Abstract
Entry into sporulation in Bacillus subtilis is characterized by the formation of a polar septum, which asymmetrically divides the developing cell into forespore (the smaller cell) and mother cell compartments, and by migration of replication origin regions to extreme opposite poles of the cell. Here we show that polar septation is closely correlated with movement of replication origins to the extreme poles of the cell. Replication origin regions were visualized by the use of a cassette of tandem copies of lacO that had been inserted in the chromosome near the origin of replication and decorated with green fluorescent protein-LacI. The results showed that extreme polar placement of replication origin regions is not under sporulation control and occurred in stationary phase under conditions under which entry into sporulation was prevented. On the other hand, the formation of a polar septum, which is under sporulation control, was almost invariably associated with the presence of a replication origin region in the forespore. Moreover, cells in which the polar placement of origin regions was perturbed by deletion of the gene (smc) for the structural maintenance of chromosomes (SMC) protein were impaired in polar division. A small proportion (≈1%) of the mutant cells were able to undergo asymmetric division, but the forespore compartment of these exceptional cells was generally observed to contain a replication origin region. Immunofluorescence microscopy experiments indicated that the block in polar division caused by the absence of SMC occurred at or prior to the step of bipolar Z-ring formation by the cell division protein FtsZ. A model is discussed in which polar division is under the dual control of sporulation and an event associated with the placement of a replication origin at the cell pole.
The spore-forming bacterium Bacillus subtilis exhibits two principal modes of cell division. Under conditions of vegetative growth, the bacterium undergoes binary fission, in which a septum is formed at the midcell position. Binary fission gives rise to two equal-size progeny cells. In contrast, during sporulation, the developing cell undergoes a process of asymmetric division in which a septum is formed near one pole of the cell. This results in the formation of dissimilar-size progeny, called the forespore (the smaller cell) and the mother cell (18). This switch from symmetric to asymmetric division upon entry into sporulation is governed by the master regulator for sporulation, the response regulator Spo0A (14). An unknown gene or genes under the control of Spo0A causes the site of formation of the cytokinetic Z ring, which is composed of the tubulin-like protein FtsZ (1), to shift from the midcell position to sites near both poles of the cell. Subsequent events in sporulation allow cytokinesis to occur at only one of the two polar Z rings, resulting in the formation of a single polar septum. Both symmetric division and asymmetric division involve the partitioning of a chromosome to each progeny cell, but some of the features of chromosome segregation in these two processes differ sharply. The first step in chromosome segregation in both cases involves the movement of newly duplicated replication origin regions toward opposite poles of the cell (7, 15, 22). This is followed by, or concurrent with, condensation of the chromosomes in a manner that may be mediated by a bacterial homologue of the SMC (structural maintenance of chromosomes) family of chromosome-condensing proteins (8). SMC proteins are present in eukaryotes and prokaryotes and are essential for chromosome condensation and segregation (19), probably by introducing positive writhe into DNA (13). In growing cells, the medial septum is formed after a chromosome has been largely or completely partitioned to the nascent progeny. In contrast, in sporulating cells, cytokinesis occurs before a complete chromosome is partitioned to the forespore. Instead, following polar septation, only the origin-proximal one-third of the chromosome is located in the forespore; the remainder of the chromosome is pumped across the septum from the mother cell into the forespore by the DNA translocase SpoIIIE, which is itself located in the septum (23–25).
Here we are concerned with the initial events of asymmetric division and the coordination of the polar placement of replication origin regions with the formation of an asymmetrically positioned septum. Our results show that the process by which replication origin regions become localized at the extreme poles of the cell is not under sporulation control. Nonetheless, the formation of the sporulation septum is closely correlated with the presence of a replication origin region at an extreme polar position. We also report experiments in which the normal polar localization of the replication origin is perturbed by the use of a null mutant lacking SMC. Such a mutant is conditionally lethal but is capable of growth at low temperatures (2, 9, 17), under which conditions spore formation occurs but at a low efficiency. Here we show that this block occurs extremely early in sporulation, prior to asymmetric division and prior to the formation of bipolar Z rings, and that spore formation and polar septation in SMC mutant cells are closely correlated with, and possibly dependent on, the extreme polar placement of a replication origin at the cell pole at which the sporulation septum will form. The significance of our findings is discussed, including the possible existence of a checkpoint mechanism that links polar division to the polar placement of a replication origin.
MATERIALS AND METHODS
Bacterial strains.
Strain PG63 (lacO cassette at 359° pveg-gfp-lacI smc::kan) was constructed by transformation of AT63 (lacO cassette at 359° pveg-gfp-lacI) (20) with chromosomal DNA from PGΔ388 (smc::kan) (9). Strain PG7 (lacO cassette at 359° pveg-gfp-lacI spo0A::spec) was created by transformation of strain AT63 (20) with chromosomal DNA from RL2242 (spo0A::spec) (5). Strain PG11 (lacO cassette at 359° pveg-gfp-lacI divIVa::spec) was constructed by transformation of AT63 with chromosomal DNA from FG22 (divIVA::spec; a gift from F. Gueiros Filho, Harvard University). Strain FG24, which contains a translational fusion of spoIIE to the gene (gus) for β-glucuronidase (gus) (spoIIE-gus) located at amyE, was obtained from F. Gueiros Filho. PG3 (smc::kan spoIIE-gus) was created by transformation of FG24 with chromosomal DNA from PGΔ388 (smc::kan) (9). Strain PG13 (spoIIE-gus spo0A::spec) was constructed by transformation of strain FG24 with chromosomal DNA from RL2242 (spo0A::spec) (5).
Conditions for growth and sporulation.
Because some of the experiments described in this report were carried out with cells with mutated smc and because such cells are unable to grow at 37°C, all experiments, that is, those with both smc+ cells and cells with mutant smc, were carried out at the permissive temperature of 23°C.
With the exception of the experiment whose results are shown in Fig. 1C (in which Luria-Bertani [LB] medium was used), all of the experiments reported here were carried out with Difco Sporulation (DS) medium. T0 is defined as the end of exponential growth in DS medium. In parallel work (data not shown), similar results were obtained in all cases by using cells that had been induced to sporulate by suspension in CH medium (10).
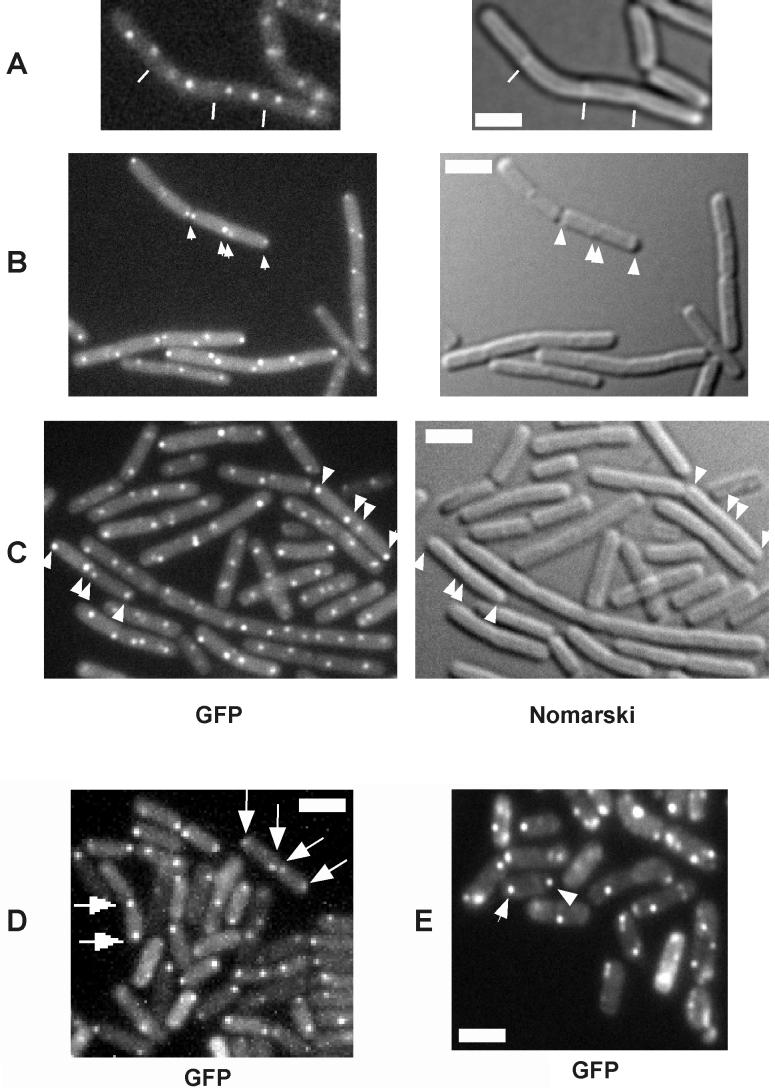
FIG. 1.
Fluorescence and Nomarski differential interference contrast microscopy of cells harboring a lacO cassette near the origin of replication and producing GFP-LacI. Unless indicated otherwise, the cells were grown and sporulated in DS medium. Representative fluorescent foci are indicated by arrows, and representative septa are indicated by white lines. Scale bars (white rectangles), 2 μm; A, cells of strain AT63 (wild type) during exponential growth; B, cells of strain PG7 (spo0A::spec) at hour 3 of sporulation; C, cells of AT63 at 3 h after the end of exponential-phase growth in nonsporulation (LB) medium; D, AT63 cells at hour 3 of sporulation; E, PG63 cells (smc::kan) at hour 3 of sporulation.
Measurement of heat-resistant spores was carried out by heating the cells to 80°C for 20 min (10) at 60 h after the start of sporulation. A time course experiment showed that at 23°C, the maximum number of heat-resistant spores is not reached until at least 48 h (compared to 14 h for sporulation carried out at 37°C).
Fluorescence microscopy.
An Olympus BX60 microscope and a Princeton Instruments MicroMax charge-coupled device camera were used for image acquisition. Metamorph software was used for image processing and measurement of distances. FM4-64 (Molecular Probes) was used for membrane staining at 1 nM, and 4′,6′-diamidino-2-phenylindole (DAPI) was used at 2 μg/ml for staining of DNA.
Measurement of β-glucuronidase activity.
The activity of β-glucuronidase was measured as described for the assay of β-galactosidase activity (16), except that the substrate was p-nitrophenyl-β-d-glucuronide (6 mM).
RESULTS
Strategy.
The purpose of this investigation was to study the connection between the process of polar septation during sporulation and the extreme polar localization of DNA replication origins. As part of our strategy, we took advantage, in certain experiments described below, of the mislocalization of replication origins (8) observed in cells with mutant SMC protein. Because cells lacking SMC are unable to grow at 37°C, all experiments with both smc+ cells and cells with mutant smc were carried out at the permissive temperature of 23°C.
Polar placement of replication origins is not under sporulation control.
Following duplication, replication origins move apart, toward opposite poles of the cell but do so in a more extreme manner in cells that have entered sporulation than in cells that are in the exponential phase of growth (7, 15, 22). This can be seen through a comparison of Fig. 1A and D, which display fluorescent images of cells (strain AT63) harboring a lacO cassette inserted into the chromosome near the origin of replication and decorated with green fluorescent protein (GFP)-LacI. Florescent foci corresponding to origin regions were well separated from each other in both growing cells and cells under sporulation conditions but were further apart and in closer proximity to the extreme poles of the cells in the latter. We calculated that the average separation between origins in growing cells was 1.37 μm (among 120 cells counted), whereas the separation at the start of sporulation was 2.3 μm (among 122 cells counted). In contrast, regions near the terminus of replication localized to midcell positions (21, 22; data not shown). We investigated whether the extreme polar placement of origin regions was a specific feature of entry into sporulation by examining the localization of the lacO cassette in cells mutant for spo0A, a transcription factor that governs entry into sporulation. Figure 1B shows that fluorescent foci corresponding to the origin region exhibited extreme polar localization in cells of a spo0A mutant (strain PG7). Among 300 mutant cells observed that exhibited bipolar fluorescent foci, 77% exhibited extreme polar localization, compared to 78% for the wild type (among 450 cells counted). Similar results were also obtained with a spo0H mutant when Spo0J-GFP was used as a marker for origin localization (data not shown). However, the spo0H mutant often produced long cells that contained four origin signals at a time when wild-type cells showed only two signals per cell (data not shown). This finding could indicate that the last round of medial cell division during entry into sporulation is partially dependent on ςH, as discussed previously in the context of ςH-directed transcription of the ftsA-ftsZ cell division operon (6). Interestingly, extreme polar localization of origin regions was also observed at the transition to stationary phase in cells of the wild type (AT63) that had been grown in a rich (LB) medium that does not support sporulation (Fig. 1C), although the frequency was lower (45%) than that observed in sporulation medium (≈80%). We concluded that the movement of origin regions to the extreme poles of cells is not under sporulation control but rather is a feature of cells that have entered stationary phase.
Polar septation and polar localization of replication origin regions are coordinated.
To investigate the coordination of polar septation with the polar localization of the replication origin region, cells of strain AT63 were treated with the vital membrane stain FM4-64 at the onset of sporulation. Among 5,000 cells examined with clearly visible fluorescent foci, no case was observed of a sporangium with a polar septum in which both fluorescent foci were located outside of the forespore (Fig. 2A). That is, among sporangia in which two fluorescent foci could be detected (95% of the cells), the presence of a polar septum was always associated with the presence of a fluorescent focus (replication origin) in the forespore compartment.
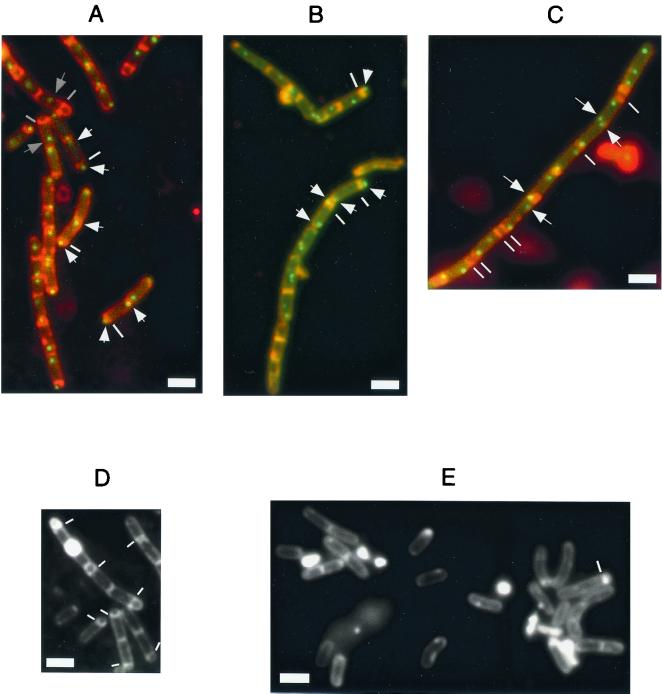
FIG. 2.
Fluorescence microscopy of cells harboring a lacO cassette near the origin of replication and producing GFP-LacI during sporulation. A to C, merged images of GFP fluorescence and FM4-64 staining; D and E, FM4-64 staining; A, wild type cells (AT63) at hour 5; B, smc mutant cells (PG63) at hour 5; C, divIVA mutant cells (PG11) at hour 3; D, wild-type cells at hour 7; E, smc mutant cells at hour 7. Scale bars (white rectangles), 2 μm. The white lines indicate polar septa, except in panel C, where the lines indicate double septa, which occur during exponential growth of the divIVA mutant. The white arrows indicate polar origin signals. Gray lines indicate septa in sporangia that had reached the stage of engulfment, and gray arrows identify origins that were no longer located at an extreme polar position in such sporangia. Scale bars (white rectangles), 2 μm.
Time course experiments showed that the percentage of cells with extreme bipolar localization of origin regions was highest 3.5 h after initiation of sporulation at 23°C (76%; data not shown). In agreement with the observations of Webb et al. (22), origin regions moved away from the poles following polar septation. In cells that had proceeded to engulfment (gray bars in Fig. 2A), the origin in the mother cell was generally no longer found at the extreme pole of the cell (signals in the forespore were no longer visible at this point). Similarly, following medial septation in spo0A mutant cells, origin signals were observed at random positions in the cells (data not shown). These findings indicate that extreme polar localization of origin regions is coordinated with polar septation but is transient.
Polar localization of origins does not require the presence of septa. One possible explanation for the extreme polar placement of replication origins is that during entry into sporulation newly duplicated chromosomal origin regions become captured at, and attached to, the poles of the sporangium. Alternatively, origin regions may simply move further apart at the onset of sporulation than during exponential growth, until they reach the cell poles. To distinguish between these possibilities, we investigated the localization of origin regions in a mutant that is defective in septation. Deletion of the cell division gene divIVA causes the formation of long, aseptate filaments during growth (3, 4) and an approximately 100-fold reduction in sporulation efficiency. We introduced a divIVA null mutation into a strain carrying chromosomal origins tagged with GFP-LacI, generating strain PG11. During exponential growth, regularly spaced origin signals were observed in long, aseptate filaments (data not shown). The average distance measured by using METAMORPH software was found to be similar to that found in wild-type cells (≈1.4 μm). Additionally, the mutant cells produced minicells that generally did not contain origin signals (data not shown).
At the onset of sporulation, origin signals separated an average distance of ≈2.5 μm in divIVA mutant cells. This is similar to the average distance of separation (2.3 μm) measured for wild-type cells, although, in contrast to wild-type cells, the divIVA mutant formed filaments that lacked septa. Interestingly, a pair of origin foci, each from a separate segregating pair of chromosomes, were frequently observed next to each other in the absence of a septum between the two origin regions (Fig. 2C, arrows; observed in 42 of 50 filaments analyzed). We interpreted these findings to indicate that the extreme separation of origins at the onset of sporulation does not depend on septation and that origin regions are not, or least need not be, captured at the cell poles.
Coordination of axial filament formation and polar Z rings with polar localization of replication origin regions.
Two of the earliest morphologic markers for entry into sporulation are axial filament formation and the appearance of polar Z rings. Following entry into sporulation but prior to asymmetric division (stage I), the nucleoid adopts a structure known as the axial filament, which extends to both poles of the sporangium (14). Examples of axial filaments (labeled A) are presented in the DAPI-stained cells of Fig. 3. In contrast, growing cells contain one or two nucleoids that are more condensed than axial filaments and generally do not extend to the extreme poles of the cell (Fig. 3, labeled N). Also, following entry into sporulation, the site of ring formation by FtsZ switches from the midcell position to sites near the cell poles. We investigated whether axial filament formation and polar Z-ring formation are accompanied by the localization of origins to the extreme poles. We used wild-type cells (strain AT63) carrying a lacO cassette to visualize origin regions. To visualize Z rings, we carried out immunofluorescence microscopy by using anti-FtsZ antibodies after fixing the cells. Polar Z rings are indicated with white lines in Fig. 3, and medial rings are indicated with the letter M. Fluorescent foci from GFP-LacI were more difficult to observe in the fixed cells than in living cells. Nevertheless, we were able to collect images on a significant number of cells that showed both FtsZ and GFP signals. We distinguished bipolar origins from those located at the extreme cell poles by visual inspection (compare gray with white arrows in Fig. 3) and by measuring the distances between fluorescent foci. Distances between origins in cells with axial filaments varied from 1.6 to 2.0 μm (average, 1.83 μm), whereas the distance between origins in cells with central FtsZ rings was between 0.6 and 1.5 μm (average, 1.1 μm).
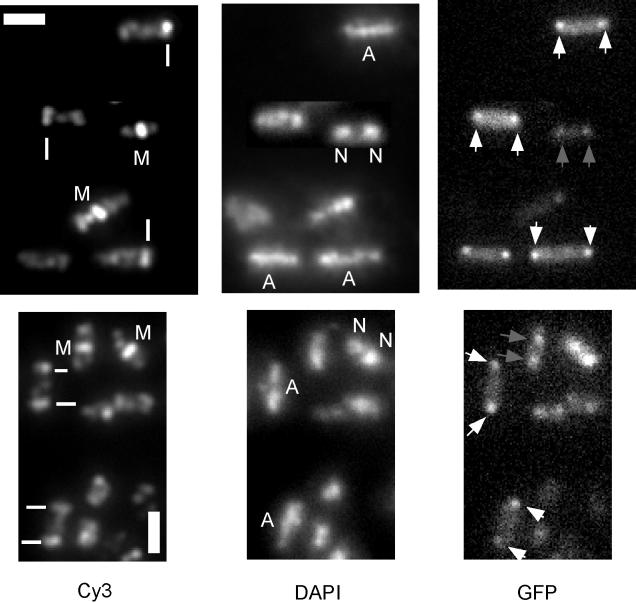
FIG. 3.
Immunofluorescence microscopy of wild-type cells (AT63) cells harboring a lacO cassette near the origin of replication and producing GFP-LacI at hour 3 of sporulation. Cells were stained for FtsZ with anti-FtsZ antibodies and Cy3-conjugated secondary antibodies and for DNA with DAPI. Scale bars (white rectangles), 2 μm; white lines, polar FtsZ rings; M, medial FtsZ rings; N, nucleoids in growing cells; A, axial filaments in sporulating cells. White arrows identify origins located at the extreme cell poles in sporulating cells, and gray arrows indicate bipolar origins in growing cells.
In 30 out of 31 cases of cells that had one or two polar Z rings (indicated by white bars) and an axial filament, chromosomal origins were localized to the extreme poles. In 46 out of 51 cases of cells that had axial filaments but no visible FtsZ rings, origins were also localized at extreme polar positions. In contrast, in 38 out of 39 cases of cells with a medial FtsZ ring and two separated nucleoids, origins were not located at the extreme cell poles. These findings suggest that at the onset of sporulation, axial filament formation (stage I) is generally accompanied by extreme polar positioning of origins and by polar positioning of FtsZ rings.
Sporulation defect in cells carrying a deletion of the smc gene.
A deletion of the smc gene results in chromosome decondensation, slow and temperature-dependent growth (2, 9, 17), and a defect in sporulation (2). In addition, the arrangement of the chromosome is perturbed in growing cells of an smc mutant (8). Because the extreme separation of origin regions is characteristic of cells that have entered sporulation, we wondered whether the defect in chromosome arrangement caused by an smc mutation would impede sporulation at an early stage. By measuring the production of heat-resistant spores, we found that the mutant sporulated at an efficiency of 0.8%, compared to 76% for the wild type. Use of the vital membrane stain FM4-64 revealed that the mutant cells were blocked prior to the stage of asymmetric division; only about 0.3% of the cells had a polar septum (Fig. 2E) at a time when about 65% of the wild-type cells showed a polar septum or had proceeded to later stages of development (Fig. 2D).
Next, we investigated the localization of origin regions in smc mutant cells at the onset of sporulation. In contrast to wild-type cells, which showed two origin signals localized at extreme opposite poles of the sporangium (Fig. 1D), mutant cells variably contained one to four signals and these were localized at more or less random positions in the sporangium (Fig. 1E). Thus, in the absence of SMC, the arrangement of the chromosome is perturbed not only during growth (8) but also during initiation of sporulation. Nonetheless, at a low frequency (about 1.2% of 210 cells counted), mutant cells were observed that contained two signals close to or at opposite cell poles (arrows, Fig. 1E).
Presence of polar septa is associated with polar origin in smc mutant cells.
In spite of their defect in chromosome arrangement, smc mutant cells are able to sporulate at a low frequency. We wondered whether mutant cells that were capable of sporulating were those in which a replication origin was located at the extreme forespore pole. To investigate this smc mutant, cells carrying origin regions decorated with GFP-LacI were stained with FM4-64 after the initiation of sporulation so that both origin foci and polar septa could be visualized. We observed over 20,000 cells at times ranging from 3 to 8 h after entry into sporulation. About 1% of these cells had polar septa (indicated by white bars in Fig. 2B), and of these, 84 exhibited clear fluorescent foci (arrows, Fig. 2B). Of the 84 cells, 81 showed a fluorescent focus in the forespore, with the other signal generally close to or at the other pole of the cell (Fig. 2B), although the origin in the mother cell was found at various positions in some cells (e.g., the upper cell indicated in Fig. 2B). These findings are consistent with the idea that the formation of a polar septum is dependent upon the presence of a replication origin region near the pole of the cell.
smc mutant cells are defective in Z-ring switching.
The correlation between polar localization of replication origins and polar septation in smc mutant cells prompted us to ask whether septum formation was blocked prior to the formation of polar Z rings. We performed immunofluorescence microscopy (14) on wild-type and mutant cells by using affinity-purified anti-FtsZ antibodies. The results showed that among wild-type cells in which a Z ring(s) was present (82 out of 340 cells), only medial Z rings were observed at hour 2 of sporulation. At hour 3, 120 cells exhibited polar or bipolar Z rings and 108 exhibited medial rings out of a total of 440 cells observed. At hours 4 to 5, the pattern was predominantly polar Z rings, and by hour 6, the pattern was almost exclusively polar Z rings (Fig. 4A, indicated by white bars). By hour 6, 10% of the cells exhibited a condensed forespore chromosome (Fig. 4A, indicated by arrows), which is characteristic of sporangia that had replaced the polar Z rings with a polar septum. Polar Z rings became less frequent by hour 7.
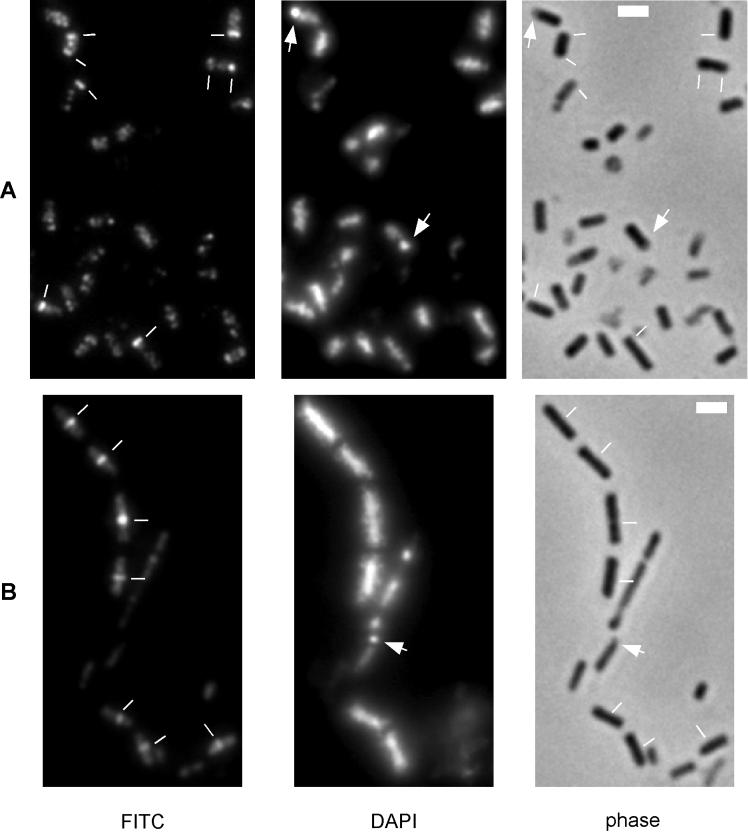
FIG. 4.
Immunofluorescence microscopy of wild-type (A) and smc mutant (B) cells at hour 4 of sporulation at 23°C. FITC secondary antibodies were used that resulted in staining of cells indistinguishable from Cy3-coupled secondary antibodies. Scale bars (white rectangles), 2 μm; white lines, positions of Z rings; arrows, condensed forespore nucleoids.
In contrast, polar septa were rarely detected in sporulating cells of the smc mutant. Among 1,250 mutant cells observed between hours 3 and 7, only 28 exhibited Z rings close to the cell poles and 388 showed a medial Z ring. At hour 6, Z rings were still predominantly close to the midcell position (Fig. 4B) and rarely close to the poles. Even from 12 to 18 h, polar rings were rarely observed, in contrast to medial Z rings. We concluded that the defect in polar septation in smc mutant cells occurs at or before the formation of polar Z rings. In agreement with the finding that about 1% of smc mutant cells were able to sporulate, some mutant cells could be detected that exhibited a condensed forespore chromosome (Fig. 4B, arrow), although the abundance of such sporangia was less than 1% at hours 6 to 8 of sporulation.
Spo0A activity in smc mutant cells.
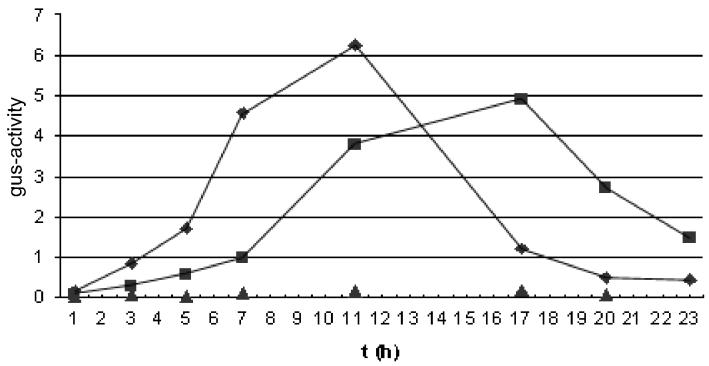
It is known that Z-ring switching depends on the activation of Spo0A (14), which acts in part by turning on the transcription of spoIIE (12). Conceivably, the defect in chromosome organization caused by the smc mutation causes a block in Spo0A activation. To investigate this possibility, we introduced a fusion of the gus reporter to spoIIE into the wild type and an smc mutant to create strains PG1 and PG5, respectively. Figure 5 shows that Spo0A-directed expression of spoIIE (see Materials and Methods) was modestly lower in PG5 than in PG1 and that the time course of expression was slower in the mutant than in the wild type. Thus, the smc mutation does have a modest effect on Spo0A activity, but it seems unlikely that this effect is adequate to explain the defect in Z-ring switching and polar septation caused by the absence of SMC.
FIG. 5.
Induction of a spoIIE-gus fusion during sporulation at 23°C of wild-type cells (FG24; ♦), of smc mutant cells (PG3; ▪), and of spo0A mutant cells (PG13; ▴).
DISCUSSION
This work provides three principal contributions to the problem of chromosome segregation at the onset of sporulation. First, we have shown that the movement of replication origins to the extreme poles of the sporangium is not under sporulation control. It occurs in a mutant (spo0A) blocked in entry into sporulation and in wild-type cells grown under nonsporulation conditions. These findings show that extreme separation of origins is a consequence of the last round of chromosome segregation before the cessation of growth. Interestingly, extreme separation of origins occurred even in filaments in the absence of septa, indicating that origin regions are separated as far as possible but are not held at or attached to the cell poles. Second, we have found that formation of the sporulation septum is closely coordinated with the extreme polar placement of a replication origin. Among 5,000 sporangia examined, we failed to detect a case in which both replication origin regions were located outside of the forespore. This observation indicates the existence of a mechanism for linking of asymmetric division to polar placement of the origin region. The accuracy of this process is comparable to that of chromosome segregation during normal growth, where only 1 in 10,000 cells does not receive a chromosome complement (11). The presence of polar FtsZ rings was generally accompanied by axial filament formation and extreme separation of origin regions, suggesting that coordination of chromosome structure and polar division may occur at the stage of FtsZ ring switching.
Third, and consistent with this idea, we obtained evidence that polar septation depends on the movement of an origin to the extreme pole of the sporangium. This evidence was obtained by the use of an smc mutant in which the normal layout of the chromosome is perturbed (8), such that during entry into sporulation, replication origins were found at extreme polar positions in only a small proportion of the cells. smc mutant cells are defective in sporulation (2), and we have shown that this defect occurs prior to the formation of the polar septum and, indeed, prior to the switch in the site of Z-ring formation from the midcell to the poles of the cell. Importantly, however, a small proportion of mutant cells were able to undergo asymmetric division and in these exceptional cases a replication origin was found at the extreme pole. This correlation between polar division and polar placement of the replication origin is consistent with the hypothesis that polar division depends on the proper organization of the chromosome.
We favor the idea that a checkpoint mechanism exists that delays the formation of polar Z rings until and unless a replication origin reaches the extreme pole of the sporangium. This is an attractive idea because it would help to explain the high fidelity of the segregation of a chromosome into the forespore. Were septation to occur prior to the movement of an origin to the pole, then the subsequent SpoIIIE-dependent process of DNA translocation (23) could not take place and the resulting forespore would be devoid of a chromosome. Indeed, minicells that occur in divIVA mutant cells during growth (3, 4), where origins are not separated to the extreme cell poles (21), did not contain origin signals or other chromosomal DNA. An alternative possibility is that disorganization of the chromosome caused by the absence of SMC is sensed by the phosphorelay, resulting in impaired activation of Spo0A. It is known that Z-ring switching depends on Spo0A-directed gene transcription (14), and hence, a defect in Spo0A activation could, in principle, account for the block in polar septation. We investigated this hypothesis by monitoring Spo0A activity and found that the smc mutation caused only a modest impairment of Spo0A-directed transcription. We therefore favor the view that polar division is somehow coupled to the movement of replication origins to the extreme poles. Distinguishing definitively between the checkpoint hypothesis and an effect on Spo0A activity will, however, require the identification of the gene or genes under Spo0A control that mediate the switch in the site of Z-ring formation. In any event, the formation of the polar septum appears to be under the control of dual mechanisms: a sporulation-specific control mediated through the activation of Spo0A and a non-sporulation-specific mechanism involving the machinery for chromosome partitioning.
ACKNOWLEDGMENTS
We thank F. Gueiros Filho for the gift of unpublished strains and J. Kemp for the gift of affinity-purified anti-FtsZ antibodies.
This work was supported by NIH grant GM15868 to R.L. P.L.G. was a postdoctoral fellow of the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Beall B, Lutkenhaus J. FtsZ in Bacillus subtilis is required for vegetative septation and for asymmetric septation during sporulation. Genes Dev. 1991;5:447–455. doi: 10.1101/gad.5.3.447. [DOI] [PubMed] [Google Scholar]
- 2.Britton R A, Lin D C, Grossman A D. Characterization of a prokaryotic SMC protein involved in chromosome partitioning. Genes Dev. 1998;12:1254–1259. doi: 10.1101/gad.12.9.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cha J H, Stewart G C. The divIVA minicell locus of Bacillus subtilis. J Bacteriol. 1997;179:1671–1683. doi: 10.1128/jb.179.5.1671-1683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edwards D H, Errington J. The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division. Mol Microbiol. 1997;24:905–915. doi: 10.1046/j.1365-2958.1997.3811764.x. [DOI] [PubMed] [Google Scholar]
- 5.Fawcett P, Eichenberger P, Losick R, Youngman P. The transcriptional profile of early to middle sporulation in Bacillus subtilis. Proc Natl Acad Sci USA. 2000;97:8063–8068. doi: 10.1073/pnas.140209597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gholamhoseinian A, Shen Z, Wu J J, Piggot P. Regulation of transcription of the cell division gene ftsA during sporulation of Bacillus subtilis. J Bacteriol. 1992;174:4647–4656. doi: 10.1128/jb.174.14.4647-4656.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glaser P, Sharpe M E, Raether B, Perego M, Ohlsen K, Errington J. Dynamic, mitotic-like behavior of a bacterial protein required for accurate chromosome partitioning. Genes Dev. 1997;11:1160–1168. doi: 10.1101/gad.11.9.1160. [DOI] [PubMed] [Google Scholar]
- 8.Graumann P L. Bacillus subtilis SMC is required for proper arrangement of the chromosome and for efficient segregation of replication termini but not for bipolar movement of newly duplicated origin regions. J Bacteriol. 2000;182:6463–6471. doi: 10.1128/jb.182.22.6463-6471.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graumann P L, Losick R, Strunnikov A V. Subcellular localization of Bacillus subtilis SMC, a protein involved in chromosome condensation and segregation. J Bacteriol. 1998;180:5749–5755. doi: 10.1128/jb.180.21.5749-5755.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harwood C R, Cutting S M. Molecular biological methods for Bacillus. New York, N.Y: John Wiley & Sons, Inc.; 1990. [Google Scholar]
- 11.Ireton K, Gunther A D, Grossman A D. spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J Bacteriol. 1994;176:5320–5329. doi: 10.1128/jb.176.17.5320-5329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khvorova A, Zhang L, Higgins M L, Piggot P J. The spoIIE locus is involved in the Spo0A-dependent switch in the location of FtsZ rings in Bacillus subtilis. J Bacteriol. 1998;180:1256–1260. doi: 10.1128/jb.180.5.1256-1260.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimura K, Rybenkov V V, Crisona N J, Hirano T, Cozzarelli N R. 13S condensin actively reconfigures DNA by introducing global positive writhe: implications for chromosome condensation. Cell. 1999;98:239–248. doi: 10.1016/s0092-8674(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 14.Levin P A, Losick R. Transcription factor Spo0A switches the localization of the cell division protein FtsZ from a medial to a bipolar pattern in Bacillus subtilis. Genes Dev. 1996;10:478–488. doi: 10.1101/gad.10.4.478. [DOI] [PubMed] [Google Scholar]
- 15.Lin D C-H, Levin P A, Grossman A D. Bipolar localization of a chromosome partition protein to Bacillus subtilis. Proc Natl Acad Sci USA. 1997;94:4721–4726. doi: 10.1073/pnas.94.9.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 17.Moriya S, Tsujikawa E, Hassan A K, Asai K, Kodama T, Ogasawara N. A Bacillus subtilis gene encoding protein homologous to eukaryotic SMC motor protein is necessary for chromosome partition. Mol Microbiol. 1998;29:179–187. doi: 10.1046/j.1365-2958.1998.00919.x. [DOI] [PubMed] [Google Scholar]
- 18.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 19.Strunnikov A V, Jessberger R. Structural maintenance of chromosomes (SMC) proteins: conserved molecular properties for multiple biological functions. Eur J Biochem. 1999;263:6–13. doi: 10.1046/j.1432-1327.1999.00509.x. [DOI] [PubMed] [Google Scholar]
- 20.Teleman A A, Graumann P L, Lin D C H-, Grossman A D, Losick R. Chromosome arrangement within a bacterium. Curr Biol. 1998;8:1102–1109. doi: 10.1016/s0960-9822(98)70464-6. [DOI] [PubMed] [Google Scholar]
- 21.Webb C D, Graumann P L, Kahana J, Teleman A A, Silver P, Losick R. Use of time-lapse microscopy to visualize rapid movement of the replication origin region of the chromosome during the cell cycle in Bacillus subtilis. Mol Microbiol. 1998;28:883–892. doi: 10.1046/j.1365-2958.1998.00808.x. [DOI] [PubMed] [Google Scholar]
- 22.Webb C D, Teleman A, Gordon S, Straight A, Belmont A, Lin D C-H, Grossman A D, Wright A, Losick R. Bipolar localization of the replication origin regions of chromosomes in vegetative and sporulating cells of B. subtilis. Cell. 1997;88:667–674. doi: 10.1016/s0092-8674(00)81909-1. [DOI] [PubMed] [Google Scholar]
- 23.Wu L J, Errington J. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science. 1994;264:572–575. doi: 10.1126/science.8160014. [DOI] [PubMed] [Google Scholar]
- 24.Wu L J, Errington J. Septal localization of the SpoIIIE chromosome partitioning protein in Bacillus subtilis. EMBO J. 1997;16:2161–2169. doi: 10.1093/emboj/16.8.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu L J, Errington J. Use of asymmetric cell division and spoIIIE mutants to probe chromosome orientation and organization in Bacillus subtilis. Mol Microbiol. 1998;27:777–786. doi: 10.1046/j.1365-2958.1998.00724.x. [DOI] [PubMed] [Google Scholar]