Abstract
Background and Aims
Vitamins are bioactive compounds naturally found in many different types of food and required by the human body for many biological functions and enzymatic activities. Due to their antioxidant properties, certain vitamin derivatives have been synthesized for inclusion in many cosmetics, thus leading to an increasing incidence of allergic contact dermatitis (ACD) cases. Therefore, the present review may be helpful to provide an insight into the sensitizing role of at least certain vitamins and may also offer possible patch test alternatives for definitive diagnosis.
Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) guidelines. Literature search regarding ACD cases to vitamins was performed using the Medline, PubMed, Scopus, EMBASE, and Google Scholar databases from January 1940 up to June 2021.
Results
A total of 4494 articles matched the keywords used for the researched. Records removed before screening included 15 duplicate articles and 3429 not eligible articles (e.g., not written in English, studies on animals, not relevant to the topic). A total of 1050 articles underwent the screening phase and 258 were therefore excluded as they were not primary studies. Subsequentially, 792 articles were considered eligible for the review and 688 of them were finally excluded as they did not report the outcome of interest. Therefore, 104 articles were definitely included in the present review.
Conclusion
ACD to vitamins is still probably an underestimated issue in cosmetology, as many vitamins are considered “natural” and therefore “safe” ingredients. On the contrary, according to current literature, almost all vitamins contained in topical products are able to induce allergic reactions, with the exception of vitamin B2 and vitamin B9. Patch tests are not standardized, thus leading to difficulties in diagnosis.
Keywords: allergic contact dermatitis, fat‐soluble vitamins, hydro‐soluble vitamins, lymphocyte transformation test, multivitamins, patch test
1. INTRODUCTION
Vitamins are organic compounds that are essential for numerous human metabolic functions in many cellular, enzymatic, and biological pathways. However, the human body cannot produce, or else produces very little of most of them so a rich, highly differentiated diet is fundamental for their correct intake. 1 , 2 Some vitamins occur naturally in food and each vitamin has been identified and industrially produced, both for pharmaceutical purposes and food supplementation, although commercial vitamins are not completely identical to their natural forms. 3 Synthetic vitamins offer many advantages compared to natural ones, as they can be administered not only orally but also intramuscularly or intravenously. Furthermore, synthetic vitamins can be stored for longer periods, and are not influenced by food deterioration.
Vitamin derivatives are currently available in cosmetics and in food excipients. Vitamins A, E, B3, B5, B6, and C and their derivatives are used for their antioxidant and antiaging properties in cosmetology 4 but also as excipients, preservatives or dyes in food, juices, and drinks. 5 , 6 Due to their widespread use, allergic contact dermatitis (ACD) to vitamins is rapidly increasing. 7 However, unlike hydro soluble vitamins (B1–B9, B12, and C), which are more likely to induce immediate‐type reactions, ACD is more frequently induced by fat soluble vitamins (A, D, E, and K). 8 Occupational ACD from vitamins is rarely reported, 8 as also ACD caused by topical use of cosmetics containing vitamin derivatives or their pure forms. 9
Besides ACD, vitamins can cause contact urticaria, 10 , 11 anaphylaxis, 12 photoallergic reactions, 13 , 14 , 15 , 16 erythema multiforme‐like eruptions, 17 airborne contact dermatitis, 18 , 19 and atopic dermatitis‐like lesions. 20 Moreover, vitamins can be responsible for diffuse ACD, 21 , 22 , 23 , 24 , 25 , 26 together with local flare‐up after systemic intake 21 , 22 , 24 , 27 , 28 and systemic allergy. 8 , 29 The latter imposes a burden on these patients since vitamins are contained in food supplements and tablets.
The beneficial properties, recommended amounts, and food sources of the essential vitamins are reported in Table 1, while all denominations and chemical structures are listed in Table 2.
Table 1.
Benefits, recommended amount, and food sources of vitamins
| Vitamins | Benefits | Recommended amount (daily RDA or daily AI) | Food sources |
|---|---|---|---|
| Vitamin A | Essential for sight. Lycopene may lower prostate cancer risk. Keeps tissues and skin healthy. Plays an important role in bone growth and the immune system. Diets rich in the carotenoids alpha carotene and lycopene seem to lower lung cancer risk. Carotenoids act as antioxidants. Foods rich in the carotenoids lutein and zeaxanthin may protect against cataract | M: 900 μg (3000 IU) | Sources of retinoids: beef liver, eggs, shrimp, fish, fortified milk, butter, cheddar cheese, Swiss cheese |
| F: 700 μg (2333 IU) | Sources of β‐carotene: sweet potatoes, carrots, pumpkins, squash, spinach, mangoes, turnip greens | ||
| Vitamin B1 | Helps convert food into energy. Needed for healthy skin, hair, muscles, and brain and critical for nerve function | M: 1.2 mg | Pork chops, brown rice, ham, soymilk, watermelons, acorn squash |
| F: 1.1 mg | |||
| Vitamin B3 | Helps convert food into energy. Essential for healthy skin, blood cells, brain, and nervous system | M: 16 mg | Meat, poultry, fish, fortified and whole grains, mushrooms, potatoes, peanut butter |
| F: 14 mg | |||
| Vitamin B5 | Helps convert food into energy. Helps make lipids (fats), neurotransmitters, steroid hormones, and hemoglobin | M: 5 mg | Wide variety of nutritious foods, including chicken, egg yolk, whole grains, broccoli, mushrooms, avocados, tomato products |
| F: 5 mg | |||
| Vitamin B6 | Aids in lowering homocysteine levels and may reduce the risk of heart disease. Helps convert tryptophan to niacin and serotonin, a neurotransmitter that plays key roles in sleep, appetite, and moods. Helps make red blood cells Influences cognitive abilities and immune function | 31–50 years old: M: 1.3 mg; F: 1.3 mg | Meat, fish, poultry, legumes, tofu and other soy products, potatoes, noncitrus fruits such as bananas and watermelons |
| 51+ years old: M: 1.7 mg; F: 1.5 mg | |||
| Vitamin B12 | Aids in lowering homocysteine levels and may lower the risk of heart disease. Assists in making new cells and breaking down some fatty acids and amino acids. Protects nerve cells and encourages their normal growth. Helps make red blood cells and DNA | M: 2.4 μg | Meat, poultry, fish, milk, cheese, eggs, fortified cereals, fortified soymilk |
| F: 2.4 μg | |||
| Vitamin C | Aliments rich in vitamin C may lower the risk for some cancers, including those of the mouth, esophagus, stomach, and breast. Long‐term use of supplemental vitamin C may protect against cataract. Helps make collagen, a connective tissue that knits together wounds and supports blood vessel walls. Helps make the neurotransmitters serotonin and norepinephrine. Acts as an antioxidant, neutralizing unstable molecules that can damage cells. Bolsters the immune system | M: 90 mg | Fruits and fruit juices (especially citrus), potatoes, broccoli, bell peppers, spinach, strawberries, tomatoes, Brussels sprouts |
| F: 75 mg | |||
| smokers: add 35 mg | |||
| Vitamin D | Helps maintain normal blood levels of calcium and phosphorus, which strengthen bones. Helps form teeth and bones. Supplements can reduce the number of non‐spinal fractures | 31–70: 15 μg (600 IU) | Fortified milk or margarine, fortified cereals, fatty fish |
| 71+: 20 μg (800 IU) | |||
| Vitamin E | Acts as an antioxidant, neutralizing unstable molecules that can damage cells. Protects vitamin A and certain lipids from damage. Diets rich in vitamin E may help prevent Alzheimer's disease | M: 15 mg; F: 15 mg (15 mg equals about 22 IU from natural sources of vitamin E and 33 IU from synthetic vitamin E) | Wide variety of foods, including vegetable oils, salad dressings, and margarines made with vegetable oils, wheat germ, leafy green vegetables, whole grains, nuts |
| Vitamin K | Activates proteins and calcium essential to blood clotting. May help prevent hip fractures | M: 120 μg | Cabbage, liver, eggs, milk, spinach, broccoli, sprouts, kale, collards, and other green vegetables |
| F: 90 μg |
Table 2.
Name, chemical formula, and chemical structure of vitamins
| Names | Chemical formula | Chemical structure | |
|---|---|---|---|
| Vitamin B1 | Thiamine/thiamin/vitamin B1 | C12H17N4OS | Aminopyrimidine ring linked to a thiazolium ring by a methylene bridge; methyl and hydroxyethyl side chains are present instead of a thiazole |
| Thiamine pyrophosphate (TPP/co‐carboxylase), thiamine hydrochloride (TH‐HC), thiamine mononitrate (TH‐MN) | |||
| Vitamin B3 |
Vitamin B3/vitamin PP/Niacin/Nicotinic acid |
C6H5NO2 | Derivate of pyridine with a carboxyl group at the 3‐position |
| Niacinamide/amide nicotinamide | |||
| Vitamin B5 | Vitamin B5/pantotheic acid | C9H17NO5 | Combination of pantoic acid and beta‐alanine |
| Panthenol (pro‐vitamin B5)2; syntetic variants (panthenyl ethyl ether, panthenyl triacetate, calcium and sodium pantothenate) | |||
| Vitamin B6 | Vitamin B6/Pyridoxine (PN) | C8H11NO3 | 3‐Hydroxy‐4,5‐ hydroxymethyl‐2‐methylpyridine |
| Pyridoxamine (PM), pyridoxal (PL)/pyridoxine hydrochloride | |||
| Vitamin B7 | Vitamin B7/Vitamin H/Biotin | C10H16N203S | Heterocyclic, sulfur‐containing, monocarboxylic acid with two rings (imidazolidone and tetrahydrothiophene moieties) fused together |
| Vitamin B12 | Vitamin B12/Cobalamin | C63H88CON14014P | Planar group containing a corrin ring cetered by a cobalt ion and a nucleotide set |
| Cyanocobalamin (the most stable; used in pharmaceuticals), hydroxocobalamin, adenosylcobalamin (active), methylcobalamin (active) | |||
| Vitamin C | Vitamin C/(l‐)Ascorbic acid (AA)/Ascorbate | C6H806 | 2‐Oxo‐l‐theo‐hexono‐4‐lactone‐2,3‐enediol |
| Dehydroascorbate (DHA), d‐arabo‐ascorbic acid, l‐arabo–ascorbic acid | |||
| Vitamin A | Vitamin A/Retinoids | C20H30O (retinol), C20H28O (retinal), C20H28O2 (retinoid acid) | 20‐Carbon molecule made up of a trimethylated cyclohexenyl ring, an isoprenoid side chain with four double bonds (the retinyl group), and a polar carbon–oxygen functional group |
| Retinol (alcohol isoform)/retinal (the aldehyde isoform)/retinoid acid (the irreversibly oxidized form of retinol; main metabolite)/carotenoids (provitamins A)/Retinyl palmitate (RP; storage form) | |||
| Vitamin D | Vitamin D/secosteroids | C27H44O (cholecalciferol), C28H44O (ergocalciferol), C27H44O3 (calcitriol) | Compared with cholesterol, vitamin D has only three intact rings, being the A ring not rigidly fused to the B ring. In solution it may exhibit two spatial conformations: the 6‐s‐cis form, i.e., the steroid‐like shape, and the 6‐s‐trans, i.e., the extended shape |
| Cholecalciferol (previtamin D; vitamin D3; originated in the skin after sun exposure and found in animal meat), ergocalciferol (vitamin D2; found in vegetables and plants), calcitriol (active metabolite) | |||
| Vitamin E | Vitamin E/alpha tocopherol | C28H48O2 | Basilar structure with a head made up of a chromanol ring and a tail represented by a phytyl side chain, attached at the 2‐position |
| Beta tocopherol, gamma tocopherol, delta tocopherol, tocotrienols | |||
| Vitamin K | Vitamin K/phytonadione | C28H48O2 | Common 2‐methyl‐1,4 naftoquinone core, called menadione, and a phytyl side chain at the 3‐position. This side chain, which is an isoprenoid structure, may display different lengths and degrees of saturation depending on the specific isoform |
| Phylloquinone, menaquinone |
The aim of this review is to update the role of vitamins as inducers of ACD, focusing on clinical manifestations and possible cross‐reactivity among them.
2. METHODS
This study was conducted in accordance with the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) guidelines. Literature search was performed using the Medline, PubMed, Scopus, EMBASE, and Google Scholar databases from January 1940 up to June 2021, inserting the following search terms: allergic contact dermatitis; contact hypersensitivity; photoallergic contact dermatitis; photoallergic contact dermatitis; patch test; photopatch test; fat‐soluble vitamins; hydro‐soluble vitamins; multivitamins; vitamins; vitamin B complex; vitamin a; vitamin b; vitamin c; allergy; hypersensitivity
2.1. Inclusion criteria
Original articles, clinical trials, case reports, review articles, posters, and editorial letters were included.
Only articles written in English were included.
Only articles relevant to the main topic were included.
Only articles regarding primary studies on humans were included.
2.2. Exclusion criteria
Articles written in another language different from English were excluded.
Articles not relevant to the main topic were excluded.
Articles regarding laboratory studies or studies on animals were excluded.
2.3. Data quality assessment and management
Title and abstract were used to screen all articles. Relevant abstracts and articles without abstracts were selected for subsequent review. Data on first author, country of origin, publication year, study design, number of cases, age, sex, source of exposure, epicutaneous reaction, patch test materials, and reaction to vitamins were extracted. MS Excel version 2019 (build 14430.20298) was used for statistical analyses.
2.4. Results
A total of 4494 articles matched the keywords used for the researched. Records removed before screening included 15 duplicate articles and 3429 not eligible articles (e.g., not written in English, studies on animals, not relevant to the topic). A total of 1050 articles underwent the screening phase and 258 were therefore excluded as they were not primary studies. Subsequentially, 792 articles were considered eligible for the review and 688 of them were finally excluded as they did not report the outcome of interest. Therefore, only 104 articles were definitely included in the present review (Figure 1).
Figure 1.
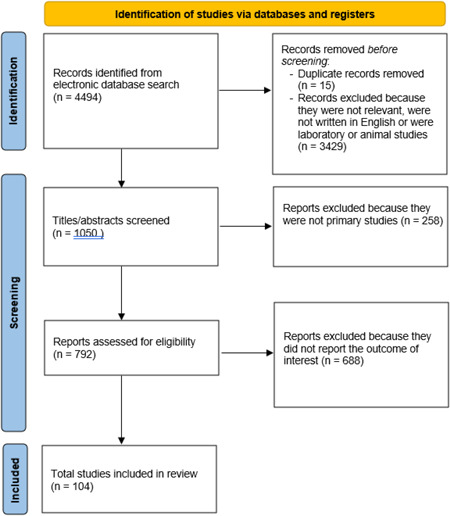
Flow diagram of research screening
3. REVIEW
Literature data on ACD to vitamins, with detailed descriptions of patch testing (tested substances, vehicles, and concentrations) and clinical manifestations, are reported in Table 3.
Table 3.
Allergic contact dermatitis to vitamins reported in literature
| Name | Cases | Vehicle and concentrations | Peculiar clinical manifestations | |
|---|---|---|---|---|
| Vitamin B1 | Thiamine | 3 | Pure thiamine (20,32)a | |
| 1 | 0.1% aq (32) | |||
| 2 | 1% aq (19, 32) | |||
| 1 | 5% aq (19) | |||
| 2 | 10% aq (19, 32) | |||
| TPP | 1 | 10% aq (32) | ||
| 1 | 1% aq (32) | |||
| Thiamine hydrochloride | 2 | 10% aq (19, 21)a | Systemic allergy | |
| Vitamin B3 | Benzyl nicotinate | 1 | 2.5% pet (33) | |
| 1 | 1% aq (35) | Burning mouth syndrome | ||
| Butoxyethyl nicotinate | 1 | 0.1% pet (34) | ||
| 1 | 1% pet (34) | |||
| 1 | 2.5% pet (33) | |||
| Propyl nicotinate | 1 | 1% aq (35) | ||
| Vitamin B5 | Panthenyl ethyl ether | 2 | 30% pet (38, 48) | |
| Panthenol | 26 | 5% pet (23, 42, 46, 48) | ||
| 1 | 30% pet (8)b | Contact urticaria | ||
| Panthenol cream | 1 | ND (44) | ||
| d‐panthenol 75 W | 1 | 30% aq (43) | ||
| dexpanthenol | 1 | 0.5% aq (41) | ||
| 1 | 5% aq (40) | |||
| 29 | 5% pet (22, 25, 44, 47) | |||
| Calcium panthotenate | 1 | 5% pet (49) | ||
| Vitamin B6 | Pyridoxine hydrochloride | 1 | 1% pet (55)b | |
| 1 | 10% pet (55)b | |||
| Pyridoxine | 1 | ND (53) | ||
| 1 | 1% HCl (52) | |||
| 2 | 1% pet (56)b | |||
| 2 | 5% pet (56)b | |||
| Pyridoxal | 1 | 1% HCl (52) | ||
| 1 | 1% PO4 (52) | |||
| Vitamin B7 | (3aS,6aR) hexahydro‐1,3‐dibenzyl‐6‐hydroxyfurano[3,4‐d]imidazol‐2,4‐dione | 1 | 0.1% pet (16) | |
| 1 | 0.5% pet (16) | |||
| 1 | 1% pet (16) | |||
| 1 | 5% pet (16) | |||
| 1 | 10% pet (16) | |||
| Vitamin B12 | Vitamin B12 | 1 | 10% pet (59)a | |
| Vitamin C | Ascorbic acid | 1 | Undiluted (30) | |
| 1 | 5% aq (61) | |||
| 3‐O‐ethyl‐l‐ascorbic acid | 1 | 0.05% pet (62) | ||
| 1 | 0,1% pet (62) | |||
| 1 | 0.5% pet (62) | |||
| 1 | 1% pet (62) | |||
| 2 | 5% pet (62, 66) | |||
| 1 | 1% aq (64) | |||
| 1 | 10% aq (65) | |||
| Vitamin A | All‐trans retinoic acid | 2 | 0.01 pet/alchohol/gel (71) | |
| 2 | 0.05% pet (commercial cream) (71) | |||
| 2 | 0.00625 diluted with ethyl alcohol (commercial lotion) (71) | |||
| Vitamin A acid | 3 | cream ND (72) | ||
| 2 | cream (Roche) (72) | |||
| 1 | 0.05% soft paraffin (72) | |||
| 3 | 0.05% abs alcohol (72) | |||
| 3 | 0.005% abs alcohol (72) | |||
| Retinoic acid | 3 | Cream ND (72) | ||
| 1 | 0.02% in ethyl alcohol (76) | |||
| 1 | 0.01% in ethyl alcohol (76) | |||
| 1 | 0.02% in acetone (76) | |||
| 1 | 0.01% in acetone (76) | |||
| Retin‐A liquid | 1 | 0.025% in ethyl alcohol (76) | ||
| 1 | 0.0065% in ethyl alcohol (76) | |||
| Tretinoin | 1 | 0.05% pet (73) | ||
| 1 | 0.05% eth (73) | |||
| Retrieve cream® | 1 | “as is” (75) | ||
| Glaan cream® | 1 | “as is” (78) | ||
| Retinol palmitate | 1 | 1% MEK (78) | ||
| 1 | 10% MEK (78) | |||
| 1 | 5% pet (79) | |||
| 1 | 5% PCL (79) | |||
| Vitamin A | 1 | 200,000 IU/100 g (80) | ||
| Vitamin A acetate | 1 | 0.1% pet (17) | ||
| 1 | 0.5% pet (17) | |||
| 1 | 1% pet (17) | |||
| 1 | 5% pet (17) | |||
| 1 | 10% pet (17) | |||
| Vitamin D | Tacalcitol | 1 | Ointment (83) | |
| 1 | 0.0002% eth (83) | |||
| Calcitriol | 1 | 0,0002% eth (83) | ||
| Psorcutan® Salbe | 1 | “as is” (84) | ||
| Calcipotriol | 1 | 0.08 μg/ml in isopropyl alcohol (84) | ||
| 1 | 0.4 μg/ml in isopropyl alcohol (84) | |||
| 1 | 0.2 μg/ml in isopropyl alcohol (84) | |||
| 1 | 10 μg/ml in isopropyl alcohol (84) | |||
| 1 | 50.0 μg/ml in isopropyl alcohol (84) | |||
| 3 | Ointment “as is” (85) | |||
| 3 | 50 μg/ml in isopropranolol (85) | |||
| 3 | 10 μg/ml in isopropranolol (85) | |||
| 2 | 5 μg/ml in isopropranolol (85) | |||
| 1 | 2 μg/ml in isopropranolol (85) | |||
| 1 | 0.4 μg/ml in isopropranolol (85) | |||
| 1 | 50 μg/ml in pet (85) | |||
| 1 | 10 μg/ml in pet (85) | |||
| 1 | 5 μg/ml in pet (85) | |||
| Vitamin E | Glaan cream® as is | 1 | “as is” (78) | |
| Vitamin E | 1 | “pure” at 2,5% (80) | ||
| 1 | Pure vitamin E oil formulation 1 (91) | |||
| 1 | Pure vitamin E oil formulation 1 (91) | |||
| Tocopheryl acetate | 5 | 10% pet (78, 94) | ||
| 1 | 20% pet (78) | |||
| Tocopheryl acetate nicotinate | 1 | 1% pet (93) | ||
| Alpha‐tocopherol | 2 | “as is” (88, 92) | ||
| Tocopherol acetate | 1 | 1% aq (98) | Systemic allergy | |
| 1 | 0.25% pet (99) | Systemic allergy | ||
| 0.5% pet (99) | ||||
| 5% pet (100) | ||||
| ϒ‐tocopherol | 1 | 0.002% ϒ‐tocopherol skin lotion (100) | ||
| Tocopherol | 1 | 10% pet (9) | ||
| 1 | “as is” (18) | Atopic dermatisi‐like ACD | ||
| dl‐alpha‐tocopherol nicotinate | 1 | 0.1% pet (106) | ||
| Vitamin K | Vitamin K1 | 1 | 1% pet (110) | |
| 1 | 10% pet (110) | |||
| 2 | 1 mg/ml aq (111) | |||
| 1 | Eye contour cream “as is” (116) | |||
| 1 | Konakion (14)b | |||
| Vitamin K1 oxide | 2 | 1% pet (115) | ||
| 2 | 5% pet (15) | Erythema‐multiforme like ACD | ||
| Phytonadione epoxide | 1 | 1% pet (116) | ||
| 1 | 5% pet (116) | |||
| 1 | Martiderm® cream “as is” (116) | |||
| Vitamin K3 sodium bisulfite | 2 | 0.1% aq (117) | ||
| 1 | 1% aq (117) | |||
| 1 | 0.1% pet (119) | |||
| 1 | 0.01% pet (119) | |||
Occupational exposure.
Negative PT and positive photo‐PT.
3.1. ACD to hydro‐soluble vitamins
All hydro‐soluble vitamins have been reported to be responsible for ACD, except for vitamin B2 and vitamin B9. Due to their increasing role in cosmetics and as topical therapeutic agents, 4 the incidence of ACD induced by hydro‐soluble vitamins seems to be rising.
3.1.1. Vitamin B1
ACD to thiamine has been rarely described, mainly in occupational settings and pharmaceutical industries. 21 , 22 Fingers, hands, and wrists are the sites most frequently involved, with a possible further spread to forearms and other body regions. 22 The face (eyelids, periorbital, and perioral regions) may also be involved through airborne contact or self‐contamination. Moreover, oral assumption of vitamin B1 or multivitamins containing vitamin B1 may cause systemic allergy. 21 , 22 , 23
Patch tests using different thiamine epitopes showed that the whole molecule is highly immunogenic, with a possible cross‐reactivity between thiamine and thiamine pyrophosphate (TPP or co‐carboxylase). 30 Oral challenge test may be required in uncertain cases. 31
3.1.2. Vitamin B3
ACD has been reported more frequently following the topical use of nicotinic acid esters than of pure niacin or niacinamide. 31 Besides classical eczematous lesions on the site of application, 32 a case of allergic contact mucositis to a toothpaste with benzyl nicotinate and propyl nicotinate, presenting as a burning mouth syndrome, was also described. 33
Patch tests to nicotinic acid and its esters are not yet fully standardized, and probably the pyridine ring is the true immunogenic site. 31 Therefore, since a pyridine ring is present in the chemical structure of various drugs, such as isoniazid, cross‐sensitization may be expected. However, Sasseville et al. 34 demonstrated that cross‐reactivity among different pyridine derivatives and nicotinic acid is rare. 34 The pyridine ring is also contained in the basic structure of vitamin B6, but no cross‐reactivity between the two vitamins has been reported.
3.1.3. Vitamin B5
Vitamin B5 and its derivatives, widely used in pharmaceutical and cosmetic compounds, such as hair and skin conditioning agents, 35 have been reported as causative agents of ACD ranging from classic localized eczematous lesions at the site of contact 36 to generalized eczematous eruptions. 31 , 37 Interestingly, a single case of contact urticaria induced by dexpathenol has been reported, 10 as well as an exacerbation of a pre‐existent ACD to dexpanthenol after the systemic ingestion of pantothenic acid. 27 Moreover, an increased risk for anaphylaxis has been documented following multivitamin intake in patients sensitized to dexpanthenol through routine contact with dairy products. 12
Dexpanthenol contained in hair lotions and conditioners, 36 sunscreens, 8 , 9 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 facial moisturizing preparations, 41 , 42 make‐up‐remover wipes, 43 and cream applied after a permanent tattoo 44 has been reported as responsible for ACD. Leg ulcers and stasis dermatitis have been identified as the main risk factors for ACD to dexpanthenol. 45
To date, there is no standardization regarding the best concentrations and vehicles for patch testing dexpanthenol, 46 , 47 considered a strong sensitizer that does not cross‐react with d‐panthenyl ethyl ether. 46 , 47 , 48
Vitamin B5 in cosmetics as calcium pantothenate was reported to be a relevant sensitizer. 49 However, due to the complexity of vitamin B5 metabolic pathways, it is also possible that pantothenate may require metabolic activation. Metabolic pathways activated by microsomes play an important role in biochemical modifications of the hapten. 50 Even if some authors identified the beta‐alanine group as the antigenic determinant of pantothenic acid, 25 , 36 biochemical studies have demonstrated that the whole molecule displays immunogenic properties. 51
3.1.4. Vitamin B6
Vitamin B6, its vitamers and their phosphorylated derivatives, have been demonstrated to be able to induce ACD. 52 , 53 It has been suggested that increased levels of the B6 vitamers and their synthetic derivatives can generate UV toxic photoproducts. 54 Therefore, most of the hypersensitivity reactions to vitamin B6 are, in fact, photoallergic dermatitis after oral or parental intake. 4 , 55 , 56 Wundrak et al. 13 identified the 3‐hydroxypyridine chromophores as the photosensitizing products, confirming the true role of vitamin B6 as a photosensitizing agent. 13 Moreover, the excessive consumption of vitamin B6 seems to be a risk factor for the development of photoallergic reactions. 14 , 15
3.1.5. Vitamin B7
Biotin, also known as vitamin B7 and vitamin H, is produced by human enteric bacteria and is found in a wide variety of foods. However, only a single case of occupational airborne ACD was described, involving a pharmaceutical‐plantation exposed to a biotin precursor (hexahydro‐1,3‐dibenzyl‐6‐hydroxyfurano[3,4‐d]imidazole‐2,4‐dione). 18 Patch testing to the biotin precursor was positive at different concentrations in pet. (0.1%, 0.5%, 1%, 5%, 10%), while patch tests with other substances used to produce it resulted negative.
3.1.6. Vitamin B12
Vitamin B12, also known as cobalamin, is one of the few organo‐metallic compounds occurring in nature, identified in 1948 as the last hydro‐soluble vitamin. Its peculiar biochemical structure mainly includes a carbon‐metal bond and a cobalt atom in its core corrin ring. 57 The biologically active forms of vitamin B12 found in nature are adenosyl cobalamin and methylcobalamin, while cyanocobalamin is the most stable synthetic form, frequently used for pharmaceutical purposes. 57 It is currently unclear whether there is a risk of systemic allergy when a patient sensitized to cobalt receives oral vitamin B12. 28 , 38 , 39 However, relapsing cheilitis may occur in patients sensitized to cobalt after systemic ingestion of vitamin B12. 58 Interestingly, only one case of pure ACD to vitamin B12 has been reported, with positive patch tests to vitamin B12 (10% pet.), and negative results to cobalt chloride (1% pet.), suggesting that the cobalt ion in the corrin ring is not the only immunogenic site. 59 Furthermore, Malten et al. 28 postulated that only the free form of the cobalt atom, that is, the one usually originating after vitamin B12 catabolism and not binding cobalamin, displays allergenic properties. 57 , 60
3.1.7. Vitamin C
Due to its antioxidant properties, vitamin C is widely used as an excipient in food and drinks. Fatty acid esters of ascorbic acid such as ascorbylpalmitate and stearate are also authorized for use as food additives. Other synthetic esters of ascorbic acid have been produced (tetra‐hexyldecyl‐ascorbate), as well as other lipophilic compounds (3‐O‐ethylascorbic acid, ascorbyl‐tetraisopalmitate), mainly used in cosmetics and antiaging products. 4 , 61
After the introduction of ascorbic acid semisynthetic esters in cosmetics, 62 ACD of the face and neck was reported in middle‐aged female patients. Also 3‐O‐ethylascorbic acid proved to be an emerging contact allergen due to its increasing diffusion as a whitening and antioxidant agent in cosmetics. 63 , 64 Since the first case of ACD to ascorbic acid described in 1980, 29 several cases of ACD to vitamin C, demonstrated through patch testing at different concentrations and vehicles, have been described. 65 , 66 , 67 Vitamin C patch testing was reported, with a weak but persistent response at D7 or D9 despite immunosuppressive therapy with methotrexate. 64 However, a ROAT with the suspected ingredient of a cosmetic product can be very useful in doubtful cases, also considering that patch testing with diluted ingredients in different vehicles may yield false negatives. 68 Moreover, neither the exact concentration nor the right vehicle have yet been established. 68
3.2. Lipid‐soluble vitamins
Fat‐soluble vitamins seem to be more sensitizing than hydro‐soluble ones due to their high lipophilia. However, the ingestion of fat‐soluble vitamins does not seem to induce local flare‐up of ACD, as observed with hydro‐soluble vitamins. Humans have a three‐months' storage of fat‐soluble vitamins, and so suffer less frequently from a deficiency of these, and their consumption is lower. 1 , 2 On the other hand, fat‐soluble vitamins are more likely to cause vitamin intoxication. 69 , 70 For these reasons, their concentration in multivitamin supplements is low. Furthermore, fat‐soluble vitamins can be partially produced by the body, 2 thus inducing a certain level of immune tolerance. Finally, fat‐soluble vitamins can be absorbed only during a caloric fatty meal, an aspect which should always be taken into account when performing an oral challenge test with these vitamins. 2
3.2.1. Vitamin A
Due to their beneficial properties as antioxidant agents and to their role in reversing solar skin damage, vitamin A derivatives have been added to cosmetics. 6 , 60 However, besides their cosmetic use, vitamin A esters, and above all beta‐carotene, are also approved as food colorants by both the European Authorities and the FDA. 5
The first case of ACD elicited by topical all‐trans‐retinoic acid (TRA), and confirmed by leukocyte migration inhibition test, was reported by Jordan et al. in 1975. 71 Interestingly, since patch testing was positive for retinoic acid (at different concentrations and in different vehicles) and negative for retinol, retinal, and retinol palmitate, the terminal polar carbon–oxygen functional group of retinoic acid was considered to be the allergen, without cross‐reactivity with other vitamin A derivatives. 71
Due to its use in cosmetics and topical treatments, tretinoin has been related to many cases of ACD, mainly involving the facial area in young patients. 72 , 73 , 74 , 75 Patch testing with tretinoin is not always easy to perform; ethyl alcohol is probably a suitable vehicle. 76 Avoidance of oral administration of isotretinoin after contact sensitization to tretinoin has been suggested, due to the possible cross‐sensitivity. 77
Topical retinol palmitate was related to some cases of ACD, 78 also in combination with tocopheryl acetate (vitamin E). 79 , 80 Moreover, a single case of occupational ACD to retinyl acetate with relapsing airborne eczematous lesions was reported, confirmed by positive patch tests with retinyl acetate at different concentrations (0.1%, 0.5%, 1%, 5%, and 10% in pet). 19
Moreover, many cases of irritant contact dermatitis have been reported. 6 , 60 , 81 Tazarotene and less frequently adapalene are common irritant agents, but are generally considered to be very rare sensitizers. In fact, only a single case of ACD to a topical product containing both adapalene and benzoyl peroxide was described. However, systemic retinoids have been reported to facilitate the development of ACD to other topical products due to their skin barrier‐damaging properties. 81
3.2.2. Vitamin D
Vitamin D analogs have been successfully used to treat secondary hyperparathyroidism in patients with chronic kidney disease, while other synthetic derivatives (calcipotriol, calcitol, and maxacalcitol) are widely used as topical therapeutics for psoriasis, with possible ACD. 82 Calcipotriol seems to be more likely to induce ACD than calcitol 83 or maxacalcitol, probably due to its wider marketing. 84 Moreover, no cross‐sensitivity between calcipotriol and calcitriol has been demonstrated. 85
3.2.3. Vitamin E
Alpha‐tocopherol occurs in nature as a single stereoisomer, while synthetic vitamin E is usually a mixture of eight stereochemical isomers. Therefore, chemically synthesized alpha‐tocopherol is not identical to its natural form. 86
Due to its poor water solubility, new esters of alpha‐tocopherol have been synthesized to increase their water dispersion, as well as their stability and shelf‐life. 4 , 87 Besides their role as dietary supplements, vitamin E esters are largely used also as antioxidant and antiaging agents in cosmetics. 4
The first case of ACD to vitamin E was reported in 1965 by Brodkin et al. 88 in a patient with an earlobe dermatitis after the local application of a cream containing vitamin E. 88 Since then, several cases of ACD to vitamin E have been described, contained in deodorants, 89 , 90 body oils, 91 , 92 hair lotions, 93 and moisturizers. 78 , 94 Vitamin E was the third most common contact allergen present in 151 of 276 samples of moisturizers (55%), after fragrances (68%) and parabens (62%). 95 d,l‐Alpha‐tocopherol (synthetic origin) induces dermatitis more frequently than d‐alpha‐tocopherol (natural origin). Moreover, the higher the concentration of vitamin E in its pure oils, the greater the risk of them causing ACD. 96
ACD to vitamin E exhibits two main clinical patterns: an eczematous dermatitis involving the face and/or the extremities, and a generalized eruption spreading from the site of vitamin E application. 97 However, generalized skin eruptions may show different morphological patterns such as systemic allergy, 98 , 99 erythema multiforme‐like, 92 , 93 , 100 and contact urticaria. 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26
Nevertheless, according to some authors, contact allergy to vitamin E is debatable. 101 In fact, patch tests with vitamin E are not standardized and different vehicles and concentrations were used, 12 , 94 , 99 sometimes testing undiluted oral drops 26 or the topical formulations as is. 26 , 91 Moreover, vitamin E is sensitive to both light and temperature, easily undergoing oxidation and thus releasing different degradation products, such as alpha‐tocopheryl quinine and tocopheryl acetate. 102 , 103 , 104 In two cases of sensitization to VEA® lipogel, a cosmetic formulation containing tocopherol acetate, the culprit agent was a tocopherol acetate oxide product that presumably originated from incorrect storage. For these reasons, correct storage of vitamin E‐based cosmetics is important to avoid the development of degradation products, thus suggesting that patch testing or ROAT should be performed with patients' products only if optimally stored. 104 Furthermore, tocopheryl seems to be a stronger sensitizer than tocopherol, and probably acts as an adjuvant, since contact allergy to vitamin E is often associated to other sensitizations such as to vitamin A, 78 , 80 glycyrrhetinic acid, 100 decyl oleate, 98 and Aloe vera. 105 The vitamin E esterification seems to influence its immunogenic properties, since cross‐reactivity among different esters was not elicited by patch testing. 93 , 94 , 97 , 106 Given its increasing use in cosmetics and topical products, it has been suggested that vitamin E should be included in routine patch test series, 96 , 97 although the optimal concentration and vehicle are not known. Currently, there is no evidence that dietary vitamin E intake may induce a flare‐up of skin lesions in topically sensitized patients, although some authors recommend these patients to avoid vitamin E intake. 11 , 90
3.2.4. Vitamin K
Vitamin K occurs naturally in two forms, vitamin K1 and vitamin K2, while vitamins K3–K7 are synthetic. Vitamin K1, also known as phylloquinone or phytonadione, can be generally found in plants, while vitamin K2 is synthesized by Gram‐positive bacteria in the human large intestine. 107 Although synthetic versions of vitamins K1, K3, K4, and K5 have been used in clinical practice, vitamin K1 is the predominant dietary form, and also the most widely used preparation for both intravenous and oral administration.
The first description of an ACD induced by the topical application of vitamin K1 dates back to 1942. 108 Two sources of sensitization have been identified: cosmetic products and occupational exposure. Since vitamin K1 is contained in many different cosmetics such as creams against either hyperpigmentation or rosacea, ACD to vitamin K1 generally manifests with eczematous lesions and localized edema mainly involving the periorbital area and the eyelids. 109 , 110 , 111 , 112 , 113 For these reasons, in November 2009, vitamin K1 use in cosmetics and other topical formulations was forbidden in Europe. 114 Vitamin K1 was then replaced by its oxide, 2,3‐epoxy phytylmenaquinone, even if cases of ACD to vitamin K1 oxide have also been reported. Although vitamin K1 oxide seems to display a weaker sensitizing action compared to vitamin K1 itself, Garcia‐Gavin et al. reported three patients who developed ACD due to the use of a cream containing vitamin K1. 114 , 115 , 116 Moreover, a high cross‐reactivity between vitamin K1 and its oxide has been reported, as ACD can be provoked by its oxide in patients previously sensitized to vitamin K1. 115 , 116 For these reasons, since vitamin K1 oxide is not provided by manufacturers, vitamin K1 as is can be used for patch testing. 10 Vitamin K1 may also cause erythema multiforme‐like contact dermatitis 17 and photoallergic reactions. 16
Occupational sensitization is mainly to vitamin K3 sodium bisulfite, affecting patients working in both veterinary and pharmaceutical industries. 117 , 118 , 119 Vitamin K3 sodium bisulfate may lead to the development of eczematous lesions on hands, face, neck, and eyelids, sometimes associated with gastrointestinal symptoms. 119 However, since vitamin K3 sodium bisulfite is a well‐known irritant compound, serial dilutions (0.01% and 0.1% in aq.) have been suggested. 117 , 118 , 119 , 120
Even if a case of cross‐sensitivity between sodium bisulfite and vitamin K4 was reported, 117 sodium bisulfite 5% in aq. resulted negative and without cross‐reactivity with vitamin K1. 120
4. RESULTS
ACD to vitamins is still probably an underestimated issue in cosmetology, as many vitamins are considered “natural” and therefore “safe” ingredients. On the contrary, according to current literature, almost all vitamins contained in topical products are able to induce allergic reactions, with the exception of vitamin B2 and vitamin B9.
From the careful review of literature and considering the fragmentation of collected data, mainly based on single case reports extrapolated from literature, it is evident that the ACD to vitamins are induced by synthetic forms of vitamins, whose formulations allow their use as topical products.
Previously, indeed, the onset of an ACD to vitamins involved subjects working in the pharmaceutical industry and occupationally exposed to vitamins or their intermediates originated from the process of synthetic production. 18 , 19 , 30 Moreover, it has been suggested that water‐soluble vitamins are more likely to induce immediate type reactions like urticaria or anaphylaxis 8 whereas lipid‐soluble vitamins are more prone to cause ACD for their own greater lipophilia, above all, when used in cosmetics or moisturizers. 37 The example of ACD to vitamin E in USA is paradigmatic, given its wide use in topical products and the consequent high incidence of alpha tocopherol‐induced ACD in that country. 97 However, the increased cosmetic use of water‐soluble vitamins such as vitamin B5 10 , 36 , 40 , 41 , 49 or vitamin C derivatives 62 , 63 , 64 , 65 , 66 , 67 , 68 is quickly gaining the gap in inducing ACD by such a group of vitamins and some clinicians have focused their attention on such substances, suggesting to include them in patch tests series for cosmetics 47 , 67 or to perform investigations on larger cohorts of patients with ACD. 46 The drugs originated from vitamins represent another riddle, for the potential allergic cross‐reaction between the synthetic drug‐derivative and the naïve vitamin. Such an occurrence has been poorly investigated, as in the cases of vitamin A 77 and D 85 only. Yet, it is interesting to evidence the possibility of a dermatitis flare‐up following the ingestion of the culprit vitamin, above all as assumed in a multivitamin preparation, that is always a synthetic vitamin form 20 , 30 but may involve even vitamins naturally present in foodstuff, 27 , 29 leading patients to follow a low‐content diet for the responsible vitamin, although that circumstance seems to occur exceptionally. Lastly, the review of literature has evidenced the scarce and not yet optimal standardization of vitamins patch tests, as illustrated in Table 2, while cutaneous tests are the gold standard to diagnose an ACD.
5. CONCLUSION
The increasing use of vitamins in cosmetics and daily consumer products is responsible for rising rates of ACD in the general population. Even if ACD to vitamins is not frequent, the etiologic diagnosis is mandatory to establish correct prevention measures, and in this setting patch testing is the gold standard. Nevertheless, diagnosis is not always easy due to the absence of standardization in patch testing, as also in other in vivo methods.
ACD from vitamins is an issue for dermatologists, considering that sensitization from cosmetics or other skin products containing vitamins may induce systemic reactions when vitamins are orally administered in apparently innocent multivitamin dietary supplements. In fact, semisynthetic vitamins are administered orally and parenterally for therapeutic purposes and are increasingly marketed: it has been estimated that more than 90,000 dietary supplementation products containing vitamins and minerals are available on the USA market. 121 Nowadays, almost 52% of the adult USA population regularly take vitamins and multivitamins, 121 whereas in Europe and Latin America vitamin intake is less common and the lowest percentage (13%–17%) is registered in France, Italy, and Spain probably due to the more balanced diet. 122
AUTHOR CONTRIBUTION
All authors have read and approved the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICAL STATEMENT
All the authors declare that the present work has been carried out in accordance with the Wiley's Best Practice Guidelines on Publishing Ethics and that is has been performed in an ethical and responsible way, with no research misconduct. The article has not been previously published and is not currently submitted elsewhere.
TRANSPARENCY STATEMENT
Prof. Caterina Foti, MD affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Foti C, Calogiuri G, Nettis E, et al. Allergic contact dermatitis from vitamins: a systematic review. Health Sci Rep. 2022;5:e766. 10.1002/hsr2.766
DATA AVAILABILITY STATEMENT
Prof. Caterina Foti, corresponding author, had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis. The authors confirm that the data supporting the findings of this study are available within the article.
REFERENCES
- 1. Truswell AS. ABC of nutritions. Vitamins I. Br Med J (Clin Res Ed). 1985;291(6501):1033‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Trustwell AS. ABC of nutritions. Vitamin II. Br Med J (Clin Res Ed). 1985;291(6502):1103‐1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Das JK, Salam RA, Kumar R, Bhutta ZA. Micronutrient fortification of food and its impact on woman and child health: a systematic review. Syst Rev. 2013;2:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Manela‐Azulay M, Bagatin E. Cosmeceuticals vitamins. Clin Dermatol. 2009;27(5):469‐474. [DOI] [PubMed] [Google Scholar]
- 5. Lucas CD, Hallagan JB, Taylor SL. The role of natural color additives in food allergy. Adv Food Nutr Res. 2001;43:195‐216. [DOI] [PubMed] [Google Scholar]
- 6. Beckenbach L, Baron JM, Merk HF, Löffler H, Amann PM. Retinoids treatments of skin diseases. Eur J Dermatol. 2015;25(5):384‐391. [DOI] [PubMed] [Google Scholar]
- 7. Stingeni L, Bianchi L, Hansel K, et al. Italian guidelines in patch testing—adapted from the European Society of Contact Dermatitis (ESCD). G Ital Dermatol Venereol. 2019;154(3):227‐253. [DOI] [PubMed] [Google Scholar]
- 8. Calogiuri G, Garvey LH, Nettis E, et al. Hypersensitivity to vitamins with a focus on immediate‐type reactions: food or drug allergy? Endocr Metab Immune Disord Drug Targets. 2021;21(10):1804‐1816. [DOI] [PubMed] [Google Scholar]
- 9. Cameli N, Zanniello R, Mariano M, Cristaudo A. Vitaminn K1‐photo induced reaction during treatment with cetuximab. Contact Dermatitis. 2020;82(3):189‐190. [DOI] [PubMed] [Google Scholar]
- 10. Schalock PC, Storrs FJ, Morrison L. Contact urticaria from panthenol in hair conditioner. Contact Dermatitis. 2000;43(4):223. [DOI] [PubMed] [Google Scholar]
- 11. Sanz‐Sánchez T, Núñez‐Acevedo B, Rubio Flores C, Díaz‐Díaz RM. Contact urticaria caused by tocopherol. Contact Dermatitis. 2018;79(6):395. [DOI] [PubMed] [Google Scholar]
- 12. Röckmann S, Goerdt S, Bayerl C. Anaphylaxis after dexpanthenol exposure by multivitamin tablets. Clin Exp Dermatol. 2005;30(6):714‐716. [DOI] [PubMed] [Google Scholar]
- 13. Wondrak GT, Roberts MJ, Jacobson MK, Jacobson EL. 3‐Hydroxypyridine chromophores are endogenous sensitizers of photo‐oxidative stress in human skin cells. J Biochem Chem. 2004;16 279(29):30009‐30020. [DOI] [PubMed] [Google Scholar]
- 14. Baer RL, Stillman MA. Cutaneous skin changes probably due to pyridoxine abuse. J Am Acad Dermatol. 1984;10(3):527‐528. [DOI] [PubMed] [Google Scholar]
- 15. Friedman MA, Resnick JS, Baer RL. Sub‐epidermal vesicular dermatosis and sensory peripheral neuropathy caused by pyridoxine abuse. J Am Acad Dermatol. 1986;14(5 Pt 2):915‐917. [DOI] [PubMed] [Google Scholar]
- 16. Cameli N, Zanniello R, Mariano M, Cristaudo A. Vitaminn K(1) photo induced reaction during treatment with cetuximab. Contact Dermatitis. 2020;82(3):189‐190. [DOI] [PubMed] [Google Scholar]
- 17. Pastor‐Nieto MA, Gatica‐Ortega ME, Melgar‐Molero V, et al. Erythema multiforme‐like reaction resulting from vitamin K1 oxide (phytomenadione epoxide). Contact Dermatitis. 2017;77(5):343‐345. [DOI] [PubMed] [Google Scholar]
- 18. Nishioka K, Seguchi T, Kaniwa M, Suetomi Y. Occupational contact dermatitis due to biotin precursor. Contact Dermatitis. 1998;39(1):49‐51. [DOI] [PubMed] [Google Scholar]
- 19. Heidenheim M, Jemec GBE. Occupational allergic contact dermatitis from vitamin A acetate. Contact Dermatitis. 1995;33(6):439. [DOI] [PubMed] [Google Scholar]
- 20. Chen R, Raffi J, Murase JE. Tocopherol allergic dermatitis masquerading as lifelong atopic dermatitis. Dermatitis. 2020;31(1):e3‐e4. [DOI] [PubMed] [Google Scholar]
- 21. Ingemann‐Larsen A, Jepsen JR, Thulin H. Allergic contact dermatitis from thiamine. Contact Dermatitis. 1989;20(5):387‐388. [DOI] [PubMed] [Google Scholar]
- 22. Combes FC, Groopman J. Contact dermatitis due to thiamine. Report of two cases. Arch Derm Syphilol. 1950;61(5):858‐859. [DOI] [PubMed] [Google Scholar]
- 23. Arruti N, Bernedo N, Audicana MT, Villarreal O, Uriel O, Muñoz D. Systemic allergic dermatitis caused by thiamine after iontophoresis. Contact Dermatitis. 2013;69(6):375‐376. [DOI] [PubMed] [Google Scholar]
- 24. Miroux‐Catarino A, Silva L, Amaro C, Viana I. Allergic contact dermatitis caused by dexpanthenol. But is that all? Contact Dermatitis. 2019;81(5):391‐392. [DOI] [PubMed] [Google Scholar]
- 25. Fernandes S, Macias V, Cravo M, Amaro C, Santos R, Cardoso J. Allergic contact dermatitis caused by dexpanthenol: report of two cases. Contact Dermatitis. 2012;66(3):160‐161. [DOI] [PubMed] [Google Scholar]
- 26. Mitchell JC. Contact urticaria from vitamin E preparation (vitamin E vegetable oil) in two siblings. Int J Dermatol. 1975;14:246‐247. [Google Scholar]
- 27. Hemmer W, Bracun R, Wolf‐Abdolvahab S, Focke M, Götz M, Jarisch R. Maintenance of hand eczema by oral pantothenic acid in a patient sensitised to dexpanthenol. Contact Dermatitis. 1997;37(1):51. [DOI] [PubMed] [Google Scholar]
- 28. Malten EK. Flare reaction due to vitamin B12 in a patient with psoriasis and contact eczema. Contact Dermatitis. 1975;1(5):325‐326. [DOI] [PubMed] [Google Scholar]
- 29. Metz J, MetzHundertmark U, Pevny I. Vitamin C allergy of the delayed type. Contact Dermatitis. 1980;6(3):172‐174. [DOI] [PubMed] [Google Scholar]
- 30. Hjorth N. Contact dermatitis from vitamin B1 (thiamine); relapse after ingestion of thiamine, cross‐sensitization to cocarboxylase. J Invest Dermatol. 1958;30(5):261‐264. [DOI] [PubMed] [Google Scholar]
- 31. Audicana M, Schmidt R, de Corres LF. Allergic contact dermatitis from nicotinic acid esters. Contact Dermatitis. 1990;22(1):60‐61. [DOI] [PubMed] [Google Scholar]
- 32. Bilbao I, Aguirre A, Zabala R, González R, Ratón J, Diaz Pérez JL. Allergic contact dermatitis from butoxyethyl nicotinic acid and Centella asiatica extract. Contact Dermatitis. 1995;33(6):435‐436. [DOI] [PubMed] [Google Scholar]
- 33. Haustein UF. Burning mouth syndrome due to nicotinic acid esters and sorbic acid. Contact Dermatitis. 1988;19(3):225‐226. [DOI] [PubMed] [Google Scholar]
- 34. Ebner F, Heller A, Rippke F, Tausch I. Topical use of dexpanthenol in skin disorders. Am J Clin Dermatol. 2002;3(6):427‐433. [DOI] [PubMed] [Google Scholar]
- 35. Sasseville D, Kwong P, Yu K. Narrow spectrum of cross‐sensitization with pyridine derivatives. Contact Dermatitis. 1998;38(4):212‐214. [DOI] [PubMed] [Google Scholar]
- 36. van Ketel WG. Hair lotion dermatitis with sensitization to D‐panthenyl ethyl ether. Contact Dermatitis. 1984;10(1):48. [DOI] [PubMed] [Google Scholar]
- 37. Ramírez Santos A, Fernández‐Redondo V, Pérez Pérez L, Concheiro Cao J, Toribio J. Contact dermatitis from vitamins in cosmetic products. Dermatitis. 2008;19(3):154‐156. [PubMed] [Google Scholar]
- 38. Brescoll J, Daveluy S. A review of vitamin B12 in dermatology. Am J Clin Dermatol. 2015;16(1):27‐33. [DOI] [PubMed] [Google Scholar]
- 39. Fisher AA. Contact dermatitis at home and abroad. Cutis. 1972;10:719‐723. [Google Scholar]
- 40. Jeanmougin M, Manciet JR, Moulin JP, Blanc P, Pons A, Civatte J. Contact allergy to dexpanthenol in sunscreens. Contact Dermatitis. 1988;18(4):240. [DOI] [PubMed] [Google Scholar]
- 41. Roberts H, Williams J, Tate B. Allergic contact dermatitis to panthenol and cocamidopropyl PG dimonium chloride phosphate in a facial hydrating lotion. Contact Dermatitis. 2006;55(6):369‐370. [DOI] [PubMed] [Google Scholar]
- 42. Stables GI, Wilkinson SM. Allergic contact dermatitis due to panthenol. Contact Dermatitis. 1998;38(4):236‐237. [DOI] [PubMed] [Google Scholar]
- 43. Chin MF, Hughes TM, Stone NM. Allergic contact dermatitis caused by panthenol in a child. Contact Dermatitis. 2013;69(5):321‐322. [DOI] [PubMed] [Google Scholar]
- 44. Bregnbak D, Johansen JD, Zachariae C. Contact dermatitis caused by panthenol use for aftercare treatment of a new tattoo. Contact Dermatitis. 2016;75(1):50‐52. [DOI] [PubMed] [Google Scholar]
- 45. Gollhausen R, Przybilla B, Ring J. Contact allergy to dexpanthenol. Contact Dermatitis. 1985;13(1):38. [DOI] [PubMed] [Google Scholar]
- 46. Clerens I, Goossens A. Allergic contact dermatitis caused by panthenol: a rare but relevant sensitizer. Contact Dermatitis. 2017;76(2):122‐123. [DOI] [PubMed] [Google Scholar]
- 47. Fernandes RA, Santiago L, Gouveia M, Gonçalo M. Allergic contact dermatitis caused by dexpanthenol—probably a frequent allergen. Contact Dermatitis. 2018;79(5):276‐280. [DOI] [PubMed] [Google Scholar]
- 48. Foti C, Romita P, Bufano T, Antelmi A. Allergic contact dermatitis caused by panthenyl ethyl ether in a patient with psoriasis. Contact Dermatitis. 2017;76(3):181‐182. [DOI] [PubMed] [Google Scholar]
- 49. Pastor‐Nieto MA, Gatica‐Ortega ME, Sánchez‐Herreros C, et al. Calcium pantothenate is present in cosmetics and may cause allergic contact dermatitis. Contact Dermatitis. 2021;84(3):201‐203. [DOI] [PubMed] [Google Scholar]
- 50. Hahn C, Röseler S, Fritzsche R, Schneider R, Merk HF. Allergic contact reaction to dexpanthenol: lymphocyte transformation test and evidence for microsomal‐dependent metabolism of the allergen. Contact Dermatitis. 1993;28(2):81‐83. [DOI] [PubMed] [Google Scholar]
- 51. Miller JW, Rucker RB. Panthotenic acid. In: Eirdman JW, McDonald IA, Zeiser SH, eds. Present knowledge in nutrition. 10th ed. Wiley Publisher; 2012:375‐390. [Google Scholar]
- 52. Yoshikawa K, Watanabe K, Mizuno N. Contact allergy to hydrocortisone 17‐butyrate and pyridoxine hydrochloride. Contact Dermatitis. 1985;12(1):55‐56. [DOI] [PubMed] [Google Scholar]
- 53. Córdoba S, Martínez‐Morán C, García‐Donoso C, Borbujo J, Gandolfo‐Cano M. Non‐occupational allergic contact dermatitis from pyridoxine hydrochloride and ranitidine hydrochloride. Dermatitis. 2011;22(4):236‐237. [PubMed] [Google Scholar]
- 54. Maeda T, Taguchi H, Minami H, et al. Vitamin B6 phototoxicity induced by UVA radiation. Arch Dermatol Res. 2000;292(11):562‐567. [DOI] [PubMed] [Google Scholar]
- 55. Morimoto K, Kawada A, Hiruma M, Ishibashi A. Photosensitivity from pyridoxine hydrochloride (vitamin B6). J Am Acad Dermatol. 1996;35(2Pt2):304‐305. [DOI] [PubMed] [Google Scholar]
- 56. Murata Y, Kumano K, Ueda T, Araki N, Nakamura T, Tani M. Photo‐sensitive dermatitis caused by pyridoxine hydrochloride. J Am Acad Dermatol. 1998;39(2 Pt 2):314‐317. [DOI] [PubMed] [Google Scholar]
- 57. Green R, Miller JW. Vitamin B12. In: Zempleni J, Rucker RB, McCormicke DB, Suttie JW, eds. Handbook of Vitamins. 4th ed. CRC Press—Taylor & Francis Group; 2007:413‐459. [Google Scholar]
- 58. Price ML, McDonald MD. Cheilitis and cobalt allergy related to ingestion of vitamin B12. Contact Dermatitis. 1981;7(6):352. [DOI] [PubMed] [Google Scholar]
- 59. Rodriguez A, Echechipía S, Alvarez M, Muro MD. Occupational contact dermatitis to vitamin B12. Contact Dermatitis. 1994;31(4):271. [DOI] [PubMed] [Google Scholar]
- 60. Ross AC, Harrison AH. Vitamin A: nutritional aspects of retinoids and carotenoidsIn: Zempleni J, Rucker RB, McCormicke DB, Suttie JW, eds. Handbook of Vitamins. 4th ed. CRC Press—Taylor & Francis Group; 2007:1‐30. [Google Scholar]
- 61. Pullar JM, Carr AC, Vissers MCM. The roles of vitamin C in skin health. Nutrients. 2017;9(8):866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Belhadjali H, Giordano‐Labadie F, Bazex J. Contact dermatitis from vitamin C in a cosmetic anti‐aging cream. Contact Dermatitis. 2001;45(5):317. [DOI] [PubMed] [Google Scholar]
- 63. Yagami A, Suzuki K, Morita Y, Iwata Y, Sano A, Matsunaga K. Allergic contact dermatitis caused by 3‐o‐ethyl‐L‐ascorbic acid (vitamin C ethyl). Contact Dermatitis. 2014;70(6):376‐377. [DOI] [PubMed] [Google Scholar]
- 64. Hamanaka M, Kanto H, Mikai H, et al. A rare case of allergic contact dermatitis caused by 3‐O‐ethyl‐L‐ascorbic acid in skin‐whitening cosmetics identified under immunosuppressive therapy. Contact Dermatitis. 2020;83(6):520‐521. [DOI] [PubMed] [Google Scholar]
- 65. Numata T, Kobayashi Y, Ito T, Harada K, Tsuboi R, Okubo Y. Two cases of allergic contact dermatitis due to skin‐whitening cosmetics. Allergol Int. 2015;64(2):194‐195. [DOI] [PubMed] [Google Scholar]
- 66. Victoria‐Martínez AM, Mercader‐García P. Allergic contact dermatitis to 3‐O‐ethyl‐L‐ascorbic acid in skin‐lightening cosmetics. Dermatitis. 2017;28(1):89. [DOI] [PubMed] [Google Scholar]
- 67. Romita P, Foti C, Barlusconi C, Mercurio S, Hansel K, Stingeni L. Allergic contact dermatitis to 3‐O‐ethyl‐L‐ascorbic acid: an underrated allergen in cosmetics? Contact Dermatitis. 2020;83(1):63‐64. [DOI] [PubMed] [Google Scholar]
- 68. Mamodaly M, Dereure O, Raison‐Peyron N. A new case of allergic contact dermatitis caused by 3‐O‐ethyl ascorbic acid in facial anti‐ageing cosmetics. Contact Dermatitis. 2019;81(4):315‐316. [DOI] [PubMed] [Google Scholar]
- 69. Penniston KL, Tanumihardjo SA. The acute and chronic toxic effects of vitamin A. Am J Clin Nutr. 2006;83(2):191‐201. [DOI] [PubMed] [Google Scholar]
- 70. Jacobsen RB, Hronek BW, Schmidt GA, Schilling ML. Hypervitaminosis D associated with a vitamin D dispensing error. Ann Pharmacother. 2011;45(10):e52. [DOI] [PubMed] [Google Scholar]
- 71. Jordan WP, Higgins M, Dvorak J. Allergic contact dermatitis to all‐trans retionoic acid: epicutaneous and leukocyte migration inhibiting testing. Contact Dermatitis. 1975;1(5):306‐310. [DOI] [PubMed] [Google Scholar]
- 72. Lindgren S, Groth O, Molin L. Allergic contact response to vitamin A. Contact Dermatitis. 1976;2(4):212‐217. [DOI] [PubMed] [Google Scholar]
- 73. Balato N, Patruno C, Lembo G, Cuccurullo FM, Ayala F. Allergic contact dermatitis from retinoic acid. Contact Dermatitis. 1995;32(1):51. [DOI] [PubMed] [Google Scholar]
- 74. Serup J. Allergic contact dermatitis from retinoic acid? Contact Dermatitis. 1995;33(2):142. [DOI] [PubMed] [Google Scholar]
- 75. Anderson A, Gerbauer K. Perorbital allergic contact dermatitis resulting from topical retinoic acid use. Australas J Dermatol. 2014;55(2):152‐153. [DOI] [PubMed] [Google Scholar]
- 76. Nordqvist BC, Meher K. Allergic contact dermatitis to retinoic acid. Contact Dermatitis. 1977;3(1):55‐56. [DOI] [PubMed] [Google Scholar]
- 77. Tomb R, Dolfus A, Couppie P. Eczema caused by contact allergy to tretinoin. Ann Dermatol Venereol. 1992;119(10):761‐764. [PubMed] [Google Scholar]
- 78. Manzano D, Aguirre A, Gardeazabal J, Eizaguirre X, Pérez JLD. Allergic contact dermatitis from tocopheryl acetate (vitamin E) and retinol palmitate (vitamin A) in a moisturizing cream. Contact Dermatitis. 1994;31(5):324. [DOI] [PubMed] [Google Scholar]
- 79. Clemensen A, Thormann J, Andersen KE. Allergic contact dermatitis from retinyl palmitate. Contact Dermatitis. 2007;56(5):288‐289. [DOI] [PubMed] [Google Scholar]
- 80. Bazzano C, de Angeles S, Kleist G, Macedo N. Allergic contact dermatitis from topical vitamins A and E. Contact Dermatitis. 1996;35(4):261‐262. [DOI] [PubMed] [Google Scholar]
- 81. Foti C, Romita P, Borghi A, Angelini G, Bonamonte D, Corazza M. Contact dermatitis to topical acne drugs: a review of the literature. Dermatol Ther. 2015;28(5):323‐329. [DOI] [PubMed] [Google Scholar]
- 82. Maestro M, Molnàr F, Carlberg C. Vitamin D and its synthetic analogs. J Med Chem. 2019;62(15):6854‐6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kimura K, Katayama I, Nishioka K. Allergic contact dermatitis from tacalcitol. Contact Dermatitis. 1995;33(6):441‐442. [DOI] [PubMed] [Google Scholar]
- 84. Frosch PJ, Rustmeyer T. Contact allergy to calcipotriol does exist: an unequivocal case and review of literature. Contact Dermatitis. 1999;40(2):66‐71. [DOI] [PubMed] [Google Scholar]
- 85. Foti C, Carnimeo L, Bonamonte D, Conserva A, Casulli C, Angelini G. Tolerance to calcitriol and tacalcitol in three patients with allergic contact dermatitis to calcipotriol. J Drugs Dermatol. 2005;4(6):756‐759. [PubMed] [Google Scholar]
- 86. Weber P, Bendich A, Machlin LJ. Vitamin E and human health: rationale for determining recommended intake levels. Nutrition. 1997;13(5):450‐460. [DOI] [PubMed] [Google Scholar]
- 87. Netscher T. Synthesis of vitamin E. Vitam Horm. 2007;76:155‐202. [DOI] [PubMed] [Google Scholar]
- 88. Brodkin RH, Bleiberg J. Sensitivity to topically applied vitamin E. Arch Dermatol. 1965;92(1):76‐77. [PubMed] [Google Scholar]
- 89. Aeling JL, Panagotacos PJ, Andreozzi RJ. Allergic contact dermatitis to vitamin E aerosol deodorant. Arch Dermatol. 1973;108(4):579‐580. [DOI] [PubMed] [Google Scholar]
- 90. Minkin W, Cohen HJ, Frank SB. Contact dermatitis from deodorants. Arch Dermatol. 1973;107(5):774‐775. [PubMed] [Google Scholar]
- 91. Goldman MP, Rapaport M. Contact dermatitis to vitamin E oil. J Am Acad Dermatol. 1986;14(1):133‐134. [DOI] [PubMed] [Google Scholar]
- 92. Saperstein H, Rapaport M, Rietschel RL. Topical vitamin E as a cause of erythema multiforme‐like eruption. Arch Dermatol. 1984;120(7):906‐908. [PubMed] [Google Scholar]
- 93. Navarro‐Triviño FJ, Linares‐González L, Ayén‐Rodríguez Á, Ruiz‐Villaverde R. Allergic contact dermatitis caused by tocopheryl nicotinate. Contact Dermatitis. 2021;84(6):479‐480. [DOI] [PubMed] [Google Scholar]
- 94. De Groot AC, Berretty PJ, Van Ginkel CJ, den Hengst CW, van Ulsen J, Weyland JW. Allergic contact dermatitis from tocopheryl acetate in cosmetic creams. Contact Dermatitis. 1991;25(5):302‐304. [DOI] [PubMed] [Google Scholar]
- 95. Zirwas MJ, Stechschulte SA. Moisturizer allergy. Diagnosis and management. J Clin Aesthet Dermatol. 2008;1(4):38‐44. [PMC free article] [PubMed] [Google Scholar]
- 96. Kosari P, Alikhan A, Sockolov M, Feldman SR. Vitamin E and allergic contact dermatitis. Dermatitis. 2010;21(3):148‐153. [PubMed] [Google Scholar]
- 97. Adams AK, Connolly SM. Allergic contact dermatitis from vitamin E: the experience at Mayo Clinic Arizona, 1987 to 2007. Dermatitis. 2010;21(4):199‐202. [PubMed] [Google Scholar]
- 98. García‐Bravo H, Mozo P. Generalized contact dermatitis from vitamin E. Contact Dermatitis. 1992;26(4):280. [DOI] [PubMed] [Google Scholar]
- 99. Matsumura T, Nakada T, Iijima M. Widespread contact dermatitis from tocopherol acetate. Contact Dermatitis. 2004;51(4):211‐212. [DOI] [PubMed] [Google Scholar]
- 100. Ohko K, Ito A, Ito M. Allergic contact dermatitis syndrome due to tocopherol acetate, in addition to glycyrrhetinic acid. J Cosmet Dermatol Sci Appl. 2012;2:38‐40. [Google Scholar]
- 101. Corazza M, Minghetti S, Borghi A, Bianchi A, Virgili A. Vitamin E contact allergy: a controversial subject. Dermatitis. 2012;23(4):167‐169. [DOI] [PubMed] [Google Scholar]
- 102. Traber MG. Vitamin E. In: Zempleni J, Rucker RB, McCormicke DB, Suttie JW, eds. Handbook of Vitamins. 4th ed. CRC Press—Taylor & Francis Group; 2007:153‐174. [Google Scholar]
- 103. Nada AH, Zaghloul AA, Hedaya MA, et al. Stability of vitamin E and vitamin E acetate containing cosmetic preparations. J Glob Pharm Technol. 2012;4:1‐8. [Google Scholar]
- 104. Milanesi N, Gola M, Francalanci S. Allergic contact dermatitis caused by VEA® lipogel: compound allergy? Contact Dermatitis. 2016;75(4):243‐244. [DOI] [PubMed] [Google Scholar]
- 105. Hunter D, Frumkin A. Adverse reactions to vitamin E and aloe vera preparations after dermabrasion and chemical peel. Cutis. 1991;47(3):193‐196. [PubMed] [Google Scholar]
- 106. Oshima H, Tsuji K, Oh‐I T, Koda M. Allergic contact dermatitis due to DL‐alpha‐tocopheryl nicotinate. Contact Dermatitis. 2003;48(3):167‐168. [DOI] [PubMed] [Google Scholar]
- 107. Suttie WJ. Vitamin K. In: Zempleni J, Suttie JW, Gregory JF III, Stover PJ, Suttie WJ, eds. Handbook of Vitamins. V ed. CRC Press; 2013. [Google Scholar]
- 108. Page RC, Bercovitz Z. Dermatitis from topical application of 2‐methyl‐1,4 naphthoquinone (synthetic vitamin K1 analogue). Am J Med Sci. 1942;203:566‐569. [Google Scholar]
- 109. Veneziano L, Silvani S, Voudouris S, Tosti A. Contact dermatitis due to topical cosmetic use of vitamin K. Contact Dermatitis. 2005;52(2):113‐114. [DOI] [PubMed] [Google Scholar]
- 110. Serra‐Baldrich E, Dalmau J, Pla C, Muntañola AA. Contact dermatitis due to clarifying cream. Contact Dermatitis. 2005;53(3):174‐175. [DOI] [PubMed] [Google Scholar]
- 111. Ruiz‐Hornillos FJ, Prieto A, De Castro FJ, et al. Allergic contact dermatitis to vitamin K1 contained in a cosmetic cream. Contact Dermatitis. 2006;55(4):246‐247. [DOI] [PubMed] [Google Scholar]
- 112. Pigatto PD, Bigardi A, Fumagalli M, Altomare GF, Riboldi A. Allergic dermatitis from parenteral vitamin K. Contact Dermatitis. 1990;22(5):307‐308. [DOI] [PubMed] [Google Scholar]
- 113. Wong DA, Freeman S. Cutaneous allergic reaction to intramuscular vitamin K1. Australas J Dermatol. 1999;40(3):147‐152. [DOI] [PubMed] [Google Scholar]
- 114. Lopez‐Lerma I, Villaplana J. Contact dermatitis to vitamin K1 in an eye cream. Ann Allergy Asthma Immunol. 2013;111(3):227‐228. [DOI] [PubMed] [Google Scholar]
- 115. Garcia‐Gavin J, Goossens A, Tennstedt D. Allergic contact dermatitis due to cosmetics containing vitamin K1 oxide. Contact Dermatitis. 2010;62(4):248‐250. [DOI] [PubMed] [Google Scholar]
- 116. Schneller‐Pavelescu L, Ochando‐Ibernón G, Vergara‐de Caso E, Silvestre‐Salvador JF. “Crossed” allergic contact dermatitis caused by oxidized vitamin K1 in a patient previously sensitized to non‐oxidized vitamin K1. Contact Dermatitis. 2019;80(1):64‐65. [DOI] [PubMed] [Google Scholar]
- 117. Romaguera C, Grimalt F, Conde‐Salazar L. Occupational dermatitis from vitamin K3 sodium bisulphite. Contact Dermatitis. 1980;6(5):355‐356. [DOI] [PubMed] [Google Scholar]
- 118. Dinis A, Brandaõ M, Faria A. Occupational contact dermatitis from vitamin K3 sodium bisulphite. Contact Dermatitis. 1988;18(3):170‐171. [DOI] [PubMed] [Google Scholar]
- 119. Camarasa JG, Barnadas M. Occupational dermatosis by vitamin K3 sodium bisulphite. Contact Dermatitis. 1982;8(4):268. [DOI] [PubMed] [Google Scholar]
- 120. Bianchi L, Hansel K, Tramontana M, Assalve D, Stingeni L. Occupational allergic contact dermatitis with secondary spreading from vitamin K3 sodium bisulphite in a pig farmer. Dermatitis. 2015;26(3):150‐151. [DOI] [PubMed] [Google Scholar]
- 121. Manson JE, Bassuk SS. Vitamin and mineral supplements: what clinicians need to know. JAMA. 2018;319(9):859‐860. [DOI] [PubMed] [Google Scholar]
- 122. Gahche J, Bailey R, Burt V, et al. Dietary supplement use among U.S. adults has increased since NHANES III (1988–1994). NCHS Data Brief. 2011;61:1‐8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Prof. Caterina Foti, corresponding author, had full access to all of the data in this study and takes complete responsibility for the integrity of the data and the accuracy of the data analysis. The authors confirm that the data supporting the findings of this study are available within the article.


