Abstract
Current in vitro three-dimensional (3D) models of liver tissue have been limited by the inability to study the effects of specific extracellular matrix (ECM) components on cell phenotypes. This is in part due to limitations in the availability of chemical modifications appropriate for this purpose. For example, hyaluronic acid (HA), which is a natural ECM component within the liver, lacks key ECM motifs (e.g. arginine–glycine–aspartic acid (RGD) peptides) that support cell adhesion. However, the addition of maleimide (Mal) groups to HA could facilitate the conjugation of ECM biomimetic peptides with thiol-containing end groups. In this study, we characterized a new crosslinkable hydrogel (i.e. HA-Mal) that yielded a simplified ECM-mimicking microenvironment supportive of 3D liver cell culture. We then performed a series of experiments to assess the impact of physical and biochemical signaling in the form of RGD peptide incorporation and transforming growth factor ß (TGF-ß) supplementation, respectively, on hepatic functionality. Hepatic stellate cells (i.e. LX-2) exhibited increased cell–matrix interactions in the form of cell spreading and elongation within HA-Mal matrices containing RGD peptides, enabling physical adhesions, whereas hepatocyte-like cells (HepG2) had reduced albumin and urea production. We further exposed the encapsulated cells to soluble TGF-ß to elicit a fibrosis-like state. In the presence of TGF-ß biochemical signals, LX-2 cells became activated and HepG2 functionality significantly decreased in both RGD-containing and RGD-free hydrogels. Altogether, in this study we have developed a hydrogel biomaterial platform that allows for discrete manipulation of specific ECM motifs within the hydrogel to better understand the roles of cell–matrix interactions on cell phenotype and overall liver functionality.
Keywords: maleimide, liver, microenvironment, extracellular matrix, hyaluronic acid, RGD
1. Introduction
Bioengineered liver models have become useful tools in drug development and liver disease modeling. In particular, the use of hydrogel biomaterials has allowed for the suspension of hepatic cells in three-dimensions (3D), where the cells are able to interact with both cellular and liver-like extracellular matrix (ECM) components in all directions, while still being able to receive nutrients via diffusion, and conduct paracrine signaling [1]. Several investigators have demonstrated success with the culturing of hepatic cell lines (e.g. HepG2) and primary hepatocytes, either in monoculture or co-culture with non-parenchymal cells (e.g. hepatic stellate cells (HSCs), Kupffer cells, liver sinusoidal endothelial cells) [2–11]. The benefits of these 3D bioengineered models in comparison to traditional two-dimensional (2D) cell culture methods, for creating physiologically relevant liver models have been well studied and previously described [12, 13].
Over the past 30 years, a number of new materials for use within tissue engineering have been developed, with focused efforts on enabling control over fiber or particle size and shape, stiffness, porosity, and texture [14]. There has also been tremendous advances in the development of organ-specific ECM-like hydrogels, or ‘bioinks’ to support a variety of cell types [15–17]. Yet, most materials ideal for forming structurally controlled hydrogels (i.e. polyacrylamide, polyethylene glycol (PEG), alginate) are not well-suited for cell–matrix interactions and lack cell-adhesive ECM components [18]. To improve these biomaterials for the mechanistic study of cell–ECM interactions driving disease pathologies, chemical addition of ECM components or biomimetic peptides is needed.
Today, the majority of 3D hydrogels for bioengineered liver models use hyaluronic acid (HA) or collagen as the base biomaterial due to their biologic relevance and ability to replicate the in vivo tissue microenvironment. HA, or hyaluronan, is a non-sulfated linear glycosaminoglycan composed of two repeating disaccharide units, D-glucuronic acid and N-acetyl glucosamine, with native molecular weight ranging between 1000 and 10 000 kDa in most tissue. It is integral to many tissue components and functions such as contributing to ECM architecture, and influencing cell motility, cell adhesion, and proliferation via CD44 and receptor for hyaluronan-mediated motility cell receptor interactions [19]. Changes in the material composition and fabrication of the hydrogel, including HA-based hydrogels, can lend to differences in mechanical properties and porosity allowing for the cells to receive nutrients from the culture media, conduct paracrine signaling, and interact with the physical microenvironment [1]. However, HA lacks the cell adhesion motifs that induce strong cell–matrix interactions.
The lack of cell adhesive components required for integration of cells in a hydrogel, even HA-based hydrogels, is a major limitation of current 3D bioengineered organoids. One method for mediating this limitation is through the addition of adhesive peptide sequences. For example, specific amino acid sequence motifs found on natural ECM components, that do not fully replace the entire ECM protein sequence and are often referred to as biomimetic ECM peptides [20, 21]. These can be beneficial as specific, amino acid sequence and corresponding protein receptor interactions can be studied in a controlled manner; in comparison to the in vivo scenario where a milieu of contributing factors are pressing, and changes in cell genotype, phenotype, or function can be hard to attribute to particular ECM components [22]. A commonly used peptide sequence for 2D and 3D cell culture applications is the arginine–glycine–aspartic acid (RGD) sequence. This sequences is found in many different cell adhesion proteins, but has been most closely associated with fibronectin (FN) [23]. FN specifically helps form the fibrillar ECM through interactions with cell surface receptors and secreted FN [24]. It has been shown to play a major role in development, tissue repair, cancer progression and invasion, and inflammation [25]. FN has also been shown to be upregulated in the diseased liver microenvironment, making the addition of RGD-peptides important for understanding how cell–FN interactions influence disease development, including hepatic fibrosis [26, 27]. Despite RGD peptides having been used often with other tissue types, few studies have been conducted to determine the specific impact of RGD cell adhesion sites on hepatocyte or hepatocyte-like cell function [28]. Their potential for creating an adhesive surface for cells, and contributing to a fibrotic disease-like microenvironment makes them an important ECM component to study.
Within the context of the liver, HSCs have been shown to play a major role in the development of liver cirrhosis, fibrosis, microenvironment remodeling and long-term viability of in vitro 3D liver models [29, 30]. While normal HSCs in the healthy liver are mesenchymal and do not significantly impact liver function [30] upon activation, they transform into myofibroblast-like cells, and upregulate the expression of α-smooth muscle actin and fibroblast activation protein (FAP), synthesize and deposit ECM (including FN), synthesize pro-inflammatory cytokines, and interact with the surrounding ECM to heal tissue [31] Importantly, HSCs are known to activate in response to both physical and biochemical signals. For example, upregulation of transforming growth factor ß (TGF-ß) will activate HSCs and subsequently lead to a more fibrotic cell phenotype and increased cellular activity and ECM deposition [32–35]. Undoubtedly, there is an important interaction between the HSCs, hepatocytes and the tissue microenvironment (i.e. ECM) which needs to be better understood. Exploiting a hydrogel system that incorporates the cell-adhesive RGD peptide sequence can potentially provide greater insights on the cell–ECM interactions influencing cell phenotype and liver functionality under normal and disease conditions.
A variety of models of liver fibrosis have been described in recent years. Each offer unique approaches towards modeling this pathology, but most come with some shortcomings as the field still has many hurdles to overcome before realizing a liver model that truly recapitulates native liver. Spheroid models comprised of HepaRG and primary human HSCs have been described [36]. These spheroids support cytochrome p450 functionality, albumin secretion, and expression of key hepatic genes. Importantly, they could be driven towards a fibrotic state via treatment with allyl alcohol, methotrexate, and acetaminophen. However, these spheroids lack any ECM components [36]. Matrigel-supported induced pluripotent stem cell-derived hepatic organoids have been developed [37], which were used to model fibrosis through engineering a genetic mutation that causes the autosomal recessive polycystic kidney disease [38]. While certainly a novel way to model this disease, the reliance of Matrigel—an undefined murine sarcoma-derived ECM biomaterial—results in introduction of countless potentially confounding variables that are impossible to account for [39, 40]. Models of liver fibrosis comprised of defined ECM components also exist. For example, Brovold et al described the use of activated HSCs in defined collagen-based organoids in which fibrosis was initiated by TGF-β or methotrexate and resulting collagen architectures were analyzed quantitatively [41]. These examples describe multiple distinct approaches to modeling liver fibrosis—each with a unique research question. Our objective was to distill the physical environment of our model system into one that is cell-supportive, but comprised of defined components in a relatively simple matrix formulation. We previously demonstrated the use of decellularized liver ECM as a supplement to our HA-based hydrogels to support primary human hepatocyte cultures [17, 42–44], but have recently moved towards more defined hydrogel platforms [16] that can be customized in a modular fashion to support different cell types.
Towards our goal of evaluating cell–ECM interactions, modulation of synthetic polymers such as PEG, as well as natural polymers such as HA, via ECM biomimetic peptides has increased their utility for cell culture (2D and 3D) [45–47]. In particular, maleimide-functionalized HA (HA-Mal) has been documented for applications in tissue engineering [48–51], but studies in which adhesion peptides are grafted to the HA-Mal are remarkably few [52]. Moreover, studies in which grafting of biomimetic peptides such as RGD onto polymers in order to study their effects on hepatic function and phenotype have largely been limited to 2D systems in which biological assays are performed only within several days of initiating culture [53, 54]. In this study, we show the utility of an 3D HA-Mal hydrogel system for probing hepatic phenotype by functionalizing the hydrogel network with RGD biomimetic peptides containing cysteine end groups to yield HA-Mal-RGD. We cultured hepatocyte-like cells (HepG2) and HSCs (LX-2) within different formulations of HA-Mal-RGD hydrogels in order to study the role of RGD peptides within the liver, specifically in simulated healthy and diseased microenvironments. Specifically, we employed co-cultures of LX-2 and HepG2 cells in HA-Mal and HA-Mal-RGD hydrogels, with and without the addition of the inflammatory cytokine TGF-ß to create experimental normal and diseased liver-based models and evaluate their subsequent effects of cell viability and function.
2. Results
2.1. HA-Mal synthesis characterization
Synthesis of the HA-Mal was carried out using N-hydroxysuccinimide (NHS) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) chemistry and utilization of 1-(2-aminoethyl) maleimide hydrochloride (figure 1(A)). While utilization of NHS and EDC chemistry with HA has been previously documented in the literature for modification of maleimides [48–51], studies in which adhesion peptides are grafted to the HA-Mal are limited [52]. The covalent addition of maleimide groups to the HA polysaccharide backbone was confirmed through the use of proton nuclear magnetic resonance (1H-NMR), specifically indicated by the peak shown at 6.92 ppm (2H), representing the maleimide, and the peak at 2.06 ppm (3H), representing the pendent acetyl protons on the HA backbone (figure 1(B)). Utilizing high performance liquid chromatography (HPLC), the molecular weight of both modified (HA-Mal) and unmodified HA was found and showed an approximate 0.1 × 105 g mol−1 increase in molecular weight (figure 1(C)). Although these values are within one standard deviation of each other, the minimal increase was expected in the modified material given the size of the maleimide molecule. Further, Rh(z) indicated z-average hydrodynamic radius remains approximately the same between the two materials, as does Rh(w) (weight average hydrodynamic radius), both of which were expected as the polymer size was not been substantially modified (figure 1(C)). Free maleimides were quantified and it was found that, per mole of HA-Mal, 0.0814 ± 0.0007 moles of free maleimides were present (figure 1(D)), and that 8% of the carboxyl groups available on the HA were modified to maleimides. When 1 mM RGD was added to the HA-Mal, the average moles of free maleimides reduced to 0.0616 ± 0.007 moles, as expected. This change indicated that the linear RGD functionalization occurred successfully through maleimide-thiol reactions, while maintaining enough free maleimides for subsequent crosslinking. With this data final concentrations for formation of the hydrogel were 2.5% w/v HA-Mal (0.0432 M), 1% w/v 3.4 kDa PEG di-thiol (0.00294 M), and in 1 mM linear RGD (0.001 M).
Figure 1.

HA-Mal synthesis and characterization. (A) Hydrogel synthesis was carried out in four steps. HA was initially reacted with NHS and EDC to add a carbodiimide to each HA group. Small molecule-maleimides (SM-Mal) were then reacted with the modified HA to yield maleimide functionalized hyaluronic acid (i.e. HA-Mal). After dialysis and lyophilization, HA-Mal was reacted with linear RGD-containing peptides with cysteines (thiol containing end groups) to yield the final HA-Mal-RGD hydrogel that was crosslinked using 3.4 kDa di-thiol PEG. (B) HA-Mal was shown to have a representative peak at 6.85 ppm which is characteristic of SM-Mal as shown. Red circles indicate the peak signal coming from maleimide. (C) Molecular weight (MW) was shown to increase as expected with the addition of the SM-Mal. Rh(z) and Rh(w) indicated z-average hydrodynamic radius and weight average mean square radius respectively. Standard deviation is reported as percent in parentheses for each measure. (D) Available maleimides in HA-Mal and HA-Mal-RGD revealed a significant difference between the two hydrogels (*p < 0.005, mean ± SD).
2.2. Hydrogel material testing
Mechanical, physical, and ultra-structural characterization of the RGD-modified HA-Mal hydrogels was carried out to ensure that the addition of the RGD peptides did not significantly impact the original hydrogel characteristics. With confirmation from maleimide quantification that the RGD peptides were covalently bound, results indicated that the RGD addition did not detract from the ability of the hydrogel to crosslink with di-thiol PEG. The elastic modulus was calculated for HA-Mal and HA-Mal-RGD (2.5% w/v, each) each crosslinked to form a complete hydrogel with 3.4 kDa PEG di-thiol (1% w/v) (figure 2(A)). There was no significant difference in stiffness of the two hydrogels indicating that the addition of RGD did not impact the ability of the PEG di-thiol to crosslink. Storage and loss moduli were measured after each of the materials had been crosslinked, demonstrating no significant difference between storage modulus or loss modulus between the two hydrogels (figure 2(B)). These findings further indicate that physical and mechanical properties do not vary significantly between HA-Mal and HA-Mal-RGD. Hydrogels were weighed over seven days to compare swelling ratio (SR) of the materials. We observed significantly lower SRs in the HA-Mal-RGD on day 1 compared to HA-Mal; however, by day 7 there was no difference (figure 2(C)). The initial difference is potentially due to increased hydrophobic regions associated with the RGD peptides, which could slow uptake of water. Pore size (i.e. area) and circularity of each hydrogel network were calculated using SEM images (figure 2(D)), demonstrating no significant difference in pore size measured in area between the two hydrogels (figure 2(E)). However, RGD-modified hydrogels showed a significant difference in pore circularity (range of 0–1, 1 being a perfect circle) and had more circular pores in comparison to HA-Mal alone (figure 2(F)). This difference was unexpected, but it should be noted that the difference in magnitude of pore circularity was quite small, and the statistical significance was driven largely by the incredibly small standard deviations. Yet, the addition of RGDs does effectively prevent some maleimide binding sites from binding to the PEG-dithiol cross-linker. As such, while a change in circularity was not expected, it is possible, but it is not characteristic that we would expect to play a significant role in subsequent studies. In general, size and shape were similar to other HA-based hydrogels [51].
Figure 2.

Physical analysis of HA-Mal and HA-Mal-RGD hydrogels. (A), (B) Analysis of elastic (A) and storage and loss (B) moduli show no significant differences between HA-Mal and HA-Mal-RGD hydrogels, indicating similar physical properties (mean ± SD are illustrated). (C) Swelling ratio was calculated across seven days for the hydrogels and only on day 1 was there significant difference in swelling ratio between the two (mean ± SD, *p < 0.001). (D) SEM images of the two hydrogels reveal similar microstructural pores. (E) Pore area was quantified from the SEM images and the 5th–95th percentiles are represented within the boxplots; no significant difference was found. (F) Pore circularity was quantified from the SEM images and HA-Mal-RGD hydrogel was significantly more circular in comparison to the HA-Mal (mean ± SEM, *p < 0.001).
2.3. HSCs and hepatocyte-like cell phenotypein HA-Mal
To study the growth and phenotype of liver cells in the HA-Mal hydrogels we used an immortalized HSC line, LX-2 and the hepatocyte-like cells, HepG2. We further tested the effect of TGF-ß, a prominent factor involved in liver development and disease (fibrosis), on cell growth and phenotype. Two hydrogel formulations were tested: HA-Mal and HA-Mal-RGD, and TGF-ß stimulation in order to induce a fibrotic phenotype. First, we cultured LX-2 cells individually; phenotype and cell viability within the hydrogels were evaluated through image analysis of cell clusters, collagen, FN, immunohistochemistry (IHC) for FAP, and viability across seven days (figures 3(A) and (B)). Hematoxylin and eosin (H&E) images showed cell cluster formation in all four conditions with substantial spreading in conditions with RGD peptides present (figure 3(A)). All four hydrogel formulations supported increases in LX-2 cell viability during the culture period (figure 3(B)) with the HA-Mal-RGD + TGF-ß displaying significantly greater viability on day 7 compared to HA-Mal + TGF-ß. Collagen, FN, and FAP expressing cells were counted and shown as a percent of the total population (figures 3(A) and (C)). Collagen was shown to be most abundant in both TGF-ß supplemented cultures with significantly greater expression than HA-Mal and HA-Mal-RGD. FN was also shown to be greatest in the HA-Mal + TGF-ß supplemented hydrogel and was significantly greater than the HA-Mal and HA-Mal-RGD. FAP expression was found for HA-Mal, HA-Mal + TGF-ß, and HA-Mal-RGD + TGF-ß but all conditions had low expression. Cluster area analysis showed that addition of RGD supported larger clusters than cultures formed with HA-Mal alone (figure 3(D)). In addition, inclusion of RGD induced cell elongation compared with HA-Mal (figure 3(E)). Addition of TGF-ß had a moderate effect on circularity. Taken together, RGD-modification of HA-Mal and supplementation of TGF-ß significantly impact LX-2 phenotype inside the hydrogels, and LX-2 cells within HA-Mal-RGD + TGF-ß are behaving in a disease like manner.
Figure 3.

Characterization of LX-2 phenotype in HA-Mal hydrogels. (A) LX-2 cells encapsulated in the different hydrogels were analyzed after seven days of culture in the presence or absence of TGF-ß, as indicated. Sections of the cell-containing hydrogels were stained with H&E, and co-stained with either collagen, fibronectin, or fibroblast activation protein (FAP) (magenta) and DAPI (blue, nuclei). (B) Cell viability was measured across seven days for each of the conditions (mean ± SD). Data was normalized to HA-Mal hydrogel on day 1. HA-Mal + TGF-ß was significantly lower than all other conditions on day 7. (C) The number of cells expressing collagen, fibronectin, and FAP was quantified from the images in panel (A), and using fluorescence images (cells with co-localization of nuclei and stain/number of nuclei) (mean ± SD). Cluster area (D) and circularity (E) of LX-2 encapsulated in the different hydrogels, as indicated, were measured from the H&E images in panel (A), using custom MATLAB script (mean ± SEM). *p < 0.05, **p < 0.01, $p < 0.001, #p < 0.0001.
Next, HepG2s were cultured in HA-Mal and HA-Mal-RGD with and without TGF-ß supplementation. H&E images showed cell cluster formation in all four conditions with substantial spreading in conditions when RGD peptides were present (figure 4(A)). When exposed to TGF-ß, the cluster sizes appeared to be smaller. Quantification of adenosine triphosphate (ATP) across seven days showed comparable cell viability for cells encapsulated in all conditions with a moderate increase in cell viability over time (figure 4(B)), and similar results were observed for albumin secretion (figure 4(C)). Initially, one might think that a lack of RGD peptides would negatively impact HepG2 viability. However, it should be noted that HepG2 cells are highly capable of existing in culture primarily through cell–cell interactions rather than cell–matrix interactions. They readily form clumps in adhesion cultures and aggregates/spheroids in suspension cultures with no loss in viability. As such we would not expect a decrease in HepG2 viability in conditions without RGD added, and have verified such in subsequent ongoing studies. Urea concentrations were less distinct among the different conditions, with cells encapsulated in HA-Mal-RGD demonstrating the highest urea concentrations (figure 4(D)). Clusters within HA-Mal-RGD were significantly larger than all other groups (figures 4(A) and (E)). Circularity of clusters varied among conditions with cell clusters encapsulated in HA-Mal-RGD + TGF-ß the most circular and cells in HA-Mal + TGF-ß the least circular (figure 4(F)). Although the addition of RGD peptides increased availability of cell adhesion sites, thereby changing size and circularity of the clusters, increased presence of RGD did not increase hepatic functionality, as measured by urea secretion, under (basal) normal conditions. Urea secretion, however, was significantly higher in the HA-Mal-RGD cultures compared to Ha-Mal when both were exposed to TGF-ß.
Figure 4.

Characterization of HepG2 phenotype in HA-Mal hydrogels. HepG2 cells were encapsulated in the different hydrogels and analyzed after seven days of culture in the presence or absence of TGF-ß, as indicated. (A) Sections of the cell-containing hydrogels stained with hematoxylin and eosin (H&E). (B) Cell viability was consistent across all conditions (mean ± SD).(C) Albumin and (D) urea production (mean ± SD) were measured after seven days of culture. HepG2 cells cultured in HA-mal exhibited the highest albumin concentrations, whereas HepG2 cells cultured in HA-mal-RGD displayed the highest urea levels. (E) HepG2 cluster area and (F) cell cluster circularity was quantified from the images in (A) (mean ± SEM). The presence of RGD peptides was associated with larger cluster area sizes, and when exposed to TGF-ß resulted in more circular clusters. *p < 0.05, **p < 0.01, $p < 0.001, #p < 0.0001.
2.4. Co-culture of HSC and hepatocyte-like cells in HA-Mal hydrogels
In order to create a more complex and accurate model of hepatic fibrosis, HepG2 and LX-2 cells were co-cultured in HA-Mal hydrogels in a ratio of 1:3. H&E were performed to assess cell aggregation and spreading (figure 5(A)). Similar to before, co-culture of HepG2 and LX-2 cells in the HA-Mal-RGD hydrogel resulted in the greatest cell spreading. Additionally, IHC staining was used to delineate between cell types, consequently counting the number of epithelial cell adhesion molecule (EpCAM) positive (i.e. HepG2 cells) and negative (i.e. LX-2) cells within each cluster (figure 5(A)). Cell viability at seven days showed that cells encapsulated in HA-Mal-RGD hydrogel had a significantly higher viability compared to other conditions and the addition of TGF-ß to this culture resulted in a significantly lower cell viability (figure 5(B)). The addition of TGF-ß to these hydrogels resulted in significantly lower albumin secretion (figure 5(C)). There was no significant difference in urea production between the different hydrogels (figure 5(D)).
Figure 5.
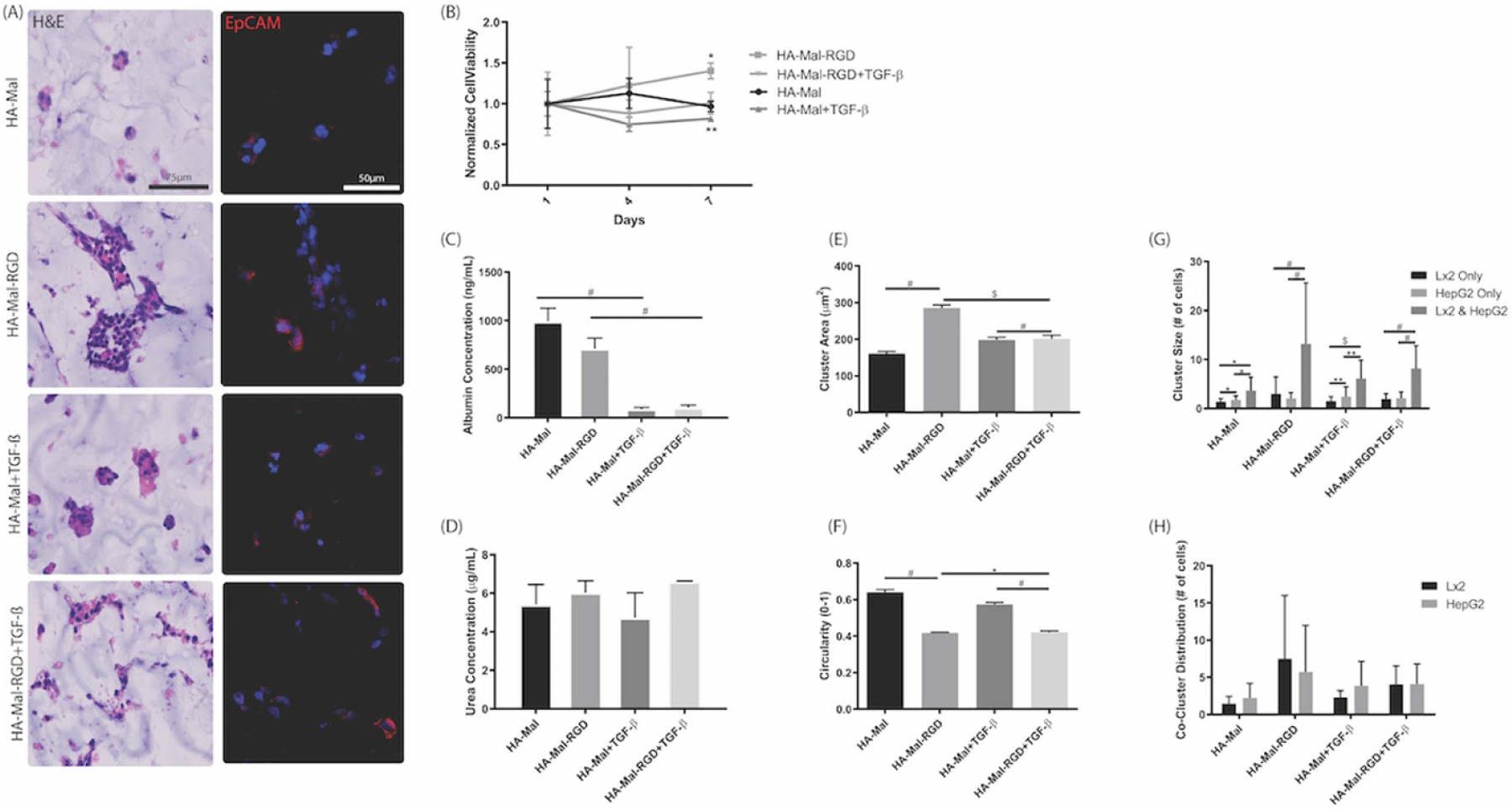
Characterization of LX-2 and HepG2 co-cultures in HA-Mal hydrogels. HepG2 and LX-2 cells were co-encapsulated in a 1:3 ratio in each hydrogel and analyzed after seven days of culture in the presence or absence of TGF-ß, as indicated. (A) Sections of the cell-containing hydrogels were stained with H&E, and co-stained with EpCAM (red) and DAPI (blue, nuclei). Purple areas represent overlap between EpCAM and DAPI signals. (B) Cell viability gradually increased over time in HA-Mal-RGD hydrogel (data normalized to day 1HA-Mal only viability). (C) Albumin secretion was significantly higher in HA-Mal and HA-Mal-RGD hydrogels compared with cultures with TGF-ß exposure (mean ± SD). (D) Urea concentration remained consistent across all hydrogels (mean ± SD). (E) Cluster area measured from H&E images was significantly greater and (F) circularity significantly smaller in the HA-Mal-RGD condition compared to all others (mean ± SEM). Cluster size by number of cells and cell type (G) was determined using EpCAM (specific to HepG2 cells) stained images (mean ± SD). In all conditions, clusters containing both cell types were larger compared with clusters of each individual cell type. Hydrogels with linear RGD peptides had the most significant difference in co-culture versus single cell type clusters. (H) Average number of HepG2 and LX-2 cells per clusters containing both cell types were not significantly different between hydrogel types (mean ± SD). *p < 0.05, **p < 0.01, $p < 0.001, #p < 0.0001.
Images from H&E and IHC staining were used to quantify cluster size by counting the number of cell nuclei in each cluster (# cells) as well as the number of EpCAM positive (i.e. HepG2 cells) and negative (i.e. LX-2) cells (figures 5(A) and (E)–(H)). In general, cells encapsulated in HA-Mal-RGD had the largest cluster area (figure 5(E)) and were significantly less circular than all other conditions (figure 5(F)). We then counted the number of cells in the cluster and their type was recorded in order to determine average cluster size for each cell type (figure 5(G)). In each of the hydrogels, co-cultures resulted in cell clusters that were significantly larger than in single cell type cultures. The clusters containing both cell types were further analyzed to demonstrate that LX-2 or HepG2 cells were evenly distributed in the hydrogel (figure 5(H)). These results suggest that RGD-like cell adhesion sites may play a role in LX-2-HepG2 organization, however, the presence of LX-2 cells and introduction of TGF-ß may be more influential in driving HepG2 cell spatial organization and function.
3. Discussion
Hydrogels have played an essential role in the advancement of tissue engineering research broadly, and in vitro/ex vivo tissue modeling research more specifically, over the past 30 years [55]. One important aspect of hydrogels has been their ability to support 3D culture systems that better replicate the in vivo tissue microenvironment in comparison to traditional 2D cell culture or biomaterial sandwich cultures [13]. Numerous methods have been employed to create hydrogels for 3D cell culture, especially for the liver, that allow cells to better integrate into the hydrogel matrix and ultimately form liver-like models for the study of liver function, disease modeling, and treatment responses in drug screening studies [56]. Many of these models, however, broadly employ multiple biological components—some of which are not well defined [39, 40]—and lack the ability to determine the impact each component has on the viability, functionality, and organization of the culture.
To overcome this deficiency, we have synthesized HA-Mal using a well characterized synthesis method utilizing EDC-NHS activation chemistry to modify the carboxyl group of the D-glucuronic acid moiety of HA with maleimide (Mal) groups. The HA-Mal can then be further functionalized using thiol group-containing peptides and crosslinked with thiolated crosslinkers through maleimide-thiol reactions. These reactions are known to have rapid kinetics at neutral pH, which allow thiol-containing peptide components and crosslinking molecules to spontaneously react with HA-Mal and form a crosslinked hydrogel in the presence of cells [57, 58]. This approach resulted in a simple hydrogel system that allows for discrete manipulation via modular RGD peptide add-ons that allows direct observation of the effects of physical ECM elements, such as adhesion peptides or proteins, without confounding variables. HA was chosen as the base hydrogel component, as it is naturally found in the liver microenvironment—and most other tissues—and has been widely employed as a structural backbone material in hydrogel systems for tissue engineering applications by our group and others [16, 17, 51, 59–63]. A small molecule (SM) containing a maleimide end group was covalently bound to the carboxyl groups of the HA polysaccharide chains to create reactive groups. The reactive maleimide groups are stable at neutral pH (pH 7.4) and have preferential reactivity to thiols. This high reactivity allows for stepwise addition of thiol-containing compounds. Characterization of the newly synthesized hydrogel indicated that the hydrogel physical properties remain largely unchanged when linear RGD peptides are added.
Physical RGD cell adhesion sites were added to the HA-Mal hydrogel to support liver cells within the hydrogel to determine the impact of soluble TGF-ß biochemical signaling on ECM-bound and unbound cells. Specifically, in this study, we focused on a liver model using established cell lines of hepatoma (HepG2—employed as a model for hepatocytes) and HSCs (LX-2), respectively. We should note that we acknowledge the limitations of the HepG2 line, including the source being hepatoma, decreased cytochrome p450 activity, and its proliferative capacity. As such, our team typically employs HepG2 cells in studies using a new hydrogel, and shifts to employing primary human hepatocytes in subsequent studies, as we have demonstrated in previous systems, and are continuing in ongoing studies [16, 42, 44, 64, 65]. We further used TGF-ß to simulate HSC activation and progression towards a fibrotic environment [41, 66]. Results showed that addition of TGF-ß alone had a measurable impact on cellular collagen and FN expression, but did not impact cell–matrix interactions, as measured by cell cluster circularity and cluster size. This suggests that HSCs are able to maintain their TGF-ß induced activated state in the presence of TGF-ß while their interaction with cell adhesion sites were not inhibited. In the presence of RGD adhesive sites, HepG2 function was significantly reduced in comparison to the control HA-Mal hydrogel that lacks cell binding capability. It has been previously shown that upregulation of FN is common in diseased liver, and thus these RGD adhesive sites, derived from FN, may be replicating the diseased liver microenvironment and causing a reduction in functionality of the cultures. This suggests that future studies should include additional cell adhesion peptides such as Tyr–Ile–Gly–Ser–Arg (YIGSR) and Ile-Lys-Val-ala-Val (IKVAV) to simulate upregulation of laminin cell adhesion motifs in contrast to FN/RGD. These results are supported by a previous study indicating that an increase in RGD sites may reduce functionality as measured by albumin and urea secretion [28]. The administration of TGF-ß in both RGD-containing and control HA-Mal cultures significantly reduced HepG2 function, as indicated by albumin production. These results indicate that the regardless of being ECM-bound, HepG2 cells respond to TGF-ß as an inflammatory signal. Using the FN-like RGD adhesive sites, the model behaves most similarly to a disease state in the ECM-binding and TGF-ß supplemented condition. LX-2 and HepG2 cells were also co-cultured within each of the microenvironment conditions and results suggest that in the presence of FN-like RGD adhesive sites, LX-2 cells are able to prevent a reduction in HepG2 function in comparison to HepG2 culture alone. However, when TGF-ß is also administered, the LX-2 cells take on an elongated activated phenotype, as shown in the monoculture studies, and may be unable to support HepG2 function in both HA-Mal and HA-M-RGD conditions, suggesting that ECM-binding cannot alter HepG2 function under conditions simulating disease such as inflammation or fibrosis. We should acknowledge that cell morphology and functional outputs such as albumin alone are surface level indications of hepatic functions. In future studies based around primary human hepatocytes and stellate cells, perhaps also paired with Kupffer cells and endothelial cells, more nuanced functional outputs will be deployed. These include hepatocyte viability/cytotoxicity using alpha-glutathione-S-transferase for hepatocyte specific stresses, drug metabolism, secreted and expressed cytokine analyses to evaluate respective stellate cell and Kupffer cell-driven inflammation, and quantification of newly deposited fibrotic ECM components as we have described [44, 65, 67].
Hydrogel systems have been used extensively as tissue engineering scaffolds, representing simplified versions of the in vivo microenvironment in models of normal tissue behavior and disease [68]. The hydrogel system described herein allows for the determination of the impact of isolated elements on cell and construct function, without the confounding influence of additional variables that can be found in more complex and undefined materials in which unknown quantities of ECM and cytokines are present. We focused our current study on the functions of the RGD peptide found in FN; however, other sequences representative of laminin, collagen I and collagen IV could also be evaluated for their impact on the function of healthy and diseased liver tissue. The simple hydrogel system developed here can be used to study a multitude of other tissues and the impact of ECM components on cell phenotype and functionality. While the full scope of the liver microenvironment is not represented in this material, it is advantageous for studying simple interactions and breaking down complex cellular responses into individual components.
4. Materials and methods
4.1. HA-Mal synthesis
Synthesis of HA-Mal polymer took place in two parts (figure 1(A)). Monodisperse HA (0.5 g, 1.2 × 10−3 mol, 1.0 eq) (100 kDa, LifeCore Biomedical, Chaska, MN) was dissolved in nanopure water at a concentration of 1% w/v for 15 min. NHS (1.2 eq) and EDC (1.2 eq) were each added sequentially to the solution and allowed to react for 1 h. 1-(2-aminoethyl) maleimide (SM-Mal) (1.2 eq) was added and allowed to react for ~18 h at room temperature. The solution was transferred to a dialysis bag (10 kDa) and dialyzed in deionized water for 72 h with dialysate being changed every 4–6 h. After dialysis, the solution was placed into 50 ml conicals and frozen at −20 °C overnight. The material was lyophilized for 72 h and weighed to determine final dry weight. The final product was referred to as HA-Mal.
4.2. 1H-NMR chemical characterization
Samples of HA-Mal were taken after lyophilization of the product in addition to HA 100 kDa HA and 1-(2-aminoethyl) maleimide. Each of the materials were re-suspended in deuterated water (D2O) and using 1H NMR (Bruker, Billerica, MA), spectra for analysis were produced. To determine if the 1-(2-aminoethyl) maleimide had been appropriately added to the HA, peaks at 6.92 ppm (2H, s) and 2.06 ppm (3H, s) were analyzed. The first peak represents the maleimide protons and the second peak represents the pendant acetyl protons on the HA backbone. Sources have confirmed these peaks are essential when modifying materials with maleimides [51, 69, 70].
4.3. Cell-free hydrogel formation
Cysteine-containing linear RGD specific peptides (G-R-G-D-S-P-C) were purchased (AAPPTec, Louisville, KY) lyophilized with HCl. The peptide was suspended in 1× phosphate buffered saline (PBS) at 100 mM and stored at −20 °C. Lyophilized HA-Mal was re-suspended in 1× PBS and neutralized to pH 7.0 using 1 N NaOH. The RGD peptide was added to the solution to yield a final molarity of 1 mM peptide. HA-Mal not functionalized with RGD was further diluted with 1× PBS (final concentration of 2.5% w/v HA-Mal). Single aliquots of the mixture were placed into wells and 3.4 kDa di-thiol PEG (Creative PEGWorks, Durham, NC) was combined with each (HA-Mal or HA-MAL-RGD) to yield a final concentration of 1% w/v PEG. A small volume of 1 N NaOH was added to each mixture to neutralize the solution to pH 7.4 and each was mixed using a pipet tip. Crosslinking of the organoids occurred within 10 s.
4.4. Maleimide quantification
Maleimide quantification in the HA-Mal and HA-Mal-RGD solutions was carried out using a colorimetric maleimide assay kit (MAK162-1KT, Sigma-Aldrich, St. Louis, MS). The number of maleimides available in a known volume of sample was calculated as the difference in the initial number of thiols added and those that remain unreacted after complete reaction of the maleimides. The hydrogel mixture was made as described in cell-free hydrogel formation without the addition of the di-thiol PEG. There each of the two conditions were each run in triplicate with duplicate assay samples.
4.5. Molecular weight quantification
HA (100 kDa) and HA-Mal (synthesized using 100 kDa HA) were individually dissolved in 1× PBS and filtered by centrifugation through a 0.45 μm nylon filter unit. Samples were injected into the HPLC system by an autosampler. The HPLC system was composed of a Waters 717 plus autosampler, two Waters model 510 chromatography pumps, and three detectors in series (MALS detector at 661 nm, differential refractive index detector at 658 nm, UV/Vis detector monitoring at 214 nm). Data acquisition and analysis were performed using Wyatt ASTRA software version 6.1.5.22.
4.6. Rheology quantification
Elastic modulus of the final crosslinked hydrogel was determined using a Discovery HR-2 Hybrid Rheometer and standard calculations. Compression data was collected through outputs of axial force and height, and the surface area of the hydrogels was calculated using ImageJ. Stress was calculated by dividing force by surface area (stress = F/A) and strain was calculated by change in height divided by initial height of organoids (strain = Δh/h). Stress and strain (y, x) were plotted and the elastic modulus was determined by analyzing the linear portion of the plot. Storage and loss modulus were found using the previously referenced rheometer and a shear-stress sweep test (0.1–10.0 Pa, 1.0 Hz, 25 °C) was applied to crosslinked HA-Mal or HA-MAL-RGD. The experiment was repeated twice, each time in triplicate and the average values for the storage and loss moduli, G′ and G″ respectively, for each condition were found.
4.7. Pore size and circularity quantification
SEM was used to image cell-free hydrogels. The methods described above were used to create cell-free hydrogels that were then frozen in −20 °C overnight. The hydrogels were lyophilized for 48 h, broken in half, placed onto SEM pucks and gold sputter coated. Images were taken via SEM (FlexSEM 1000, Hitachi, Tokyo, Japan) at various magnifications. Images of the hydrogel interior were taken of four representative areas within each hydrogel. Images were exported and ImageJ FUJI suite was used to find pore area and pore circularity for each hydrogel.
4.8. SR
Cell-free HA-Mal and HA-Mal-RGD were made in triplicate using the methods previously described. Hydrogels were frozen in −20 °C overnight and lyophilized for 48 h. The dry weight of each hydrogel was measured (Wdry) and then each was placed into 1.5 ml of 1× PBS for 24 h. The hydrogels were removed from PBS, gently tapped against tissue paper to remove excess water and weighed. The weight of the swelled sample was documented (Wwet) and the SR was calculated. Hydrogels were placed back into PBS (changed daily) and weighed again on days 3, 5, and 7
4.9. HSC and HepG2 organoid formation
LX-2 (HSCs, 1.5 × 107 cells ml−1), HepG2 cells (0.5 × 107 cells ml−1), or LX-2 and HepG2 cells together (1.5 × 107 and 0.5 × 107 cells ml−1, (i.e. 3:1 ratio of LX2:HepG2) respectively) were combined with HA-Mal or HA-Mal-RGD. After thorough mixing, 7.4 pH neutralized di-thiol PEG crosslinker was added, yielding a crosslinked cell-encapsulating hydrogel construct with a final volume of 25 μl. High glucose Dulbecco’s Modified Eagle’s Medium (DMEM), 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% L-glutamine were combined to make DMEM-10, and added to each construct. The cultures were maintained at 37 °C through seven days with media changed on day 4.
For experimentation, microenvironment and culture conditions were as follows: HA-Mal, HA-Mal-RGD (1 mM RGD), HA-Mal + TGF-ß via media supplementation (10 ng ml−1), or HA-Mal-RGD + TGF-ß via media supplementation [71]. All cultures were maintained over the course of seven days. The constructs were removed from media and fixed with 4% paraformaldehyde (PFA) or used directly for functionality and viability assays. Media was sampled for additional analysis.
4.10. Cell viability
On days 1, 4, and 7 cell viability was measured using the CellTiter-Glow® 3D Cell Viability Assay (Pro-mega, Madison, WI), and ATP was quantified as per the manufacturer’s protocol. Data was normalized to day 1 luminescence for each of the conditions. Comparison between each of the groups on day 4 and day 7 was done using a two-tailed Student’s t-test.
4.11. H&E staining
Constructs were fixed in 4% PFA for 6 h, embedded in paraffin, and cut into 5 μm sections and placed on glass slides. H&E staining was conducted on all organoid types (LX-2 alone, HepG2 alone, LX-2 and HepG2 combined) and the stained sections were imaged in brightfield (Leica DM4000B microscope, Leica, Wetzlar, Germany) at 10× (HepG2, LX-2 and HepG2, n = 3 organoids per hydrogel type and cell condition) or 20× (LX-2, n = 4 organoids per hydrogel type and cell condition).
4.12. Quantification of cell clusters
H&E images were converted to gray scale and thresholding with value 128 was used on all images in Adobe Photoshop. A custom MATLAB program was employed to determine cell cluster size (area, μm2) and cluster circularity (ranging from 0–1, with 1 representing perfect circle). Data for n = 3 or n = 4 samples were combined and data was normalized to mean values on day 1. Normalized data was analyzed using a two-tailed Student’s t-test.
4.13. IHC
Sections of LX-2 constructs were stained and imaged in triplicate for ECM and fibrotic specific markers (collagen, FN, FAP). Sections for staining were deparaffinized and hydrated using a slide autostainer and antigen retrieval was done using 10 mM sodium citrate pH 6.0. Sections were permeabilized for 5 min using PBS Triton-X (0.1%) then washed and blocked with protein block for 30 min. Primary antibodies anti-collagen I, FN, and FAP (ab34710, ab2413, ab207178, Abcam, Cambridge, UK) were placed on separate slides each at a 1:200 dilution in antibody diluent (Abcam) and left at 4 °C overnight. The stain was removed, and the sections were washed prior to the addition of secondary antibodies (#20152, Biotium, Fremont, CA) at a 1:200 dilution at room temperature for 1 h. The secondary antibody stain was removed, sections were washed, and 4′,6-diamidino-2-phenylindole (DAPI) was placed onto the sections at a 1:300 dilution in PBS for 10 min. After stain removal and washing, Prolong Gold was used to mount cover slips.
Fluorescence imaging was conducted using an Olympus Bx63 upright microscope. Three representative frames of 3 × 3 tiled, 10× magnification images were captured for each stain and each LX-2 construct condition. The number of nuclei with ECM component co-localization was counted as well as the total number of nuclei in each frame. Positive expression was calculated by number of positive nuclei divided by total nuclei for each stain and hydrogel condition. A two-tailed Student’s t-test was used to compare between hydrogel conditions for each stain.
LX-2 and HepG2 co-culture sections from each culture condition were stained and imaged for EpCAM. Sections of each were prepared in triplicate for staining as described above. The primary antibody for EpCam (ab71916, Abcam) was diluted 1:200 in antibody diluent and incubated overnight at 4 °C. Washing, the addition of DAPI and coverslips, and imaging were each carried out as described above.
The number of nuclei per cluster with EpCAM co-localization (considered HepG2) and without co-localization (considered LX-2) were counted and values were collected for each cluster for each condition. Size of HepG2 only clusters, LX-2 only clusters, and combined (HepG2 and LX-2) clusters were considered. For the co-culture model, the number of HepG2 versus LX-2 on average per cluster was analyzed for each hydrogel condition.
4.14. Albumin and urea assays
Media was collected on day 7 for determination of albumin and urea secretion in each culture condition. After media collection, vials were stored at −80 °C until assays were conducted. A Human Albumin ELISA Kit (Bethyl Laboratories, Inc. Montgomery, TX) was used for albumin quantification of all samples. For urea quantification, a QuantiChrom™ Urea Assay Kit (BioAssay Systems, Hayward, CA) was used. Manufacturer’s protocol was followed for both assays. A two-tail Student’s t-test was used to compare results between each hydrogel and treatment condition for either HepG2 alone or HepG2 and LX-2 co-culture.
4.15. Statistical analysis
To assess the effect of linear RGD peptides on hepatic function, comparisons were made between organoids fabricated with HA-Mal and HA-Mal-RGD. To assess the effect of an inflammatory biochemical signal, both hydrogels (i.e. HA-Mal and HA-Mal-RGD) were exposed to TGF-β. All quantitative measures had a minimum sample size of n = 3 constructs. When representative images were taken within organoid sections, n = 3 images per culture were taken. All data was tested for normal distributions; data with non-normal distributions were normalized or transformed prior to statistical analysis. Two-tailed, Student’s t-tests were conducted to determine differences between hydrogel and treatment conditions; a p-value <0.05 was considered statistically significant. GraphPad Prism was used for all statistical calculations.
5. Conclusions
Described herein is the synthesis and characterization of a modular HA-based hydrogel for use in elucidating the role of physical and chemical signals within the liver microenvironment. Through maleimide-thiol ‘click’ chemistry, ECM biomimetic RGD peptides were incorporated into HA-Mal and the material was crosslinked to create a user friendly hydrogel environment. The methods for peptide addition also allow for customization of the tissue microenvironment and invite further modulations to the ECM. This system allows for direct querying of microenvironmental variables, which we demonstrated through the formation of LX-2, HepG2, and co-cultured hepatic 3D culture models to evaluate the roles of specific physical (RGD adhesion sites) and biochemical (TGF-ß) components on tissue functionality. We anticipate that this platform will prove useful for future studies of ECM component roles in liver tissue, as well as other tissues and pathological conditions.
Acknowledgments
We would like to acknowledge funding by the Government under Other Transactions Number W81XWH-15-9-0001 (Skardal and Soker); The views and conclusions contained herein are those of the authors and should not be interpreted as necessarily representing the official policies or endorsements, either expressed or implied, of the U.S. Government. We would also like to acknowledge funding by NIH/NHLBI K25 HL133611-01A1 and K25 HL133611-04S1 (Rahbar), The Ohio State University Comprehensive Cancer Center (Skardal), and the Mike and Lucy Robbins Fellowship from the Wake Forest Baptist Comprehensive Cancer Center (Mazzocchi).
Footnotes
Conflict of interest
The authors have no competing interest to disclose.
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary files).
References
- [1].Tibbitt MW and Anseth KS 2009. Hydrogels as extracellular matrix mimics for 3D cell culture Biotechnol. Bioeng 103 655–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wei G, Wang J, Lv Q, Liu M, Xu H, Zhang H, Jin L, Yu J and Wang X 2018. Three-dimensional coculture of primary hepatocytes and stellate cells in silk scaffold improves hepatic morphology and functionality in vitro J. Biomed. Mater. Res. A 106 2171–80 [DOI] [PubMed] [Google Scholar]
- [3].Nguyen TV, Ukairo O, Khetani SR, McVay M, Kanchagar C, Seghezzi W, Ayanoglu G, Irrechukwu O and Evers R 2015. Establishment of a hepatocyte-Kupffer cell coculture model for assessment of proinflammatory cytokine effects on metabolizing enzymes and drug transporters Drug Metab. Dispos 43 774–85 [DOI] [PubMed] [Google Scholar]
- [4].Rose KA, Holman NS, Green AM, Andersen ME and LeCluyse EL 2016. Co-culture of hepatocytes and Kupffer cells as an in vitro model of inflammation and drug-induced hepatotoxicity J. Pharm Sci 105 950–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Pingitore P, Sasidharan K, Ekstrand M, Prill S, Lindén D and Romeo S 2019. Human multilineage 3D spheroids as a model of liver steatosis and fibrosis Int. J. Mol. Sci 20 1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yap KK, Gerrand Y-W, Dingle AM, Yeoh GC, Morrison WA and Mitchell GM 2020. Liver sinusoidal endothelial cells promote the differentiation and survival of mouse vascularised hepatobiliary organoids Biomaterials 251 120091. [DOI] [PubMed] [Google Scholar]
- [7].Pettinato G. et al. Generation of fully functional hepatocyte-like organoids from human induced pluripotent stem cells mixed with endothelial cells. Sci. Rep. 2019;9:8920. doi: 10.1038/s41598-019-45514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pang Y, Sutoko S, Wang Z, Horimoto Y, Montagne K, Horiguchi I, Shinohara M, Danoy M, Niino T and Sakai Y 2020. Organization of liver organoids using Raschig ring-like micro-scaffolds and triple co-culture: toward modular assembly-based scalable liver tissue engineering Med. Eng. Phys 76 69–78 [DOI] [PubMed] [Google Scholar]
- [9].Larkin AL, Rodrigues RR, Murali TM and Rajagopalan P 2013. Designing a multicellular organotypic 3D liver model with a detachable, nanoscale polymeric Space of Disse Tissue Eng. C 19 875–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Miranda JP, Rodrigues A, Tostões RM, Leite S, Zimmerman H, Carrondo MJT and Alves PM 2010. Extending hepatocyte functionality for drug-testing applications using high-viscosity alginate-encapsulated three-dimensional cultures in bioreactors Tissue Eng. C 16 1223–32 [DOI] [PubMed] [Google Scholar]
- [11].Bokhari M, Carnachan RJ, Cameron NR and Przyborski SA 2007. Culture of HepG2 liver cells on three dimensional polystyrene scaffolds enhances cell structure and function during toxicological challenge J. Anat 211 567–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Baker BM and Chen CS 2012. Deconstructing the third dimension: how 3D culture microenvironments alter cellular cues J. Cell Sci 125 3015–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Mazzocchi A. et al. Pleural effusion aspirate for use in 3D lung cancer modeling and chemotherapy screening. ACS Biomater. Sci. Eng. 2019;5:7. doi: 10.1021/acsbiomaterials.8b01356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mitragotri S and Lahann J 2009. Physical approaches to biomaterial design Nat. Mater 8 15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Maloney E. et al. Immersion bioprinting of tumor organoids in multi-well plates for increasing chemotherapy screening throughput. Micromachines. 2020;11:208. doi: 10.3390/mi11020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mazzocchi A, Devarasetty M, Huntwork R, Soker S and Skardal A 2018. Optimization of collagen type I-hyaluronan hybrid bioink for 3D bioprinted liver microenvironments Biofabrication 11 015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Skardal A et al. 2015. A hydrogel bioink toolkit for mimicking native tissue biochemical and mechanical properties in bioprinted tissue constructs Acta Biomater. 25 e53606. [DOI] [PubMed] [Google Scholar]
- [18].Caliari SR and Burdick JA 2016. A practical guide to hydrogels for cell culture Nat. Methods 13 405–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Naor D. Editorial: interaction between hyaluronic acid and its receptors (CD44, RHAMM) regulates the activity of inflammation and cancer. Front. Immunol. 2016;7:39. doi: 10.3389/fimmu.2016.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lam J, Truong NF and Segura T 2014. Design of cell–matrix interactions in hyaluronic acid hydrogel scaffolds Acta Biomater. 10 1571–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen S. et al. A laminin mimetic peptide SIKVAV-conjugated chitosan hydrogel promoting wound healing by enhancing angiogenesis, re-epithelialization and collagen deposition. J. Mater. Chem. B. 2015;3:7. doi: 10.1039/c5tb00842e. [DOI] [PubMed] [Google Scholar]
- [22].Sottile J, Hocking DC and Langenbach KJ 2000. Fibronectin polymerization stimulates cell growth by RGD-dependent and -independent mechanisms J. Cell Sci 113 4287–99 [DOI] [PubMed] [Google Scholar]
- [23].Sobers CJ, Wood SE and Mrksich M 2015. A gene expression-based comparison of cell adhesion to extracellular matrix and RGD-terminated monolayers Biomaterials 52 385–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sechler JL, Cumiskey AM, Gazzola DM and Schwarzbauer JE 2000. A novel RGD-independent fibronectin assembly pathway initiated by alpha4beta1 integrin binding to the alternatively spliced V region J. Cell Sci 113 1491–8 [DOI] [PubMed] [Google Scholar]
- [25].Takahashi S et al. 2007. The RGD motif in fibronectin is essential for development but dispensable for fibril assembly J. Cell Biol 178 167–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hahn E, Wick G, Pencev D and Timpl R 1980. Distribution of basement membrane proteins in normal and fibrotic human liver: collagen type IV, laminin, and fibronectin Gut 21 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Younesi S and Parsian H 2019. Diagnostic accuracy of glycoproteins in the assessment of liver fibrosis: a comparison between laminin, fibronectin, and hyaluronic acid Turk. J. Gastroenterol 30 524–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Christoffersson J, Aronsson C, Jury M, Selegård R, Aili D and Mandenius C-F 2018. Fabrication of modular hyaluronan-PEG hydrogels to support 3D cultures of hepatocytes in a perfused liver-on-a-chip device Biofabrication 11 015013. [DOI] [PubMed] [Google Scholar]
- [29].Agarwal T, Subramanian B and Maiti TK 2019. Liver tissue engineering: challenges and opportunities ACS Biomater. Sci. Eng 5 5. [DOI] [PubMed] [Google Scholar]
- [30].Friedman SL 2008. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver Physiol. Rev 88 125–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Heindryckx F and Gerwins P 2015. Targeting the tumor stroma in hepatocellular carcinoma World J. Hepatol 7 165–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Agostoni C, Mazzocchi A, Leone L, Ciappolino V, Delvecchio G, Altamura CA and Brambilla P 2017. The first model of keeping energy balance and optimal psycho affective development: breastfed infants J. Affective Disorders 224 10–15 [DOI] [PubMed] [Google Scholar]
- [33].Hellerbrand C, Stefanovic B, Giordano F, Burchardt ER and Brenner DA 1999. The role of TGFbeta1 in initiating hepatic stellate cell activation in vivo J. Hepatol 30 77–87 [DOI] [PubMed] [Google Scholar]
- [34].Tsuchida T and Friedman SL 2017. Mechanisms of hepatic stellate cell activation Nat. Rev. Gastroenterol. Hepatol 14 397–411 [DOI] [PubMed] [Google Scholar]
- [35].Busso N, Chesne C, Delers F, Morel F and Guillouzo A 1990. Transforming growth-factor-beta (TGF-beta) inhibits albumin synthesis in normal human hepatocytes and in hepatoma HepG2 cells Biochem. Biophys. Res. Commun 171 647–54 [DOI] [PubMed] [Google Scholar]
- [36].Leite SB, Roosens T, El Taghdouini A, Mannaerts I, Smout AJ, Najimi M, Sokal E, Noor F, Chesne C and van Grunsven LA 2016. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro Biomaterials 78 1–10 [DOI] [PubMed] [Google Scholar]
- [37].Guan Y. et al. Human hepatic organoids for the analysis of human genetic diseases. JCI Insight. 2017;2:e94954. doi: 10.1172/jci.insight.94954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Guan Y. et al. A human multi-lineage hepatic organoid model for liver fibrosis. Nat. Commun. 2021;12:6138. doi: 10.1038/s41467-021-26410-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Aisenbrey EA and Murphy WL 2020. Synthetic alternatives to Matrigel Nat. Rev. Mater 5 539–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nguyen EH. et al. Versatile synthetic alternatives to Matrigel for vascular toxicity screening and stem cell expansion. Nat. Biomed. Eng. 2017;1:0096. doi: 10.1038/s41551-017-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Brovold M, Keller D and Soker S 2020. Differential fibrotic phenotypes of hepatic stellate cells within 3D liver organoids Biotechnol. Bioeng 117 2516–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Devarasetty M, Wang E, Soker S and Skardal A 2017. Mesenchymal stem cells support growth and organization of host-liver colorectal-tumor organoids and possibly resistance to chemotherapy Biofabrication 9 021002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Skardal A, Devarasetty M, Kang H-W, Seol Y-J, Forsythe SD, Bishop C, Shupe T, Soker S and Atala A 2016. Bioprinting cellularized constructs using a tissue-specific hydrogel bioink J. Vis. Exp 110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Skardal A, Smith L, Bharadwaj S, Atala A, Soker S and Zhang Y 2012. Tissue specific synthetic ECM hydrogels for 3D in vitro maintenance of hepatocyte function Biomaterials 33 4565–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Leach JB, Bivens KA, Collins CN and Schmidt CE 2004. Development of photocrosslinkable hyaluronic acid-polyethylene glycol-peptide composite hydrogels for soft tissue engineering J. Biomed. Mater. Res. A 70 74–82 [DOI] [PubMed] [Google Scholar]
- [46].Teng B et al. 2021. A chondrogenesis induction system based on a functionalized hyaluronic acid hydrogel sequentially promoting hMSC proliferation, condensation, differentiation, and matrix deposition Acta Biomater. 122 145–59 [DOI] [PubMed] [Google Scholar]
- [47].Roy T, James BD and Allen JB 2021. Anti-VEGF-R2 aptamer and RGD peptide synergize in a bifunctional hydrogel for enhanced angiogenic potential Macromol. Biosci 21 e2000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Yao Y, Wang P, Li X, Xu Y, Lu G, Jiang Q, Sun Y, Fan Y and Zhang X 2020. A di-self-crosslinking hyaluronan-based hydrogel combined with type I collagen to construct a biomimetic injectable cartilage-filling scaffold Acta Biomater. 111 197–207 [DOI] [PubMed] [Google Scholar]
- [49].Fan M, Ma Y, Zhang Z, Mao J, Tan H and Hu X 2015. Biodegradable hyaluronic acid hydrogels to control release of dexamethasone through aqueous Diels–Alder chemistry for adipose tissue engineering Mater. Sci. Eng. C 56 311–7 [DOI] [PubMed] [Google Scholar]
- [50].Rodell CB, Wade RJ, Purcell BP, Dusaj NN and Burdick JA 2015. Selective proteolytic degradation of guest-host assembled, injectable hyaluronic acid hydrogels ACS Biomater. Sci. Eng 1 277–86 [DOI] [PubMed] [Google Scholar]
- [51].Holloway JL, Ma H, Rai R and Burdick JA 2014. Modulating hydrogel crosslink density and degradation to control bone morphogenetic protein delivery and in vivo bone formation J. Control. Release 191 63–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Ren Y, Zhang H, Qin W, Du B, Liu L and Yang J 2020. A collagen mimetic peptide-modified hyaluronic acid hydrogel system with enzymatically mediated degradation for mesenchymal stem cell differentiation Mater. Sci. Eng. C 108 110276. [DOI] [PubMed] [Google Scholar]
- [53].de Bartolo L et al. 2005. Biotransformation and liver-specific functions of human hepatocytes in culture on RGD-immobilized plasma-processed membranes Biomaterials 26 4432–41 [DOI] [PubMed] [Google Scholar]
- [54].Pinkse GG, Jiawan-Lalai R, Bruijn JA and de Heer E 2005. RGD peptides confer survival to hepatocytes via the beta1-integrin-ILK-pAkt pathway J. Hepatol 42 87–93 [DOI] [PubMed] [Google Scholar]
- [55].Vashist A and Ahmad S 2015. Hydrogels in tissue engineering: scope and applications Curr. Pharm. Biotechnol 16 606–20 [DOI] [PubMed] [Google Scholar]
- [56].Ye S, Boeter JWB, Penning LC, Spee B and Schneeberger K 2019. Hydrogels for liver tissue engineering Bioengineering 6 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Baldwin AD and Kiick KL 2011. Tunable degradation of maleimide-thiol adducts in reducing environments Bioconjugate Chem. 22 1946–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Jansen LE, Negron-Pineiro LJ, Galarza S and Peyton SR 2018. Control of thiol-maleimide reaction kinetics in PEG hydrogel networks Acta Biomater. 70 120–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Aleman J, George SK, Herberg S, Devarasetty M, Porada CD, Skardal A and Almeida-Porada G 2019. Deconstructed microfluidic bone marrow on-a-chip to study normal and malignant hemopoietic cell–niche interactions Small 15 e1902971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Aleman J and Skardal A 2018. A multi-site metastasis-on-a-chip microphysiological system for assessing metastatic preference of cancer cells Biotechnol. Bioeng 116 936–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Forsythe S, Mehta N, Devarasetty M, Sivakumar H, Gmeiner W, Soker S, Votanopoulos K and Skardal A 2019. Development of a colorectal cancer 3D micro-tumor construct platform from cell lines and patient tumor biospecimens for standard-of-care and experimental drug screening Ann. Biomed. Eng 48 940–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Rajan SAP et al. 2020. Probing prodrug metabolism and reciprocal toxicity with an integrated and humanized multi-tissue organ-on-a-chip platform Acta Biomater. 106 124–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ouyang L, Armstrong JPK, Lin Y, Wojciechowski JP, Lee-Reeves C, Hachim D, Zhou K, Burdick JA and Stevens MM 2020. Expanding and optimizing 3D bioprinting capabilities using complementary network bioinks Sci. Adv 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Forsythe SD, Devarasetty M, Shupe T, Bishop C, Atala A, Soker S and Skardal A 2018. Environmental toxin screening using human-derived 3D bioengineered liver and cardiac organoids Front. Public Health 6 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Skardal A. et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci. Rep. 2017;7:8837. doi: 10.1038/s41598-017-08879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dominijanni A, Devarasetty M and Soker S 2020. Manipulating the tumor microenvironment in tumor organoids induces phenotypic changes and chemoresistance iScience 23 101851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Skardal A. et al. Drug compound screening in single and integrated multi-organoid body-on-a-chip systems. Biofabrication. 2020;12:025017. doi: 10.1088/1758-5090/ab6d36. [DOI] [PubMed] [Google Scholar]
- [68].Liaw CY, Ji S and Guvendiren M 2018. Engineering 3D hydrogels for personalized in vitro human tissue models Adv Healthc Mater 7 [DOI] [PubMed] [Google Scholar]
- [69].Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS and Burdick JA 2013. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels Nat. Mater 12 458–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ananda K, Nacharaju P, Smith PK, Acharya SA and Manjula BN 2008. Analysis of functionalization of methoxy-PEG as maleimide-PEG Anal. Biochem 374 231–42 [DOI] [PubMed] [Google Scholar]
- [71].Lin X-L, Liu M, Liu Y, Hu H, Pan Y, Zou W, Fan X and Hu X 2018. Transforming growth factor beta1 promotes migration and invasion in HepG2 cells: epithelial-to-mesenchymal transition via JAK/STAT3 signaling Int. J. Mol. Med 41 129–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data that support the findings of this study are included within the article (and any supplementary files).


