Abstract
Despite accumulating evidence that supports the beneficial effects of physical exercise in inhibiting cancer progression, whether exercise modulates its effects through systemic and cellular changes in iron metabolism and immune-tumor crosstalk is unknown. Cancer cells have greater metabolic requirements than normal cells, with their survival and proliferation depending largely on iron bioavailability. Although iron is an essential mineral for mitogenesis, it also participates in a form of iron-dependent programmed cell death termed ferroptosis. In this short hypothesis paper, we speculate that modulating iron bioavailability, transport and metabolism with regular exercise can have significant implications for tumor and stromal cells in the tumor microenvironment, by affecting multiple tumor-autonomous and stromal cell responses.
Keywords: Cytokines, Macrophages, Tumor Microenvironment, Metabolic Networks and Pathways, Tumor Biomarkers
Introduction
Regular participation in moderate-intensity physical activity improves survival in patients with breast, colorectal and prostate cancers.1 2 Some probable anticancer mechanisms involve exercise-mediated changes in: (1) immune cell subset distributions, as well as their enhanced tumor-suppressive properties, (2) muscle-derived factors (myokines),3 and finally, (3) micronutrients may also be relevant in cancer survivorship.4 The role of iron in cancer has been demonstrated in epidemiological studies, where higher iron stores were associated with increased cancer risk. In a recent meta-analysis, increased consumption of dietary heme iron was associated with a greater relative risk of breast cancer,2 while increased dietary intake of iron was also associated with higher relative risk of breast cancer in a prospective cohort study.5 In the prospective study, a decreased relative risk of breast cancer was observed in women supplemented with vitamins C and E, β-carotene, selenium and zinc, compared with women in the placebo group. Presumably, the reported chemoprotective effects of dietary antioxidants are linked to decreased lipid peroxidation.
In this hypothesis, the focus will be on the potential role of aerobic exercise in modulating some aspects of dysregulated iron biology in cancer. It is acknowledged that the systemic effects of exercise cannot be reduced to a single mechanism to fully explain its protective role in cancer development. As it is beyond the scope of this hypothesis paper to comprehensively address all potential mechanisms and available evidence on exercise-induced cancer cell death, we thus sought to discuss the beneficial effects of exercise training on cancer biology that have not been studied, including changes in iron transport, storage and metabolism, as well as their mediating role in direct or indirect killing of cancer cells. Thus, this exciting area of research awaits further experimental investigation.
Aberrant iron trafficking, storage and metabolism drive cancer progression
The relationship between iron trafficking, storage and metabolism, and cancer biology has been reviewed extensively6 and therefore, will not be mentioned. Here, we discuss the roles of key iron transporters such as the cellular iron exporter protein, ferroportin (Fpn), the cellular iron importer protein, transferrin receptor 1 (TFR1), and hepcidin, a liver-derived hormone that regulate systemic iron availability. Cancer progression has been shown to be driven, in part, by the downregulation of protein concentrations of Fpn in breast,7 prostate,8 and ovarian cancer cells.9 Further, decreased tumoral Fpn protein expression was associated with higher histological grade and lymph node infiltration in breast cancer survivors.7 In the same study, decreased Fpn gene expression predicted metastatic outcomes in four longitudinal cohorts, suggesting a pivotal role of iron in breast cancer progression. In high-grade serous ovarian carcinoma, malignant tissue from patients demonstrated significant decreased protein expression of Fpn compared with normal ovarian epithelial tissue from healthy volunteers.9 The other key iron transporter, TFR1, also demonstrates increased protein concentrations in breast and ovarian cancer.9 Finally, when there is excess systemic iron, hepcidin will bind to Fpn and mediate its degradation.10 Hepcidin mRNA expression is increased in breast cancer cells and predicts poor prognosis in the presence of low mRNA expression of Fpn. These examples support the notion that increased iron storage and uptake in cancer cells may be related to a malignant phenotype.7
Iron uptake in tumor-associated macrophages
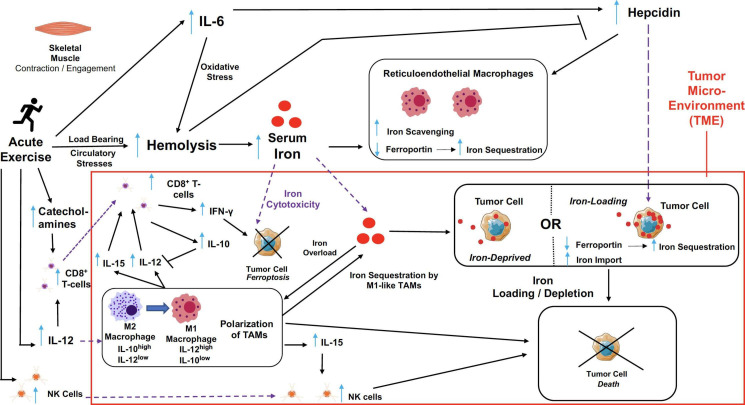
The tumor microenvironment (TME) consists of stromal cells that aid in cancer progression. Well-known players include tumor-associated macrophages (TAMs), which either suppress or promote tumor growth, depending on their polarized phenotype—M1, being tumor-suppressive and M2, being tumor-permissive. M1 macrophages sequester iron by absorbing iron-loaded transferrin through TFR1.11 12 M2 macrophages also have high expression of TFR1, as well as increased Fpn gene (by 10-fold) and protein expressions (by 2.5-fold), thus facilitating increased iron uptake and releasing capability, and decreased ferritin compared with M1 macrophages.13–15 As mentioned previously, Fpn facilitates iron export from the cell. Hence, by displaying increased expression of Fpn, this unique characteristic of M2 macrophages has earned them a nickname as ‘iron donors’ in promoting tumor growth. In advanced stages of cancer development, most macrophages polarize to pro-tumor M2 macrophages.16 Thus, in ensuring the regression of cancer cells, it is critical for M2 macrophages to be repolarized to M1 macrophages (figure 1).
Figure 1.
T The purported effects of acute exercise on the dynamic interplay between iron metabolism and immuno-oncological modulations in the system and particularly, the tumor microenvironment. Exercise causes myokines such as IL-6 to be released from the skeletal muscle. The load bearing and circulatory stresses resulting from exercise increases hemolysis, leading to a rise in serum iron. The elevation of both IL-6 and serum iron stimulate the upregulation in hepcidin activity. In the tumor microenvironment, hepcidin may inhibit Fpn of tumor cells and this increases iron sequestration by these cells. Thus, iron-loading of the tumor cells may occur, which we postulate to in turn cause tumor cell death. Alternatively, the rise in hepcidin, which also inhibits Fpn of macrophages, results in increased iron-scavenging typically by M1 macrophages. If such an effect occurs in the tumor microenvironment, this may cause tumor cells to be iron-deprived, which consequentially leads to their death. With the possibility of a rise in circulating iron affecting the tumor microenvironment, the abundance of iron may cause tumor cell death from iron toxicity. We postulate that exercise may result in the polarization of TAMs to favor an M1 phenotype that may eventually lead to tumor repression/suppression. In turn, the recruitment of CD8+ T-cells and NK cells through the secretion of cytokines (e.g. IL-12 and IL-15) by TAMs in the tumor microenvironment may destroy tumor cells. IL-6, interleukin-6; Fpn, ferroportin; TAMs, tumour-associated macrophages; NK cells, natural killer cells; IL-10, interleukin-10; IL-12, interleukin-12; IL-15, interleukin-15.
Ferroptosis
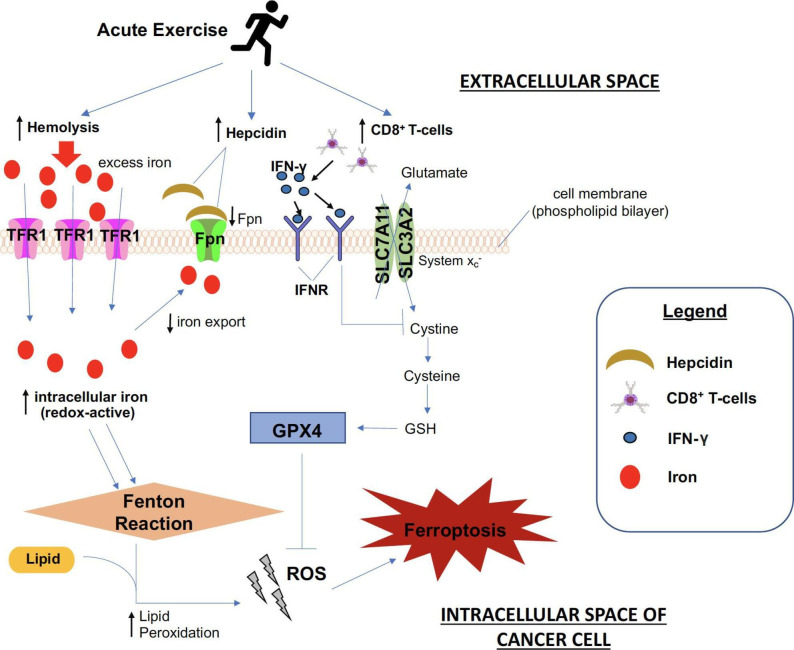
Although the increase in iron availability is known to drive cancer proliferation, a novel form of iron-dependent programmed cell death, termed ferroptosis, has shown to cause cancer cell death. Of note, many cancer types—lung, liver and breast cancer cells—are susceptible to ferroptosis.4 17 As depicted in figure 2, ferroptosis may be triggered by, (1) failure of system Xc− (a cystine/glutamate antiporter system involved in glutathione production by importing cystine and exporting glutamate made up of subunits solute carrier family 3 member 2 (SLC3A2) and solute carrier family 7 member 11 (SLC7A11)), and (2) glutathione peroxidase 4 (GPX4) activity. An example of this is evident via the endogenous anti-oncogenic mechanisms downstream of p53, which can inhibit system Xc− uptake of cystine by downregulating the expression of SLC7A11.4 In turn, this affects GPX4 activity, increasing lipid peroxides, leading to ferroptosis. Renal carcinoma cell lines with downregulated GPX4 expression were more sensitive to ferroptosis, whereas upregulation of GPX4 expression inhibited the cell killing.17
Figure 2.
The purported effects of acute exercise in inducing ferroptosis in cancer cells. Hemolysis, as well as increased concentrations of hepcidin and CD8+ T-cells, are postulated to increase as a result of exercise. Increased hemolysis augments iron levels in the systemic circulation, which is taken up by TFR1 in cancer cells. Higher hepcidin levels decrease Fpn expression, and together with greater iron import, this leads to higher intracellular iron, contributing to greater ROS accumulation. Enhanced ROS accumulation stems from increased lipid peroxidation that attributes to a higher Fenton reaction rate. When coupled with greater CD8+ T-cell recruitment in the tumor milieu, higher concentrations of IFN-γ secreted is posited to cause considerably more hindrance to cystine uptake, which eventually leads to a lower production of the anti-oxidant enzyme, GPX4, to counteract ROS accumulation. Collectively, greater ROS production stemming from the effect of exercise ultimately amounts to a heightened propensity for cancer cell destruction via ferroptosis. TFR1, transferrin receptor 1; Fpn, ferroportin; ROS, reactive oxygen species; IFN-γ, interferon gamma; GPX4, glutathione peroxidase 4.
In figure 1, our model proposes how acute aerobic exercise might modulate the sensitivity of cancer cells to ferroptosis through pathways associated with iron metabolism. Here, we discuss the impact of acute aerobic exercise on iron metabolism both directly (via exercise-induced ferroptosis in cancer cells) and indirectly (via exercise-induced changes in macrophages sequestration of iron).
A bidirectional relationship: immuno-oncological modulations & iron metabolism
Regular aerobic exercise training reduces serum concentrations of iron in healthy human adults.18 We speculate that regular aerobic exercise training inhibits cancer growth via direct and indirect mechanisms on iron metabolism, with the former involving (1) reactive oxygen species (ROS)-driven ferroptosis and (2) iron efflux in cancer cells, and the latter via iron-mediated changes in macrophage polarization toward an anticancer phenotype, which sequesters iron away from cancer cells.
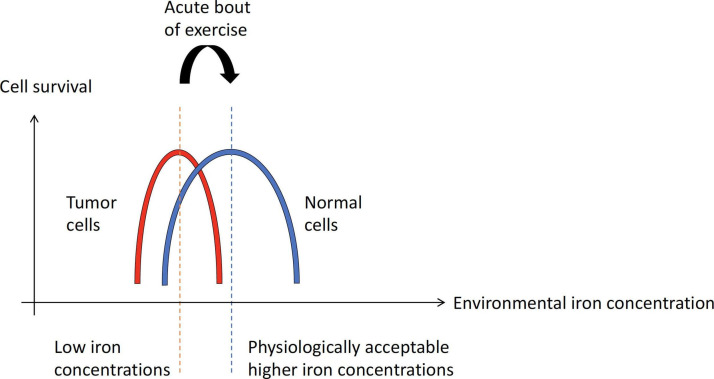
As tumor cells express high TFR1 compared with normal cells, we hypothesize that an acute bout of exercise would result in an environmental iron concentration conducive for the optimal survival of normal cells, but not for tumor cells (figure 3). At low iron concentrations, conditions are favorable for both tumor cells and normal cells to survive (figure 3). From the perspective of ferroptosis based on TFR1 expression levels, normal cells would tend to be more resistant against ferroptosis with increases in iron concentrations, while elevated iron concentrations would hinder the survival of tumor cells that are more susceptible to ferroptosis.
Figure 3.
Hypothetical graph to show tumor cells versus normal cells survivability across environmental iron concentration. The hypothetical increase in environmental iron concentration with an acute bout of exercise (red dotted line to blue dotted line). Red, and blue solid graph represents an inverted “U” response of tumor cells, and normal cells, respectively. At a low iron concentration environment, conditions are favorable for both tumor cells and normal cells to survive. With exercise, normal cells have a buffer against the increase in environmental iron concentration due to their lower number of TFR1 (iron importer) compared with tumor cells which possess more TFR1. We hypothesize that an acute bout of exercise would result in an environmental iron concentration conducive enough for the optimal survival of normal cells. From the perspective of ferroptosis based on the TFR1 levels, normal cells would tend to be more resistant against ferroptosis with increases in iron concentrations, while elevated iron concentrations would hinder the survival of tumor cells that are more susceptible to ferroptosis.
The upregulation of hepcidin activity is primarily mediated by interleukin-6 (IL-6), an inflammatory cytokine19 (figure 1). The rise in systemic concentrations of iron following acute aerobic exercise is commonly observed20 and may be due to a direct consequence of hemolysis resulting from the load-bearing and circulatory stresses incurred from acute exercise.20 In addition, microstructural damage to skeletal muscle during exercise may release iron-containing hemoglobin21 and myoglobin into the circulation,22 thus contributing to the pool of systemic (free) iron. In general, free iron is bound to transferrin in the systemic pool and subsequently, iron-loaded transferrin forms a complex with the membrane TFR1 of the cell to be endocytosed (figure 2).
Moreover, acute exercise mediates the release of myokines from contracting skeletal muscles, with the prototypical myokine being IL-6.3 Increased systemic IL-6 during acute exercise may thus upregulate hepcidin concentrations and lead to a greater amount of free iron taken up by macrophages. The increased presence of hepcidin leads to the degradation of Fpn, which in turn causes macrophages to sequester iron.19 During acute exercise, systemic elevations in catecholamines initiate leukocyte egress into peripheral blood, resulting in increased concentrations of innate (neutrophils, monocytes) and adaptive immune cells, including CD8+ T cells that can potentially enhance immune surveillance against cancer3 (figure 1). Interestingly, immunotherapy-activated CD8+ T cells demonstrate increased ferroptosis-specific lipid peroxidation in tumor cells, contributing to its antitumor efficacy.23 This increased tumoricidal activity results from the downregulation of SLC3A2 and SLC7A11 by interferon gamma (IFN-y) produced by CD8+ T cells, which consequentially impairs cystine uptake by tumor cells, promoting tumor cell lipid peroxidation and ferroptosis. Moreover, Gomes-Santos et al24 have recently reported that exercise synergizes with immunotherapy and improves IFN-y activity in CD8+ T cells, wherein an increased infiltration of these cells in the breast cancer TME was evident.24 Hence, we hypothesize on the enhanced propensity for inducing ferroptosis in tumor cells, which may be augmented by increased systemic concentrations of CD8+ T cells resulting from physical exercise, that could concomitantly affect local concentrations of CD8+ T cells within the TME (figure 1).
Long-term exercise training decreases lipid peroxidation in various organs including the liver, adipose tissue, skeletal muscle, heart and brain.25–27 It is hence tempting to think that exercise may reduce lipid peroxidation in cancer cells. Here, a point of contention may lie in the period of exercise training. We suggest that during each acute bout of exercise, enhanced lipid peroxidation would occur, potentially amounting to ferroptosis in tumor cells. This mechanism could partially explain the benefits of regular exercise: the cumulative effect of acute ferroptotic responses in tumor cells from repetitive bouts of acute exercise overtime selectively destroys tumor cells but not healthy stromal cells. This notion on the beneficial cumulative effect of acute changes with repeated exercise bouts is supported by a number of studies/literatures on exercise and cancer.28–30 Although long-term exercise training also provides adaptative benefits such as the global decrease in lipid peroxidation, its mechanisms are not the focus of the current hypothesis. We thus speculate that, regardless of training status, acute bouts of exercise would increase lipid peroxidation that may promote exercise-induced ferroptotic effects in an intermittent manner, leading to tumor cell death.
Granted that exercise-induced iron deficiency anemia (IDA) is common in young women, especially those participating in endurance sports—the prevalence of female marathon runners with IDA is 28% compared with 11% with the healthy female population,31 we speculate that this may be a case of chronic overtraining. As proposed earlier, it is the cumulative effect of acute changes in iron metabolism from repetitive bouts of acute exercise overtime that may account for the purported mechanisms (as illustrated in our paper) in support of our hypothesis.32 33 We also acknowledge that cancer and cancer treatment may cause anemia or pseudoanemia in patients with cancer. Curiously however, as demonstrated by Furrer and colleagues,34 exercise training ameliorates tumor-associated anemia. In this work, exercise-trained C57BL/6J mice bearing Lewis lung carcinoma demonstrated a later onset of tumor-induced anemia, as well as promotion of red blood cell survival, compared with in non-exercised, tumor-bearing mice.34 A clinical trial by Drouin and colleagues35 also support these findings; patients with breast cancer participating in 7 weeks of aerobic exercise training while undergoing radiation therapy had significantly higher erythrocyte counts and increased hematocrit and hemoglobin concentrations, compared with patients in the placebo (stretching) arm.
The antitumorigenic irony: iron overload or depletion?
Macrophages are pivotal in establishing a delicate balance in iron homeostasis. Iron overload induces macrophage polarization to a proinflammatory phenotype by supporting ROS production, as well as increasing both p300/CBP acetyltransferase activity and p53 acetylation.36 Iron-loaded TAM infiltration has been linked to tumor regression in patients with non-small cell lung carcinoma.37 Similarly, an iron delivery system targeted at TAMs was demonstrated to be an effective ancillary therapy to augment tumor-suppressive responses.37 Moreover, it has been shown that exposing TAMs to hemolytic red blood cells or iron-containing nanoparticles caused the polarization of TAMs from an M2-like to a proinflammatory M1-like phenotype, which consequently killed lung cancer cells both in vitro and in vivo.27 Hence, a similar mechanism to curtail tumor development could be extended to cancer and driven by an acute bout of aerobic exercise, by potentially manipulating the interaction between immuno-oncological agents and iron metabolism to counteract the tumor-promoting TME. Paradoxically, in spite of the threat posed to normal cells, we propose that such a phenomenon is suggestive of a novel mechanism to destroy tumor cells38 (figure 1).
On the contrary, tumor growth is repressed by depriving cancer cells of iron through the administration of iron-sequestering drugs. This induces a greater demand for iron, thereby subjecting cancer cells to adapt to this metabolic need through modulating their protein expression of iron transporters, including hepcidin and Fpn; both of which contribute to the cellular supply and export of iron. Hepcidin disturbance can greatly affect iron export and lead to iron sequestration in tumor cells. There is evidence to demonstrate the differential regulation of this hormone in cancer and non-cancerous tissues.39 In treating cancer, it is possible that regulating hepcidin levels can decrease iron availability in neoplastic cells.39 This mechanism positions the manipulation of iron homeostasis as a potential therapeutic target in cancer. To this end, more studies, especially those in vivo, are needed to understand the specific contribution of both liver and local hepcidin to global hepcidin levels in the TME.
In essence, we have discussed two potential iron-depriving mechanisms for tumor regression. First, by depriving tumors of iron via the chelation of free iron by macrophages—an antimitogenic effect that reduces survival, growth and proliferation rates. Second, we described the tumoricidal effect through iron sequestration by M1 macrophages in the TME. Drawing from these examples of iron deprivation in tumor growth repression, we speculate whether the effect of acute aerobic exercise on iron metabolism could imply similar mechanisms by macrophages to induce tumor cell death. We further propose that aerobic exercise-induced hemolysis shifts TAMs from an M2 to an M1 phenotype.
To date, most of the iron chelation/deprivation intervention strategies are directed at tumor cells, and more work is required to address iron deprivation in the TME. Knowing that solid tumors are largely infiltrated by macrophages which are instrumental in iron metabolism, targeting these immune cells to counteract tumor development is promising. One way to speculate on the type of iron metabolism response would be based on the concept that ‘hot’ tumors are usually enriched in immune cell infiltration and extremely rich in iron availability within the microenvironment; conversely, ‘cold’ tumors are often very poor in immune cells mainly due to immune exclusion.
We propose that an iron-rich TME may induce iron toxicity (iron overload) leading to ferrototic cancer cell death. Consistent with this hypothesis, Bordini and colleagues40 reported that iron toxicity through the in vivo administration of iron at high dose inhibited prostate cancer cell proliferation. Such toxicity implied oxidative stress which mainly involve lipids that promoted ferroptosis.41
One important question to ask is whether the high concentrations of iron involved in ferroptosis of prostate cancer cells, as shown by Bordini and colleagues, is physiologically achievable with a bout of exercise. This requires more research to elucidate. In addition, many forms of iron exist in vivo compared with in vitro models that have demonstrated how ferroptosis has been induced generally by (a) particular form(s) of iron being studied. It may be that an in vivo model requires different forms of iron and possibly the involvement of other organs or molecules to overcome the need for high in vitro concentrations (eg, coactivators, enzymes) for ferroptosis to result.
Conclusions and future perspectives
More clinical studies need to be conducted to probe the mechanisms underlying iron metabolism and the human immune response in relation to acute aerobic exercise, ultimately to translate clinical benefits from bench to bedside. Furthermore, considering the (1) myriad of physiological effects induced by various types of exercises (eg, aerobic vs resistance), (2) time-dependent nature of the ferroptotic responses induced by exercise, as well as (3) degree of susceptibility of different cancer types toward ferroptosis, this begs the question as to the optimal exercise program for different cancer types. In addition, does the ferroptotic effect have an inverted U dose–effect relationship in cancer?
The ideal study model would be a patient with early-stage cancer who has completed curative surgery and received adjuvant treatment. Such a patient would be deemed physically fit to undergo exercise programs, including those that are of higher intensity. Agents that modify iron metabolism and modulators of the human immune system may be incorporated into such clinical studies to help refine the role and impact of exercise in patients with cancer.
Footnotes
Contributors: Idea conception: JS, JG; drafting of manuscript: JS, ZXL; revising of manuscript: JS, BK, ELH, JG, ZXL.
Funding: This work was funded by NUHS (A-0002469-00-00) Interventions for Healthy Longevity grant.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc 2019;51:2375–90. 10.1249/MSS.0000000000002116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang VC, Cotterchio M, Khoo E. Iron intake, body iron status, and risk of breast cancer: a systematic review and meta-analysis. BMC Cancer 2019;19:1–28. 10.1186/s12885-019-5642-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kruk J, Kotarska K, Aboul-Enein BH. Physical exercise and catecholamines response: benefits and health risk: possible mechanisms. Free Radic Res 2020;54:105–25. 10.1080/10715762.2020.1726343 [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Zhang C, Wang J, et al. The regulation of ferroptosis by tumor suppressor p53 and its pathway. Int J Mol Sci 2020;21:8387. 10.3390/ijms21218387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diallo A, Deschasaux M, Partula V, et al. Dietary iron intake and breast cancer risk: modulation by an antioxidant supplementation. Oncotarget 2016;7:79008–16. 10.18632/oncotarget.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Torti SV, Torti FM. Iron and cancer: 2020 vision. Cancer Res 2020;80:5435–48. 10.1158/0008-5472.CAN-20-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinnix ZK, Miller LD, Wang W, et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci Transl Med 2010;2:ra56. 10.1126/scitranslmed.3001127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tesfay L, Clausen KA, Kim JW, et al. Hepcidin regulation in prostate and its disruption in prostate cancer. Cancer Res 2015;75:2254–63. 10.1158/0008-5472.CAN-14-2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Basuli D, Tesfay L, Deng Z, et al. Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene 2017;36:4089–99. 10.1038/onc.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torti SV, Torti FM. Ironing out cancer. Cancer Res 2011;71:1511–4. 10.1158/0008-5472.CAN-10-3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pyle CJ, Azad AK, Papp AC, et al. Elemental ingredients in the macrophage cocktail: role of ZIP8 in host response to Mycobacterium tuberculosis. Int J Mol Sci 2017;18. 10.3390/ijms18112375. [Epub ahead of print: 09 Nov 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayadi A, Nguyen A-T, Bard FA, et al. Zip14 expression induced by lipopolysaccharides in macrophages attenuates inflammatory response. Inflamm Res 2013;62:133–43. 10.1007/s00011-012-0559-y [DOI] [PubMed] [Google Scholar]
- 13.Recalcati S, Locati M, Marini A, et al. Differential regulation of iron homeostasis during human macrophage polarized activation. Eur J Immunol 2010;40:824–35. 10.1002/eji.200939889 [DOI] [PubMed] [Google Scholar]
- 14.Cairo G, Recalcati S, Mantovani A, et al. Iron trafficking and metabolism in macrophages: contribution to the polarized phenotype. Trends Immunol 2011;32:241–7. 10.1016/j.it.2011.03.007 [DOI] [PubMed] [Google Scholar]
- 15.Corna G, Campana L, Pignatti E, et al. Polarization dictates iron handling by inflammatory and alternatively activated macrophages. Haematologica 2010;95:1814–22. 10.3324/haematol.2010.023879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, Yao G, Zhang Y, et al. M2-polarized tumor-associated macrophages are associated with poor prognoses resulting from accelerated lymphangiogenesis in lung adenocarcinoma. Clinics 2011;66:1879–86. 10.1590/S1807-59322011001100006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014;156:317–31. 10.1016/j.cell.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kortas J, Ziemann E, Antosiewicz J. Effect of HFE gene mutation on changes in iron metabolism induced by nordic walking in elderly women. Clin Interv Aging 2020;15:663–71. 10.2147/CIA.S252661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest 2004;113:1271–6. 10.1172/JCI200420945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lippi G, Sanchis-Gomar F. Epidemiological, biological and clinical update on exercise-induced hemolysis. Ann Transl Med 2019;7:270. 10.21037/atm.2019.05.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sentürk UK, Gündüz F, Kuru O, et al. Exercise-induced oxidative stress leads hemolysis in sedentary but not trained humans. J Appl Physiol 2005;99:1434–41. 10.1152/japplphysiol.01392.2004 [DOI] [PubMed] [Google Scholar]
- 22.Nishiie-Yano R, Hirayama S, Tamura M, et al. Hemolysis is responsible for elevation of serum iron concentration after regular exercises in judo athletes. Biol Trace Elem Res 2020;197:63–9. 10.1007/s12011-019-01981-3 [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Green M, Choi JE, et al. CD8+ T cells regulate tumour ferroptosis during cancer immunotherapy. Nature 2019;569:270–4. 10.1038/s41586-019-1170-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes-Santos IL, Amoozgar Z, Kumar AS, et al. Exercise training improves tumor control by increasing CD8+ T-cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade. Cancer Immunol Res 2021;9:765–78. 10.1158/2326-6066.CIR-20-0499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delwing-de Lima D, Ulbricht ASSF, Werlang-Coelho C, et al. Effects of two aerobic exercise training protocols on parameters of oxidative stress in the blood and liver of obese rats. J Physiol Sci 2018;68:699–706. 10.1007/s12576-017-0584-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanzelli AS, Medeiros A, Rolim N, et al. Integrative effect of carvedilol and aerobic exercise training therapies on improving cardiac contractility and remodeling in heart failure mice. PLoS One 2013;8:e62452. 10.1371/journal.pone.0062452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morris RT, Laye MJ, Lees SJ, et al. Exercise-induced attenuation of obesity, hyperinsulinemia, and skeletal muscle lipid peroxidation in the OLETF rat. J Appl Physiol 2008;104:708–15. 10.1152/japplphysiol.01034.2007 [DOI] [PubMed] [Google Scholar]
- 28.Devin JL, Hill MM, Mourtzakis M, et al. Acute high intensity interval exercise reduces colon cancer cell growth. J Physiol 2019;597:2177–84. 10.1113/JP277648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dethlefsen C, Lillelund C, Midtgaard J, et al. Exercise regulates breast cancer cell viability: systemic training adaptations versus acute exercise responses. Breast Cancer Res Treat 2016;159:469–79. 10.1007/s10549-016-3970-1 [DOI] [PubMed] [Google Scholar]
- 30.Dethlefsen C, Pedersen KS, Hojman P. Every exercise bout matters: linking systemic exercise responses to breast cancer control. Breast Cancer Res Treat 2017;162:399–408. 10.1007/s10549-017-4129-4 [DOI] [PubMed] [Google Scholar]
- 31.Mettler S, Zimmermann MB. Iron excess in recreational marathon runners. Eur J Clin Nutr 2010;64:490–4. 10.1038/ejcn.2010.16 [DOI] [PubMed] [Google Scholar]
- 32.Devin JL, Hill MM, Mourtzakis M, et al. Acute high intensity interval exercise reduces colon cancer cell growth. J Physiol 2019;597:2177–84. 10.1113/JP277648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dethlefsen C, Lillelund C, Midtgaard J, et al. Exercise regulates breast cancer cell viability: systemic training adaptations versus acute exercise responses. Breast Cancer Res Treat 2016;159:469–79. 10.1007/s10549-016-3970-1 [DOI] [PubMed] [Google Scholar]
- 34.Furrer R, Jauch AJ, Nageswara Rao T, et al. Remodeling of metabolism and inflammation by exercise ameliorates tumor-associated anemia. Sci Adv 2021;7:eabi4852. 10.1126/sciadv.abi4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Drouin JS, Young TJ, Beeler J, et al. Random control clinical trial on the effects of aerobic exercise training on erythrocyte levels during radiation treatment for breast cancer. Cancer 2006;107:2490–5. 10.1002/cncr.22267 [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y, Que K-T, Zhang Z, et al. Iron overloaded polarizes macrophage to proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer Med 2018;7:4012–22. 10.1002/cam4.1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costa da Silva M, Breckwoldt MO, Vinchi F, et al. Iron induces anti-tumor activity in tumor-associated macrophages. Front Immunol 2017;8:1479. 10.3389/fimmu.2017.01479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goh J, Kirk EA, Lee SX, et al. Exercise, physical activity and breast cancer: the role of tumor-associated macrophages. Exerc Immunol Rev 2012;18:158-76. [PubMed] [Google Scholar]
- 39.Vela D, Vela-Gaxha Z. Differential regulation of hepcidin in cancer and non-cancer tissues and its clinical implications. Exp Mol Med 2018;50:e436. 10.1038/emm.2017.273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bordini J, Morisi F, Elia AR, et al. Iron induces cell death and strengthens the efficacy of antiandrogen therapy in prostate cancer models. Clin Cancer Res 2020;26:6387–98. 10.1158/1078-0432.CCR-20-3182 [DOI] [PubMed] [Google Scholar]
- 41.Bordini J, Morisi F, Cerruti F, et al. Iron causes lipid oxidation and inhibits proteasome function in multiple myeloma cells: a proof of concept for novel combination therapies. Cancers 2020;12. 10.3390/cancers12040970. [Epub ahead of print: 14 04 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]