Abstract
Ustilago maydis, the causal agent of corn smut disease, acquires and transports ferric ion by producing the extracellular, cyclic peptide, hydroxamate siderophores ferrichrome and ferrichrome A. Ferrichrome biosynthesis likely proceeds by hydroxylation and acetylation of l-ornithine, and later steps likely involve covalently bound thioester intermediates on a multimodular, nonribosomal peptide synthetase. sid1 encodes l-ornithine N5-oxygenase, which catalyzes hydroxylation of l-ornithine, the first committed step of ferrichrome and ferrichrome A biosynthesis in U. maydis. In this report we characterize sid2, another biosynthetic gene in the pathway, by gene complementation, gene replacement, DNA sequence, and Northern hybridization analysis. Nucleotide sequencing has revealed that sid2 is located 3.7 kb upstream of sid1 and encodes an intronless polypeptide of 3,947 amino acids with three iterated modules of an approximate length of 1,000 amino acids each. Multiple motifs characteristic of the nonribosomal peptide synthetase protein family were identified in each module. A corresponding iron-regulated sid2 transcript of 11 kb was detected by Northern hybridization analysis. By contrast, constitutive accumulation of this large transcript was observed in a mutant carrying a disruption of urbs1, a zinc finger, GATA family transcription factor previously shown to regulate siderophore biosynthesis in Ustilago. Multiple GATA motifs are present in the intergenic region between sid1 and sid2, suggesting bidirectional transcription regulation by urbs1 of this pathway. Indeed, mutation of two of these motifs, known to be important to regulation of sid1, altered the differential regulation of sid2 by iron.
Iron is an essential trace element that plays a key role in many cellular functions, such as respiration, DNA synthesis and damage repair, and detoxification of free radicals (13, 14). Despite iron being one of the most abundant elements on earth, its availability is largely limited to living organisms due to its extreme insolubility at physiological pH (19). Many specialized systems have evolved in microorganisms to efficiently assimilate iron from the environment. One such mechanism is the production and secretion of a family of low-molecular-weight, ferric ion-chelating compounds, termed siderophores, in response to iron starvation (27, 29, 30). Various studies have demonstrated that the production of siderophores is negatively regulated by iron (9).
Ustilago maydis, the causal agent of common smut of corn, is a basidiomycete belonging to the order Ustilaginales. In response to iron starvation, U. maydis produces two cyclic, hexapeptide, hydroxamate siderophores, ferrichrome and ferrichrome A (Fig. 1) (10). Two ferrichrome biosynthesis-related genes, sid1 and urbs1, encoding l-ornithine N5-oxygenase and a GATA family transcription factor, respectively, have been previously identified and characterized. sid1 is responsible for the first committed step, the hydroxylation of l-ornithine, in ferrichrome and ferrichrome A biosynthesis (28, 42). Northern hybridization analysis has shown that the accumulation of sid1 transcript is negatively affected by iron concentration in the growth medium (28). Furthermore, two lines of evidence suggest that this regulation requires the presence of both a functional copy of the urbs1 gene and cis elements upstream of the sid1 promoter. Disruption of urbs1, a zinc finger GATA family transcription factor, leads to constitutive expression of sid1 and unregulated production of the ferrichromes (2). A similar deregulated phenotype can be generated by mutating the two GATA motifs 2.5 kb upstream of the sid1 translation start site, suggesting that urbs1 may directly interact with the sid1 promoter to repress sid1 expression when sufficient iron is present in the growth medium (2). In vitro, Urbs1 protein has been shown to specifically interact with one of the GATA motifs (2).
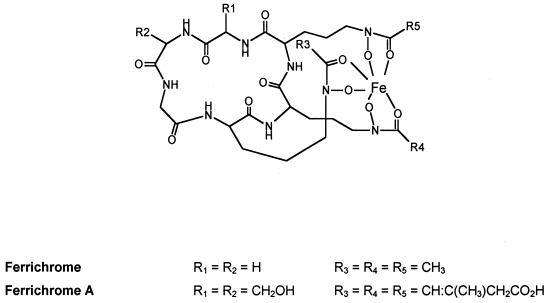
FIG. 1.
Structure of ferrichrome and ferrichrome A. Both siderophores have a hexapeptide ring containing three residues of δ-N-acyl-N-hydroxy-ornithine and a tripeptide of neutral amino acids consisting of three glycine residues in ferrichrome or one glycine and two serine residues in ferrichrome A.
The hexapeptide ring structure of ferrichrome and ferrichrome A contains three δ-N-acyl-N-hydroxy-ornithine and three neutral amino acid residues consisting of glycines in ferrichrome and one glycine and two serines in ferrichrome A (10) (Fig. 1). Aside from the initial hydroxylation of l-ornithine, which is catalyzed by sid1, other steps of siderophore biosynthesis at the molecular level are poorly understood (31). Based on biochemical studies of several microorganisms, these steps have been assumed to proceed via thioester intermediates on a multimodular nonribosomal peptide synthetase complex. This assumption has been confirmed in Aspergillus quadricintus by purification of ferrichrome synthetase and its subsequent characterization as a peptide synthetase consisting of repeated subunits (24). A phosphopantetheine cofactor was found to covalently link the substrate to each of the subunits, suggesting a thiotemplate mechanism for ferrichrome biosynthesis.
In this report, we describe the identification and characterization of the second biosynthetic gene, sid2, which encodes a nonribosomal peptide synthetase that is located in the ferrichrome biosynthetic gene cluster in U. maydis. The analysis of sid2 regulation by iron indicates a common mechanism of regulation exists for the divergently transcribed sid1 and sid2 genes.
MATERIALS AND METHODS
Media and strains.
Minimal, complete, and plate media used in these studies were adapted from Holliday (22) as previously described (41). Low-iron medium, E medium, and EM medium were previously described (42). Escherichia coli DH5alpha (Bethesda Research Laboratories, Bethesda, Md.) was used for all DNA manipulations. The Salmonella enterica serovar Typhimurium LT-2 mutant, enb-7, was a gift from J. B. Neilands (University of California, Berkeley). The U. maydis strains used in this study are listed in Table 1.
TABLE 1.
List of U. maydis strains
| Strain | Relevant characteristics | Source or reference |
|---|---|---|
| 518 | Wild type; a2 b2 | R. Holliday |
| 521 | Wild type; a1 b1 | R. Holliday |
| 288 | a1 b1 pan1-1 inos-3 nar1-1 rec1-1 | R. Holliday |
| 227 | a2 b2 ade1-1 met1-2 nar1-6 rec2-1 | R. Holliday |
| S1 | NTG mutant from 518; Fc−a2 b2 | 42 |
| UMS049 | Derived from cross between S1 and 521; Fc−a2 b2 | This work |
| UMS053 | Derived from cross between UMS049 and 288 | This work |
| UMS062 | Derived from cross between S1 and 521; Fc−a2 b2 | This work |
| UMSD05 | Diploid UMS053 × 227 | This work |
| SHr008 | Hygr Fc− FcA− | 28 |
| UMS2rH1 | Fc−a2 b2 sid2::Hygr (Hyg cassette in the 4.2-kb KpnI fragment) | This work |
| UMS2rH2 | Fc−a2 b2 sid2::Hygr (Hyg cassette in the 4.2-kb KpnI fragment) | This work |
| UMS2rH3 | Fc−a2 b2 sid2::Hygr (Hyg cassette in the 4.2-kb KpnI fragment) | This work |
| UMS2rH5 | Fc−a2 b2 sid2::Hygr (Hyg cassette in the 4.8-kb KpnI fragment) | This work |
| UMS2rH7 | Fc−a2 b2 sid2::Hygr (Hyg cassette in the 4.8-kb KpnI fragment) | This work |
| UMCO15 | a2 b2 urbs1::phleor | 2 |
| UMS204 | a2 b2 sid1− 204::GUS phleor | 2 |
Genetic manipulations.
Genetic crosses and construction of a diploid strain were done as described previously (26).
Assay for siderophores.
Production of ferrichrome by U. maydis colonies was routinely assessed by the ability of colonies to cross feed the Salmonella serovar Typhimurium LT-2 mutant, as described by Wang (42). To confirm the production of ferrichrome, an XAD-4 resin-based extraction procedure was used. Briefly, a column was loaded with saturated XAD-4 resin (Sigma, St. Louis, Mo.), which was then washed with distilled H2O, methanol, and distilled H2O again. The iron complexes of siderophores in the culture supernatant were formed by the addition of 2% aqueous FeCl3. The ferric complexes of siderophores were loaded onto the column and subsequently eluted with methanol (G. Winkelmann, personal communication). The volume of eluant was reduced by rotary evaporation and then analyzed by thin-layer chromatography with authentic standards (10).
DNA procedures.
Plasmid DNA was purified from E. coli by the boiling minipreparation protocol (18, 23) or by using a Qiagen midi-plasmid extraction kit (Qiagen, Valencia, Calif.). U. maydis genomic DNA was isolated by the glass bead method (26). Digestion of DNA and analysis of fragments were done by standard methods (6, 32). For subcloning, DNA fragments were isolated from SeaKem LE gels by running the DNA fragments through 3M filter paper, which was then centrifuged to extract DNA (6), or by using a QIAquick gel-extraction kit. Fragments to be labeled with 32P were isolated with a Gene Clean kit (Bio 101, Inc., La Jolla, Calif.) or with a QIAquick gel-extraction kit. DNA fragments were labeled with a random oligonucleotide kit (Pharmarcia, Piscataway, N.J.) or with a nick translation kit (Bethesda Research Laboratories). Transformation of E. coli, Southern hybridization, and colony blotting were done as described previously (28, 32). Vectors for subcloning were pHL1 (41), pJW42, pANUMV1 (1), pANUMV2 (1), pUC18, and pBluescript II KS(+) (Stratagene, La Jolla, Calif.). For complementation and gene replacement in U. maydis, self-replicative (pJW42, pANUMVII) and integrative (pHL1, pANUMVI) vectors were used. These vectors were also used to prepare genomic libraries of U. maydis wild-type strain 518. DNA transformation of U. maydis was carried out as described by Wang et al. (41) as modified by Voisard et al. (40).
DNA sequencing and sequence analysis.
Nucleotide sequencing of double-stranded DNA fragments cloned in pANUMVI, pUC18, and pBluescript II KS(+) was done by the dideoxy method using the Sequenase kit (U.S. Biochemicals, Cleveland, Ohio) and by automated cycling sequencing using the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction kit with AmpliTaq DNA polymerase FS (Perkin-Elmer, Foster City, Calif.). Both universal primers T7 and T3 and oligonucleotide primers designed from previously determined sequences and synthesized by the Applied Biosystems model 392 DNA/RNA synthesizer were used in sequencing. For cycle sequencing, we used the Perkin-Elmer model 480 DNA Thermal Cycler following the ABI PRISM Ready Reaction kit protocol, with the exception that the number of cycles was increased from 25 to 30. The initial sequence analysis was done using DNALysis (version 0.9) software. The sequence reads generated from automated sequencing reactions were also submitted for similarity searches to the BLAST (Best Local Alignment Search Tool) web server maintained at the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/BLAST). A more detailed analysis was carried out using the GCG package (Genetics Computer Group, Madison, Wis.).
RNA isolation and Northern hybridization analysis.
U. maydis total RNA was extracted by first grinding cell pellets in liquid nitrogen with a mortar and pestle. Total RNA was extracted from the cell powder by using the RNeasy Max kit (Qiagen). From 2 g (wet weight) of cells, 2 to 4 mg of total RNA can typically be obtained using this procedure. For Northern hybridization analysis, RNA samples were denatured with formaldehyde and fractionated by electrophoresis in a Reliant RNA gel system (FMC Bioproducts, Rockland, Maine). RNA was transferred to Hybond-XL nylon membrane (Amersham Pharmacia Biotech, Amersham, United Kingdom) and then hybridized and washed under stringent conditions (28, 32).
Primer extension mapping and cloning the 5′ end of sid2 mRNA.
Primer extension with primers 1, 2, and 3 (see Fig. 4) was performed as described previously (28) on total RNA extracted from U. maydis cells grown in low (<5 μM Fe detected) or high (∼35 μM Fe) iron media. The 5′ end of the sid2 gene was cloned using a 5′ RACE (rapid amplification of cDNA ends) strategy (16, 17). The total RNA isolated from cells grown under the low iron condition was directly reverse transcribed using the sid2 internal primer 4 (see Fig. 4). The 5′ RACE system (GIBCO BRL/Life Technologies, Grand Island, Md.) was then used for both single-stranded cDNA isolation and tailing with poly(A) and a subsequent second-strand synthesis. The double-stranded cDNA was amplified by PCR using the adaptor from the deoxythymidine primer-adaptor and internal primer 5 (see Fig. 4). The PCR conditions were chosen as described in the GIBCO RACE system manual. The PCR products were purified using QIAquick PCR Purification kits (Qiagen) and subcloned in a pBluescript II KS(+) vector at the SalI and SacI sites.
FIG. 4.
Nucleotide and deduced amino acid sequence of sid2, including 5′ and 3′ untranslated regions. The number, which refers to the nucleotide sequence, is based on the deduced ORF of sid2 from +1 at the adenine of the translational initiation codon (ATG). The transcription start sites are marked in bold. Primers used for primer extension and 5′ RACE are indicated as arrows with the respective primer number. Some putative transcription binding sites, including GATA boxes, are underlined.
RESULTS
Genetic analysis of the sid2 mutant.
Mutagenesis of U. maydis with nitrosoguanidine treatment previously led to the isolation of several classes of siderophore auxotrophic mutants (42). The ferrichrome− (Fc−) phenotype was assigned to the mutant S1, a derivative of strain 518 (a2b2), because no ferrichrome was detected by high-pressure liquid chromatography in the culture supernatants when the mutant was grown in low iron medium for at least 4 days (42). To determine the genetic basis of the siderophore-nonproducing phenotype, S1 was independently crossed with the compatible wild-type strain 521 (a1b1) and the auxotrophic mutant 288 (a1b1 pan1-1 inos1-3 nar1-1 rec 1-1) and siderophore production of the basidiospore segregants was examined by assessing their ability to support the growth of the enterobactin-deficient Salmonella serovar Typhimurium LT2 strain enb-7 on E medium. The segregation ratio of 1:1 for the siderophore mutation versus the wild-type allele suggested that the Fc− phenotype is controlled by a single gene lesion (data not shown).
Fc− strain UMS049 was isolated after three backcrosses of strain S1 with strain 521. Fc− strain UMS062 was isolated after two additional backcrosses with strain 521. Diploid cells (UMD05) were constructed by fusing an Fc− progeny (UMS053) obtained from a cross between UMS049 and strain 288 with strain 227 (a2b2 ade1-1 met1-2 nar1-6 rec2-1). After purification on minimal medium, the resultant diploids were tested for siderophore production by using the Salmonella serovar Typhimurium LT2 bioassay and by high-pressure liquid chromatography. All diploids tested exhibited wild-type ferrichrome production, suggesting that the mutation in S1 and in its backcross derivatives is recessive and that the wild-type allele is dominant. The sid2 genotype was assigned to this series of Fc− mutants.
Genetic analysis of the linkage between sid2 and sid1 and location of sid2.
We investigated possible linkage between sid2 and sid1 by crossing strain UMS062 with strain SHr008, a mutant whose sid1 gene has been disrupted by a gene cassette conferring resistance to hygromycin (28). Both strains are Fc−, and a ratio of 1:3 for segregation of the Fc− phenotype versus the wild-type phenotype was expected if sid1 and sid2 were not genetically linked. Only 14 of 200 progenies tested were Fc+, and they were all Hygs, suggesting a tight linkage. We therefore investigated the possibility of complementing the sid2 mutation with cosmid clones containing sid1. This strategy led to the successful cloning of the a mating-type locus of U. maydis, which is tightly linked to pan-1 (5.3% recombination). These two loci were only a few kilobases apart on the same cosmid clone (15).
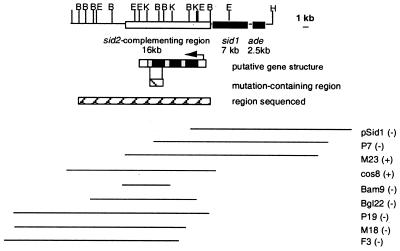
We first tested the cosmids from which we had originally isolated sid1 (42) and found that these did not complement sid2. Overlapping cosmids were identified by using the ends of the pSid1 cosmid insert as probes to a genomic library generated in replicative vector pJW42 with DNA extracted from wild-type strain 518. Selected cosmids were used to transform sid2− UMS062, and transformants were analyzed for siderophore production by using the Salmonella serovar Typhimurium LT2 bioassay and chemical analyses. Cosmid 8 was able to restore ferrichrome production in all transformants, suggesting that the mutated gene sid2− had been complemented in trans. Cosmid 8 was partially mapped but the ends of the gene could not be defined. Another genomic DNA library of 518 was prepared in the improved replicative vector pANUMV2, which contains NotI sites flanking the cloning site (1). Additional cosmid clones were identified by colony hybridization and allowed us to place the gene within a 16-kb region (Fig. 2).
FIG. 2.
The ferrichrome biosynthesis gene cluster of U. maydis. The cosmids used to localize the sid2-complementing region by transformation of the ferrichrome mutant are at the bottom. The hatched-box region contains the mutation. Sequence analysis demonstrates that sid2 shares homology with members of the peptide synthetase protein family. The pSid1 DNA insert is in pJW42. All other inserts are in pANUMV2. trans complementation results are shown in parentheses: (+), complementation; (−), no complementation. Only BamHI (B), EcoRV (E), and KpnI (K) sites are indicated on the restriction map. One end of the map is defined by a HindIII site (H).
Subclones of cosmid 8 were generated in the integrative vector pANUMV1 (1) to define the minimal region required for complementation in cis of the mutation harbored in sid2 by UMS062. DNA preparations of the subclones were digested with various restriction enzymes to release different-sized inserts from the cosmid vector. UMS062 was then cotransformed with the vector, which carries a hygromycin selectable marker and the released insert DNA. Transformants were screened for ferrichrome production.
Molecular analyses of ferrichrome-positive transformants showed that a 23-kb BglII insert restored production of ferrichrome in mutant UMS062. Two deletion derivatives of the BglII subclone and an overlapping 9-kb BamHI insert also restored the production of ferrichrome by homologous recombination. The minimum region capable of restoring ferrichrome production by homologous integration was localized to a 1.8-kb BamHI-KpnI fragment (Fig. 2).
Gene disruption.
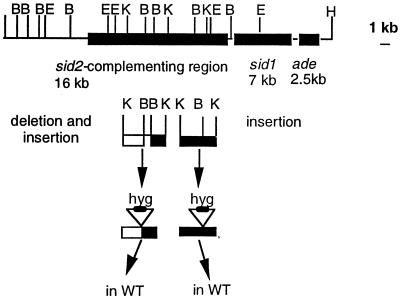
Two constructs were devised to demonstrate the isolation of sid2 by gene disruption. The 4.5- and 4.8-kb KpnI fragments of cosmid 8 were subcloned in pBS′, a pBluescript II KS(+) derivative in which the BamHI restriction site has been destroyed by Klenow fill-in. A BamHI digest released the 0.7-kb BamHI fragment contained in the 4.8-kb KpnI fragment, and a XbaI-HindIII fragment of pHLI carrying the hygromycin B resistance gene was inserted in place of the missing BamHI fragment, yielding a 6.4-kb KpnI fragment (Fig. 3) in the resulting subclone pKΔB::Hyg. Similarly, we inserted the hygromycin B resistance cassette into the unique BamHI site of the 4.2-kb KpnI fragment harbored in pBS′, thus obtaining a 6.5-kb KpnI fragment giving rise to the resulting subclone pK::Hyg. On the basis of the restriction map of the complementing DNA, this deletion and these insertions were expected to disrupt the putative sid2 gene. Each of the two altered KpnI fragments was transformed into wild-type strain 518.
FIG. 3.
Gene disruption of sid2 in U. maydis. A hygromycin B resistance cassette was either inserted directly at the BamHI site in the 4.2-kb KpnI fragment (right-hand side) or was used to replace a 1-kb BamHI in the 4.8-kb KpnI fragment (left-hand side).
Seven hygromycin B-resistant transformants were obtained with the 6.4-kb KpnI fragment; three of them failed to feed the Salmonella serovar Typhimurium LT-2 mutant enb-7, whereas the other four displayed a wild-type phenotype for ferrichrome production. With the other construct, pK::Hyg, more than 200 hygromycin B-resistant transformants were obtained; 87 were tested for ferrichrome production using serovar Typhimurium LT-2 bioassay, and only 3 of 87 had a wild-type phenotype. Southern hybridization analysis of KpnI-digested genomic DNA of a selected set of the potentially disrupted transformants with the 6.4-kb KpnI fragment and the 6.5-kb KpnI fragment as probes showed the correct exchange of the wild-type fragment with the mutated allele containing the hygromycin B resistance cassette (data not shown).
Genetic analysis of the disrupted mutants.
Both sid2 gene-disruption mutants were crossed with the compatible U. maydis prototrophic wild-type isolate 521 (UM002, a1b1). Basidiospore segregants were examined for ferrichrome production by assessing their ability to support the growth of serovar Typhimurium LT2 enb-7 on E medium, and the segregation patterns (Table 2) showed a 1:1 ratio of Fc+ and Fc− as would be expected for the segregation of alternative alleles of a single locus. Hygromycin B resistance cosegregated with the absence of ferrichrome biosynthesis. Southern hybridization analysis of genomic DNA from five segregants chosen from each cross confirmed that the mutant phenotype cosegregated with the expected restriction fragment polymorphisms (data not shown).
TABLE 2.
Segregation of the hygromycin B resistance gene inserted into sid2 and ferrichrome production
| Cross | Ratio of Fc−/Fc+ segregantsa | Ratio of Hygr/Hygs segregants |
|---|---|---|
| 518 × 521 | 0/100 | 0/100 |
| UMS2rH2 × 521 | 46/54b | 46/54b |
| UMS2rH5 × 521 | 41/59b | 41/59b |
Determined by bioassay with Salmonella serovar Typhimurium LT2 enb-7.
P > 0.05 for chi-square analysis of a 1:1 segregation model.
Mapping and cloning of the mRNA 5′ ends.
The transcription initiation sites of sid2 were defined by primer extension with primers 1, 2, and 3 (Fig. 4). The sid2 transcripts were shown to initiate at two different sites, −84 bp and −246 bp, upstream of the putative ATG start codon (Fig. 4). Interestingly, when total RNA extracted from iron-replete and starved cells was used, elevated levels of the extension product were observed from the iron-starved cells, suggesting that sid2 transcription is iron regulated (data not shown). A 5′ RACE strategy was used to confirm the results obtained from the primer extension experiments and to check for the presence of introns at the mRNA 5′ ends. To clone the 5′ end of sid2 mRNA, the cDNAs obtained from reverse transcription with primer 4 (Fig. 4) were amplified by PCR and subcloned into a pBluescript vector. Positive clones were identified by colony hybridization with a sid2 DNA probe, and 12 of those clones were chosen for sequencing. Sequencing results showed that no introns were present at the 5′ end of the sid2 mRNA, as the DNA sequence of the cDNAs was identical to the corresponding regions of the genomic DNA fragment. Consistent with the primer extension data, there were two transcript sizes which differed by 162 bp in length at the 5′ end in the cDNA population, suggesting that sid2 transcription initiation sites were heterogeneous.
Nucleotide sequence and predicted protein structure of sid2.
The DNA sequence of both strands of the 13.8-kb genomic DNA region corresponding to the sid2 gene and the deduced amino acid sequence are shown in Fig. 4 (GenBank accession no. UMU62738). DNA sequence inspection revealed a single open reading frame (ORF) with a length of 11,841 bp which we have designated sid2. The putative translation initiation site (CAACATGTCCG) matches the consensus sequence [CCA(C/A)(C/A)ATGGC] for a fungal translation initiation site. The two most conserved nucleotides, an A at position −3 and a C at position +5 (underlined in the sequences above), relative to the ATG start codon (in bold) are present in the sid2 sequence.
The CodonUsage program (CodonUse 3.5, Peter A. Stockwell, University of Otago, Box 56, Dunedin, New Zealand) was used to determine the coding preference of the deduced sid2 ORF. Previously sequenced genes from U. maydis were used to compile a codon frequency table as a parameter to CodonUsage (Z. An and S. A. Leong, unpublished result). In this analysis, it was shown that the ORF exhibited U. maydis codon bias throughout the 11,841 bp (data not shown) and no evidence was found to support the presence of introns in the sid2 gene. This coding potential was further confirmed with a second program, TestCode, which is part of the GCG (Genetics Computer Group, Madison, Wis.) sequence analysis package (data not shown). The putative sid2 polypeptide contains 3,947 amino acid residues, with a predicted molecular mass of 432,650 Da and an isoelectric point of 6.42.
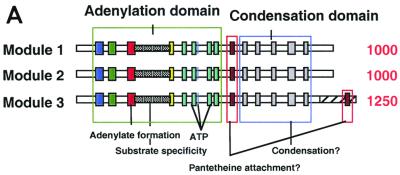
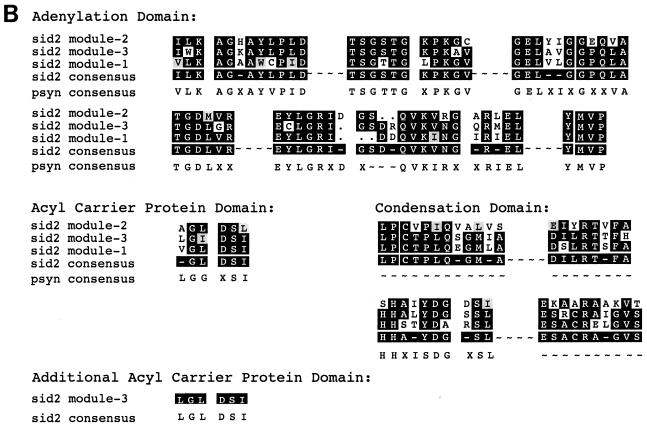
A database search using the deduced amino acid sequence of sid2 led to the identification of multiple motifs which are characteristic of the nonribosomal peptide synthetase family. The number of appearances and the distribution of the motifs in the putative protein sequence led to the prediction that sid2 contains three similar modules of 1,000 amino acids each. To confirm this prediction, the first 1,500 amino acids of sid2 were used to compare with the following residues by using the Compare program (GCG). The results showed that ∼1,000 amino acids in the query sequence shared similarity with two distinct segments of approximately equivalent length in the remainder of the protein. The three segments, termed modules 1, 2, and 3, were graphically represented using the DotPlot program from GCG (data not shown). Pair-wise alignments between the three modules were carried out using the GCG programs BestFit and Gap. The three domains, known as adenylation, acyl carrier protein (ACP), and condensation domains, within each module were delineated (Fig. 5A). The core motifs in the modules were further demonstrated by their alignment using PileUp (GCG) and comparison to the compiled peptide synthetase consensus sequence (Fig. 5B). Interestingly, an additional ACP motif was identified at the end of the last module (Fig. 5A and B). This motif has a sequence (QLGLDS∗ISAIR) that is almost identical to the default motif (RLGIDS∗ISAIR). Blast searches of each ACP domain identified by pfam (Protein Families Database) (www.ebi.ac.uk) revealed similarity to both ACP domains found in polyketide synthetases and the peptidyl carrier protein domains found in nonribosomal peptide synthetases. In all cases, the majority of matches found among the top 10 were with the peptidyl carrier protein domain.
FIG. 5.
Multimodular structure of sid2 and multiple sequence alignment of key motifs in the modules. (A) A schematic representation of the highly conserved and ordered modular organization of the sid2 gene. (B) Sequence alignment of the modules. Conserved motifs are shaded and grouped according to the domain they reside in. The sequences were aligned using the PileUp program in the GCG package, with a gap penalty of 8 and a gap extension penalty of 2. The consensus sequence of the alignment was further calculated using Pretty in GCG.
Transcriptional regulation of the sid1 and sid2 ferrichrome biosynthetic gene cluster in U. maydis.
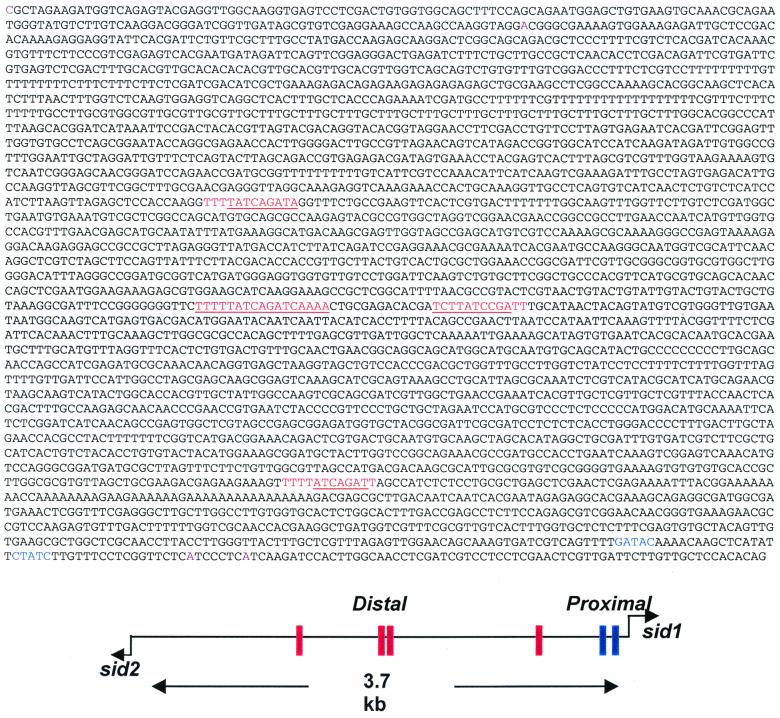
The two ferrichrome biosynthetic genes, sid1 and sid2, are divergently transcribed from a 3.7-kb intergenic region (Fig. 6). Like sid1, the 1.6 kb of the 5′ nontranscribed region of sid2 does not contain an apparent TATA box, but an array of putative transcription factor binding sites is present (Find Pattern Analysis) (data not shown) (Fig. 4). Multiple GATA motifs are present in the 3.7-kb intergenic region, including a 12-bp palindromic motif (Fig. 6) which is present four times. The two palindromic GATA motifs in the middle have been previously shown to be essential for urbs1-mediated, iron-responsive regulation of the sid1 gene, as either deletion or replacement of these motifs led a constitutive expression of sid1 (3). A purine track between −506 and −476 bp (Fig. 6) may contribute to the initiation of transcription, as is the case for the other genes in filamentous fungi (21).
FIG. 6.
sid1/sid2 intergenic region. Conserved 12-bp GATA motif repeats are in red. The palindromic sequence within the repeat unit is underlined. The upstream “proximal” GATA (2, 28) in sid1 are in blue. The transcription start sites of sid1 and sid2 are indicated by dark rose coloring.
Regulation of sid2 by iron.
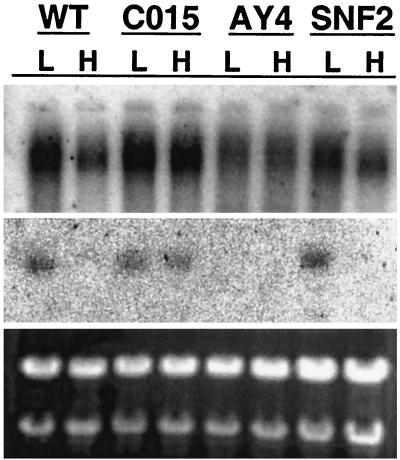
To determine whether iron affects the accumulation of sid2 mRNA, Northern hybridization analysis of total RNA extracted from wild-type and three mutant strains grown in low-iron medium with or without 40uM FeSO4 was performed (Fig. 7). A higher level (2.5-fold) of the 11-kb transcript was observed in wild-type cells grown in low-iron medium than in cells grown in the presence of iron. By contrast, approximately the same levels of the 2.3-kb transcript were observed in CO15 (2) (urbs1) and S204 (5′ 3′ distal GATA mutant AY4) (2) cells grown in either low- or high-iron conditions. A truncated transcript was detected in total RNA isolated from the sid2 mutant, and the accumulation pattern of this truncated transcript was similar to that of the wild type (data not shown).
FIG. 7.
Northern hybridization analysis of sid2 total RNA. Total RNA (0.5 μg/well) was denatured in formamide and electrophoresed on a 1.2% SeaKem gold agarose gel in MOPS (morpholinepropanesulfonic acid) buffer with no denaturants. Ribosomal RNAs stained with ethidium bromide are shown in the lower panel to show equivalence of RNA loading. The RNA was transferred to nylon and hybridized sequentially with the sid1 (middle panel) or sid2 (upper panel) genomic DNA probes. Shown are RNA samples from L (low iron grown) and H (high iron grown): WT, wild type; CO15, urbs1 disruption mutant; AY4, sid1 deletion mutant UMS204; SNF2, umsnf2 disruption mutant of U. maydis.
DISCUSSION
Following the initial hydroxylation of ornithine, ferrichrome biosynthesis has been postulated to proceed via covalently bound thioester intermediates on a multifunctional enzyme complex (33). This mechanism has been supported by biochemical data where hydroxyornithine-ATP-PPi exchange activity was observed in extracts prepared from ornithine hydroxamate siderophore-producing fungi (4, 5). The genetic and molecular data presented herein indicate that sid2 is essential for ferrichrome biosynthesis. sid2 encodes a putative multifunctional nonribosomal peptide synthetase of a predicted molecular mass of 432,650 Da. As expected, the transcription accumulation of sid2 in the cell is regulated by iron.
Based on several lines of evidence, sid2 encodes a single genomic 11.8-kb ORF with no apparent introns. First, codon preference analysis showed a high codon potential throughout the entire 11.8-kb ORF. Second, the sequence of the cDNA which covers 800 bp of the 5′ end of the sid2 gene was identical to that of the corresponding genomic DNA. Third, no apparent intron consensus sequences were identified in the sid2 sequence. Only one intron-containing nonribosomal peptide synthetase has been identified among the known peptide synthetases (7, 25). This finding is consistent with the hypothesis that many of the eukaryotic peptide synthetases are of prokaryotic origin.
Sequence analysis of sid2 revealed the presence of three iterated modules of approximately 1,000 amino acid residues each. The three modules of deduced sid2 protein sequence show significant sequence similarity to other known nonribosomal peptide synthetases from fungi and bacteria (20, 34, 39). The presence of three modules suggests that this synthetase may be involved in synthesis of one of the tripeptide components of ferrichrome as the structure of ferrichrome is composed of two “homotripeptides” (Gly-Gly-Gly and N-acetyl-N-OHOrn-N-acetyl-N-OHOrn-N-acetyl-N-OHOrn). Intuitively, having three modules do the same job seems inefficient. Moreover, the low overall similarity shared among the three modules may further indicate that all three modules activate different amino acid residues. Siegmund et al. (33) postulated that only two different amino-acid-activating units might trimerize and be capable of synthesizing ferrichrome. Thus, we hypothesize the repeated use of one or more modules of sid2 during ferrichrome biosynthesis.
Work from the laboratories of Brick and Marahiel has led to the crystal structure of the N-terminal 556-amino-acid residue segment of the gramicidin S synthetase A (GrsA), which is known to be a single-modular nonribosomal peptide synthetase and activates the amino acid l-phenylalanine (12). Key amino acid residues at the active site and a substrate-binding pocket were identified. By comparing this structure with sequences of adenylation domains to those with known amino acid specificity, a set of rules was established to predict the specificity of the adenylation domain (37). Using a predictive program (11) (http://ravnam.chm.jhu.edu/∼nrps), the amino acid specificity of the modules of Sid2 could not be definitively predicted; ornithine, modified ornithine, hydrophobic amino acids such as glycine or alanine, or the acidic amino acid aspartate might be charged by the domains of Sid2 (Hans von Doehren, personal communication). The repeated use of a single domain to charge different amino acids in order to complete the entire synthesis of the ferrichrome peptide remains an open possibility. It is noteworthy that a second potential peptidyl carrier protein domain is present in module 3 (Fig. 5). Future protein structure prediction, sequence alignment, and biochemical analysis in combination with site-directed mutagenesis will be required to provide a more definitive test of this repetitive module model.
Besides the additional putative acyl carrier protein domain motif, the third module of sid2 is 250 amino acids larger than the other two (Fig. 5A). Since Orn is not only activated but also hydroxylated and acetylated, the additional sequence of the third domain could be involved in side chain modification such as the acetylation step. Interestingly, Hummel and Diekmann (24) first showed that a partially purified protein fraction also catalyzed transacetylation of hydroxyornithine from acetyl coenzyme A, hence implying that the peptide synthesizing activity may be part of a multienzyme complex. Later Siegmund et al. (33) managed to separate the transacetylase activity from the amino-acid-activating and the ferrichrome synthetase activities. Depending on the state of purification, the ferrichrome synthetase can thus be dissociated into functional subunits with partial activities. Efforts are under way to purify Sid2 and determine the biochemical activity of each module.
In many microbial systems genes responsible for secondary metabolites are often clustered (8, 25, 35, 36, 38). Here we have shown this also to be the case for ferrichrome biosynthesis in U. maydis. sid1 and sid2 are divergently transcribed from a 3.7-kb intergenic region which likely contains all the information necessary for the cis regulation of both genes. Previous studies led to the identification and characterization of a zinc finger GATA family transcription factor, urbs1, which is responsible for iron-mediated regulation of sid1 expression (2, 3, 40).
Northern hybridization analysis was performed to test whether urbs1 also regulates sid2 expression in a similar manner. High levels of a large transcript (>9.5 kb) were detected in wild-type cells grown in an iron-deficient medium, suggesting sid2 is also transcriptionally regulated by iron (Fig. 7). To test the role of urbs1 in this regulation, two mutant strains were included in this analysis, UMCO15 and UMS204 (Table 1). UMCO15 contains a nonfunctional copy of urbs1, whereas UMSO24 has two mutated cis elements involved in iron-mediated regulation of sid1. In contrast to the wild type, constitutive accumulation of high levels of the >9.5-kb transcript was detected, suggesting that urbs1 may simultaneously regulate both sid genes in response to the iron concentration in the environment and the cell. A truncated transcript of about 9.0 kb was observed in the total RNA from the other mutant, UMS2H5, which has a hygromycin cassette inserted at the last domain of sid2. Future work involving site-directed mutagenesis will likely reveal other potential cis regulatory elements. In particular, the four highly conserved palindromic GATA motifs bear further examination. Preliminary efforts to determine the role of chromatin in the regulation of this gene cluster indicate that iron does modulate the pattern of nuclease hypersensitivity in the 3.7-kb intergenic region and that these changes also depend on Urbs1 (M. W. Yuan and S. A. Leong, unpublished results).
To delineate the borders of this ferrichrome biosynthetic gene cluster, the genomic DNA sequence immediately flanking the sid1 and sid2 genes has been determined. A yeast ADE6 homolog was identified downstream of sid1, and disruption of this gene resulted in an adenine requirement on minimal medium (M. Warriner, A. D. Budde, and S. A. Leong, unpublished results). A yeast SNF2 transcription factor homolog was identified immediately downstream of sid2, the function of which still needs to be determined. Disruption of this gene led to no obvious phenotypic effect on sid gene expression (M. W. Yuan, B. Bride, and S. A. Leong, unpublished results).
While our knowledge of the molecular mechanisms of iron uptake and storage in prokaryotes has greatly advanced in recent years, a similar understanding of these mechanisms in fungal systems is lacking. This is due to the greater difficulty of working with fungi and the lack of efficient cloning systems for many of these organisms. Using U. maydis as a model system, rapid progress has been made to genetically identify and characterize these mechanisms at the molecular level and hence iron-mediated transcriptional regulation is becoming clearer. Future studies will center on identifying other structural genes in the ferrichrome biosynthetic pathway, determining the substrate specificity of modules in sid2, and lastly, developing a better understanding of how the sid genes are regulated in response to iron conditions.
ACKNOWLEDGMENTS
We are very grateful to ZhiQiang An and Hans von Doehren for their helpful discussions and critical review of the manuscript and Gunther Winkelmann for providing the figure of the structure of the ferrichromes.
This work was supported by the USDA-ARS and NIH grant GM33716 to S.A.L.
M. W. Yuan and Guillaume D. Gentil contributed equally to the work described in this paper.
REFERENCES
- 1.An Z, Farman M L, Taura S, Leong S A. New cosmid vectors for library construction, systematic chromosome walking and rapid restriction mapping in filamentous fungi. Gene. 1996;176:93–96. doi: 10.1016/0378-1119(96)00225-9. [DOI] [PubMed] [Google Scholar]
- 2.An Z, Mei B, Yuan W, Leong S A. The distal GATA sequences of the sid1 promoter of Ustilago maydis mediate repression of siderophore production and interact directly with Urbs1, a GATA family transcription factor. EMBO J. 1997;16:1742–1750. doi: 10.1093/emboj/16.7.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.An Z, Zhao Q, McEvoy J, Yuan W, Markley J, Leong S A. The C-terminal finger domain of Urbs1 is required for iron-mediated regulation of siderophore biosynthesis in Ustilago maydis. Proc Natl Acad Sci USA. 1997;94:5882–5887. doi: 10.1073/pnas.94.11.5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anke H, Anke T, Diekmann H. Biosynthesis of sideramines in fungi. Fusigen synthetase from extracts of Fusarium cubense. FEBS Lett. 1973;36:323–325. doi: 10.1016/0014-5793(73)80401-6. [DOI] [PubMed] [Google Scholar]
- 5.Anke T, Diekmann H. Biosynthesis of sideramines in fungi. Rhodotorulic acid synthetase from extracts of Rhodotorula glutinis. FEBS Lett. 1972;27:259–262. doi: 10.1016/0014-5793(72)80635-5. [DOI] [PubMed] [Google Scholar]
- 6.Ausubel F M, et al., editors. Current protocols in molecular biology. New York, N.Y: John Wiley; 1994. [Google Scholar]
- 7.Bailey A M, Kershaw M J, Hunt B A, Patterson I C, Charnley A K, Reynolds S E, Clarkson J M. Cloning and sequence analysis of an intron-containing domain from a peptide synthetase-encoding gene of the entomopathogenic fungus Metarhizium anisopliae. Gene. 1996;173:195–197. doi: 10.1016/0378-1119(96)00212-0. [DOI] [PubMed] [Google Scholar]
- 8.Brakhage A A. Molecular regulation of penicillin biosynthesis in Aspergillus (Emericella) nidulans. FEMS Microbiol Lett. 1997;148:1–10. doi: 10.1111/j.1574-6968.1997.tb10258.x. [DOI] [PubMed] [Google Scholar]
- 9.Braun V, Killmann H. Bacterial solutions to the iron-supply problem. Trends Biochem Sci. 1999;24:104–109. doi: 10.1016/s0968-0004(99)01359-6. [DOI] [PubMed] [Google Scholar]
- 10.Budde A D, Leong S A. Characterization of siderophores from Ustilago maydis. Mycopathologia. 1989;108:125–133. doi: 10.1007/BF00436063. [DOI] [PubMed] [Google Scholar]
- 11.Challis G L, Ravel J, Townsend C A. Predictive, structure-based model of amino acid recognition by nonribosomal peptide synthetase adenylation domains. Chem Biol. 2000;7:211–224. doi: 10.1016/s1074-5521(00)00091-0. [DOI] [PubMed] [Google Scholar]
- 12.Conti E, Stachelhaus T, Marahiel M A, Brick P. Structural basis for the activation of phenylalanine in the non-ribosomal biosynthesis of gramicidin S. EMBO J. 1997;16:4174–4183. doi: 10.1093/emboj/16.14.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crichton R R, Ward R J. Iron metabolism—new perspectives in view. Biochemistry. 1992;31:11255–11264. doi: 10.1021/bi00161a001. [DOI] [PubMed] [Google Scholar]
- 14.Crichton R R, Ward R J. Iron homeostasis. Metal Ions Biol Syst. 1998;35:633–665. [PubMed] [Google Scholar]
- 15.Froeliger E H, Leong S A. The a mating-type alleles of Ustilago maydis are idiomorphs. Gene. 1991;100:113–122. doi: 10.1016/0378-1119(91)90356-g. [DOI] [PubMed] [Google Scholar]
- 16.Frohman M A, Downs T R, Chomczynski P, Frohman L A. Cloning and characterization of mouse growth hormone-releasing hormone (GRH) complementary DNA: increased GRH messenger RNA levels in the growth hormone-deficient lit/lit mouse. Mol Endocrinol. 1989;3:1529–1536. doi: 10.1210/mend-3-10-1529. [DOI] [PubMed] [Google Scholar]
- 17.Frohman M A. On beyond classic RACE (rapid amplification of cDNA ends) PCR Methods Appl. 1994;4:S40–S58. doi: 10.1101/gr.4.1.s40. [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Marquez J, Freire M, Segade F. A simple procedure for large-scale purification of plasmid DNA. Gene. 1987;54:255–259. doi: 10.1016/0378-1119(87)90494-x. [DOI] [PubMed] [Google Scholar]
- 19.Griffiths E. Iron in biological systems. In: Bullen J J, editor. Iron and infection. New York, N.Y: John Wiley & Sons; 1987. pp. 1–25. [Google Scholar]
- 20.Guenzi E, Galli G, Grgunna I, Gross D C, Grandi G. Characterization of the syringomycin synthetase gene cluster. A link between prokaryotic and eukaryotic peptide synthetases. J Biol Chem. 1998;273:32857–32863. doi: 10.1074/jbc.273.49.32857. [DOI] [PubMed] [Google Scholar]
- 21.Hamer J E, Timberlake W E. Functional organization of the Aspergillus nidulans trpC promoter. Mol Cell Biol. 1987;7:2352–2359. doi: 10.1128/mcb.7.7.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holliday R. Ustilago maydis. In: King R C, editor. Handbook of genetics. New York, N.Y: Plenum Press; 1974. pp. 273–287. [Google Scholar]
- 23.Holmes D S, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 24.Hummel W, Diekmann H. Preliminary characterization of ferrichrome synthetase from Aspergillus quadricinctus. Biochim Biophys Acta. 1981;657:313–320. doi: 10.1016/0005-2744(81)90317-x. [DOI] [PubMed] [Google Scholar]
- 25.Kleinkauf H, Von Doehren H. A nonribosomal system of peptide biosynthesis. Eur J Biochem. 1996;236:335–351. doi: 10.1111/j.1432-1033.1996.00335.x. [DOI] [PubMed] [Google Scholar]
- 26.Kronstad J W, Leong S A. Isolation of two alleles of the b locus of Ustilago maydis. Proc Natl Acad Sci USA. 1989;86:978–982. doi: 10.1073/pnas.86.3.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leong S A, Neilands J B. Siderophore production by phytopathogenic microbial species. Arch Biochem Biophys. 1982;218:351–359. doi: 10.1016/0003-9861(82)90356-3. [DOI] [PubMed] [Google Scholar]
- 28.Mei B, Budde A D, Leong S A. sid1, a gene initiating siderophore biosynthesis in Ustilago maydis: molecular characterization, regulation by iron, and role in phytopathogenicity. Proc Natl Acad Sci USA. 1993;90:903–907. doi: 10.1073/pnas.90.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Neilands J B. Siderophores. Arch Biochem Biophys. 1993;302:1–3. doi: 10.1006/abbi.1993.1172. [DOI] [PubMed] [Google Scholar]
- 30.Neilands J B. Siderophores: structure and function of microbial iron transport compounds. J Biol Chem. 1995;270:26723–26726. doi: 10.1074/jbc.270.45.26723. [DOI] [PubMed] [Google Scholar]
- 31.Ong D E, Emery T F. Ferrichrome biosynthesis: enzyme catalyzed formation of the hydroxamic acid group. Arch Biochem Biophys. 1972;148:77–83. doi: 10.1016/0003-9861(72)90117-8. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 33.Siegmund K D, Plattner H J, Diekmann H. Purification of ferrichrome synthetase from Aspergillus quadricinctus and characterization as a phosphopantetheine containing multienzyme complex. Biochim Biophys Acta. 1991;1076:123–129. doi: 10.1016/0167-4838(91)90228-r. [DOI] [PubMed] [Google Scholar]
- 34.Smith D J, Earl A J, Turner G. The multifunctional peptide synthetase performing the first step of penicillin biosynthesis in Penicillium chrysogenum is a 421,073 dalton protein similar to Bacillus brevis peptide antibiotic synthetases. EMBO J. 1990;9:2743–2750. doi: 10.1002/j.1460-2075.1990.tb07461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith D J, Burnham M K, Bull J H, Hodgson J E, Ward J M, Browne P, Brown J, Barton B, Earl A J, Turner G. Beta-lactam antibiotic biosynthetic genes have been conserved in clusters in prokaryotes and eukaryotes. EMBO J. 1990;9:741–747. doi: 10.1002/j.1460-2075.1990.tb08168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith D J, Burnham M K, Edwards J, Earl A J, Turner G. Cloning and heterologous expression of the penicillin biosynthetic gene cluster from Penicillum chrysogenum. Biotechnology (New York) 1990;8:39–41. doi: 10.1038/nbt0190-39. [DOI] [PubMed] [Google Scholar]
- 37.Stachelhaus T, Mootz H D, Marahiel M A. The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem Biol. 1999;6:493–505. doi: 10.1016/S1074-5521(99)80082-9. [DOI] [PubMed] [Google Scholar]
- 38.Turner G. Genes for the biosynthesis of beta-lactam compounds in microorganisms. Ciba Found Symp. 1992;171:113–124. [PubMed] [Google Scholar]
- 39.van Liempt H, von Doehren H, Kleinkauf H. delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Aspergillus nidulans. The first enzyme in penicillin biosynthesis is a multifunctional peptide synthetase. J Biol Chem. 1989;264:3680–3684. [PubMed] [Google Scholar]
- 40.Voisard C, Wang J, McEvoy J, Xu P, Leong S A. urbs1, a gene regulating siderophore biosynthesis in Ustilago maydis, encodes a protein similar to the erythroid transcription factor GATA-1. Mol Cell Biol. 1993;13:7091–7100. doi: 10.1128/mcb.13.11.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, Holden D W, Leong S A. Gene transfer system for the phytopathogenic fungus Ustilago maydis. Proc Natl Acad Sci USA. 1988;85:865–869. doi: 10.1073/pnas.85.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang J, Budde A D, Leong S A. Analysis of ferrichrome biosynthesis in the phytopathogenic fungus Ustilago maydis: cloning of an ornithine-N5-oxygenase gene. J Bacteriol. 1989;171:2811–2818. doi: 10.1128/jb.171.5.2811-2818.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]