To the Editor:
Long-term ambient air pollution exposure has been associated with reduced child lung function (1). Although some studies have demonstrated that early-life exposure has a persistent effect on child lung function, others have shown that more recent exposures have a greater impact, and improvements in air quality may reverse or attenuate these effects (2–6). We previously found that living close to a major roadway and past-year and lifetime exposure to particulate matter ⩽2.5 μm in aerodynamic diameter (PM2.5) were associated with lower lung function among elementary school–aged children in the Boston area (2). In this follow-up study, we evaluate pollution exposures at different time points and associations with adolescent lung function. As PM2.5 concentrations have been reduced, we also examined if change in PM2.5 concentration during follow-up was associated with lung function growth.
Project Viva is a prospective prebirth cohort study of mother–child pairs followed from pregnancy to adolescence. We recruited 2,128 women in early pregnancy between 1999 and 2003 from Atrius Harvard Vanguard Medical Associates, a group practice in eastern Massachusetts. Children completed spirometry at a midchildhood visit (median age, 7.7 years; n = 510) and an early adolescent visit completed between 2013 and 2016 (median age, 12.8 years; n = 844). Details of the exposures, spirometry measurements, and study design have been described elsewhere (2, 7, 8). Participants’ home addresses were collected at each visit, and any changes in address were estimated to have occurred halfway between the two visits. On the basis of published findings that traffic-related pollutants decay to background concentrations exponentially with distance from a freeway (9), we calculated the natural logarithm of the distance from each participant’s home to the nearest major roadway. Annual and lifetime PM2.5 exposures were estimated using a satellite model, as previously described (2). We analyzed associations of residential proximity to a major roadway (at birth, midchildhood, and adolescence) and PM2.5 exposure (for the first year of life, the year before spirometry, average from birth to midchildhood, average since midchildhood, and lifetime at adolescence) with adolescent lung function (forced expiratory volume in 1 second [FEV1], forced vital capacity [FVC], and FEV1/FVC ratio) and lung function growth between visits. Finally, we examined if change in PM2.5 between visits was associated with change in FEV1 and FVC between visits.
All associations were analyzed using linear regression. We adjusted for child age, race or ethnicity, sex, height, household income, household smoking status, date of visit, season (as the sine and cosine of visit date), temperature and humidity the day before spirometry, and census tract medium income and education. As in our previous analysis in this cohort (2), participants with missing covariate data (n = 41 [4.9%]) were excluded. We tested for effect modification by sex, race (White vs. non-White), household income (⩽$70,000 vs. >$70,000), and current asthma.
Among participants with exposure and outcome data, 50.7% were female, 64.9% identified as White and 16.1% as Black, and 15.3% had asthma. Seventy-seven percent of households had annual incomes greater than $70,000, and 6.5% included at least one smoker. Mean FEV1 was 2.7 L (97.9% predicted; standard deviation [SD], 12.9%), and mean FVC was 3.2 L (100.9% predicted; SD, 11.9%). Mean annual increases in FEV1 and FVC between visits were 246 ml/yr (SD, 76.8 ml/yr) and 287 ml/yr (SD, 84.3 ml/yr), respectively. Median distance to a major roadway in adolescence was 1,350 m (interquartile range [IQR], 2,459 m). Median annual PM2.5 exposure was 11.2 μg/m3 (IQR, 1.5 μg/m3) for the first year of life, 9.9 μg/m3 (IQR, 1.3 μg/m3) for the year before the midchildhood visit, 7.8 μg/m3 (IQR, 0.8 μg/m3) for the year before the adolescent visit, 8.0 μg/m3 (IQR, 1.2 μg/m3) for the years between visits, and 9.3 μg/m3 (IQR, 1.1 μg/m3) for lifetime exposure up to the adolescent visit. The logarithms of distance to a major roadway and annual average PM2.5 preceding the adolescent visit were not correlated (Pearson r = −0.18).
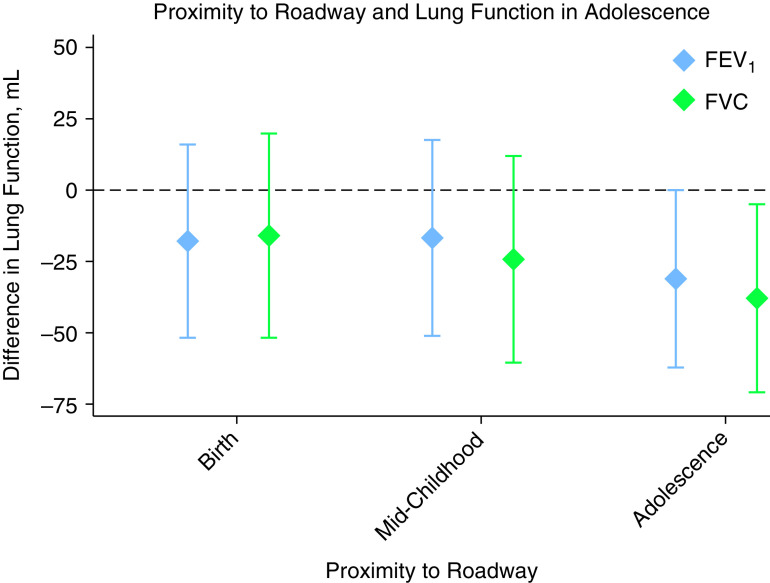
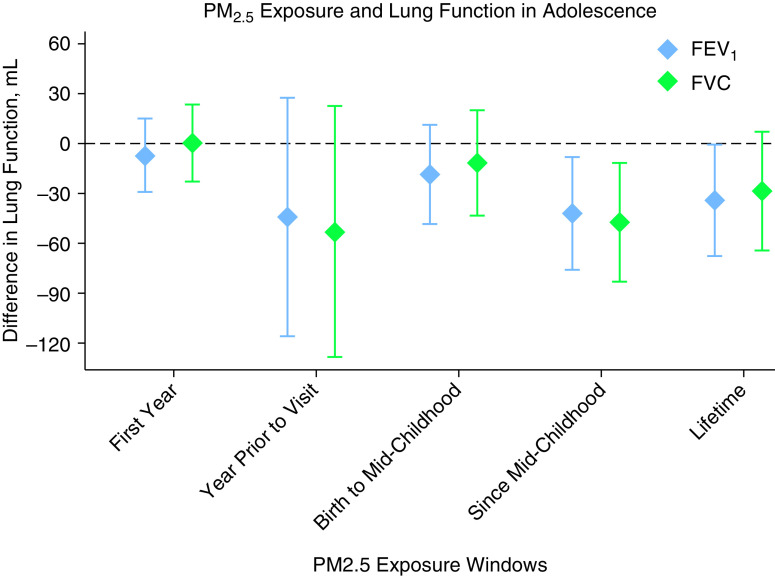
Living closer to a major roadway at the adolescent visit was associated with lower adolescent FEV1 (−31.2 ml; 95% confidence interval [CI], −62.5 to −0.01 ml) and FVC (−38.2 ml; 95% CI, −71.3 to −5.1 ml) per IQR difference in distance (log scale) (Figure 1). Lifetime PM2.5 exposure was associated with lower FEV1 (−34.2 ml; 95% CI, −67.9 to −0.5 ml), and average PM2.5 since midchildhood was associated with lower FEV1 (−42.5 ml; 95% CI, −76.4 to −8.5 ml) and FVC (−47.6 ml; 95% CI, −83.4 to −11.8 ml) per 1 μg/m3 (Figure 2). There were no associations of proximity to a roadway at birth or midchildhood, PM2.5 exposure during the first year of life, or PM2.5 exposure in the prior year with adolescent lung function. There were no associations of these exposures with subsequent change in lung function between the two visits. Reduction in average annual PM2.5 exposure between the midchildhood and early adolescent visits was associated with faster lung function growth: the mean difference in annual PM2.5 between the visits of 0.4 μg/m3 was associated with a 17.5 ml/yr (95% CI, 6.5 to 28.4 ml/yr) faster growth in FEV1 and a 14.5 ml/yr (95% CI, 2.7 to 26.3 ml/yr) faster growth in FVC.
Figure 1.
Associations of proximity to a major roadway and lung function in early adolescence. Results are scaled from the 75th to the 25th percentile of the log-transformed distance to a major roadway. All models are adjusted for child age, sex, race/ethnicity, and height; household income; household smoking; season (as sine and cosine terms); temperature and humidity the day before the spirometry examination; and census tract median household income and education. FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity.
Figure 2.
PM2.5 exposure and lung function in adolescence. Results are scaled per 1 μg/m3. All models are adjusted for child age, sex, race/ethnicity, height; household income; household smoking; season (as sine and cosine terms); temperature and humidity the day before the spirometry examination; and census tract median household income and education. FEV1 = forced expiratory volume in 1 second; FVC = forced vital capacity; PM2.5 = particulate matter ⩽2.5 μm in aerodynamic diameter.
Associations between pollution and adolescent lung function differed by sex. Living closer to a roadway and PM2.5 exposure in the prior year, since midchildhood, and lifetime were negatively associated with lung function for boys but were null among girls (Pinteraction < 0.05). Among non-White participants, living closer to a road at birth was associated with lower FEV1 in adolescence (Pinteraction < 0.05). Associations did not differ by household income or asthma.
In this follow-up study, we found that lifetime PM2.5 exposure and traffic-related pollution in adolescence were associated with lower adolescent lung function. Recent reduction in PM2.5 exposure was also associated with more rapid lung function growth during adolescence.
Consistent with our prior findings regarding midchildhood lung function, exposures occurring closer to spirometry completion were more consistently associated with lower lung function compared with exposures in early life around the time of birth. Exposures in midchildhood had similar effect estimates as adolescent exposures, indicating a possible issue with power rather than a lack of association. In our study and the Children’s Health Study in southern California, improvements in air quality were associated with greater lung function growth, suggesting at least a partial reversibility of the harmful effects of pollution and the importance of more recent exposures on adolescent lung function (3, 10). A study in the Netherlands and the Children’s Health Study both revealed decrements in FEV1 in adolescence and slower FEV1 growth in association with greater long-term PM2.5 exposure (5, 11, 12). Notably, these studies were conducted in areas with higher PM2.5 concentrations than our study. Our findings suggest that respiratory health benefits of improved air quality can be realized with small changes in PM2.5 at even lower pollution concentrations within current U.S. Environmental Protection Agency standards.
Several of the exposures in our study were associated with reduced lung function in boys, with no effect in girls. Similar effect modification for boys has been reported in other studies (3, 6), though results have been mixed and there is yet to be conclusive evidence of differing susceptibility to the detrimental effects of pollution on the basis of sex (1). There was no consistent difference in associations on the basis of race in our study.
There are several limitations to our study. Proximity to a roadway may not reflect actual pollution exposures and may incorporate nonpollution exposures such as noise and social stress. In addition, our analyses evaluating changes in lung function used only two time points for each child, and we were unable to assess trajectories in early childhood.
In conclusion, we found that more recent air pollution exposures may have a greater effect on adolescent lung function than early-life exposure, and improving air quality, even at already low air pollution concentrations, appears to be beneficial to adolescent respiratory health.
Footnotes
Supported by National Institute of Environmental Health Sciences grants K23ES026204, R01ES031252, P30ES000002, and P01-ES009825; Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R01 HD034568; National Institute of Allergy and Infectious Diseases grants R01AI102960 and UH3 OD023286; and U.S. Environmental Protection Agency grants R832416 and RD834798.
Author Contributions: S.A.M. developed the data analysis plan under the supervision of M.B.R., conducted the data analysis with assistance of M.B.R. and L.N., and wrote the first version of the manuscript. J.E.S., S.L.R.-S., H.L.-G., E.O., and D.R.G. advised on the data analysis. S.L.R.-S. assisted with creating variables for the study. E.O. and D.R.G. supervised the collection and quality control of data in the Viva Cohort study and obtained funding. All authors contributed to the interpretation of the data, revised the manuscript, and approved the final manuscript.
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Schultz ES, Litonjua AA, Melén E. Effects of long-term exposure to traffic-related air pollution on lung function in children. Curr Allergy Asthma Rep . 2017;17:41. doi: 10.1007/s11882-017-0709-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rice MB, Rifas-Shiman SL, Litonjua AA, Oken E, Gillman MW, Kloog I, et al. Lifetime exposure to ambient pollution and lung function in children. Am J Respir Crit Care Med . 2016;193:881–888. doi: 10.1164/rccm.201506-1058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, et al. Association of improved air quality with lung development in children. N Engl J Med . 2015;372:905–913. doi: 10.1056/NEJMoa1414123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gauderman WJ, Gilliland GF, Vora H, Avol E, Stram D, McConnell R, et al. Association between air pollution and lung function growth in southern California children: results from a second cohort. Am J Respir Crit Care Med . 2002;166:76–84. doi: 10.1164/rccm.2111021. [DOI] [PubMed] [Google Scholar]
- 5. Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, et al. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med . 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 6. Schultz ES, Hallberg J, Bellander T, Bergström A, Bottai M, Chiesa F, et al. Early-life exposure to traffic-related air pollution and lung function in adolescence. Am J Respir Crit Care Med . 2016;193:171–177. doi: 10.1164/rccm.201505-0928OC. [DOI] [PubMed] [Google Scholar]
- 7. Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, et al. Cohort profile: project viva. Int J Epidemiol . 2015;44:37–48. doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cardenas A, Sordillo JE, Rifas-Shiman SL, Chung W, Liang L, Coull BA, et al. The nasal methylome as a biomarker of asthma and airway inflammation in children. Nat Commun . 2019;10:3095. doi: 10.1038/s41467-019-11058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhu Y, Hinds WC, Kim S, Sioutas C. Concentration and size distribution of ultrafine particles near a major highway. J Air Waste Manag Assoc . 2002;52:1032–1042. doi: 10.1080/10473289.2002.10470842. [DOI] [PubMed] [Google Scholar]
- 10. Avol EL, Gauderman WJ, Tan SM, London SJ, Peters JM. Respiratory effects of relocating to areas of differing air pollution levels. Am J Respir Crit Care Med . 2001;164:2067–2072. doi: 10.1164/ajrccm.164.11.2102005. [DOI] [PubMed] [Google Scholar]
- 11.Gauderman WJ, Vora H, McConnell R, Berhane K, Gilliland F, Thomas D, et al. Effect of exposure to traffic on lung development from 10 to 18 years of age: a cohort study. Lancet. 2007;369:571–577. doi: 10.1016/S0140-6736(07)60037-3. [DOI] [PubMed] [Google Scholar]
- 12. Milanzi EB, Koppelman GH, Smit HA, Wijga AH, Oldenwening M, Vonk JM, et al. Air pollution exposure and lung function until age 16 years: the PIAMA birth cohort study. Eur Respir J . 2018;52:1800218. doi: 10.1183/13993003.00218-2018. [DOI] [PubMed] [Google Scholar]