Abstract
Background and Aims
Kidney stone disease is a crystal concretion formed usually within the kidneys. The worldwide prevalence of kidney stones could be affected by numerous differences in socioeconomic, and environmental factors. The purpose of this study is to investigate kidney stones among Iranian adults aged between 35 and 70 years and the prevalence and evaluation of socioeconomic inequalities.
Methods
In this, a cohort‐based cross‐sectional study was carried out among 20,427 participants of ArNCDs aged 35 and 70 years old. Kidney stone was defined as a dependent (and binary) variable while demographic and socioeconomic factors were considered independent variables. Multivariable logistic regression was used to identify the key socioeconomic factors affecting kidney stone prevalence in Ardabil.
Results
The overall prevalence of kidney stones was 17.6 (95% confidence interval [CI]: 16.1–19.2) out of which 21.53% and 14.36% pertained to men and women and 14.18% and 23.17% pertained to the poorest and richest groups, respectively. There was a significant difference in the prevalence of kidney stones between men and women in terms of age, marital status, education level, chronic disease, body mass index, and socioeconomic status (<0.001). The prevalence of kidney stones had a positive correlation with age (1.7, 95% [CI]: 1.42–2.04) and socioeconomic status (1.5, 95% [CI]: 1.34–1.69), where the odds of kidney stones increased significantly by increasing age and socioeconomic status. Moreover, the kidney stone concentration index showed a pro‐rich distribution wherein it was more common among wealthy people (higher socioeconomic status) 0.062 (95% [CI]: 0.051–0.072).
Conclusion
The results of this study showed that there is significant inequality in the prevalence of kidney stones, where it was more common among the richest people. In addition, being men and old age are significantly related to kidney stones, so policymakers and physicians should consider these factors.
Keywords: age‐dependency, kidney stone, prevalence, socioeconomic status
1. INTRODUCTION
The formation of stones in the urinary system is a problem that has plagued humans since the beginning of medical history. 1 Urinary stones are currently the third most common disease of the urinary tract, wherein its incidence has steadily increased over the past few decades. 2 The probability of the formation of kidney stones varies in different parts of the world. It is estimated to be 1%–5% in Asia, 5%–9% in Europe, 3% in North America, and 20% in Saudi Arabia 3 , 4 Countries on the African–American belt (stretches from Egypt and Sudan to the Middle East, India, Pakistan, Thailand, Indonesia, and the Philippines) and tropical and subtropical regions reported high rates of kidney stones. 5 In various studies, the prevalence of kidney stones in Iran was 1.9%–5.7% with a higher prevalence in western provinces. 6 , 7 Kidney stones are an important health issue that sometimes leads to surgery. 8 Kidney stones pose a significant health burden to a working‐age population. 9 Besides the physical problems, kidney stones cost a lot accounting for 9% of hospitalization in the United States with an average length of 3 days and $1.83 billion a year. 10 As a result, control of this situation, especially in developing countries, relies heavily on preventing the growth of old stones and formation of new ones. 11
Indeed, socioeconomic status including social classes based on family income, occupation and education, living in different parts of a city, and housing status have a significant effect on the occurrence and prevalence of diseases. 12 Studies have demonstrated that various epidemiological and demographic factors as predisposing factors are involved in kidney stone incidence. As per results, the socioeconomic and lifestyle factors have an active role in the risk of kidney stones. 13 Data from various studies show an association between kidney stone disease and weight gain, body mass index (BMI), and diabetes. On the other hand, the diversity in genetics, ethnicity, and diet in different regions has been considered the cause of kidney stones. 14 Furthermore, the increased incidence of kidney stones has also been linked to habitat changes, arid climate, obesity, food, dehydration, and global warming. 15 Kidney stones have been associated with an increased risk of chronic kidney diseases, end‐stage renal failure, cardiovascular diseases, diabetes, and hypertension. 16 , 17 , 18 It has been suggested that kidney stones may be a systemic disorder linked to metabolic syndrome. 19 Nephrolithiasis is responsible for 2%–3% of end‐stage renal cases if it is associated with nephrocalcinosis. 18 Therefore, identifying the associated risk factors could be useful in diagnosing and treating pathogenesis. 20 In patients with a high risk of kidney stone recurrence, an extensive metabolic workup is recommended to identify stone risk factors and to initiate a specific diet and medical treatment for each patient. 21 , 22 In general, patients with kidney stones are advised to consume a lot of fluids, a lot of fruits and vegetables, limited sodium and enough calcium. In addition, all patients should be encouraged to have adequate physical activity and maintain a normal weight. 23 To our knowledge, no study has been carried out to examine the socioeconomic inequality of kidney stone prevalence in Ardabil province, Iran. In addition, no historical research has used concentration indices to analyze aspects of inequality in the prevalence of kidney stones. This study filled this gap in the literature by calculating the socioeconomic differences in the prevalence of kidney stones in Iran using a rich data set with a national representation.
2. METHODS
2.1. Study setting and sample
This population‐based cross‐sectional analysis was performed in Ardabil, the capital of Ardabil province in northwest Iran, with approximately 610,000 inhabitants. 24 The data was collected from the Iran Prospective Epidemiological Research Studies, 25 and analyzed to provide the sense required to reform health care policy in the context of noncommunicable diseases. One of the 18 geographically distinct PERSIAN cohort research areas is the Ardabil Non‐Communicable Disease (ArNCD) Cohort Study. It is important to locate the specifics of the sampling design in someplace. 25
Participants in the sample featured people from predominantly Azeri ethnicities. A total of 20,525 adults between the ages of 35–70 of both sexes living in Ardabil were enrolled as PERSIAN cohort targets and its rigorous protocol from May 2017 to February 2020. The study included only individuals living in Ardabil with Iranian citizenship. The PERSIAN cohort omitted deaf, mute, palsy, and individuals with neurological disabilities, mental retardation, and any psychiatric condition in an acute stage. The qualified interviewers conducted the cohort questionnaire. Considering the missing details, the final research sample size was 20,427 people.
2.2. Data and variables
Kidney stone, the dependent (and binary) variable of this study, was measured based on current smoking status. The current smokers were defined as those who smoked one or more cigarettes a day for at least a month, or who registered to stop smoking for less than 1 month. The independent variable was age (categorized from 35 to 70 years), gender (male/female), marital status (single/married/divorced/other), education status (illiterate/primary/tips/diploma/academic degree), BMI (underweight/normal/overweight/obesity), and occupational status, and data on noncommunicable diseases (cardiovascular disease, diabetes, and hypertension). These data were collected based on the self‐declaration of the participants, the outcomes of clinical studies, and the invitation to collect their clinical records. The wealth index was also calculated as the participant's socioeconomic status based on their self‐reported wealth, and it was divided into five quintiles from 1st quintile as the poorest to 5th quintile as the richest group. The assets, homeownership, home area, and car price were considered for the calculation of the wealth index.
2.3. Statistical analysis
The data were analyzed using Stata software, version14.0.0. The findings were dichotomized to have no kidney stones for the study. Kolmogorov–Smirnov test was used before running models to detect the normality of prevalence and data distribution. The categorical variables were defined as proportions, with their respective 95% confidence intervals (95% CI). The means as dispersion scales for the empirical factors, the standard deviation, and interquartile intervals were measured. Poisson regression was used to obtain the crude and modified prevalence and their corresponding 95% CI. To measure the correlation and odds ratio based on multivariate logistic regression, demographic variables, and chronic disease status were included in the model.
3. RESULTS
Totally, data from 20,427 participants of ArNCDs were included in this study. Table 1 shows the prevalence of kidney stones based on the imported variables and calculated subgroup comparison. The overall adjusted prevalence of kidney stones in the Ardabil population was 17.6% (95% CI: 16.1–19.2). The adjusted prevalence of kidney stone in men (21.53, 95% CI: 20.7–22.3) were higher than in women (14.36, 95% CI: 13.6–14.93). The weighted prevalence was increased with age in both sexes, where the prevalence of kidney stones for under 40 years old and above 65 years old groups of men was 17.72 (95% CI: 15.9–19.6) and 21.36 (95% CI: 18.1–25.0). In the case of women, they were 11.47 (95% CI: 10.2–12.7) and 18.28 (95% CI: 15.1–21.8), respectively. There was a significant difference between married men and women regarding kidney stone prevalence (p < 0.001). The prevalence of kidney stones in both men and women decreased with an increasing level of education. The prevalence of kidney stones for illiterate men and women was 22.07% (95% CI: 20.6–23.6) and 14.33% (95% CI: 13.2–15.5), respectively. A significant difference was detected among all education levels, between men and women (p < 0.001), where men showed a higher prevalence of kidney stones in all education groups. The results showed that kidney stone prevalence was approximately the same in men and women with different underlying diseases. The prevalence of kidney stones was significantly higher in underweight individuals. The prevalence of kidney stones in men and women of this group was respectively 28.88% (95% CI: 17.5–43.6) and 18.18 (95% CI: 10.0–30.6), and significantly difference (χ 2 = 185.17, p < 0.001). On the other hand, there was a significant difference between the poorest and richest groups in the prevalence of kidney stones (χ 2 = 38.24, p < 0.001), where the prevalence was higher in the richest people with 23.17 (95% CI: 21.6–24.7) in men and 14.18 (95% CI: 12.4–16.1) in women. A significant difference was also observed in kidney stone prevalence between men and women in all socioeconomic groups (p < 0.001).
Table 1.
Percent prevalence of kidney stones by population characteristic and calculating the difference between subgroups in terms of kidney stone prevalence
| Male | Female | p value | |||||
|---|---|---|---|---|---|---|---|
| n | proportion | (95% CI) | n | proportion | (95% CI) | ||
| Age categories | |||||||
| <40 | 278 | 17.72 | 15.91–19.69 | 283 | 11.47 | 10.27–12.79 | <0.001 |
| 40–44 | 273 | 19.75 | 17.73–21.93 | 245 | 13.61 | 12.10–15.28 | <0.001 |
| 45–49 | 425 | 21.90 | 20.12–23.80 | 315 | 15.00 | 13.54–16.60 | <0.001 |
| 50–54 | 386 | 22.38 | 20.48–24.41 | 260 | 14.19 | 12.66–15.86 | <0.001 |
| 55–59 | 312 | 24.18 | 21.92–26.59 | 217 | 15.18 | 13.41–17.14 | <0.001 |
| 60–64 | 230 | 24.67 | 22.01–27.54 | 168 | 17.74 | 15.43–20.30 | <0.001 |
| >65 | 116 | 21.36 | 18.11–25.01 | 94 | 18.28 | 15.17–21.86 | 0.120 |
| Marital status | |||||||
| Single | 27 | 16.87 | 11.83–23.49 | 27 | 15.42 | 10.79–21.56 | 0.105 |
| Married | 1813 | 21.71 | 20.84–22.61 | 1402 | 14.10 | 13.43–14.80 | <0.001 |
| divorced | 111 | 20.10 | 16.97–23.66 | 103 | 17.08 | 14.28–20.29 | 0.325 |
| Other | 31 | 19.74 | 14.24–26.71 | 24 | 15.48 | 10.59–22.06 | 0.333 |
| Education | |||||||
| Illiterate | 646 | 22.07 | 20.60–23.60 | 503 | 14.33 | 13.20–15.52 | <0.001 |
| Primary | 437 | 21.32 | 19.60–23.15 | 354 | 14.37 | 13.04–15.81 | <0.001 |
| Tips | 308 | 22.20 | 20.09–24.47 | 248 | 15.28 | 13.61–17.11 | <0.001 |
| Diploma | 331 | 20.66 | 18.74–22.71 | 238 | 13.27 | 11.78–14.92 | <0.001 |
| Academic degree | 260 | 20.66 | 18.51–22.99 | 214 | 14.39 | 12.69–16.26 | <0.001 |
| Chronic disease | |||||||
| Have diabetes | 234 | 21.80 | 19.43–24.37 | 193 | 15.26 | 13.38–17.35 | <0.001 |
| Have hypertension | 404 | 21.12 | 19.35–23.01 | 322 | 14.37 | 12.98–15.88 | <0.001 |
| Have cardiac ischemic | 163 | 21.47 | 18.69–24.54 | 145 | 15.74 | 13.53–18.24 | 0.004 |
| BMI categorized | |||||||
| Underweight | 13 | 28.88 | 17.57–43.63 | 10 | 18.18 | 10.07–30.60 | 0.526 |
| Normal weight | 308 | 21.73 | 19.66–23.96 | 234 | 13.59 | 12.05–15.29 | <0.001 |
| Overweight | 834 | 21.60 | 20.33–22.93 | 627 | 14.14 | 13.14–15.20 | <0.001 |
| obesity | 825 | 21.20 | 19.95–22.52 | 684 | 14.69 | 13.70–15.74 | <0.001 |
| Socioeconomic status (SES) | |||||||
| Poorest | 231 | 18.06 | 16.04–20.26 | 401 | 14.27 | 13.02–15.61 | <0.001 |
| Poor | 286 | 20.60 | 18.55–22.81 | 383 | 14.15 | 12.89–15.51 | <0.001 |
| Middle | 352 | 21.87 | 19.92–23.96 | 372 | 15.01 | 13.65–16.47 | <0.001 |
| Rich | 506 | 21.72 | 20.09–23.44 | 236 | 13.40 | 11.89–15.08 | <0.001 |
| Richest | 639 | 23.17 | 21.63–24.78 | 188 | 14.18 | 12.41–16.17 | <0.001 |
Abbreviations: BMI, body mass index; CI, confidence interval; ref, Reference group; SES, socioeconomic status.
Associations between kidney stones and age, gender, level of education, marital status, noncommunicable disease, BMI groups, and socioeconomic status were observed using multivariate logistic regression (Table 2). Based on the results, the prevalence of kidney stones was significantly increased by age, where the probability of kidney stones in the 65 years and older group was about 1.7 (95% CI: 1.42–2.04) times higher than the youngest group, as the reference group. The probability of kidney stones was 1.07 (95% CI: 1.42–2.04) in males, but there was no significant association between sex and the probability of kidney stones (p = 0.051). Besides, insignificant associations were found between kidney stones and marital status, educational level, and chronic diseases such as diabetes, hypertension, and cardiac ischemia (p > 0.05). In contrast, a significant association was detected between kidney stones and socioeconomic status wherein the probability of kidney stones was significantly increased by increasing the socioeconomic status level. As per the results, the probability of kidney stones among the richest group was about 1.5 (95% CI: 1.34–1.69) times higher than the poorest group, as the reference group.
Table 2.
Multivariable logistic regression model for the association between socio‐demographic factors and Kidney stone among Ardabil population
| Odds ratio | ||||
|---|---|---|---|---|
| Crude (95% CI) | p value | Adjusted (95% CI) | p value | |
| Age categories | ||||
| <40 (ref.) | 1 | – | 1 | – |
| 40–44 | 1.20 (1.05–1.37) | 0.005 | 1.22 (1.07–1.40) | 0.002 |
| 45–49 | 1.38 (1.23–1.56) | <0.001 | 1.42 (1.26–1.61) | <0.001 |
| 50–54 | 1.37 (1.21–1.55) | <0.001 | 1.42 (1.26–1.62) | <0.001 |
| 55–59 | 1.49 (1.31–1.70) | <0.001 | 1.58 (1.38–1.80) | <0.001 |
| 60–64 | 1.66 (1.44–1.91) | <0.001 | 1.80 (1.55–2.08) | <0.001 |
| >65 | 1.53 (1.28–1.82) | <0.001 | 1.70 (1.42–2.04) | <0.001 |
| Sex | ||||
| male | 1.07 (0.99–1.15) | 0.058 | 1.07 (0.99–1.16) | 0.051 |
| Marital status | ||||
| Single (ref.) | 1 | – | 1 | – |
| Married | 1.10 (0.82–1.48) | 0.486 | 1.11 (0.82–1.49) | 0.478 |
| divorced | 1.18 (0.85–1.63) | 0.311 | 1.15 (0.83–1.60) | 0.386 |
| Other | 1.11 (0.73–1.67) | 0.609 | 1.08 (0.72–1.64) | 0.690 |
| Education | ||||
| Illiterate (ref.) | 1 | – | 1 | – |
| Primary | 0.97 (0.88–1.08) | 0.667 | 0.99 (0.90–1.10) | 0.964 |
| Tips | 1.04 (0.93–1.16) | 0.464 | 1.06 (0.94–1.19) | 0.305 |
| Diploma | 0.92 (0.82–1.03) | 0.176 | 0.94 (0.83–1.05) | 0.290 |
| Academic degree | 0.96 (0.85–1.08) | 0.503 | 0.98 (0.86–1.11) | 0.771 |
| Chronic disease | ||||
| Have diabetes | 1.05 (0.94–1.17) | 0.370 | 1.05 (0.92–1.18) | 0.433 |
| Have hypertension | 0.98 (0.90–1.08) | 0.816 | 0.96 (0.86–1.06) | 0.435 |
| Have cardiac ischemic | 1.05 (0.92–1.20) | 0.415 | 1.05 (0.91–1.120) | 0.453 |
| BMI categorized | ||||
| Normal weight (ref.) | 1 | – | 1 | – |
| Underweight | 1.43 (0.88–2.30) | 0.139 | 1.41 (0.87–2.28) | 0.152 |
| Overweight | 1.02 (0.91–1.14) | 0.665 | 1.00 (0.90–1.12) | 0.862 |
| obesity | 1.02 (0.92–1.14) | 0.624 | 1.00 (0.89–1.12) | 0.992 |
| Socioeconomic quintiles | ||||
| Poorest (ref.) | 1 | – | 1 | – |
| Poor | 1.06 (0.94–1.20) | 0.274 | 1.10 (0.97–1.24) | 0.115 |
| Middle | 1.17 (1.04–1.32) | 0.006 | 1.23 (1.09–1.39) | 0.001 |
| Rich | 1.21 (1.07–1.36) | 0.001 | 1.29 (1.15–1.46) | <0.001 |
| Richest | 1.38 (1.23–1.55) | <0.001 | 1.50 (1.34–1.69) | <0.001 |
Abbreviations: BMI, body mass index; CI, confidence interval; ref, Reference group.
The results related to inequality in kidney stone prevalence in the study population based on the socioeconomic level and gender are reported in Table 3 and Figure 1. The estimated Cn was 0.062 (95% CI: 0.051–0.072) for the entire population, and was 0.083 (95% CI: 0.072–0.093) for the age group. This estimation showed that kidney stone was more common among people with higher socioeconomic status. The socioeconomic inequality in kidney stone was significant for age groups where kidney stones are common among older people. Based on the results of the focus curves, the kidney stone concentration curves for the study population and age groups were below the equality line, indicating that kidney stone was more common among wealthy people (Figure 1).
Table 3.
Normalized concentration indices for kidney stones among Ardabil PERSIAN Cohort participants based on Wagstaff norm between SES quintiles and age groups
| Concentration index | |||
|---|---|---|---|
| Normalized concentration index | 95% CI | p value | |
| Socioeconomic status | 0.062 | 0.051–0.072 | <0.001 |
| Age | 0.083 | 0.072–0.093 | <0.001 |
Note: No. of Obs. = number of observations.
Abbreviations: BMI, body mass index; CI, confidence interval.
Figure 1.
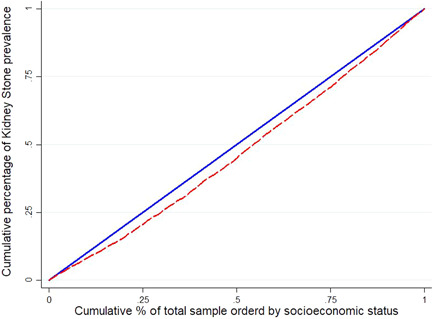
Concentration curve for kidney stone prevalence among total samples (A) and age groups (B) based on the wealth index
4. DISCUSSION
This study showed that the incidence of kidney stones in patients aged 35–70 years was 17.6% during 2017–2018. The findings revealed that the prevalence of kidney stone disease in Ardabil was significantly higher than the average prevalence in Iran (13.6%). 26 This value varies from 3.5% to 18.5%, depending on the countries or regions. 27 , 28 For instance, it was reported to be 14.8% in Turkey, approximately 10% in the United States, and 5.9%–10.6% in China. 29 , 30 , 31
The results of the present study showed that the kidney stone prevalence was, respectively, 21.53% and 14.36% in men and women. The results were consistent with the hypothesis that men are more likely to develop kidney stones, possibly due to high protein and salt intake compared to women, and that older men are more likely to develop the disease due to male hormones.
As per several studies, the average prevalence of kidney stones in men and women is between 7%–15% and 3%–6%, respectively. 32 The prevalence ratio of kidney stones in men to women has been generally higher than one, 33 for example, it has been reported to be 1.5 in Turkey and 5 in Saudi Arabia. 29 , 34 As per some empirical information, the higher prevalence of kidney stones in men could result from the effect of sex hormones on a number of kidney stone risk factors. Reportedly, androgen can increase the urinary excretion of oxalate and its deposition in the kidney whereas estrogen acts inversely. 35 Still, this requires further studies with large sample sizes.
In this study, the prevalence of kidney stones in men and women significantly increased with age, which was in line with other studies' findings. 34 , 36 The age of onset of kidney stones has been reported to be 30–50 years old in various studies. 37
At the same time, the prevalence of kidney stones was reduced with an increase in education level. Although insignificant, both men and women with higher education levels had a lower chance of developing kidney stones. As an illustration, in the Tefekli et al. study in Turkey in 2005, most of the patients with kidney stones were at nonuniversity education levels. 37 Additionally, Konjengbam's et al. 38 reported that higher academic education was associated with a reduced risk of developing kidney stones. The probable cause of the reduced risk of developing stones with an increased level of education could be due to the differences in their diet and behavioral habits. this is because as the level of education improves, the level of health literacy of individuals also increases, which in turn reduces the risk of noncommunicable diseases, including kidney stones. 38
The results exposed a disproportionate distribution of kidney stone prevalence in participants in terms of BMI wherein the prevalence was low in obese people, but the highest was in overweight people. Although the differences between men and women in the prevalence of kidney stones were significant as per BMI, no significant relationship was found between the increased BMI and the prevalence of kidney stones. In contrast, several studies have reported the elevated risk of kidney stones in association with obesity, weight gain, and BMI. 35 , 39 From a physiological point of view, obesity is associated with increased renal excretion of calcium and uric acid, thus increased urinary acidity all of which the risk of stone formation is increasing. 40 , 41 In a study by Taylor et al. 42 it was found that obesity and weight gain increased the risk of developing kidney stones, higher in women than in men. In another study, Sarıca et al. showed that obesity can boost the excretion of stone constituents into the urine. 43 On the contrary, the urinary calcium secretion in obese people was lower than in ordinary people. Two hypotheses concerning the role of obesity in developing kidney stones can be proposed: (1) Obesity itself is a risk factor for kidney stones, and (2) Obesity is associated with another risk factor for developing urinary stones, such as increased calcium or oxalate secretion. 43 Assuming obesity as a symptom of metabolic syndrome, which is epidemiologically and physiologically related to the risk of kidney stones, the high rate of obesity in Ardabil, especially among women, could be an explanation for the significant increase in the incidence of kidney stones. The mechanism of stone formation includes nucleation of stone constituent crystals, their growth or aggregation to a size that can interact with some intrarenal structure, their retention within the kidney or renal collecting system and further aggregation and/or secondary nucleation to form the clinical stone. 44 The crystals form either in renal tubular fluid or in the renal interstitial fluid that is supersaturated with respect to these constituents, which in turn may be a consequence of increased excretion of stone constituent molecules, reduced urine volume, an alteration in urine pH, or a combination of these factors. 45 , 46
The socioeconomic inequality indicators employed in the present study showed that there was a severe prorich inequality in the prevalence of kidney stones. As per the results, the probability of kidney stone occurrence among the richest group was about 1.5 times higher than in the poor group where the distance between the richest and poorest groups was 8.47%. One of the important factors could be the fact that rich and poor individuals follow different lifestyles and nutrition habits. As per studies, nutrition habit plays an important role in the onset and recurrence of kidney stone disease. 33 For instance, some studies have shown that vegetarians faced a lower risk of developing kidney stones than those on a high‐meat diet. 47 The available scientific evidences supports the harmful effects of high consumption of red meat and low calcium diets, while the high portion of fruits and vegetables along with the balanced consumption of low‐fat dairy products result in the lowest risk of kidney stones. 48 Therefore, since the accessibility of rich people to high‐protein foods could be higher than the poor, the prevalence of kidney stones showed a higher rate in the rich group.
This is the first study in Iran, as a developing country, that has investigated the disparity in kidney stones as one of the most common kidney diseases. The findings of this study provide important implications for patients with kidney stones in Ardabil. The results of this study suggest that various factors can be useful in preventing kidney stones. The rapid change in the prevalence of kidney stones implies that an effort should be made to prevent recurrence. 48 , 49
The significance of the present study was the large size of the population sample in the PERSIAN Cohort. According to the standard data collection protocol, all the required information was recorded and analyzed with high accuracy. In addition, this study is among the rare studies investigating socioeconomic inequality in the prevalence of kidney stones at the global and national levels. The only limitation of the present study was using the self‐reported method to diagnose kidney stones in participants. However, the reports were confirmed by checking the final ultrasound results of the participants.
5. CONCLUSIONS
The prevalence of kidney stones in the population of 35–70 years old in northwest Iran (Ardabil) was significantly high. The results of this study showed that kidney stone incidence can be affected by socioeconomic factors which were more common among men, the elderly, and individuals with high socioeconomic status. Furthermore, socioeconomic inequality was among the most influential factors in the prevalence of the disease. The socioeconomic inequality in kidney stones was significant for age groups where kidney stones are common among older people. Economic status and access to high‐protein nutrition can be one of the main factors affecting socioeconomic inequalities in the prevalence of kidney stones. New curricula and policies must be developed in health systems to reduce these inequalities.
In our study, the results of logit regression predicted the probability of kidney stones in Iran. The results of this study are a background for future studies. It is suggested that in future studies, the diet of people with urinary stones and those without stones should be investigated as a case‐control study and the causes of stone formation should be investigated based on socioeconomic inequality. Also, the reasons for not visiting a doctor in people with stones should be investigated.
AUTHOR CONTRIBUTIONS
Telma Zahirian Moghadam: conceptualization; data curation; investigation; resources; validation; writing—original draft. Farhad Pourfarzi: data curation; funding acquisition; methodology; supervision; validation; writing—review & editing. Hamed Mohseni Rad: conceptualization; data curation; funding acquisition; project administration; resources. Hamed Zandian: conceptualization; formal analysis; methodology; software; visualization; writing—original draft.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS STATEMENT
This study has been approved by the ethical committee of Ardabil University of Medical Sciences (ARUMS) with code IR.ARUMS.REC.1399.072. The Ardabil PERSIAN cohort data was used where all participants signed the informed consent.
TRANSPARENCY STATEMENT
The lead author Hamed Mohseni Rad, Hamed Zandian affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
ACKNOWLEDGMENTS
This project was financially supported by National Institute for Medical Research Development (NIMAD: 962249). In addition, this work was funded by the Ardabil University of Medical Sciences (ARUMS). The funder had no forms of support and financial involvement (e.g., employment, consultancies, honoraria, stock ownership and options, expert testimony, grants or patents received or pending, royalties) and the nonfinancial relationships (personal, political, or professional) that may potentially influence the writing of the manuscript (study design, data analysis, decision to publish, or preparation of the manuscript).
Zahirian Moghadam T, Pourfarzi F, Mohseni Rad H, Zandian H. Kidney stones among Iranian adults: prevalence and socioeconomic inequality assessment in a cohort‐based cross‐sectional study. Health Sci Rep. 2022;5:e877. 10.1002/hsr2.877
Contributor Information
Hamed Mohseni Rad, Email: sirhamed2@gmail.com.
Hamed Zandian, Email: Zandian.hamed899@gmail.com.
DATA AVAILABILITY STATEMENT
All data will be available by formal requests from corresponding authors and approving the request in the core committee of the PERSIAN cohort study.
REFERENCES
- 1. Sohgaura A, Bigoniya P. A review on epidemiology and etiology of renal stone. Am J Drug Discov Dev. 2017;7(2):54‐62. [Google Scholar]
- 2. Jung JH, Park J, Kim WT, et al. The association of benign prostatic hyperplasia with lower urinary tract stones in adult men: a retrospective multicenter study. Asian J Urol. 2018;5(2):118‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Khan Z, Siddiqui N, Alastal Y, Sodeman T, Nawras A. P182 epidemiology of nephrolithiasis in hospitalized patients of inflammatory bowel disease: national inpatient sample analysis 2002–2014. Inflamm Bowel Dis. 2018;24(suppl_1):S70‐S71. [Google Scholar]
- 4. Ziemba JB, Matlaga BR. Epidemiology and economics of nephrolithiasis. Investig Clin Urol. 2017;58(5):299‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delvecchio FC, Preminger GM. Medical management of stone disease. Curr Opin Urol. 2003;13(3):229‐233. [DOI] [PubMed] [Google Scholar]
- 6. Nikpay S, Moradi K, Azami M, Babashahi M, Otaghi M, Borji M. Frequency of kidney stone different compositions in patients referred to a lithotripsy center in Ilam, West of Iran. J Ped Nephrol. 2016;4(3):102‐107. [Google Scholar]
- 7. Rafiei H, Malekpoor F, Amiri M, Madiseh MR. Kidney stone development among older adults in Iran. J Indian Acad Geriatr. 2014;10(24):10‐13. [Google Scholar]
- 8. Anderson RA. A complementary approach to urolithiasis prevention. World J Urol. 2002;20(5):294‐301. [DOI] [PubMed] [Google Scholar]
- 9. Ferraro PM, Ticinesi A, Meschi T, et al. Short‐term changes in urinary relative supersaturation predict recurrence of kidney stones: a tool to guide preventive measures in urolithiasis. J Urol. 2018;200(5):1082‐1087. [DOI] [PubMed] [Google Scholar]
- 10. Assimos D, Krambeck A, Miller NL, et al. Surgical management of stones: American urological association/endourological society guideline, PART I. J Urol. 2016;196(4):1153‐1160. [DOI] [PubMed] [Google Scholar]
- 11. Canvasser NE, Alken P, Lipkin M, et al. The economics of stone disease. World J Urol. 2017;35(9):1321‐1329. [DOI] [PubMed] [Google Scholar]
- 12. Sortsø C, Lauridsen J, Emneus M, Green A, Jensen PB. Socioeconomic inequality of diabetes patients' health care utilization in Denmark. Health Econ Rev. 2017;7(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khongji P, Socio economic and demographic differentials in the prevalence of chronic diseases across states in North East India. 2017.
- 14. Aune D, Mahamat‐Saleh Y, Norat T, Riboli E. Body fatness, diabetes, physical activity and risk of kidney stones: a systematic review and meta‐analysis of cohort studies. Springer; 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson RJ, Stenvinkel P, Jensen T, et al. Metabolic and kidney diseases in the setting of climate change, water shortage, and survival factors. J Am Soc Nephrol. 2016;27(8):2247‐2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aleebrahim‐Dehkordi E, Soleiman‐Dehkordi E, Saberianpour S, Hasanpour‐Dehkordi A, Hasanpour Dehkordi A. Care and prevention during the COVID‐19 pandemic quarantine: sedentary lifestyle and increased risk of kidney stones. Przegl Epidemiol. 2021;75:45‐50. [DOI] [PubMed] [Google Scholar]
- 17. Hyrich KL, Machado PM. Rheumatic disease and COVID‐19: epidemiology and outcomes. Nat Rev Rheumatol. 2021;17(2):71‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alelign T, Petros B. Kidney stone disease: an update on current concepts. Adv Urol. 2018;2018:3068365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khalili P, Jamali Z, Sadeghi T, et al. Risk factors of kidney stone disease: a cross‐sectional study in the southeast of Iran. BMC Urol. 2021;21(1):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ferraro PM, et al., Urolithiasis/Endourology Dietary and Lifestyle Risk Factors Associated with Incident Kidney Stones in Men and Women. [DOI] [PMC free article] [PubMed]
- 21. Somani BK. After COVID‐19: planning postpandemic care of patients with kidney stones. Nat Rev Urol. 2021;18(9):511‐512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aouissi HA, Ababsa M, Leveau CM, et al. Beyond vaccination: a cross‐sectional study of the importance of behavioral and native factors on COVID‐19 infection and severity. medRxiv. 2022:1‐24. 10.1101/2022.01.23.22269214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tavasoli S, Borumandnia N, Basiri A, Taheri M. Effects of COVID‐19 pandemics on urinary metabolites in kidney stone patients: our kidney stone prevention clinic experience. Environ Health Prev Med. 2021;26(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Statistical Center of Iran . Population and Household of the Country by Province and Sub‐province in Population and Housing Censuses, 2017 . 2017; Statistical Center of Iran. [Google Scholar]
- 25. Poustchi H, Eghtesad S, Kamangar F, et al. Prospective epidemiological research studies in Iran (the PERSIAN Cohort Study): rationale, objectives, and design. Am J Epidemiol. 2017;187(4):647‐655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pourmand G, Pourmand B. Epidemiology of stone disease in Iran, in Urolithiasis . Springer; 2012:85‐87. [Google Scholar]
- 27. Tadayyon F, Sabbagh M. The prevalence of kidney stone different composition in patients referred to the lithotripsy wards. J Isfahan Med Sch. 2011;28(122):1‐11. [Google Scholar]
- 28. Safarinejad MR. Adult urolithiasis in a population‐based study in Iran: prevalence, incidence, and associated risk factors. Urol Res. 2007;35(2):73‐82. [DOI] [PubMed] [Google Scholar]
- 29. Karabacak OR, Dilli A, Saltaş H, Yalçınkaya F, Yörükoğlu A, Sertçelik MN. Stone compositions in Turkey: an analysis according to gender and region. Urology. 2013;82(3):532‐538. [DOI] [PubMed] [Google Scholar]
- 30. Scales CD, Jr. , Smith AC, Hanley JM, Saigal CS. Prevalence of kidney stones in the United States. Eur Urol. 2012;62(1):160‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zeng G, Mai Z, Xia S, et al. Prevalence of kidney stones in China: an ultrasonography based cross‐sectional study. BJU Int. 2017;120(1):109‐116. [DOI] [PubMed] [Google Scholar]
- 32. Lewandowski S, Rodgers AL. Idiopathic calcium oxalate urolithiasis: risk factors and conservative treatment. Clin Chim Acta. 2004;345(1‐2):17‐34. [DOI] [PubMed] [Google Scholar]
- 33. Shoag J, Tasian GE, Goldfarb DS, Eisner BH. The new epidemiology of nephrolithiasis. Adv Chronic Kidney Dis. 2015;22(4):273‐278. [DOI] [PubMed] [Google Scholar]
- 34. Alkhunaizi AM. Urinary stones in Eastern Saudi Arabia. Urol Ann. 2016;8(1):6‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Taylor EN, Stampfer MJ, Curhan GC. Obesity, weight gain, and the risk of kidney stones. JAMA. 2005;293(4):455‐462. [DOI] [PubMed] [Google Scholar]
- 36. Indridason OS, Birgisson S, Edvardsson VO, Sigvaldason H, Sigfusson N, Palsson R. Epidemiology of kidney stones in Iceland. A population‐based study. Scand J Urol Nephrol. 2006;40(3):215‐220. [DOI] [PubMed] [Google Scholar]
- 37. Vupputuri S, Soucie JM, McClellan W, Sandler DP. History of kidney stones as a possible risk factor for chronic kidney disease. Ann Epidemiol. 2004;14(3):222‐228. [DOI] [PubMed] [Google Scholar]
- 38. Konjengbam H, Meitei SY. Association of kidney stone disease with dietary factors: a review. Anthropological Review. 2020;83(1):65‐73. [Google Scholar]
- 39. Taylor EN, Stampfer MJ, Curhan GC. Diabetes mellitus and the risk of nephrolithiasis. Kidney Int. 2005;68(3):1230‐1235. [DOI] [PubMed] [Google Scholar]
- 40. Ekeruo WO, Tan YH, Young MD, et al. Metabolic risk factors and the impact of medical therapy on the management of nephrolithiasis in obese patients. J Urol. 2004;172(1):159‐163. [DOI] [PubMed] [Google Scholar]
- 41. Spatola L, Ferraro PM, Gambaro G, Badalamenti S, Dauriz M. Metabolic syndrome and uric acid nephrolithiasis: insulin resistance in focus. Metabolism. 2018;83:225‐233. [DOI] [PubMed] [Google Scholar]
- 42. Turney BW, Appleby PN, Reynard JM, Noble JG, Key TJ, Allen NE. Diet and risk of kidney stones in the Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur J Epidemiol. 2014;29(5):363‐369. [DOI] [PubMed] [Google Scholar]
- 43. Sarıca K, Altay B, Erturhan S. Effect of being overweight on stone‐forming risk factors. Urology. 2008;71(5):771‐774. [DOI] [PubMed] [Google Scholar]
- 44. Ratkalkar VN, Kleinman JG. Mechanisms of stone formation. Clinical reviews in bone and mineral metabolism. 2011;9(3):187‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lemann J, Jr. , Pleuss JA, Worcester EM, Hornick L, Schrab D, Hoffmann RG. Urinary oxalate excretion increases with body size and decreases with increasing dietary calcium intake among healthy adults. Kidney Int. 1996;49(1):200‐208. [DOI] [PubMed] [Google Scholar]
- 46. Rodgers AL. Physicochemical mechanisms of stone formation. Urolithiasis. 2017;45(1):27‐32. [DOI] [PubMed] [Google Scholar]
- 47. Assimos DG. Re: diet and risk of kidney stones in the Oxford cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC). J Urol. 2014;192:1714‐1715. [DOI] [PubMed] [Google Scholar]
- 48. Escribano J, Balaguer A, Pagone F, Feliu A, Roqué I Figuls M. Pharmacological interventions for preventing complications in idiopathic hypercalciuria. Cochrane Database Syst Rev. 2009(1):004754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin B‐B, Lin ME, Huang RH, Hong YK, Lin BL, He XJ. Dietary and lifestyle factors for primary prevention of nephrolithiasis: a systematic review and meta‐analysis. BMC Nephrol. 2020;21(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data will be available by formal requests from corresponding authors and approving the request in the core committee of the PERSIAN cohort study.


