Abstract
Introduction
Patients with low levels of knowledge, skills and confidence to manage their health and well-being (activation) are more likely to have unmet health needs, delay seeking healthcare and need emergency care. National Health Service England estimates that this may be applicable to 25%–40% of patients with long-term health conditions. Volunteer peer coaching may support people to increase their level of activation. This form of intervention may be particularly effective for people with low levels of activation.
Methods and analysis
This single site, two-arm randomised controlled trial has been designed to assess the feasibility of conducting a definitive trial of volunteer peer health and well-being coaching for people with long-term health conditions (multiple sclerosis, rheumatic diseases or chronic pain) and low activation. Feasibility outcomes include recruitment and retention rates, and intervention adherence. We will measure patient activation, mental health and well-being as potential outcomes for a definitive trial. These outcomes will be summarised descriptively for each time point by allocated group and help to inform sample size calculation for the definitive trial. Criteria for progression to a full trial will be used.
Ethics and dissemination
Ethical approval has been granted by the London - Surrey Research Ethics Committee, reference 21/LO/0715. Results from this feasibility trial will be shared directly with participants, presented at local, regional and national conferences and published in an open-access journal.
Trial registration number
ISRCTN12623577.
Keywords: Protocols & guidelines, Quality in health care, Neurology, Rheumatology
STRENGTHS AND LIMITATIONS OF THIS STUDY.
It specifically targets patients with low levels of patient activation.
It utilises a novel, codesigned, volunteer peer coaching intervention for outpatients with long-term conditions based on an evidence-based and manualised training programme delivered online.
The research team includes academics, clinical service members and public contributors.
As a single-site study, the transferability of the trial’s findings to other sites may be limited.
Introduction
National Health Service (NHS) England estimates that 25%–40% of patients in England have low patient activation, defined as poor knowledge, skills and confidence to manage health and well-being (level 1 or 2 on the Patient Activation Measure (PAM)).1 These patients are more likely to have unmet health needs, delay seeking healthcare and need emergency care. Activation level is a modifiable factor, and it is likely that people with low activation have most to gain from an intervention designed to increase patient activation levels.2 Supporting self-management in people with a health condition is 1 of 6 key components of the NHS Personalised Care Model (PCM) to address low activation.3 The PCM focuses on an individual’s strengths and assets alongside working towards improvements in health conditions based on a ‘what matters to me’ approach.
One emerging approach from the literature to support self-management is health and well-being coaching.4 Nationally, programmes have been developed primarily to support patients with lifestyle changes.5 These recommend health professionals deliver coaching alongside their clinical work. However, national roll-out and adoption of these programmes has been slow, which may be in part due to increasing demand on services and lack of resources due to stagnating budgets.6 An alternative approach to staff delivery of coaching services is to involve patients with lived experience as coaches (peer coaches) especially if they are highly activated (PAM levels 3 and 4). There is an expanding body of research exploring the effectiveness of peer coaching provided via a range of delivery modes; in-person7 8; telephone9 10 and digital.11 Recent randomised controlled trials (RCTs) of peer coaching have included people with diabetes9 12 13 and chronic pain.8 14 15 These studies have demonstrated improvements in perceived physical activity (PA),9 quality of life (QoL),9 13 pain9 and depression.12 13 In contrast, Matthias et al reported no statistically significant between-group differences in pain at 6 (estimate(SE) 0.01 (0.23), 95% CI (−0.45 to 0.46)) or 9 months (estimate(SE) 0.07 (0.24), 95% CI (−0.40 to 0.54)) following their effectiveness trial of a peer coach-delivered pain self-management intervention versus controls who received a class on pain and pain self-management.8 However, several trials have reported barriers to implementing this kind of intervention which guides towards methods to minimise or overcome potential barriers.
A number of studies have highlighted potential challenges of peer coaching such as coach well-being,14 low intervention adherence and high drop-out rates.8 9 13 A recent feasibility RCT of peer mentorship for people with osteoarthritis in the UK reports a mixed picture with challenges in matching coaches to peers and difficulties with coach retention alongside positive reports of coach enjoyment and satisfaction.7 16 We have not located any studies of peer coaching that have targeted peer coaching interventions at patients reporting low levels of activation. People with low levels of activation stand to benefit most from an intervention designed to improve confidence, problem solving and ability to manage their healthcare and well-being.2 This may in turn impact use of health and social cares resources, and could feasibly be delivered by peers (others with long-term conditions) with high levels of activation to negate the issues of resource within the NHS.
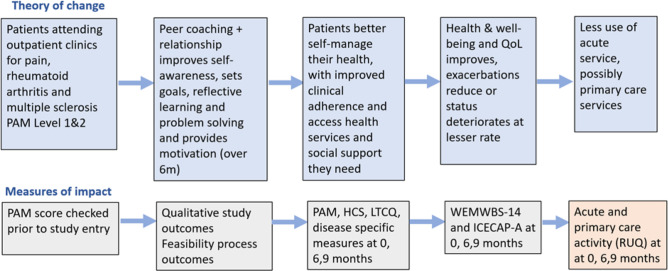
This paper describes the trial protocol for the PEER CONNECT study, a two-arm randomised controlled feasibility trial of peer coaching for people receiving outpatient care for one of three long-term health conditions; multiple sclerosis, a rheumatic disease or chronic pain. The peer coaching service will only be offered to people with low levels of patient activation. It provides up to 14 coaching sessions delivered over 6 months which decrease in frequency over time. Volunteer peer coaches (confirmed to have high levels of activation) will attend a comprehensive training programme that follows a manualised coaching approach and includes independent and group learning sessions delivered online. In addition, they will receive regular individual and group supervision. The logic model for the intervention is illustrated in figure 1.
Figure 1.
Volunteer peer coaching logic model. HCS, Health Confidence Score; ICECAP-A, ICEpop CAPability measure for Adults; LTCQ, Long-Term Conditions Questionnaire; PAM, Patient Activation Measure; QoL, Quality of Life; RUQ, Resource Use Questionnaire; WEMWBS, Warwick Edinburgh Mental Well-Being Scale.
Objectives
Our research question is:
Is it feasible to undertake a future definitive multi-centre RCT to determine the effectiveness of a targeted peer coaching intervention on the health and well-being of people with long-term health conditions and low activation attending outpatient services?
Our trial feasibility objectives are:
Are we able to identify, recruit, retain and follow-up eligible volunteer coaches and peers?
What is a sustainable number of peers per volunteer coach?
Are trial procedures acceptable to participants (peers and volunteer coaches)?
To estimate parameters needed to inform future sample size calculation.
Are trial outcome measures acceptable to participants (peers)?
Does the trial demonstrate evidence to suggest that the coaching holds promise as an effective intervention?
Definitions
Within this paper the following key definitions are used:
Peers: Participants eligible to receive coaching.
Volunteer peer coaches: Participants eligible to train to deliver coaching to peers.
Methods and analysis
Study design
This research is a single-site, two-arm, pragmatic randomised controlled feasibility trial. Eligible peers will be randomised 1:1 to either the intervention arm which includes (up to) 14 sessions of peer coaching over 6 months and their usual care, or the control arm who receive usual care only. Embedded within this feasibility study is a qualitative component that will include individual interviews with volunteer coaches and peers, clinic and peer coaching staff, and people who decline to take part in the interventional aspect of the study. All aspects of the trial protocol have been approved by the London - Surrey Research Ethics Committee, reference 21/LO/0715.
Participants
Eligibility criteria (peers and coaches)
Eligible participants will:
Be aged 18 years or older (peers and volunteer coaches).
Attend a rheumatology, pain or multiple sclerosis out-patient clinic (peers and volunteer coaches).
Score PAM level 1 or 2 (peers), PAM level 3 or 4 (volunteer coaches).
Be willing and able to engage in the 6-month intervention (peers and volunteer coaches).
Be willing and able to commit to undertaking assessments at baseline, 6 and 9 months (peers).
Have capacity to provide informed consent (peers and volunteer coaches).
Have sufficient fluency in English to be able to engage with the intervention and trial material (peers and volunteer coaches).
Not be participating in any other observational or interventional research trial.
Recruitment
This trial aims to recruit 15 volunteer coaches and 60 peers to take part in the intervention. This feasibility sample size was selected by a team of experienced researchers and clinicians and was based on predicted recruitment within time frame and resource, parameters of the population size, modelling of coach to peer matching and is in line with recommendations.17 The sample size of 60 peers will allow overall retention rate to be estimated to within a 95% CI of approximately ±13%. Coaches, peers, clinic and service delivery staff, and people who decline to take part in the study will also be invited to take part in the qualitative component of the research.
Recruitment of volunteer coaches and peers
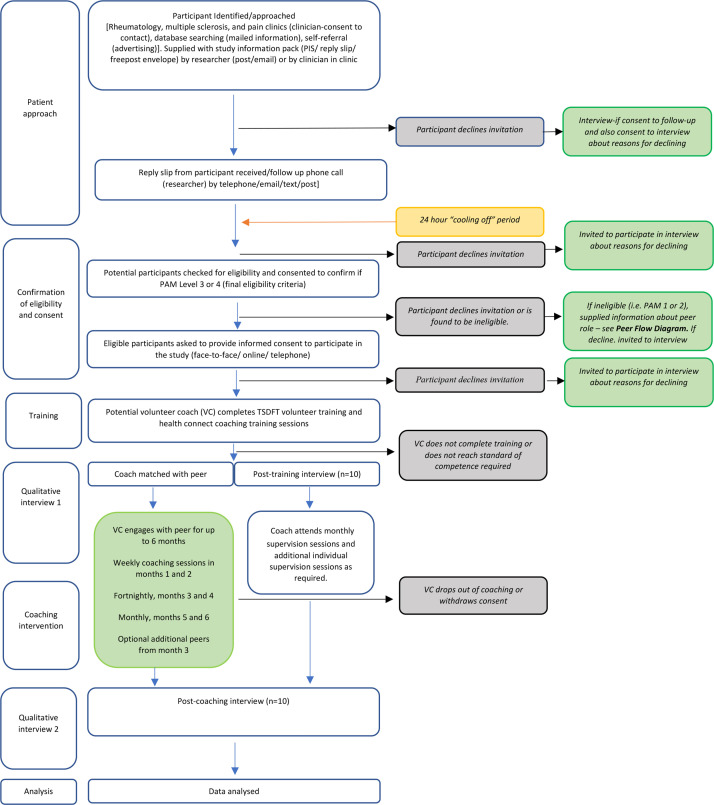
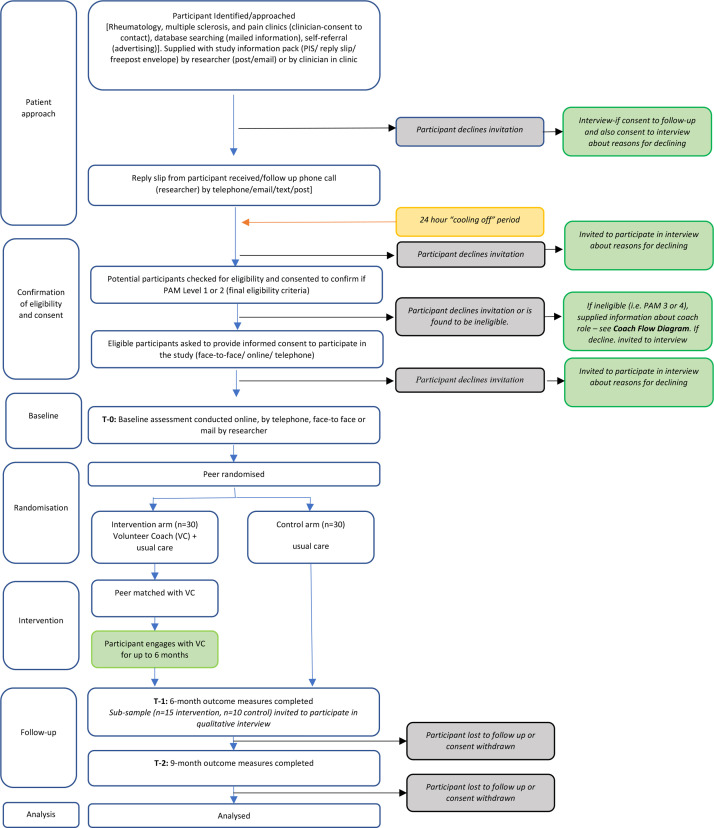
Potential volunteer coaches and peers will be recruited from the multiple sclerosis, rheumatology and chronic pain out-patient clinics at a single NHS Trust (Torbay and South Devon NHS Foundation Trust (TSDFT)). In addition, the relevant study information will be sent to patients with a recorded PAM score on the clinical team’s database. Patients known to multiple sclerosis, rheumatology and chronic pain clinics may also respond directly to adverts placed at a range of healthcare and community venues. Recruitment is planned to commence in November 2021 and continue for 6 months. figures 2 and 3 indicate the research journey of eligible participants. Following initial telephone screening, potential participants will provide consent to complete the PAM to confirm eligibility as a volunteer coach or peer.
Figure 2.
Trial flow diagram: volunteer coach.
Figure 3.
Trial flow diagram—peer.
Consent
Participants will be offered a choice of four options for providing informed consent:
In-person signed form with scanned copy stored electronically on a TSDFT secure drive.
Video-recorded using MS Teams and stored securely as above.
Completed via Jisc (https://www.onlinesurveys.ac.uk/) with exported record stored securely as above.
Postal signed consent form, scanned on receipt and stored as above.
Randomisation
Following baseline data collection, eligible peers will be randomised to either the intervention or control arm on a 1:1 ratio using random permuted blocks, stratified by outpatient clinic. The randomisation list will be generated and stored by a statistician not involved in the trial, and allocation will be accessed through a web-portal hosted by the University of Plymouth Peninsula Clinical Trials Unit.
Blinding
Blinding of participants will not be possible due to the nature of the intervention. Due to restricted capacity not all members of the research team will be blinded. The trial statistician will be blinded to allocation.
Intervention and setting
Setting
All participants will be recruited from TSDFT, a district general hospital in the South West of the UK.
Control arm
Usual care is defined as access to services and treatment provided as routine care, examples of which include attending out-patient clinic appointments, referral to therapies, and signposting to community or support services as required.
Intervention arm
The intervention includes up to 14 sessions with a volunteer coach delivered over 6 months. Sessions are expected to last from 15 to 60 minutes and will be provided in a COVID-19 secure environment either on-line, by telephone or face-to-face. A flexible framework for the coaching will be used to facilitate a personalised approach with a suggested format of one session per week for the first 2 months, followed by fortnightly sessions for 2 months and monthly sessions thereafter. Peers will be supported to produce a coaching plan with associated goals at the end of each session. A brief summary of the content, duration and mode of coaching delivery will also be recorded. Missed planned sessions (non-attendance) will be recorded by the volunteer coach. In addition, peers will be asked to report any adverse events (AEs) they have experienced and rate their experience of being coached.
Volunteer coach training
Volunteer peer coach training will include 8 structured 90 minute live sessions supported by interactive online learning tasks (homework). Training will be delivered by the TSDFT volunteer peer health and well-being coaching service, the ‘Health Connect Coaching Programme’. Sessions will draw on evidence-based behavioural change methods,18 motivational strategies19 and communication techniques. The content will also draw on evidence-based materials to improve health and well-being such as Making Every Contact Count,20 Five Ways to Well Being21 and NHS health coaching programmes.5 The intervention will emphasise22:
A patient-centred approach where patients determine their goals.
Active learning or self-discovery.
A problem-solving focus to work towards goals.
Regular peer feedback on implementing the coaching plan.
Training will initially be completed virtually using Microsoft Teams, with a view to offering face-to-face training in the future should COVID-19 restrictions allow. Each 90 minute session will include a break. There will be 2 training sessions each week for 4 consecutive weeks. Training will total a minimum of 15 hours for each volunteer coach (12 hours of live sessions and around 3 hours homework) and will include practical sessions and on-line modules.
The training content covers:
Background to personalised care and why it matters.
How this volunteer role has been developed and why.
Stages of behaviour change and how this relates to managing long-term condition(s).
Exploring beliefs and boundaries.
Insight and awareness of the drama triangle and what impact this can have.
Exploring each of the core coaching skills (open questions, empathy, value of silence, reflection, recognising change).
Using confidence and/or importance scaling and practising how to embed use of these in coaching conversations.
Skills practice throughout using pair and group activities.
Understanding the flow of coaching conversations.
How to use appropriate resource tools to support conversations.
Using Microsoft Teams and Patient Knows Best platforms.
Awareness of appropriate signposting and increasing confidence in how to signpost well.
Goal setting and goal follow-up.
By the end of the course, volunteer coaches will be confident and competent to:
Understand their role, boundaries and how to seek help and guidance.
Use technology to contact and engage with peers.
Use health coaching conversational skills to work with peers on what matters to them, to support motivation for positive behaviour change to improve their health, well-being, and self-management of their condition.
Be aware of local services and have the confidence to signpost to appropriate services.
Know when and how to use the Health Connect Coaching Programme coordinators to support them in their role, and their peer on their journey.
Training will also include learning to use a range of behaviour change techniques, which may include supporting peers to self-monitor, develop healthy habits, focus on past successes and set goals. Following successful completion of all training sessions and competence assessment by the coach trainers, coaches will be carefully matched to a peer. Matching will be completed by the programme coordinators and will be based on criteria including: having a shared or similar health condition or symptoms, social deprivation (based on postcode) and other factors that peers feel are important to them which will be explored in an initial telephone conversation with the coordinator. Volunteer coaches will be supervised and supported through monthly peer coaching group meetings and one-to-one supervision sessions with the coach coordinators as required. All coaches will complete a Disclosure and Barring Service check prior to working with peers.
Outcomes
Primary outcomes
The primary outcomes of this trial are feasibility outcomes.
Recruitment
Recruitment of peers and volunteer coaches will be calculated as follows:
Peer recruitment (%) = number of peers recruited/potentially eligible cohort (indicated by the number of information packs distributed) × 100.
Coach recruitment (%) = number of volunteer coaches recruited/potentially eligible cohort (indicated by the number of information packs sent or handed out) × 100.
Retention and follow-up
Follow-up will be online. Peer retention and follow-up will be calculated as the proportion of peers completing all questionnaires at 6 months (post-intervention) and 9 months (follow-up).
Coach retention will be calculated as the proportion of coaches who complete the training programme and coach at least 1 peer (defined as providing at least 2 coaching sessions).
Adherence
Adherence will be calculated as the number of sessions attended out of the total planned and mutually agreed coaching sessions (as long as this is at least 2 sessions).
Qualitative outcomes
We will report themes relevant to the experience of participating in the trial from peers, volunteer coaches and service provider staff, including feasibility of progressing to a full-scale trial. These will include experience of: referral and recruitment to the trial, randomisation, questionnaire completion, interview participation, and burden and reward for participation in the trial. In addition, reasons for not wanting to take part will be collated and reported where such information is provided on reply slips and/or in decliner interviews.
Secondary outcomes
Peers will complete a sociodemographic and health questionnaire (including items such as diagnosis, time since diagnosis, comorbidity, place of residence, level of mobility and occupation) at baseline. The following health, well-being and resource use outcomes will be completed at baseline, post-intervention (6 months) and follow-up (9 months) time points.
Patient Activation Measure (PAM): This is a validated, 13-item licensed tool that has been extensively tested in many studies.1 It measures the spectrum of knowledge, skills and confidence for managing health and healthcare.
Warwick Edinburgh Mental Well-being Scale (WEMWBS) : This validated scale assesses mental well-being within the adult population using 14 questions.23 The scale measures positive mental well-being in terms of both feeling good (hedonia) and functioning well (eudaimonia).
ICEpop CAPability measure for adults (ICECAP-A): The ICECAP-A is a measure of capability in the adult population that can be used for economic evaluation.24 It includes 5 items 1 for each domain: stability, attachment, autonomy, achievement and enjoyment. Each item includes 4 possible responses. A tariff value for an overall state is calculated using an ICECAP algorithm and is used to calculate well-being adjusted life-years.
Health Confidence Score (HCS): The HCS is a short, generic, person-reported measure of people’s perceived confidence in managing aspects of their own health and care. It has 4 items covering health knowledge, capability to self-manage, access to help and shared decisions.25
Long-Term Conditions Questionnaire (LTCQ): This 20-item questionnaire assesses outcomes in patients with either single or multiple long term conditions (physical and/or mental health condition(s)) in health and social care contexts.26 It measures across 3 broad concepts: impact of LTCs, experience of services and support, and self-care.
Resource use questionnaire: Details of health service utilisation including health, social and broader care provision and support (eg, outpatient, Accident and Emergency and General Practitioner visits, community care worker visits, voluntary sector support and informal care) will be captured using a questionnaire developed by members of the research team for use in other trials.
Session Rating Scale 3.0.27: This is a 4-item, client-completed measure of session experience.
Disease-specific symptom measures: Participants will additionally be asked to complete 1 disease specific questionnaire. This will be selected based on their clinical diagnosis from the 5 options below.
Brief Pain Inventory (BPI): The BPI includes 9 items and was developed to assess the severity of pain and the impact of pain on functioning.28
Multiple Sclerosis Impact Scale(MSIS-29v2): This is a 29-item condition specific measure of health-related QoL, devised specifically for people with multiple sclerosis.29
The EULAR Psoriatic Arthritis Impact of Disease (PsAID9) for clinical trials (PsAID9): The 9-item PsAID is a questionnaire validated to assess the impact of Psoriatic Arthritis on patients' lives.30
Bath AS Disease Activity Index (BASDAI): This 6-item questionnaire assesses the impact of the 5 major symptoms of Ankylosing Spondylitis.31
Rheumatoid Arthritis Impact of Disease (RAID) questionnaire: The RAID questionnaire comprises 7 domains of disease impact.32
Qualitative secondary outcomes
We will gather the views of peers and coaches about the volunteer coach training, matching process, intervention, coach–peer relationship, perceived impact on health and well-being and overall participation in the trial using a combination of semi-structured interviews, observations and analysis of coaching plans. Purposive sampling will ensure interviewees are representative of the cohorts’ range of demographic characteristics, degree of engagement with the programme and in the case of coaches, will include coaches who coach a different numbers of peers and who use online or face-to-face delivery. We will also capture barriers to trial participation by interviewing decliners, volunteer coaches and peers who drop out. Peer, volunteer coach, staff and decliner interviews will explore the barriers and facilitators of set up and delivering the peer coaching service, its active ingredients in relation to the 4 elements of coaching outlined above and elements of the peer–coach relationship that facilitate behavioural change.
We will observe the training and monthly coaching supervision to understand, explore, and describe the intervention. Brief session notes will be recorded by the coach coordinators who lead the supervision sessions that will be used by the research team to summarise issues discussed. Analysis will be framed around a conceptual model of coaching adapted from Matthias et al, which includes motivation, strategies and finding what works.33
Patient and public involvement statement
To ensure procedures and intervention delivery are acceptable and relevant to participants, they were developed with input from a patient and public involvement (PPI) group that included people with lived experience of the targeted conditions (n=7, 2 women). Members of the group had either attended a TSDFT codesign event in 2019 and had continued to be part of the intervention development or were recruited from local condition-specific support groups. The group was established and convened twice during the setup phase of the trial. Key objectives of the PPI group included but were not limited to: trial materials development; questionnaire design and delivery; disease-specific questionnaire selection; adaptations to intervention format, content and delivery; data collection processes; interview topic guide development; and the minimising of burden and maximising of engagement and retention through identification of barriers and facilitators. Further consultation is planned to consider the interpretation of findings, dissemination strategy and the study’s next steps. All PPI consultation has been, and will be completed in line with the National Institute of Health and Care Research (NIHR) guidelines, including financial reimbursement.
Data analysis
Quantitative
A period of 5 months has been allocated for data analysis, write up and dissemination. A detailed statistical analysis plan will be finalised before the trial database is locked. A Consolidated Standards of Reporting Trials (CONSORT) diagram will show information from screening, recruitment and follow-up and feasibility outcomes will be summarised with recruitment and retention rates presented with 95% CIs. All quantitative data for this feasibility trial are self-reported and outcomes will be used and scored in line with author guidance. PAM scores will be calculated using the algorithm from Insignia Health (https://www.insigniahealth.com/products/pam-survey). Descriptive statistics will be presented for secondary outcomes at baseline, 6 and 9 months by allocated group. Between-group differences of the change in scores between baseline and each follow-up time point will be presented but no inferential analysis will be performed, in accordance with CONSORT guidance.34
Sample size estimation
To inform sample size estimation for a future trial, we will calculate the SD of the secondary outcomes of patient activation, mental well-being and QoL. To estimate plausible between group differences for a primary outcome in a future definitive trial, namely change in scores on key secondary outcome measures from pre-intervention to post-intervention, we will calculate the between group difference (with 95% CIs) in change score between baseline and follow-up (9 months).
Qualitative
We will use thematic framework analysis35 following the 5 steps of analysis (familiarisation, identifying a thematic framework, indexing, charting, and mapping and interpretation) to explore qualitative data with themes identified and discussed between a minimum of 2 researchers. The process will use a combination of inductive and deductive framing, using the conceptual model of the intervention as a guide. Analysis will be completed using NVivo V.12 (QSR International, 2018). PPI input will help clarify and interpret identified themes within the framework.
Progression criteria
At the end of this feasibility trial the following criteria, developed in line with Avery et al36 will be used to determine progression to a full trial application. We shall progress to a full trial application if minimum success criteria are achieved in key feasibility areas. These criteria will be discussed with the trial management group (TMG) and trial steering committee (TSC), but may include:
Target peer population (n=60) plus sufficient coaches recruited within 9-month recruitment window (<60% stop, 60%–80% discuss, 80%+ go).
Adherence (a ‘dose’ of coaching is defined as attending at least 2 of the mutually agreed number of coaching sessions37 (which may range from 2 to 14 sessions) of participants randomised to coaching (<40% of peers attend stop, 40%–60% discuss, 60%+ go).
Completion of outcome measures (scored PAM at 9-month follow-up) (<60% stop, 60%–80% discuss, 80%+ go).
Evidence to suggest efficacy that is, that the coaching holds promise as an effective intervention (indicated by examination of the CIs of the between group differences in PAM at 9 months and qualitative data).
Any issues that arise during this feasibility trial will be discussed with our PPI group members to consider possible action. Changes may be implemented within this feasibility trial or be evident on trial completion which will inform the feasibility, and optimum delivery, for a potential definitive trial.
Ethics and dissemination
Safety monitoring
Throughout the trial, all possible precautions will be taken to ensure participant safety and well-being. Experienced professional coaches will deliver the volunteer coaching training and will ensure that volunteer coaches are trained and supervised to an appropriate level in order to deliver the coaching independently and safely. All AEs will be reported by participants to the health connect coaching coordinators via their volunteer coach. This information will be shared with the research team who will assess any relation to the intervention. All serious AEs (SAEs) will be reported to the CI within 24 hours of identification and the trial sponsor will be informed. All AEs and SAEs will be reported to the TMG on a monthly basis. In addition, a summary of this information will be shared with the TSC every 6 months.
Data management and monitoring
Confidentiality
Any identifiable information will be stored in a shared drive on TSDFT computers. All self-reported data will be collected via Jisc platform (https://www.onlinesurveys.ac.uk/). This anonymised data will be exported to and stored on a password protected and encrypted University computer. Interview recordings will be transcribed with any identifiable information removed. The recordings will be destroyed after transcription and the transcripts containing non-identifiable information will be retained. At the end of the trial all anonymised research information held on university computers will be returned to the sponsor (NHS trust) for storage on a TSDFT drive for a minimum of 5 years. As members of the research team also hold honorary contracts with TSDFT no other data sharing agreements are necessary. All information will be handled in compliance with the General Data Protection Regulations (2018).
Data monitoring
Data will be managed independently from the Sponsor and research funder. As this is a feasibility trial a data monitoring committee has not been deemed necessary, as there will be insufficient data to establish benefits or harms of the intervention worthy of invoking early stopping rules.
Trial management and oversight
Two committees are involved in the set up and management of this trial.
The TMG comprises the university research team and members of the NHS Trust peer coaching service. It will meet monthly throughout the course of the trial via web-based platforms such as Microsoft Teams or face-to-face should COVID-19 restrictions allow. The group is responsible for development of the protocol and other trial documentation and ensuring smooth and safe running of the trial.
The TSC is made up of an independent chair, an independent statistician, a person with lived experience and an independent health economist. The role of the group is to provide overall supervision for the trial on behalf of the sponsor and funder and to ensure that the trial is conducted according to the rigorous standards set out in the Department of Health’s Research Governance Framework for Health and Social Care and the Guidelines for Good Clinical Practice. The group will continue to meet twice a year across the trial timeline.
Post-trial care
Participants in the control arm will be offered priority access to the intervention after final data collection has taken place. All participants will have access to their usual healthcare as routine practice.
Dissemination
Results from this feasibility trial will be shared directly with participants once they are available. In addition, results will be presented at local, regional and national conferences. Further, the protocol and trial findings will be published in an open-access journal and a final report will be presented to the funders and sponsor.
Supplementary Material
Acknowledgments
The authors would like to acknowledge Annette Thom for her assistance with the initial literature review. In addition, the study PPI group and trial steering committee for their involvement in the design of this study.
Footnotes
Twitter: @DrTomPThompson
Contributors: AS, JE, WC, TT, JH and HD-C developed the study. RD, AS, JE, WC, TT, JH, HD-C, KB and OW are responsible for the conduct of the study. Each of the named authors contributed to the reporting of this work.
Funding: This work is funded by Torbay Medical Research Fund grant number 137.
Disclaimer: The Funder has no role in trial design, conduct, data analyses and interpretation, manuscript writing, or dissemination of results.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, conduct, reporting, and dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Hibbard JH, Stockard J, Mahoney ER, et al. Development of the patient activation measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res 2004;39:1005–26. 10.1111/j.1475-6773.2004.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff 2013;32:207–14. 10.1377/hlthaff.2012.1061 [DOI] [PubMed] [Google Scholar]
- 3.NHS England: Comprehensive model of personalised care, 2019. Available: https://www.england.nhs.uk/personalisedcare/comprehensive-model-of-personalised-care/
- 4.Sforzo GA, Kaye MP, Todorova I, et al. Compendium of the health and wellness coaching literature. Am J Lifestyle Med 2018;12:436–47. 10.1177/1559827617708562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NHS England and NHS Improvement 2020: Health Coaching . Implementation and quality summary guide support self-management. Available: https://www.england.nhs.uk/publication/health-coaching-summary-guide-and-technical-annexes/
- 6.Robertson R, Wenzel L, Thompson J. Understanding NHS financial pressures: how are they affecting patient care? The Kings Fund, 2017. Available: https://www.kingsfund.org.uk/publications/understanding-nhs-financial-pressures
- 7.Anderson AM, Lavender EC, Dusabe-Richards E, et al. Peer mentorship to improve self-management of hip and knee osteoarthritis: a randomised feasibility trial. BMJ Open 2021;11:e045389. 10.1136/bmjopen-2020-045389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matthias MS, Bair MJ, Ofner S, et al. Peer support for self-management of chronic pain: the evaluation of a peer Coach-Led intervention to improve pain symptoms (eclipse) trial. J Gen Intern Med 2020;35:3525–33. 10.1007/s11606-020-06007-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andreae SJ, Andreae LJ, Richman JS, et al. Peer-Delivered cognitive behavioral training to improve functioning in patients with diabetes: a cluster-randomized trial. Ann Fam Med 2020;18:15–23. 10.1370/afm.2469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Small N, Blickem C, Blakeman T, et al. Telephone based self-management support by 'lay health workers' and 'peer support workers' to prevent and manage vascular diseases: a systematic review and meta-analysis. BMC Health Serv Res 2013;13:533. 10.1186/1472-6963-13-533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortuna KL, Naslund JA, LaCroix JM, et al. Digital peer support mental health interventions for people with a lived experience of a serious mental illness: systematic review. JMIR Ment Health 2020;7:e16460. 10.2196/16460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andreae SJ, Andreae LJ, Richman JS, et al. Peer-delivered cognitive behavioral Therapy-based intervention reduced depression and stress in community Dwelling adults with diabetes and chronic pain: a cluster randomized trial. Ann Behav Med 2021;55:970–80. 10.1093/abm/kaab034 [DOI] [PubMed] [Google Scholar]
- 13.Khodneva Y, Safford MM, Richman J, et al. Volunteer peer support, diabetes, and depressive symptoms: results from the encourage trial. J Clin Transl Endocrinol 2016;4:38–44. 10.1016/j.jcte.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matthias MS, Daggy J, Ofner S, et al. Exploring peer coaches' outcomes: findings from a clinical trial of patients with chronic pain. Patient Educ Couns 2020;103:1366–72. 10.1016/j.pec.2020.02.007 [DOI] [PubMed] [Google Scholar]
- 15.Shue SA, McGuire AB, Matthias MS. Facilitators and barriers to implementation of a peer support intervention for patients with chronic pain: a qualitative study. Pain Med 2019;20:1311–20. 10.1093/pm/pny229 [DOI] [PubMed] [Google Scholar]
- 16.Lavender EC, Dusabe-Richards E, Anderson AM, et al. Exploring the feasibility, acceptability and value of volunteer peer mentors in supporting self-management of osteoarthritis: a qualitative evaluation. Disabil Rehabil 2021:1–11. 10.1080/09638288.2021.1964625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sim J, Lewis M. The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. J Clin Epidemiol 2012;65:301–8. 10.1016/j.jclinepi.2011.07.011 [DOI] [PubMed] [Google Scholar]
- 18.Simmons LA, Wolever RQ. Integrative health coaching and motivational interviewing: synergistic approaches to behavior change in healthcare. Glob Adv Health Med 2013;2:28–35. 10.7453/gahmj.2013.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundahl BW, Kunz C, Brownell C, et al. A meta-analysis of motivational interviewing: twenty-five years of empirical studies. Res Soc Work Pract 2010;20:137–60. 10.1177/1049731509347850 [DOI] [Google Scholar]
- 20.Health Education England . Making every contact count. Available: https://www.makingeverycontactcount.co.uk/
- 21.Aked J, Marks N, Cordon C. Five ways to wellbeing; communicating the evidence. Available: https://neweconomics.org/2008/10/five-ways-to-wellbeing
- 22.Wolever RQ, Simmons LA, Sforzo GA, et al. A systematic review of the literature on health and wellness coaching: defining a key behavioral intervention in healthcare. Glob Adv Health Med 2013;2:38–57. 10.7453/gahmj.2013.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Putz R, O’hara K, Taggart F. Using WEMWBS to measure the impact of your work on mental wellbeing: a practice-based user guide. Warwick Medical School, Coventry City Council, NHS Coventry, 2012. [Google Scholar]
- 24.Al-Janabi H, Flynn TN, Coast J. Development of a self-report measure of capability wellbeing for adults: the ICECAP-A. Qual Life Res 2012;21:167–76. 10.1007/s11136-011-9927-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benson T, Potts HWW, Bark P, et al. Development and initial testing of a health confidence score (HCS). BMJ Open Qual 2019;8:e000411. 10.1136/bmjoq-2018-000411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potter CM, Batchelder L, A'Court C, et al. Long-Term conditions questionnaire (LTCQ): initial validation survey among primary care patients and social care recipients in England. BMJ Open 2017;7:e019235. 10.1136/bmjopen-2017-019235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller SD, Duncan BL, Johnson LD. The session rating scale 3.0. Chicago, IL: Authors, 2000. [Google Scholar]
- 28.Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singap 1994;23:129–38. [PubMed] [Google Scholar]
- 29.Hobart J, Lamping D, Fitzpatrick R, et al. The multiple sclerosis impact scale (MSIS-29): a new patient-based outcome measure. Brain 2001;124:962–73. 10.1093/brain/124.5.962 [DOI] [PubMed] [Google Scholar]
- 30.Gossec L, de Wit M, Kiltz U, et al. A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the psoriatic arthritis impact of disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis 2014;73:1012–9. 10.1136/annrheumdis-2014-205207 [DOI] [PubMed] [Google Scholar]
- 31.Garrett S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath ankylosing spondylitis disease activity index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- 32.Duarte C, Santos EJF, Ferreira RJO, et al. Validity and reliability of the EULAR instrument RAID.7 as a tool to assess individual domains of impact of disease in rheumatoid arthritis: a cross-sectional study of 671 patients. RMD Open 2021;7:e001539. 10.1136/rmdopen-2020-001539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matthias MS, Daggy J, Adams J, et al. Evaluation of a peer coach-led intervention to improve pain symptoms (eclipse): rationale, study design, methods, and sample characteristics. Contemp Clin Trials 2019;81:71–9. 10.1016/j.cct.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 34.Eldridge SM, Chan CL, Campbell MJ, et al. Consort 2010 statement: extension to randomised pilot and feasibility trials. BMJ 2016;355:i5239. 10.1136/bmj.i5239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritchie J, Spencer L. Qualitative Data Analysis for Applied Policy Research. In: Bryman A, Burgess R, eds. Analyzing qualitative data. London: Routledge, 1994: 173–94. [Google Scholar]
- 36.Avery KNL, Williamson PR, Gamble C, et al. Informing efficient randomised controlled trials: exploration of challenges in developing progression criteria for internal pilot studies. BMJ Open 2017;7:e013537. 10.1136/bmjopen-2016-013537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor A, Thompson TP, Ussher M, et al. Randomised controlled trial of tailored support to increase physical activity and reduce smoking in smokers not immediately ready to quit: protocol for the trial of physical Activity-assisted reduction of smoking (tars) study. BMJ Open 2020;10:e043331. 10.1136/bmjopen-2020-043331 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.