Abstract
Objective
Ulcerative colitis (UC) is a chronic inflammatory disease of the large intestine. At present, the significance of appendiceal orifice inflammation (AOI) in UC prognosis is still controversial. This prospective observational study investigated the importance of AOI in UC diagnosis and prognosis. Additionally, it compared the therapeutic efficacy of treatments in UC patients with or without AOI.
Design
This study was a prospective, observational, single-centre, real-world study. Patients with AOI were included in the observation group, and patients without AOI were assigned to the control group. All patients were followed up for 1 year; the disease remission and treatment efficacy were re-examined by colonoscopy. In addition, the clinical, endoscopic and pathological features were collected before and after the treatment.
Results
Patients with endoscopic diffuse inflammatory changes in the distal colorectum accompanied by AOI had a higher positive UC diagnosis rate than those without (96.5% vs 78.0%). Also, AOI had a specificity of 95.2% and a sensitivity of 28.3% for UC diagnosis. However, no difference in the modified Mayo score (p=0.881) or Baron grading was observed between the control and observation groups, indicating that AOI does not affect the treatment outcome of UC patients.
Conclusion
In this study, the observation of AOI improved the UC diagnostic accuracy in patients with diffuse lesions in the distal colorectum. Furthermore, the presence of AOI does not affect the treatment efficacies of UC.
Trial registration number
ChiCTR1800017753.
Keywords: adult gastroenterology, inflammatory bowel disease, endoscopy
Strengths and limitations of this study.
The first reported study that concomitantly explores the significance of appendiceal orifice inflammation in the ulcerative colitis (UC) diagnosis and prognosis, which is in line with the general clinical path.
This study highlights the clinical value of an underevaluated disease, the skip UC with skip lesions.
This study is a single-centre, single-city prospective cohort study with a relatively small cohort.
This study did not adopt the random blind method.
The clinical management is not necessarily standard in other geographic locations, which compromises the results’ generalisability.
Background
Conventionally, ulcerative colitis (UC) is defined as a chronic relapsing inflammatory disorder with diffuse lesions beginning in the rectum and extending proximally without skip areas.1 The diagnosis of UC is based on clinical presentation, colonoscopic evaluation and pathological parameters in the absence of alternative aetiology.2 A correct diagnosis is imperative since it is critical for differential diagnosis and treatment option selection.2 However, due to the overlapping symptoms and pathology, it is challenging to distinguish UC from other forms of colitis, particularly infectious colitis, Crohn’s disease and intermediate colitis.3 4 In the absence of gold standard diagnostics, clinicians may face distress when diagnosing UC. UC can be generally classified into three groups, based on the inflammation extent and endoscopic features, recognised as the Montreal classification: proctitis, left-sided colitis and pancolitis.2
Additionally, several types of skip inflammation, such as the preappendical red patch or appendiceal orifice inflammation (AOI), have been reported in previous studies.2 Meanwhile, AOI accompanied by diffuse inflammation in the distal rectum has been reported in UC patients.5 However, there is no substantial evidence that this skip lesion can directly aid in diagnosing UC.
With the progression in endoscopic technology, the presence of AOI in UC patients has attracted the attention of clinical researchers. Although some investigators suggest that AOI does not significantly affect UC prognosis,1 6–8 an increasing number of studies have indicated that the presence of AOI possesses potential clinical implications. For instance, UC patients with AOI have better prognoses, minor disease progressions and fewer medical intervention demands than those without AOI.9 In addition, UC patients who received appendectomies had a lower recurrence rate and disease activity than those who did not, and AOI has been considered a marker of active UC.10 However, there are currently limited qualitative studies regarding the prognosis of UC with AOI. Also, whether the therapeutic effects of varying treatment options are different in UC patients with and without AOI is still elusive.
This prospective observational study investigated the significance of this skip lesion of the appendiceal orifice for UC diagnosis. Moreover, it explored the difference in treatment efficacies between UC patients with or without AOI.
Patients and methods
Patient and public involvement
All the patients were recruited from the outpatient clinic. Their experiences and related clinical needs drove the research question, and no patients were involved in the study design, recruitment, conduct or analysis. The results were released to study participants after the clinical trial. The aggregate data will be made publicly available. During the examination, we found that some UC patients may have been misdiagnosed; therefore, those patients were not included in the UC prognosis analysis. In this study, the patients evaluated the burden of the intervention by themselves.
Patient population
This study was a prospective, observational, single-centre, real-world study. The patients were recruited from a gastroenterology department at the West China Hospital. A total of 497 patients exhibiting colonoscopic diffuse inflammation in the distal colorectal region from January 2018 to December 2020 were recruited. Moreover, patients with AOI were included in the observation group, and patients without AOI were recruited to the control group.
Patient inclusion criteria: (1) 14–80 years of age; (2) main clinical manifestations were at least one of persistent or recurrent diarrhoea, bloody mucopurulent stool, abdominal pain and/or tenesmus over 4 weeks; (3) endoscopic distal diffuse colitic lesion, which was defined as follows: (A) diffuse mucosal friability, loss of vascularity, erythema, or oedema involving distal colon in a continuous manner regardless of rectal sparing, (B) diffuse, shallow ulcers and (C) absence of contradictory colonoscopic findings, such as stricture, fissure, classical longitudinal ulcers along mesocolic side or circular ulcers. (4) Agreement to participate in the study and a signed informed consent form.
Patient exclusion criteria: (1) diagnosed with intestinal inflammation of known aetiology; (2) pregnancy; (3) prior or concurrent malignancies; (4) previously received appendectomy and (5) previously received colectomy.
Methods
All patients received an endoscopic evaluation performed by a specially assigned endoscopist. During the examination, biopsies were obtained from multiple sites. In addition, the colonoscope tip was required to reach the terminal ileum to characterise the disease at the macroscopic level according to the Montreal classification.6 Furthermore, biopsies were obtained if inflammatory lesions were found in the terminal ileum or appendiceal orifice. Meanwhile, the diagnosis of UC was made by a gastroenterologist based on the patient’s clinical presentation (symptoms lasting more than 6 months), colonoscopic evaluation (twice), as well as the pathological features with the exclusion of alternative causes according to the guidelines in the Chinese Consensus on Diagnosis and Treatment in Inflammatory Bowel Disease (2018 Beijing).6 Also, as a real-world study, the endoscopy procedure, treatment, data collection and data analysis were all performed by different researchers. Both investigators and patients were blinded to the study group assignment. The grouping information was concealed until the end of the study while all data were collected, verified and filed.
In addition, the therapeutic strategies for all diagnosed UC patients were selected based on the up-to-date guidelines from the Chinese Consensus on Diagnosis and Treatment in Inflammatory Bowel Disease (2018 Beijing.6 Specifically, the treatments were selected based on the patient’s disease severity and the regimens were identical for patients with or without AOI6 as follows:
Mild UC: (1) mesalamine (2 g, per os, once daily (QD)); and (2) prednisone (0.75 mg/kg, per os, QD) adopted for patients who failed to respond to mesalamines, especially those with more extensive lesions.
Moderate UC: (1) mesalamine (4 g, per os, QD); (2) prednisone (1.0 mg/kg, per os, QD) after 2–4 weeks of mesalamine for patients with poor symptom control (bloody stool was not attenuated), especially those with more extensive lesions (gradually reduced dosage to discontinuation when symptom relief was achieved) and (3) azathioprine (1.5–2.5 mg/kg, per os, QD) for patients who were hormone-ineffective or hormone-dependent.
Severe UC: (1) methylprednisolone (40–60 mg/day, per os, QD) or hydrocortisone (300–400 mg, per os, QD); (2) cyclosporine (2–4 mg/kg, per os, QD) for patients who did not respond to hormones; and (3) infliximab (3 mg/kg, intravenous injection, every 4 weeks) when the above drugs were ineffective.
Follow-up
All patients underwent follow-up assessments after their first coloscopy examination. The follow-up periods lasted 6 months to 1 year for the final diagnosis and therapeutic efficacy determination. On follow-up evaluation, all patients received a re-examination by colonoscopy. The clinical manifestations, colonoscopic results and pathological examination results were collected before and after treatment.
Disease scaling and assessment
Disease severity and therapeutic efficacy were evaluated extensively. First, the significance of the appearance of AOI regarding the UC diagnosis was determined by evaluating its specificity and sensitivity. Moreover, multiple widely used scoring systems were adopted in this study.
Primary measurements (modified Mayo Scoring system)
The prognosis for UC patients with AOI was evaluated by the modified Mayo score. The Mayo Score, also known as the Mayo Clinic Score and Disease Activity Index, is one of the most popular and commonly adopted scoring systems in clinical practices.6 Therefore, patients were evaluated before and after treatment to determine disease severity and therapeutic efficacy (table 1).11 The scores range from 0 (no disease) to 12 (high disease activity). Moreover, a patient who meets any of the following criteria was considered to have a positive treatment response: (1) exhibits over 30% reduction of the mayo score; (2) displays decreased score in rectal bleeding and (3) endoscopic subscore was 0 or 1.
Table 1.
Mayo scoring system for evaluating ulcerative colitis
| Rectal bleeding (score represent the most severe bleeding of the day) |
0 = No blood observed 1 = Fringe of blood with faeces less than half of the time 2 = Obvious blood with faeces more than half of the time 3 = Blood passed |
| Stool frequency | 0 = Normal 1 = One-to-two times more than normal 2 = Three-to-four times more than normal 3 = Five times more than normal |
| Endoscopic features (based on the presence of erythema, vascular pattern, tissue friability and observation of erosion or ulceration) |
0 = Normal or inactive disease 1 = Mild disease 2 = Moderate disease 3 = Severe disease |
| Gastroenterologist’s general evaluation | 0 = Normal 1 = Mild disease 2 = Moderate disease 3 = Severe disease |
Secondary measurements (Baron grading system and histopathological grading)
Endoscopic disease activity was evaluated using a four-point (grade 0–3) system based on the severity of rectal bleeding and histopathological changes according to the Baron grading system12: grade 0, no spontaneous rectal bleeding; grade 1, abnormal but not haemorrhagic, mucosal contact bleeding observed; grade 2, mild haemorrhagic, no spontaneous bleeding detected at the initial examination and grade 3, severely haemorrhagic, spontaneous bleeding observed ahead of the instrument at the initial inspection. Additionally, histology changes in the biopsies were compared, and the quantified results were reflected as histology scores as previously reported.12 Histopathological grading: grade 0, no polymorphs; grade 1, small number of polymorphs in lamina propria with minimal infiltration of crypts; grade 2, prominent polymorphs in the lamina propria with infiltration of >50% of crypts; grade 3, florid polymorph infiltrate with crypt abscesses; and grade 4, florid acute inflammation with ulceration.
The pretreatment and post-treatment endoscopic Baron grading results (describing the bleeding status of the lesions under the endoscope), histopathological grading (reflecting the histological changes of the inflamed lesions), and the biopsy pathology before and after treatment were collected.
Statistical methods
SPSS V.22.0 statistical software was used to process and analyse the data. Enumerated data were expressed as percentages (%), and measurement data were expressed as mean±SD. In addition, comparative enumeration data were calculated by chi-square test, pairwise comparisons were made using the unpaired Student’s t-test, and Fisher’s exact test was used to determine the significance. A value of p<0.05 was considered statistically significant.
Results
Patient baseline clinical and disease characteristics
Data from 120 patients exhibiting endoscopic distal diffuse lesions in our hospital from January 2018 to December 2020 were collected (figure 1). Among, 29 patients (24.2%) with AOI were recruited to the observation group, including 16 males and 13 females (39.24±11.63 years old). Meanwhile, 91 patients (75.8%) without skip lesions were included in the control group, including 55 males and 36 females (42.95±12.76 years old). Moreover, after profound clinical investigation, 99 patients were finally diagnosed with UC, including 28 patients from the observation group (15 males and 13 females, average age 39.42±11.80 years) and 71 patients from the control group (43 males and 28 females, average age 44.35±12.25 years), respectively. There was no significant difference between the two groups regarding gender, age and Montreal classification6 (p>0.05). In contrast, the modified Mayo score of the control group was higher than that of the observation group (p=0.043; table 2). Interestingly, 96.5% of patients with AOI were finally diagnosed with UC; however, the diagnostic rate in patients without AOI was only 78%. This finding suggests that the presence of AOI significantly increased the diagnostic rate of UC (96.5% vs 78.0%, p=0.024). More importantly, AOI had a specificity of 95.2%, a sensitivity of 28.3%, a positive predictive value of 96.5%, and a negative predictive value of 21.9% for UC diagnosis in patients with endoscopic diffuse inflammation of the descending colon and rectum. Furthermore, one out of the 29 patients (3.5%) with AOI was diagnosed with infectious colitis. In contrast, among the 91 patients without AOI, 5 (5.5%) were diagnosed with Crohn’s disease, 10 (11.0%) were diagnosed with infectious colitis, 2 (2.2%) were diagnosed with colonic neoplasia (tubular adenoma) and 3 (3.3%) were diagnosed with intestinal tuberculosis(table 3). Moreover, among all 99 patients diagnosed with UC, 75.0% (21/28) and 88.7% (63/71) patients responded to treatment in the observation and control groups, respectively. There was no significant difference in the treatment response rate between the two groups (p=0.119). Treatment outcomes of the UC patients were evaluated using both the modified Mayo scoring system and the revised Baron grading system. According to the modified Mayo scoring system, the treatment outcome between the two groups was not significantly different (p=0.881). Meanwhile, the modified Baron endoscopic grades and endoscopy subscores of the Mayo scoring system before and after treatment were also evaluated in patients from both groups. The results revealed that the disease was attenuated after treatment in both groups and the improvements were not significantly different (table 4).
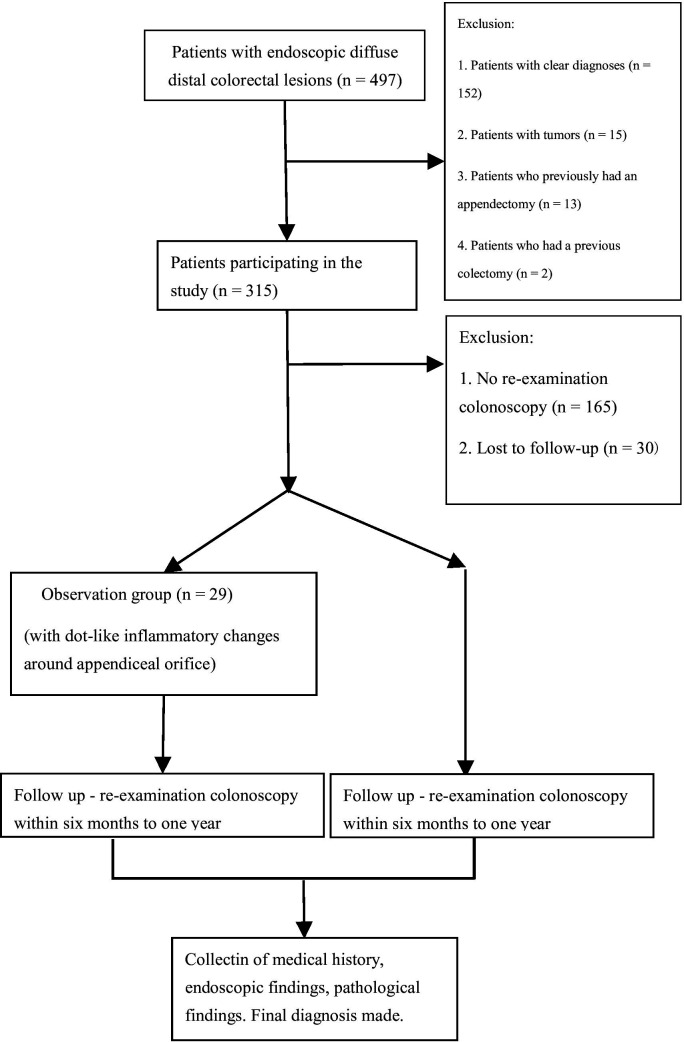
Figure 1.
Flow chart for patient recruitment strategy.
Table 2.
Baseline characteristics of the two study groups
| Observation (n=29) | Control (n=91) | P value | |
| Gender, n (%) | |||
| Male | 16 (55.0) | 55 (60.0) | 0.667 |
| Female | 13 (45.0) | 36 (40.0) | |
| Age (years) | 39.24±11.63 | 42.95±12.76 | 0.167 |
| Smoking history, n (%) | 11 (37.9) | 48 (52.7) | 0.165 |
| Drinking history, n (%) | 13 (44.8) | 49 (53.8) | 0.397 |
| Complications, n (%) | |||
| Diabetes | 2 (6.9) | 8 (8.8) | 1.000 |
| Hypertension | 4 (13.8) | 15 (16.5) | 1.000 |
| Renal insufficiency | 2 (6.9) | 7 (7.7) | 1.000 |
| Chronic obstructive pulmonary disease | 3 (12.6) | 12 (13.2) | 1.000 |
| Lesion range, n (%) | |||
| E1 | 10 (34.5) | 25 (27.5) | 0.282 |
| E2 | 6 (20.7) | 34 (37.4) | |
| E3 | 13 (44.8) | 32 (35.1) | |
| Modified Mayo Scoring | 6.79±1.42 | 7.44±1.35 | 0.043 |
E1: endoscopic lesion was limited to the rectum; E2: endoscopic lesion involved the left colon; E3: splenic flexure was engaged near the whole colon.
Table 3.
Comparison between the two groups of patients postfollow-up
| Observation (n=29) | Control (n=91) | P value | |
| Diagnosed as ulcerative colitis | 28 | 71 | 0.024 |
| Diagnosed with other diseases | 1 | 20 | |
| Crohn’s disease, n (%) | 0 (0.0) | 5 (5.5) | 0.334 |
| Infectious colitis, n (%) | 1 (3.5) | 10 (11.0) | 0.293 |
| Colonic neoplasia, n (%) | 0 (0.0) | 2 (2.2) | 1.000 |
| Intestinal tuberculosis, n (%) | 0 (0.0) | 3 (3.3) | 1.000 |
Table 4.
Comparison of the therapeutic efficacies between study groups
| Observation (n=28) | Control (n=71) | P value | |||
| Therapeutic effect, n (%) | |||||
| Treatment response | 21 (75.0) | 63 (88.7) | 0.119 | ||
| No treatment response | 7 (25.0) | 8 (11.3) | |||
| Change in Modified Mayo score | −3 (−4 to −2) | −3 (−4 to −3) | 0.881 | ||
| First diagnosis | Follow-up | First diagnosis | Follow-up | ||
| Modified Mayo score | 6.79±1.42* | 3.42±2.25* | 7 (7–8)* | 4 (3–5)* | |
| Revised Baron grading | 1 (1–4)* | 1 (0–1)* | 1 (1–2)* | 1 (1–1)* | |
*Comparison between groups. First diagnosis versus follow-up, before and after treatment, showed a significant difference (p<0.05).
Histopathological evaluation was also used to determine disease progression and treatment outcomes in diagnosed UC patients. Interestingly, the results revealed that the tissue damage and inflammation were significantly reduced in the observation group (p<0.001) compared with the control group (p=0.079; table 5 and table 6).
Table 5.
Comparison of the pathological stages before and after the treatment in the observation group
| Pathological stages | 0 | I | II | III | IV |
| Before treatment, n | 0 | 5 | 11 | 11 | 1 |
| After treatment, n | 1 | 8 | 19 | 0 | 0 |
| χ² | 17.049 | ||||
| P value | <0.001 |
Table 6.
Comparison of the pathological stages before and after the treatment in the control group
| Pathological stages | 0 | I | II | III | IV |
| Before treatment, n | 0 | 7 | 36 | 25 | 3 |
| After treatment, n | 1 | 9 | 38 | 22 | 1 |
| χ² | 2.391 | ||||
| P value | 0.709 |
AOI was not observable in 26 patients (92.8%) in the observation group during the follow-up endoscopic examination. Therefore, the tissues around the appendiceal orifice were not biopsied. AOI was observed in two patients (2.8%) during endoscopic re-examination in the control group. To determine if the presence or disappearance of AOI affected UC treatment, the modified Mayo Scores in these 28 patients (26 cases of AOI disappearance and two cases of AOI new occurrence) were compared. No significant difference was observed regarding the responses to medical treatment between these two groups (changes of the modified Mayo score: −2.72±1.56 vs -1.50±0.70, p=0.283, AOI disappearance vs AOI new occurrence).
Discussion
UC is characterised by diffuse and continuous inflammatory changes extending proximally from the rectum.1 It is one of the major types of inflammatory bowel disease, and clinical presentation, endoscopy findings and mucosal biopsy evaluation are commonly used for UC diagnosis.13 The presence of AOI has been reported in UC patients, and its significance in UC has been investigated. However, the results are controversial.1 6–9 14–16 In this study, the significance of AOI in UC diagnosis and prognosis was prospectively studied.
The identification of AOI improved the diagnostic accuracy of UC in patients with diffuse lesions in the distal colorectum. In alignment with these findings, AOI was considered to precede UC development, suggesting its potential for UC diagnosis.7 Moreover, a large cohort study found that combining proctitis and AOI features could improve UC diagnosis and facilitate physicians to identify this disorder among various conditions.16 Furthermore, the current study found that UC patients with AOI developed less severe disease than those without, as reflected by lower modified Mayo scores, and these findings were supported by previous reports.8 9 Skip lesions were previously thought only to be characteristic of Crohn’s disease. At the same time, periappendiceal inflammatory changes with diffuse inflammatory changes in the distal rectum may be observed in UC.15 It has been reported that AOI was correlated with the proximal extension of inflammation in patients with proctitis.15 Thus, endoscopic periappendiceal inflammatory lesions with continuous inflammatory changes in the distal colon and rectum may be a particular type of UC. Therefore, UC should be preferably considered if periappendiceal inflammatory lesions are found in future clinical work.
In the current study, 21 of 120 recruited patients were diagnosed with disorders other than UC, including 11 cases of infectious colitis, which may be related to the fact that patients with inflammatory lesions involving the splenic flexure near and even the entire colon were not excluded. In addition, colonic inflammation can be observed in patients with other specific etiologies. For instance, one previous study reported ruptured appendicitis in 17.1% (6/35) of patients with infectious pancolitis, radiation pancolitis and ischaemic pancolitis. However, the appendix was not involved in 29 cases of non-pancolitis.10
To maintain the two groups’ treatment consistency, it was recommended to administer treatment only according to the guidelines.6 However, there are some differences in clinical characteristics and treatments for UC between Chinese and foreign hospitals. In China, the first-line medications for UC are aminosalicylates and glucocorticoids for mild-to-moderate and moderate-to-severe UC, respectively.17–20 This study found that the therapeutic effect of treatments in UC patients with AOI was similar to those without, which is consistent with previously reported results.21 22
Furthermore, skip lesions disappeared in most patients in the observation group after treatment. On the other hand, two patients in the control group developed new AOI. However, due to the limited sample size, it is difficult to conclude whether the disappearance or development of AOI changes the condition of UC.
The appendix contains abundant lymphoid tissue and exerts immune functions similar to lymphoid organs. As an autoimmune disease, UC can rarely be associated with inflammatory changes around the appendiceal orifice.23 At present, there is no study to prove whether the inflammatory changes around the appendiceal orifice can reflect the severity of UC. In this study, although the treatment outcome was similar between the two groups, the post-treatment pathological condition was improved in patients with AOI. At the same time, this study found that the disease severity in UC with skip lesions was lower than that without AOI at the first diagnosis according to the modified Mayo score, which was also comparable to previous reports.24
Although the ‘skipping’ lesion is traditionally considered a characteristic of Crohn’s disease,25 an increasing body of evidence has revealed ‘patchy’ inflammation at the appendiceal orifice in UC.5 Studies have found that in cases of UC, the CD4+/CD8+ T cell ratio is increased in periappendiceal tissue, and CD4+, CD69+, and CD62+ T cell infiltration is evident along with increased rates of plasma cell proliferation.26 27 Since the appendix and rectum accumulate many lymphoid follicles, these collecting lymphoid nodules, mesenteric lymph nodes and independent lymphoid follicles constitute gut-associated lymphoid tissues. Meanwhile, they possess an essential immune effect on harmful antigens and invading microorganisms. This lymphoid follicular inflammation may be the initial site of UC. The appendix and rectum are also sites where microorganisms tend to accumulate. The immune response produced by microbial accumulation is an important cause of UC, which explains why skip lesions often appear around the appendiceal orifice.28 29
This study has the following limitations: (1) it is a single-centre, single-city prospective cohort study; (2) it does not adopt the random blind method and may have bias; (3) the cohort is small due to the rarity of skip UC cases and (4) the therapy used is not necessarily standard in other geographic locations, which limits the generalisability of the results
In conclusion, it was shown that identification of AOI improved the diagnostic accuracy of UC in patients with diffuse lesions in the distal colon and rectum. Furthermore, it was found that the disease severity in UC patients with AOI was lower compared with UC patients without AOI, but the therapeutic efficacies were similar in all patients.
Supplementary Material
Footnotes
Contributors: PD and CP were responsible for the data curation; PY analysed data; PD and SZ drafted the manuscript, design the study and interpreted the data; XL and JW were responsible for the project administration; SZ was responsible for the supervision. All authors gave intellectual input to the study and approved the final version of the manuscript. PD is the guarantor.
Funding: This study was supported by Sichuan Science and Technology Program: 2018SZ0270.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data may be obtained from a third party and are not publicly available. The data were deposited to the Chinese Clinical Trial Registry (ChiCTR) database and are available at http://www.chictr.org.cn/usercenter.aspx.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Ethics Committee on Biomedical Research at West China Hospital of Sichuan University (Ethics No: 2018-Review 134). Participants gave informed consent to participate in the study before taking part.
References
- 1.Rubin DT, Rothe JA. The peri-appendiceal red patch in ulcerative colitis: review of the University of Chicago experience. Dig Dis Sci 2010;55:3495–501. 10.1007/s10620-010-1424-x [DOI] [PubMed] [Google Scholar]
- 2.Kaenkumchorn T, Wahbeh G. Ulcerative colitis: making the diagnosis. Gastroenterol Clin North Am 2020;49:655–69. 10.1016/j.gtc.2020.07.001 [DOI] [PubMed] [Google Scholar]
- 3.Herfarth H. Can oct be used to distinguish accurately between patients with ulcerative colitis and those with crohn's disease? Nat Clin Pract Gastroenterol Hepatol 2005;2:172–3. 10.1038/ncpgasthep0144 [DOI] [PubMed] [Google Scholar]
- 4.Feakins RM, British Society of Gastroenterology . Inflammatory bowel disease biopsies: updated British Society of gastroenterology reporting guidelines. J Clin Pathol 2013;66:1005–26. 10.1136/jclinpath-2013-201885 [DOI] [PubMed] [Google Scholar]
- 5.Deng P, Wu J. Meta-analysis of the association between appendiceal orifice inflammation and appendectomy and ulcerative colitis. Rev Esp Enferm Dig 2016;108:401–10. 10.17235/reed.2016.4176/2015 [DOI] [PubMed] [Google Scholar]
- 6.Inflammatory Bowel Disease Group, CSoGCMA . Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing). J Dig Dis 2021;22:298–317. 10.1111/1751-2980.12994 [DOI] [PubMed] [Google Scholar]
- 7.Park SH, Yang SK, Kim MJ, et al. Long term follow-up of appendiceal and distal right-sided colonic inflammation. Endoscopy 2012;44:95–8. 10.1055/s-0031-1291443 [DOI] [PubMed] [Google Scholar]
- 8.Perry WB, Opelka FG, Smith D, et al. Discontinuous appendiceal involvement in ulcerative colitis: pathology and clinical correlation. J Gastrointest Surg 1999;3:141–4. 10.1016/S1091-255X(99)80023-7 [DOI] [PubMed] [Google Scholar]
- 9.Hokama A, Ihama Y, Chinen H, et al. Appendiceal orifice inflammation in ulcerative colitis. Dig Dis Sci 2010;55:1189. 10.1007/s10620-009-0840-2 [DOI] [PubMed] [Google Scholar]
- 10.Cosnes J, Carbonnel F, Beaugerie L, et al. Effects of appendicectomy on the course of ulcerative colitis. Gut 2002;51:803–7. 10.1136/gut.51.6.803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jixi L, Zhenhai S, Xiaoti W, et al. The clinical significance of appendiceal orifice inflammation in the distal ulcerative colitis [In Chinese]. Chin j hepatol 2013;22:74–6. [Google Scholar]
- 12.Pullan RD, Rhodes J, Ganesh S, et al. Transdermal nicotine for active ulcerative colitis. N Engl J Med 1994;330:811–5. 10.1056/NEJM199403243301202 [DOI] [PubMed] [Google Scholar]
- 13.Collins P, Rhodes J. Ulcerative colitis: diagnosis and management. BMJ 2006;333:340–3. 10.1136/bmj.333.7563.340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Park J, Kang EA, et al. Prognostic value of terminal ileal inflammation in patients with ulcerative colitis. Gut Liver 2021;15:858–66. 10.5009/gnl20294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anzai H, Hata K, Kishikawa J, et al. Appendiceal orifice inflammation is associated with proximal extension of disease in patients with ulcerative colitis. Colorectal Dis 2016;18:O278–82. 10.1111/codi.13435 [DOI] [PubMed] [Google Scholar]
- 16.Zhan DQ, Chen X, Wang R, et al. Diagnostic evaluation of appendiceal orifice inflammation in ulcerative colitis. Turk J Gastroenterol 2016;27:444–9. 10.5152/tjg.2016.16215 [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto T, Nakamura S, Shimizu M, et al. Significance of appendiceal involvement in patients with ulcerative colitis. Gastrointest Endosc 2002;55:180–5. 10.1067/mge.2002.121335 [DOI] [PubMed] [Google Scholar]
- 18.Matsushita M, Takakuwa H, Matsubayashi Y, et al. Appendix is a priming site in the development of ulcerative colitis. World J Gastroenterol 2005;11:4869–74. 10.3748/wjg.v11.i31.4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng P, Gan T, Wu J. The importance of endoscopic discontinuous appendiceal orifice inflammation accompanied with left-sided colitis in ulcerative colitis diagnosis. West China Medical Journal 2014;29:249–51. [Google Scholar]
- 20.Lan Y, Wang S, Liu X, et al. The role of the skip inflammatory lesions at appendiceal orifice in the non-pancolitis diagnosis. Clinical Focus 2008:943–4. [Google Scholar]
- 21.Farkas SA, Hornung M, Sattler C, et al. Preferential migration of CD62L cells into the appendix in mice with experimental chronic colitis. Eur Surg Res 2005;37:115–22. 10.1159/000084543 [DOI] [PubMed] [Google Scholar]
- 22.Kawachiya T, Oshitani N, Jinno Y, et al. Significance of increased proliferation of immature plasma cells in the appendix of patients with ulcerative colitis. Int J Mol Med 2005;15:417–23. 10.3892/ijmm.15.3.417 [DOI] [PubMed] [Google Scholar]
- 23.Yamagishi N, Iizuka B, Nakamura T, et al. Clinical and colonoscopic investigation of skipped periappendiceal lesions in ulcerative colitis. Scand J Gastroenterol 2002;37:177–82. 10.1080/003655202753416849 [DOI] [PubMed] [Google Scholar]
- 24.Lucci E, Lattuneddu A, Valpiani D, et al. Skip lesion of the cecum associated with proctitis: an atypical case of ulcerative colitis. Dig Liver Dis 2004;36:847–51. 10.1016/j.dld.2004.01.029 [DOI] [PubMed] [Google Scholar]
- 25.Odze R. Diagnostic problems and advances in inflammatory bowel disease. Mod Pathol 2003;16:347–58. 10.1097/01.MP.0000064746.82024.D1 [DOI] [PubMed] [Google Scholar]
- 26.Chiba M, Yamano H, Fujiwara K, et al. Lymph folliculitis in ulcerative colitis. Scand J Gastroenterol 2001;36:332–6. 10.1080/003655201750074753 [DOI] [PubMed] [Google Scholar]
- 27.Wehkamp J, Stange EF. Recent advances and emerging therapies in the non-surgical management of ulcerative colitis. F1000Res 2018;7. 10.12688/f1000research.15159.1. [Epub ahead of print: 07 08 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ran W, Ouyang Q, Dong L. Retrospective analysis of 24 cases of refractory ulcerative colitis treated with azathioprine. Chinese j intern med 2012:613–7. [PubMed] [Google Scholar]
- 29.Hui D, Jiaming Q, Keshu S. Adverse effects of azathioprine in the treatment of inflammatory bowel disease. Chin J Gastroenterol 2011;23:40–2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data may be obtained from a third party and are not publicly available. The data were deposited to the Chinese Clinical Trial Registry (ChiCTR) database and are available at http://www.chictr.org.cn/usercenter.aspx.