Abstract
Objective
The aim of this systematic review is to evaluate the various modalities available for extended ECG monitoring in the detection of atrial fibrillation (AF) following a cryptogenic stroke.
Methods
MEDLINE (Ovid), EMBASE (Ovid), Cochrane Central Register of Controlled Trials (CENTRAL) were searched from January 2011 to November 2021. All randomised controlled trials and prospective cohort studies including the use of extended ECG monitoring >24 hours with a minimum duration of AF of 30 s in patients with either cryptogenic strokes or transient ischaemic attacks were included. A random-effects model was used to pool effect estimates of AF detection rates from different ECG modalities.
Results
3924 studies were identified, of which 47 were included reporting on a pooled population of 6448 patients with cryptogenic stroke. The pooled AF rate for implantable loop recorders (ILRs) increased from 4.9% (3.0%–7.9%) at 1 month to 38.4% (20.4%–60.2%) at 36 months. Mobile cardiac outpatient telemetry (MCOT) had a significantly higher pooled AF detection rate of 12.8% (8.9%–17.9%) versus 4.9% (3.0%–7.9%) for ILR at 1 month (p<0.0001). Predictors for AF detection include duration of monitoring (p<0.0001) and age (p<0.0001) for ILRs, but only age for MCOTs (p<0.020).
Conclusion
MCOT has a higher rate of detection at 1 month and is less invasive. Beyond 1 month, compliance becomes a significant limitation for MCOT. MCOT may be a reasonable alternative AF screening tool for patients with cryptogenic stroke if ILR is not available.
PROSPERO registration number
CRD42022297782.
Keywords: atrial fibrillation, stroke, electrophysiology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Implantable loop recorders (ILRs) are currently the preferred method of atrial fibrillation (AF) screening after cryptogenic stroke due to the longer duration of monitoring possible. The role of mobile cardiac outpatient telemetry (MCOT) in the detection of AF after cryptogenic stroke is currently unclear.
WHAT THIS STUDY ADDS
We found that at 1 month of monitoring, the rate of AF detection was higher with MCOT than with ILRs. Beyond 1 month, compliance becomes a major limiting factor for MCOT.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
In patients with sufficient cognitive and physical ability to carry out ECG monitoring daily, a 1-month duration of MCOT can capture a significant proportion of AF and can be considered in place of ILRs.
Introduction
Stroke is one of the leading causes of death and disability worldwide. Despite advancements in medical technology, the cause of ischaemic stroke remains undetermined in approximately 26% of cases.1 This is also termed as cryptogenic stroke. An important cause of cryptogenic stroke is atrial fibrillation (AF), with up to 10% of patients have AF detected within a year following a cryptogenic stroke.2
Current methods of extended ECG monitoring include invasive methods such as implantable loop recorders (ILRs),2 and non-invasive methods such as prolonged inpatient telemetry, Holter monitoring and outpatient monitoring strategies, collectively known as mobile cardiac outpatient telemetry (MCOT).3 Compared with conventional follow-up with scheduled ECG monitoring, both ILRs and MCOT have far higher rates of AF detection.2 4 5 ILRs can be left in place for up to 36 months, as compared with MCOTs which are typically only used up to 1 month in duration due to limitations in compliance. With longer duration of monitoring, ILRs are typically considered the gold standard of ECG monitoring after cryptogenic stroke.
However, ILRs have several limitations. According to the 2021 American Heart Association/American Stroke Association guidelines for the management of acute ischaemic stroke, the optimal duration of extended ECG monitoring after acute ischaemic stroke is uncertain.6 The need for an invasive procedure and long-follow up required may not be acceptable to some patients.7 ILRs are also more costly and are only considered cost-effective if continuously used over a 3-year period.8
Existing systematic reviews mainly focus on assessing efficacy of ILRs3 9 with a relative scarcity of literature exploring the use of MCOT after cryptogenic stroke or directly comparing efficacy of ILRs with MCOT. However, some individual studies have shown promising data regarding the efficacy of MCOT, suggesting a role for MCOT replacing or used in conjunction with ILRs.3 4 The primary aim of this systematic review is to identify the AF detection rates of different modalities and compare the difference between ILRs and MCOTs in the detection of AF following a cryptogenic stroke. Secondary outcomes include identifying factors influencing the rate of AF detection rate between different modalities to prioritise patients who would benefit most from MCOT.
Methods
Search strategy
The systematic review and meta-analysis were reported in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.10 This review was registered in January 2022 on the International Prospective Register of Systematic Reviews (PROSPERO) under the ID CRD42022297782.
A comprehensive search was performed in November 2021 including studies from January 2011 to November 2021 on the following electronic databases: MEDLINE (Ovid), EMBASE (Ovid) and Cochrane Central Register of Controlled Trials (CENTRAL). The search consisted of keywords of ‘Electrocardiography’, ‘Stroke’, ‘Transient Ischemic Attack’ and ‘Atrial Fibrillation’. The full search strategy can be found in online supplemental appendix I. The retrieved papers were then exported into the systematic review managing software Covidence (Veritas Health Innovation) where duplicates were removed.
openhrt-2022-002081supp001.pdf (1.1MB, pdf)
Screening of studies
Two independent reviewers (HJ, SYT) independently screened each title, abstract and full text according to the inclusion and exclusion criteria (table 1). All randomised controlled trials and prospective cohort studies including the use of extended ECG monitoring >24 hours with a minimum duration of AF of 30 s in patients with either cryptogenic strokes or transient ischaemic attacks were included. Studies published in languages other than English and studies published before January 2011 were excluded. Any discrepancies at either the screening of titles and abstract, or full-text stages were adjudicated by consensus or by consulting with a third reviewer (CY). The references of included studies were subsequently screened to identify other potential studies. The quality of studies was assessed with a tool developed specifically for studies looking at disease prevalence.11
Table 1.
Full inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
| Studies including participants with either cryptogenic stroke or TIAs | Any review papers, editorials, opinion pieces or other reviews |
| Studies including the use of any form of extended ECG monitoring >24 hours | Studies published in languages other than English |
| Studies which defined the duration of AF detected as >30 s | Studies published before January 2011 |
| RCTs and prospective cohort studies | Studies in which the minimum duration of AF was unspecified or <30 s |
AF, atrial fibrillation; RCTs, randomised controlled trials; TIA, transient ischaemic attack.
The data extracted from the papers include publication details (year of publication, study design), ECG monitoring modality used, inclusion and exclusion criteria, total number of participants, prevalence of AF detected, median age of participants, duration of monitoring, minimum duration of AF to be detected, duration after stroke for device implantation, CHA2DS2-VASc score, National Institutes of Health Stroke Scale (NIHSS) score, modified Rankin scale and mean left ventricular ejection fraction (LVEF). The data were extracted independently by two reviewers (HJ, SYT) using a standardised extraction protocol into a common data extraction sheet on Microsoft Excel, 2013 (Microsoft). Any disagreements were adjudicated by consensus or by consulting with a third reviewer (CY).
Data analysis
Data analysis was performed via R statistical software with RStudio (R V.4.1.2). As we anticipated significant between-study heterogeneity, a random-effects meta-analysis of the prevalence of AF detected was performed with inverse variance method. The measure of effect was the proportion of patients with AF. The restricted maximum likelihood estimator was used to calculate the heterogeneity variance τ2. The Knapp-Hartung adjustments were used to calculate the CIs around the pooled effect. Factors potentially contributing to the variance of the AF detection rates were evaluated by meta-regression. Heterogeneity was assessed using the I2 statistic on Cochrane’s Q statistic. I2 values of <25%, 25%–75% and >75% represent low, medium and high heterogeneity, respectively. Clinical factors potentially contributing to the variance in study results were assessed via mixed-effects meta-regression. Publication bias was assessed via visualisation of funnel plot and Egger’s regression test. The Trim and Fill method was used to determine a bias-corrected estimate of AF detection rate. Outliers and influential studies were detected via leave-one-out analysis with influence analysis and visualisation of Baujat plots. All statistical tests were two-tailed, and statistical significance was defined as p <0.05.
Patient and public involvement
Patients and the public were not involved.
Results
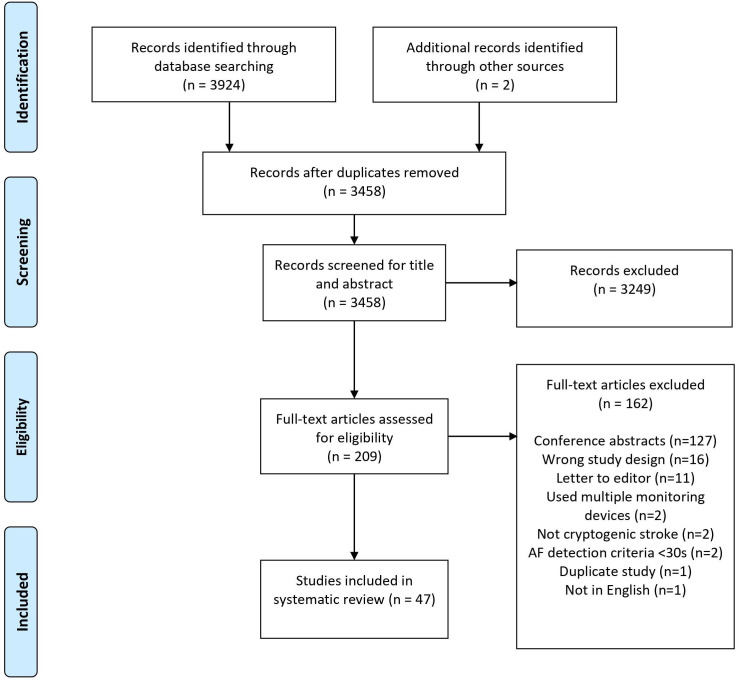
Three thousand nine hundred and twenty-four studies were identified from database searching, and two additional studies were found via screening through references. The title and abstract of 3458 studies were screened after removing 466 duplicates. Among these, 3245 studies were excluded, and 209 studies were assessed for full-text eligibility. One hundred and sixty-four studies were excluded, and 47 studies were included. Forty studies (85.1%) were classified as having low risk of bias, and seven studies (14.9%) were classified as having moderate risk of bias.12–18 The quality assessment of each study can be found in online supplemental appendix VII. The reasons for exclusion are depicted in figure 1. An overview of the general characteristics can be found in online supplemental appendix III (online supplemental table S1).
Figure 1.
PRISMA flow chart on study selection process. AF, atrial fibrillation; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
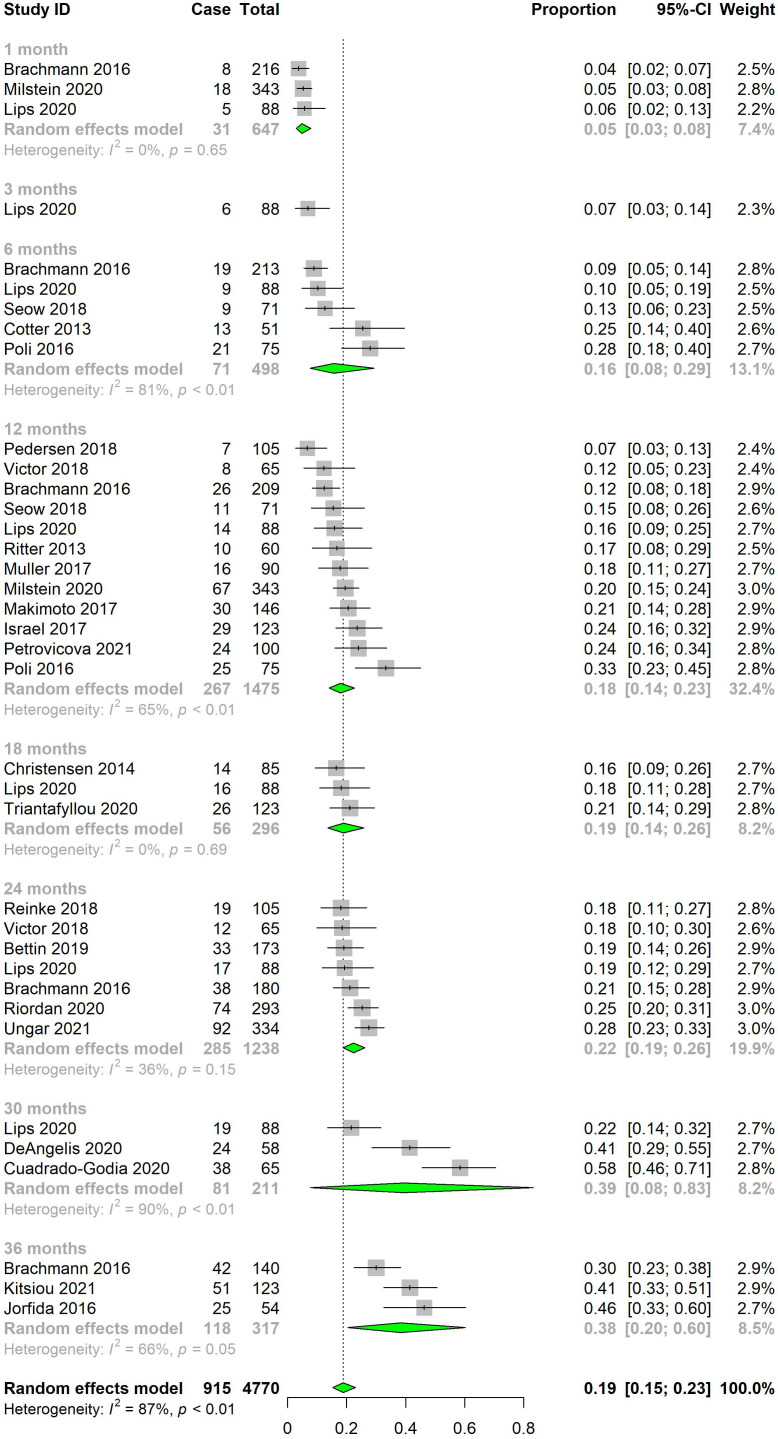
The pooled rate of AF detection by ILR monitoring was 4.9% (95% CI 3.0% to 7.9%, I2=0.0%) at 1 month, 15.7% (95% CI 7.7% to 29.3%, I2=81.3%) at 6 months, 18.0% (95% CI 14.0% to 22.7%, I2=64.9%) at 12 months, 19.0% (95% CI 13.7% to 25.8%, I2=0.0%) at 18 months, 22.3% (95% CI 18.9% to 26.1%, I2=36.0%) at 24 months, 39.4% (95% CI 7.8% to 83.3%, I2=90.2%) at 30 months and 38.4% (95% CI 20.4% to 60.2%, I2=66.2%) at 36 months (figure 2). There was a significant increase in AF detection rate with duration of monitoring (p<0.0001). An overview of the pooled AF detection rates can be found in table 2 and online supplemental figure S1, online supplemental appendix II.
Figure 2.
Pooled atrial fibrillation detection rates for implantable loop recorders by duration of monitoring.
Table 2.
Overview of pooled AF detection rates
| Subgroup | No of studies | No of patients | Pooled AF detection rate (95% CI) | Heterogeneity | ||
| Cases | Total | I2 (%) | P value | |||
| ILR monitoring | 23 | 701 | 2870 | 24.5% (20.0% to 29.6%) | 81.4 | <0.0001 |
| Duration of monitoring | ||||||
| 1 month | 3 | 31 | 647 | 4.9% (3.0% to 7.9%) | 0.0 | 0.65 |
| 3 months | 1 | 6 | 88 | 6.8% (3.0% to 14.4%) | – | – |
| 6 months | 5 | 71 | 498 | 15.7% (7.7% to 29.3%) | 81.3 | <0.0001 |
| 12 months | 12 | 267 | 1475 | 18.0% (14.0% to 22.7%) | 64.9 | <0.0001 |
| 18 months | 3 | 56 | 296 | 19.0% (13.7% to 25.8%) | 0.0 | 0.69 |
| 24 months | 7 | 285 | 1238 | 22.3% (18.9% to 26.1%) | 36.0 | 0.15 |
| 30 months | 3 | 81 | 211 | 39.4% (7.8% to 83.3%) | 90.2 | <0.0001 |
| 36 months | 3 | 118 | 317 | 0.3% (0.1% to 0.7%) | 66.2 | 0.052 |
| AF cut-off | ||||||
| 30 s | 8 | 181 | 867 | 20.8% (17.4% to 24.6%) | 23.4 | 0.24 |
| 60 s | 1 | 38 | 65 | 58.5% (46.2% to 69.7%) | – | – |
| 120 s | 13 | 457 | 1884 | 24.0% (18.3% to 30.7%) | 79.1 | <0.0001 |
| 300 s | 1 | 25 | 54 | 46.3% (33.6% to 59.5%) | – | – |
| Time of device implant from stroke/TIA | ||||||
| <7 days | 2 | 105 | 408 | 36.6% (0.0% to 100%) | 97.3 | <0.0001 |
| <28 days | 4 | 109 | 411 | 24.5% (11.4% to 45%) | 85.4 | <0.0001 |
| >28 days | 11 | 281 | 1219 | 22.5% (15.9% to 31%) | 79.4 | <0.0001 |
| MCOT monitoring | 19 | 262 | 2094 | 11.2% (8.2% to 15.1%) | 72.3 | <0.0001 |
| Duration of monitoring | ||||||
| <2 days | 1 | 4 | 72 | 5.6% (2.1% to 13.9%) | – | – |
| <14 days | 7 | 58 | 640 | 9.4% (5.7% to 15.2%) | 54.4 | 0.040 |
| <28 days | 14 | 228 | 1621 | 12.8% (8.9% to 17.9%) | 70.6 | <0.0001 |
| AF cut-off | ||||||
| 30 s | 19 | 218 | 1834 | 10.5% (7.4% to 14.6%) | 71.6 | <0.0001 |
| 60 s | 1 | 12 | 114 | 10.5% (6.1% to 17.6%) | – | – |
| 120 s | 1 | 32 | 146 | 21.9% (16.0% to 29.4%) | – | – |
| Time of device use from stroke/TIA | ||||||
| <7 days | 4 | 68 | 419 | 14.9% (6.5% to 30.8%) | 73.4 | 0.010 |
| <28 days | 4 | 22 | 343 | 7.0% (2.4% to 18.3%) | 37.5 | 0.19 |
| >28 days | 5 | 65 | 540 | 10.9% (6.2% to 18.5%) | 42.9 | 0.14 |
| Device type | ||||||
| Wireless recorder | 9 | 116 | 996 | 11.2% (8.1% to 15.3%) | 43.2 | 0.079 |
| Patch | 3 | 19 | 221 | 9.1% (3.3% to 22.6%) | 6.4 | 0.34 |
| Chest belt | 5 | 111 | 670 | 15.5% (7.0% to 30.9%) | 81.9 | 0.0002 |
| Handheld device | 3 | 31 | 272 | 8.6% (0.3% to 74.5%) | 82.4 | 0.0034 |
| Inpatient monitoring | 7 | 175 | 1484 | 12.6% (7.3% to 21.0%) | 89.2 | <0.0001 |
AF, atrial fibrillation; ILR, implantable loop recorder; MCOT, mobile cardiac outpatient telemetry; TIA, transient ischaemic attack.
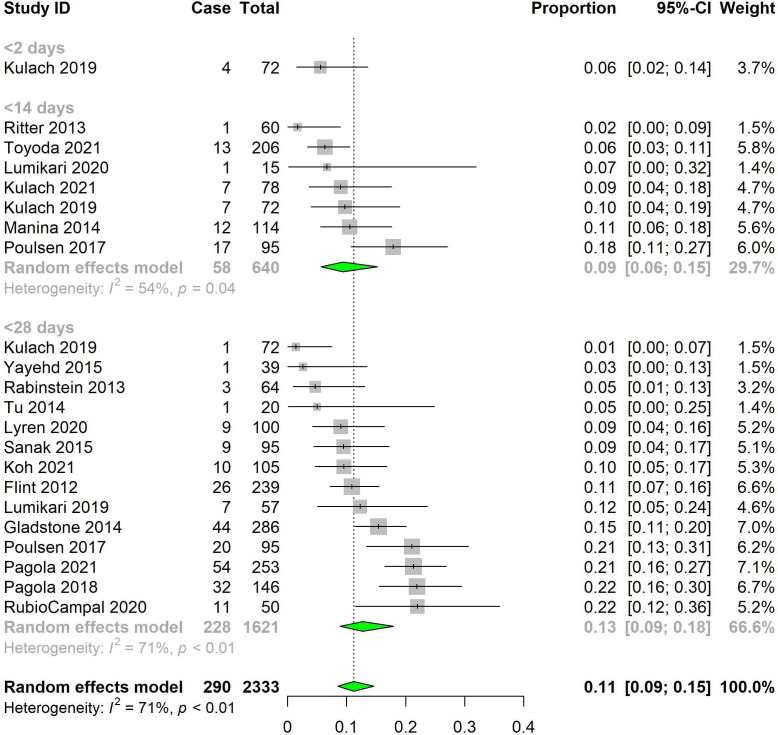
The pooled rate of AF for MCOTs was 9.4% (95% CI 5.7% to 15.2%, I2=54.4%) at <14 days and 12.8% (95% CI 8.9% to 17.9%, I2=70.6%) at <28 days (figure 3). There was no significant difference in AF detection rates with changes in duration of monitoring (p=0.31). At the same duration of monitoring (1 month), MCOTs had a significantly higher pooled AF detection rate than ILRs (12.8% vs 4.9%, p<0.0001) (online supplemental appendix III figure S2). However, the study population of MCOT had a significantly higher average age (67.3±6.4) as compared with ILRs (65.0±4.0), as well as a significantly shorter timing of device implantation after stroke (20.7±36.4 days for MCOT, 38.8±48.2 days for ILRs). The MCOT population also had a significantly lower average CHA₂DS₂-VASc score (2.2±1.2) as compared with the ILR population (3.3±1.5) (online supplemental appendix IV table S1). The pooled rate of AF for inpatient monitoring was 12.6% (95% CI 7.3% to 21.0%, I2=89.2%) (online supplemental appendix III figure S3).
Figure 3.
Pooled atrial fibrillation (AF) detection rates for mobile cardiac outpatient telemetry by AF duration of monitoring.
In terms of varying the cut-off duration, the pooled rate of AF for ILRs was 20.8% (95% CI 17.4% to 24.6%, I2=23.4%) for a cut-off of 30 s, and 23.96% (95% CI 18.3% to 30.7%, I2=79.1%) for a cut-off of 120 s (online supplemental appendix III figure S4). There was no significant difference between a cut-off of 30 s versus 120 s (p=0.18). The pooled rate of AF for MCOT was 10.5% (95% CI 7.4% to 14.6%, I2=71.6%) for a cut-off of 30 s (online supplemental appendix III figure S5). There were insufficient data for a meaningful subgroup analysis for AF cut-off duration for MCOT and inpatient monitoring.
In terms of varying the timing of device implantation, the pooled rate of AF for ILRs was 36.6% (95% CI 0.0% to 100.0%, I2=97.3%) at <7 days poststroke, 24.5% (95% CI 11.4% to 45.0%, I2=85.4%) at <28 days poststroke, 22.5% (95% CI 15.9% to 31.0%, I2=79.4%) at >28 days poststroke (online supplemental appendix III figure S6). There was no significant difference in AF detection rates between the different timings of device implantation (p=0.73). The pooled rate of AF for MCOT was 14.9% (95% CI 6.5% to 30.8%, I2=73.4%) at the timing of device implantation at <7 days poststroke, 7.0% (95% CI 2.4% to 18.3%, I2=37.5%) at <28 days poststroke and 10.9% (95% CI 6.2% to 18.5%, I2=42.9%) at >28 days poststroke (online supplemental appendix III figure S7). There was a significant increase in AF detection rates when the timing of device implantation was <7 days versus <28 days (p=0.031), but no difference in AF detection rate between a timing of implantation of <7 days and >28 days (p=0.19) or between <28 days and >28 days (p=0.22).
A wide variety of models and configurations of MCOTs were employed. Nine studies (52.9%) employed wireless recorders, five studies (29.4%) employed chest belts, three studies (17.6%) employed patches and three studies (17.6%) employed handheld devices. The pooled rate of AF was 11.2% (95% CI 8.1% to 15.1%, I2=43.2%) for wireless recorders, 9.1% (95% CI 3.3% to 22.6%, I2=6.4%) for ECG patch monitoring, 15.5% (95% CI 7.0% to 30.9%, I2=81.9%) for chest belts and 8.6% (95% CI 0.3% to 74.5%, I2=82.4%) for handheld devices (online supplemental appendix III figure S8).
At 28 days of monitoring, most studies (n=10, 71.4%) used continuous ECG monitoring, stopping only for showering or to recharge batteries. Three studies (21.4%) used twice daily ECG recordings, while one study (7.1%) used thrice daily ECG recordings. The pooled AF detection rate was 13.8% (95% CI 9.2% to 20.0%, I2=69.1%) for continuous ECG monitoring over 28 days, and 8.4% (95% CI 0.3% to 74.0%, I2=82.9%) for twice-daily ECG recordings over 28 days. There was no significant difference in AF detection rates (p=0.49) (online supplemental appendix III figure S9).
Of the 19 studies reporting on MCOTs, 13 studies (68.4%) reported on compliance.4 12 13 17 19–27 Compliance to using MCOTs was recorded as the percentage of the total ideal duration of monitoring. The mean compliance was 75.6%, with a range of 33.3%–96.4%. Five studies (26.3%) reported varying incidences of minor adverse skin reactions ranging from <1% to as high as 35%.4 21 22 25 26 No other adverse side effects were mentioned in studies on MCOTs. Of the 23 studies reporting on ILRs, 4 studies (17.4%) reported side effects.14 28–30 Side effects included infection, pocket erosion, pain and mild discomfort while wearing bras in 2%–6% of the patient population.
Meta-regression was performed and found that length of monitoring (r2=54.0%, p<0.0001) and mean age of patients (r2=39.7%, p<0.0001) were significant contributors to variations in AF detection proportion. For MCOT (r2=46.3%, p=0.021) and inpatient monitoring (r2=64.9%, p=0.029), only mean age of patients significantly contributed to variations. In particular, the mean age of patients accounted for most of the variation in AF detection proportions at 64.9%. No significant predictors were found in CHA₂DS₂-VASc, NIHSS, Rankin and LVEF scores (table 3; online supplemental appendix V figures S1–S3).
Table 3.
Univariate meta-regression analysis
| Variable | Univariate meta-regression analysis | ||||
| ECG modality | n | R2 | Coefficient (95% CI) | P value | |
| Days of monitoring | ILR | 23 | 54.0% | 0.0013 (0.0006 to 0.0020) | <0.0001 |
| MCOT | 19 | 29.3% | 0.0297 (–0.0045 to 0.0638) | 0.085 | |
| Inpatient | 7 | 0.0% | 0.0874 (–0.2226 to 0.3974) | 0.50 | |
| Age (years) | ILR | 23 | 39.7% | 0.0675 (0.0253 to 0.1098) | 0.0032 |
| MCOT | 19 | 46.3% | 0.0378 (0.0065 to 0.0692) | 0.021 | |
| Inpatient | 7 | 64.9% | 0.1037 (0.0156 to 0.1919) | 0.029 | |
| CHA₂DS₂-VASc | ILR | 21 | 3.9% | 0.1930 (–0.1311 to 0.5172) | 0.23 |
| MCOT | 11 | 2.2% | 0.2669 (–0.4402 to 0.9740) | 0.42 | |
| Inpatient | 4 | 0.0% | −0.2806 (–2.3491 to 1.7878) | 0.62 | |
| NIHSS | ILR | 9 | 25.5% | 1.8005 (–0.0525 to 0.3878) | 0.11 |
| MCOT | 9 | 23.4% | −0.0891 (–0.2424 to 0.0643) | 0.21 | |
| Inpatient | 4 | 87.4% | 0.1212 (–0.0714 to 0.3137) | 0.11 | |
| Modified Rankin scale | MCOT | 3 | 0.0% | −0.1646 (–11.4354 to 11.1061) | 0.88 |
| LVEF | ILR | 5 | 45.2% | 0.2669 (–0.1940 to 0.7278) | 0.16 |
| MCOT | 6 | 0.0% | 0.0003 (–0.0345 to 0.0351) | 0.98 | |
Values in bold are statistically significant (<0.05).
ILR, implantable loop recorder; LVEF, left ventricular ejection fraction; MCOT, mobile cardiac outpatient telemetry; NIHSS, National Institutes of Health Stroke Scale.
A few outliers were identified which may have influenced the pooled AF detection rates31–34 (online supplemental appendix VI figures S1–S9). Removal of these outliers resulted in a fall in pooled AF detection rate for ILRs to 17.7% (95% CI 14.5% to 21.3%, I2=83.4%) and for MCOT to 10.6% (95% CI 7.8% to 14.4%, I2=67.4%) (online supplemental appendix VIII table S1).
Discussion
In line with other meta-analysis looking at AF prevalence in cryptogenic stroke, the detection rate increased with increased duration of monitoring for ILRs.3 Rates of AF detection continued to increase up till 30 months of ECG monitoring, hence it is reasonable to keep patients with cryptogenic stroke on ILRs for as long as possible or until AF is detected. However, AF detection rates for MCOTs did not increase significantly with duration of monitoring. While longer duration of monitoring is likely to increase AF detection rates in MCOTs, there is a limitation in terms of the maximum duration of monitoring due to compliance issues. Given the short duration of monitoring possible with MCOTs (1 month), compared with ILRs (36 months), there is a limited range of data available to identify any statistically significant difference in AF detection rate with the duration of monitoring.
Interestingly, there is a higher AF detection rate with a cut-off value of 120 s as compared with 30 s for ILRs, although this result was not statistically significant (p=0.18). It has been postulated that the difference is due to different device capabilities in detecting AF. While the 30 s cut-off is generally determined by expert consensus, the 120 s cut-off is related to AF detection algorithms within devices.3 In our study, we chose a minimum AF duration cut-off of 30 s following the CRYSTAL-AF study.2
AF detection rates were not correlated with any patient characteristic except for age, agreeing the findings of similar studies.35 Age is the most consistent predictor of AF in studies looking at AF rates in patients with cryptogenic stroke.3 Other factors such as the CHA₂DS₂-VASc score, NIHSS, Rankin score and LVEF were not found to be correlated with AF detection rate. While other meta-analyses have found a correlation with the CHA₂DS₂-VASc, the correlation was not strong and one study did not perform multivariable analysis to look for any confounding effect of age.3 36 Furthermore, as we performed meta-regression for each ECG modality rather than for all included studies, there may not be enough studies in each subgroup to prove a relationship between CHA₂DS₂-VASc score and AF detection rate.
The higher rates of AF detection with MCOTs (12.8%) as compared with ILRs (4.9%) at 1 month were consistent with other reviews that reported an AF detection rate of 11%–14% with 1 month of MCOT monitoring3 37 and an AF detection rate of 4.1% for ILRs.3 However, the MCOT population had a significantly higher average age and shorter time to device implantation. It has also been suggested that patients with ILRs undergo a more extensive work-up for AF.3 These factors imply that the gap between MCOT and ILRs at 1 month may not be as large as predicted. Nonetheless, this still suggests a role for MCOTs given their lower cost and being less invasive. Other reviews have also supported a stepwise approach to work-up for stroke, incorporating both inpatient and outpatient modalities, as well as both MCOT and ILRs to maximise AF detection while minimising cost.38
Compliance is one of the main factors limiting the effectiveness of MCOTs.3 The use of MCOTs depends on the patients’ conscious effort to use it every day, sometimes even multiple times a day. Furthermore, some extended ECG modalities require poststroke patients with possible neurological disability to be able to place two fingers from each hand on electrodes for 30 s to record the ECG.20 Despite this, studies examining handheld ECG monitoring devices have found that such devices have good compliance,20 even better than chest belts once patients have mastered the handheld devices.27 A majority of patients reported that twice daily monitoring of handheld devices over a 4-week period is feasible.39 Nonetheless, some patients would be unable to handle the device because of cognitive or physical impairments.39 For such patients, an alternative form of extended ECG monitoring such as ILRs should be considered.
There are some MCOTs which are not validated for use in patients with cryptogenic stroke and are hence not included in this study. MyDiagnostick is a widely used handheld MCOT which has not been evaluated for use in cryptogenic stroke yet. Most of the studies regarding this device were looking at its role in AF screening in primary care, with a reported sensitivity of 87%–100% and a specificity of 85%–97%.40 41 Other models such as DigiO2 Cardio Care ECG recorder, Zenicor-EKG and CardioBip have been described for the detection of AF in other settings but not postcryptogenic stroke.5 Given the wide range of MCOTs available and the differences in usability, sensitivity and specificity, there is a need to further assess each model for cryptogenic stroke.
Outliers
Cuadrado-Godia 2020 had an AF detection rate of 58.5% using ILRs at 30 months.32 Factors potentially contributing to this unusually high rate of AF include an older mean age of participants (76.1±8.8 vs mean of 65.0±4.0), and an early implantation of <7 days after stroke.32 Kitsiou 2021 also had a high rate of AF detected at 41.4%, mainly attributable to the long duration of monitoring of 36 months.31 Among studies looking at MCOTs, Pagola 2021 was an outlier with an AF detection rate of 21.3% at 28 days of monitoring due to the large sample size of 253 participants (vs 50–146 in other studies) and high mean age of participants (74.4±9.1 vs mean of 67.3±6.4).
Strengths and limitations
This study provides the most up-to-date information regarding AF detection rates in patients with cryptogenic stroke. As far as we are aware, this is the only study providing in-depth analysis comparing MCOTs and ILRs. This study includes only peer-reviewed articles to ensure high-quality data for our analysis, with a comprehensive search strategy to ensure all relevant articles would be included. Furthermore, this study accounted for factors that were previously unaccounted for in other meta-analyses, including compliance, timing of device implantation and frequency of monitoring.3 37 42
However, while we attempted to factor in the heterogeneity via our meta-regression, there exists significant unexplained heterogeneity even within subgroups. Other similar meta-analysis have also found significant heterogeneity within the papers included.3 36 This likely stems from differences in characteristics of study participants, the extent of work-up done for diagnosis of cryptogenic stroke, and the sensitivities between various models of ECG monitoring devices. There was also poor reporting of features of the study population, such as the CHA2DS2-VASc score and other characteristics, making it difficult to perform multivariable analysis for these variables. Furthermore, publication bias analysis suggests that there is a risk underestimation of AF detection rates in MCOT studies. Hence, further study is warranted.
Conclusion
In patients with sufficient cognitive and physical ability to carry out ECG monitoring daily, a 1-month duration of MCOT can capture a significant proportion of AF and should be considered in place of ILRs. The choice of specific model should be made based on cost, patient’s preferences and clinical judgement. ILRs can be considered in patients where a prolonged duration of monitoring is anticipated, if MCOT fails to detect any AF after 4 weeks of monitoring or if there are anticipated issues with compliance. Further research is needed regarding the use of MCOT for the detection of AF in patients with cryptogenic stroke.
Footnotes
Contributors: CY conceived of the idea for the review. HJ and SYT performed study screening, data extraction and quality assessment of studies. HJ, JKW and JL performed data analysis of the studies. HJ, SYT, JKW and JL wrote the manuscript. JL, CY, TMT and VHT critically appraised the manuscript and provided feedback. All authors had full access to all the data in the study and had final responsibility in the decision to submit the manuscript for publication. CY is responsible for the overall content as guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data sharing not applicable as no datasets generated and/or analysed for this study.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1.Ornello R, Degan D, Tiseo C, et al. Distribution and temporal trends from 1993 to 2015 of ischemic stroke subtypes: a systematic review and meta-analysis. Stroke 2018;49:814–9. 10.1161/STROKEAHA.117.020031 [DOI] [PubMed] [Google Scholar]
- 2.Sanna T, Diener H-C, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–86. 10.1056/NEJMoa1313600 [DOI] [PubMed] [Google Scholar]
- 3.Noubiap JJ, Agbaedeng TA, Kamtchum-Tatuene J, et al. Rhythm monitoring strategies for atrial fibrillation detection in patients with cryptogenic stroke: a systematic review and meta-analysis. Int J Cardiol Heart Vasc 2021;34:100780. 10.1016/j.ijcha.2021.100780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gladstone DJ, Spring M, Dorian P, et al. Atrial fibrillation in patients with cryptogenic stroke. N Engl J Med 2014;370:2467–77. 10.1056/NEJMoa1311376 [DOI] [PubMed] [Google Scholar]
- 5.Hermans ANL, Gawalko M, Dohmen L, et al. Mobile health solutions for atrial fibrillation detection and management: a systematic review. Clin Res Cardiol 2022;111:479–91. 10.1007/s00392-021-01941-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kleindorfer DO, Towfighi A, Chaturvedi S, et al. 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline from the American heart association/american stroke association. Stroke 2021;52:e364–467. 10.1161/STR.0000000000000375 [DOI] [PubMed] [Google Scholar]
- 7.Seet RCS, Friedman PA, Rabinstein AA. Prolonged rhythm monitoring for the detection of occult paroxysmal atrial fibrillation in ischemic stroke of unknown cause. Circulation 2011;124:477–86. 10.1161/CIRCULATIONAHA.111.029801 [DOI] [PubMed] [Google Scholar]
- 8.Chew DS, Rennert-May E, Quinn FR, et al. Economic evaluation of extended electrocardiogram monitoring for atrial fibrillation in patients with cryptogenic stroke. Int J Stroke 2021;16:809–17. 10.1177/1747493020974561 [DOI] [PubMed] [Google Scholar]
- 9.Edwards SJ, Wakefield V, Jhita T, et al. Implantable cardiac monitors to detect atrial fibrillation after cryptogenic stroke: a systematic review and economic evaluation. Health Technol Assess 2020;24:1–184. 10.3310/hta24050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. 10.1136/bmj.b2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol 2012;65:934–9. 10.1016/j.jclinepi.2011.11.014 [DOI] [PubMed] [Google Scholar]
- 12.Kulach A, Majewski M, Gasior Z. Efficacy of 72-hour Holter monitoring, 7-day Holter monitoring and 30-day intermittent patient-activated heart rhythm recording in detecting arrhythmias in cryptogenic stroke patients. Kardiologia Polska 2019;77:24–5. 10.1515/med-2020-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lumikari TJ, Pirinen J, Putaala J, et al. Prolonged ECG with a novel recorder utilizing electrode belt and mobile device in patients with recent embolic stroke of undetermined source: a pilot study. Ann Noninvasive Electrocardiol 2020;25:e12802. 10.1111/anec.12802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen KB, Madsen C, Sandgaard NCF, et al. Subclinical atrial fibrillation in patients with recent transient ischemic attack. J Cardiovasc Electrophysiol 2018;29:707–14. 10.1111/jce.13470 [DOI] [PubMed] [Google Scholar]
- 15.Rubio Campal JM, García Torres MA, Sánchez Borque P, et al. Detecting atrial fibrillation in patients with an embolic stroke of undetermined source (from the daf-esus registry). Am J Cardiol 2020;125:409–14. 10.1016/j.amjcard.2019.10.050 [DOI] [PubMed] [Google Scholar]
- 16.Seow S-C, How A-K, Chan S-P, et al. High incidence of occult atrial fibrillation in Asian patients with cryptogenic stroke. J Stroke Cerebrovasc Dis 2018;27:2182–6. 10.1016/j.jstrokecerebrovasdis.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 17.Yayehd K, Irles D, Akret C, et al. Detection of paroxysmal atrial fibrillation by prolonged electrocardiographic recording after ischaemic stroke in patients aged<60years: a study with 21-day recording using the SpiderFlash(®) monitor. Arch Cardiovasc Dis 2015;108:189–96. 10.1016/j.acvd.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 18.Triantafyllou S, Katsanos AH, Dilaveris P, et al. Implantable cardiac monitoring in the secondary prevention of cryptogenic stroke. Ann Neurol 2020;88:946–55. 10.1002/ana.25886 [DOI] [PubMed] [Google Scholar]
- 19.Flint AC, Banki NM, Ren X, et al. Detection of paroxysmal atrial fibrillation by 30-day event monitoring in cryptogenic ischemic stroke: the stroke and monitoring for PAF in real time (smart) registry. Stroke 2012;43:2788–90. 10.1161/STROKEAHA.112.665844 [DOI] [PubMed] [Google Scholar]
- 20.Koh KT, Law WC, Zaw WM, et al. Smartphone electrocardiogram for detecting atrial fibrillation after a cerebral ischaemic event: a multicentre randomized controlled trial. Europace 2021;23:1016–23. 10.1093/europace/euab036 [DOI] [PubMed] [Google Scholar]
- 21.Lumikari TJ, Putaala J, Kerola A, et al. Continuous 4‐week ECG monitoring with adhesive electrodes reveals AF in patients with recent embolic stroke of undetermined source. Ann Noninvasive Electrocardiol 2019;24:e12649. 10.1111/anec.12649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pagola J, Juega J, Francisco-Pascual J, et al. Yield of atrial fibrillation detection with textile wearable Holter from the acute phase of stroke: pilot study of Crypto-AF registry. Int J Cardiol 2018;251:45–50. 10.1016/j.ijcard.2017.10.063 [DOI] [PubMed] [Google Scholar]
- 23.Pagola J, Juega J, Francisco-Pascual J, et al. Predicting atrial fibrillation with high risk of embolization with atrial strain and NT-proBNP. Transl Stroke Res 2021;12:735–41. 10.1007/s12975-020-00873-2 [DOI] [PubMed] [Google Scholar]
- 24.Poulsen MB, Binici Z, Dominguez H, et al. Performance of short ECG recordings twice daily to detect paroxysmal atrial fibrillation in stroke and transient ischemic attack patients. Int J Stroke 2017;12:192–6. 10.1177/1747493016669883 [DOI] [PubMed] [Google Scholar]
- 25.Miyazaki Y, Toyoda K, Iguchi Y, et al. Atrial fibrillation after ischemic stroke detected by chest Strap-Style 7-day Holter monitoring and the risk predictors: EDUCATE-ESUS. J Atheroscler Thromb 2021;28:544–54. 10.5551/jat.58420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu HT, Spence S, Kalman JM, et al. Twenty-eight day Holter monitoring is poorly tolerated and insensitive for paroxysmal atrial fibrillation detection in cryptogenic stroke. Intern Med J 2014;44:505–8. 10.1111/imj.12410 [DOI] [PubMed] [Google Scholar]
- 27.Magnusson P, Lyren A, Mattsson G. Diagnostic yield of chest and thumb ECG after cryptogenic stroke, transient ECG assessment in stroke evaluation (tease): an observational trial. BMJ Open 2020;10:e037573. 10.1136/bmjopen-2020-037573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brachmann J, Morillo CA, Sanna T, et al. Uncovering atrial fibrillation beyond short-term monitoring in cryptogenic stroke patients: three-year results from the cryptogenic stroke and underlying atrial fibrillation trial. Circ Arrhythm Electrophysiol 2016;9:e003333. 10.1161/CIRCEP.115.003333 [DOI] [PubMed] [Google Scholar]
- 29.Christensen LM, Krieger DW, Højberg S, et al. Paroxysmal atrial fibrillation occurs often in cryptogenic ischaemic stroke. final results from the surprise study. Eur J Neurol 2014;21:884–9. 10.1111/ene.12400 [DOI] [PubMed] [Google Scholar]
- 30.Ritter MA, Kochhäuser S, Duning T, et al. Occult atrial fibrillation in cryptogenic stroke: detection by 7-day electrocardiogram versus implantable cardiac monitors. Stroke 2013;44:1449–52. 10.1161/STROKEAHA.111.676189 [DOI] [PubMed] [Google Scholar]
- 31.Kitsiou A, Rogalewski A, Kalyani M, et al. Atrial fibrillation in patients with embolic stroke of undetermined source during 3 years of prolonged monitoring with an implantable loop recorder. Thromb Haemost 2021;121:826–33. 10.1055/a-1346-2899 [DOI] [PubMed] [Google Scholar]
- 32.Cuadrado-Godia E, Benito B, Ois A, et al. Ultra-early continuous cardiac monitoring improves atrial fibrillation detection and prognosis of patients with cryptogenic stroke. Eur J Neurol 2020;27:244–50. 10.1111/ene.14061 [DOI] [PubMed] [Google Scholar]
- 33.Pagola J, Juega J, Francisco J, et al. Abstract P624: high detection of atrial fibrillation by 90 days Textil Holter monitoring in patients with cryptogenic stroke. Stroke 2021;52. 10.1161/str.52.suppl_1.P624 [DOI] [Google Scholar]
- 34.Jung M, Kim J-S, Song JH, et al. Usefulness of P wave duration in embolic stroke of undetermined source. J Clin Med 2020;9:1134. 10.3390/jcm9041134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsivgoulis G, Katsanos AH, Köhrmann M, et al. Duration of implantable cardiac monitoring and detection of atrial fibrillation in ischemic stroke patients: a systematic review and meta-analysis. J Stroke 2019;21:302–11. 10.5853/jos.2019.01067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lu Y, Diao S-S, Huang S-J, et al. Insertable cardiac monitors for detection of atrial fibrillation after cryptogenic stroke: a meta-analysis. Neurol Sci 2021;42:4139–48. 10.1007/s10072-021-05104-6 [DOI] [PubMed] [Google Scholar]
- 37.Diederichsen SZ, Haugan KJ, Kronborg C, et al. Comprehensive evaluation of rhythm monitoring strategies in screening for atrial fibrillation: insights from patients at risk monitored long term with an implantable loop recorder. Circulation 2020;141:1510–22. 10.1161/CIRCULATIONAHA.119.044407 [DOI] [PubMed] [Google Scholar]
- 38.Sposato LA, Cipriano LE, Saposnik G, et al. Diagnosis of atrial fibrillation after stroke and transient ischaemic attack: a systematic review and meta-analysis. Lancet Neurol 2015;14:377–87. 10.1016/S1474-4422(15)70027-X [DOI] [PubMed] [Google Scholar]
- 39.Magnusson P, Lyren A, Mattsson G. Patient-reported feasibility of chest and thumb ECG after cryptogenic stroke in Sweden: an observational study. BMJ Open 2020;10:e037360. 10.1136/bmjopen-2020-037360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaes B, Stalpaert S, Tavernier K, et al. The diagnostic accuracy of the MyDiagnostick to detect atrial fibrillation in primary care. BMC Fam Pract 2014;15:113. 10.1186/1471-2296-15-113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yeo C, Mon AA, Tan VH, et al. Validation of MyDiagnostick tool to identify atrial fibrillation in a multi-ethnic Asian population. Singapore Med J 2022. 10.11622/smedj.2022028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramkumar S, Nerlekar N, D'Souza D, et al. Atrial fibrillation detection using single lead portable electrocardiographic monitoring: a systematic review and meta-analysis. BMJ Open 2018;8:e024178. 10.1136/bmjopen-2018-024178 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2022-002081supp001.pdf (1.1MB, pdf)
Data Availability Statement
Data sharing not applicable as no datasets generated and/or analysed for this study.