |
| CV1524 | XL1B/pCV309 | This work |
| CV1528 | CV1445/pCV311 | This work |
| CV1529 | XL1B/pCV312 | This work |
| CV1530 | XL1B/pCV313 | This work |
| CV1534 | CV1445/pTRC99A | This work |
| CV1535 | CV1008/pTRC99A | This work |
| CV1536 | CV1008/pCV311 | This work |
| GHICS91 | Contains plasmid pCS91 | ATCC |
| P90C | F−ara thi Δ(lac-pro) | 36 |
| XL1B | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10] | Stratagene |
| H. influenzae | ||
| Rd | Parent strain | ATCC |
| CV1526 | lrfB::kan | This work |
| Plasmids | ||
| pCS91 | pUC18 containing lrfB | ATCC |
| pCV309 | 1.2-kb kan cassette from pUC4K inserted into XmnI site of pCS91 | This work |
| pCV311 | pTRC99A with lrfB gene behind trc promoter | This work |
| pCV312 | pQE30 containing lrfB | This work |
| pCV313 | pQE30 containing lrfB with kan insertion | This work |
| pQE30 | Plasmid used to prepare six-His-tagged proteins | Qiagen |
H. influenzae Rd (ATCC 51907) was grown in brain heart infusion medium (Difco) supplemented with 3 μg of NAD per ml 10 μg of hemin per ml, and 10 μg of histidine per ml (sBHI) or in MIc defined medium (2). Growth was monitored by turbidimetry at A550 or by measuring protein in samples of cells.
American Type Culture Collection (ATCC) strain GHICS91 has the 498-bp coding region of lrfB (HI1596) together with 356 bp of upstream and 891 bp of downstream DNA cloned into the SmaI site of plasmid pUC18. We designated this plasmid pCS91.
Recombinant DNA methods.
The following methods were performed by the indicated published procedures: isolation of chromosomal DNA from H. influenzae by the quick miniprep method of Barcak et al. (2), transformation of H. influenzae (2), and DNA sequencing by the Sanger method (35).
Construction of plasmids and strains.
H. influenzae strain CV1526 containing a disrupted lrfB gene was prepared as follows. The 1.2-kb HindII DNA fragment carrying the kanamycin gene cassette from pUC4K (Pharmacia) was ligated to a full-length DNA fragment resulting from an XmnI partial digest of plasmid pCS91, and transformants of strain XL1B (Stratagene) were selected on LB plates containing both 50 μg of kanamycin and 100 μg of ampicillin per ml. Plasmid pCV309, isolated from one of the transformants (CV1524), contained the kanamycin cassette within the lrfB gene, as determined by restriction mapping. A 1,750-bp EcoRI/BamHI fragment of plasmid pCV309 was purified by gel electrophoresis and introduced into H. influenzae Rd by transformation, selection being for growth on sBHI agar plates containing 10 μg of kanamycin per ml. Transformants were tested by PCR using primers HIB-4 (5′ GGAATTCGCGGTTTTTCATTCTCTTCGTTC) and HIB-5 (5′CGGATCCGTTAGAGCATGCCATTGACTGTTC) to verify that the disrupted gene (3-kb PCR fragment) had replaced the resident gene (1.8-kb PCR fragment). One of the amplified fragments was sequenced to confirm that the kanamycin cassette was inserted in the expected position within lrfB, between nucleotides 317 and 318 (A of ATG start codon is position 1). The protein encoded by this construct should have the N-terminal 106 amino acids of LrfB fused to 12 additional amino acids.
Plasmid pCV311 containing the lrfB gene under the control of the trc promoter was constructed as follows. The open reading frame of lrfB was amplified by PCR from template pCS91 using primers HIB-8 (5′ CCTTTCCATGGGCAAAGAAATAAAGA) and HIB-9 (5′ CGGGATCCTTATTTCAATACAAGGAA). The resulting fragment was purified by gel electrophoresis, digested by NcoI and BamHI, ligated to plasmid pTRC99A (Pharmacia) digested with the same enzymes, and transformed into E. coli strain CV1445 (yielding strain CV1528). Sequencing demonstrated that plasmid pCV311 had a complete wild-type lrfB gene downstream from the trc promoter and the ribosome binding site of the vector.
pCV312, a plasmid encoding an LrfB having a six-His tag at its N terminus (6×His-LrfB), was constructed as follows. The lrfB coding region was amplified by PCR using primers HI-10 (5′ TTCCTTGGATCCAGCAAAGAAATAAAGAAAATGG) and HI-11 (5′TTGTTTAAGCTTATTATTTCAATACAAGG) and plasmid pCS91 DNA as the template. The amplified fragment was cut with BamHI and HindIII, ligated to plasmid pQE30 (Qiagen) cut with the same enzymes, and transformed into strain XL1B, yielding strain CV1529. Strain CV1530 containing plasmid pCV313 (identical to pCV312 except for having a kan insertion in lrfB) was prepared as described above using plasmid pCV309 as the template in the PCR.
DNA mobility shift experiments.
The reaction conditions were as described in reference 38, except that calf thymus DNA was omitted. The DNA used in these experiments was a 276-bp XbaI/EcoRI fragment containing six binding sites for Lrp upstream from the ilvIH promoter (fragment I in reference 38). Fragments were labeled with 32P at their ends using reverse transcriptase and dCTP or dATP. 6×His-Lrp was purified as described previously (9). To prepare 6×His-LrfB and 6×His-LrfBΔ60 (LrfB lacking 60 C-terminal amino acids), strains CV1529 and CV1530 were grown at 37°C with shaking in LB medium containing ampicillin and at an A600 of 0.7 to 0.8, isopropylthiogalactoside was added to a final concentration of 50 μM. After further incubation for 12 h, cells were harvested by centrifugation and six-His-tagged proteins were purified by affinity chromatography on nickel-nitrilotriacetic acid columns as recommended by the manufacturer (Qiagen).
Western blotting.
Western blotting was performed as previously described (22) using antibodies against E. coli Lrp (40) and against H. influenzae LrfB. The latter were prepared by the Cornell Center for Research Animal Resources. A rabbit was inoculated with adjuvant containing 0.35 mg of six-His–LrfB; after 2 weeks the rabbit was reinjected with 0.35 mg of protein without adjuvant, and serum was collected 4 weeks after the second injection.
H. influenzae cells were grown in MIc medium to late log phase, and samples were analyzed for total protein and by Western blotting for the amount of LrfB. For the latter, previously described procedures were used with a 1:4,000 dilution of rabbit anti-LrfB antibody and a 1:10,000 dilution of peroxidase-conjugated goat anti-rabbit immunoglobulin G (22). Similar determinations were made for E. coli strain CV1536, grown in sSSA medium.
2D gel electrophoresis of proteins.
Stationary-phase cultures grown in sBHI medium were diluted to an A550 of 0.15 in defined MIc medium lacking methionine (MIc-m) and grown to an A550 of 0.4 in the same medium. Following another round of dilution and growth, 1-ml cultures were shaken for 5 min at 37C with [35S]methionine (100 mCi, 1,000 Ci/mmol; Amersham) and then for 3 min following the addition of 17 μmol of unlabeled methionine. The cells were centrifuged for 2 min in an Eppendorf centrifuge at 4°C, washed once with 1 ml of a cold solution containing 17 μmol of methionine and 10 mM Tris, pH 7.5, and stored at −80°C. Electrophoretic analysis of 35S-labeled proteins from H. influenzae was performed by Kendrick Laboratories, Inc. (Madison, Wis.). Prior to electrophoresis, samples were boiled for 5 min in 50 μl of a solution containing 5% sodium dodecyl sulfate, 5% β-mercaptoethanol, 10% glycerol, and 60 mM Tris, pH 6.8, and centrifuged for 5 min. Samples containing 40 to 100 μg of protein (about 40 μCi) were analyzed by two-dimensional (2D) electrophoresis following the procedure of O'Farrell (30). Isoelectric focusing was performed with 2% pH 4 to 8 ampholines for 9,600 V-h, and slab electrophoresis in gels containing 10% acrylamide was carried out for about 4 h. Samples were run in duplicate, fixed, dried, silver stained, and exposed to Kodak XAR film for 2 and 15 h. Quantitation of protein spots was performed by initial manual analysis followed by laser scanning and computerized comparisons with Phoretix software.
Protein and β-galactosidase assays.
Total protein in samples was determined using the Bio-Rad protein assay kit. For bacterial cultures, cells were centrifuged, washed in 50 mM Tris-Cl, pH 7.5, taken up in an equal volume of Tris buffer, and 0.2 to 1.0 ml samples were sonicated on ice for 2 min using a Sonifier cell disruptor 350 (Branson Sonic Power Co.) equipped with a microtip.
β-Galactosidase was assayed by the method of Miller (26) with modifications as described previously (22).
Computer analysis of amino acid sequences.
Amino acid sequence comparisons were made using the Gap and Pileup programs of the Genetics Computer Group software package (12).
RESULTS
A survey of Lrp-related genes.
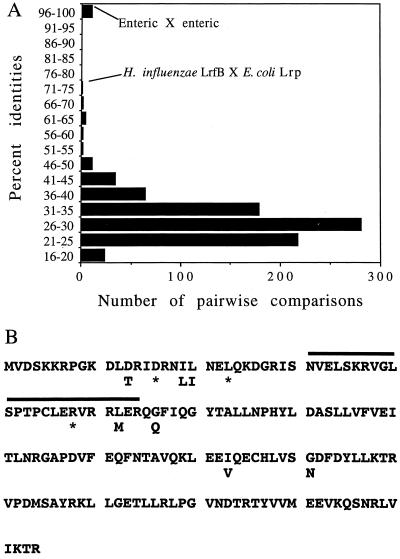
A BLAST search of nonredundant databases (1) suggests that Lrp-related proteins are limited to the domains Archaea and Bacteria. Clearly, not all members of these groups have genes encoding Lrp-related proteins (Mycoplasma genitalium, Mycoplasma pneumonia, Synechocystis sp., and Helicobacter pylori have none), whereas some have the potential to encode several different Lrp-related proteins (H. influenzae Rd and E. coli each have three and Methanococcus jannaschii has two). At the time of this writing, 46 different sequences in E. coli and other organisms showed clear amino acid sequence similarity to Lrp. A summary of percent amino acid identities for pairwise comparisons of these sequences is given in Fig. 1A. Somewhat arbitrarily, three categories can be recognized: pairwise comparisons yielding very high identities (97% or greater), associated with Lrps from a group of enteric bacteria; a relatively small number of pairwise comparisons giving amino acid identities in the range of 50 to 75% (11 comparisons in total); and the remainder, which had less than 50% identities and represent the vast bulk of the comparisons (809 of a total of 820 comparisons). For this last group, the average amino acid identity was 29%, with a standard deviation of 5.9. Thus, in comparison with Lrps from enteric bacteria, Lrp-related proteins do not generally show a high degree of sequence identity. A similar conclusion is seen from Fig. 1B, which shows positions within Lrp-related proteins that are highly conserved. Only three positions are completely invariant, and only seven other positions are nearly invariant, with but two amino acids observed.
FIG. 1.
Comparison of Lrp-related proteins from a number of microorganisms. (A) Amino acid identities for pairwise combinations of sequences. Amino acid sequences of the following Lrp-related proteins were compared in pairwise combinations using the Genetics Computer Group GAP alignment program (12). (∼; see below): Agrobacterium rhizogenes, Orf142∼, M61151; A. tumefaciens, Prp (PutR)∼, U39263; B. subtilis, AzlB, Y11043; B. subtilis, YdaI, AB001488; B. subtilis, YddO, AB001488; B. subtilis, YugG, Z93934; B. subtilis, YwrC, Z93767; Bradyrhizobium japonicum, Orf153∼, U85623; Enterobacter aerogenes, Lrp, U02272; E. coli, AsnC∼, P03809; E. coli, Lrp∼, P19494; E. coli, YbaO∼, D82943; H. influenzae, HI0224∼, P44580; H. influenzae, HI0563∼, P44337; H. influenzae, HI1596∼, P45265; Klebsiella pneumoniae, Lrp, P37424; M. jannaschii, Orf148j∼, U67472; M. jannaschii, Orf156∼, U67519; Mycobacterium tuberculosis, Orf150∼, Z92771; M. tuberculosis, Orf148t, Z79702; Proteus mirabilis, Lrp, Y10417; P. putida, BkdR∼, P42179; P. putida, MdeR∼, D89015; Pyrococcus furiosus, Orf141∼, M97860; Ralstonia eutropha, PdhR (Orf147)∼, X91878; Rhizobium meliloti, Orf127∼, S43966; R. meliloti, Orf169, gil2182625; R. capsulatus, PutR∼, X78346; Rhodobacter sphaeroides, Orf154∼, L07247; Rhodococcus erythropolis, Orf170∼, U42216; Salmonella enterica serovar Typhimurium. Lrp, P37403; Serratia marcescens, Lrp, P37425; Sulfolobus solfataricus, Orf c01007∼, Y08256; Z. mobilis, Grp∼, S52279; and Z. mobilis, Zrp, L34333∼. The protein designations of Belitsky et al. (4) were used here where possible. In addition, 12 other Lrp-related proteins identified in unfinished sequencing projects were included: Vibrio cholerae (two sequences), Pseudomonas aeruginosa (four sequences), Archaeoglobus fulgidus (three sequences), Deinococcus radiodurans (two sequences), and M. tuberculosis (one sequence in addition to the two above). The pairwise comparisons for the enterics (E. coli, K. pneumoniae, P. mirabilis, S. enterica serovar, Typhimurium, and E. aerogenes) were made separately and gave values of 97% or higher. All other pairwise comparisons involved E. coli as a representative of the enteric bacteria together with the other 42 other sequences listed above. The pairwise comparisons that gave <97% and >50% identities are E. coli Lrp × H. influenzae LrfB (75%), E. coli AsnC × H. influenzae HI0563 (68%), E. coli AsnC × V. cholerae LrfB (68%), E. coli YbaO × V. cholerae LrfA (65%), R. eutropha PdhR × P. putida BkdR (64%), H. influenzae AsnC × V. cholerae LrfB (62%), E. coli Lrp × P. aeruginosa LrfA (62%), E. coli Lrp × B. japonicum Orf153 (58%), P. aeruginosa LrfA × B. japonicum Orf153 (58%), P. aeruginosa LrfA × H. influenzae LrfB (55%), A. tumefaciens Prp × R. capsulatus PutR (52%). (B) Highly conserved positions in Lrp-related proteins. The Genetics Computer Group Pileup program (12) was used to align sequences identified in the legend above by a tilde. The sequence of E. coli Lrp is shown, with asterisks designating amino acids that are completely conserved among the 23 proteins. Cases in which a position is limited to only two amino acids are also shown. The helix-turn-helix region is identified with an overline.
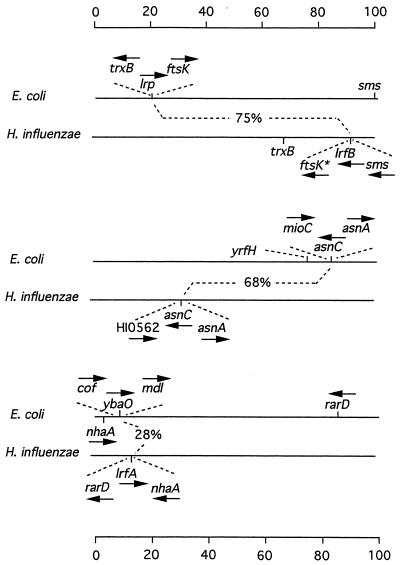
Comparison of lrp-related genes in E. coli and H. influenzae.
Both E. coli and H. influenzae have three lrp-like genes. Their map positions and their flanking genes are compared in Fig. 2. To distinguish between E. coli and H. influenzae genes, we denote H. influenzae genes by including HI identification numbers in parentheses (16). asnC and asnC (HI0563) are orthologs based upon criteria established by Tatusov et al. (37), having both high predicted amino acid sequence identity and a homologous flanking region, at least on one side (see the legend to Fig. 2 for criteria and for a definition of ortholog). The other two lrp-related H. influenzae genes were given gene designations lrfA (HI0224) and lrfB (HI1596) (pneumonic lrp-related family of regulatory proteins). lrfB (HI1596) shows only minimal homology with ybaO (28% amino acid identity), and the two have no flanking regions in common. On the other hand, phylogenetic analysis shows lrfA (HI0224) to be relatively closely related to ybaO and grp (4, 41).
FIG. 2.
Genes related to lrp in E. coli and H. influenzae. Each horizontal line represents the genome of E. coli or H. influenzae on a scale of 100 units. Arrows represent direction of transcription. The lrp-related genes lrp, asnC, ybaO, lrfA (HI0224), and lrfB are shown together with flanking genes. The percent predicted amino acid identities of proteins encoded by pairs of lrp-related genes is indicated. ftsK* (HI1592, HI1593, HI1594, and HI1595) is related to E. coli ftsK as follows: HI1595 is weakly related to the N-terminal amino acids of FtsK (unfiltered BLAST score, 110), and HI1592 is highly related to the C terminus of FtsK (BLAST score, 1358). Orthologs are homologous genes in different organisms that encode proteins with the same function and that have evolved by direct, vertical descent. Two genes are considered to be orthologs if their predicted amino acid sequences fulfill the following criteria (37): they show higher similarity to each other than to other proteins in either organisms, they show a higher similarity to each other than to homologs from phylogenetically more distant organisms, and they align throughout most of their lengths. In addition, an orthologous relationship between two genes is strengthened if the two are flanked on one or both sides by orthologous gene pairs.
lrfB (HI1596) and lrp fit all of the criteria for being orthologs. In addition to a high degree of predicted amino acid sequence identity (75%), a common evolutionary relationship is inferred from the fact that both are adjacent to ftsK, a gene involved in cell division (3). However, we argue below that these two genes are in one sense not true orthologs, because their functions have diverged significantly.
Phenotypes associated with an H. influenzae lrfB null allele.
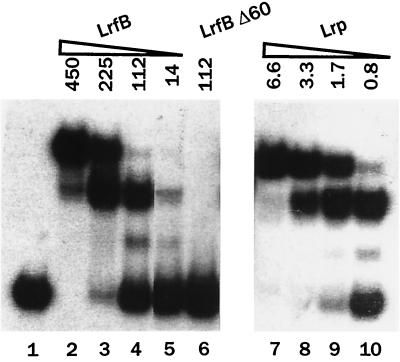
H. influenzae strain CV1526 containing a kan insertion in the lrfB gene was created in a transformation experiment employing plasmid pCV309 cut with BamHI/EcoRI. The kan insertion in strain CV1526 is between nucleotides 317 and 318 of the coding region of the lrfB gene and a truncated protein having 118 amino acids should be produced (106 amino acids from LrfB plus 12 others encoded by the insertion) rather than the full-length polypeptide of 166 amino acids. The results of the experiments described below suggest that the truncated LrfB protein has no biological activity. Six-His-tagged derivatives of LrfB and the truncated LrfB were prepared and purified by affinity chromatography on nickel-nitrilotriacetic acid columns. In vitro, Lrp and 6×His-LrfB both bound with high affinity to sites upstream of the ilvlH promoter, but the truncated 6×His-LrfBΔ60 showed no such binding (Fig. 3, compare lanes 4 and 6). In the absence of specific DNA binding, it is highly unlikely that a truncated LrfB would retain any function in vivo.
FIG. 3.
Binding of Lrp and LrfB to sites upstream of the ilvIH promoter as visualized by gel retardation. A 32P-labeled 276-bp DNA fragment from a region upstream of the E. coli ilvIH promoter containing six binding sites for Lrp was mixed with no protein (lane 1), purified 6×His-LrfB (lanes 2 to 5), purified 6×His-LrfBΔ60 (lacking 60 C-terminal amino acids) (lane 5), and purified 6×His-Lrp (lanes 7 to 10), and samples were fractionated by electrophoresis. The concentrations (nanomolar) of proteins are indicated. For the experiment with Lrp, the faster-moving band contains Lrp bound to sites 1 and 2, and the slower-moving band contains Lrp bound to sites 1, 2, 3, 4, 5, and 6 (38).
H. influenzae strain CV1526 (lrfB::kan) and its parent grew in defined MIc medium with the same doubling time (about 75 min), but the final yield of CV1526 cells was only about 60% that of the parent, as judged by turbidimetry or protein measurements. Microscopic examination showed that strain CV1526 (lrfB::kan) grew in log phase as long filaments rather than as discrete cells (data not shown). This phenotype might be related to some cis effect of the kan insertion on the ftsK-related region located immediately downstream of lrfB, since some ftsK mutations in E. coli lead to filamentation (3, 14).
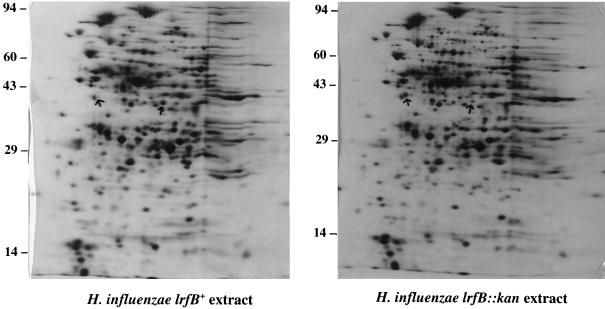
To search for other phenotypes associated with the disrupted lrfB gene, we performed a 2D gel analysis of proteins in extracts of wild-type and mutant H. influenzae strains after cells were grown in MIc medium and pulse-labeled with [35S]methionine. Only two spots were found to reproducibly differ in intensity (Fig. 4). One of them was more intense in the mutant than the parent (16-fold in one experiment and 5-fold in another; estimated pI 5.57; estimated molecular mass, 44 to 54 kDa), whereas the other was more intense in the parent (5-fold in each of two experiments; pI 6.32; 43 to 51 kDa). The former was visible in gels stained with silver but not Coomassie blue. The latter, abundant enough to be visible in Coomassie-stained gels, was identified after proteolysis and mass spectrometry as asparagine synthetase (EC 6.3.1.1).
FIG. 4.
2D gel analysis of proteins in extracts of wild-type (A) and lrfB::kan (B) strains of H. influenzae. For the isoelectric focusing dimension, the anode is on the left. Cells were grown in MIc medium and pulse-labeled with [35S]methionine. Arrows indicate the positions of the two spots that were reproducibly different in the two strains.
To summarize, strain CV1526 grows in a defined medium with an undiminished growth rate but a reduced yield, shows filamentation, and produces at least two proteins in amounts different than the parent. The filamentation may be due to a cis effect of the kan insertion.
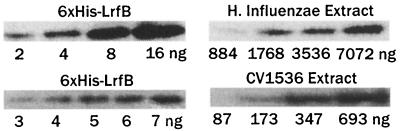
LrfB abundance in H. influenzae.
Western blotting was employed to measure the abundance of LrfB in crude extracts of H. influenzae, using antibodies raised against purified six-His-LrfB (Fig. 5A). About 0.073% of the total protein was LrfB, corresponding to about 130 dimers per cell (see the legend to Fig. 5 for assumptions used in making these estimates). By comparison, the numbers for Lrp in E. coli are 0.122% of the total protein, corresponding to about 3,000 dimers per cell (40). The sizeable difference between estimates based upon total protein (1.7-fold higher abundance of Lrp) and upon dimers per cell (23-fold higher abundance of Lrp) is due to the estimated difference in volumes of the two organisms (E. coli is about 14 times larger than H. influenzae).
FIG. 5.
Amount of LrfB in H. influenzae (A) and in E. coli strain CV1536 (B) as determined by Western blotting. A standard curve prepared using 6×His-LrfB was employed to estimate that 0.073% of the total protein in H. influenzae is LrfB. The number of dimers per cell was calculated assuming that the total protein per unit volume is the same for E. coli and H. influenzae. The following parameters were used in the calculation: total protein per E. coli cell, 0.156 pg (28); volume of an E. coli cell, 0.87 × 10−12 ml, calculated from the weight of a cell together with the densities of the components of a cell (28) (a similar number is derived from assuming that the dimensions of an E. coli cell are 0.75 by 2 μm); volume of an H. influenzae cell, 0.636 × 10−12 ml, calculated assuming dimensions of 0.3 by 1 μm (27); LrfB dimer molecular mass, 37.8 kDa.
lrfB partially activates expression from the E. coli ilvIH promoter and represses expression from the lrp promoter.
The H. influenzae lrfB gene was cloned behind the trc promoter and introduced into different E. coli strains to determine whether lrfB influenced expression from promoters known to be regulated by Lrp.
Strains CV975, CV1535, and CV1536 (Table 2) contain a transcriptional fusion of the ilvIH promoter to lacZ. As demonstrated previously (34), expression from the ilvIH promoter was more than 30-fold lower in a strain lacking Lrp than in the parent strain (Table 2). Furthermore, consistent with earlier results (11, 34), expression from the ilvIH promoter was repressed about 10-fold when cells were grown with exogenous leucine (Table 2). In a strain containing LrfB but not Lrp, expression from the ilvIH promoter was obviously stimulated by LrfB (about 14-fold), but not to the extent observed for an Lrp-containing strain (Table 2). Thus, LrfB can at least partially substitute for Lrp as an activator of ilvIH expression. Furthermore, that activation is reversed in part by exogenous leucine, as is the case for Lrp-mediated activation of ilvIH expression (Table 2).
TABLE 2.
LrfB activates expression from the E. coli ilvIH promoter and represses expression from the lrp promoter
| Straina |
lrp or lrfB allele on:
|
Promoter-lacZ fusion | Addition to mediumb | Sp act of β-galactosidasec | Relative sp act (%) | |
|---|---|---|---|---|---|---|
| Chromosome | Plasmid | |||||
| CV975 | lrp+ | PilvIH | 359 ± 20 | 100 | ||
| CV975 | lrp+ | PilvIH | Leu | 37.4 ± 2.6 | 10 | |
| CV1535 | lrp-35::Tn10 | PilvIH | 3.0 ± 2.1 | 0.83 | ||
| CV1536 | lrp-35::Tn10 | lrfB | PilvIH | 43.3 ± 9.1 | 12 | |
| CV1536 | lrp-35::Tn10 | lrfB | PilvIH | Leu | 6.6 ± 0.34 | 1.8 |
| CV1534 | lrp-35::Tn10 | Plrp | 774 ± 53 | 100 | ||
| CV1528 | lrp-35::Tn10 | lrfB | Plrp | <0.5 | <0.06 | |
Strains CV975, CV1535, and CV1536 were grown to log phase in sSSA minimal medium containing 50 μg of ampicillin per ml (except for CV975). Strains CV1534 and CV1528 were grown in LB medium containing 100 μg of ampicillin per ml.
When present, leucine was at 50 μg/ml.
Specific activity is in Miller units. Data are averages ± standard deviations of at least two different experiments, each performed in duplicate.
Lrp serves as a repressor of transcription from the lrp promoter (39), although it is not a particularly effective repressor, as judged from the fact that Lrp is a moderately abundant protein in E. coli (40). To determine whether LrfB can repress transcription from the lrp promoter, we introduced lrfB into a strain containing the lrp promoter fused to a lacZ gene. Overproduction of LrfB effectively eliminated transcription from the lrp promoter (Table 2).
An estimate of the amount of LrfB in one of the strains in Table 2 (CV1536) was made by Western blotting (Fig. 5B). LrfB was about 2.3% of total protein, an amount that is nearly 20 times higher than that of Lrp in wild-type strains of E. coli. In summary, when produced in high levels in E. coli cells, LrfB weakly activates transcription from the ilvIH promoter and strongly represses transcription from the lrp promoter.
LrfB binds in vitro upstream of ilvIH and lrp DNA.
In vitro, Lrp binds to six sites upstream from the ilvIH promoter (called sites 1 through 6, with site 6 being closest to the promoter). In DNA mobility shift experiments, two major bands are observed, representing cooperative binding to sites 1 and 2 and to sites 1, 2, 3, 4, 5, and 6 (38). We compared binding of purified 6×His-Lrp and purified 6×His-LrfB to a DNA fragment containing these six sites. As shown in Fig. 3, all three proteins gave the same pattern of two bands, suggesting that they all bind to the same sites. The dissociation constant for 6×His-LrfB, estimated from these titration experiments (concentration of protein at which 50% of the DNA was converted to any complex), was about 135 times higher than for Lrp.
DISCUSSION
From analysis of DNA sequences, 46 proteins evolutionarily related to E. coli Lrp are presumed to exist in a variety of eubacteria and archaea. In pairwise sequence comparisons, they show on average 29% amino acid sequence identity, and in a Pileup analysis of 23 sequences there were only three amino acid positions that were entirely conserved. One of those conserved positions, an arginine at position 48 of Lrp, is within a helix-turn-helix region that has been shown to be important for binding of Lrp to DNA (33). A helix-turn-helix region has 24 amino acids, and the relevant arginine is at position 18. This arginine may represent a signature for Lrp-related proteins, since only 3% of 609 tabulated helix-turn-helix regions have an arginine at that position (32). While the conserved arginine at position 18 of the helix-turn-helix is the most striking departure from the tabulated values, the distribution is skewed at other positions also, including position 8 (60% valine in Lrp-related proteins, 6% among 609 proteins with a helix-turn-helix region), position 13 (39% threonine versus 6% expected), position 14 (35% proline versus 2% expected), and position 15 (52% cysteine versus 2% expected).
When viewed against the average pairwise amino acid identity of 29%, the group of enteric bacteria having 97% or greater identity stand out as a special class. That high identity almost certainly involves strong selection, as we have argued earlier (17), and selection, in turn, may result from the fact that Lrp affects expression of many operons (more than 30 in E. coli). A corollary is that most Lrp-related proteins may have more limited roles, regulating the expression of only one or a few operons, with the relatively low sequence identity among them a reflection of a relaxed selection pressure. In this context we chose to investigate LrfB from H. influenzae, a protein having 75% amino acid identity with Lrp. Our results, summarized below, suggest that LrfB does not affect expression of a large number of genes.
In a 2D gel analysis of wild type and lrp mutant strains of E. coli, Ernsting et al. observed differences in 20 proteins for cells grown in a medium containing leucine and 10 other differences for cells grown without leucine (15). In our similar experiments with wild-type and lrfB mutant strains of H. influenzae grown in MIc medium (which contains leucine), we observed a difference in the rate of synthesis of only two proteins. It might be argued that the H. influenzae genome is only 4/10 the size of the E. coli genome, but even including this factor, a difference of eight proteins might be expected (20 × 0.4) if LrfB were a global regulator of a smaller genome. We tried to grow wild-type and lrfB mutant strains of H. influenzae in MIc medium lacking leucine, but both strains stopped growing after nutrients in the inoculum were depleted.
We were surprised to learn that the protein that consistently had a lower rate of synthesis in the lrfB mutant strain was asparagine synthetase (encoded by asnA). In E. coli, the transcription of asnA is regulated by asnC, a gene that is immediately upstream and transcribed divergently (13, 21), and since asnC and asnA are similarly organized in H. influenzae, we assumed a similar regulatory relationship. It remains to be determined whether LrfB directly affects expression of asnA in H. influenzae, or whether it affects it indirectly in some way, for example, by affecting the expression of asnC.
Another phenotype that resulted from a kan insertion in lrfB was filamentous growth. This phenotype could reflect an effect of LrfB upon some gene involved in cell division, but it might be related instead to the location of lrfB upstream of four genes (HI1592, HI1593, HI1594, and HI1595) that together represent ftsK in E. coli. The latter encodes a large putative polypeptide (147 kDa) with predicted membrane spanning regions within an N-terminal domain and a predicted nucleotide binding consensus sequence within a C-terminal domain (3). One mutation near the proximal end of E. coli ftsK causes a temperature-sensitive block at a late stage in cell division and leads to filamentation (3) and a cat insertion near the middle of the gene gives the same phenotype (14). There is some question about the location of the promoter(s) for ftsK (3, 7), but the results of complementation studies suggest that, at least under some conditions, transcription of ftsK may originate upstream from lrp. Furthermore, some unpublished work suggests that overproduction of FtsK may inhibit cell division (L. Gregg-Jolly, personal communication). Thus, the kan cassette insertion in lrfB could lead to filamentation either by reducing transcription of the downstream ftsK region (HI1592, HI1593, HI1594, and HI1595) or by increasing the transcription of this region possibly via a promoter within the kan cassette.
The abundance of LrfB in H. influenzae is noticeably lower than that of Lrp in E. coli (3.5 versus 5.7 μM), especially when expressed as dimers per cell (130 versus 3,000). Our recent experiments indicate that 15 to 50% of Lrp in E. coli cells is free (not bound to DNA) (S. Chen, Z. Hao, E. Bieniek, and J. Calvo, unpublished data) and that free Lrp in cells exists primarily as an 8-mer or a 16-mer (S. Chen and J. Calvo, unpublished data) rather than the previously reported dimer (40). If the association state of LrfB is higher than a dimer, then H. influenzae cells are expected to contain even fewer than 130 molecules per cell. It is generally held that the amount of a regulatory protein must be in reasonable excess over the total number of specific target sites (23). With but 130 or fewer LrfB molecules per cell, it seems unlikely that LrfB acts effectively at a relatively large number of promoters, given that some substantial fraction of LrfB is expected to be either bound nonspecifically to DNA or free.
The ilvIH operon is a member of the Lrp regulon (7). When produced in E. coli, LrfB functioned to a limited extent as an activator of ilvIH expression. The limited ability to complement might result from low occupancy due to poor binding or to reduced efficiency of bound LrfB in activating transcription (in comparison with Lrp, the binding affinity of LrfB is about 100-fold lower and the amount of LrfB per cell is about 20 times higher). For the case of LrfB acting on lrp expression, the concentration of LrfB produced in these E. coli cells was sufficiently high that expression from the lrp promoter was effectively eliminated. It is not surprising that LrfB functioned more effectively as a repressor than as an activator, because the latter may require specific interactions with RNA polymerase.
It is worth noting that the aforementioned ability of Lrp-related proteins to complement one another is not an isolated case. Lrp partially complemented BkdR action in P. putida (25), and, likewise, BkdR partially complemented Lrp action in E. coli (B. Tyler and J. M. Calvo, unpublished data). Grp, an Lrp-related protein from Zymomonas mobilis, was a reasonably effective activator of ilvIH expression in E. coli (31), whereas LrpC from B. subtilis was a modest repressor of this same operon (5). In addition, an Lrp-related protein from Bradyrhizobium japonicum partially activated ilvIH expression in E. coli, and King and O'Brian speculated that it also competed with Lrp for interaction with the E. coli opp operon (20). All of these examples suggest that there is some overlap in the specificity of binding of Lrp and Lrp-related proteins. Indeed, our demonstration here that LrfB binds in vitro to sites upstream of the ilvIH promoter provides direct evidence that this is the case.
In conclusion, we present comments on the broader implications of this work, including implications for the role of Lrp in E. coli. Some workers have implied that one or another Lrp-related protein has a global regulatory function merely on the basis of amino acid sequence comparisons to Lrp. We prefer to emphasize the point made by Belitsky et al. that “…only the Lrp protein of E. coli … is known to be involved in global regulation of cellular metabolism” (4). We show here that even for LrfB, the protein most closely resembling Lrp, a more limited regulatory role is likely. Thus, our work supports a stronger conclusion that only in enteric bacteria do Lrp-related proteins likely have a global regulatory role. In our view, Lrp evolved as a regulatory protein that could interact with and be influenced by various amino acids, including branched-chain amino acids (BkdR and AzlB), proline (PutR), glutamate (Grp), asparagine (AsnC), and methionine (MdeR) (4, 8, 18, 19, 21, 25, 31). Presumably, with a limited number of target operons (as with most other regulatory proteins), there were few restraints upon the evolution of new Lrp-related regulatory proteins and that accounts for the observed family of proteins. By this view, Lrp evolved a special global regulatory role in enteric bacteria that helped them adapt to their environment. E. coli lives within a sometimes nutritionally rich intestinal environment or in a presumably less rich environment outside a host, and a postulated role of Lrp has been to help the organism make the transition between these environments (7). Most other microorganisms do not live in two distinct environments. For example, H. influenzae normally inhabits the upper respiratory tract of humans, a presumably more constant nutritional environment than that of the intestine, and thus may have no need for the global regulatory function of Lrp.
Newman and colleagues have considered Lrp not so much a regulatory protein per se but a member of a class of abundant proteins (HU, HNS, IHF, and Fis) whose functions include organizing DNA and, in some cases, regulation (10). While it is true that any protein that binds to DNA can be considered a DNA-organizing protein, we think that considering Lrp a DNA-organizing protein may be stretching the concept. The abundance of Lrp dimers per cell, about 3,000, is only about 1/10 of that of the other members of this class. Furthermore, as we have argued above, Lrp-related proteins are basically regulatory proteins like others that may be more familiar, such as members of the LysR family. It seems unlikely that in E. coli Lrp evolved to have a role that was primarily structural.
Sequence comparisons, while commonly used to assign orthologous relationships, are not sufficient for that purpose and the Lrp/LrfB example is a particularly good case in point. For this example, there is even additional experimental evidence for relatedness that we summarize as follows: each protein binds to antibodies raised against the other (data not shown); both proteins bind to similar DNA sequences in vitro; in vitro binding of both proteins to DNA is reduced by leucine; both proteins activate transcription from the ilvIH promoter in vivo; and both proteins repress transcription from the lrp promoter in vivo. Nevertheless, despite all of these similarities, our data strongly suggest that Lrp and LrfB have fundamentally different functions.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grant GM48861.
We thank W. Ghiorse for help with photography, S. Calvo and I. Friedberg for assistance with computer programs, M. A. Gawinowicz and K. Williams for performing mass spectrometric analyses, and R. J. Redfield, L. Macfadyen, L. Gregg-Jolly, and N. Kendrick for helpful discussions.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Barcak G J, Chandler M S, Redfield R J, Tomb J F. Genetic systems in Haemophilus influenzae. Methods Enzymol. 1991;204:321–342. doi: 10.1016/0076-6879(91)04016-h. [DOI] [PubMed] [Google Scholar]
- 3.Begg K J, Dewar S J, Donachie W D. A new Escherichia coli cell division gene, ftsK. J Bacteriol. 1995;177:6211–6222. doi: 10.1128/jb.177.21.6211-6222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belitsky B R, Gustafsson M C U, Sonenshein A L, Wachenfeldt C V. An lrp-like gene of Bacillus subtilis involved in branched-chain amino acid transport. J Bacteriol. 1997;179:5448–5457. doi: 10.1128/jb.179.17.5448-5457.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beloin C, Ayora S, Exley R, Hirschbein L, Ogasawara N, Kasahara Y, Alonso J C, Hegarat F L. Characterization of an lrp-like (lrpC) gene from Bacillus subtilis. Mol Gen Genet. 1997;256:63–71. doi: 10.1007/s004380050546. [DOI] [PubMed] [Google Scholar]
- 6.Calvo J M, Freundlich M, Umbarger H E. Regulation of branched-chain amino acid biosynthesis in Salmonella typhimurium: isolation of regulatory mutants. J Bacteriol. 1969;97:1272–1282. doi: 10.1128/jb.97.3.1272-1282.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calvo J M, Matthews R G. The leucine-responsive regulatory protein (Lrp), a global regulator of metabolism in Escherichia coli. Microbiol Rev. 1994;58:466–490. doi: 10.1128/mr.58.3.466-490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho K, Winans S C. The putA gene of Agrobacterium tumefaciens is transcriptionally activated in response to proline by an Lrp-like protein and is not autoregulated. Mol Microbiol. 1996;22:1025–1033. doi: 10.1046/j.1365-2958.1996.01524.x. [DOI] [PubMed] [Google Scholar]
- 9.Cui Y, Midkiff M A, Wang Q, Calvo J M. The leucine-responsive regulatory protein (Lrp) from Escherichia coli. Stoichiometry and minimal requirements for binding to DNA. J Biol Chem. 1996;271:6611–6617. doi: 10.1074/jbc.271.12.6611. [DOI] [PubMed] [Google Scholar]
- 10.D'Ari R, Lin R T, Newman E B. The leucine-responsive regulatory protein: more than a regulator. Trends Biochem Sci. 1993;18:260–263. doi: 10.1016/0968-0004(93)90177-o. [DOI] [PubMed] [Google Scholar]
- 11.DeFelice M, Levinthal M. The acetohydroxy acid synthase III isoenzyme of Escherichia coli K-12: regulation of synthesis by leucine. Biochem Biophys Res Commun. 1997;79:82–87. doi: 10.1016/0006-291x(77)90063-8. [DOI] [PubMed] [Google Scholar]
- 12.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Wind N, de Jong M, Meijer M, Stuitje A R. Site-directed mutagenesis of the Escherichia coli chromosome near oriC: identification and characterization of asnC, a regulatory element in E. coli asparagine metabolism. Nucleic Acids Res. 1985;13:8797–8811. doi: 10.1093/nar/13.24.8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diez A A, Farewell A, Nannmark U, Nystrom T. A mutation in the ftsK gene of Escherichia coli affects cell-cell separation, stationary-phase survival, stress adaptation, and expression of the gene encoding the stress protein UspA. J Bacteriol. 1997;179:5878–5883. doi: 10.1128/jb.179.18.5878-5883.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ernsting B R, Atkinson M R, Ninfa A J, Matthews R G. Characterization of the regulon controlled by the leucine-responsive regulatory protein in Escherichia coli J. Bacteriol. 1992;174:1109–1118. doi: 10.1128/jb.174.4.1109-1118.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleischmann R D, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae Rd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 17.Friedberg D, Platko J V, Tyler B, Calvo J M. The amino acid sequence of Lrp is highly conserved in four enteric microorganisms. J Bacteriol. 1995;177:1624–1626. doi: 10.1128/jb.177.6.1624-1626.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye H, Inagaki K, Eriguchi S-I, Tamura T, Esaki N, Soda K, Tanaka H. Molecular characterization of the mde operon involved in l-methionine catabolism of Pseudomonas putida. J Bacteriol. 1997;179:3956–3962. doi: 10.1128/jb.179.12.3956-3962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keuntje B, Masepohl B, Klipp W. Expression of the putA gene encoding proline dehydrogenase from Rhodobacter capsulatus is independent of NtrC regulation but requires an Lrp-like activator protein. J Bacteriol. 1995;177:6432–6439. doi: 10.1128/jb.177.22.6432-6439.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King N D, O'Brian M R. Identification of the lrp gene in Bradyrhizobium japonicum and its role in regulation of δ-aminolevulinic acid uptake. J Bacteriol. 1997;179:1828–1831. doi: 10.1128/jb.179.5.1828-1831.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kolling R, Lother H. AsnC: an autogenously regulated activator of asparagine synthetase A transcription in Escherichia coli. J Bacteriol. 1985;164:310–315. doi: 10.1128/jb.164.1.310-315.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Landgraf J R, Wu J, Calvo J M. The effect of nutrition and growth rate upon Lrp levels in Escherichia coli. J Bacteriol. 1996;178:6930–6936. doi: 10.1128/jb.178.23.6930-6936.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewin B. Genes, V. P. 432–435. Oxford, United Kingdom: Oxford University Press; 1994. [Google Scholar]
- 24.Madhusudhan K T, Huang N, Sokatch J R. Characterization of BkdR-DNA binding in the expression of the bkd operon of Pseudomonas putida. J Bacteriol. 1995;177:636–641. doi: 10.1128/jb.177.3.636-641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madhusudhan K T, Lorenz D, Sokatch J R. The bkdR gene of Pseudomonas putida is required for expression of the bkd operon and encodes a protein related to Lrp of Escherichia coli. J Bacteriol. 1993;175:3934–3940. doi: 10.1128/jb.175.13.3934-3940.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 352–355. [Google Scholar]
- 27.Moxon E R. Haemophilus influenzae. In: Mandell G, Douglas R, Bennett J, editors. Principles and practice of infectious diseases. 3rd ed. New York, N.Y: John Wiley & Sons; 1985. pp. 1274–1279. [Google Scholar]
- 28.Neidhardt F C. Chemical composition of Escherichia coli. In: Neidhardt F C, Ingraham J L, Low K B, Magasanik B, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Vol. 1. Washington, D.C.: American Society for Microbiology; 1987. pp. 3–6. [Google Scholar]
- 29.Newman E B, Lin T T, D'Ari R. The leucine/Lrp regulon. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1513–1525. [Google Scholar]
- 30.O'Farrell P. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 31.Peekhaus N, Tolner B, Poolman B, Kramer R. The glutamate uptake regulatory protein (Grp) of Zymomonas mobilis and its relatin to the global regulator Lrp of Escherichia coli. J Bacteriol. 1995;177:5140–5147. doi: 10.1128/jb.177.17.5140-5147.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietrokovski S. Searching databases of conserved sequence regions by aligning protein multiple-alignments. Nucleic Acids Res. 1996;24:3836–3845. doi: 10.1093/nar/24.19.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Platko J V, Calvo J M. Mutations affecting the ability of Escherichia coli Lrp to bind DNA, activate transcription, or respond to leucine. J Bacteriol. 1993;175:1110–1117. doi: 10.1128/jb.175.4.1110-1117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Platko J V, Willins D A, Calvo J M. The ilvIH operon of Escherichia coli is positively regulated. J Bacteriol. 1990;172:4563–4570. doi: 10.1128/jb.172.8.4563-4570.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 37.Tatusov R L, Mushegian A R, Bork P, Brown N P, Hayes W S, Borodovsky M, Rudd K E, Koonin E V. Metabolism and evolution of Haemophilus influenzae deduced from a whole-genome comparison with Escherichia coli. Curr Biol. 1996;6:279–291. doi: 10.1016/s0960-9822(02)00478-5. [DOI] [PubMed] [Google Scholar]
- 38.Wang Q, Calvo J M. Lrp, a global regulatory protein of E. coli, binds cooperatively to multiple sites and activates transcription of ilvIH. J Mol Biol. 1993;229:306–318. doi: 10.1006/jmbi.1993.1036. [DOI] [PubMed] [Google Scholar]
- 39.Wang Q, Wu J, Friedberg D, Platko J, Calvo J M. Regulation of the Escherichia coli lrp gene. J Bacteriol. 1994;176:1831–1839. doi: 10.1128/jb.176.7.1831-1839.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Willins D A, Ryan C W, Platko J V, Calvo J M. Characterization of Lrp, an Escherichia coli regulatory protein that mediates a global response to leucine. J Biol Chem. 1991;266:10768–10774. [PubMed] [Google Scholar]
- 41.Zeng Q, Summers A O. A glutamate uptake regulatory protein (Grp) in Escherichia coli? Mol Microbiol. 1997;24:231–232. [PubMed] [Google Scholar]