Abstract
Background
Antipsychotic‐induced weight gain is an extremely common problem in people with schizophrenia and is associated with increased morbidity and mortality. Adjunctive pharmacological interventions may be necessary to help manage antipsychotic‐induced weight gain. This review splits and updates a previous Cochrane Review that focused on both pharmacological and behavioural approaches to this problem.
Objectives
To determine the effectiveness of pharmacological interventions for preventing antipsychotic‐induced weight gain in people with schizophrenia.
Search methods
The Cochrane Schizophrenia Information Specialist searched Cochrane Schizophrenia's Register of Trials on 10 February 2021. There are no language, date, document type, or publication status limitations for inclusion of records in the register.
Selection criteria
We included all randomised controlled trials (RCTs) that examined any adjunctive pharmacological intervention for preventing weight gain in people with schizophrenia or schizophrenia‐like illnesses who use antipsychotic medications.
Data collection and analysis
At least two review authors independently extracted data and assessed the quality of included studies. For continuous outcomes, we combined mean differences (MD) in endpoint and change data in the analysis. For dichotomous outcomes, we calculated risk ratios (RR). We assessed risk of bias for included studies and used GRADE to judge certainty of evidence and create summary of findings tables. The primary outcomes for this review were clinically important change in weight, clinically important change in body mass index (BMI), leaving the study early, compliance with treatment, and frequency of nausea. The included studies rarely reported these outcomes, so, post hoc, we added two new outcomes, average endpoint/change in weight and average endpoint/change in BMI.
Main results
Seventeen RCTs, with a total of 1388 participants, met the inclusion criteria for the review. Five studies investigated metformin, three topiramate, three H2 antagonists, three monoamine modulators, and one each investigated monoamine modulators plus betahistine, melatonin and samidorphan. The comparator in all studies was placebo or no treatment (i.e. standard care alone). We synthesised all studies in a quantitative meta‐analysis. Most studies inadequately reported their methods of allocation concealment and blinding of participants and personnel. The resulting risk of bias and often small sample sizes limited the overall certainty of the evidence. Only one reboxetine study reported the primary outcome, number of participants with clinically important change in weight. Fewer people in the treatment condition experienced weight gains of more than 5% and more than 7% of their bodyweight than those in the placebo group (> 5% weight gain RR 0.27, 95% confidence interval (CI) 0.11 to 0.65; 1 study, 43 participants; > 7% weight gain RR 0.24, 95% CI 0.07 to 0.83; 1 study, 43 participants; very low‐certainty evidence). No studies reported the primary outcomes, 'clinically important change in BMI', or 'compliance with treatment'. However, several studies reported 'average endpoint/change in body weight' or 'average endpoint/change in BMI'.
Metformin may be effective in preventing weight gain (MD −4.03 kg, 95% CI −5.78 to −2.28; 4 studies, 131 participants; low‐certainty evidence); and BMI increase (MD −1.63 kg/m2, 95% CI −2.96 to −0.29; 5 studies, 227 participants; low‐certainty evidence). Other agents that may be slightly effective in preventing weight gain include H2 antagonists such as nizatidine, famotidine and ranitidine (MD −1.32 kg, 95% CI −2.09 to −0.56; 3 studies, 248 participants; low‐certainty evidence) and monoamine modulators such as reboxetine and fluoxetine (weight: MD −1.89 kg, 95% CI −3.31 to −0.47; 3 studies, 103 participants; low‐certainty evidence; BMI: MD −0.66 kg/m2, 95% CI −1.05 to −0.26; 3 studies, 103 participants; low‐certainty evidence). Topiramate did not appear effective in preventing weight gain (MD −4.82 kg, 95% CI −9.99 to 0.35; 3 studies, 168 participants; very low‐certainty evidence). For all agents, there was no difference between groups in terms of individuals leaving the study or reports of nausea. However, the results of these outcomes are uncertain given the very low‐certainty evidence.
Authors' conclusions
There is low‐certainty evidence to suggest that metformin may be effective in preventing weight gain. Interpretation of this result and those for other agents, is limited by the small number of studies, small sample size, and short study duration. In future, we need studies that are adequately powered and with longer treatment durations to further evaluate the efficacy and safety of interventions for managing weight gain.
Keywords: Humans, Antipsychotic Agents, Antipsychotic Agents/adverse effects, Betahistine, Betahistine/therapeutic use, Famotidine, Famotidine/therapeutic use, Fluoxetine, Fluoxetine/therapeutic use, Melatonin, Melatonin/therapeutic use, Metformin, Metformin/therapeutic use, Nausea, Nausea/drug therapy, Nizatidine, Nizatidine/therapeutic use, Ranitidine, Ranitidine/therapeutic use, Reboxetine, Reboxetine/therapeutic use, Schizophrenia, Schizophrenia/drug therapy, Schizophrenia/prevention & control, Topiramate, Topiramate/therapeutic use, Weight Gain
Plain language summary
How effective are medications given alongside antipsychotics at preventing weight gain in people with schizophrenia?
Key messages
‐ Metformin may be effective in preventing weight gain caused by antipsychotics.
‐ H2 antagonists and monoamine modulators may be slightly effective in preventing weight gain caused by antipsychotics.
‐ Future studies should include more people and evaluate them for longer.
What are antipsychotics?
Antipsychotics are medications used to treat symptoms of psychosis, such as hallucinations, delusions and agitation. They are often used to treat people with schizophrenia. Examples of antipsychotics are haloperidol (Haldol), chlorpromazine (Thorazine), olanzapine (Zyprexa) and risperidone (Risperdal).
Schizophrenia and weight gain
People with schizophrenia are twice as likely as the general population to be overweight, perhaps due to a poor diet and an inactive lifestyle. Excess weight can lead to other health conditions, such as heart disease, stroke and diabetes.
Unfortunately, an unwanted effect of antipsychotics is weight gain. Sometimes, people with schizophrenia are given medications alongside antipsychotics (‘add‐on’ medications) to prevent this additional weight gain. These add‐on medications may stop people feeling hungry or help them feel full. Usually, they are medications developed for other purposes, such as metformin, which is a medicine to treat diabetes, and fluoxetine, which is an antidepressant.
What did we want to find out?
We wanted to find out whether add‐on medications to prevent weight gain caused by antipsychotics were effective in people with schizophrenia.
What did we do?
We searched for studies that examined any medicine given alongside antipsychotics to prevent weight gain in people with schizophrenia. Study participants could be any age or sex but had to have a diagnosis of schizophrenia or a schizophrenia‐like illness. They had to be chosen at random to receive either the weight‐prevention medicine, or a placebo (a dummy medicine) or no add‐on medicine (standard treatment).
We compared and summarised the results of the studies and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
We found 17 studies with 1388 people that examined the effects of add‐on medications to prevent weight gain caused by antipsychotics. The add‐on medications were metformin, topiramate, H2 antagonists, monoamine modulators, monoamine modulators plus betahistine, melatonin, and samidorphan. Studies were short, lasting between 6 and 24 weeks. And they were small, with only 63 people on average ‐ the smallest included only 14 people, the largest 561 people.
Studies done to date suggest that:
‐ metformin may be effective in preventing weight gain and is well‐tolerated compared to standard treatment (5 studies, 232 participants);
‐ H2 antagonists, such as nizatidine, famotidine and ranitidine, or monoamine modulators, such as reboxetine and fluoxetine may be potentially effective in preventing weight gain caused by antipsychotics;
‐ topiramate is probably not effective in preventing weight gain caused by antipsychotics.
What are the limitations of the evidence?
Our confidence in the evidence is limited because we found only a small number of studies for each add‐on medication. Studies included few people, and lasted only a short time.
How up to date is this evidence?
The evidence is up to date to February 2021.
Summary of findings
Summary of findings 1. Metformin compared to placebo or no treatment for prevention of weight gain in people with schizophrenia.
| Metformin compared to placebo or no treatment for prevention of weight gain in people with schizophrenia | ||||||
| Patient or population: people with schizophrenia with antipsychotic‐induced weight gain Setting: inpatients or outpatients Intervention: metformin Comparison: placebo or no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with metformin | |||||
| Weight: clinically important change in weight (kg) | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
| Weight: average endpoint/change in weight (kg) | Average endpoint/change in weight ranged from 5.88 to 6.87 | MD 4.03 kg lower (5.78 lower to 2.28 lower) | ‐ | 131 (4 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| Weight: clinically important change in BMI (kg/m2) | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
|
Weight Average endpoint/change in BMI (kg/m2) |
Average endpoint/change in BMI ranged from 1.93 to 2.26 | MD 1.63 lower (2.96 lower to 0.29 lower) | ‐ | 227 (5 RCTs) | ⊕⊕⊝⊝ Lowc,b | |
| Leaving the study early: for any reason | Study population | RR 1.02 (0.25 to 4.13) | 137 (4 RCTs) | ⊕⊝⊝⊝ Very lowa,c | ||
| 58 per 1000 | 59 per 1000 (14 to 239) | |||||
| Compliance with treatment | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
| Reports of nausea | Study population | RR 2.38 (0.28 to 19.95) | 69 (2 RCTs) | ⊕⊕⊝⊝ Lowa | ||
| 57 per 1000 | 136 per 1000 (16 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe total number of participants included in the review is less than the number of participants required for a single, adequately powered study. Also, the confidence interval is extremely wide. bWe deemed one of the included studies in this outcome to have high risk of bias. cHeterogeneity of the results was quite high making interpretation uncertain.
Summary of findings 2. H2 antagonists compared to placebo for prevention of weight gain in people with schizophrenia.
| H2 antagonists compared to placebo for prevention of weight gain in people with schizophrenia | ||||||
| Patient or population: people with schizophrenia with antipsychotic‐induced weight gain Setting: inpatients or outpatients Intervention: H2 antagonists Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with H2 antagonists | |||||
| Weight: clinically important change in weight (kg) | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
| Weight: average endpoint/change in body weight (kg) | Average endpoint/change in body weight ranged from 4.18 to 4.88 | MD 1.32 lower (2.09 lower to 0.56 lower) | ‐ | 248 (3 RCTs) | ⊕⊕⊝⊝ Lowa,b | |
| Weight: clinically important change in BMI (kg/m2) | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
| Weight: average endpoint/change in BMI (kg/m2) | Average endpoint/change in BMI was 1.82 | MD 0.66 lower (0.99 lower to 0.33 lower) | ‐ | 79 (2 RCTs) | ⊕⊝⊝⊝ Very lowa,b | |
| Leaving the study early: for any reason | Study population | RR 1.07 (0.72 to 1.57) | 189 (2 RCTs) | ⊕⊕⊝⊝ Lowa,b | ||
| 343 per 1000 | 367 per 1000 (247 to 539) | |||||
| Compliance with treatment | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
| Reports of nausea | Study population | RR 1.13 (0.34 to 3.68) | 175 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | ||
| 67 per 1000 | 75 per 1000 (23 to 245) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe risk of bias is uncertain for most studies included in this comparison. bThe total number of participants included in the review is less than the number of participants required for a single, adequately powered study.
Summary of findings 3. Monoamine modulators compared to placebo for prevention of weight gain in people with schizophrenia.
| Monoamine modulators compared to placebo for prevention of weight gain in people with schizophrenia | ||||||
| Patient or population: people with schizophrenia with antipsychotic‐induced weight gain Setting: inpatients or outpatients Intervention: monoamine modulators Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with monoamine modulators | |||||
| Weight: clinically important change in weight (kg) | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
| Weight: average endpoint/change in body weight (kg) | Average endpoint/change in body weight ranged from 4.91 to 5.5 | MD 1.89 lower (3.31 lower to 0.47 lower) | ‐ | 103 (3 RCTs) | ⊕⊕⊝⊝ Lowa | |
| Weight: clinically important change in BMI (kg/m2) | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
| Weight: average endpoint/change in BMI (kg/m2) | Average endpoint/change in BMI ranged from 0.86 to 1.12 | MD 0.66 lower (1.05 lower to 0.26 lower) | ‐ | 103 (3 RCTs) | ⊕⊕⊝⊝ Lowa | |
| Leaving the study early: for any reason | Study population | RR 1.05 (0.56 to 1.94) | 115 (3 RCTs) | ⊕⊕⊝⊝ Lowa | ||
| 257 per 1000 | 255 per 1000 (149 to 435) | |||||
| Compliance with treatment | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
| Reports of nausea | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe total number of participants included in the review is less than the number of participants required for a single, adequately powered study.
Summary of findings 4. Topiramate compared to placebo or no treatment for prevention of weight gain in people with schizophrenia.
| Topiramate compared to placebo or no treatment for prevention of weight gain in people with schizophrenia | ||||||
| Patient or population: people with schizophrenia with antipsychotic‐induced weight gain Setting: inpatients or outpatients Intervention: topiramate Comparison: placebo or no treatmentL | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with topiramate | |||||
| Weight: clinically important change in weight (kg) | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
| Weight: average endpoint/change in weight (kg) | Average endpoint/change in weight was 4.02 | MD 4.82 lower (9.99 lower to 0.35 higher) | ‐ | 168 (3 RCTs) | ⊕⊝⊝⊝ Very lowa,b | |
| Weight: clinically important change in BMI (kg/m2) | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
| Weight: average endpoint/change in BMI (kg/m2) | Average endpoint/change in BMI was 22.5 | MD 2.68 lower (4.10 lower to 1.26 lower) | ‐ | 120 (2 RCTs) | ⊕⊝⊝⊝ Very lowb,c | |
| Leaving the study early: for any reason | Study population | RR 1.09 (0.85 to 1.41) | 132 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c | ||
| 379 per 1000 | 413 per 1000 (322 to 534) | |||||
| Compliance with treatment | Study population | Not estimable | (0 RCTs) | ‐ | No data available | |
| Not estimable | Not estimable | |||||
| Reports of nausea | Study population | RR 1.20 (0.26 to 5.44) | 120 (2 RCTs) | ⊕⊕⊝⊝ Lowb,c | ||
| 29 per 1000 | 91 per 1000 (10 to 830) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BMI: body mass index; CI: confidence interval; MD: mean difference; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
aThe risk of bias is uncertain or high for most studies included in this comparison. bThe total number of participants included in the review is less than the number of participant required for a single, adequately powered study. cThe risk of bias is high for most studies included in this comparison.
Background
Description of the condition
Schizophrenia and weight gain
Schizophrenia is a complex and severe neuropsychiatric disorder characterised by delusions, hallucinations, disorganised behaviour and progressive cognitive deficits (Keshavan 2008; Van Os 2009). It is also a heterogeneous disorder with psychopathology varying across patients and over the course of the illness (Seaton 2001). The onset is typically in late adolescence or early adulthood and is marked by episodes of psychosis and severe functional disability (Liversedge 2011). The complexity, phenotypic heterogeneity, and polygenic nature of the genetic risk for schizophrenia make it a challenge to treat and investigate, and the etiopathogenesis (the cause and development of a disease or abnormal condition) of schizophrenia is yet to be fully understood (Keshavan 2011). The severity of the disability and lack of knowledge into its aetiology makes it the most disabling among all psychiatric disorders requiring a disproportionate share of mental health services (Mueser 2004); it is the costliest among severe mental disorders in terms of human suffering and expenditure incurred by society (Van Os 2009). The disability and cost to society are compounded by the common presence of comorbid obesity in this population, a problem that has been exacerbated more recently with the increased use of second‐generation antipsychotics, many of which are associated with the risk of weight gain and metabolic disturbances such as diabetes and metabolic syndrome (Allison 1999; Casey 2004; De Hert 2011; Homel 2002; Rajkumar 2017).
The World Health Organization (WHO) defines overweight and obesity as an "abnormal or excessive fat accumulation that may impair health". A person who has a body mass index (BMI) of more than 25 is overweight and those with a BMI of more than 30 are obese (WHO 2013). The prevalence of obesity in people with schizophrenia has been reported to be anywhere from 1.5 times to 4 times higher than the general population (ADA/APA 2004; Coodin 2001; Gurpegui 2012; Silverstone 1988); the risk may be even higher for long‐term inpatients (Ringen 2018). For people with schizophrenia, there is a marked increase in standardised mortality ratios for both natural and unnatural causes of death, and much of this increment may be attributed to the increased prevalence of coronary heart disease risk (Cohn 2004; Goff 2005; Henderson 2005b; Mackin 2005; Saari 2005; Westman 2017), and related obesity in this population (Annamalai 2017; Coodin 2001; Daumit 2003; Susce 2005). Obesity doubles the risk of all‐cause mortality, coronary heart disease, stroke and type 2 diabetes, increases the risk of some cancers, musculoskeletal problems and loss of function, and carries negative psychological consequences (DoH 2004). Being an obese or overweight adult is associated with increases in early mortality and large decreases in life expectancy, and these decreases are similar to those seen with smoking (Peeters 2003). The significance and recognition of this prevalence and its impact on premature mortality and morbidity has led to the development of consensus statements (ADA/APA 2004; De Nayer 2005), and guidelines (Cooper 2016), on its management. Despite this, evidence from a systematic review suggests that the all‐cause standardised mortality ratio between people with schizophrenia and the general population has risen steadily since the 1970s (Saha 2007). In stark contrast to the well‐recognised risk of metabolic comorbidity in schizophrenia, studies have repeatedly shown extremely low rates of intervention for these risk factors (De Hert 2011; Lappin 2018). Extremely low rates of intervention for what would be considered 'modifiable' cardiovascular risk factors are also apparent in young, first‐episode populations (Correll 2014). In turn, a concurrent body of literature suggests that metabolic risk is accrued early on in illness (De Hert 2006; Ward 2015), later shaving off 15 to 20 years of life due to cardiovascular disease (Hoang 2011; Newcomer 2007).
Beyond effects on cardiovascular morbidity and mortality, growing evidence in non‐psychiatric populations also suggests that obesity can be associated with structural brain changes, brain perfusion changes and cognitive deficits (Jagust 2007; Sellbom 2012), with observations supporting some similarities to those noted in schizophrenia (Reichenberg 2007). The clinical implications of being overweight or obese on cognitive function in addition to the deficits observed in schizophrenia, remains a relatively unexplored area of research. Emerging evidence has linked cognitive impairment in schizophrenia to metabolic dysfunction (Bora 2017; Friedman 2010; Lindenmayer 2012), which might suggest that interventions to reduce obesity and cardio‐metabolic risk could have dual health benefits on cardiovascular outcomes and illness‐related functional disability. Quality of life is further reduced for people with schizophrenia with a high BMI (Bueno‐Antequera 2018; Faulkner 2007a; Kurzthaler 2001; Strassnig 2003), and those gaining weight (Allison 2003). Furthermore, Weiden and colleagues reported a significant, positive association between obesity, subjective distress from weight gain and medication non‐compliance in a sample of people with schizophrenia (Weiden 2004). Many people with schizophrenia face the combined challenges of living with the illness, obesity and related illnesses. This combination is a major public health problem (Bueno‐Antequera 2018; Wirshing 2004), and carries considerable cost to human life. Recognition of this has led to growing concern with how best to intervene (Birt 2003; Bueno‐Antequera 2018; Catapana 2004; Cooper 2016; Green 2000; Le Fevre 2001; Osborn 2001).
Mechanisms of weight gain in schizophrenia
To date, there is no consensus on what pharmacological factors may be involved in this weight gain, particularly regarding the newer antipsychotics. As reviewed elsewhere (Ananth 2004; Jin 2008; Reynolds 2010; Reynolds 2017), a range of potential weight gain‐inducing mechanisms such as dopaminergic blockage, increased appetite due to the interaction of antipsychotic medication with dopamine, serotonin, and histamine neuronal receptors, increased leptin, and increased systemic levels of various cytokines and soluble cytokine receptors could be implicated. Whether gender influences antipsychotic‐related weight gain susceptibility remains a topic of debate; while there are clinical data suggesting that women may be more susceptible to atypical antipsychotic‐associated weight gain (Aichhorn 2007; Gebhardt 2009), others have failed to demonstrate this (Basson 2001; Ratzoni 2002). The weight gain story may be further complicated through genetic or epigenetic mechanisms, or both, which may modulate risk. In this regard, among others, dopamine, serotonin, and leptin gene polymorphisms have emerged as genetic candidates for antipsychotic‐related cardio‐metabolic side effects (Correll 2011). In addition, it is important to note that obesity was commonly reported before antipsychotics were widely introduced (Baptista 2002). Compared to the general population, people with schizophrenia also have a poor diet (Dipasquale 2013; McCreadie 1998; Strassnig 2003), and a physically inactive lifestyle (Brown 1999; Cohn 2004; Daumit 2005; Vancampfort 2017), and these lifestyle factors will contribute to weight gain. However, pharmacological intervention strategies may still treat or minimise weight gain associated with poor lifestyle.
Description of the intervention
Pharmacological agents that have been approved for weight loss in the general population, and other medications that may suppress appetite, increase satiety, or increase thermogenesis have been studied to prevent weight gain in people with schizophrenia. These include metformin, topiramate, H2 antagonists such as famotidine and nizatidine, and antidepressants such as fluoxetine and reboxetine. Most clinical trials have been between six and 12 weeks long. Very few have been for 24 weeks or longer. However, clear evidence regarding the optimal duration of such interventions is lacking (Cooper 2016).
Metformin is a biguanide and is a first‐line anti‐diabetic agent. It is usually prescribed in a dose ranging from 500 mg to 2500 mg and is usually administered in divided doses twice a day. Topiramate is an anticonvulsant that has recently been approved by the Food and Drug Administration (FDA) in combination with phentermine for weight loss. The dose ranges from 100 mg to 200 mg given in divided doses twice a day. Famotidine (20 mg to 40 mg once a day) and nizatidine (150 mg to 300 mg once a day) are both commonly used in the treatment of peptic ulcer disease and gastroesophageal reflux disease as they block the histamine H2 receptor. Fluoxetine (20 mg once a day) and reboxetine (4 mg once a day) are antidepressants that have also been investigated for their weight loss promoting properties. Reboxetine is a norepinephrine reuptake inhibitor approved as an antidepressant in parts of Europe. Loss of appetite is a side effect of this medication prompting investigation as a weight loss agent. More recently, samidorphan, an opioid modulator that preferentially antagonises the μ‐opioid receptor, is being used for the prevention of olanzapine‐induced weight gain (Correll 2020a). It is taken orally with a usual dose of 10 mg/day. Common side effects include nausea, sedation and dizziness.
How the intervention might work
Pharmacological interventions may operate on a range of potential mechanisms such as suppressing appetite, increasing satiety, or increasing thermogenesis by modifying central nervous system neurotransmission of norepinephrine, dopamine and serotonin.
Antidiabetic agents are a class of drugs investigated for weight management. Metformin lowers liver glucose production and improves whole‐body insulin sensitivity. It is associated with weight loss in non‐psychiatrically ill populations, and may prevent continual weight gain while improving insulin resistance (Hundal 2003). Hence, it is commonly understood as a peripheral insulin sensitiser. Topiramate is an anticonvulsant, and weight loss is a common side effect of the drug. Its weight‐management properties come from its ability to reduce appetite, affect taste sensation, and control leptin and cortisol levels via GABA‐mediated mechanisms in the central nervous system (Velazquez 2018).
Another class of drugs used for weight loss includes agents that work on the monoamine system, and particularly the serotonin and norepinephrine systems. Fluoxetine is a selective serotonin reuptake inhibitor (SSRI) commonly used as an antidepressant. Early studies have shown serotonin blockade to be an effective anorectic strategy (Goldstein 1994). Therefore, fluoxetine has been studied for weight gain attenuation seen with antipsychotic use. Reboxetine is a norepinephrine reuptake inhibitor approved as an antidepressant in parts of Europe. Loss of appetite is a side effect of this medication prompting investigation as a weight loss agent.
The histaminergic system has also been a candidate for managing weight loss. Used in treatment of gastroesophageal reflux disease (GERD), famotidine, nizatidine and ranitidine are H2 receptor antagonists that work to decrease acid production, and that have associated weight‐reducing properties. With respect to H2 receptor antagonists, it is unclear whether the weight loss action is a direct result of gastric histamine receptor antagonism or if other factors play a role. Histamine is known to mediate leptin action and is involved in energy and feeding regulation (Lett 2012). H2 receptor antagonists can therefore plausibly interact with these medicators to effect weight loss. Betahistine is a histamine enhancer with H1 agonistic/H3 antagonistic properties that has been associated with weight‐reducing properties.
A combination of olanzapine and samidorphan is now available in the USA for the treatment of schizophrenia or bipolar disorder in adults. This agent offers the therapeutic efficacy of olanzapine while also mitigating olanzapine‐induced weight gain through opioid receptor antagonism; it is presently the only FDA‐approved drug for this specific indication. A recent evidence‐based review of the pharmacokinetics, safety, and efficacy of olanzapine plus samidorphan indicates that the effectiveness of the agent is equivalent to that of olanzapine, along with the advantage of lesser weight gain.
Melatonin is a molecule with diverse physiological functions, which through its receptors may improve components of the metabolic syndrome and reduce obesity. As such, it is being explored for the attenuation of antipsychotic‐induced weight gain.
Preventing weight gain avoids all the negative outcomes associated with weight gain and may help engender a healthy lifestyle. Furthermore, sustained changes in health behaviours as a result of such interventions may reduce risk of mortality and morbidity independent of any weight loss (Wei 1999). Indeed, prevention of weight gain has been an area of active enquiry and both older interventions such as metformin (de Silva 2016), and newer molecules such as samidorphan may be useful in achieving this goal (Silverman 2018).
Why it is important to do this review
In the seminal meta‐analysis highlighting atypical antipsychotic‐related weight gain, every antipsychotic medication except ziprasidone and molindone were associated with some degree of weight increase after just 10 weeks of treatment (Allison 1999). The effects were greatest with olanzapine and clozapine, which increased body weight by approximately 4 kg to 4.5 kg, followed by risperidone (mean weight gain 2 kg). Notably, these data were assembled from chronic populations characterised by many years of exposure to medications and illness‐related effects. What has become clearer is that factors related to illness chronicity likely result in an underestimation of the impact of antipsychotics on weight gain, and an overestimation of differences between agents. Collectively, data involving both short‐term and long‐term evidence comparing olanzapine or risperidone in chronic patients to those experiencing a first episode, demonstrate a three to four times larger magnitude of weight gain in those early on in the illness (Alvarez‐Jimenez 2008). Furthermore, no antipsychotic medication appears to be devoid of weight gain risk in people with little prior antipsychotic exposure. For example, one 12‐week cohort study enrolling antipsychotic‐naive youth assigned to aripiprazole, quetiapine or olanzapine, demonstrated substantial weight gain not only with olanzapine (average 8.5 kg), but also with risperidone, quetiapine and aripiprazole (average 4.4 kg; Correll 2009). These findings have since been replicated, including in a recent meta‐analysis (Bak 2014). Interestingly, data in previously medication‐unexposed individuals also suggests that agents classified as being metabolically neutral may exhibit a more delayed onset of weight gain, with treatments differing by pattern, and not always the final amount of weight increase (Findling 2010; Perez‐Iglesias 2008; Zipursky 2005). Moreover, results from a nationwide, register‐based analysis suggest that all antipsychotics contribute to the risk of diabetes, independently of class (Rajkumar 2017). Obesity is also one of the most important risk factors for the development of dyslipidaemia, diabetes and cardiovascular diseases, leading to premature death (Alberti 2009). Taken together, these emerging data highlight the susceptibility, particularly of first‐episode patients, to antipsychotic‐related weight gain. This highlights the case for implementing early effective strategies to prevent or decrease metabolic risk accrual, which may occur early in the treatment of the illness (Ward 2015).
A previous Cochrane Review, published in 2007, covered both pharmacological and non‐pharmacological strategies for preventing weight gain in people with schizophrenia (Faulkner 2007). We believe there is a sufficient volume of material to split Faulkner 2007 into separate reviews focusing on pharmacological and behavioural interventions independently. Furthermore, given the vast number of pharmacological interventions tried for prevention and treatment of weight gain, we have chosen to split the review on pharmacological interventions to focus on prevention of weight gain and reduction of weight gain in two separate reviews. The current review focuses on pharmacological interventions for the prevention of weight gain. While previous reviews have systematically analysed the role of metformin in preventing weight gain (de Silva 2016), no systematic review examining all available pharmacological interventions in a preventive role has been published. This is important, as what we consider effective treatments for adult obesity produce modest weight loss (approximately 2 kg to 5 kg) compared to no treatment or usual care. While this degree of weight loss may have a meaningful impact, it is not sufficient to reverse the weight increases associated with antipsychotic treatment (e.g. average 8.5 kg increase in antipsychotic‐naive patients starting olanzapine; Correll 2009). In this regard, prevention strategies may represent the most useful strategy. We are interested in identifying and including all randomised controlled trials (RCTs) of pharmacological agents to prevent antipsychotic‐induced weight gain in all people with schizophrenia or schizophrenia‐like illnesses.
Objectives
To determine the effectiveness of pharmacological interventions for preventing antipsychotic‐induced weight gain in people with schizophrenia.
Methods
Criteria for considering studies for this review
Types of studies
We considered all relevant RCTs that met our inclusion criteria and reported useable data. We considered both open‐label and double‐blind studies, in which randomisation was implied; studies at high risk of bias for these categories were removed in a sensitivity analysis (see Sensitivity analysis). We excluded quasi‐randomised studies, such as those that allocated intervention by alternate days of the week.
Types of participants
People diagnosed with schizophrenia or schizophrenia‐like illnesses (such as schizoaffective disorder, schizophreniform disorder, and delusional disorder) using any diagnostic criteria irrespective of age, nationality or sex of participants. We included studies regardless of the length of the participant's illness, stage of illness, treatment setting, current clinical state, or symptom cluster. Diagnostic tools to determine diagnosis included the Diagnostic and Statistical Manual of Mental Health Disorders, 4th edition (DSM IV; APA 2000), the International Statistical Classification of Diseases (ICD‐10; WHO 2016), and the Chinese Classificaton of Mental Disorders (CCMD‐3; CSP 2003).
Types of interventions
Pharmacological interventions for preventing weight gain
For people with schizophrenia, 'weight prevention' interventions are typically 'adjunctive' (add‐on) interventions that are co‐initiated with other routinely prescribed medications such as antipsychotics before any antipsychotic‐induced weight gain is experienced. For instance, co‐prescription of metformin at olanzapine initiation would be an example of an adjunctive agent prescribed for the purposes of preventing olanzapine‐induced weight gain.
We considered all types of adjunctive pharmacological interventions for preventing weight gain. These can include those currently licensed for weight loss, an off‐label therapy, withdrawn from the market, or an isolated nutritive supplement. During article screening, a non‐prevention study could be identified if the adjunctive pharmacological intervention was being initiated in individuals that had already experienced significant antipsychotic‐induced weight gain (i.e. the agent was being prescribed for the purposes of treating weight gain, not preventing weight gain). Prevention studies were identified as those in which the pharmacological agent was prescribed around the same time as antipsychotic initiation.
Standard care
We defined this as the care that all participants received in the study, which typically includes regular visits with their psychiatrist and continuing antipsychotic medications.
Non‐standard care: other behavioural interventions
We considered an intervention where an additional pharmacological intervention was combined with a behavioural intervention (i.e. diet or exercise, or both). We only considered interventions that compare such a combined intervention strategy with a behavioural intervention alone in order to assess the additive effect of using a pharmacological adjunct.
In accordance with the above definitions, the planned or expected comparisons were as follows.
Drug 1 plus standard care (e.g. antipsychotics, diet advice) versus placebo or no pharmacological weight gain prevention treatment plus standard care
Drug 1 plus standard care (e.g. antipsychotics, diet advice) versus drug 2 (active control) plus standard care
Drug 1 plus non‐standard care (e.g. behavioral intervention) versus placebo or no pharmacological weight gain prevention treatment plus non‐standard care
Drug 1 plus non‐standard care (e.g. behavioral intervention) versus drug 2 (active control) plus non‐standard care
Types of outcome measures
We aimed to report binary outcomes recording clear and clinically meaningful degrees of change (e.g. global impression of much improved, or more than 50% improvement on a rating scale* ‐ as defined within the studies) before any others. Thereafter, we will list other binary outcomes and then those that are continuous.
* For types of scales we extracted data from, please see (Data extraction and management).
Primary outcomes
1. Weight or BMI
Clinically important change in weight
Average endpoint/change in weight
Clinically important change in BMI
Average endpoint/change in BMI
2. Leaving the study early
For any reason
4. Reports of nausea
The included studies very rarely reported the primary outcomes, 'clinically important change in weight' or 'clinically important change in BMI'. As such, we added 'average endpoint/change in weight' and 'average endpoint/change in BMI' as additional primary outcomes post‐hoc. We noted this change in the Differences between protocol and review section.
Secondary outcomes
1. Weight (or another indicator of body mass (e.g. BMI, waist measurement, waist‐to‐hip ratio)
Any change in body weight
Any change in BMI
Clinically important change in waist circumference (as defined by individual studies)
Any change in waist circumference
Average endpoint/change in waist circumference
Clinically important change in waist‐to‐hip ratio (as defined by individual studies)
Any change in waist‐to‐hip ratio
Average endpoint/change in waist‐to‐hip ratio
Clinically important change in percentage body fat
Any change in percentage body fat
Similar to the primary outcomes, the included studies very rarely reported the secondary outcomes, 'clinically important change in waist circumference', 'any change in waist circumference', 'clinically important change in waist‐to‐hip ratio' or 'any change in waist‐to‐hip ratio'. As such, we added 'average endpoint/change in waist circumference' and 'average endpoint/change in waist‐to‐hip ratio' as additional secondary outcomes, post‐hoc. We noted this change in the Differences between protocol and review section.
2. Leaving the study early
For specific reason(s)
3. Global state
Clinically important change in global state (as defined by individual studies)
Any change in global state
Average endpoint/change score on global state scale
4. Mental state
Clinically important change in general mental state
Any change in general mental state
Average endpoint/change score on mental state scale
5. Well‐being
Clinically important change in well‐being
Any change in well‐being
Average endpoint/change score on well‐being scale
6. Quality of life
Clinically important change in quality of life
Any change in quality of life
Average endpoint/change score on quality‐of‐life scale
7. Adverse effects/events ‐ general or specific
-
General
At least one adverse effect/event
Average endpoint/change score on general adverse effect scale
-
Specific
Clinically important specific adverse effects (e.g. cardiovascular, gastrointestinal)
Death ‐ suicide and natural causes
8. Physiological measures
Cardiovascular measures
Laboratory measures
9. Economic costs
Direct costs
Indirect costs
Search methods for identification of studies
Electronic searches
Cochrane Schizophrenia Group's Register of Trials
On 16 June 2014, 5 August 2015, 4 September 2019, and 10 February 2021, the Information Specialist searched the register using the following search strategy:
(*Metabolic Adverse Event* in Health Care Condition) AND (*Pharmacological Interventions* in Intervention) of STUDY
In such a study‐based register, searching the major concept retrieves all the synonyms and relevant studies because all the studies have already been organised based on their interventions and linked to the relevant topics (Roberts 2021; Shokraneh 2017; Shokraneh 2021). This allows rapid and accurate searches that reduce waste in the next steps of systematic reviewing (Shokraneh 2019).
Following Cochrane methods (Lefebvre 2019), this register is compiled by systematic searches of major resources (AMED, BIOSIS, CENTRAL, CINAHL, ClinicalTrials.Gov, Embase, ISRCTN, MEDLINE, PsycINFO, PubMed, WHO ICTRP) and their monthly updates, ProQuest Dissertations and Theses A&I and its quarterly update, hand‐searches, grey literature, and conference proceedings (Shokraneh 2020; see Cochrane Schizophrenia: Register of trials). There are no language, date, document type, or publication status limitations for inclusion of records into the register.
Searching other resources
Reference searching
We inspected references of all included studies for further relevant studies.
Personal contact
We contacted the first author of each potentially eligible study that we identified in the search for which we could not find published data. We noted the outcome of this contact in the Characteristics of excluded studies or Characteristics of included studies.
Data collection and analysis
Selection of studies
Review authors NS, SMA and ZA independently inspected citations from the searches and identified relevant abstracts; MH independently re‐inspected a random 20% sample of these abstracts to ensure reliability of selection. Where disputes arose, we acquired the full report for more detailed scrutiny. NS and ZA then obtained and inspected full reports of the abstracts or reports meeting the review criteria. SMA re‐inspected a random 20% of these full reports in order to ensure reliability of selection. When it was not possible to resolve disagreement by discussion, we attempted to contact the authors of the study concerned for clarification.
Data extraction and management
Extraction
Review authors NS, MD, ZA, and JL extracted data from all included studies. In addition, to ensure reliability, SMA independently extracted data from a random sample of these studies, comprising 10% of the total. We attempted to extract data presented only in graphs and figures whenever possible, but included them only if two review authors independently obtained the same result. If studies were multi‐centre, then where possible we extracted data relevant to each centre. We discussed any disagreement and documented our decisions. Where necessary, we attempted to contact study authors through an open‐ended request in order to obtain missing information or for clarification.
Management
Forms
We extracted data onto standard, predesigned, simple forms.
Scale‐derived data
We included continuous data from rating scales only if:
the psychometric properties of the measuring instrument had been described in a peer‐reviewed journal (Marshall 2000);
the measuring instrument had not been written or modified by one of the study authors for that particular study; and
the instrument was a global assessment of an area of functioning, not subscores, which are not, in themselves, validated or shown to be reliable. However there were exceptions, we included subscores from mental state scales that measure positive and negative symptoms of schizophrenia.
Ideally, the measuring instrument was either:
a self‐report; or
completed by an independent rater or relative (not the therapist).
We realise that this is not often reported clearly. We note whether this was the case or not in Description of studies.
Scales used
Global measures
Clinical Global Impression Scale (CGI) provides an assessment of an individual’s clinical status in light of the severity of their illness and level of clinical improvement (Guy 1976). An individual is scored according to standardised criteria based on others with the same diagnosis. Assigned scores range on a 7‐point scale with lower scores indicating minimal clinical improvement or reduced illness severity, or both.
Mental state
Brief Psychiatric Rating Scale (BPRS) provides an indication of an individual's psychological functioning in light of psychosis (Overall 1962). This 18‐item scale (revised version ‐ the original contained 16 items) includes a 7‐point scale for each question (ranging from 0‐6 or 1‐7). Scores within this measure may vary from 0‐126 and reflect either the presence or absence of psychological abnormalities (ratings range from "not present" to "extremely severe"). In this scale, elevated scores evidence an increasingly disordered mental state.
Positive and Negative Syndrome Scale (PANSS) can be administered as a whole or as three separate components (1 ‐ severity of general psychopathology, 2 ‐ positive symptoms (PANSS‐P), 3 ‐ negative symptoms (PANSS‐N); Kay 1986). This scale is a 30‐item measure rating on a scale from 1‐7 (absent‐severe) with higher scores reflecting greater symptom severity.
Scale for the Assessment of Negative Symptoms (SANS) is used in patients with psychosis to assess the symptom severity of negative symptoms using a 6‐point scale, with lower scores being indicative of a reduced number of symptoms (Andreasen 1989). Aspects of psychopathology measured by this scale include: affective blunting, alogia, avolition/apathy and anhedonia/asociality.
Scale for the Assessment of Positive Symptoms (SAPS) is used in patients with psychosis to assess the symptom severity of positive symptoms using a 6‐point scale, with lower scores being indicative of a reduced number of symptoms (Andreasen 1989). Aspects of psychopathology measured by this scale include: hallucinations, delusions, disorganisation, and formal thought disorder.
Hamilton Rating Scale for Depression (HAM‐D) is a 17‐item scale that uses a 5‐point rating system for each question but in cases where it is especially difficult to categorise the individual, a 3‐point scale may be used (Hamilton 1960). Here, lower scores reflect a less serious state of depression, whereas higher ones result for more intense depressive symptomology. The HAM‐D assesses various domains, including depressed mood, suicide, work and loss of interest, retardation, agitation, gastro‐intestinal symptoms, general somatic symptoms, hypochondria, loss of insight, and loss of weight. Interrater reliability is of particular value in this assessment given the difficulty of its administration; in this case, an individual will be scored based on the sum of both ratings.
Adverse effects
Barnes Akathisia Scale (BAS) has three main components: reckless movements, agitation and distress ‐ all of which are assessed by the BAS in both objective and subjective domains (Barnes 1989). This measure also includes a 5‐item global severity rating; otherwise, items are scored on a scale of 0 to 3, with higher ratings being indicative of severe akathisia.
Simpson Angus Scale (SAS) provides an assessment of drug‐induced Parkinsonism; a temporary movement disorder often prompted by the use of pharmacological agents (Simpson 1970). This scale includes 10 items with a 0 to 4 rating system. A high score on this measure is indicative of a high degree of Parkinsonian symptoms.
Endpoint versus change data
There are advantages of both endpoint and change data: change data can remove a component of between‐person variability from the analysis. However, calculation of change needs two assessments, baseline and endpoint, which can be difficult to obtain in unstable and difficult‐to‐measure conditions such as schizophrenia. We decided to use endpoint data primarily, and only use change data if the former were not available. We combined endpoint and change data in the analysis as we prefer to use mean differences (MDs) rather than standardised mean differences (SMDs) throughout (Deeks 2021).
Skewed data
Continuous data on clinical and social outcomes are often not normally distributed. To avoid the pitfall of applying parametric tests to non‐parametric data, we applied the following standards to relevant continuous data before inclusion.
For endpoint data from studies including fewer than 200 participants:
when a scale starts from the finite number zero, we subtracted the lowest possible value from the mean, and divided this by the standard deviation (SD). If this value was lower than one, it strongly suggested that the data were skewed and we excluded these data. If this ratio was higher than one but less than two, there was a suggestion that the data were skewed. We entered these data and tested whether their inclusion or exclusion would change the results substantially. If such data changed the results, we entered these as 'other data'. Finally, if the ratio was larger than two we included these data, because it is less likely that they were skewed (Altman 1996; Higgins 2021).
if a scale starts from a positive value (such as the Positive and Negative Syndrome Scale (PANSS), which can have values from 30 to 210 (Kay 1986)), we modified the calculation described above to take the scale starting point into account. In these cases skewed data were present if 2 SD > (S − S min), where S is the mean score and 'S min' is the minimum score.
Please note: we entered all relevant data from studies of more than 200 participants in the analysis irrespective of the above rules, because skewed data pose less of a problem in large studies. We also entered all relevant change data, as when continuous data are presented on a scale that includes a possibility of negative values (such as change data), it is difficult to tell whether or not data are skewed.
Common measurement
To facilitate comparison between studies we aimed to convert variables that could be reported in different metrics, such as days in hospital (mean days per year, per week or per month) to a common metric (e.g. mean days per month).
Conversion of continuous to binary
Where possible, we made efforts to convert outcome measures to dichotomous data. This was done by identifying cut‐off points on rating scales and dividing participants accordingly into 'clinically improved' or 'not clinically improved'. It is generally assumed that if there is a 50% reduction in a scale‐derived score such as the Brief Psychiatric Rating Scale (BPRS; Overall 1962), or the PANSS (Kay 1986), this could be considered as a clinically significant response (Leucht 2005a; Leucht 2005b). If data based on these thresholds were not available, we used the primary cut‐off presented by the original authors.
Direction of graphs
Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for pharmacological intervention for prevention of weight gain. Where keeping to this made it impossible to avoid outcome titles with clumsy double‐negatives (e.g. 'not un‐improved') we reported data where the left of the line indicated an unfavourable outcome and noted this in the relevant graphs.
Assessment of risk of bias in included studies
Review authors NS, SMA and ZA worked independently to assess risk of bias by using criteria described in the Cochrane Handbook for Systematic Reviews of Interventions to assess study quality (Higgins 2011). This set of criteria is based on evidence of associations between potential overestimation of effect and the level of risk of bias of the article that may be due to aspects of sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting, or the way in which these 'domains' are reported.
If the raters disagreed, we made the final rating by consensus. Where inadequate details of randomisation and other characteristics of studies are provided, we attempted to contact authors of the studies in order to obtain further information. We reported non‐concurrence in quality assessment, but if disputes arose regarding the category to which a study was to be allocated, we resolved this by discussion.
Measures of treatment effect
Binary data
For binary outcomes we calculated a standard estimation of the risk ratio (RR) and its 95% confidence interval (CI), as it has been shown that RR is more intuitive than odds ratios (Boissel 1999); and that odds ratios tend to be interpreted as RRs by clinicians (Deeks 2000). Although the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH), with their CIs, are intuitively attractive to clinicians, they are problematic to calculate and interpret in meta‐analyses (Hutton 2009). For binary data presented in the summary of findings tables we calculated illustrative comparative risks where possible.
Continuous data
For continuous outcomes we estimated MD between groups. We preferred not to calculate effect size measures (SMD). However if scales of very considerable similarity were used, we presumed there was a small difference in measurement, and calculated effect size and transformed the effect back to the units of one or more of the specific instruments.
Unit of analysis issues
Cluster studies
Studies increasingly employ 'cluster randomisation' (such as randomisation by clinician or practice), but analysis and pooling of clustered data poses problems. Authors often fail to account for intra‐class correlation in clustered studies, leading to a unit‐of‐analysis error whereby P values are spuriously low, CIs unduly narrow and statistical significance overestimated (Divine 1992). This causes type I errors (Bland 1997; Gulliford 1999).
Had we found any studies where clustering was incorporated into the analysis, we would have presented these data as if from a non‐cluster randomised study, but would have adjusted for the clustering effect. We did not identify any cluster‐RCTs to include in this review.
Cross‐over studies
A major concern of cross‐over studies is the carry‐over effect. This occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, participants can differ significantly from their initial state at entry to the second phase, despite a wash‐out phase. For the same reason, cross‐over studies are not appropriate if the condition of interest is unstable (Elbourne 2002). As both carry‐over and unstable conditions are very likely in severe mental illness, we decided to only use data from the first phase of cross‐over studies. We did not identify any cross‐over studies to include in this review.
Studies with multiple treatment groups
Where a study involved more than two treatment arms, if relevant, we presented the additional treatment arms in comparisons. If data were binary, we simply added these and combined within the two‐by‐two table. If data were continuous, we combined data following the formula in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). Where additional treatment arms were not relevant, we did not reproduce these data.
Dealing with missing data
Overall loss of credibility
At some degree of loss of follow‐up, data must lose credibility (Xia 2009). We chose, for any particular outcome, not to reproduce data or use them within analyses should more than 50% of data be unaccounted for. If, however, more than 50% of those in one arm of a study were lost, but the total loss was less than 50%, we chose to address this within the summary of findings tables by down‐rating certainty. Finally, we also downgraded certainty within the summary of findings tables if the loss was 25% to 50% in total.
Binary data
Where attrition for a binary outcome was between 0% and 50% and where these data were not clearly described, we presented data on a 'once‐randomised‐always‐analyse' basis (an intention‐to‐treat (ITT) analysis). Those leaving the study early are all assumed to have the same rates of negative outcome as those who completed. We used the rate of those who stayed in the study ‐ in that particular arm of the study ‐ and applied this also to those who did not. We undertook a sensitivity analysis to test how prone the primary outcomes were to change when data only from people who completed the study to that point were compared to the ITT analysis using the above assumptions.
Continuous data
Attrition
We used data where attrition for a continuous outcome was between 0% and 50%, and reported data only from people who completed the study to that point.
Standard deviations
If SDs were not reported, we tried to obtain the missing values from the authors. If these were not available, where there were missing measures of variance for continuous data, but an exact standard error (SE) and CIs available for group means, and either P value or t value available for differences in mean, we calculated SDs according to the rules described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021). When only the SE was reported, we calculated SDs by the formula SD = SE * √(n). The Cochrane Handbook for Systematic Reviews of Interventions presents detailed formulae for estimating SDs from P, t or F values, CIs, ranges or other statistics (Higgins 2021). If these formulae did not apply, we calculated the SDs according to a validated imputation method which was based on the SDs of the other included studies (Furukawa 2006). Although some of these imputation strategies can introduce error, the alternative would have been to exclude a given study’s outcome and thus lose information. Nevertheless, if this were to be the case, we decided we would examine the validity of the imputations in a sensitivity analysis that excludes imputed values.
Assumptions about participants who left the studies early or were lost to follow‐up
Various methods are available to account for participants who left the studies early or were lost to follow‐up. Some studies just present the results of study completers; others use the method of last observation carried forward (LOCF); while more recently, methods such as multiple imputation or mixed‐effects models for repeated measurements (MMRM) have become more of a standard. While the latter methods seem to be somewhat better than LOCF (Leon 2006), we feel that the high percentage of participants leaving the studies early and differences between groups in their reasons for doing so is often the core problem in randomised schizophrenia studies. We therefore did not exclude studies based on the statistical approach used. However, by preference we used the more sophisticated approaches, that is, we preferred to use MMRM or multiple‐imputation to LOCF, and we only presented completer analyses if some kind of ITT data were not available at all. Moreover, we addressed this issue in the item 'Incomplete outcome data' of the risk of bias tool (Higgins 2011).
Assessment of heterogeneity
Clinical heterogeneity
We considered all included studies initially, without seeing comparison data, to judge clinical heterogeneity. We simply inspected all studies for participants who were clearly outliers or situations that we had not predicted would arise and, where found, discussed such situations or participant groups.
Methodological heterogeneity
We considered all included studies initially, without seeing comparison data, to judge methodological heterogeneity. We simply inspected all studies for clearly outlying methods that we had not predicted would arise, and discussed any such methodological outliers.
Statistical heterogeneity
Visual inspection
We inspected graphs visually to investigate the possibility of statistical heterogeneity.
Employing the I² statistic
We investigated heterogeneity between studies by considering the I² statistic (Higgins 2003), alongside the Chi² P value. The I² statistic provides an estimate of the percentage of inconsistency thought to be due to chance. The importance of the observed value of the I² statistic depends on the magnitude and direction of effects as well as the strength of evidence for heterogeneity (e.g. P value from Chi² test, or a confidence interval for I² statistic). We interpreted an I² statistic estimate of 50% or more, accompanied by a statistically significant Chi² statistic, as evidence of substantial heterogeneity (Deeks 2021). When substantial levels of heterogeneity were found in the primary outcome, we explored reasons for heterogeneity (Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in section 10.1 of the Cochrane Handbook for Systemic reviews of Interventions (Sterne 2011).
Protocol versus full study
We tried to locate protocols of included RCTs. If the protocol was available, we compared outcomes in the protocol and in the published report. If the protocol was not available, we compared outcomes listed in the methods section of the study report with reported results.
Funnel plot
We are aware that funnel plots may be useful in investigating reporting biases but are of limited power to detect small‐study effects. We did not use funnel plots for outcomes where there were 10 or fewer studies, or where all studies were of similar size. In other cases, where funnel plots were possible, we sought statistical advice in their interpretation.
Data synthesis
We understand that there is no closed argument for preference for use of fixed‐effect or random‐effects models. The random‐effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This often seems to be true to us and the random‐effects model takes into account differences between studies, even if there is no statistically significant heterogeneity. There is, however, a disadvantage to the random‐effects model: it puts added weight onto small studies, which often are the most biased ones. Depending on the direction of effect, these studies can either inflate or deflate the effect size. We chose to use a random‐effects model for all analyses.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses
Primary outcomes
We only undertook subgroup analyses for the primary outcome 'average endpoint/change in weight'. Subgroup analyses were only done for comparisons in which we observed high heterogeneity and the studies could be divided into subgroups (decided on post‐hoc) to potentially explain and reduce the source of heterogeneity.
Investigation of heterogeneity
We reported if inconsistency was high. Firstly, we investigated whether data were entered correctly. Secondly, if data were correct, we inspected the graph visually and removed outlying studies successively to see if homogeneity was restored. When unanticipated clinical or methodological heterogeneity was obvious we simply stated hypotheses regarding these for future reviews or versions of this review. If homogeneity could not be achieved with the first two approaches, heterogeneity was left unresolved.
Sensitivity analysis
We conducted sensitivity analyses where relevant, based on the evidence found for each intervention comparison. Sensitivity analyses were only done for primary outcomes related to weight (e.g. average endpoint/change in body weight). If there were substantial differences in the direction or precision of effect estimates in any of the sensitivity analyses listed below, we did not add data from the lower‐quality studies to the results of the higher‐quality studies, but presented these data within a subcategory. If their inclusion did not result in a substantive difference, they were kept in the analyses.
Implication of randomisation
If studies were described in some way as to imply randomisation, we compared data from the studies that were randomised with those where randomisation was implied.
Assumptions for lost binary data
Where assumptions had to be made regarding people lost to follow‐up (see Dealing with missing data), we compared the findings when we used our assumption with completer data only. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
Assumptions for lost continuous data
Where assumptions have to be made regarding missing SDs (see Dealing with missing data), we compared the findings when we used our assumption with data that were not imputed. If there was a substantial difference, we reported results and discussed them but continued to employ our assumption.
Risk of bias
We analysed the effects of excluding studies that were at high risk of bias across one or more of the domains (see Assessment of risk of bias in included studies).
Imputed values
We also undertook a sensitivity analysis to assess the effects of including data from studies where we used imputed values for ICC in calculating the design effect in cluster‐RCTs.
Fixed‐effect and random‐effects models
We synthesised data using a random‐effects model; however, we also synthesised data for the primary outcome using a fixed‐effect model to evaluate whether this altered the significance of the results.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach to interpret findings (Schünemann 2021); and used GRADEpro GDT to export data from our review to create summary of findings tables. These tables provide outcome‐specific information concerning the overall certainty of evidence from each included study in the comparison, the magnitude of effect of the interventions examined, and the sum of available data on all outcomes we rated as important to patient care and decision making. We selected the following main outcomes for inclusion in the summary of findings tables.
Weight: clinically important change in weight
Weight: average endpoint/change in weight (post‐hoc)
Weight: clinically important change in BMI
Weight: average endpoint/change in BMI (post‐hoc)
Leaving the study early: for any reason
Compliance with treatment
Reports of nausea
Given the sparse data available for the prespecified primary outcomes, we added post‐hoc primary and secondary outcomes and reported them in the summary of findings tables. This change in protocol was made post‐hoc and is described in Types of outcome measures and Differences between protocol and review.
Results
Description of studies
A brief overview of the included and excluded studies is presented below. For substantive descriptions of studies see Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies. Additional information on studies awaiting assessment is presented below.
Results of the search
Database searching identified a total of 991 records (on 16 June 2014, 5 August 2015, 4 September 2019 and 10 February 2021). After screening titles and abstracts for inclusion in the review, we selected 142 studies for full‐text assessment. From this, we excluded 121 full‐text articles, with reasons (Characteristics of excluded studies). Two studies are awaiting classification and two are ongoing. We included 17 studies in the quantitative meta‐analysis of this review.
Please see also Figure 1 for details (Moher 2009).
1.
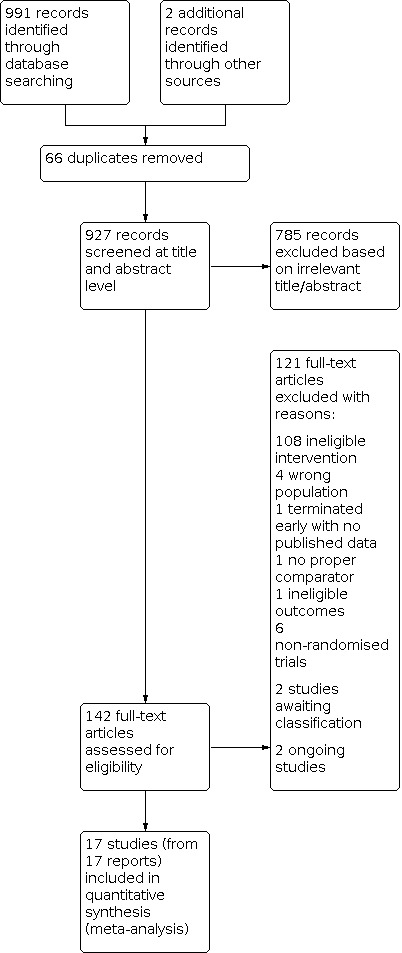
Study flow diagram
Included studies
Design and duration
All studies were randomised. Fourteen interventions were double‐blind, two were open‐label (Kim 2006; Vishnupriya 2016), and one had unclear blinding (Liu 2011). The duration of the studies ranged between 6 weeks (Poyurovsky 2003; Poyurovsky 2004; Poyurovsky 2007; Poyurovsky 2013), and 24 weeks (Correll 2020a; Rado 2016; Vishnupriya 2016). The average duration was approximately 12 weeks. Hence, we were able to provide information on outcomes over the short term and were not able to measure any outcome measure over the medium or long term.
Participants
Most studies included people diagnosed with schizophrenia, schizoaffective disorder or schizophreniform psychosis, while one study included patients with bipolar disorder and major depressive disorder as well (Rado 2016). The majority of studies employed DSM IV criteria for diagnosis (APA 2000), while others (Narula 2010; Liu 2011), used ICD‐10 (WHO 2016), and Sun 2007 used CCMD‐3 (CSP 2003).
The current review comprises an analysis of 1388 individuals. Only one study was conducted in children, with a mean age of 10.9 years; all other studies were conducted in adults aged between 18‐65 with a median age of 26.53 years. We pooled the study conducted in children together with the adult studies, despite known differences in treatment effect sizes between these populations; however, we expected the direction of effects to be the same. There were 737 male participants and 372 female participants, but gender was not specified for 251 individuals. When data were provided, the mean weight and BMI were 58.7 kg (in 14 studies) and 22.11 kg/m2 (in 13 studies), respectively. Nine studies indicated that they were conducted in antipsychotic naive or first episode patients (Liu 2011; Modabbernia 2014; Narula 2010; Poyurovsky 2002; Poyurovsky 2003; Poyurovsky 2004; Poyurovsky 2007; Poyurovsky 2013; Wu 2008).
Setting
Studies included in the meta‐analysis involved either inpatients or outpatients, or both. Eleven studies reported this characteristic (Arman 2008; Baptista 2006; Kim 2006; Liu 2011; Modabbernia 2014; Narula 2010; Poyurovsky 2002; Poyurovsky 2003; Poyurovsky 2004; Poyurovsky 2007; Wu 2008); there were 319 inpatients and 99 outpatients. The setting of the remaining studies is unclear.
Study size
Most studies were small (63 participants) and ranged between 14 and 561 participants.
Interventions
All 17 studies evaluated adjunctive pharmacotherapy for weight maintenance or prevention of weight gain.
Pharmacological Interventions
The included studies used the following medications and drug classes: topiramate (Liu 2011; Narula 2010; Kim 2006), metformin (Arman 2008; Baptista 2006; Rado 2016; Vishnupriya 2016; Wu 2008), monoamine modulators such as reboxetine (Poyurovsky 2003; Poyurovsky 2007; Poyurovsky 2013), and fluoxetine (Poyurovsky 2002), H2 antagonists such as nizatidine (Cavazzoni 2003), famotidine (Poyurovsky 2004), and ranitidine (Sun 2007), reboxetine plus betahistine, samidorphan (Correll 2020a), and melatonin (Modabbernia 2014).
For the comparisons in this review, we grouped together medications that use a similar mechanism of action.
Standard care
In all studies, standard care included treatment with antipsychotic medication. Other components of standard care were not explicitly outlined in all studies.
Non‐standard care: other behavioural interventions
None of the included studies looked at a combined pharmacological plus behavioural intervention.
Outcomes
All the included studies provided data for weight‐related outcomes. However, in some instances, they did not report adequate details of our primary and secondary outcomes or reported them in ways that made them unusable for the purpose of this review. For such studies, we emailed authors to provide us with more data. If we did not receive a response, we excluded the study for those outcomes (see Characteristics of excluded studies). Some studies failed to report appropriate measures of central tendency and deviation (e.g. mean and standard deviation or standard error or 95% CI). For such studies, we used the Review Manager 5 Calculator (Review Manager 2020). However, caution is needed as the values may not be a complete reflection of the actual values.
We divided all outcomes into short term (less than six months), medium term (seven to 12 months) and long term (over one year).
Primary outcomes
Weight or BMI
Most studies reported endpoint data for weight or another indicator of body mass such as BMI. Three studies reported only change data (Rado 2016; Vishnupriya 2016; Wu 2008), while four studies reported both change and end‐point measures (Poyurovsky 2002; Poyurovsky 2003; Poyurovsky 2007; Poyurovsky 2013).
Other less commonly reported measures included waist circumference, waist‐to‐hip ratio and hip circumference.
Leaving the study early
Most of the studies made note of individuals who withdrew from the study early.
Compliance with treatment
None of the included studies reported compliance with treatment. No study mentioned how they confirmed compliance, and no study reported adherence to medication during follow‐up. Given the lack of reporting data, we were unable to include this measure in the meta‐analysis.
Reports of nausea
Only five studies reported the frequency of nausea (Arman 2008; Cavazzoni 2003; Liu 2011; Narula 2010; Wu 2008).
Secondary outcomes
Details of only those scales that provided usable data are shown below. Reasons for exclusion of data are given above under 'Outcomes'.
Global measures
Clinical Global Impression Scale (CGI): five studies reported data using this instrument (Correll 2020a; Poyurovsky 2003; Poyurovsky 2004; Poyurovsky 2007; Poyurovsky 2013).
Mental state
Brief Psychiatric Rating Scale (BPRS): two studies reported data using this instrument (Baptista 2006; Cavazzoni 2003).
Positive and Negative Syndrome Scale (PANSS): Four studies reported data using this instrument (Correll 2020a; Liu 2011; Modabbernia 2014; Narula 2010).
Scale for the Assessment of Negative Symptoms (SANS): five studies reported data using this instrument (Poyurovsky 2002; Poyurovsky 2003; Poyurovsky 2004; Poyurovsky 2007; Poyurovsky 2013).
Hamilton Rating Scale for Depression (HAM‐D): four studies reported data using this instrument (Poyurovsky 2002; Poyurovsky 2003; Poyurovsky 2007; Poyurovsky 2013).
Adverse effects
Barnes Akathisia Scale (BAS): four studies reported data using this scale (Correll 2020a; Poyurovsky 2003; Poyurovsky 2007; Poyurovsky 2013).
Simpson Angus Scale (SAS): five studies reported data using this scale (Cavazzoni 2003; Correll 2020a; Poyurovsky 2003; Poyurovsky 2004; Poyurovsky 2007; Poyurovsky 2013).
Other outcome measures
Cardiovascular measures: three studies reported blood pressure data (Modabbernia 2014; Narula 2010; Poyurovsky 2013).
Laboratory measures: investigating metabolic‐related laboratory measures was of importance in this review as they serve as additional indicators of drug action. However, only a limited number of studies reported on laboratory parameters. Some reported measures were fasting glucose (Baptista 2006; Correll 2020a; Liu 2011; Modabbernia 2014; Narula 2010; Rado 2016; Wu 2008), lipids (high‐density, low‐density and very low‐density lipoproteins, total cholesterol and triglycerides; Baptista 2006; Correll 2020a; Liu 2011; Modabbernia 2014; Narula 2010; Wu 2008), fasting insulin (Baptista 2006; Correll 2020a; Modabbernia 2014; Narula 2010; Wu 2008), insulin resistance (Baptista 2006; Modabbernia 2014; Rado 2016; Wu 2008), leptin (Narula 2010), and haemoglobin (HbA1c) (Correll 2020a; Rado 2016).
Unusable data: studies reported a large amount of data presented in a way not useable for this review. A fair number of studies provided their results only in graph form; additionally, many studies did not appropriately indicate measures of deviation and central tendency (e.g. mean and standard deviation or standard error or 95% CI), which rendered their results unusable within our review. These are stated in the Characteristics of included studies tables.
Missing outcomes: no studies reported on the primary outcomes, 'clinically important change in BMI' or 'compliance with treatment'. None of the studies provided a description of how they confirmed compliance, and none of the studies reported on adherence to medication during follow‐up. Therefore, we were unable to include this measure in the meta‐analysis.
Excluded studies
We excluded 121 studies from the review for the following reasons:
108 studies used an ineligible intervention (Adams 2013; Agahi 2017; Agarwal 2019; Assuncao 2006; Atmaca 2003; Atmaca 2004; Ball 2011; Baptista 2007; Baptista 2008; Baptista 2009; Barak 2010; Barak 2016; Biedermann 2014; Borba 2011; Borovicka 2002; Bushe 2010; Bustillo 2003; Carrizo 2009; Chang 2012; Chen 2010; Chen 2013; Chen 2015; Chiu 2016; Correll 2020b; Dai 2014; Danilov 2014; Deberdt 2005; de Silva 2015; Deutsch 2003; Ding 2005; Eriksson 2019; Fadai 2014; Fan 2013; Fleischhacker 2010; Ghanizadeh 2013; Goodall 1988; Graham 2005; Hebrani 2015; Heikkinen 1993; Henderson 2005a; Henderson 2007; Henderson 2009a; Henderson 2011; Holka‐Pokorska 2015; Hu 2013; IRCT20191223045870N1; Ishoy 2017; Jamilian 2018; Jarskog 2013; Jarskog 2018; Jiang 2017; Joffe 2008; Kang 2018; Kelly 2011; Khan 2020; Kim 2007; Kim 2016; Klein 2008; Ko 2005; Kwon 2006; Larsen 2017; Li 2013; Li 2020; Liu 2004; Lu 2004; Lyu 2018; Maagensen 2021; Martin 2019; Mehta 2014; Modell 1965; Muscatello 2011a; Muscatello 2011b; NCT00044187; NCT00114595; NCT00320723; NCT00512070; NCT00672464; NCT01491490; NCT03132571; Peng 2016; Pierre 2007; Qi 2014; Radulovic 2002; Ranjbar 2013; Reeves 2013; Simmons 2018; Siskind 2018; Siskind 2020; Smith 2013; Smith 2018; Strous 2007; Sulejmanpasic 2019; Talaei 2016; Tavakoli 2014; Taveira 2014; Tek 2014; Terevnikov 2013; Tiihonen 2005; Wang 2009; Wang 2010; Wang 2012; Wang 2020a; Weber 2006; Weiner 2012; Whicher 2021; Wu 2012; Yagcioglu 2005; Yoon 2008);
six studies study were non‐randomised studies or reviews (Correll 2009; Correll 2013; De Hert 2006; Hadley 2009; Henderson 2009b; Hoffmann 2012);
four studies included an ineligible population (Egger 2007; Faghihi 2012; Klein 2006; McElroy 2012);
one study terminated early with no published data (CTRI/2013/05/003685 2013);
one study did not report eligible outcomes (NCT00425815); and
one study did not have a proper comparator group (Wang 2020b).
These reasons are also presented in Characteristics of excluded studies table.
Studies awaiting assessment
We were unable to find abstracts or full publications for two studies, and there was no available contact information to gain access to these publications to determine eligibility (Ginsberg 2004; Mondal 2014).
Ongoing studies
Two studies identified as being potentially eligible are still ongoing (NCT04524403; NL8440).
Risk of bias in included studies
For graphical representations of our judgements of risk of bias, please refer to Figure 2 and Figure 3. Full details of judgements are seen in the ’Risk of bias’ tables.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
All included studies were reported as randomised. Nine studies clearly mentioned their random sequence generation procedure; five studies used a computer‐generated randomisation code (Baptista 2006; Rado 2016; Sun 2007; Vishnupriya 2016; Wu 2008), three studies used a table of random numbers (Poyurovsky 2003; Poyurovsky 2007; Poyurovsky 2013), and one study used a block randomisation procedure (Modabbernia 2014). The remaining studies did not provide any details on their randomisation methods (Arman 2008; Cavazzoni 2003; Correll 2020a; Kim 2006; Liu 2011; Narula 2010; Poyurovsky 2002; Poyurovsky 2004).
Concealment of allocation has repeatedly been shown to be of key importance in excluding selection biases. Only three of the included studies explicitly mentioned the procedure followed to conceal allocation (Baptista 2006; Vishnupriya 2016; Wu 2008), so we judged them to be at low risk of bias. We deemed six studies to be at high risk of selection bias due to a combined lack of reporting on details relating to randomisation, allocation concealment, and blinding (Arman 2008; Cavazzoni 2003; Kim 2006; Narula 2010; Poyurovsky 2004; Vishnupriya 2016). We rated the remaining eight studies as unclear risk for allocation concealment as the study did not provide enough details on this point to make a judgement (Correll 2020a; Liu 2011; Modabbernia 2014; Poyurovsky 2002; Poyurovsky 2003; Poyurovsky 2007; Rado 2016; Sun 2007).
Blinding
Thirteen studies included in this review were double‐blind, two were open‐label (Kim 2006; Vishnupriya 2016), and one had unclear blinding procedures (Liu 2011). However, most of the studies did not report any test of blinding, and we therefore rated them as unclear for performance bias (Arman 2008; Baptista 2006; Cavazzoni 2003; Liu 2011; Narula 2010; Poyurovsky 2002; Poyurovsky 2003; Poyurovsky 2004). The two open‐label studies were deemed high risk of bias while all remaining studies were deemed to be low risk.
We judged 10 studies to be at low risk of detection bias as most outcomes (weight and other metabolic parameters) were objective in nature and unlikely to be biased by blinding. However, the remaining studies did not explicitly state their methods of blinding outcome assessments, so it wasn't clear that detection bias was absent. In these instances, we judged them to be at unclear risk of bias for this domain (Arman 2008; Baptista 2006; Cavazzoni 2003; Narula 2010; Poyurovsky 2002; Poyurovsky 2013; Wu 2008).
Incomplete outcome data
We did not include studies where more than 50% of data were missing. We judged 12 studies to have low risk of attrition bias as they had a small dropout rate that was fairly distributed between groups, and they stated methods on how missing data were imputed. One study was found to have a high risk of bias because of the high rates of dropout and only 62% of the sample being included in the analyses (Arman 2008). However, a sensitivity analysis excluding this study from the analysis did not change the findings significantly. Hence, we included it in the review. We judged the remaining four studies as unclear in this domain because of the lack of information on number of dropouts in the study or the analysis methods accounting for missing data (Cavazzoni 2003; Correll 2020a; Poyurovsky 2013; Sun 2007).
Inclusion and exclusion of studies that performed an ITT or LOCF analysis did not change the results of the review significantly. Hence, we judged attrition bias to be low for all comparisons.
Selective reporting
We deemed risk of bias due to selective reporting to be low in 10 studies. These studies reported all prespecified outcomes in their study protocol or comprehensive methods section. We judged the remaining seven studies as unclear because there was no clinical trial protocol available, and a lack of information on other outcomes that may have been measured (Arman 2008; Cavazzoni 2003; Poyurovsky 2002; Poyurovsky 2003; Poyurovsky 2004; Poyurovsky 2007; Wu 2008).
Other potential sources of bias
Due to the comprehensive nature of the bias categorisation, we deemed most studies as low risk in this category. We rated only one study as unclear in this category because it was translated from Chinese and therefore language constraints made it difficult to assess any other biases (Liu 2011).
All of the included studies had a duration of six months or less, thus, care needs to be taken in interpreting the long‐term effects of the treatment. However, we were unable to judge publication bias since the number of studies was 10 or fewer in each of the comparisons.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Quantitative synthesis
Metformin versus placebo or no treatment
See Table 1.
Five studies compared metformin with standard care (Arman 2008; Baptista 2006; Rado 2016; Vishnupriya 2016; Wu 2008).
The following primary outcomes were not reported in any of the studies included in this comparison: 'clinically important change in weight', 'clinically important change in BMI' and 'compliance with treatment'. The following secondary outcomes were also not reported in any of the studies included in this comparison: 'global state', 'well‐being', 'quality of life', 'adverse effects/events ‐ general or specific', or 'economic costs'.
Primary outcomes
Weight or BMI
Clinically important change in weight: no data to report
Average endpoint/change in weight (post‐hoc): metformin may prevent weight gain (MD −4.03 kg, 95% CI −5.78 to −2.28; I2 = 0%; 4 studies, 131 participants; Analysis 1.1); however, the certainty of evidence for this outcome is low.
Clinically important change in BMI: no data to report
Average endpoint/change in BMI (post‐hoc)
Metformin may be effective in preventing increases in BMI (MD −1.63 kg/m2, 95% CI −2.96 to −0.29; I2 = 90.25%; 5 studies, 227 participants; Analysis 1.2); however, the certainty of evidence for this outcome is low.
1.1. Analysis.

Comparison 1: Metformin versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 1: Weight: average endpoint/change in body weight
1.2. Analysis.

Comparison 1: Metformin versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 2: Weight: average endpoint/change in body mass index
Leaving the study early
For any reason: there was no difference between groups in terms of individuals leaving the study (RR 1.02, 95% CI 0.25 to 4.13; I2 = 0%; 4 studies, 137 participants; Analysis 1.4); however, certainty of evidence for this outcome is very low.
1.4. Analysis.

Comparison 1: Metformin versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 4: Leaving the study early: for any reason
Compliance with treatment ‐ as defined by individual studies
No data to report
Reports of nausea
Metformin does not appear to cause more nausea than placebo (RR 2.38, 95% CI 0.28 to 19.95; I2 = 40%; 2 studies, 69 participants; Analysis 1.5). The certainty of this outcome is very low, however, given the small number of studies reporting this adverse event.
1.5. Analysis.

Comparison 1: Metformin versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 5: Reports of nausea
Secondary outcomes
Weight: average endpoint/change in waist circumference (post‐hoc)
Metformin does not appear to have an effect on waist circumference (MD −1.13 cm, 95% CI −4.28 to 2.02; I2 = 98%; 5 studies, 232 participants; Analysis 1.3).
1.3. Analysis.

Comparison 1: Metformin versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 3: Weight: average endpoint/change in waist circumference
Leaving the study early
For specific reasons: no data to report
Global state
No data to report
Mental state
No effect was observed on the following mental state outcome measures: SANS (MD −0.05, 95% CI −1.38 to 1.28; 1 study, 37 participants), SAPS (MD 0.09, 95% CI −0.67 to 0.85; 1 study, 37 participants) and BPRS (MD −1.80, 95% CI −6.50 to 2.90; 1 study, 37 participants; Analysis 1.6). However, given that only one study reported on each of these outcomes, the certainty of evidence is very low and the effect is uncertain.
1.6. Analysis.

Comparison 1: Metformin versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 6: Mental state
Well‐being
No data to report
Quality of life
No data to report
Adverse effects/events ‐ general or specific
No data to report
Physiological: laboratory measures
In the studies that reported these parameters, it does not appear that metformin has an effect on laboratory measures including fasting blood glucose, HDL cholesterol, LDL cholesterol, total cholesterol, triglycerides, insulin, and insulin resistance (Analysis 1.7). This finding is uncertain, however, given the small number of studies that reported this measure.
1.7. Analysis.

Comparison 1: Metformin versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 7: Physiological: laboratory measures
Economic costs
No data to report
H2 antagonists versus placebo
See Table 2.
We included three RCTs in this comparison, one examined two doses of nizatidine (Cavazzoni 2003), one examined famotidine (Poyurovsky 2004), and one examined ranitidine (Sun 2007). None of the studies included in this comparison reported the primary outcomes: 'clinically important change in weight', 'clinically important change in BMI' and 'compliance with treatment'. The following secondary outcomes were also not reported in any of the studies included in this comparison: 'weight (or other indicator of body mass)', 'leaving the study early for specific reasons', 'global state', 'well‐being', 'quality of life', 'physiological measures' or 'economic costs'.
Primary outcomes
Weight or BMI
Clinically important change in weight: no data to report
Average endpoint/change in weight (post‐hoc): H2 antagonists may be effective in preventing weight gain (MD −1.32 kg, 95% CI −2.09 to −0.56; I2 = 0%; 3 studies, 248 participants; Analysis 2.1); however, the certainty of evidence for this outcome is low.
Clinically important change in BMI: no data to report
Average endpoint/change in BMI (post‐hoc): H2 antagonists may be effective in preventing any increases in BMI (MD −0.66 kg/m2, 95% CI −0.99 to −0.33; I2 = 0%; 2 studies, 79 participants; Analysis 2.2); however, given the small number of studies in the comparison, the certainty of evidence for this outcome is very low.
2.1. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 1: Weight: average endpoint/change in body weight
2.2. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 2: Weight: average endpoint/change in body mass index
Leaving the study early
For any reason: there was no difference between groups in terms of individuals leaving the study (RR 1.07, 95% CI 0.72 to 1.57; I2 = 0%; 2 studies, 189 participants; Analysis 2.3); however, the certainty of evidence for this outcome is low.
2.3. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 3: Leaving the study early: for any reason
Compliance with treatment ‐ as defined by individual studies
No data to report
Reports of nausea
There was no report of increased incidence of nausea in the intervention groups compared with placebo (RR 1.13, 95% CI 0.34 to 3.68; 1 study, 175 participants; Analysis 2.4); however, the effect is uncertain as the certainty of evidence is low.
2.4. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 4: Reports of nausea
Secondary outcomes
Weight (or other indicator of body mass)
No data to report
Leaving the study early
For specific reasons: no data to report
Global state
No data to report
Mental state
There was no evidence of effect of H2 antagonists on mental state, as reported by various mental state scales: CGI (MD 0.10, 95% CI −0.74 to 0.94; 1 study, 14 participants), SANS (MD −1.90, 95% CI −4.97 to 1.17; 1 study, 14 participants), SAPS (MD −0.10, 95% CI −4.09 to 3.89; 1 study, 14 participants) (Analysis 2.6) and BPRS (MD 2.32, 95% CI −0.89 to 5.53; 1 study, 169 participants; Analysis 2.5). However, the effect of these agents on mental state is uncertain as the certainty of evidence is very low.
2.6. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 6: Mental state: various scales
2.5. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 5: Mental state: BPRS (higher = worse)
Well‐being
No data to report
Quality of life
No data to report
Adverse effects/events ‐ general or specific
There was no report of increased incidence of any adverse effects in the intervention groups compared with placebo. However, the effect is uncertain as the certainty of evidence is very low.
Headache (RR 1.06, 95% CI 0.28 to 4.08; 1 study, 175 participants; Analysis 2.7)
Dry mouth (RR 3.60, 95% CI 0.67 to 19.38; 1 study, 175 participants; Analysis 2.8)
Anxiety (RR 1.04, 95% CI 0.37 to 2.90; 1 study, 175 participants; Analysis 2.9);
Depression (RR 3.69, 95% CI 0.68 to 19.97; 1 study, 175 participants; Analysis 2.10 );
Dizziness (RR 0.61, 95% CI 0.21 to 1.73; 1 study, 175 participants; Analysis 2.11);
Increased appetite (OR 0.47, 95% CI 0.19 to 1.16; 1 study, 175 participants; Analysis 2.12);
Somnolence (RR 0.72, 95% CI 0.47 to 1.10; I2 = 0%; 2 studies, 189 participants; Analysis 2.13);
SAS (MD −0.48, 95% CI −1.86 to 0.90; I2 = 50%; 2 studies, 183 participants; Analysis 2.14).
2.7. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 7: Adverse effect: headache
2.8. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 8: Adverse effect: dry mouth
2.9. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 9: Adverse effect: anxiety
2.10. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 10: Adverse effect: depression
2.11. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 11: Adverse effect: dizziness
2.12. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 12: Adverse effect: increased appetite
2.13. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 13: Adverse effect: somnolence
2.14. Analysis.

Comparison 2: H2 antagonists versus placebo, Outcome 14: Adverse effect: SAS
Physiological: laboratory measures
No data to report
Economic costs
No data to report
Monoamine modulators versus placebo
See Table 3.
We included three RCTs in this comparison, two examined reboxetine alone (Poyurovsky 2003; Poyurovsky 2007), and one examined fluoxetine (Poyurovsky 2002). None of these studies reported the primary outcomes, 'clinically important change in weight', 'clinically important change in BMI' and 'compliance with treatment'. The following secondary outcomes were also not reported in any of the studies included in this comparison: 'weight (or other indicator of body mass)', 'leaving the study early for specific reasons', 'global state', 'well‐being', 'quality of life', 'physiological measures' or 'economic costs'.
Primary outcomes
Weight or BMI
Clinically important change in weight: no data to report
Average endpoint/change in weight (post‐hoc): these monoamine modulators may have an effect on preventing increases in body weight (MD −1.89 kg, 95% CI −3.31 to −0.47; I2 = 12%; 3 studies, 103 participants; Analysis 3.1); however, the certainty of evidence for this outcome is low.
Clinically important change in BMI: no data to report
Average endpoint/change in BMI (post‐hoc): these monoamine modulators may have an effect on preventing increases in BMI (MD −0.66 kg/m2, 95% CI −1.05 to −0.26; I2 = 0%; 3 studies, 103 participants; Analysis 3.2); however, the certainty of evidence for this outcome is low.
3.1. Analysis.

Comparison 3: Monoamine modulators versus placebo, Outcome 1: Weight: average endpoint/change in body weight
3.2. Analysis.

Comparison 3: Monoamine modulators versus placebo, Outcome 2: Weight: average endpoint/change in body mass index (BMI)
Leaving the study early
For any reason: there was no difference between groups in terms of individuals leaving the study (RR 1.05, 95% CI 0.56 to 1.94; I2 = 0%; 3 studies, 103 participants; Analysis 3.3); however, the certainty of evidence for this outcome is low.
3.3. Analysis.

Comparison 3: Monoamine modulators versus placebo, Outcome 3: Leaving the study early: for any reason
Compliance with treatment ‐ as defined by individual studies
No data to report
Reports of nausea
No data to report
Secondary outcomes
Weight (or other indicator of body mass)
No data to report
Leaving the study early
For specific reasons: no data to report
Global state
No data to report
Mental state
Monoamine modulators showed improvements in the HAM‐D scale (MD −2.12, 95% CI −4.22 to −0.01; I2 = 37%; 2 studies, 79 participants; Analysis 3.7), but there were no between‐group differences in other mental state scores such as the SANS (MD −0.14, 95% CI −1.98 to 1.71; I2 = 0%; 3 studies, 103 participants; Analysis 3.4), SAPS (MD 0.09, 95% CI −2.01 to 2.19; I2 = 54%; 3 studies, 103 participants; Analysis 3.5), or CGI (MD 0.13, 95% CI −0.28 to 0.54; I2 = 0%; 2 studies, 79 participants; Analysis 3.6).
3.7. Analysis.

Comparison 3: Monoamine modulators versus placebo, Outcome 7: Mental state: HAM‐D (higher = worse)
3.4. Analysis.

Comparison 3: Monoamine modulators versus placebo, Outcome 4: Mental state: SANS (higher = worse)
3.5. Analysis.

Comparison 3: Monoamine modulators versus placebo, Outcome 5: Mental state: SAPS (higher = worse)
3.6. Analysis.

Comparison 3: Monoamine modulators versus placebo, Outcome 6: Mental state: CGI (higher = worse)
Well‐being
No data to report
Quality of life
No data to report
Adverse effects/events ‐ general or specific
Only one study reported the following adverse effects.
Change in BAS score (MD −0.18, 95% CI −0.65 to 0.29; 1 study, 59 participants)
Change in SAS score (MD 0.26, 95% CI −1.00 to 1.52; 1 study, 59 participants)
Increased appetite (MD −0.68, 95% CI −1.19 to −0.17; 1 study, 59 participants)
It is difficult to draw firm conclusions given the very low certainty of the evidence (Analysis 3.8).
3.8. Analysis.

Comparison 3: Monoamine modulators versus placebo, Outcome 8: Adverse effects
Physiological: laboratory measures
No data to report
Economic costs
No data to report
Topiramate versus placebo or no treatment
See Table 4.
We included three RCTs in this comparison (Kim 2006; Liu 2011; Narula 2010). We deemed the overall certainty of evidence to be very low for all outcomes.
None of the studies in this comparison reported the primary outcomes, 'clinically important change in weight', 'clinically important change in BMI' and 'compliance with treatment'.
The following secondary outcomes were also not reported in any of the studies included in this comparison: 'weight (or other indicator of body mass)', 'leaving the study early for specific reasons', 'global state', 'well‐being', 'quality of life', or 'economic costs'.
Primary outcomes
Weight or BMI
Clinically important change in weight: no data to report
Average endpoint/change in weight (post‐hoc): topiramate did not show a significant effect in preventing weight gain (MD −4.82 kg, 95% CI −9.99 to 0.35; I2 = 74%; 3 studies, 168 participants; Analysis 4.1), however, the certainty of evidence for this outcome is very low.
Clinically important change in BMI: no data to report
Average endpoint/change in BMI (post‐hoc): topiramate may prevent increases in BMI (MD −2.68 kg/m2, 95% CI −4.10 to −1.26; I2 = 0%; 2 studies, 120 participants; Analysis 4.2), however, the certainty of evidence for this outcome is very low.
4.1. Analysis.

Comparison 4: Topiramate versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 1: Weight: average endpoint/change in body weight
4.2. Analysis.

Comparison 4: Topiramate versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 2: Weight: average endpoint/change in body mass index
Leaving the study early
For any reason: there was no difference between groups in terms of individuals leaving the study (RR 1.09, 95% CI 0.85 to 1.41; I2 = 0%; 2 studies, 132 participants; Analysis 4.3) but it is difficult to draw firm conclusions given the low certainty of the evidence.
4.3. Analysis.

Comparison 4: Topiramate versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 3: Leaving the study early: for any reason
Compliance with treatment ‐ as defined by individual studies
No data to report
Reports of nausea
The rates of gastrointestinal and neurological adverse effects were not different between groups (Analysis 4.4); however, the certainty of evidence for this outcome is low.
4.4. Analysis.

Comparison 4: Topiramate versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 4: Reports of nausea
Secondary outcomes
Weight (or other indicator of body mass)
No data to report
Leaving the study early
For specific reasons: no data to report
Global state
No data to report
Mental state
Topiramate may slightly decrease PANSS total scores (MD −2.08, 95% CI −3.07 to −1.10; I2 = 0%; 2 studies, 120 participants) and PANSS General Psychopathology Scale (MD −1.53, 95% CI −2.16 to −0.90; I2 = 0%; 2 studies, 120 participants; Analysis 4.5).
4.5. Analysis.

Comparison 4: Topiramate versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 5: Mental state
Well‐being
No data to report
Quality of life
No data to report
Adverse effects/events ‐ general or specific
There was no report of increased incidence of any adverse effects in the intervention groups compared to the placebo groups. However, the effect is uncertain as the certainty of the evidence is very low (Analysis 4.6).
4.6. Analysis.

Comparison 4: Topiramate versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 6: Adverse effects
Physiological measures
Cardiovascular measures: participants in the topiramate arm had lower systolic (MD −4.62, 95% CI −8.14 to −1.10; 1 study, 67 participants) and diastolic (MD −3.47, 95% CI −6.12 to −0.82; 1 study, 67 participants) blood pressure compared to those in the placebo arm but the certainty of evidence is very low (Analysis 4.7).
Laboratory measures: there was no difference between groups in terms of total cholesterol (MD −11.69, 95% CI −31.86 to 8.48; I2 = 90%; 2 studies, 120 participants), LDL cholesterol (MD −7.99, 95% CI −21.82 to 5.84; I2 = 80%; 2 studies, 120 participants), HDL cholesterol (MD 0.36, 95% CI −1.59 to 2.31; I2 = 0%; 2 studies, 120 participants) and triglycerides (MD −5.12, 95% CI −18.70 to 8.46; I2 = 52%; 2 studies, 120 participants). However, topiramate did appear to improve fasting blood glucose (MD −9.22, 95% CI −12.59 to −5.86; I2 = 0%; 2 studies, 120 participants). Results in this comparison are uncertain as the certainty of the evidence is very low (Analysis 4.8).
4.7. Analysis.

Comparison 4: Topiramate versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 7: Physiological: cardiovascular measures
4.8. Analysis.

Comparison 4: Topiramate versus placebo/no‐pharmacological weight gain prevention treatment, Outcome 8: Physiological: laboratory measures
Economic costs
No data to report
Melatonin versus placebo
Only one study examined the use of melatonin for preventing antipsychotic induced weight gain in an eight‐week long intervention (Modabbernia 2014).This study did not report on the primary outcomes 'clinically important change in weight', 'clinically important change in BMI' and 'compliance with treatment'. The following secondary outcomes were also not reported in this study: 'leaving the study early for specific reasons', 'global state', 'well‐being', 'quality of life', or 'economic costs'.
Primary outcomes
Weight or BMI
Clinically important change in weight: no data to report
Average endpoint/change in weight (post‐hoc): melatonin was associated with significantly less weight gain (MD 3.20 kg, 95% CI −5.86 to −0.54; Analysis 5.1) compared to placebo.
Clinically important change in BMI: no data to report
Average endpoint/change in BMI (post‐hoc): melatonin was associated with a significantly less increase in BMI (MD −1.10 kg/m2, 95% CI −1.99 to −0.21; Analysis 5.2).
5.1. Analysis.

Comparison 5: Melatonin versus placebo, Outcome 1: Weight: average endpoint/change in body weight
5.2. Analysis.

Comparison 5: Melatonin versus placebo, Outcome 2: Weight: average endpoint/change in body mass index
Leaving the study early
For any reason: there was no difference between groups in terms of individuals leaving the study. The study initially randomised 48 patients (24 to each group). Following dropouts, 36 participants (18 in each group) were included in the ITT analysis (Analysis 5.6).
5.6. Analysis.

Comparison 5: Melatonin versus placebo, Outcome 6: Leaving the study early: for any reason
Compliance with treatment ‐ as defined by individual studies
No data to report
Reports of nausea
No data to report
Secondary outcomes
Weight (or other indicator of body mass)
Average endpoint/change in weight, BMI, or other measures (post‐hoc): melatonin was associated with a significant decrease in waist circumference (MD −2.80 cm, 95% CI −5.43 to −0.17; Analysis 5.3). No significant effect on hip circumference, or waist‐to‐hip ratio was observed (Analysis 5.4; Analysis 5.5).
5.3. Analysis.

Comparison 5: Melatonin versus placebo, Outcome 3: Weight: average endpoint/change in waist circumference
5.4. Analysis.

Comparison 5: Melatonin versus placebo, Outcome 4: Weight: average endpoint/change in hip circumference
5.5. Analysis.

Comparison 5: Melatonin versus placebo, Outcome 5: Weight: average endpoint/change in waist/hip ratio
Leaving the study early
For specific reasons: no data to report
Global state
No data to report
Mental state
There seemed to be a positive effect of melatonin on mental state scores. PANSS total (MD −12.90, 95% CI −22.59 to −3.21) and general psychopathology (MD −7.50, 95% CI −12.65 to −2.35) scores were reduced significantly more in the melatonin group compared to placebo Analysis 5.7).
5.7. Analysis.

Comparison 5: Melatonin versus placebo, Outcome 7: Mental state
Well‐being
No data to report
Quality of life
No data to report
Adverse effects/events ‐ general or specific
No data to report
Physiological measures
Cardiovascular measures: no data to report
Laboratory measures: the melatonin group had lower total cholesterol at endpoint than the placebo group (MD −25.40, 95% CI −49.04 to −1.76). There were no significant differences between groups in terms of any of the other physiological laboratory outcomes. (Analysis 5.8).
5.8. Analysis.

Comparison 5: Melatonin versus placebo, Outcome 8: Physiological: laboratory measures
Economic costs
No data to report
Reboxetine plus betahistine versus placebo
Only one study examined the use of reboxetine plus betahistine for preventing antipsychotic‐induced weight gain in a six‐week‐long intervention (Poyurovsky 2013). This study did not report on the primary outcomes 'clinically important change in weight', 'clinically important change in BMI' 'reports of nausea' and 'compliance with treatment'. The following secondary outcomes were also not reported in this study: 'weight (or other indicators of body mass', 'leaving the study early for specific reasons', 'global state', 'well‐being', 'quality of life', 'physiological measures', or 'economic costs'.
Primary outcomes
Weight or BMI
Clinically important change in weight: fewer participants in the reboxetine group reported more than 5% weight gain (RR 0.27, 95% CI 0.11 to 0.65; 43 participants; Analysis 6.1) and more than 7% weight gain (RR 0.24, 95% CI 0.07 to 0.83; 43 participants; Analysis 6.2) than the placebo group.
Average endpoint/change in weight (post‐hoc): participants in the reboxetine plus betahistine group experienced less weight gain than those in the placebo group (MD −2.75 kg, 95% CI −4.62 to −0.88; Analysis 6.3).
Clinically important change in BMI: no data to report
Average endpoint/change in BMI (post‐hoc): there was also less increase in BMI in the reboxetine plus betahistine group versus the placebo group (MD −0.88 kg/m2, 95% CI −1.47 to −0.29; Analysis 6.4).
6.1. Analysis.

Comparison 6: Reboxetine + betahistine versus placebo, Outcome 1: Weight: clinically important change in weight > 5%
6.2. Analysis.

Comparison 6: Reboxetine + betahistine versus placebo, Outcome 2: Weight: clinically important change in weight > 7%
6.3. Analysis.

Comparison 6: Reboxetine + betahistine versus placebo, Outcome 3: Weight: average endpoint/change in body weight
6.4. Analysis.

Comparison 6: Reboxetine + betahistine versus placebo, Outcome 4: Weight: average endpoint/change in body mass index
Leaving the study early
For any reason: there was no difference between groups in terms of number of individuals who left the study. There were seven dropouts from 29 participants in the reboxetine plus betahistine group and four dropouts from 14 participants in the placebo group (RR 0.84, 95% CI 0.30 to 2.41; Analysis 6.5).
6.5. Analysis.

Comparison 6: Reboxetine + betahistine versus placebo, Outcome 5: Leaving the study early: for any reason
Compliance with treatment ‐ as defined by individual studies
No data to report
Reports of nausea
No data to report
Secondary outcomes
Weight (or other indicator of body mass)
No data to report
Leaving the study early
For specific reasons: no data to report
Global state
No data to report
Mental state
There were no significant differences between the reboxetine plus betahistine group and the placebo group in terms of mental state scores on the SANS, HAM‐D, SAPS and CGI (Analysis 6.6).
6.6. Analysis.

Comparison 6: Reboxetine + betahistine versus placebo, Outcome 6: Mental state
Well‐being
No data to report
Quality of life
No data to report
Adverse effects/events ‐ general or specific
The groups did not differ in terms of neurological adverse effects, such as change in BAS (MD 0.06, 95% CI −0.21 to 0.33) or SAS (MD 0.55, 95% CI −0.28 to 1.38; Analysis 6.7).
6.7. Analysis.

Comparison 6: Reboxetine + betahistine versus placebo, Outcome 7: Adverse events
Physiological measures
Cardiovascular measures: no data to report
Laboratory measures: no data to report
Economic costs
No data to report
Samidorphan plus olanzapine versus olanzapine alone
Only one study examined the use of samidorphan plus olanzapine versus olanzapine alone (Correll 2020a). This study did not report on the primary outcomes 'clinically important change in BMI', 'average endpoint/change in BMI', 'reports of nausea' and 'compliance with treatment'. The following secondary outcomes were also not reported in this study: 'leaving the study early for specific reasons', 'global state', 'well‐being', 'quality of life', or 'economic costs'.
Primary outcomes
Weight or BMI
Clinically important change in weight: significantly lower proportions of participants in the olanzapine plus samidorphan group experienced 10% or higher weight gain (RR 0.59, 95% CI 0.43 to 0.81; Analysis 7.1) and 7% or higher weight gain (RR 0.64, 95% CI 0.51 to 0.82; Analysis 7.2) than in the olanzapine group.
Average endpoint/change in weight (post‐hoc): there were no between‐group differences in body weight at endpoint (MD −2.35, 95% CI −4.80 to 0.10; Analysis 7.3).
Clinically important change in BMI: no data to report
Average endpoint/change in BMI (post‐hoc): no data to report
7.1. Analysis.

Comparison 7: Olanzapine/samidorphan versus olanzapine only, Outcome 1: Weight: clinically important change in weight > 10%
7.2. Analysis.

Comparison 7: Olanzapine/samidorphan versus olanzapine only, Outcome 2: Weight: clinically important change in weight > 7%
7.3. Analysis.

Comparison 7: Olanzapine/samidorphan versus olanzapine only, Outcome 3: Weight: average endpoint/change in body weight (kg)
Leaving the study early
For any reason: a total of 352 (64%) participants completed treatment, with similar completion rates in the two treatment groups (RR 0.98, 95% CI 0.79 to 1.23; Analysis 7.5). The most common reasons for discontinuation with olanzapine plus samidorphan and with olanzapine alone were adverse events (12.0% and 9.8%, respectively), withdrawal by participant (8.4% and 9.8%, respectively), and loss to follow‐up (8.0% and 9.4%, respectively).
7.5. Analysis.

Comparison 7: Olanzapine/samidorphan versus olanzapine only, Outcome 5: Leaving the study early: for any reason
Compliance with treatment ‐ as defined by individual studies
No data to report
Reports of nausea
No data to report
Secondary outcomes
Weight (or other indicator of body mass)
Average endpoint/change in waist circumference: participants in the samidorphan plus olanzapine group had less increase in waist circumference than the olanzapine group (MD −2.11, 95% CI −3.64 to −0.58; Analysis 7.4).
7.4. Analysis.

Comparison 7: Olanzapine/samidorphan versus olanzapine only, Outcome 4: Weight: average endpoint/change in waist circumference
Leaving the study early
For specific reasons: no data to report
Global state
No data to report
Mental state
The PANSS total score improved similarly in both groups (MD 1.20, 95% CI −0.81 to 3.21; Analysis 7.6). Reductions in CGI‐S score from baseline to week 24 were similar between the two treatment groups (MD 0.07, 95% CI −1.12 to 1.26; Analysis 7.6).
7.6. Analysis.

Comparison 7: Olanzapine/samidorphan versus olanzapine only, Outcome 6: Mental state
Well‐being
No data to report
Quality of life
No data to report
Adverse effects/events ‐ general or specific
Adverse events were reported in 74.1% and 82.2% of the olanzapine plus samidorphan group and the olanzapine group, respectively, with no significant between‐group differences. The most commonly reported adverse event in the two groups was weight increase (24.8% and 36.2%, respectively), with significant between‐group differences. Somnolence (21.2% and 18.1%), dry mouth (12.8% and 8.0%), and increased appetite (10.9% and 12.3%) were other commonly reported adverse events but with no between‐group differences (Analysis 7.8). There were no clinically meaningful changes or differences observed in vital signs, ECG results, or movement disorder scale scores for participants in the two treatment groups.
7.8. Analysis.

Comparison 7: Olanzapine/samidorphan versus olanzapine only, Outcome 8: Adverse events
Physiological measures
Cardiovascular measures: no data to report
Laboratory measures: there were no significant between‐group differences in terms of physiological laboratory measures such as fasting blood glucose, insulin, HbA1c, LDL‐C, HDL‐C, total cholesterol and triglycerides (Analysis 7.7).
7.7. Analysis.

Comparison 7: Olanzapine/samidorphan versus olanzapine only, Outcome 7: Physiological: laboratory measures
Economic costs
No data to report
Subgroup analyses and investigation of heterogeneity
No subgroup analyses were completed for the outcome average endpoint/change in weight for any of the comparisons.
Sensitivity analyses
We only performed sensitivity analyses for the following weight outcomes, 'average endpoint/change in body weight' or 'average endpoint/change in BMI'. The following preplanned sensitivity analyses did not apply to our review: exclusion of studies with unclear randomisation methods, lost binary data, lost continuous data or imputed values.
Metformin versus placebo or no treatment
Exclusion of studies with high risk of bias
We judged Arman 2008 to be high risk of bias for allocation concealment (selection bias) and incomplete outcome data (attrition bias). Excluding this study did not change the overall results (MD −4.18 kg, 95% CI −5.95 to −2.42; I2 = 0%, 3 studies, 99 participants; Analysis 8.1).
8.1. Analysis.

Comparison 8: Sensitivity analyses, Outcome 1: Metformin vs placebo excluding studies with high risk of bias: weight: change in body weight
Fixed‐effect model
When we applied a fixed‐effect model, the overall results did not change (MD −4.03 kg, 95% CI −5.78 to −2.28; I2 = 0%; I2 = 0%; 4 studies, 131 participants; Analysis 8.2).
8.2. Analysis.

Comparison 8: Sensitivity analyses, Outcome 2: Metformin vs placebo, fixed‐effect model: weight, change in body weight
H2 Antagonists versus placebo
Fixed‐effect model
When we applied a fixed‐effect model, the overall results did not change (MD −1.32 kg, 95% CI −2.09 to −0.56; I2 = 0%; 3 studies, 248 participants;Analysis 8.3).
8.3. Analysis.

Comparison 8: Sensitivity analyses, Outcome 3: H2 antagonists vs placebo, fixed‐effect model: weight, change in body weight
Monoamine modulators versus placebo
Fixed‐effect model
When we applied a fixed‐effect model, the overall results did not change (MD ‐1.84 kg, 95% CI ‐3.01 to ‐0.67; I2 = 12%; 3 studies; 103 participants Analysis 8.4).
8.4. Analysis.

Comparison 8: Sensitivity analyses, Outcome 4: Monoamine modulators vs placebo, fixed‐effect model: weight, change in body weight
Topiramate versus placebo or no treatment
Exclusion of studies with high risk of bias
We deemed Kim 2006 to be high risk of bias for allocation concealment (selection bias) and incomplete outcome data (attrition bias). After excluding this study, only two studies remained in the analysis (Narula 2010; Liu 2011); this analysis shows that topiramate may be effective in preventing weight gain (MD −7.63 kg, 95% CI −12.01 to −3.25; I2 = 0%; 2 studies; 120 participants; Analysis 8.5).
8.5. Analysis.

Comparison 8: Sensitivity analyses, Outcome 5: Topiramate vs placebo excluding studies with high risk of bias: weight, change in body weight
Fixed‐effect model
When we applied a fixed‐effect model, the overall results did not change (MD −1.83 kg, 95% CI −3.03 to −0.63; I2 = 74%; 3 studies; 168 participants; Analysis 8.6).
8.6. Analysis.

Comparison 8: Sensitivity analyses, Outcome 6: Topiramate vs placebo, fixed‐effect model: weight: change in body weight
Publication bias
Due to the small number of included studies we did not perform a funnel plot analysis.
Discussion
Summary of main results
General summary
This review included 17 RCTs with 1388 participants that examined adjunctive pharmacological interventions for the prevention of antipsychotic‐induced weight gain in people with schizophrenia or schizophrenia‐like illnesses. All of the included studies were published between 2002 and 2020 and varied in duration (interventions ranged from six weeks to six months).
This review found that pharmacological options may offer promise in preventing weight gain associated with antipsychotic use. Of the studied drugs, metformin may be slightly effective in preventing weight gain. We are uncertain about the other agents used in a preventative role because the certainty of evidence is very low.
This review found the medications to have a favourable tolerability profile; none of the drugs studied had a higher number of dropouts in the active arm compared with the placebo arm or no treatment. Moreover, none of the agents had an associated negative impact on mental state.
The pharmacological interventions with the most evidence and promise and the specifics of their effect on weight and other outcome measures are discussed below.
Treatment effects
Metformin verus placebo or no treatment
Used as a first‐line antidiabetic agent for more than five decades, metformin has a very good established safety and tolerability profile. As a preventative strategy, combined results from five studies involving 227 participants, show that co‐initiation of metformin treatment along with an antipsychotic may lead to less weight gain and reduction in BMI. There were no differences between metformin and placebo in terms of number of participants leaving the study early for any reason. for number of reports of nausea, or for any mental state outcomes.
Interestingly, only one study included in this comparison was conducted in first‐episode or antipsychotic‐naive patients (Wu 2008). The effect of metformin on weight in this population appeared to be similar to that observed in more chronic populations.
Our findings are in line with recent meta‐analyses and systematic reviews that have examined the utility of metformin in the prevention and treatment of antipsychotic‐induced weight gain (de Silva 2016), or in the context of clozapine use alone (Liu 2015; Siskind 2016).
H2 antagonists versus placebo
We included three RCTs that evaluated H2 antagonists as preventative agents. One examined two doses of nizatidine (Cavazzoni 2003), one examined famotidine (Poyurovsky 2004), and one examined ranitidine (Sun 2007). Overall, this medication class may be slightly effective in mitigating the amount of weight gained during antipsychotic treatment. Additionally, there were no differences between H2 antagonists and placebo in terms of number of participants leaving the study early for any reason or for number of reports of nausea or other adverse effects. However, we need more studies with a greater sample size to fully understand the effects of these medications, as the current certainty of evidence is very low.
Monoamine modulators versus placebo
We included three RCTs that evaluated monoamine modulators as preventative agents. Two examined reboxetine alone (Poyurovsky 2003; Poyurovsky 2007), and one examined fluoxetine (Poyurovsky 2002). Overall, this class may be effective in preventing weight gain. There were no differences in the number of dropouts and frequency of adverse events. Important to note is that all studies were conducted in populations that were fairly antipsychotic‐naive or in their first episode of psychosis.
Of the pharmacological agents studied in this review that act on monoamine systems to prevent weight gain in people with schizophrenia, the most evidence is available for reboxetine. Two RCTs studied reboxetine's role as an agent for controlling weight gain. The dose of reboxetine was 4 mg/day in both studies Reboxetine alone may be effective in preventing weight gain with antipsychotics. Reboxetine is a noradrenaline reuptake inhibitor approved for treating depression. The antidepressant properties were also evident in our review, where we found the active groups to score significantly lower on depression‐rating scales. These findings agree with a meta‐analysis of the effect of noradrenaline reuptake inhibitors such as reboxetine, atomoxetine and mazindol on various aspects of pathology in schizophrenia (Kishi 2015). Noradrenaline reuptake inhibitors were found to decrease depressive symptoms and lower weight, effects driven largely by the reboxetine studies.
Topiramate versus placebo or no treatment
We included three RCTs that studied topiramate as a preventative agent for antipsychotic‐induced weight gain in a total of 175 participants (Kim 2006; Liu 2011; Narula 2010). There did not appear to be an effect of topiramate in preventing weight gain; however, two RCTs showed that topiramate (100 mg/d to 200 mg/d) may prevent increase in BMI following initiation of olanzapine, and may improve fasting blood glucose levels (Liu 2011; Narula 2010). Both these studies were conducted in an antipsychotic‐naive or first‐episode population. There were no differences in the number of dropouts and reports of nausea. Only two of the included studies reported on physiological laboratory measures and mental state outcomes (Liu 2011; Narula 2010); therefore, we judged the certainty of evidence for this comparison to be very low and the effects of topiramate on these outcomes are uncertain. Topiramate may also have favourable effects on psychopathology, as demonstrated by improved scores on the PANSS; however, results of the outcome are uncertain.
Overall, this review is in line with a recent review of topiramate efficacy, which had less stringent inclusion criteria and included all RCTs that involved topiramate use in schizophrenia (Okuyama 2016). They found topiramate to be effective, but significantly more participants were found to suffer from paraesthesia and concentration difficulties. These side effects were numerically more common in our review as well. However, while these are preliminary findings from small studies, more research needs to be conducted to better characterise its effects. Moreover, the cognitive effects of topiramate need to evaluated in this population.
Other comparisons: melatonin versus placebo; reboxetine plus betahistine versus placebo; and olanzapine plus samidorphan versus olanzapine alone
We found only one study for each of the following interventions: melatonin versus placebo; reboxetine plus betahistine versus placebo; and olanzapine plus samidorphan versus olanzapine alone. Given the limited evidence, we cannot draw any conclusions for these interventions. We need more RCTs to determine the treatment effects with greater certainty.
Melatonin versus placebo
Modabbernia 2014 examined the effects of melatonin versus placebo in a group of first‐episode or antipsychotic‐naive patients. They found that melatonin may be effective in limiting weight gain and increase in BMI and waist circumference. There was also no significant difference between groups in terms of number of individuals discontinuing or leaving the study early. Melatonin appeared to have favourable effects on total cholesterol levels and PANSS scores. However, there were no significant differences between the two groups in terms of changes in other laboratory measures (e.g. cholesterol, insulin and blood sugar) or mental state scores.
Reboxetine plus betahistine versus placebo
Poyurovsky 2013 combined 4 mg/d of reboxetine with 48 mg/d betahistine. Significantly fewer participants gained more than 5% and more than 7% of their bodyweight in the reboxetine plus betahistine group than in the placebo group. There was also less increase in body weight and BMI in the experimental than placebo group, with no significant differences in mental state outcomes and adverse events. Betahistine is a histamine enhancer with H1 agonistic/H3 antagonistic properties. Recent studies in people with schizophrenia (Barak 2016; Smith 2018), have demonstrated betahistine to be well‐tolerated and effective in reducing weight gain following the initiation of olanzapine. Given olanzapine's strong action on the histaminergic receptors and their probable importance in relation to weight gain, betahistine seems like an interesting lead worth pursuing.
Olanzapine plus samidorphan versus olanzapine alone
Correll 2020a compared the effectiveness of two doses of the combination agent olanzapine plus samidorphan (10 mg/10 mg and 20 mg/10 mg) with olanzapine alone. The chosen doses represent the lowest and highest approved maintenance dosages of olanzapine for schizophrenia treatment and the intended therapeutic fixed dosage of samidorphan that has been determined to have the most optimal weight and safety profile when combined with olanzapine. Samidorphan is an opioid receptor antagonist. This combination agent has recently gained popularity in the USA following its FDA approval for the treatment of schizophrenia and bipolar disorder in adults. Results from this single study indicated that participants gained less weight on olanzapine plus samidorphan compared to olanzapine alone. Furthermore, the risk of clinically significant weight gain (of ≥ 7% and ≥ 10%) was reduced by 50% relative to olanzapine. Mental state scores demonstrated similar improvements in both groups, and there was no significant difference between the groups in terms of number of reported adverse events. These findings are in line with an earlier study, Martin 2019, that studied the efficacy of olanzapine plus 5 mg of samidorphan to treat schizophrenia compared to olanzapine alone (the primary outcome of this study was to determine its therapeutic effect on primary symptoms of schizophrenia).
Sensitivity analyses
The results of the outcome 'change in weight' were not much different in a series of pre‐planned sensitivity analyses in which we excluded studies with high risk of bias regarding blinding or incomplete outcome data, or when a fixed‐effect model instead of a random‐effects model was applied. Nevertheless, the statistical power of these analyses was low.
Overall completeness and applicability of evidence
Extending the previous version of this review (Faulkner 2007), we were able to include studies that involved people with schizophrenia from a wide range of settings including both inpatient and outpatients from North and South America, Europe, Asia, and Australia. The 15 years since the last version of the review was published has seen more studies being published overall; the number of studies that evaluated a pharmacological intervention for the prevention of antipsychotic‐induced weight gain has gone up to 17, compared to six in the previous review. Taken together, these studies provide enough information to answer the question we set out to answer. However, given the low number of studies for most of the interventions, the low to very low certainty of evidence limits our ability to draw substantive conclusions. Another limitation is that there were a few studies for which we were not able to obtain the full text and therefore could not include in the meta‐analysis. Furthermore, the broad geographical representativeness of the included studies (we were able to include studies from each of the continents except Africa), the variety of clinical settings and disease severity of the included participants increases the generalisability of the evidence with the obvious caveat that much more needs to be done before firm conclusions can be drawn about most of the agents studied in this review.
Quality of the evidence
In this review, we have summarised evidence from 17 double‐blind or open‐label RCTs that together studied 1388 individuals with schizophrenia and related disorders. However, many of the shortcomings of studies individually and in the field generally that were identified in the previous version of this review (Faulkner 2007), still apply. For many of the pharmacological comparisons there was only a single study. Additionally, there was variability in the dosage of the interventions, study duration and follow‐up, and other essential aspects of methodology. Most studies did not describe adequate randomisation and blinding procedures, and not all studies included an ITT analysis. Many studies did not use structured scales to evaluate side effects and tolerability systematically. In addition, while clinical guidelines suggest that weight loss medications should only be used in combination with lifestyle counselling, the majority of pharmacological studies did not include, or did not describe, such a component. Finally, there was an absence of long‐term studies that would allow for evaluation of longer‐term effectiveness and safety of the interventions.
We deemed the risk of bias to be high in a large number of the studies that contributed to this review as they did not describe essential elements of an RCT in the methods. As such, we downgraded the certainty of evidence for all outcomes by one level in most of the comparisons. Additionally, for most of the comparisons, we downgraded the certainty of the evidence one level further to 'low', since the total number of participants included in the comparisons was less than the number of participants generated by a conventional sample size calculation for a single, adequately powered study. For several outcomes, in most comparisons, we downgraded the certainty of evidence by one further level to 'very low' as the heterogeneity of the results was quite high making interpretation uncertain. For most agents analysed in the meta‐analysis, interpretation is limited by the small number of studies and small sample sizes.
Potential biases in the review process
For studies registered yet incomplete due to early termination or loss to follow‐up, we could not obtain any data. Hence, this review presents a potential bias towards published data. Contributing to this point is that our search was primarily based on the Cochrane Schizophrenia Register of Trials, which searches only published literature. Given the limited number of studies in each comparison, we could not complete funnel plot analyses to assess publication bias. Lastly, the majority of comparisons in this review are based on only one or two studies, thus, the results cannot indicate the true effect of the medication. Further studies on a number of agents giving positive results are warranted before we can draw firm conclusions.
Agreements and disagreements with other studies or reviews
Systematic reviews that have examined individual agents that are included in this meta‐analysis have been discussed under respective sections in the summary of main results, above. This section discusses other studies or reviews that have dealt with multiple agents for the purposes of preventing antipsychotic‐induced weight gain. Notably, to the best of our knowledge, only the previous version of this review (Faulkner 2007), has been published that compares various pharmacological interventions in a preventative role. Despite the number of studies that have been published since examining many other pharmacological interventions, there has yet to be another review published comparing the most up‐to‐date literature. This emphasises the importance of this present review.
In agreement with Faulkner 2007, which looked at only six RCTs for the prevention of weight gain in people with schizophrenia, we also found that there was a significant treatment effect of adjunctive pharmacological agents for achieving modest prevention of weight gain and that this can be achieved in a safe manner. The previous review asserted that reboxetine and topiramate were effective at weight prevention, although this must be interpreted with caution given only one study was available at the time for each agent. In contrast, however, the present review, which adds to and extends the previous pool of evidence, suggests that metformin may be the most effective in modestly preventing weight gain. The present review also reports on olanzapine plus samidorphan for the first time, which is a novel combination drug that has received recent FDA approval for the treatment of schizophrenia and bipolar disorder in the USA and aims to mitigate the amount of weight gain experienced with olanzapine. Only one study was available to be included in this review on this agent, thus the evidence is quite limited. Nonetheless, it suggests that there is another novel agent that may be effective in preventing the risk of clinically significant weight gain (of ≥ 7% and ≥ 10%) with olanzapine, an antipsychotic with one of the greatest metabolic liabilities.
Authors' conclusions
Implications for practice.
For people with schizophrenia
An increase in weight during treatment with antipsychotics is a common problem experienced by individuals with schizophrenia. As a preventative strategy, starting metformin treatment along with an antipsychotic may lead to lesser weight gain and may not cause other adverse effects. Evidence about the use of most other adjunctive pharmacological agents is limited by the small number of studies utilising the agents, variability in the studies testing the same agent, and variability in the duration and intensity of the studies using the same interventional agent. Individuals with schizophrenia are advised to seek the support and advice of their clinical team for weight management and appraising the evidence in this review, and collaborate with their physician to decide a mutually acceptable approach to weight gain prevention. We urge individuals with schizophrenia to work with researchers and clinicians to help discover better evidence of the effects of various interventional agents than is currently available. This is very important as people with mental illness are systematically excluded from studies of efficacy of weight‐reducing agents in the general population.
For clinicians
The evidence for weight prevention interventions is limited for most of the agents described in this review. Metformin is the only treatment with some evidence to suggest it may be a well‐tolerated and effective agent in preventing weight gain when started together with an antipsychotic. However, it is important to realise that the first strategy in preventing weight gain is to carefully appraise the metabolic risks associated with an antipsychotic agent, although treatment of the mental illness should be prioritised (Faulkner 2007). Thereafter, the patients, their families and their care circles should be informed about the metabolic profiles of the prescribed antipsychotic agents and be given advice regarding maintaining a healthy lifestyle with adequate diet and exercise. In order to keep track of an individual's weight and metabolic profile, baseline and pre‐planned monitoring should be conducted as has been summarised in relevant guidelines. Treatment with weight loss or weight maintenance‐promoting agents, such as metformin, may be considered to prevent weight gain. Furthermore, as far as possible, treatment with an interventional agent should be in addition to lifestyle interventions and not as a substitute for them.
For policy makers
The metabolic disturbance and weight gain that is experienced in people with schizophrenia is associated with reduced quality of life, as well as premature morbidity and mortality. In the long term, the impact of increased obesity rates will cause significant economic strain. Only modest reductions in weight gain have been observed with current strategies that aim to prevent weight gain in people with schizophrenia. As most evidence of pharmacological agents for preventing weight gain have been reported in short‐term studies, it is imperative that effectiveness of these interventions is demonstrated in longer‐term studies (longer than six months). At present, metformin emerges as the only agent that may cause few adverse effects and be effective in preventing weight gain when started with an antipsychotic. Important to note is that treatment with any pharmacological agent must always be accompanied by lifestyle interventions. As individuals with schizophrenia frequently contact their mental health service providers, it should be noted that frequent reinforcement may be essential for attaining long‐term adoption of physical activity on a regular basis, as well as dietary modification. Furthermore, professionals who are trained to be sensitive and supportive in regard to the mental health‐specific barriers to dietary modification and physical activity can address these issues in individuals more effectively (Richardson 2005). The cost‐effectiveness of any of the pharmacological strategies has not been formally established.
Implications for research.
General
Data reporting was not consistent among the studies included in this review. In order to improve the quality of comparisons, future studies should follow a standardised method of measuring and reporting outcomes (Moher 2001).
Specific
Larger randomised controlled trials of longer treatment durations are required to determine the true effects of these adjunctive pharmacological agents for prevention of weight gain in schizophrenia. The studies that we included in this review were completed over a short time period (up to six months), and therefore limit our ability to determine the long‐term effectiveness of these medications. Moreover, reporting would be more efficient if researchers included baseline and final outcomes, along with their mean differences and standard deviations. Binary outcomes such as the number of participants losing more than 7% of their initial body weight would also be helpful and easier to interpret.
As noted in the 'Standard care' section (see Types of interventions), these studies did not explicitly outline what other components were included in their interventions, beyond the prescribed medications. Behavioural lifestyle interventions should be used in all pharmacological studies and should be a part of all arms of the design. Structured scales should be used for the assessments of side effects and medication tolerability. Studies should aim to use intention‐to‐treat analysis and describe in detail the interventions and characteristic of participants who were randomised into the study as well as those lost to follow‐up along with their outcome. In order to reduce the risk of bias and improve the certainty of the evidence, study authors should clearly describe the generation and concealment of participant allocation. Most of our included studies did not use or describe adequate methods of allocation concealment. Furthermore, the lack of description or usage of adequate allocation techniques may influence the degree of interventional effects.
In terms of outcome measures, future studies could add to their study results by including other measures of body mass, in addition to body weight and BMI. For instance, waist circumference may be the single best indicator for cardiovascular risk factors and a good alternative for identifying the need for weight management.
For a suggested design, please see Table 5.
1. Suggested design for future studies.
| Methods | Randomisation: random Allocation: concealed Blinding: double‐blind Duration: 12 months Setting: inpatients or outpatients |
| Participants | Diagnosis: patients with schizophrenia, or any schizophrenia‐like illness Gender: male and female Age: mean 30 years, range: 18‐65 (adult population) |
| Interventions | Pharmacological interventions for preventing weight gain including those currently licensed for weight loss, an off‐label therapy, withdrawn from the market, or an isolated nutritive supplement Samidorphan ‐ standalone + combination therapy with olanzapine Pharmacological adjunct plus behavioural intervention versus behavioural intervention alone |
| Outcomes | Clinically important change in weight (e.g. binary outcomes such as ≥ 7% weight loss)
Clinically important change in BMI Waist‐to‐hip ratio |
| Notes | Look at effects of factors such as stage of illness and ethnicity Dose effects |
| BMI: body mass index | |
What's new
| Date | Event | Description |
|---|---|---|
| 20 December 2022 | Amended | Background (Description of the intervention), Analysis (7.8.4), Discussion (Summary of main results) amended |
History
Protocol first published: Issue 6, 2019 Review first published: Issue 10, 2022
| Date | Event | Description |
|---|---|---|
| 8 October 2019 | Amended | The reviewers requested for 38 full studies (including 75 reports). Their studies were added into the review. |
| 4 September 2018 | Amended | Search was run and 369 reports were sent to the reviewers for screening. |
Acknowledgements
The authors also wish to thank Ms. Sabrina Santos for her assistance with data entry and validation, and proof‐reading this review.
The Cochrane Schizophrenia Editorial Base, situated across the University of Melbourne, Australia, the Technical University of Munich, Germany, and the University of Nottingham, UK, produces and maintains standard text for use in the Methods section of their reviews. We have used this text as the basis of what appears here and adapted it as required.
Cochrane Schizophrenia supported the authors in the development of this review.
The following people conducted the editorial process for this article.
Sign‐off Editor (final editorial decision): Mahesh Jayaram, University of Melbourne
Managing Editor (selected peer reviewers, provided editorial guidance to authors, edited the article, conducted editorial policy checks ): Hui Wu, Technical University of Munich
Contact Editor: Johannes Schneider‐Thoma, Technical University of Munich
Copy Editor (copy‐editing and production): Denise Mitchell, Evidence, Production & Methods Directorate, Cochrane Central Executive
Information Specialist (search strategy and results): Farhad Shokraneh, Systematic Review Consultants
Peer‐reviewers* (provided comments and recommended an editorial decision): Wei Li, Shanghai Jiao Tong University School of Medicine, Huijuan Zhang, Shanghai Jiao Tong University School of Medicine (clinical and content review)
The previous Cochrane Schizophrenia also supported this work: Co‐ordinating Editor, Clive Adams (before 2020), Managing Editor, Claire Irving (before 2020), Assistant Managing Editor, Ghazaleh Aali (before April 2021).
*Peer‐reviewers are members of Cochrane Schizophrenia, and provided peer‐review comments on this article, but they were not otherwise involved in the editorial process or decision making for this article.
Data and analyses
Comparison 1. Metformin versus placebo/no‐pharmacological weight gain prevention treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Weight: average endpoint/change in body weight | 4 | 131 | Mean Difference (IV, Random, 95% CI) | ‐4.03 [‐5.78, ‐2.28] |
| 1.2 Weight: average endpoint/change in body mass index | 5 | 227 | Mean Difference (IV, Random, 95% CI) | ‐1.63 [‐2.96, ‐0.29] |
| 1.3 Weight: average endpoint/change in waist circumference | 4 | 232 | Mean Difference (IV, Random, 95% CI) | ‐1.13 [‐4.28, 2.02] |
| 1.4 Leaving the study early: for any reason | 4 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.25, 4.13] |
| 1.5 Reports of nausea | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 2.38 [0.28, 19.95] |
| 1.6 Mental state | 2 | 111 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.63, 0.67] |
| 1.6.1 SANS (higher = worse) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐1.38, 1.28] |
| 1.6.2 SAPS (higher = worse) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.67, 0.85] |
| 1.6.3 BPRS (higher = worse) | 1 | 37 | Mean Difference (IV, Random, 95% CI) | ‐1.80 [‐6.50, 2.90] |
| 1.7 Physiological: laboratory measures | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.7.1 Fasting blood glucose (mg/dL) | 4 | 195 | Mean Difference (IV, Random, 95% CI) | ‐10.04 [‐28.27, 8.19] |
| 1.7.2 HDL cholesterol (mg/dL) | 2 | 58 | Mean Difference (IV, Random, 95% CI) | 2.43 [‐4.07, 8.92] |
| 1.7.3 Insulin (mIU/mL) | 2 | 74 | Mean Difference (IV, Random, 95% CI) | ‐2.82 [‐9.53, 3.89] |
| 1.7.4 Insulin resistance index | 3 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.62, 0.02] |
| 1.7.5 LDL cholesterol (mg/dL) | 2 | 57 | Mean Difference (IV, Random, 95% CI) | ‐3.54 [‐39.77, 32.68] |
| 1.7.6 Total cholesterol (mg/dL) | 2 | 57 | Mean Difference (IV, Random, 95% CI) | ‐6.71 [‐30.35, 16.92] |
| 1.7.7 Triglycerides (mg/dL) | 2 | 59 | Mean Difference (IV, Random, 95% CI) | ‐17.18 [‐45.31, 10.94] |
Comparison 2. H2 antagonists versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Weight: average endpoint/change in body weight | 3 | 248 | Mean Difference (IV, Random, 95% CI) | ‐1.32 [‐2.09, ‐0.56] |
| 2.1.1 Nizatidine 150 mg, twice daily | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.62 [‐2.68, 1.44] |
| 2.1.2 Nizatidine 300 mg, twice daily | 1 | 85 | Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐3.01, 1.23] |
| 2.1.3 Famotidine 40 mg, once daily | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 2.40 [‐6.09, 10.89] |
| 2.1.4 Ranitidine 150 mg, twice daily | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐1.58 [‐2.48, ‐0.68] |
| 2.2 Weight: average endpoint/change in body mass index | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐0.99, ‐0.33] |
| 2.3 Leaving the study early: for any reason | 2 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.72, 1.57] |
| 2.3.1 Nizatidine 150 mg, twice daily | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.59, 1.87] |
| 2.3.2 Nizatidine 300 mg, twice daily | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.64, 1.83] |
| 2.3.3 Famotidine 40 mg, once daily | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | Not estimable |
| 2.4 Reports of nausea | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.34, 3.68] |
| 2.4.1 Nizatidine 150 mg, twice daily | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.08, 3.55] |
| 2.4.2 Nizatidine 300 mg, twice daily | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.40, 8.18] |
| 2.5 Mental state: BPRS (higher = worse) | 1 | 169 | Mean Difference (IV, Random, 95% CI) | 2.32 [‐0.89, 5.53] |
| 2.5.1 dose= 150 mg, twice daily | 1 | 84 | Mean Difference (IV, Random, 95% CI) | 2.20 [‐2.22, 6.62] |
| 2.5.2 dose= 300 mg, twice daily | 1 | 85 | Mean Difference (IV, Random, 95% CI) | 2.45 [‐2.22, 7.12] |
| 2.6 Mental state: various scales | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.6.1 CGI (higher = worse) | 1 | 14 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.74, 0.94] |
| 2.6.2 SANS (higher = worse) | 1 | 14 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐4.97, 1.17] |
| 2.6.3 SAPS (higher = worse) | 1 | 14 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐4.09, 3.89] |
| 2.7 Adverse effect: headache | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.28, 4.08] |
| 2.7.1 Nizatidine 150 mg, twice daily | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.11, 2.45] |
| 2.7.2 Nizatidine 300 mg, twice daily | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [0.47, 9.14] |
| 2.8 Adverse effect: dry mouth | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 3.60 [0.67, 19.38] |
| 2.8.1 Nizatidine 150 mg, twice daily | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 4.81 [0.27, 86.47] |
| 2.8.2 Nizatidine 300 mg, twice daily | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 3.10 [0.39, 24.61] |
| 2.9 Adverse effect: anxiety | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.37, 2.90] |
| 2.9.1 Nizatidine 150 mg, twice daily | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.20, 5.42] |
| 2.9.2 Nizatidine 300 mg, twice daily | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.28, 3.85] |
| 2.10 Adverse effect: depression | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 3.69 [0.68, 19.97] |
| 2.10.1 Nizatidine 150 mg, twice daily | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 2.63 [0.32, 21.51] |
| 2.10.2 Nizatidine 300 mg, twice daily | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 6.83 [0.40, 117.31] |
| 2.11 Adverse effect: dizziness | 1 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.21, 1.73] |
| 2.11.1 Nizatidine 150 mg, twice daily | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.11, 2.45] |
| 2.11.2 Nizatidine 300 mg, twice daily | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.16, 2.88] |
| 2.12 Adverse effect: increased appetite | 1 | 175 | Odds Ratio (M‐H, Random, 95% CI) | 0.47 [0.19, 1.16] |
| 2.12.1 Nizatidine 150 mg, twice daily | 1 | 87 | Odds Ratio (M‐H, Random, 95% CI) | 0.38 [0.09, 1.53] |
| 2.12.2 Nizatidine 300 mg, twice daily | 1 | 88 | Odds Ratio (M‐H, Random, 95% CI) | 0.55 [0.17, 1.81] |
| 2.13 Adverse effect: somnolence | 2 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.47, 1.10] |
| 2.13.1 Nizatidine 150 mg, twice daily | 1 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.30, 1.38] |
| 2.13.2 Nizatidine 300 mg, twice daily | 1 | 88 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.37, 1.43] |
| 2.13.3 Famotidine 40 mg, once daily | 1 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.36, 1.77] |
| 2.14 Adverse effect: SAS | 2 | 183 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.86, 0.90] |
| 2.14.1 Nizatidine 150 mg, twice daily | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐1.60, 1.58] |
| 2.14.2 Nizatidine 300 mg, twice daily | 1 | 85 | Mean Difference (IV, Random, 95% CI) | 0.39 [‐1.25, 2.03] |
| 2.14.3 Famotidine 40 mg, once daily | 1 | 14 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐3.84, ‐0.16] |
Comparison 3. Monoamine modulators versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Weight: average endpoint/change in body weight | 3 | 103 | Mean Difference (IV, Random, 95% CI) | ‐1.89 [‐3.31, ‐0.47] |
| 3.1.1 Reboxetine | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐3.07, ‐0.72] |
| 3.1.2 Fluoxetine | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 6.40 [‐7.56, 20.36] |
| 3.2 Weight: average endpoint/change in body mass index (BMI) | 3 | 103 | Mean Difference (IV, Random, 95% CI) | ‐0.66 [‐1.05, ‐0.26] |
| 3.2.1 Reboxetine | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.08, ‐0.28] |
| 3.2.2 Fluoxetine | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐2.35, 4.55] |
| 3.3 Leaving the study early: for any reason | 3 | 115 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.56, 1.94] |
| 3.3.1 Reboxetine | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.47, 1.82] |
| 3.3.2 Fluoxetine | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.43, 9.32] |
| 3.4 Mental state: SANS (higher = worse) | 3 | 103 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐1.98, 1.71] |
| 3.4.1 Reboxetine | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐2.67, 1.55] |
| 3.4.2 Fluoxetine | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 1.24 [‐2.54, 5.02] |
| 3.5 Mental state: SAPS (higher = worse) | 3 | 103 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐2.01, 2.19] |
| 3.5.1 Reboxetine | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐2.42 [‐8.87, 4.04] |
| 3.5.2 Fluoxetine | 1 | 24 | Mean Difference (IV, Random, 95% CI) | 1.09 [‐0.36, 2.54] |
| 3.6 Mental state: CGI (higher = worse) | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.28, 0.54] |
| 3.6.1 Reboxetine | 2 | 79 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.28, 0.54] |
| 3.7 Mental state: HAM‐D (higher = worse) | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐4.22, ‐0.01] |
| 3.7.1 Reboxetine | 2 | 79 | Mean Difference (IV, Random, 95% CI) | ‐2.12 [‐4.22, ‐0.01] |
| 3.8 Adverse effects | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.8.1 Neurological: change in BAS | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.65, 0.29] |
| 3.8.2 Neurological: change in SAS | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐1.00, 1.52] |
| 3.8.3 Gastrointestinal: increased appetite | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.19, ‐0.17] |
Comparison 4. Topiramate versus placebo/no‐pharmacological weight gain prevention treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Weight: average endpoint/change in body weight | 3 | 168 | Mean Difference (IV, Random, 95% CI) | ‐4.82 [‐9.99, 0.35] |
| 4.2 Weight: average endpoint/change in body mass index | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐2.68 [‐4.10, ‐1.26] |
| 4.3 Leaving the study early: for any reason | 2 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.85, 1.41] |
| 4.4 Reports of nausea | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.26, 5.44] |
| 4.5 Mental state | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.5.1 Average score: PANSS general psychopathology scale (higher = worse) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐1.53 [‐2.16, ‐0.90] |
| 4.5.2 Average score: PANSS positive (higher = worse) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.02, 0.09] |
| 4.5.3 Average score: PANSS negative (higher = worse) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐0.79, 0.08] |
| 4.5.4 Average score: PANSS total (higher = worse) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐2.08 [‐3.07, ‐1.10] |
| 4.6 Adverse effects | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.6.1 Gastrointestinal: constipation | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.03, 1.67] |
| 4.6.2 Gastrointestinal: dry mouth | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.27, 1.37] |
| 4.6.3 Gastrointestinal: increased appetite | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.10, 0.66] |
| 4.6.4 Gastrointestinal: nausea/vomiting/diarrhoea | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 3.09 [0.34, 28.23] |
| 4.6.5 Gastrointestinal: weight gain | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.07, 0.26] |
| 4.6.6 Neurological: asthenia | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.37, 2.20] |
| 4.6.7 Neurological: concentration/attention/memory difficulty | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 8.97 [1.17, 68.63] |
| 4.6.8 Neurological: dizziness | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.18, 0.91] |
| 4.6.9 Neurological: fatigue | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.53, 1.96] |
| 4.6.10 Neurological: insomnia | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.05, 5.41] |
| 4.6.11 Neurological: paraesthesia | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 7.21 [0.39, 134.32] |
| 4.6.12 Neurological: psychomotor slowing | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 9.26 [0.52, 165.60] |
| 4.6.13 Neurological: somnolence | 1 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.88, 1.70] |
| 4.7 Physiological: cardiovascular measures | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.7.1 Cardiovascular measure: diastolic blood pressure [mm Hg] | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐3.47 [‐6.12, ‐0.82] |
| 4.7.2 Cardiovascular measure: systolic blood pressure [mm Hg] | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐4.62 [‐8.14, ‐1.10] |
| 4.8 Physiological: laboratory measures | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.8.1 Fasting blood glucose (mg/dL) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐9.22 [‐12.59, ‐5.86] |
| 4.8.2 HDL cholesterol (mg/dL) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | 0.36 [‐1.59, 2.31] |
| 4.8.3 Insulin (ulU/mL) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐3.66, 3.58] |
| 4.8.4 Insulin resistance Index | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐1.10, 0.56] |
| 4.8.5 LDL cholesterol (mg/dL) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐7.99 [‐21.82, 5.84] |
| 4.8.6 Leptin (ng/mL) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐3.84 [‐8.52, 0.84] |
| 4.8.7 Total cholesterol (mg/dL) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐11.69 [‐31.86, 8.48] |
| 4.8.8 Triglycerides (mg/dl) | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐5.12 [‐18.70, 8.46] |
| 4.8.9 VLDL cholesterol (mg/dL) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐6.09, 3.49] |
Comparison 5. Melatonin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Weight: average endpoint/change in body weight | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2 Weight: average endpoint/change in body mass index | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.3 Weight: average endpoint/change in waist circumference | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.4 Weight: average endpoint/change in hip circumference | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.5 Weight: average endpoint/change in waist/hip ratio | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.6 Leaving the study early: for any reason | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.7 Mental state | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.7.1 Average score: PANSS general psychopathology scale (higher = worse) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.7.2 Average score: PANSS positive (higher = worse) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.7.3 Average score: PANSS negative (higher = worse) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.7.4 Average score: PANSS total (higher = worse) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.8 Physiological: laboratory measures | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.8.1 Fasting blood glucose (mg/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.8.2 HDL cholesterol (mg/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.8.3 Insulin (ulU/mL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.8.4 Insulin resistance index | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.8.5 LDL cholesterol (mg/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.8.6 Total cholesterol (mg/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.8.7 Triglycerides (mg/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 6. Reboxetine + betahistine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Weight: clinically important change in weight > 5% | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.2 Weight: clinically important change in weight > 7% | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.3 Weight: average endpoint/change in body weight | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.4 Weight: average endpoint/change in body mass index | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.5 Leaving the study early: for any reason | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6.6 Mental state | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.6.1 Average score: SAPS (higher = worse) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.6.2 Average score: SANS (higher = worse) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.6.3 Average score: HAM‐D (higher = worse) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.6.4 Average score: CGI (higher = worse) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.7 Adverse events | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.7.1 Neurological: BAS | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.7.2 Neurological: SAS | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. Olanzapine/samidorphan versus olanzapine only.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Weight: clinically important change in weight > 10% | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.2 Weight: clinically important change in weight > 7% | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.3 Weight: average endpoint/change in body weight (kg) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.4 Weight: average endpoint/change in waist circumference | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.5 Leaving the study early: for any reason | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.6 Mental state | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.6.1 Average score: PANSS total (higher = worse) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.6.2 Average score: CGI (higher = worse) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.7 Physiological: laboratory measures | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.7.1 Fasting blood glucose (mg %) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.7.2 HDL cholesterol (mg %) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.7.3 Insulin (ulU/mL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.7.4 LDL cholesterol (mg/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.7.5 Total cholesterol (mg/dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.7.6 Triglycerides (mg /dL) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.7.7 HbA1c [%] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.8 Adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.8.1 Weight increased | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.8.2 Somnolence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.8.3 Dry mouth | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7.8.4 Increased appetite | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. Sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Metformin vs placebo excluding studies with high risk of bias: weight: change in body weight | 3 | 99 | Mean Difference (IV, Random, 95% CI) | ‐4.18 [‐5.95, ‐2.42] |
| 8.2 Metformin vs placebo, fixed‐effect model: weight, change in body weight | 4 | 131 | Mean Difference (IV, Fixed, 95% CI) | ‐4.03 [‐5.78, ‐2.28] |
| 8.3 H2 antagonists vs placebo, fixed‐effect model: weight, change in body weight | 3 | 248 | Mean Difference (IV, Fixed, 95% CI) | ‐1.32 [‐2.09, ‐0.56] |
| 8.3.1 Nizatidine 150 mg, twice daily | 1 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐2.68, 1.44] |
| 8.3.2 Nizatidine 300 mg, twice daily | 1 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐0.89 [‐3.01, 1.23] |
| 8.3.3 Famotidine 40 mg, once daily | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 2.40 [‐6.09, 10.89] |
| 8.3.4 Ranitidine 150 mg, twice daily | 1 | 65 | Mean Difference (IV, Fixed, 95% CI) | ‐1.58 [‐2.48, ‐0.68] |
| 8.4 Monoamine modulators vs placebo, fixed‐effect model: weight, change in body weight | 3 | 103 | Mean Difference (IV, Fixed, 95% CI) | ‐1.84 [‐3.01, ‐0.67] |
| 8.4.1 Reboxetine | 2 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐3.07, ‐0.72] |
| 8.4.2 Fluoxetine | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 6.40 [‐7.56, 20.36] |
| 8.5 Topiramate vs placebo excluding studies with high risk of bias: weight, change in body weight | 2 | 120 | Mean Difference (IV, Random, 95% CI) | ‐7.63 [‐12.01, ‐3.25] |
| 8.6 Topiramate vs placebo, fixed‐effect model: weight: change in body weight | 3 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐1.83 [‐3.03, ‐0.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arman 2008.
| Study characteristics | ||
| Methods | Randomisation: randomised, no other details
Blinding: double‐blind Duration: 12‐weeks Country: Iran |
|
| Participants | Diagnosis: schizophrenia or schizoaffective disorder N = 49 Age: 10.09 years (< 20 years) Sex: male and female Setting: inpatients History: < 20 years of age, taking risperidone (2‐6 mg/d according to their responses to treatment), creatinine level < 1.4 mg/dL, normal liver function test Excluded: treatment with antipsychotic earlier, current substance abuse or significant medical illness, untreated hypertension, history of intolerance to metformin, receiving weight loss agents, glaucoma, heart disease, abnormal ECG, asthma, combination of antipsychotics, or treatment with anti‐migraine agents containing serotonin agonists |
|
| Interventions |
|
|
| Outcomes |
Able to use:
Unable to use:
|
|
| Notes | 61.4% study completers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After completing baseline assessments, the subjects were randomly assigned to metformin and placebo." Pg 1131 Comment: randomisation methods are unavailable |
| Allocation concealment (selection bias) | High risk | Comment: information is unavailable. Combined with the lack of details about randomisation and complete absence of information about allocation concealment, the risk of bias is quite high. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "...Identical appearing placebo pills..." Pg 1131 Comment 1: the study indicates the presence of identical placebo pills, however, it is unclear which personnel were blinded in the study. Comment 2: various adverse effects were reported in the treatment group, which may have broken blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information is unavailable |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "17 were excluded due to incomplete use of drugs or side‐effects of the drugs. 2 of these patients have experienced diarrhoea at the second week. 3 patients had nausea and vomiting in metformin group, which were excluded from study too." Pg 1132 Comment: excluded participants were not included in the analysis, hence only ~62% of the study population was analysed. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is unavailable |
| Other bias | Low risk | No obvious bias |
Baptista 2006.
| Study characteristics | ||
| Methods | Randomisation: randomised, computer‐generated Blinding: double‐blind Duration: 14 weeks Country: Venezuela |
|
| Participants | Diagnosis: schizophrenia or schizoaffective disorder (criteria unavailable) N = 40 Age: 47.65 years Sex: male and female Setting: inpatients History: participants had severe schizophrenia or related disorder who had been stabilised for > 5 years with conventional antipsychotic drugs. Excluded: chronic disease and hormone replacement therapy, abnormal physical and lab exam results |
|
| Interventions |
Standard care included maintaining a balanced diet (of 2500‐3000 kcal/d) |
|
| Outcomes |
Able to use:
|
|
| Notes | A balanced diet, 2500 to 3000 KCal/d was provided 92.5% study completers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "...the computer‐based random allocation of patients to either the Olanzapine (10mg daily at bedtime) plus Metformin group (850 to 1750 mg daily, N =20) or the Olanzapine plus placebo group ..." Pg 193 |
| Allocation concealment (selection bias) | Low risk | Quote: "...the computer‐based random allocation of patients to either the Olanzapine (10mg daily at bedtime) plus Metformin group (850 to 1750 mg daily, N =20) or the Olanzapine plus placebo group ..." Pg 193 Comment: as a computer‐generated program is used for treatment assignment, likely low risk of selection bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding methods (e.g. use of identical placebo pills) not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not enough information provided to assess the risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Two patients taking placebo and one taking Metformin dropped out of the study owing to change in residence" Pg 193 Comment: valid dropout reasons. No other dropouts |
| Selective reporting (reporting bias) | Low risk | Have reported on all outcomes mentioned in the 'Methods' section. |
| Other bias | Low risk | No obvious bias |
Cavazzoni 2003.
| Study characteristics | ||
| Methods | Randomisation: randomised, no other details Blindness: double‐blind Duration: 16 weeks Country: USA |
|
| Participants | Diagnosis: DSM‐IV schizophrenia, schizoaffective or schizophreniform disorder N = 175 Age: 18‐65 years Sex: male and female Setting: in‐ or outpatients History: participants had fairly chronic disease, with a mean of ~14 years since onset. Excluded: treatment with any atypical antipsychotics or other drugs with central nervous system activity within the past 3 months, known physical illness that could affect body weight loss programme, or had a BMI of ≥ 40 or weight ≥ 250 pounds (114 kg) |
|
| Interventions |
|
|
| Outcomes |
Able to use:
Unable to use:
|
|
| Notes | 96.6% study completers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...all eligible patients...were randomised to receive placebo (Plc), 150 mg nizatidine b.i.id (Niz 150), or 300 mg nizatidine b.i.d (Niz 300)." Pg 82 Comment: randomisation methods are unavailable |
| Allocation concealment (selection bias) | High risk | Information is unavailable. The risk of bias is cumulatively judged to be high as important information regarding randomisation, allocation concealment, blinding, and attrition has not been provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information on identical placebo pills and which personnel were blinded is unavailable. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information is unavailable |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "The analysis included 169 patients since their measurement of change in weight from baseline was available" Pg 82 Comment: although > 90% of the study population is analysed, it is unclear what proportion of the individuals in the study were completers. Moreover, the method for filling the missing data is unavailable |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is unavailable |
| Other bias | Low risk | No obvious bias |
Correll 2020a.
| Study characteristics | ||
| Methods | Randomisation: randomised, no other details Blindness: double blind. Duration: 24 weeks Country: USA |
|
| Participants | Diagnosis: DSM‐5 criteria for schizophrenia. N = 561 Age: 18‐55 years Sex: male and female Setting: no details Excluded: history of treatment‐resistant schizophrenia, 1 year elapsed since initial onset of symptoms, naive to antipsychotic medication, active alcohol or substance use disorders (excluding marijuana/tetrahydrocannabinol), or any clinically significant or unstable medical illness (e.g. diabetes, hypo‐ or hypertension, thyroid dysfunction, and history of seizure disorder or brain tumour) that might compromise patient safety or study endpoint assessments or interfere with the ability to fulfil study requirements. Opioid agonist use within 14 days of screening, opioid antagonist use within 60 days of screening, or anticipated need for opioid treatment during the study were exclusionary, as was the use of the olanzapine in the 60 days before screening. |
|
| Interventions | 1. Olanzapine (10 mg)/samidorphan (10 mg) 2. Olanzapine (20 mg)/samidorphan (10 mg) 3. Olanzapine alone |
|
| Outcomes |
Able to use:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not indicated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome unlikely to be biased by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | > 20% dropout in both groups (35.8% in olanzapine/samidorphan group and 36.2% in olanzapine only group). Missing weight assessments were imputed by multiple imputation sequentially for each visit, using a regression method. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting upon comparison with protocol |
| Other bias | Low risk | No other bias detected |
Kim 2006.
| Study characteristics | ||
| Methods | Randomisation: randomised, no other details
Blinding: open‐label Duration: 12 weeks |
|
| Participants | Diagnosis: DSM‐IV criteria for schizophrenia
N = 14
Age: 18–55 years
Sex: male Setting: outpatients History: treated with a second‐generation antipsychotic for at least 8 weeks, with the same dose for at least 4 weeks; clinically stable; and to have a BMI ≥ 30 kg/m2, or ≥ 27 kg/m2 plus adult treatment; panel III hyperlipidaemia or hypertriglyceridaemia Excluded: diagnosis of DSM‐IV substance abuse within the last month or DSM‐IV substance dependence within the last 6 months; cannabis use more than once weekly; Calgary Depression Rating Scale (CDS) total score > 7; suicidality or hospitalisation for depression in prior 6 months; the use of any medication known to alter weight or appetite; and pregnant or nursing women |
|
| Interventions |
Both groups on olanzapine, 10 mg/d, increasing but not to exceed 20 mg/d |
|
| Outcomes |
Able to use:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods not reported |
| Allocation concealment (selection bias) | High risk | Allocation concealment not indicated; open‐label study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Weight outcomes unlikely to be biased by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data were analysed in accordance with ITT methodology |
| Selective reporting (reporting bias) | Low risk | Primary outcome measure was reported |
| Other bias | Low risk | No obvious bias |
Liu 2011.
| Study characteristics | ||
| Methods | Randomisation: randomised, no other details
Blinding: no details Duration: 12 weeks |
|
| Participants | Diagnosis: ICD‐10 criteria for first‐episode schizophrenia
N = 60
Age: 18–40 years
Sex: male and female Setting: inpatients History: adult with first‐episode schizophrenia admitted to hospital with an ICD‐10 diagnosis of schizophrenia. Informed consent given by guardian or substitute decision maker Excluded: use of atypical antipsychotics or other central nervous system drugs in the past 3 months; smokers or drinkers; pregnant women; currently participating in or have participated in a clinical study in the past 3 months; BMI > 30 kg/m2; medical conditions or drug allergies |
|
| Interventions |
|
|
| Outcomes |
Able to use:
|
|
| Notes | Translated from Chinese as best possible using Google translate app. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation methods unclear. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment methods unclear. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of participants and assessors unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes unlikely to be biased by blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "53 cases completed 12 weeks of treatment (27 cases in the experimental group, 26 cases in the control group)." < 20% dropout in full sample. |
| Selective reporting (reporting bias) | Low risk | No protocol, however, measures are objective and therefore risk of bias low. |
| Other bias | Unclear risk | Difficult to determine given language constraints. |
Modabbernia 2014.
| Study characteristics | ||
| Methods | Randomisation: randomised, blocked procedures Blinding: double‐blind, participant, caregiver, investigator, outcomes assessor Duration: 8 weeks Country: Iran |
|
| Participants | Diagnosis: schizophrenia (DSM IV‐TR and SCID‐1) N = 48 Age: 18‐65 years Sex: male and female Setting: academic psychiatric hospital (Shafa Hospital, affiliated with Guilan University of Medical Sciences, Rasht, Iran) Excluded: married women who were at reproductive age (unless they used a reliable non‐hormonal contraception method), history of taking olanzapine in the recent 3 months, history of allergy or intolerance to olanzapine, history of significant head trauma (causing loss of consciousness > 5 min or neurological or cognitive sequels), liver and kidney impairment, symptomatic cerebrovascular or cardiovascular disease, diabetes mellitus, metabolic syndrome (based on National Heart, Lung, and Blood Institute/American Heart Association definition), cancer, use of antiepileptic, antihypertensive, anticoagulant, or antiplatelet drugs, using inhibitors or stimulants of hepatic isoenzymes that metabolise melatonin or olanzapine (e.g. omeprazole, rifampin, fluvoxamine, ciprofloxacin, carbamazepine, modafinil), delirium, need for administration of other antipsychotics, and addictive disorders |
|
| Interventions |
Standard care included sleep‐enhancing agents |
|
| Outcomes |
Able to use:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to 2 groups by blocked randomisation procedures |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not indicated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind (participant, caregiver, investigator, outcomes assessor) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome unlikely to be biased by blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up suggested; all participants in both arms completed the study |
| Selective reporting (reporting bias) | Low risk | There is no evidence of selective reporting upon comparing clinical trial registry information with the published study. |
| Other bias | Low risk | No obvious bias |
Narula 2010.
| Study characteristics | ||
| Methods | Randomisation: randomised, no other details Blinding: double‐blind, no other details Duration: 12 weeks Country: India |
|
| Participants | Diagnosis: WHO ICD‐10 schizophrenia N = 72 Age: 18‐65 years, mean: 31.1 years Sex: male and female Setting: inpatients or outpatients History: drug‐naive, first‐episode schizophrenia Excluded: history of any other neuropsychiatric illness; use of SSRIs, mood stabilisers or any other weight‐influencing drug; substance abuse diagnosis in last 3 months; significant medical disorder; pregnancy |
|
| Interventions |
|
|
| Outcomes |
Able to use:
|
|
| Notes | 93‐94.4% study completers | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "[Participants] were randomly assigned" Comment: no specific method of blinding offered |
| Allocation concealment (selection bias) | High risk | No specific discussion of allocation concealment. Combined with lack of details about allocation concealment and blinding, the cumulative risk of bias is likely to be high. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No specific discussion of blinding of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No specific discussion of outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No bias in attrition during study |
| Selective reporting (reporting bias) | Low risk | No protocol, however, measures are objective and therefore risk of bias low |
| Other bias | Low risk | No obvious bias |
Poyurovsky 2002.
| Study characteristics | ||
| Methods | Randomisation: randomised, no other details Blinding: double‐blind, no other details Duration: 8 weeks Country: Israel |
|
| Participants | Diagnosis: DSM‐IV schizophrenia N = 30 Age: 25.5 years Sex: male and female Setting: inpatients History: < 4 weeks of antipsychotics exposure Excluded: unco‐operative, aggressive, and suicidal patients and patients with medical illnesses that could affect body weight |
|
| Interventions |
|
|
| Outcomes |
Able to use
|
|
| Notes | Performed ITT analysis including dropouts. 74.2% study completers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information is unavailable |
| Allocation concealment (selection bias) | Unclear risk | Information is unavailable |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information is unavailable |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information is unavailable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Six subjects withdrew from the study within the first 4 weeks because of lack of response (2 patients receiving fluoxetine) and psychotic exacerbation (2 fluoxetine and 2 placebo patients)." Pg 1058 Comment: only 80% of the study population completed the study, which may effect results due to the small sample size (N = 31), however, ITT analyses reported by the study authors provides similar results as study completers. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is unavailable |
| Other bias | Low risk | No obvious bias |
Poyurovsky 2003.
| Study characteristics | ||
| Methods | Randomisation: randomised, random numbers table Blinding: double‐blind, no other details Duration: 6 weeks Country: Israel |
|
| Participants | Diagnosis: DSV‐IV schizophrenia N = 26 Age: 30.55 years Sex: male and female Setting: inpatients History: participants had < 4 weeks of antipsychotic drug exposure in the preceding 6 months, no previous olanzapine treatment, recommendation for olanzapine treatment by treating physician Excluded: specific details are not available |
|
| Interventions |
|
|
| Outcomes |
Able to use:
|
|
| Notes | Meals were served 3 times/d, and participants were not placed on a special diet or physical exercise programme for weight reduction 77% study completers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were allocated according to entries on a table of random numbers to receive..." Pg 298 |
| Allocation concealment (selection bias) | Unclear risk | Information is unavailable |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Indication of identical placebo pills is absent, and information on which personnel was blinded is unavailable |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All weight measurements were performed by a research nurse who was blind to the patients' treatment assignment" Pg 298 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Six of the 26 study patients (three in each group) withdrew during first week (before the second assessment) because of agitation that could have been related to the switch from typical antipsychotics to olanzapine." Pg 299 Comment: the study authors have analysed only ~70% of the study population's data. However, number and reasons from dropouts are similar in both groups. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is unavailable |
| Other bias | Low risk | No obvious bias |
Poyurovsky 2004.
| Study characteristics | ||
| Methods | Randomisation: randomised, no other details Blinding: double‐blind, no other details Duration: 6 weeks Country: Israel |
|
| Participants | Diagnosis: DSM‐IV schizophrenia N = 14 Age: no details. Sex: male and female Setting: inpatients History: patients hospitalised for a first episode of acute psychosis. Excluded: major mood disorders, substance‐induced psychoses, medical illness that could affect body weight (e.g. diabetes mellitus, hypothyroidism), patients with BMI ≥ 30 kg/m2 |
|
| Interventions |
|
|
| Outcomes |
Able to use:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information is unavailable |
| Allocation concealment (selection bias) | High risk | Information is unavailable. The risk of bias is cumulatively judged to be high as important information regarding randomisation, allocation concealment, blinding, and attrition has not been provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Information is unavailable |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All weight measurements were performed by a research nurse blinded to the patient's treatment assignment." Pg 333 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All participants completed the 6‐week trial" Pg 333 |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is unavailable |
| Other bias | Low risk | No obvious bias |
Poyurovsky 2007.
| Study characteristics | ||
| Methods | Randomisation: randomised, random numbers table Blinding: double‐blind, no other details Duration: 6 weeks Country: Israel |
|
| Participants | Diagnosis: DSM‐IV schizophrenia or schizophreniform disorder N = 59 Age: 29.9 years Sex: male and female Setting: inpatients History: participants had none or < 4 weeks of antipsychotic drug exposure and a recommendation for olanzapine treatment by the treating physician. Excluded: major mood disorder, aggressive or suicidal behaviour, medical illness that could affect body weight (e.g. diabetes mellitus and hypothyroidism) and obesity (BMI > 30 kg/m2) |
|
| Interventions |
|
|
| Outcomes |
Able to use:
Unable to use:
|
|
| Notes | None of the participants were placed on a special diet or physical exercise programme for weight reduction. 69.5% study completers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were allocated according to entries on a table of random numbers to receive..." Pg 442 |
| Allocation concealment (selection bias) | Unclear risk | Information is unavailable |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All the study medications were dispensed in identical capsules, and patients received two capsules per day." Pg 443 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All weight measurements were performed by a research assistant blinded to the patient's treatment assignment." Pg 443 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "The primary statistical analysis was by intention to treat and included all randomised participants." Pg 443 Quote: "Nine patients in each group discontinued the study medication..." Pg 444 Comment: > 90% of the data was analysed with ~70% study completers using an ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is unavailable |
| Other bias | Low risk | No obvious bias |
Poyurovsky 2013.
| Study characteristics | ||
| Methods | Allocation: randomised, no other details Blindness: double‐blind, no other details Duration: 6 weeks Country: Israel |
|
| Participants | Diagnosis: DSM‐IV schizophrenia or schizophreniform disorder N = 43. Age: 32.1 years Sex: male and female Setting: inpatients History: first‐episode drug‐naive participants for whom olanzapine was indicated were included in the study; participants had no exposure to antipsychotic agents or < 4 weeks of exposure to antipsychotic drugs. They were also recommended for olanzapine treatment by the treating physician. Excluded: participants with obesity (BMI ≥ 30 mg/m2), affective disorders, aggressive or suicidal behaviour were excluded. Also, individuals who had medical illness that could affect body weight (e.g. diabetes mellitus and hypothyroidism) or were taking anti‐depressants or mood stabilisers were excluded |
|
| Interventions |
|
|
| Outcomes |
Able to use:
|
|
| Notes | Participants were not placed on a special diet or physical exercise programme for weight reduction. 74.4% study completers |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were allocated according to entries on a table of random numbers" Pg 617 Comment: randomisation achieved using table of random number hence low risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Unclear reporting of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All study medications were dispensed in identical capsules, and all patients received three capsules per day. Clinical and research staff and patients were unaware of and could not determine the study drug assignment by appearance or otherwise" Pg 617 Comment: identical placebo pills were used and adverse effects were similar across groups hence likely a low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information is unavailable. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Using intent‐to‐treat analysis and LOCF as a method of imputation of the missing data, we found that reboxetine/ betahistine co‐administration with olanzapine resulted in significantly less weight gain compared to olanzapine placebo treatment as reflected in a mean between‐group difference of 2.75 kg by the end of the trial." Pg 620 Comment: LOCF and ITT were used however it is unclear whether all randomised individuals were included in analysis and how much data was imputed. |
| Selective reporting (reporting bias) | Low risk | Measures relevant to the primary outcome were reported |
| Other bias | Low risk | No obvious bias. |
Rado 2016.
| Study characteristics | ||
| Methods | Randomisation: randomised, computer‐generated Blinding: double‐blind, no other details Duration: 24 weeks Country: no details |
|
| Participants | Diagnosis: schizophrenia, schizoaffective disorder, bipolar disorder, major depression with psychotic features N = 25 Age: ≥ 18 years Sex: male and female Setting: in‐ and outpatients Excluded: patients with history of diabetes mellitus or a baseline fasting blood glucose level > 126 mg/dL, or 2 random blood glucose levels > 200 mg/dL, or a blood glucose level > 200 mg/dL on a 2‐h oral glucose tolerance test (OGTT); haemoglobin A1c (HbA1c) > 7.0%; baseline serum glutamic oxaloacetic transaminase, serum glutamic pyruvic transaminase, or alkaline phosphatase > 3 times the normal; alcohol dependence or abuse; abnormal kidney function as measured by the Modification of Diet in Renal Disease < 60 mL/1.73 m2 (as an estimation of glomerular filtration rate); unstable medical problems in the opinion of the primary investigators; QTc prolongation > 430 ms on baseline ECG; history of lactic acidosis or hypoglycaemia; current treatment with antidiabetic agents; treatment with antihyperlipidaemic agents within 3 months of randomisation (to better assess the impact of metformin on lipid profile); concurrent treatment with an antipsychotic other than olanzapine; administration of oral corticosteroids; current treatment with topiramate, phentermine, sibutramine, orlistat, or other over‐the‐counter weight‐loss agent; or patients with active homicidal or suicidal ideation; urine pregnancy test was used to exclude pregnant women; patients on lithium or thyroid replacement therapy or with documented thyroid disease underwent serum thyroid‐stimulating hormone testing ‐ those with abnormal values were excluded. |
|
| Interventions |
|
|
| Outcomes |
Able to use:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned (without any restriction or stratification) through a computer‐based algorithm to 1 of 2 treatments (olanzapine plus metformin or olanzapine plus placebo) |
| Allocation concealment (selection bias) | Low risk | Quote: "Metformin and placebo medications were identical in appearance and were provided in coded containers by a separate research pharmacy for each patient according to their randomisation assignment." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were randomised in double‐blind fashion |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome unlikely to be biased by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the olanzapine/metformin group, 1 dropout was for drowsiness, and the other was for insomnia. No dropouts occurred in the olanzapine/placebo group |
| Selective reporting (reporting bias) | Low risk | Measures relevant to the primary outcome were reported |
| Other bias | Low risk | No obvious bias |
Sun 2007.
| Study characteristics | ||
| Methods | Randomisation: randomised, digital table Blinding: double‐blind, participant, investigator Duration: 10 weeks Country: no details |
|
| Participants | Diagnosis: schizophrenia (CCMD‐3) Age: 19‐48 years Sex: male and female Setting: no details Excluded: nervous system disorders and other mental illnesses; obesity disorders, history of diabetes, hypertension, heart disease; drug or alcohol dependence |
|
| Interventions |
|
|
| Outcomes |
Able to use:
|
|
| Notes | This study was translated from Chinese using an app | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using digital table method |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not indicated |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind (participant, investigator) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome unlikely to be biased by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up not indicated |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but all metabolic measures indicated in methods section were reported on |
| Other bias | Low risk | No obvious bias |
Vishnupriya 2016.
| Study characteristics | ||
| Methods | Randomisation: randomised, computer‐generated Blinding: open‐label, no other details Duration: 24 weeks Country: no details |
|
| Participants | Diagnosis: first episode schizophrenia patients (DSM‐IV) N = 96 Age: 18‐40 years Sex: male and female Setting: no details History: on treatment with risperidone 2 mg twice/d for ≥ 2 months Excluded: unco‐operative and aggressive patients; patients with suicidal tendency; pregnant and lactating women; patients with history of liver disease, renal disease, cardiovascular disease, diabetes mellitus, hypertension, dyslipidaemia, substance abuse,seizure disorder, malignancy; patients with diagnosis other than schizophrenia; patients with mental retardation; patients taking other drugs that may affect body weight (carbamazepine, lithium, and topiramate, antidepressants, valproate and hormone replacement therapy); patients on a special diet and who do exercise for weight loss |
|
| Interventions |
|
|
| Outcomes |
Able to use:
|
|
| Notes | We contacted study authors to obtain additional data on body weight and laboratory measures that were not included in the published paper. We did not receive a response from them. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants who were initiated on risperidone 2 mg orally twice daily for ≤ 2 months for first‐episode schizophrenia were randomised using computer‐generated table into 2 groups |
| Allocation concealment (selection bias) | High risk | No allocation concealment mentioned, however, the open‐label nature of the study puts it at high risk. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome unlikely to be biased by blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All the participants completed the study, and the results were analysed. |
| Selective reporting (reporting bias) | Low risk | Study protocol unavailable, but all outcomes indicated in the methods section were reported on. |
| Other bias | Low risk | No obvious bias |
Wu 2008.
| Study characteristics | ||
| Methods | Randomisation: randomised, computer‐generated
Blinding: double‐blind
Duration: 12 weeks Country: China |
|
| Participants | Diagnosis: DSM‐IV schizophrenia N = 40 Age: 18‐50 years; mean: 25.1 years Sex: male and female Setting: inpatients History: first‐episode psychotic, no use of any antipsychotic or recreational drugs for at least 3 months before enrolment Excluded: pregnant or lactating women, patients with mental retardation, addictive disorder, specific systemic disease, other medical condition e.g. diabetes mellitus, dyslipidaemia, cardiovascular disorder, hypertension |
|
| Interventions |
Standard care included providing lifestyle counselling to patients. |
|
| Outcomes |
Able to use:
Unable to use:
|
|
| Notes | Only completer data are provided, however study authors did an ITT analysis which was found to have similar results. 92.7% study completers. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomly assigned through a computer generated table..." Pg 353. |
| Allocation concealment (selection bias) | Low risk | Quote: "To ensure concealment of the randomisation, which was conducted independently of the investigators by a research pharmacist at a separate facility, medication was provided in coded containers containing the identical appearing pills of metformin or placebo supplies by manufacturer." Pg 353. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical appearing placebo pills were used and incidence of adverse effects were similar in both study groups. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Information is unavailable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | >90% of the study population is analysed, with 92.5% study completers. |
| Selective reporting (reporting bias) | Unclear risk | Study protocol is unavailable. |
| Other bias | Low risk | No obvious bias. |
BAS: Barnes Akathisia Scale; BMI: body mass index; BPRS: Brief Psychiatric Rating Scale; CCMD‐3: Chinese Classification of Mental Disorders; CGI: Clinical Global Impressions Scale; DSM‐IV: Diagnostic and Statistics Manual ‐ Fourth Edition; ECG: electrocardiogram; FBS: fasting blood sugar; HAM‐D: Hamilton Depression Scale; HDL: high‐density lipoprotein; HOMA‐IR: Homeostatic Model Assessment for Insulin Resistance; ITT: intention‐to‐treat; LDL: low‐density lipoprotein; PANSS: Postitive and Negative Symptom Scale; SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms; SAS: Sedation‐Agitation Scale; SSRI: selective serotonin reuptake inhibitors; TGS: triglycerides; VLDL: very low‐density lipoprotein
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 2013 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Agahi 2017 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Agarwal 2019 | Allocation: randomised Participants: people with schizophrenia (with early co‐morbid diabetes or prediabetes) Interventions: not a prevention study |
| Assuncao 2006 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Atmaca 2003 | Allocation: randomised Participants: people with schizophrenia on olanzapine monotherapy Interventions: not a prevention study |
| Atmaca 2004 | Allocation: randomised Participants: people with schizophrenia on quetiapine monotherapy Interventions: not a prevention study |
| Ball 2011 | Allocation: randomised Participants: people with schizophrenia treated with clozapine or olanzapine Interventions: not a prevention study |
| Baptista 2007 | Allocation: randomised Participants: people with schizophrenia or people with bipolar disorder under olanzapine administration Interventions: not a prevention study |
| Baptista 2008 | Allocation: randomised Participants: people with schizophrenia treated with olanzapine Interventions: not a prevention study |
| Baptista 2009 | Allocation: randomised Participants: people with schizophrenia treated with olanzapine Interventions: not a prevention study |
| Barak 2010 | Allocation: randomised Participants: people with schizophrenia treated with olanzapine Interventions: not a prevention study |
| Barak 2016 | Allocation: randomised Participants: people with schizophrenia treated with olanzapine Interventions: not a prevention study |
| Biedermann 2014 | Allocation: randomised Participants: schizophrenia outpatients Interventions: not a prevention study |
| Borba 2011 | Allocation: randomised Participants: people with schizophrenia treated with clozapine Interventions: not a prevention study |
| Borovicka 2002 | Allocation: randomised Participants: people with schizophrenia treated with clozapine Interventions: not a prevention study |
| Bushe 2010 | Allocation: randomised Participants: people with schizophrenia Intervention: compared weight measures in 2 antipsychotic groups independently; not adjunctive treatment |
| Bustillo 2003 | Allocation: randomised Participants: schizophrenia outpatients treated with olanzapine Interventions: not a prevention study |
| Carrizo 2009 | Allocation: randomised Participants: patients under prolonged clozapine administration Interventions: not a prevention study |
| Chang 2012 | Allocation: randomised Participants: clozapine‐treated patients with refractory schizophrenia Internvetion: not a prevention study. |
| Chen 2010 | Allocation: randomised Participants: people with schizophrenia treated with clozapine Intervention: clozapine + metformin + behavioural lifestyle Intervention compared with clozapine only. Cannot separate metformin effects from behavioural intervention |
| Chen 2013 | Allocation: randomised Participants: people with schizophrenia treated with clozapine Interventions: not a prevention study |
| Chen 2015 | Allocation: randomised Participants: people with schizophrenia treated with clozapine Interventions: not a prevention study |
| Chiu 2016 | Allocation: randomised Participants: people with schizophrenia treated with clozapine Interventions: not a prevention study |
| Correll 2009 | Allocation: not randomised |
| Correll 2013 | Allocation: not randomised |
| Correll 2020b | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| CTRI/2013/05/003685 2013 | Allocation: randomised Participants: people with schizophrenia Intervention: metformin Outcomes: change in weight at the end of 12 weeks Other: trial was terminated due to loss to follow‐up from 22 out of 30 participants, no published data |
| Dai 2014 | Allocation: randomised Participants: people with schizophrenia with obesity on long‐term antipsychotic treatment Interventions: not a prevention study |
| Danilov 2014 | Allocation: unclear from methods written in abstract Participants: people with schizophrenia Interventions: not a prevention study Note: only the abstract of this study was written in English and the publication could not be translated, despite best attempts; abstract did not provide sufficient detail on randomisation methods |
| Deberdt 2005 | Allocation: randomised Participants: people with schizophrenia with olanzapine treatment Interventions: not a prevention study |
| De Hert 2006 | Allocation: not randomised |
| de Silva 2015 | Allocation: randomised Participants: people with schizophrenia or schizoaffective disorder Interventions: not a prevention study |
| Deutsch 2003 | Allocation: randomised Participants: schizophrenia Interventions: not a prevention study |
| Ding 2005 | Allocation: randomised Participants: schizophrenia Interventions: not a prevention study |
| Egger 2007 | Allocation: randomised Participants: < 50% schizophrenia‐affected sample |
| Eriksson 2019 | Allocation: randomised Participants: schizophrenia Interventions: not a prevention study |
| Fadai 2014 | Allocation: randomised Participants: people with schizophrenia on olanzapine treatment Interventions: not a prevention study |
| Faghihi 2012 | Allocation: randomised Participants: < 50% schizophrenia‐affected sample |
| Fan 2013 | Allocation: randomised Participants: people with schizophrenia treated with clozapine Interventions: not a prevention study |
| Fleischhacker 2010 | Allocation: randomised Participants: people with schizophrenia treated with clozapine Interventions: not a prevention study |
| Ghanizadeh 2013 | Allocation: randomised Participants: schizophrenia Interventions: not a prevention study |
| Goodall 1988 | Allocation: randomised Participants: schizophrenia Interventions: not a prevention study |
| Graham 2005 | Allocation: randomised Participants: schizophrenia, schizoaffective, bipolar disorder patients that have gained weight with antipsychotics Interventions: not a prevention study |
| Hadley 2009 | Allocation: not a randomised study, was a review article |
| Hebrani 2015 | Allocation: randomised Participants: schizophrenia Interventions: not a prevention study |
| Heikkinen 1993 | Allocation: randomised Participants: schizophrenia Intervention: compared weight measures in 2 antipsychotic groups independently; not adjunctive treatment |
| Henderson 2005a | Allocation: randomised Participants: people with schizophrenia or schizoaffective disorder treated with olanzapine Interventions: not a prevention study |
| Henderson 2007 | Allocation: randomised Participants: people with schizophrenia or schizoaffective disorder treated with clozapine Interventions: not a prevention study |
| Henderson 2009a | Allocation: randomised Participants: people with schizophrenia and insulin resistance treated with clozapine Interventions: not a prevention study |
| Henderson 2009b | Allocation: not a randomised study; cross‐over design with no proper comparator group |
| Henderson 2011 | Allocation: randomised Participants: people with schizophrenia treated with clozapine Interventions: not a prevention study |
| Hoffmann 2012 | Allocation: not a randomised study; cross‐over design with no proper comparator group |
| Holka‐Pokorska 2015 | Allocation: randomised Participants: people with schizophrenia treated with olanzapine Interventions: not a prevention study |
| Hu 2013 | Allocation: randomised Participants: people with schizophrenia treated with olanzapine Interventions: not a prevention study |
| IRCT20191223045870N1 | Allocation: randomised Participants: schizophrenia Interventions: not a prevention study |
| Ishoy 2017 | Allocation: randomised Participants: schizophrenia spectrum patients Interventions: not a prevention study |
| Jamilian 2018 | Allocation: randomised Participants: schizophrenia inpatient and outpatients Interventions: not a prevention study |
| Jarskog 2013 | Allocation: randomised Participants: outpatients with chronic schizophrenia or schizoaffective disorder Interventions: not a prevention study |
| Jarskog 2018 | Allocation: randomised Participants: overweight schizophrenia patients Interventions: not a prevention study |
| Jiang 2017 | Allocation: randomised Participants: people with schizophrenia with metabolic syndrome Interventions: not a prevention study |
| Joffe 2008 | Allocation: randomised Participants: overweight people with schizophrenia treated with clozapine or olanzapine Interventions: not a prevention study |
| Kang 2018 | Allocation: randomised Participants: people with schizophrenia or bipolar disorder Interventions: not a prevention study |
| Kelly 2011 | Allocation: randomised Participants: people with schizophrenia or schizoaffective disorder Interventions: not a prevention study |
| Khan 2020 | Allocation: randomised Participants: schizophrenia Interventions: not a prevention study |
| Kim 2007 | Allocation: unclear from methods written in abstract Participants: people with schizophrenia Interventions: not a prevention study Note: full‐text publication could not be obtained despite best attempts to contact study authors; abstract did not provide sufficient detail on randomisation methods |
| Kim 2016 | Allocation: randomised Participants: people with schizophrenia on atypical antipsychotics Interventions: not a prevention study |
| Klein 2006 | Allocation: randomised Participants: < 50% schizophrenia‐affected sample |
| Klein 2008 | Allocation: randomised Participants: schizophrenia Interventions: not a prevention study |
| Ko 2005 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Kwon 2006 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Larsen 2017 | Allocation: randomised Participants: overweight/obese people with schizophrenia‐spectrum disorder treated with clozapine or olanzapine Interventions: not a prevention study |
| Li 2013 | Allocation: randomised Participants: schizophrenia Interventions: not a prevention study |
| Li 2020 | Allocation: randomised Participants: schizophrenia Intervention: Repetitive Transcranial Magnetic Stimulation (rTMS); not a pharmacological adjunctive agent |
| Liu 2004 | Allocation: randomised Participants: people with schizophrenia Intervention: did not study adjunctive pharmacotherapy; compared weight measures in various antipsychotic groups independently |
| Lu 2004 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Lyu 2018 | Allocation: randomised Participants: obese men with schizophrenia Interventions: not a prevention study |
| Maagensen 2021 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Martin 2019 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| McElroy 2012 | Allocation: randomised Participants: < 50% schizophrenia‐affected sample |
| Mehta 2014 | Allocation: randomised Intervention: people with first‐episode schizophrenia Interventions: not a prevention study |
| Modell 1965 | Allocation: randomised Participants: obese people with schizophrenia Interventions: not a prevention study |
| Muscatello 2011a | Allocation: randomised Participants: people with schizophrenia treated with clozapine Interventions: not a prevention study |
| Muscatello 2011b | Allocation: randomised Participants: people with schizophrenia treated with clozapine Interventions: not a prevention study |
| NCT00044187 | Allocation: randomised Participants: people with schizophrenia Intervention: not a prevention review |
| NCT00114595 | Allocation: randomised Participants: people with dyskinesia, people with schizophrenia Interventions: not a prevention study |
| NCT00320723 | Allocation: randomised Participants: people with schizophrenia Intervention: bupropion for nicotine replacement therapy; not a weight‐prevention study |
| NCT00425815 | Allocation: randomised Participants: people with schizophrenia Intervention: not a weight‐intervention study |
| NCT00512070 | Allocation: randomised Participants: people with schizophrenia, schizoaffective, bipolar, obesity, metabolic syndrome Interventions: not a prevention study |
| NCT00672464 | Allocation: randomised Particiapnts: people with schizophrenia Interventions: not a prevention study |
| NCT01491490 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| NCT03132571 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Peng 2016 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Pierre 2007 | Allocation: randomised Participants: people with schizophrenia or schizoaffective disorder Interventions: not a prevention study |
| Qi 2014 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Radulovic 2002 | Allcoation: randomised. Participants: people with schizophrenia Interventions: not a prevention study |
| Ranjbar 2013 | Allocation: randomised Participants: people with schizophrenia, schizoaffective and schizophreniform disorders who received olanzapine for the first time Interventions: not a prevention study |
| Reeves 2013 | Allocation: randomised Participants: youth who have experienced clinically significant weight gain during antipsychotic treatment Interventions: not a prevention study |
| Simmons 2018 | Allocation: randomised Participants: people with schizophrenia, schizophreniform, bipolar I disorder. Intervention: not studying adjunctive pharmacotherapy; comparing weight gain in ALKS 3831 treatment versus olanzapine |
| Siskind 2018 | Allocation: randomised Participants: people with schizophrenia with metabolic syndrome newly commenced on clozapine Interventions: not a prevention study |
| Siskind 2020 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Smith 2013 | Allocation: randomised Participants: people with schizophrenia treated with antipsychotic medication Interventions: not a prevention study |
| Smith 2018 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Strous 2007 | Allocation: randomised Participants: people with chronic schizophrenia stabilised on olanzapine Intervnetion: not a prevention study |
| Sulejmanpasic 2019 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Talaei 2016 | Allocation: randomised Participants: patients hospitalised and treated with olanzapine due to the onset of an acute episode of schizophrenia or a manic episode of bipolar I disorder Interventions: not a prevention study |
| Tavakoli 2014 | Allocation: randomised Participants: people with schizophrenia with metabolic syndrome Interventions: not a prevention study |
| Taveira 2014 | Allocation: randomised Participants: people with schizophrenia or schizoaffective disorder on a stable dose of olanzapine Interventions: not a prevention study |
| Tek 2014 | Allocation: randomised Participants: women with schizophrenia Interventions: not a prevention study |
| Terevnikov 2013 | Allocation: randomised Participants: people with first‐generation schizophrenia treated with antipsychotics Interventions: not a prevention study |
| Tiihonen 2005 | Allocation: randomised Participants: treatment‐resistant people with chronic schizophrenia Internventions: not a prevention study |
| Wang 2009 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Wang 2010 | Allocation: randomised Participants: people with schizophrenia Intervention: comparing anaesthesia for electroconvulsive therapy |
| Wang 2012 | Allocation: randomised Participants: people with first‐episode schizophrenia who gained > 7% of their weight Interventions: not a prevention study |
| Wang 2020a | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Wang 2020b | Allocation: randomised Participants: people with schizophrenia Intervention: topiramate and metformin were compared, but no placebo control or usual care group to use as a comparator |
| Weber 2006 | Allocation: randomised Participants: not indicated Intervention: no pharmacological adjunct provided, added cognitive/behavioural intervention only |
| Weiner 2012 | Allocation: randomised Participants: people with schizophrenia or schizoaffective disorder Intervention: bupropion for nicotine replacement therapy; not a weight prevention study |
| Whicher 2021 | Allocation: randomised Participants: people with schizophrenia Interventions: not a prevention study |
| Wu 2012 | Allocation: randomised
Participants: people with first‐episode schizophrenia who gained > 7% of their pre‐drug weight Interventions: not a prevention study |
| Yagcioglu 2005 | Allocation: randomised Participants: people with schizophrenia partially responsive to clozapine Intervention: not a weight loss intervention. |
| Yoon 2008 | Allocation: randomised Participants: inpatients with schizophrenia Interventions: not a prevention study |
Characteristics of studies awaiting classification [ordered by study ID]
Ginsberg 2004.
| Methods | Information not available |
| Participants | Information not available |
| Interventions | Sibutramine |
| Outcomes | Information not available |
| Notes | No abstract or full publication could be found and no available contact information to gain access to this publication and determine eligibility. It is not believed to be an ongoing study |
Mondal 2014.
| Methods | Randomised, placebo‐controlled |
| Participants | Schizophrenia N = 123 |
| Interventions |
41 participants in each group |
| Outcomes | Planned assessments included body weight, waist circumference, fasting glucose, insulin and insulin resistance, blood pressure and lipid profile, SAPS, SANS |
| Notes | Only an abstract is available but with no extractable data. We emailed study authors for more details but we did not receive a response. |
SANS: Scale for the Assessment of Negative Symptoms; SAPS: Scale for the Assessment of Positive Symptoms
Characteristics of ongoing studies [ordered by study ID]
NCT04524403.
| Study name | Study evaluating the safety, efficacy, and pharmacokinetics of miricorilant in obese adult patients with schizophrenia while taking antipsychotic medications (GRATITUDE II) |
| Methods | Phase 2, double‐blind, placebo‐controlled, randomised study |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Starting date | 24 August 2020 |
| Contact information | Study Director: Ada Lee, MD, Corcept Therapeutics Contact: Clinical Trial Lead 650‐327‐3270 study877ct.gov@corcept.com |
| Notes |
NL8440.
| Study name | The Metformin‐LIfestyle in Antipsychotic users study (MELIA): optimising the use of metformin in the management of antipsychotic‐induced weight gain |
| Methods | A randomised, double blind, multicenter, placebo‐controlled, pragmatic study |
| Participants | Study population: 2 groups of schizophrenia patients who undergo lifestyle interventions:
Patients must have a diagnosis of schizophrenia spectrum disorders according to DSM‐IV‐TR or DSM‐5 criteria. They must have been using the same antipsychotic for at least 3 months. Patients are at least 16 years of age and are overweight (BMI > 25) |
| Interventions | Metformin or placebo started at 500 mg twice daily and then increased 1000 mg twice daily after 2 weeks |
| Outcomes |
|
| Starting date | 1 January 2021 |
| Contact information | Nini de Boer n.m.deboer‐6@umcutrecht.nl 088‐7567412 |
| Notes |
Differences between protocol and review
In the original protocol, we stated that we would apply specific standards to potential skewness of data before inclusion to avoid the pitfall of applying parametric tests to non‐parametric data. However, excluding studies would also lead to loss of valuable information and bias. As such, we used the following rule to assess skewness: for all endpoint data, if the standard deviation was greater than the mean, then we would remove this study from the analysis.
Additionally, we originally stated that we would only present data that contributed less than 10% of the total weighting that contributed to the summary finding. However, we know of no supporting research for this 10% cut‐off and therefore did not apply the 10% rule for investigating heterogeneity. If homogeneity was not achieved after checking that all data were entered correctly or removing studies that were visible outliers, then heterogeneity was left unresolved.
We have clarified the definition of types of interventions included in this review. We defined weight prevention studies as those that are adjunctive (i.e. add‐on) and are co‐initiated with other routinely prescribed medications. During article screening, we could, therefore, identify a non‐prevention study because the adjunctive pharmacological intervention was initiated in people who had already experienced significant antipsychotic‐induced weight gain (i.e. the agent was being prescribed for the purposes of treating weight gain, not preventing weight gain). We identified prevention studies as those in which the pharmacological agent was prescribed around the same time as antipsychotic initiation. We defined standard care as the care that all participants received in the study. Non‐standard care included other behavioural interventions that were combined with the pharmacological intervention. We only included interventions that compared such a combined intervention strategy with a behavioural intervention alone in order to assess the additive effect of using a pharmacological adjunct. In accordance with these definitions, we included the following comparisons in this review:
Drug 1 plus standard care (e.g. antipsychotics, diet advice) vs placebo or no‐pharmacological weight gain prevention treatment plus standard care
Drug 1 plus standard care (e.g. antipsychotics, diet advice) vs drug 2 (active control) plus standard care
Drug 1 plus non‐standard care (e.g. behavioral intervention) vs placebo or no‐pharmacological weight gain prevention treatment plus non‐standard care.
Drug 1 plus non‐standard care (e.g. behavioral intervention) vs drug 2 (active control) plus non‐standard care.
The included studies rarely reported the prespecified primary outcomes, 'clinically important change in weight' or 'clinically important change in BMI'. As such, we added 'average endpoint/change in weight' and 'average endpoint/change in BMI' as additional primary outcomes post‐hoc. Similarly, the secondary outcomes, 'clinically important change in waist circumference', 'clinically important change in waist‐to‐hip ratio', 'any change in waist circumference' and 'any change in waist‐to‐hip ratio' were very rarely reported, so we added 'average endpoint/change in waist circumference' and 'average endpoint/change in waist‐to‐hip ratio' as additional secondary outcomes post‐hoc.
We added the outcomes, 'average endpoint/change in weight' and 'average endpoint/change in BMI' to the summary of findings tables.
We also included change in weight data in addition to the endpoint weight measures for studies in which only change measures were available or when baseline weight measures between groups were different. With different baseline measures, the end point measurement failed to provide a true depiction of the course of change in weight and thus we included the change score.
We contacted the first author of each potentially eligible study that we identified in the search for which we could not find published data, not the first author of each included study, as stated in the protocol.
Contributions of authors
Margaret Hahn and Sri Mahavir Agarwal updated the protocol to reflect the division between behavioural and pharmacological interventions, and between treatment and prevention of weight gain. Both contributed to the write‐up of this final review.
Nicolette Stogios updated the review to reflect the division between treatment and prevention of pharmacological interventions, and also assisted in study selection, data extraction, risk of bias assessment, and write‐up of the final version of the review.
Guy Faulkner, Tony Cohn, and Gary Remington contributed to the original protocol and review of interventions to reduce weight in schizophrenia (Faulkner 2007). Guy Faulkner initiated and conceptualised the initial review (2007).
Mark Duncan, John Lockhart, Hiroyoshi Takeuchi, Valerie H Taylor, and Zohra Ahsan assisted in writing the current protocol.
Sources of support
Internal sources
-
CAMH Foundation, Toronto, Canada
SMA is supported in part by the Discovery Fund awarded by the CAMH Foundation, Toronto, Canada
-
National Institute for Health and Care Research (NIHR), UK
provided funding for Cochrane Schizophrenia Group
External sources
-
Best and Banting Diabetes Centre (BBDC), University of Toronto (U of T), Canada
MH has partial grant support through the New Investigator Award by the BBDC, U of T, Canada
NS is supported in part by the Novo‐Nordisk Graduate Studentship by the BBDC, U of T, Canada
-
Department of Psychiatry, University of Toronto, Canada
MH and SMA are both supported in part by the Academic Scholars Award, U of T, Toronto, Canada
-
Canadian Institutes of Health Research (CIHR), Canada
NS is supported in part by the Frederick Banting and Charles Best Canada Graduate Scholarship ‐ Master's (CGS‐M) Award
MH, SMA and GR have received grant support from the CIHR, Canada
-
PSI Foundation, Ontario, Canada
MH and SMA both have partial grant support by the physicians of Ontario, PSI Foundation, Canada
-
Canada Foundation for Innovation (CFI), Canda, Canada
GR has received research support from the Research Hospital Fund–Canada Foundation for Innovation (RHF‐CFI), Canada
Declarations of interest
Hioyoshi Takeuchi: has received speaker fees from EA Pharma, Janssen Pharmaceuticals, Kyowa, Lunbeck LLC, Meiji Sieka Pharma, Otsuka Pharmaceutical Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Takeda Pharmaceutical Company, and Yoshitomiyakuhin. He has also received consultant fees from Janssen Pharmaceuticals, Mitsubishi Tanabe Pharma Corporation, and Sumitomo Dainippon Pharma Co., Ltd. However, he declares that he did not receive any direct payment for completion of this review.
Margaret Hahn: has received consultant fees from Alkermes, Inc. However, she declares that she did not receive any direct payment for completion of this review.
Gary Remington: nothing to declare
Valerie Taylor: has been on advisory boards for NovoNordisk and Valeant. She has also received honoraria from Sunovion and Shire. However, she declares that she did not receive any direct payment for completion of this review.
Tony Cohn: has received speaker fees from Pfizer Canada Inc. However, he declares that he did not receive any direct payment for completion of this review.
Guy Faulkner: nothing to declare
Sri Mahavir Agarwal: nothing to declare
Nicolette Stogios: nothing to declare
Zohra A Ahsan: nothing to declare
Jonathan T Lockwood: nothing to declare
Markus J Duncan: nothing to declare
These authors contributed equally to this work.
Edited (no change to conclusions)
References
References to studies included in this review
Arman 2008 {published data only}12757
- Arman S, Sadramely MR, Nadi M, Koleini N. A randomized, double-blind, placebo-controlled trial of metformin treatment for weight gain associated with initiation of risperidone in children and adolescents. Saudi Medical Journal 2008;29(8):1130-4. [CSZG: 16966] [PubMed] [Google Scholar]
- Farshidfar F, Koleini N, Sadramely M. Is metformin effective to prevent weight gain associated with risperidone? European Psychiatry 2011;26 Suppl 1:1713. [CSZG: 22962] [Google Scholar]
Baptista 2006 {published data only}10759
- Baptista T, Martinez J, Lacruz A, Rangel N, Beaulieu S, Serrano A, et al. Metformin for prevention of weight gain and insulin resistance with olanzapine: a double-blind placebo-controlled trial. Canadian Journal of Psychiatry. Revue Canadienne de Psychiatrie 2006;51(3):192-6. [CSZG: 12656] [DOI] [PubMed] [Google Scholar]
- Baptista T, Sandia I, Lacruz A, Rangel N, Mendoza S, Beaulieu S, et al. Insulin counter-regulatory factors, fibrinogen and C-reactive protein during olanzapine administration: effects of the antidiabetic metformin. International Clinical Psychopharmacology 2007;22:69-76. [DOI] [PubMed] [Google Scholar]
Cavazzoni 2003 {published data only}7925
- Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine may ameliorate weight gain during olanzapine treatment. European Neuropsychopharmacology 2001;11(3):279. [CSZG: 7803] [Google Scholar]
- Breier AF, Tanaka Y, Roychowdhury S. Nizatidine ameliorates olanzapine treatment-related weight gain. In: 154th Annual Meeting of the American Psychiatric Association; 2001 May 5-10; New Orleans, Louisiana, USA. Marathon Multimedia, 2001. [CSZG: 7996]
- Breier AF, Tanaka Y, Roychowdhury S. Nizatidine ameliorates olanzapine treatment-related weight gain. In: 155th Annual Meeting of the American Psychiatric Association; 2002 May 18-23; Philadelphia, Pennsylvania, USA. 2002. [CSZG: 8949]
- Cavazzoni P, Tanaka Y, Roychowdhury SM, Breier A, Allison DB. Nizatidine for prevention of weight gain with olanzapine: A double-blind placebo-controlled trial. European Neuropsychopharmacology 2003;13:81-5. [CSZG: 11078] [DOI] [PubMed] [Google Scholar]
Correll 2020a {published data only}
- Correll CU, Newcomer JW, Silverman B, DiPetrillo L, Graham C, Jiang Y, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. American Journal of Psychiatry 2020;177(12):1168-78. [DOI] [PubMed] [Google Scholar]
Kim 2006 {published data only}
- Kim J, Yim S, Nam J. A 12-week, randomized, open-label, parallel-group trial of topiramate in limiting weight gain during olanzapine treatment in patients with schizophrenia. In: 13th Biennial Winter Workshop on Schizophrenia Research; 2006 Feb 4-10; Davos, Switzerland. Elsevier Science Bv, 2006:86. [DOI] [PubMed]
- Kim JH, Yim S, Nam J. A 12-week open label trial of topiramate for limiting weight gain during olanzapine treatment in patients with schizophrenia. In: 159th annual meeting of the American Psychiatric Association; 2006 May 20-25; Toronto, Canada. 2006.
- Kim JH, Yim SJ, Nam JH. A 12-week, randomized, open-label, parallel-group trial of topiramate in limiting weight gain during olanzapine treatment in patients with schizophrenia. Schizophrenia Research 2006;82(1):115-7. [DOI] [PubMed] [Google Scholar]
Liu 2011 {published data only}
- Liu JJ. Topiramate combined olanzapine prevention second-generation antipsychotic-induced body weight and metabolic dysfunction. Contemporary Nurse (Specialist Edition) 2011;23(06):665-7. [Google Scholar]
Modabbernia 2014 {published data only}
- IRCT201107187049N1. An assessment of effects of melatonin on metabolic effects of olanzapine in patients with schizophrenia. https://www.irct.ir/trial/7480 (first received 2011-09-10).
- Modabbernia A, Heidari P, Soleimani R, Sobhani A, Roshan ZA, Taslimi S, et al. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. Journal of Psychiatric Research 2014;53(1):133-40. [DOI] [PubMed] [Google Scholar]
- NCT01593774. Melatonin for prevention of metabolic side effects of olanzapine. https://clinicaltrials.gov/ct2/show/NCT01593774 (first received 8 May 2012).
Narula 2010 {published data only}16348
- Narula PK, Rehan HS, Unni KE, Gupta N. Topiramate for prevention of olanzapine associated weight gain and metabolic dysfunction in schizophrenia: a double-blind, placebo-controlled trial. Schizophrenia Research 2010;118(1-3):218-23. [CSZG: 20722] [DOI] [PubMed] [Google Scholar]
Poyurovsky 2002 {published data only}31964
- Poyurovsky M, Isaaks I, Pashnian A, Fuchs C, Schneidman M, Faragian S, et al. Reboxetine but not fluoxetine attenuates olanzapine-induced weight gain in first-episode schizophrenia patients. European Neuropsychopharmacology 2002;12 Suppl 3:S312. [Google Scholar]
- Poyurovsky M, Pashinian A, Gil-Ad I, Maayan R, Schneidman M, Fuchs C, et al. Olanzapine-induced weight gain in patients with first-episode schizophrenia: a double-blind, placebo-controlled study of fluoxetine addition. American Journal of Psychiatry 2002;159(6):1058-60. [CSZG: 8582] [DOI] [PubMed] [Google Scholar]
Poyurovsky 2003 {published data only}5815
- Poyurovsky M, Isaacs I, Fuchs C, Schneidman M, Faragian S, Weizman RW. Attenuation of olanzapine-induced weight gain with reboxetine in patients with schizophrenia: a double-blind, placebo-controlled study. American Journal of Psychiatry 2003;160(2):297-302. [CSZG: 9260] [DOI] [PubMed] [Google Scholar]
- Poyurovsky M, Isaaks I, Fuchs C, Schneidman M, Faragian S, Weizman R, et al. Reboxetine and attenuation of olanzapine-induced weight gain in first-episode schizophrenia patients. A double-blind placebo-controlled study. International journal of neuropsychopharmacology 2002;5 Suppl 1:S171. [CSZG: 8358] [Google Scholar]
- Poyurovsky M, Isaaks I, Fuchs C, Schneidman M, Faragian S, Weizman R, et al. Reboxetine and attenuation of olanzapine-induced weight gain in first-episode schizophrenia patients. A double-blind placebo-controlled study. International Journal of Neuropsychopharmacology 2002;5 Suppl 1:S171. [CSZG: 8358] [Google Scholar]
Poyurovsky 2004 {published data only}7906
- Poyurovsky M, Tal V, Maayan R, Gil-Ad I, Fuchs C, Weizman A. The effect of famotidine addition on olanzapine-induced weight gain in first-episode schizophrenia patients: a double-blind placebo-controlled pilot study. European Neuropsychopharmacology 2004;14(4):332-6. [CSZG: 11020] [DOI] [PubMed] [Google Scholar]
Poyurovsky 2007 {published data only}16114
- Poyurovsky M, Fuchs C, Pashinian A, Levi A, Faragian S, Maayan R, et al. Attenuating effect of reboxetine on appetite and weight gain in olanzapine-treated schizophrenia patients: a double-blind placebo-controlled study. In: 62nd Annual Scientific Meeting of the Society of Biological Psychiatry. 2007:129. [CSZG: 20279] [DOI] [PubMed]
- Poyurovsky M, Fuchs C, Pashinian A, Levi A, Faragian S, Maayan R, et al. Attenuating effect of reboxetine on appetite and weight gain in olanzapine-treated schizophrenia patients: a double-blind placebo-controlled study. Psychopharmacology 2007;192(3):441-8. [CSZG: 15289] [DOI] [PubMed] [Google Scholar]
- Poyurovsky M, Maayan R, Gil-Ad I, Weizman A, Weizman R. Reboxetine attenuates weight gain and increases dehydroepiandrosterone levels in olanzapine- treated schizophrenia patients. In: 156th Annual Meeting of the American Psychiatric Association; 2003 May 17-22; San Francisco, California, USA. 2003. [CSZG: 9811]
Poyurovsky 2013 {published data only}22216
- Poyurovsky M, Fuchs C, Pashinian A, Levi A, Weizman R, Weizman A. Reducing antipsychotic-induced weight gain in schizophrenia: a double-blind placebo-controlled study of reboxetine-betahistine combination. Psychopharmacology 2013;226(3):615-22. [CSZG: 28732] [DOI] [PubMed] [Google Scholar]
Rado 2016 {published data only}
- NCT00682448. Metformin for the prevention of the metabolic side-effects of zyprexa. http://clinicaltrials.gov/show/NCT00682448 (first received 22 May 2008).
- Rado J, Ammon Cavanaugh S. A naturalistic randomized placebo-controlled trial of extended-release metformin to prevent weight gain associated with olanzapine in a US community-dwelling population. Journal of Clinical Psychopharmacology 2016;36:163-8. [DOI] [PubMed] [Google Scholar]
Sun 2007 {published data only}
- Sun J. Ranitidine prevention and treatment of olanzapine-induced weight gain and glucose metabolism disorders [Léi ní tì dīng fáng zhì ào dàn píng suǒ zhì tǐ zhòng zēng jiā jí táng dài xiè zhàng ài de yán jiū]. Chinese Journal of Nervous and Mental Disorders 2007;33(9):560-2.
Vishnupriya 2016 {published data only}
- Vishnupriya R, Ezhilramya J, Meenakshi B. Metformin in the prevention of metabolic syndrome associated with initiation of atypical antipsychotic therapy in adolescents and young adults-a randomized, open labelled, single centered study. International Journal of Pharmacy and Pharmaceutical Sciences 2016;8:200-6. [Google Scholar]
Wu 2008 {published data only}11860
- Wu R-R, Zhao J-P, Guo X-F, He Y-Q, Fang M-S, Guo W-B, et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study: correction. American Journal of Psychiatry 2008;165(4):540. [CSZG: 16163] [DOI] [PubMed] [Google Scholar]
- Wu R-R, Zhao J-P, Guo X-F, He Y-Q, Fang M-S, Guo W-B, et al. Metformin addition attenuates olanzapine-induced weight gain in drug-naive first-episode schizophrenia patients: a double-blind, placebo-controlled study. American Journal of Psychiatry 2008;165(3):352-8. [CSZG: 16115] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adams 2013 {published data only}
- Adams DH, Kinon BJ, Baygani S, Millen BA, Velona I, Kollack-Walker S, et al. A long-term, phase 2, multicenter, randomized, open-label, comparative safety study of pomaglumetad methionil (LY2140023 monohydrate) versus atypical antipsychotic standard of care in patients with schizophrenia. BMC Psychiatry 2013;13:Art ID 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Agahi 2017 {published data only}
- Agahi M, Akasheh N, Ahmadvand A, Akbari H, Izadpanah F. Effect of melatonin in reducing second-generation antipsychotic metabolic effects: a double blind controlled clinical trial. Diabetes and Metabolic Syndrome 2018;12(1):9-15. [DOI] [PubMed]
- IRCT2016051027832N1. Effect of melatonin in side effects of second-generation antipsychotic medications. https://trialsearch.who.int/?TrialID=IRCT2016051027832N1 (first received 27 September 2016).
Agarwal 2019 {published data only}
- Agarwal SM, Cost-Dookhan K, Panda R, MacKenzie N, Treen QC, Caravaggio F, et al. Metformin in schizophrenia spectrum disorders and early co-morbid prediabetes/diabetes: a double-blind randomized control trial. Neuropsychopharmacology 2019;44 Suppl 1:110-1. [Google Scholar]
Assuncao 2006 {published data only}12641
- Assuncao SS, Ruschel SI, Rosa LD, Campos JA, Alves MJ, Bracco OL, et al. Weight gain management in patients with schizophrenia during treatment with olanzapine in association with nizatidine. Revista Brasileira de Psiquiatria (Sao Paulo, Brazil : 1999) 2006;28(4):270-6. [CSZG: 13955] [PubMed] [Google Scholar]
- NCT00486005. Weight gain management in patients with schizophrenia during treatment with olanzapine in association with nizatidine. https://clinicaltrials.gov/ct2/show/NCT00486005 (first received 13 June 2007). [CSZG: 14930]
Atmaca 2003 {published data only}7148
- Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Nizatidine treatment and its relationship with leptin levels in patients with olanzapine-induced weight gain. Human Psychopharmacology 2003;18(6):457-61. [CSZG: 9841] [DOI] [PubMed] [Google Scholar]
Atmaca 2004 {published data only}7262
- Atmaca M, Kuloglu M, Tezcan E, Ustundag B, Kilic N. Nizatidine for the treatment of patients with quetiapine-induced weight gain. Human Psychopharmacology 2004;19(1):37-40. [CSZG: 10031] [DOI] [PubMed] [Google Scholar]
Ball 2011 {published data only}10382
- Ball MP, Warren KR, Feldman S, McMahon RP, Kelly DL, Buchanan RW. Placebo-controlled trial of atomoxetine for weight reduction in people with schizophrenia treated with clozapine or olanzapine. Clinical Schizophrenia & Related Psychoses 2011;5(1):17-25. [CSZG: 22862] [DOI] [PubMed] [Google Scholar]
- NCT00176436. Double-blind study of atomoxetine for weight management in patients taking olanzapine or clozapine. https://clinicaltrials.gov/ct2/show/NCT00176436 (first received 15 September 2005). [CSZG: 14590]
Baptista 2007 {published data only}11239
- Baptista T, Rangel N, Fernandez V, Carrizo E, El Fakih Y, Uzcategui E, et al. Metformin as an adjunctive treatment to control body weight and metabolic dysfunction during olanzapine administration: a multicentric, double-blind, placebo-controlled trial. Schizophrenia Research 2007;93(1-3):99-108. [CSZG: 15343] [DOI] [PubMed] [Google Scholar]
Baptista 2008 {published data only}11853
- Baptista T, Uzcategui E, Rangel N, El Fakih Y, Galeazzi T, Beaulieu S, et al. Metformin plus sibutramine for olanzapine-associated weight gain and metabolic dysfunction in schizophrenia: a 12-week double-blind, placebo-controlled pilot study. Psychiatry Research 2008;159(1-2):250-3. [CSZG: 16155] [DOI] [PubMed] [Google Scholar]
- Uzcategui E, EIFakih Y, Rangel N, Galeazzi T, Baptista T. Metformin plus sibutramine in the treatment of weight gain and metabolic dysfunction during olanzapine administration: a double-blind, placebo controlled pilot study. In: 160th Annual Meeting of the American Psychiatric Association; 2007 May 19-24; San Diego, CA. 2007. [CSZG: 18966]
Baptista 2009 {published data only}13545
- Baptista T, Rangel N, El Fakih Y, Uzcategui E, Galeazzi T, Beaulieu S, et al. Rosiglitazone in the assistance of metabolic control during olanzapine administration in schizophrenia: a pilot double-blind, placebo-controlled, 12-week trial. Pharmacopsychiatry 2009;42(1):14-9. [CSZG: 18197] [DOI] [PubMed] [Google Scholar]
Barak 2010 {published data only}
- Barak N. Betahistine safely mitigates olanzapine adverse: role of histamine receptors in weight gain and somnolence. In: 163rd Annual Meeting of the American Psychiatric Association; 2010 May 22-26; New Orleans, LA. 2010.
Barak 2016 {published data only}
- Barak N, Beck Y, Albeck JH. A randomized, double-blind, placebo-controlled pilot study of betahistine to counteract olanzapine-associated weight gain. Journal of Clinical Psychopharmacology 2016;36:253-6. [DOI] [PubMed] [Google Scholar]
Biedermann 2014 {published data only}22669
- Biedermann F, Fleischhacker WW, Kemmler G, Ebenbichler CF, Lechleitner M, Hofer A. Sibutramine in the treatment of antipsychotic-induced weight gain: a pilot study in patients with schizophrenia. International Clinical Psychopharmacology 2014;29(3):181-4. [CSZG: 29185] [DOI] [PubMed] [Google Scholar]
- Biedermann F, Lechleitner M, Hofer A, Huber R, Futhmu NE-, Ebenbichler C, et al. Sibutramine in the treatment of antipsychotic-induces weight gain: a randomized, placebo-controlled double-blind study. Schizophrenia Research 2010;117(2-3):260-1. [CSZG: 20590] [Google Scholar]
Borba 2011 {published data only}18533
- Borba CP, Fan X, Copeland PM, Paiva A, Freudenreich O, Henderson DC. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. Journal of Clinical Psychopharmacology 2011;31(5):653-8. [CSZG: 23238] [DOI: 10.1097/JCP.0b013e31822bb573] [DOI] [PMC free article] [PubMed] [Google Scholar]
Borovicka 2002 {published data only}6341
- Borovicka MC, Fuller MA, Konicki PE, White JC, Steele VM, Jaskiw GE. Phenylpropanolamine appears not to promote weight loss in patients with schizophrenia who have gained weight during clozapine treatment. Journal of Clinical Psychiatry 2002;63(4):345-8. [CSZG: 8392] [DOI] [PubMed] [Google Scholar]
Bushe 2010 {published data only}
- Bushe C, Poole-Hoffmann V, Lipkovich I. Metabolic comparisons from a 6-month randomized trial of olanzapine and quetiapine in schizophrenia. In: 62nd Annual Scientific Meeting of the Society of Biological Psychiatry. San Diego, CA, USA: Elsevier Science Inc, 2007:255.
- Bushe C, Sniadecki J, Bradley AJ, Poole Hoffmann V. Comparison of metabolic and prolactin variables from a six-month randomised trial of olanzapine and quetiapine in schizophrenia. Journal of Psychopharmacology 2010;24(7):1001-9. [DOI] [PubMed] [Google Scholar]
Bustillo 2003 {published data only}6968
- Bustillo JR, Lauriello J, Hammond R, Keith S. Treatment of weight-gain with fluoxetine in olanzapine-treated schizophrenic outpatients. In: 39th Annual Meeting of the American College of Neuropsychopharmacology; 2000 Dec 10-14; San Juan, Puerto Rico. 2000. [CSZG: 7573] [DOI] [PubMed]
- Bustillo JR, Lauriello J, Parker K, Hammond R, Rowland L, Bogenschutz M, et al. Treatment of weight gain with fluoxetine in olanzapine-treated schizophrenic outpatients. Neuropsychopharmacology 2003;28(3):527-9. [CSZG: 18501] [DOI] [PubMed] [Google Scholar]
- Lauriello J, Bustillo J, Hammond R, Bogenshutz M, Keith SJ. Treatment of weight-gain with fluoxetine in olanzapine-treated schizophrenic outpatients. Schizophrenia Research 2001;49(1-2):236. [CSZG: 9530] [DOI] [PubMed] [Google Scholar]
Carrizo 2009 {published data only}14023
- Baptista T, Carrizo E, Fernandez V, Sandia I, Connell L, Prieto D, et al. Metformin for body weight and metabolic control in patients with schizophrenia during long-term clozapine administration. In: 162nd Annual Meeting of the American Psychiatric Association; 2009 May 16-21; San Francisco, CA. 2009. [CSZG: 19068]
- Baptista TJ, Fernandez E, Carrizo E, Fernandez V, Sandia I, Prieto D, et al. Polimorphisms of the LEP- and LEPR genes, metabolic profile after prolonged clozapine administration and response to the antidiabetic metformin. In: 163rd Annual Meeting of the American Psychiatric Association; 2010 May 22-26; New Orleans, LA. 2010. [CSZG: 21597] [DOI] [PubMed]
- Carrizo E, Fernandez V, Connell L, Sandia I, Prieto D, Mogollon J, et al. Extended release metformin for metabolic control assistance during prolonged clozapine administration: a 14 week, double-blind, parallel group, placebo-controlled study. Schizophrenia Research 2009;113(1):19-26. [CSZG: 18699] [DOI] [PubMed] [Google Scholar]
- Carrizo E, Fernandez V, Sandia I, Prieto D, Mogollon J, Connel L, et al. Corrigendum to "Extended release metfomin for metabolic control assistance during prolonged clozapine administration: A 14 week, double-blind, parallel group, placebo-controlled study" Schizophr. Res. 113 (1) (2009) 19-26 (DOI:10.1016/j.schres.2009.05.007). Schizophrenia Research 2009;115(1):96. [CSZG: 19023] [DOI] [PubMed] [Google Scholar]
- Fernandez E, Carrizo E, Fernandez V, Connell L, Sandia I, Prieto D, et al. Polymorphisms of the LEP- and LEPR genes, metabolic profile after prolonged clozapine administration and response to the antidiabetic metformin. Schizophrenia Research 2010;121(1-3):213-7. [CSZG: 20827] [DOI] [PubMed] [Google Scholar]
Chang 2012 {published data only}
- Chang JS, Lee NY, Ahn YM, Kim YS. The sustained effects of aripiprazole-augmented clozapine treatment on the psychotic systems and metabolic profiles of patients with refractory schizophrenia. Journal of Clinical Psychopharmacology 2012;32(2):282-4. [DOI] [PubMed] [Google Scholar]
Chen 2010 {published data only}
- Chen JX, Wu SY, Lin R, Chen HE. Metformin combined behavioral intervention therapy of clozapine in the treatment of schizophrenia in patients with body weight, blood glucose, blood lipids. Sichuan Mental Health 2010;23(04):198-202. [Google Scholar]
Chen 2013 {published data only}22141
- Chen CH, Huang MC, Kao CF, Lin SK, Kuo PH, Chiu CC, et al. Effects of adjunctive metformin on metabolic traits in nondiabetic clozapine-treated patients with schizophrenia and the effect of metformin discontinuation on body weight: a 24-week, randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry 2013;74:e424-30. [CSZG: 28657] [DOI] [PubMed] [Google Scholar]
- NCT01300637. Effects of adjunctive metformin on metabolic profiles in clozapine-treated schizophrenic patients. https://clinicaltrials.gov/ct2/show/NCT01300637 (first received 21 February 2011). [CSZG: 22667]
Chen 2015 {published data only}
- Chen PY, Lu ML, Huang MC, Kao CF, Kuo PH, Chiu CC, et al. The relationships of obesity-related genetic variants with metabolic profiles and response to metformin in clozapine-treated patients with schizophrenia. Journal of Clinical Psychopharmacology 2015;35(5):574-8. [DOI] [PubMed] [Google Scholar]
Chiu 2016 {published data only}
- Chiu CC, Lu ML, Huang MC, Chen PY, Lin YK, Lin SK, et al. Effects of low dose metformin on metabolic traits in clozapine-treated schizophrenia patients: an exploratory twelve-week randomized, double-blind, placebo-controlled study. PloS One 2016;11(12):e0168347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02751307. Adjunctive low-dose metformin in patients with schizophrenia and metabolic abnormalities. https://clinicaltrials.gov/ct2/show/NCT02751307 (first received 26 April 2016).
Correll 2009 {published data only}
- Correll CU, Manu P, Olshanskiy V, Napolitano B, Kane JM, Malhotra AK. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA 2009;302(16):1765-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Correll 2013 {published data only}
- Correll CU, Sikich L, Reeves G, Riddle M. Metformin for antipsychotic-related weight gain and metabolic abnormalities: when, for whom, and for how long? American Journal of Psychiatry 2013;170(9):947-52. [DOI] [PubMed] [Google Scholar]
Correll 2020b {published data only}
- Correll CU, Sikich L, Reeves G, Johnson J, Keeton C, Spanos M, et al. Metformin add-on vs. antipsychotic switch vs. continued antipsychotic treatment plus healthy lifestyle education in overweight or obese youth with severe mental illness: results from the IMPACT trial. World Psychiatry 2020;19(1):69-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTRI/2013/05/003685 2013 {published data only}
- CTRI/2013/05/003685. A clinical trial to study the effects of metformin in prevention of olanzapine induced weight gain, type 2 diabetes mellitus and lipid abnormalities in patients with schizophrenia. https://trialsearch.who.int/?TrialID=CTRI/2013/05/003685 (first received 27 May 2013).
Dai 2014 {published data only}
- Dai S, Zhu L, Xiao Y. The effects of metformin on obesity, glucose and lipid metabolism caused by antipsychotic drugs in schizophrenia patients [Er jiǎ shuāng guā duì kàng jīng shén bìng yào suǒ zhì jīng shén fēn liè zhèng huàn zhě féi pàng jí táng zhī dài xiè de yǐng xiǎng]. Cháng Zhì Yī Xué Yuàn Xué Bào 2014;24(6):409-10. [Google Scholar]
Danilov 2014 {published data only}
- Danilov DS. The use of aripiprazole in the treatment of obesity associated with the administration of neuroleptics of the second generation in patients with schizophrenia. Zhurnal Nevrologii I Psikhiatrii Imeni S.S. Korsakova 2014;114(3):34-40. [PubMed] [Google Scholar]
Deberdt 2005 {published data only}7052
- Deberdt W, Cavazzoni PA, Trzaskoma Q, Bymaster FP, Winokur A, Floris M, et al. Amantadine for weight gain in olanzapine-treated patients. In: 156th Annual Meeting of the American Psychiatric Association; 2003 May 17-22; San Francisco, California, USA. 2003. [CSZG: 9779]
- Deberdt W, Trzaskoma Q, Carlson C, Bymaster F, Winokur A, Floris M. Amantadine for weight gain in olanzapine-treated patients. In: Thematic conference of the World Psychiatric Association on "Treatments in psychiatry: an update"; 2004 Nov 10-13; Florence, Italy. 2004. [CSZG: 17055]
- Deberdt W, Trzaskoma Q, Carlson C, Bymaster F, Winokur A, Floris M. Amantadine for weight gain in olanzapine-treated patients. Schizophrenia Research 2004;67(1):182. [CSZG: 11293] [Google Scholar]
- Deberdt W, Winokur A, Cavazzoni PA, Trzaskoma QN, Carlson CD, Bymaster FP, et al. Amantadine for weight gain associated with olanzapine treatment. European Neuropsychopharmacology 2005;15(1):13-21. [CSZG: 11432] [DOI] [PubMed] [Google Scholar]
De Hert 2006 {published data only}
- De Hert M, Kalnicka D, Van Winkel R, Wampers M, Hanssens L, Van Eyck D, et al. Treatment with rosuvastatin for severe dyslipidemia in patients with schizophrenia and schizoaffective disorder. Journal of Clinical Psychiatry 2006;67(12):1889-96. [DOI] [PubMed] [Google Scholar]
de Silva 2015 {published data only}
- Anonymous. Corrigendum. Journal of Psychopharmacology (Oxford, England) 2017;31(4):515. [DOI] [PubMed] [Google Scholar]
- Silva VA, Dayabandara M, Wijesundara H, Henegama T, Gunewardena H, Suraweera C, et al. Metformin for treatment of antipsychotic-induced weight gain in a South Asian population with schizophrenia or schizoaffective disorder: a double blind, randomized, placebo controlled study. Journal of Psychopharmacology (Oxford, England) 2015;29(12):1255-61. [DOI] [PubMed] [Google Scholar]
- Pinto EF, George B, Karia S, Andrade C. Metformin for antipsychotic-induced weight gain: statistical curiosities. Journal of Psychopharmacology (Oxford, England) 2017;31(4):514. [DOI] [PubMed] [Google Scholar]
Deutsch 2003 {published data only}
- Deutsch S, Schwartz B, Rosse R, Mastropaolo J, Marvel C, Drapalski A. Adjuvant topiramate administration: a pharmacologic strategy for addressing NMDA receptor hypofunction in schizophrenia. Clinical Neuropharmacology 2003;26(4):199-206. [DOI] [PubMed] [Google Scholar]
Ding 2005 {published data only}
- Ding G, Yu G, Zhang J, Liang S, Liu L, Huang P, et al. The therapeutic effects of ling gui zhu gan tang mixture in 50 psychotic patients with obesity induced by the psychoactive drugs. Journal of Traditional Chinese Medicine / Chung i Tsa Chih Ying Wen Pan 2005;25(1):25-8. [PubMed] [Google Scholar]
Egger 2007 {published data only}
- Egger C, Muehlbacher M, Schatz M, Nickel M. Influence of topiramate on olanzapine-related weight gain in women: an 18-month follow-up observation. Journal of Clinical Psychopharmacology 2007;5:475-8. [DOI] [PubMed] [Google Scholar]
Eriksson 2019 {published data only}
- Eriksson R, Broberg BV, Ishoy PL, Bak N, Andersen UB, Jorgensen NR, et al. Effects of the glucagon-like peptide-1 receptor agonist exenatide on bone status in obese, non-diabetic, antipsychotic-treated schizophrenia spectrum patients. Schizophrenia Bulletin 2019;45 Suppl 2:S198-9. [Google Scholar]
Fadai 2014 {published data only}26719
- Fadai F, Mousavi B, Beigi N, Farhang S, Hashempour S, Shahhamei N, et al. Saffron aqueous extract prevents metabolic syndrome in patients with schizophrenia on olanzapine treatment: a randomized triple blind placebo controlled study. Pharmacopsychiatry 2014;47:156-61. [CSZG: 29063] [DOI] [PubMed] [Google Scholar]
Faghihi 2012 {published data only}
- Faghihi T, Jahed A, Mahmoudi-Gharaei J, Sharifi V, Akhondzadeh S, Padideh G. Role of omega-3-fatty acids in preventing metabolic disturbances in patients on olanzapine plus either sodium valproate or lithium: A randomized double-blind placebo-controlled trial. DARU, Journal of Pharmaceutical Sciences 2012;20:43. [DOI: 10.1186/2008-2231-20-43] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fan 2013 {published data only}22243
- Fan X, Borba CP, Copeland P, Hayden D, Freudenreich O, Goff DC, et al. Metabolic effects of adjunctive aripiprazole in clozapine-treated patients with schizophrenia. Acta Psychiatrica Scandinavica 2013;127(3):217-26. [CSZG: 28759] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00345033. Aripiprazole for clozapine associated medical morbidity. https://clinicaltrials.gov/ct2/show/NCT00345033 (first received 27 June 2006). [CSZG: 14696]
Fleischhacker 2010 {published data only}10531
- CTRI/2013/02/003397. Efficacy of aripiprazole adjunctive treatment on body weight, metabolic parameters, clinical efficacy, and adverse events in people with psychotic disorders on treatment with clozapine. A randomized, double-blind, placebo-controlled trial. https://trialsearch.who.int/?TrialID=CTRI/2013/02/003397 (first received 14 February 2013). [CSZG: 28076]
- Fleischhacker W, Heikkinen ME, Olie JP, Dewaele P, McQuade R, Hennicken D, et al. Long-term effect on weight of aripiprazole-clozapine in schizophrenia patients with suboptimal response on clozapine. European Neuropsychopharmacology 2008;18:S447. [CSZG: 17328] [Google Scholar]
- Fleischhacker WW, Heikkinen ME, Olie JP, Dewaele P, McQuade R, Hennicken D, et al. Long-term effect on weight of aripiprazole-clozapine in schizophrenia patients with suboptimal response to clozapine. World Psychiatry 2009;8 Suppl 1:PO1.31. [Google Scholar]
- Fleischhacker WW, Heikkinen ME, Olie JP, Landsberg W, Dewaele P, McQuade R, et al. Weight change on aripiprazole-clozapine combination in schizophrenic patients with weight gain and suboptimal response on clozapine: 16-week double-blind study. European Psychiatry 2008;23 Suppl 2:S114-S5. [CSZG: 19932] [Google Scholar]
- Fleischhacker WW, Heikkinen ME, Olie J-P, Landsberg W, Dewaele P, McQuade RD, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. International Journal of Neuropsychopharmacology 2010;13(8):1115-25. [CSZG: 21393] [DOI] [PubMed] [Google Scholar]
- NCT00300846. A multicenter, comparative, randomized, double-blind, placebo controlled study on the effect on weight of adjunctive treatment with aripiprazole in patients with schizophrenia. https://clinicaltrials.gov/ct2/show/NCT00300846 (first recieved 10 March 2006). [CSZG: 14603]
Ghanizadeh 2013 {published data only}
- Ghanizadeh A, Nikseresht MS, Sahraian A. The effect of zonisamide on antipsychotic-associated weight gain in patients with schizophrenia: a randomized, double-blind, placebo-controlled clinical trial. Schizophrenia Research 2013;147:110-5. [DOI] [PubMed] [Google Scholar]
Goodall 1988 {published data only}
- Goodall E, Oxtoby C, Richards R, Watkinson G, Brown D, Silverstone T. A clinical trial of the efficacy and acceptability of D-fenluramine in the treatment on neuroleptic-induced obesity. British Journal of Psychiatry 1988;153:208-13. [DOI] [PubMed] [Google Scholar]
Graham 2005 {published data only}7970
- Graham KA, Gu H, Lieberman JA, Harp JB, Perkins DO. Double-blind, placebo-controlled investigation of amantadine for weight loss in subjects who gained weight with olanzapine. American Journal of Psychiatry 2005;162(9):1744-6. [CSZG: 12392] [DOI] [PubMed] [Google Scholar]
- Graham KA, Perkins DO, Gu H, Lieberman J, Harp J. Double blind placebo controlled investigation of amantadine for weight loss in subjects who have gained weight during treatment with olanzapine. Schizophrenia Research 2004;67(1):184. [CSZG: 11296] [DOI] [PubMed] [Google Scholar]
- NCT00287352. Double blind placebo controlled investigation of amantadine for retarding weight gain in first episode adult psychotic subjects beginning therapy with olanzapine. https://clinicaltrials.gov/ct2/show/NCT00287352 (first received 6 February 2006). [CSZG: 27923]
Hadley 2009 {published data only}
- Hadley S. Metformin for schizophrenia. Stanley Foundation Research Programs 2009.
Hebrani 2015 {published data only}23352
- Hebrani P, Manteghi A, Behdani F, Hessami E, Rezayat K, Marvast M, et al. Double-blind, randomized, clinical trial of metformin as add-on treatment with clozapine in treatment of schizophrenia disorder. Journal of Research in Medical Sciences 2014;20(4):364-71. [CSZG: 29861] [PMC free article] [PubMed] [Google Scholar]
Heikkinen 1993 {published data only}
- Heikkinen H, Outakoski J, Merilaeinen V, Tuomi A, Huttunen MO. Molindone and weight loss. Journal of Clinical Psychiatry 1993;54(4):160-1. [PubMed] [Google Scholar]
Henderson 2005a {published data only}7469
- Henderson DC, Copeland PM, Daley TB, Borba CP, Cather C, Nguyen DD, et al. A double-blind, placebo-controlled trial of sibutramine for olanzapine-associated weight gain. American Journal of Psychiatry 2005;162(5):954-62. [CSZG: 11470] [DOI] [PubMed] [Google Scholar]
- Henderson DC, Daley TB, Louie P. A placebo-controlled trial of sibutramine added to patients with olanzapine-induced weight gain. World Journal of Biological Psychiatry 2004;5 Suppl 1:152. [CSZG: 18867] [Google Scholar]
- Henderson DC, Nguyen D, Daley TB, Louie P, Burba C, Copeland P. Double-blind, placebo-controlled trial of sibutramine for olanzapine-associated weight gain. In: 157th Annual Meeting of the American Psychiatric Association; 2004 May 1-6; New York, USA. 2004. [CSZG: 10884]
Henderson 2007 {published data only}11286
- Henderson DC, Fan X, Copeland PM, Borba CP, Daley TB, Nguyen DD, et al. A double-blind, placebo-controlled trial of sibutramine for clozapine-associated weight gain. Acta Psychiatrica Scandinavica 2007;115(2):101-5. [CSZG: 14460] [DOI] [PubMed] [Google Scholar]
Henderson 2009a {published data only}
- Henderson DC, Fan X, Sharma B, Copeland PM, Borba CP, Boxill R, et al. A double-blind, placebo-controlled trial of rosiglitazone for clozapine-induced glucose metabolism impairment in patients with schizophrenia. Acta Psychiatrica Scandinavica 2009;119:457-65. [DOI: 10.1111/j.1600-0447.2008.01325.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00337350. A double-blind, placebo-controlled trial of rosiglitazone for clozapine induced glucose metabolism impairment: Bergman's minimal model analysis. https://clinicaltrials.gov/ct2/show/NCT00337350 (first received 15 June 2006).
Henderson 2009b {published data only}
- Henderson DC, Fan X, Copeland PM, Sharma B, Borba CP, Boxill R, et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. Journal of Clinical Psychopharmacology 2009;29(2):165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00351936. A placebo-controlled, cross-over trial of aripiprazole added to obese olanzapine-treated patients with schizophrenia. https://clinicaltrials.gov/ct2/show/NCT00351936 (first received 13 July 2006).
Henderson 2011 {published data only (unpublished sought but not used)}11955
- Henderson DC, Freudenreich O, Borba CP, Wang X, Copeland PM, Macklin E, et al. Effects of modafinil on weight, glucose and lipid metabolism in clozapine-treated patients with schizophrenia. Schizophrenia Research 2011;130(1-3):53-6. [CSZG: 23071] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hoffmann 2012 {published data only}
- Hoffmann V, Case M, Jacobson J. Algorithms including amantadine, metformin and zonisamide for mitigation of weight gain during olanzapine treatment in outpatients with schizophrenia. In: 162nd Annual Meeting of the American Psychiatric Association; 2009 May 16-21; San Francisco, CA. 2009.
- Hoffmann VP, Case M, Jacobson JG. Assessment of treatment algorithms including amantadine, metformin, and zonisamide for the prevention of weight gain with olanzapine: a randomized controlled open-label study. Journal of Clinical Psychiatry 2012;73(2):216-23. [DOI] [PubMed] [Google Scholar]
- NCT00401973. The assessment of the safety, efficacy, and practicality of an algorithm including amantadine, metformin and zonisamide for the prevention of olanzapine-associated weight gain in outpatients with schizophrenia. https://clinicaltrials.gov/ct2/show/NCT00401973 (first received 22 November 2006).
Holka‐Pokorska 2015 {published data only}9751
- Holka-Pokorska J, Radzio R, Jarema M, Wichniak A. The stabilizing effect of dehydroepiandosterone on clinical paramters of metabolic syndrome in patients with schizophrenia treated with olanzapine- a randomized, double-blind trial. Psychiatriapolska 2015;49(2):363-76. [CSZG: 29862] [DOI] [PubMed] [Google Scholar]
Hu 2013 {published data only}
- Hu X,Ouyang Z. Efficacy of metformin on serum homocysteine level in schizophrenia patients with olanzapine - induced weight gain [Er jiǎ shuāng guā duì ào dàn píng zhì tǐ zhì liáng zēng jiā de jīng shén fēn liè zhèng huàn zhě xuè qīng tóng xíng bàn guāng ān suān shuǐ píng de yǐng xiǎng]. China Journal of Health Psychology 2013;21(11):1610-11. [Google Scholar]
IRCT20191223045870N1 {published data only}
- IRCT20191223045870N1. Betahistine and weight- related and metabolic measures in schizophrenic patients treated with olanzapin or risperidone. https://www.irct.ir/trial/44644 (first received 13 March 2020).
Ishoy 2017 {published data only}
- Ebdrup BH, Broberg BV, Ishoy PL, Bak N, Andersen UB, Jorgensen NR, et al. Erratum: Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial. Diabetes, Obesity and Metabolism 2018;20(5):1327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson R, Broberg BV, Ishoy PL, Bak N, Andersen UB, Jorgensen NR, et al. Bone status in obese, non-diabetic, antipsychotic-treated patients, and effects of the glucagon-like peptide-1 receptor agonist exenatide on bone turnover markers and bone mineral density. Frontiers in Psychiatry 2018;9:781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson R, Broberg BV, Lau Ishoy P, Bak N, Andersen UB, Jorgensen NR, et al. Effects of the glucagon-like peptide-1 receptor agonist exenatide on bone status in obese, non-diabetic, antipsychotic-treated schizophrenia spectrum patients. Schizophrenia Bulletin 2019;45:S198-9. [Google Scholar]
- Ishoy PL, Fagerlund B, Broberg BV, Bak N, Knop FK, Glenthoj BY, et al. No cognitive-enhancing effect of GLP-1 receptor agonism in antipsychotic-treated, obese patients with schizophrenia. Acta Psychiatrica Scandinavica 2017;136(1):52-62. [DOI] [PubMed] [Google Scholar]
- Ishoy PL, Fagerlund B, Broberg BV, Bak N, Knop FK, Glenthoj BY, et al. No cognitive-enhancing effect of GLP-1 receptor agonism in antipsychotictreated, obese patients with schizophrenia. Dansk Psykiatrisk Selskabs Arsmode; 2-4 February 2017; Kolding, Denmark 2017.
- Ishoy PL, Knop FK, Broberg BV, Baandrup L, Fagerlund B, Jorgensen NR, et al. Treatment of antipsychotic-associated obesity with a GLP-1 receptor agonist--protocol for an investigator-initiated prospective, randomised, placebo-controlled, double-blinded intervention study: the TAO study protocol. BMJ Open 2014;4(1):e004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishoy PL, Knop FK, Broberg BV, Bak N, Andersen UB, Jorgensen NR, et al. Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial. Dansk Psykiatrisk Selskabs Arsmode; 2-4 February 2017; Kolding, Denmark 2017.
- Ishoy PL, Knop FK, Broberg BV, Bak N, Andersen UB, Jorgensen NR, et al. Effect of GLP-1 receptor agonist treatment on body weight in obese antipsychotic-treated patients with schizophrenia: a randomized, placebo-controlled trial. Diabetes, Obesity and Metabolism 2017;19(2):162-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishoy PL, Knop FK, Broberg BV, Bak N, Andersen UB, Jorgensen NR, et al. GLP-1 receptor agonist treatment in schizophrenia patients with obesity. Schizophrenia Bulletin 2017;43:S168. [Google Scholar]
- Ishoy PL, Knop FK, Broberg BV, Bak N, Andersen UB, Jorgensen NR, et al. Treatment of antipsychotic-associated obesity with a GLP-1 receptor agonist: an investigator initiated prospective, randomized, placebo-controlled, double-blinded intervention study (the TAO study). Neuropsychopharmacology 2016;41:S402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01794429. Treatment of antipsychotic-associated obesity with a GLP-1 analogue. https://clinicaltrials.gov/ct2/show/NCT01794429 (first received 18 February 2013).
Jamilian 2018 {published data only}
- Jamilian H, Shayganfard M. The effects of topiramate on weight gain in patients with schizophrenia: a double-blind randomized placebo-controlled clinical trial. Journal of Pioneering Medical Sciences 2018;8(2):55-9. [Google Scholar]
Jarskog 2013 {published data only}13295
- Ballon JS, Hamer RM, Catellier DJ, Stewart D, Lavange L, Golden L, et al. Metformin and impaired glucose tolerance in overweight persons with schizophrenia. Schizophrenia Bulletin 2011;37 Suppl 1:24. [CSZG: 22829] [Google Scholar]
- Jarskog LF, Hamer RM, Catellier DJ, Stewart DD, Lavange L, Ray N, et al. Metformin for weight loss and metabolic control in overweight outpatients with schizophrenia and schizoaffective disorder. American Journal of Psychiatry 2013;170(9):1032-40. [CSZG: 28631] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J, Jarskog LF, Hamer R, Catellier D, Stewart D, LaVange L, et al. Metformin for obesity and metabolic abnormalities in schizophrenia. In: 49th Annual meeting of the Americam College of Neuropsychopharmacology; 2010 Dec 5-9; Miami, Florida. 2010. [CSZG: 23686]
- Lieberman J, Jarskog LF, Hamer R, Catellier D, Stewart D, LaVange L, et al. Metformin for obesity and metabolic abnormalities in schizophrenia. Neuropsychopharmacology 2010;35:S225-S6. [CSZG: 24447] [Google Scholar]
- NCT00816907. The use of metformin in the treatment of antipsychotic-induced weight gain in schizophrenia (The METS Study). https://clinicaltrials.gov/ct2/show/NCT00816907 (first received 5 January 2009). [CSZG: 17385]
- Stroup S. Effect of metformin on weight in patients with schizophrenia with impaired fasting glucose. Neuropsychopharmacology 2013;38:S41. [CSZG: 28468] [Google Scholar]
Jarskog 2018 {published data only}
- Jarskog L, Stroup TS. Metformin and 5-HT2C agonist lorcaserin for weight loss in schizophrenia. Biological Psychiatry 2018;83(9 Suppl 1):S105-6.
- NCT02796144. Metformin and lorcaserin for weight loss in schizophrenia. https://clinicaltrials.gov/.
Jiang 2017 {published data only}
- Jiang YW, Jiang WH, Ma LH, Zhang TX, Han JJ, Li L. Efficacy of behavioral intervention adjunctive to rosuvastatin for antipsychotic-induced metabolic syndrome. Chinese Journal of Psychiatry 2017;50:295-301. [Google Scholar]
Joffe 2008 {published data only}22223
- Chukhin E, Takala P, Hakko H, Raidma M, Putkonen H, Rasanen P, et al. In a randomized placebo-controlled add-on study orlistat significantly reduced clozapine-induced constipation. International Clinical Psychopharmacology 2013;28(2):67-70. [CSZG: 28739] [DOI] [PubMed] [Google Scholar]
- ISRCTN65731856. Orlistat therapy in clozapine- and olanzapine-treated patients who are overweight or obese. https://www.isrctn.com/ISRCTN65731856 (first received 25 May 2006). [CSZG: 15377]
- Joffe G, Rasaoeo PK. A double-blind, placebo controlled, randomized, 16-week trial of orlistat in the prevention and management of clozapine and olanzapine-induced weight gain in 80 patients with psychotic. Stanley Foundation Research Programs 2002.
- Joffe G, Takala P, Tchoukhine E, Hakko H, Raidma M, Putkonen H, et al. Orlistat in clozapine- or olanzapine-treated patients with overweight or obesity: a 16-week randomized, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry 2008;69(5):706-11. [CSZG: 16446] [DOI] [PubMed] [Google Scholar]
- Tchoukhine E, Takala P, Hakko H, Raidma M, Putkonen H, Rasanen P, et al. Orlistat in clozapine- or olanzapine- treated patients with overweight or obesity: a 16-week open label extension phase and both phases of a randomized controlled trial. Journal of Clinical Psychiatry 2011;72(3):326-30. [CSZG: 22994] [ISRCTN65731856] [DOI] [PubMed] [Google Scholar]
Kang 2018 {published data only}
- Kang D, Jing Z, Li R, Hei G, Shao T, Li L, et al. Effect of betahistine and metformin on antipsychotic-induced weight gain: an analysis of two clinical trials. Frontiers in Psychiatry 2018;9:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan L. Betahistine for schizophrenia. Stanley Foundation Research Programs 2009.
- NCT00709202. To examine the effect of betahistine on antipsychotic induced weight gain in adolescents. https://clinicaltrials.gov/ct2/show/NCT00709202 (first received 3 July 2008).
- Vaz-Leal FJ. Metformin plus a lifestyle intervention was more effective than either alone for antipsychotic-induced weight gain: commentary. Evidence-Based Medicine 2008;13(5):146. [DOI] [PubMed] [Google Scholar]
- Wu RR, Jin H, Gao K, Shao P, Chan PK, Ou JJO, et al. Metformin for treatment of atypical antipsychotic-induced weight gain and endocrinological side effects in patients with first episode schizophrenia: results from randomized, double blind, placebo-controlled study. Schizophrenia Research 2012;136:S77. [Google Scholar]
- Wu R-R, Zhao J-P, Jin H, Shao P, Fang M-S, Guo X-F, et al. Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain: a randomized controlled trial. JAMA 2008;299(2):185-93. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Wang Y, Wang B. Efficacy of behavioral intervention therapy and metformin in the treatment of atypical antipsychotics-induced metabolic syndrome. Archives of Psychiatry 2009;22(3):195-7. [CSZG: 19242] [Google Scholar]
Kelly 2011 {published data only (unpublished sought but not used)}11944
- Kelly DL, Gorelick DA, Conley RR, Boggs DL, Linthicum J, Liu F, et al. Effects of the cannabinoid-1 receptor antagonist rimonabant on psychiatric symptoms in overweight people with schizophrenia. Journal of Clinical Psychopharmacology 2011;31(1):86-90. [CSZG: 22623] [DOI: 10.1097/JCP.0b013e318204825b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2020 {published data only}
- Khan MF, Haq SU, Murad K, Nazar Z. Effect of metformin in reduction of anti-psychotic medication induced weight gain in schizophrenia. Journal of Postgraduate Medical Institute 2020;34(1):41-4. [Google Scholar]
Kim 2007 {published data only}
- Kim SI, Sung YM, Paik KW, Yun KW, Kim YC, Lim W. The efficacy of topiramate for weight loss and psychiatric symptom severity in overweight or obese patients maintained on atypical antipsychotics. In: 160th Annual Meeting of the American Psychiatric Association; 2007 May 19-24; San Diego, CA. 2007.
Kim 2016 {published data only}
- Kim NW, Song YM, Kim E, Cho HS, Cheon KA, Kim SJ, et al. Adjunctive alpha-lipoic acid reduces weight gain compared with placebo at 12 weeks in schizophrenic patients treated with atypical antipsychotics: a double-blind randomized placebo-controlled study. International Clinical Psychopharmacology 2016;31(5):265-74. [DOI] [PubMed] [Google Scholar]
Klein 2006 {published data only}
- Cottingham EM, Morrison JA, Klein DJ, Barton BA. A randomized, double-blind placebo-controlled trial of metformin (glucophage®) for weight-gain associated with atypical antipsychotics in children and adolescents. In: 159th Annual Meeting of the American Psychiatric Association; 2006 May 20-25; Toronto, Canada. 2006.
- Klein DJ, Cottingham EM, Sorter M, Barton BA, Morrison JA. A randomized, double-blind, placebo-controlled trial of metformin treatment of weight gain associated with initiation of atypical antipsychotic therapy in children and adolescents. American Journal of Psychiatry 2006;163(12):2072-9. [DOI] [PubMed] [Google Scholar]
Klein 2008 {published data only}
- Klein DJ. Metformin treatment of weight gain associated with antipsychotic drug therapy. International Journal of Neuropsychopharmacology 2008;11 Suppl 1:32. [Google Scholar]
Ko 2005 {published data only}13507
- Ko YH, Joe SH, Jung IK, Kim SH. Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clinical Neuropharmacology 2005;28(4):169-75. [CSZG: 18128] [DOI] [PubMed] [Google Scholar]
Kwon 2006 {published data only}
- Kwon JS, Choi J-S, Bahk W-M, Kim CY, Kim CH, Shin YC, et al. Weight management program for treatment-emergent weight gain in olanzapine-treated patients with schizophrenia or schizoaffective disorder: a 12-week randomized controlled clinical trial. Journal of Clinical Psychiatry 2006;67(4):547-53. [DOI] [PubMed] [Google Scholar]
- NCT00485823. Assessment of a weight management program for weight gain in patients with schizophrenia. https://clinicaltrials.gov/ct2/show/NCT00485823 (first received 13 June 2007).
- NCT00485823. The assessment of a weight management program for treatment-emergent weight gain in patients with schizophrenia, schizophreniform disorder, and schizoaffective disorder during olanzapine therapy. https://clinicaltrials.gov/ct2/show/NCT00485823 (first received 13 June 2007).
Larsen 2017 {published data only}
- Larsen JR, Vedtofte L, Holst JJ, Oturai P, Kjaer A, Corell CU, et al. Does a GLP-1 receptor agonist change glucose tolerance in patients treated with antipsychotic medications? Design of a randomised, double-blinded, placebo-controlled clinical trial. BMJ Open 2014;4(3):e004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen JR, Vedtofte L, Jakobsen MS, Jespersen HR, Jakobsen MI, Svensson CK, et al. Effect of liraglutide treatment on prediabetes and overweight or obesity in clozapine- or olanzapine-treated patients with schizophrenia spectrum disorder: a randomized clinical trial. JAMA Psychiatry 2017;74(7):719-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson CK, Larsen JR, Vedtofte L, Jakobsen MS, Jespersen HR, Jakobsen MI, et al. One-year follow-up on liraglutide treatment for prediabetes and overweight/obesity in clozapine- or olanzapine-treated patients. Acta Psychiatrica Scandinavica 2019;139(1):26-36. [DOI] [PubMed] [Google Scholar]
- Vedtofte L, Larsen JR, Jakobsen MS, Jespersen HR, Jakobsen MI, Svensson CK, et al. The GLP-1 analog liraglutide improves glucose tolerance and reduces body weight in schizophrenia spectrum disorder patients treated with clozapine or olanzapine. Diabetes 2017;66 Suppl 1:A33-4. [Google Scholar]
Li 2013 {published data only}11957
- Li J, Li X, Liu E, Copeland P, Freudenreich O, Goff DC, et al. No effect of adjunctive, repeated dose intranasal insulin treatment on body metabolism in patients with schizophrenia. Schizophrenia Research 2013;146(1-3):40-5. [CSZG: 28091] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00575666. Intranasal insulin treatment in patients with schizophrenia. https://clinicaltrials.gov/ct2/show/NCT00575666 (first received 18 December 2007). [CSZG: 15650]
Li 2020 {published data only}
- ChiCTR1900024422. Effects and mechanism of T-cell subgroup imbalance on antipsychotic drug-induced obesity. http://www.chictr.org.cn/showprojen.aspx?proj=34285 (first received 7 October 2017).
- Li X, Yuan X, Kang Y, Pang L, Liu Y, Zhu Q, et al. A synergistic effect between family intervention and rTMS improves cognitive and negative symptoms in schizophrenia: a randomized controlled trial. Journal of Psychiatric Research 2020;126:81-91. [DOI] [PubMed] [Google Scholar]
Liu 2004 {published data only}
- Liú JB, Wú JH. Chlorpromazine, clozapine, risperidone on glucose metabolism in patients with first-episode schizophrenia, the effects of blood lipids and weight [Dàn píng lì péi tóng duì shǒu fā jīng shén fēn liè zhèng huàn zhě táng dài xiè xuè zhī hé tǐ zhòng de yǐng xiǎng]. Chinese Journal of Nervous and Mental Diseases 2004;30(4):293-5. [Google Scholar]
Lu 2004 {published data only}
- Lu M-L, Lane H-Y, Lin S-K, Chen K-P, Chang W-H. Adjunctive fluvoxamine inhibits clozapine-related weight gain and metabolic disturbances. Journal of Clinical Psychiatry 2004;65(6):766-71. [DOI] [PubMed] [Google Scholar]
Lyu 2018 {published data only}
- Lyu X, Du J, Zhan G, Wu Y, Su H, Zhu Y, et al. Naltrexone and bupropion combination treatment for smoking cessation and weight loss in patients with schizophrenia. Frontiers in Pharmacology 2018;9:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02736474. Naltrexone and bupropion combination on obese, smoking patients with schizophrenia. https://clinicaltrials.gov/ct2/show/NCT02736474 (first received 13 April 2016).
Maagensen 2021 {published data only}
- Larsen JR, Vedtofte L, Jakobsen MS, Jespersen HR, Jakobsen MI, Svensson CK, et al. The GLP-1 analog liraglutide improves glucose tolerance and reduces body weight in schizophrenia spectrum disorder patients treated with clozapine or olanzapine. Acta Neuropsychiatrica 2017;29:12-3. [Google Scholar]
- Maagensen H, Larsen JR, Jorgensen NR, Fink-Jensen A, Vilsboll T. Liraglutide does not change bone turnover in clozapine- and olanzapine-treated schizophrenia overweight patients with prediabetes - randomized controlled trial. Psychiatry Research 2021;296:113670. [DOI] [PubMed] [Google Scholar]
Martin 2019 {published data only}
- Martin WF, Correll CU, Weiden PJ, Jiang Y, Pathak S, DiPetrillo L, et al. Mitigation of olanzapine-induced weight gain with samidorphan, an opioid antagonist: a randomized double-blind phase 2 study in patients with schizophrenia. American Journal of Psychiatry 2019;176(6):457-67. [DOI] [PubMed] [Google Scholar]
McElroy 2012 {published data only}
- McElroy SL, Winstanley E, Mori N, Martens B, McCoy J, Moeller D, et al. A randomized, placebo-controlled study of zonisamide to prevent olanzapine-associated weight gain. Journal of Clinical Psychopharmacology 2012;32(2):165-72. [DOI: 10.1097/JCP.0b013e3182488758] [DOI] [PubMed] [Google Scholar]
Mehta 2014 {published data only}
- CTRI-2014-07-004740. Efficacy of ranitidine in olanzapine induced weight gain: a dose response study. http://www.ctri.nic.in/Clinicaltrials/pdf_generate.php?trialid=7044&EncHid=&modid=&compid=%27,%277044det%27 (first received 15 July 2014).
- Mehta VS, Ram D. Efficacy of ranitidine in olanzapine-induced weight gain: a dose-response study. Early Intervention in Psychiatry 2014;10(6):522-7. [DOI] [PubMed] [Google Scholar]
- Mehta VS, Ram D. Role of ranitidine in negative symptoms of schizophrenia--an open label study. Asian Journal of Psychiatry 2014;12:150-4. [DOI] [PubMed] [Google Scholar]
Modell 1965 {published data only}8723
- Modell W, Hussar AE. Failure of dextroamphetamine sulfate to influence eating and sleeping patterns in obese schizophrenic patients. Journal of American Medical Assosiation 1965;193(4):95-8. [CSZG: 11096] [DOI] [PubMed] [Google Scholar]
Muscatello 2011a {published data only}
- Millar H, Felter C, Landsberg W. The effects of aripiprazole in combination with clozapine: patient functioning results from a double-blind, 16-week study in patients with schizophrenia (CN138-170). Journal of Psychopharmacology (Oxford, England) 2008;22(5):A17. [Google Scholar]
- Muscatello MR, Bruno A, Pandolfo G, Mico U, Scimeca G, Di Nardo F, et al. Effect of aripiprazole augmentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. Schizophrenia Research 2011;123(1-3):93-9. [DOI: 10.1016/j.schres.2010.12.011] [DOI] [PubMed] [Google Scholar]
Muscatello 2011b {published data only}
- Muscatello MR, Bruno A, Mico U, Bellinghieri PM, Scimeca G, Cacciola M, et al. Topiramate augumentation of clozapine in schizophrenia: a double-blind, placebo-controlled study. Journal of Psychopharmacology 2011;25(5):667-74. [DOI: 10.1177/0269881110372548] [DOI] [PubMed] [Google Scholar]
NCT00044187 {unpublished data only}
- NCT00044187. The assessment of sibutramine for the treatment of olanzapine-associated weight gain in subjects with schizophrenia, schizophreniform disorder, schizoaffective disorder, and bipolar I disorder. https://clinicaltrials.gov/ct2/show/NCT00044187 (first received 23 August 2002).
NCT00114595 {published data only}
- NCT00114595. A double-blind, randomised, parallel-group comparison of ethyl-eicosapentaenoic acid (ethyl-epa) versus placebo as add-on medication in patients with established tardive dyskinesia. https://clinicaltrials.gov/show/NCT00114595 (first received 23 June 2005).
NCT00320723 {published data only}
- NCT00320723. Nicotine replacement therapy added to cognitive behavioral therapy for smoking cessation in patients with major mental illness. https://clinicaltrials.gov/show/NCT00320723 (first received 3 May 2006).
NCT00425815 {published data only}
- NCT00425815. A placebo-controlled trial of org 24448 (ampakine) added to atypical antipsychotics in patients with schizophrenia. https://clinicaltrials.gov/ct2/show/NCT00425815 (first received 23 January 2007).
NCT00512070 {published data only}
- NCT00512070. Melatonin metabolism abnormality in patients with schizophrenia or schizoaffective disorder treated with olanzapine and melatonin dose finding for the correction of the metabolic abnormality. https://clinicaltrials.gov/show/NCT00512070 (first received 7 August 2007).
NCT00672464 {published data only}
- NCT00672464. Cardio risk of acute schizophrenia olanzapine Duke (CRASOD). https://clinicaltrials.gov/show/NCT00672464 (first received 5 May 2008).
NCT01491490 {unpublished data only}
- NCT01491490. A randomised, double-blind, placebo-controlled parallel group, pilot study of 40:1 ratio of formulated GWP42003:GWP42004 in the treatment of iatrogenic weight gain and dyslipidaemia associated with olanzapine or other antipsychotic(s) treatment in subjects with schizophrenia or other non-affective psychosis. https://clinicaltrials.gov/ct2/show/NCT01491490 (first received 14 December 2011).
NCT03132571 {unpublished data only}
- NCT03132571. A pilot study of naltrexone – bupropion combination versus placebo combined with bupropion for weight loss in comorbid schizophrenia. https://clinicaltrials.gov/ct2/show/NCT03132571 (first received 28 April 2017).
Peng 2016 {published data only}
- Peng PJ, Ho PS, Tsai CK, Huang SY, Liang CS. A pilot study of randomized, head-to-head of metformin versus topiramate in obese people with schizophrenia. Clinical Neuropharmacology 2016;39(6):306-10. [DOI] [PubMed] [Google Scholar]
Pierre 2007 {published data only}
- Pierre JM, Peloian JH, Wirshing DA, Wirshing WC, Marder SR. A randomized, double-blind, placebo-controlled trial of modafinil for negative symptoms in schizophrenia. Journal of Clinical Psychiatry 2007;68(5):705-10. [DOI] [PubMed] [Google Scholar]
Qi 2014 {published data only}
- Pan Q, Chen F, Ouyang H. Metformin addition therapy attenuates serum levels of leptin in schizophrenia with olanzapine-induced weight gain [èr jiǎ shuāng guā duì ào dàn píng zhì tǐ zhòng zēng jiā de jīng shén fēn liè zhèng huàn zhě xuè qīng shòu sù shuǐ píng de yǐng xiǎng]. China Journal of Health Psychology 2014;22(6):826-8. [Google Scholar]
Radulovic 2002 {published data only}
- Radulovic L, Weiden PJ, Allison DB, Camille C. Sibutramine treatment of obesity in schizophrenia. In: 155th Annual Meeting of the American Psychiatric Association; 2002 May 18-23; Philadelphia, Pennsylvania, USA. 2002.
Ranjbar 2013 {published data only}
- Ranjbar F, Ghanepour A, Sadeghi-Bazargani H, Asadlo M, Alizadeh A. The effect of ranitidine on olanzapine-induced weight gain. Biomedical Research International 2013;2013:639391. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2013 {published data only}
- Reeves GM, Keeton C, Correll CU, Johnson JL, Hamer RM, Sikich L, et al. Improving metabolic parameters of antipsychotic child treatment (IMPACT) study: rationale, design, and methods. Child and Adolescent Psychiatry and Mental Health 2013;7(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simmons 2018 {published data only}
- David M, Simmons A, Jiang Y, Graham C, Silverman B. A study comparing weight gain from ALKS 3831 to olanzapine in early-illness young adults with schizophrenia, schizophreniform, or bipolar I disorder. European Neuropsychopharmacology 2017;27 Suppl 4:S954. [Google Scholar]
- EUCTR2017-000497-11-AT. Study to evaluate the efficacy of ALKS 3831 on body weight in young adults who have been recently diagnosed with schizophrenia, schizophreniform or bipolar I disorder. https://www.clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2017-000497-11-AT (first received 2017).
- NCT03187769. Study to evaluate the efficacy of ALKS 3831 on body weight in young adults who have been recently diagnosed with schizophrenia, schizophreniform, or bipolar I disorder. https://clinicaltrials.gov/ct2/show/NCT03187769 (first received 15 June 2017).
- Simmons A, McDonnell D, Jiang Y, Graham C, Silverman B. A study comparing weight gain from ALKS 3831 to olanzapine in early-illness young adults with schizophreniform, schizophrenia, or bipolar I disorder. Schizophrenia Bulletin 2018;44 Suppl 1:S413-4. [Google Scholar]
Siskind 2018 {published data only}
- ACTRN12615000524594. A pilot study on the effect of once weekly exenatide compared to treatment as usual for weight loss and glycaemic control in schizophrenia patients with obesity and diabetes. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368512 (first received 9 May 2015).
- Mayfield K, Siskind D, Winckel K, Hollingworth S, Kisely S, Russell AW. Treatment of clozapine-associated obesity and diabetes with exenatide (CODEX) in adults with schizophrenia: study protocol for a pilot randomised controlled trial. BJ Psych Open 2015;1(1):67-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskind D, Russell A, Gamble C, Winckel K, Hollingworth S, Kisely S. RCT of exenatide for clozapine-associated obesity. Schizophrenia Bulletin 2017;43:S130. [Google Scholar]
- Siskind D, Russell A, Gamble C, Winckel K, Hollingworth S, Kisely S. Treatment of clozapine-associated obesity and diabetes with exenatide (CODEX) in adults with schizophrenia: a randomised controlled trial. Schizophrenia Bulletin 2018;44 Suppl 1:S178. [Google Scholar]
- Siskind D. Treatment of clozapine-associated obesity and diabetes with exenatide (codex) in adults with schizophrenia. Australian and New Zealand Journal of Psychiatry 2017;51 Suppl 1:68. [Google Scholar]
- Siskind DJ, Russell AW, Gamble C, Winckel K, Mayfield K, Hollingworth S, et al. Treatment of clozapine-associated obesity and diabetes with exenatide in adults with schizophrenia: a randomized controlled trial (CODEX). Diabetes, Obesity and Metabolism 2018;20(4):1050-5. [DOI] [PubMed] [Google Scholar]
Siskind 2020 {published data only}
- Siskind D, Russell A, Gamble C, Baker A, Cosgrove P, Burton L, et al. Metabolic measures 12 months after a randomised controlled trial of treatment of clozapine associated obesity and diabetes with exenatide (CODEX). Journal of Psychiatric Research 2020;124:9-12. [DOI] [PubMed] [Google Scholar]
- Siskind D, Russell A, Kisely S. 12-month follow up of metabolic measures following a randomised controlled trial of treatment of clozapine associated obesity and diabetes with exenatide (Codex). Schizophrenia Bulletin 2020;46:S248. [DOI] [PubMed] [Google Scholar]
Smith 2013 {published data only}
- Smith RC, Jin H, Li C, Bark N, Shekhar A, Dwivedi S, et al. Effects of pioglitazone on metabolic abnormalities, psychopathology, and cognitive function in schizophrenic patients treated with antipsychotic medication: a randomized double-blind study. Schizophrenia Research 2013;143(1):18-24. [DOI] [PubMed] [Google Scholar]
Smith 2018 {published data only}
- Smith RC, Maayan L, Wu R, Youssef M, Jing Z, Sershen H, et al. Betahistine effects on weight-related measures in patients treated with antipsychotic medications: a double-blind placebo-controlled study. Psychopharmacology 2018;235(12):3545-58. [DOI] [PubMed] [Google Scholar]
Strous 2007 {published and unpublished data}
- Strous RD, Stryjer R, Maayan R, Gal G, Viglin D, Katz E, et al. Analysis of clinical symptomatology, extrapyramidal symptoms and neurocognitive dysfunction following dehydroepiandrosterone (DHEA) administration in olanzapine treated schizophrenia patients: a randomized, double-blind placebo controlled trial. Psychoneuroendocrinology 2007;32(2):96-105. [PMID: ] [DOI] [PubMed] [Google Scholar]
Sulejmanpasic 2019 {published data only}
- Sulejmanpasic G, Bise S, Pepic F, Toskic A. Adjunctive treatment of aripiprazole to olanzapine for weight reduction in patients with schizophrenia. European Neuropsychopharmacology 2019;29:S90. [Google Scholar]
- Sulejmanpasic G, Bise S, Toskic A, Pepic F. Olanzapine augmented with aripiprazole in treatment-resistant schizophrenia. European Neuropsychopharmacology 2019;29:S89-S90. [Google Scholar]
Talaei 2016 {published data only}
- Talaei A, Faridhosseini F, Kazemi H, Fayyazi Bordbar MR, Rezaei Ardani A. Effect of topiramate on drug associated weight gain of patients with schizophrenia and bipolar I disorders: a dose ranging randomized trial. Turkish Journal of Psychiatry [Turk psikiyatri dergisi] 2016;27(2):84-91. [PubMed] [Google Scholar]
Tavakoli 2014 {published data only}
- Tavakoli E, Rezaei O, Fadai F. A comparison on composition of celery, dill and green tea as three medicinal plants with placebo to treat schizophrenic patients' metabolic syndrome. Afinidad 2014;80(573):1118-23. [Google Scholar]
Taveira 2014 {published data only}
- Taveira TH, Wu WC, Tschibelu E, Borsook D, Simonson DC, Yamamoto R, et al. The effect of naltrexone on body fat mass in olanzapine-treated schizophrenic or schizoaffective patients: a randomized double-blind placebo-controlled pilot study. Journal of Psychopharmacology (Oxford, England) 2014;28(4):395-400. [DOI] [PubMed] [Google Scholar]
Tek 2014 {published data only}
- Tek C, Ratliff J, Reutenauer E, Ganguli R, O'Malley SS. A randomized, double-blind, placebo-controlled pilot study of naltrexone to counteract antipsychotic-associated weight gain: proof of concept. Journal of Clinical Psychopharmacology 2014;34(5):608-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tek C, Ratliff JC, Reutenauer EL, Palmese LB, Tonizzo KM, O'Malley SS. Low-dose naltrexone for antipsychotic-induced weight gain in women with schizophrenia. Obesity 2011;19 Suppl 1:S181. [Google Scholar]
- Tek C, Ratliff JC, Reutenauer EL, Palmese LB, Tonizzo KM, O'Malley SS. Naltrexone for antipsychotic-induced weight gain in women with schizophrenia. Biological Psychiatry 2011;69(9 Suppl):283. [Google Scholar]
Terevnikov 2013 {published data only}
- Terevnikov V. Clinical effects of mirtazapine added to first generation antipsychotics in schizophrenia [Dissertation]. Helsinki, Finland: Medical Faculty of the University of Helsinki, 2013. [Google Scholar]
Tiihonen 2005 {published data only}
- Tiihonen J, Halonen P, Wahlbeck K, Repo-Tiihonen E, Hyvarinen S, Eronen M, et al. Topiramate add-on in treatment-resistant schizophrenia: a randomized, double-blind, placebo-controlled, crossover trial. Journal of Clinical Psychiatry 2005;66(8):1012-15. [DOI] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang XL. Metformin with life style intervention in treatment of clozapine - induced metabolic disorders. Chinese General Practice 2009;12(5B):840-2. [Google Scholar]
Wang 2010 {published data only}
- Wang SS, Wang CF, Zhang K, Cao CB, Gu DH, Yu ZH. Propofol and etomidate mect combined low-dose aripiprazole in the treatment of schizophrenia control study of 160 cases. Medical Journal of Chinese People's Health 2010;22(3):252-5, 257. [Google Scholar]
Wang 2012 {published data only}19105
- Wang M, Tong J-h, Zhu G, Liang G-M, Yan H-f, Wang X-Z. Metformin for treatment of antipsychotic-induced weight gain: a randomized, placebo-controlled study. Schizophrenia Research 2012;138(1):54-7. [CSZG: 23834] [DOI] [PubMed] [Google Scholar]
Wang 2020a {published data only}
- Wang L, Chen Y, Sui YC, Tan XQ, Zhou Z, Li N, et al. Metformin attenuates liver fat content: finding from schizophrenia patients with olanzapine-induced weight gain. Clinical Psychopharmacology and Neuroscience 2020;18(1):67-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020b {published data only}
- Wang C, Shi W, Xu J, Huang C, Zhu J. Outcomes and safety of concomitant topiramate or metformin for antipsychotics-induced obesity: a randomized-controlled trial. Annals of General Psychiatry 2020;19:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weber 2006 {published data only}
- Weber M, Wyne K. A cognitive/behavioral group intervention for weight loss in patients treated with atypical antipsychotics. Schizophrenia Research 2006;83(1):95-101. [DOI] [PubMed] [Google Scholar]
Weiner 2012 {published data only}
- Weiner E, Ball MP, Buchholz BA, Gold JM, Evins AE, McMahon RP, et al. Buproprion sustained release added to group support for smoking cessation in schizophrenia: a new randomized trial and a meta-analysis. Journal of Clinical Psychiatry 2012;73(1):95-101. [NCT00176449] [DOI] [PubMed] [Google Scholar]
Whicher 2021 {published data only}
- EUCTR2017-004064-35-GB. Liraglutide and the management of overweight and obesity in people with schizophrenia. https://www.clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2017-004064-35-GB (first received 2017).
- ISRCTN61129760. Liraglutide and the management of overweight and obesity in people with schizophrenia. https://www.isrctn.com/ISRCTN61129760 (first received 29 November 2017).
- Whicher CA, Price HC, Phiri P, Rathod S, Barnard-Kelly K, Ngianga K, et al. The use of liraglutide 3.0 mg daily in the management of overweight and obesity in people with schizophrenia, schizoaffective disorder and first episode psychosis: results of a pilot randomised double-blind placebo-controlled trial. Diabetes, Obesity and Metabolism 2021;26(6):1262-71. [DOI] [PubMed] [Google Scholar]
- Whicher CA, Price HC, Phiri P, Rathod S, Barnard-Kelly K, Reidy C, et al. Liraglutide and the management of overweight and obesity in people with schizophrenia, schizoaffective disorder and first-episode psychosis: protocol for a pilot trial. Trials 2019;20(1):633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whicher CA, Price HC, Rathod S, Phiri P, Barnard K, Thorne K, et al. Trial design for 'liraglutide and the management of overweight and obesity in people with severe mental illness: A pilot study' in southern health NHS foundation trust. Diabetic Medicine 2019;36 Suppl 1:51. [Google Scholar]
Wu 2012 {published data only}16701
- Wu RR, Jin H, Gao K, Twamley EW, Ou JJ, Shao P, et al. Metformin for treatment of antipsychotic-induced amenorrhea and weight gain in women with first-episode schizophrenia: a double-blind, randomized, placebo-controlled study. American Journal of Psychiatry 2012;169(8):813-21. [CSZG: 24499] [DOI] [PubMed] [Google Scholar]
Yagcioglu 2005 {published and unpublished data}
- Yagcioglu AE, Akdede BB, Turgut TI, Tumuklu M, Yazici K, Alptekin K, et al. A double-blind controlled study of adjunctive treatment with risperidone in schizophrenic patients partially responsive to clozapine: efficacy and safety. Journal of Clinical Psychiatry 2005;66(1):63-72. [DOI] [PubMed] [Google Scholar]
Yoon 2008 {published data only}
- Yoon B-H, Bahk W-M, Bae A, Kim M-K, Jon D-I, Min KJ. Effects of 3-months trial of zonisamide and weight management program in obese schizophrenic inpatients. International Journal of Neuropsychopharmacology 2008;11 Suppl 1:150. [Google Scholar]
References to studies awaiting assessment
Ginsberg 2004 {published data only}
- Ginsberg DL. Add-on sibutramine for olanzapine-induced weight gain. Primary Psychiatry 2004;11(7):24. [Google Scholar]
Mondal 2014 {published data only}
- Mondal H, Paul S, Guha P. Role of metformin versus topiramate in preventing olanzapine associated weight gain and metabolic syndrome. Indian Journal of Pharmacology 2014;1:S21. [Google Scholar]
References to ongoing studies
NCT04524403 {published data only}
- NCT04524403. Study evaluating the safety, efficacy, and pharmacokinetics of miricorilant in obese adult patients with schizophrenia while taking antipsychotic medications (GRATITUDE II). https://clinicaltrials.gov/show/NCT04524403 (first received 24 August 2020).
NL8440 {published data only}
- De Boer N, Guloksuz S, Van Baal C, Willebrands L, Deenik J, Vinkers CH, et al. Study protocol of a randomized, double-blind, placebo-controlled, multi-center trial to treat antipsychotic-induced weight gain: the Metformin-Lifestyle In Antipsychotic users (MELIA) trial. BMC Psychiatry 2021;21(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ADA/APA 2004
- American Diabetes Association, the American Psychological Association, the American Association of Clinical Endocrinologists, and the North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004;27(2):596-601. [DOI] [PubMed] [Google Scholar]
Aichhorn 2007
- Aichhorn W, Marksteiner J, Walch T, Zernig G, Hinterhuber H, Stuppaeck C, et al. Age and gender effects on olanzapine and risperidone plasma concentrations in children and adolescents. Journal of Child and Adolescent Psychopharmacology 2007;17(5):665-74. [DOI] [PubMed] [Google Scholar]
Alberti 2009
- Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120(16):1640-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Allison 1999
- Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. American Journal of Psychiatry 1999;156(11):1686-9. [DOI] [PubMed] [Google Scholar]
Allison 2003
- Allison DB, Mackell JA, McDonnell DD. The impact of weight gain on quality of life among persons with schizophrenia. Psychiatric Services 2003;54:565-7. [DOI] [PubMed] [Google Scholar]
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alvarez‐Jimenez 2008
- Alvarez-Jimenez M, Gonzalez-Blanch C, Crespo-Facorro B, Hetrick S, Rodriguez-Sanchez JM, Perez-Iglesias R, et al. Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: a systematic critical reappraisal. CNS Drugs 2008;22(7):547-62. [DOI] [PubMed] [Google Scholar]
Ananth 2004
- Ananth J, Venkatesh R, Burgoyne K, Gadasalli R, Binford R, Gunatilake S. Atypical antipsychotic induced weight gain: pathophysiology and management. Annals of Clinical Psychiatry 2004;16:75-85. [DOI] [PubMed] [Google Scholar]
Andreasen 1989
- Andreasen NC. The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. British Journal of Psychiatry. Supplement 1989;155(52):49-58. [PubMed] [Google Scholar]
Annamalai 2017
- Annamalai A, Kosir U, Tek C. Prevalence of obesity and diabetes in patients with schizophrenia. World Journal of Diabetes 2017;8(8):390-6. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
APA 2000
- American Psychiatric Association (APA). Diagnostic and statistical manual of mental disorders. 4th edition. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
Bak 2014
- Bak M, Fransen A, Janssen J, Van Os J, Drukker M. Almost all antipsychotics result in weight gain: a meta-analysis. PLOS One 2014;9(4):e94112. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Baptista 2002
- Baptista T, Kin NM, Beaulieu S, Baptista EA. Obesity and related metabolic abnormalities during antipsychotic drug administration: mechanisms, management and research perspectives. Pharmacopsychiatry 2002;35:205-19. [DOI] [PubMed] [Google Scholar]
Barnes 1989
- Barnes TR. A rating scale for drug-induced akathisia. British Journal of Psychiatry 1989;154:672-6. [DOI] [PubMed] [Google Scholar]
Basson 2001
- Basson BR, Kinon BJ, Taylor CC, Szymanski KA, Gilmore JA, Tollefson GD. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. Journal of Clinical Psychiatry 2001;62:231-8. [DOI] [PubMed] [Google Scholar]
Birt 2003
- Birt J. Management of weight gain associated with antipsychotics. Annals of Clinical Psychiatry 2003;15:49-58. [DOI] [PubMed] [Google Scholar]
Bland 1997
- Bland JM. Statistics notes. Trials randomised in clusters. BMJ 1997;315(7108):600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boissel 1999
- Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, et al. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use [Aperçu sur la problématique des indices d'efficacité thérapeutique, 3: comparaison des indices et utilisation. Groupe d'Etude des Indices D'efficacite]. Therapie 1999;54(4):405-11. [PMID: ] [PubMed] [Google Scholar]
Bora 2017
- Bora E, Akdede BB, Alptekin K. The relationship between cognitive impairment in schizophrenia and metabolic syndrome: a systematic review and meta-analysis. Psychological Medicine 2017;47(6):1030-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brown 1999
- Brown S, Birtwhistle J, Roe L, Thompson C. The unhealthy lifestyle of people with schizophrenia. Psychological Medicine 1999;29:697-701. [DOI] [PubMed] [Google Scholar]
Bueno‐Antequera 2018
- Bueno-Antequera J, Oviedo-Caro MA, Munguia-Izquierdo D. Relationship between objectively measured sedentary behavior and health outcomes in schizophrenia patients: the PsychiActive Project. Schizophrenia Research 2018;197:87-92. [PMID: ] [DOI] [PubMed] [Google Scholar]
Casey 2004
- Casey DE, Haupt DW, Newcomer JW, Henderson DC, Sernyak MJ, Davidson M, et al. Antipsychotic-induced weight gain and metabolic abnormalities: implications for increased mortality in patients with schizophrenia. Journal of Clinical Psychiatry 2004;65 Suppl 7:4-18. [PubMed] [Google Scholar]
Catapana 2004
- Catapana L, Castle D. Obesity in schizophrenia: what can be done about it? Australasian Psychiatry 2004;12:23-5. [DOI] [PubMed] [Google Scholar]
Cohn 2004
- Cohn T, Prud'homme D, Streiner D, Kameh H, Remington G. Characterizing coronary heart disease risk in chronic schizophrenia: high prevalence of the metabolic syndrome. Canadian Journal of Psychiatry 2004;49:753-60. [DOI] [PubMed] [Google Scholar]
Coodin 2001
- Coodin S. Body mass index in persons with schizophrenia. Canadian Journal of Psychiatry 2001;46:549-55. [DOI] [PubMed] [Google Scholar]
Cooper 2016
- Cooper SJ, Reynolds GP, Barnes T, England E, Haddad PM, Heald A, et al. BAP guidelines on the management of weight gain, metabolic disturbances and cardiovascular risk associated with psychosis and antipsychotic drug treatment. Journal of Psychopharmacology (Oxford, England) 2016;30(8):717-48. [PMID: ] [DOI] [PubMed] [Google Scholar]
Correll 2011
- Correll CU, Lencz T, Malhotra AK. Antipsychotic drugs and obesity. Trends in Molecular Medicine 2011;17(2):97-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Correll 2014
- Correll CU, Robinson DG, Schooler NR, Brunette MF, Mueser KT, Rosenheck RA, et al. Cardiometabolic risk in patients with first-episode schizophrenia spectrum disorders: baseline results from the RAISE-ETP study. JAMA Psychiatry 2014;71(12):1350-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
CSP 2003
- Chinese Society of Psychiatry (CSP). Chinese Classification of Mental Disorders. web.archive.org/web/20061205213538/http://www.21jk.com/english/index.asp.
Daumit 2003
- Daumit GL, Clark JM, Steinwachs DM, Graham CM, Lehman A, Ford DE. Prevalence and correlates of obesity in a community sample of individuals with severe and persistent mental illness. Journal of Nervous and Mental Disease 2003;191:799-805. [DOI] [PubMed] [Google Scholar]
Daumit 2005
- Daumit GL, Goldberg RW, Anthony C, Dickerson F, Brown CH, Kreyenbuhl J, et al. Physical activity patterns in adults with severe mental illness. Journal of Nervous and Mental Disease 2005;193:641-6. [DOI] [PubMed] [Google Scholar]
Deeks 2000
- Deeks J. Issues in the selection for meta-analyses of binary data. In: 8th International Cochrane Colloquium; 2000 Oct 25-28; Cape Town. Cape Town: The Cochrane Collaboration, 2000.
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook (accessed March 2021).
De Hert 2011
- De Hert M, Detraux J, Van Winkel R, Yu W, Correll CU. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nature Reviews Endocrinology 2011;8(2):114-26. [DOI] [PubMed] [Google Scholar]
De Nayer 2005
- De Nayer A, De Hert M, Scheen A, Van Gaal L, Peuskens J. Belgian consensus on metabolic problems associated with atypical antipsychotics. International Journal of Psychiatry in Clinical Practice 2005;9:130-7. [DOI] [PubMed] [Google Scholar]
de Silva 2016
- Silva VA, Suraweera C, Ratnatunga SS, Dayabandara M, Wanniarachchi N, Hanwella R. Metformin in prevention and treatment of antipsychotic induced weight gain: a systematic review and meta-analysis. BMC Psychiatry 2016;16(1):341. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dipasquale 2013
- Dipasquale S, Pariante CM, Dazzan P, Aguglia E, McGuire P, Mondelli V. The dietary pattern of patients with schizophrenia: a systematic review. Journal of Psychiatric Research 2013;47:197-207. [DOI] [PubMed] [Google Scholar]
Divine 1992
- Divine GW, Brown JT, Frazier LM. The unit of analysis error in studies about physicians' patient care behavior. Journal of General Internal Medicine 1992;7(6):623-9. [DOI] [PubMed] [Google Scholar]
DoH 2004
- Department of Health. At Least Five a Week. A report from the Chief Medical Officer. HMSO, London, 2004. [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne D, Altman DG, Higgins JP, Curtina F, Worthingtond HV, Vaile A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140-9. [DOI] [PubMed] [Google Scholar]
Faulkner 2007
- Faulkner G, Cohn T, Remington G. Interventions to reduce weight gain in schizophrenia. Cochrane Database of Systematic Reviews 2007, Issue 1. Art. No: CD005148. [DOI: 10.1002/14651858.CD005148.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Faulkner 2007a
- Faulkner G, Cohn T, Remington G, Irving H. Body mass index, waist circumference and quality of life in individuals with schizophrenia. Schizophrenia Research 2007;90(1-3):174-8. [DOI] [PubMed] [Google Scholar]
Findling 2010
- Findling RL, Johnson JL, McClellan J, Frazier JA, Vitiello B, Hamer RM, et al. Double-blind maintenance safety and effectiveness findings from the Treatment of Early-Onset Schizophrenia Spectrum (TEOSS) study. Journal of the American Academy of Child and Adolescent Psychiatry 2010;49(6):583-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Friedman 2010
- Friedman JI, Wallenstein S, Moshier E, Parrella M, White L, Bowler S, et al. The effects of hypertension and body mass index on cognition in schizophrenia. American Journal of Psychiatry 2010;167(19):1232-9. [DOI] [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7-10. [DOI] [PubMed] [Google Scholar]
Gebhardt 2009
- Gebhardt S, Haberhausen M, Heinzel-Gutenbrunner M, Gebhardt N, Remschmidt H, Krieg JC, et al. Antipsychotic-induced body weight gain: predictors and a systematic categorization of the long-term weight course. Journal of Psychiatric Research 2009;43:620-6. [DOI] [PubMed] [Google Scholar]
Goff 2005
- Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah NA, Daumit GL, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophrenia Research 2005;80:45-53. [DOI] [PubMed] [Google Scholar]
Goldstein 1994
- Goldstein DJ, Rampey AH Jr, Enas GG, Potvin JH, Fludzinski LA, Levine LR. Fluoxetine: a randomized clinical trial in the treatment of obesity. International Journal of Obesity and Related Metabolic Disorders 1994;18(3):129-35. [PMID: ] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed December 2020. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Green 2000
- Green A, Patel J, Goisman R. Weight gain from novel antipsychotic drugs: need for action. General Hospital Psychiatry 2000;22:224-35. [DOI] [PubMed] [Google Scholar]
Gulliford 1999
- Gulliford MC. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology 1999;149(9):876-83. [DOI] [PubMed] [Google Scholar]
Gurpegui 2012
- Gurpegui M, Martínez-Ortega JM, Gutiérrez-Rojas L, Rivero J, Rojas C, Jurado D. Overweight and obesity in patients with bipolar disorder or schizophrenia compared with a non-psychiatric sample. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2012;37(1):169-75. [DOI] [PubMed] [Google Scholar]
Guy 1976
- Guy William. ECDEU assessment manual for psychopharmacology. US Department of Health, Education, and Welfare, Public Health Service, 1976. [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry 1960;23(1):56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henderson 2005b
- Henderson DC, Nguyen DD, Copeland PM, Hayden DL, Borba CP, Louie PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. Journal of Clinical Psychiatry 2005;66:1116-21. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021
- Higgins JP, Li T, Deeks JJ editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook (accessed March 2021).
Hoang 2011
- Hoang U, Stewart R, Goldacre MJ. Mortality after hospital discharge for people with schizophrenia or bipolar disorder: retrospective study of linked English hospital episode statistics, 1999-2006. BMJ (Clinical Research Ed.) 2011;343:d5422. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Homel 2002
- Homel P, Casey D, Allison DB. Changes in body mass index for individuals with and without schizophrenia. Schizophrenia Research 2002;55:277-84. [DOI] [PubMed] [Google Scholar]
Hundal 2003
- Hundal RS, Inzucchi SE. Metformin: new understandings, new uses. Drugs 2003;63(18):1879-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hutton 2009
- Hutton JL. Number needed to treat and number needed to harm are not the best way to report and assess the results of randomised clinical trials. British Journal of Haematology 2009;146(1):27-30. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jagust 2007
- Jagust W. What can imaging reveal about obesity and the brain? Current Alzheimer Research 2007;4(2):135-9. [DOI] [PubMed] [Google Scholar]
Jin 2008
- Jin H, Meyer JM, Mudaliar S, Jeste DV. Impact of atypical antipsychotic therapy on leptin, ghrelin, and adiponectin. Schizophrenia Research 2008;100(1-3):70-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kay 1986
- Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale (PANSS) Manual. North Tonawanda, NY: Multi-Health Systems, 1986. [Google Scholar]
Keshavan 2008
- Keshavan MS, Tandon R, Boutros NN, Nasrallah HA. Schizophrenia, "just the facts": what we know in 2008 Part 3: neurobiology. Schizophrenia Research 2008;106(2-3):89-107. [DOI] [PubMed] [Google Scholar]
Keshavan 2011
- Keshavan MS, Nasrallah HA, Tandon R. Schizophrenia, "Just the Facts" 6. Moving ahead with the schizophrenia concept: from the elephant to the mouse. Schizophrenia Research 2011;127(1-3):3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kishi 2015
- Kishi T, Iwata N. Efficacy and tolerability of histamine-2 receptor antagonist adjunction of antipsychotic treatment in schizophrenia: a meta-analysis of randomized placebo-controlled trials. Pharmacopsychiatry 2015;48(1):30-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kurzthaler 2001
- Kurzthaler I, Fleischhacker WW. The clinical implications of weight gain in schizophrenia. Journal of Clinical Psychiatry 2001;62 Suppl 7:32-7. [PubMed] [Google Scholar]
Lappin 2018
- Lappin JM, Wijaya M, Watkins A, Morell R, Teasdale S, Lederman O, et al. Cardio-metabolic risk and its management in a cohort of clozapine-treated outpatients. Schizophrenia Research 2018;99:367-73. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook/archive/v6.2/chapter-04. [DOI: 10.1002/9781119536604.ch4] [DOI]
Le Fevre 2001
- Le Fevre PD. Improving the physical health of patients with schizophrenia: therapeutic nihilism or realism? Scottish Medical Journal 2001;46:11-31. [DOI] [PubMed] [Google Scholar]
Leon 2006
- Leon AC, Mallinckrodt CH, Chuang-Stein C, Archibald DG, Archer GE, Chartier K. Attrition in randomized controlled clinical trials: methodological issues in psychopharmacology. Biological Psychiatry 2006;59(11):1001-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lett 2012
- Lett TA, Wallace TJ, Chowdhury NI, Tiwari AK, Kennedy JL, Muller DJ. Pharmacogenetics of antipsychotic-induced weight gain: review and clinical implications. Molecular Psychiatry 2012;17(3):242-66. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leucht 2005a
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel RR. What does the PANSS mean? Schizophrenia Research 2005;79(2-3):231-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Leucht 2005b
- Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of brief psychiatric rating scale scores. British Journal of Psychiatry 2005;187:366-71. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lindenmayer 2012
- Lindenmayer JP, Khan A, Kaushik S, Thanju A, Praveen R, Hoffman L, et al. Relationship between metabolic syndrome and cognition in patients with schizophrenia. Schizophrenia Research 2012;142(1-3):171-6. [DOI] [PubMed] [Google Scholar]
Liu 2015
- Liu Z, Zheng W, Gao S, Qin Z, Li G, Ning Y. Metformin for treatment of clozapine-induced weight gain in adult patients with schizophrenia: a meta-analysis. Shanghai Archives of Psychiatry 2015;27(6):331-40. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liversedge 2011
- Liversedge S, Gilchrist I, Everling S. The Oxford Handbook of Eye Movements. OUP Oxford, 2011. [Google Scholar]
Mackin 2005
- Mackin P, Watkinson HM, Young AH. Prevalence of obesity, glucose homeostasis disorders and metabolic syndrome in psychiatric patients taking typical or atypical antipsychotic drugs: a cross-sectional study. Diabetologia 2005;48:215-21. [DOI] [PubMed] [Google Scholar]
Marshall 2000
- Marshall M, Lockwood A, Bradley C, Adams C, Joy C, Fenton M. Unpublished rating scales: a major source of bias in randomised controlled trials of treatments for schizophrenia. British Journal of Psychiatry 2000;176:249-52. [DOI] [PubMed] [Google Scholar]
McCreadie 1998
- McCreadie R, Macdonald E, Blacklock C, Tilak-Singh D, Wiles D, Halliday J, et al. Dietary intake of schizophrenic patients in Nithsdale, Scotland: case-control study. BMJ 1998;317:784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357(9263):1191-4. [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. PLoS Medicine 6;7:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mueser 2004
- Mueser KT, McGurk SR. Schizophrenia. Lancet 2004;363(9426):2063-72. [DOI] [PubMed] [Google Scholar]
Newcomer 2007
- Newcomer JW, Hennekens CH. Severe mental illness and risk of cardiovascular disease. JAMA 2007;298(15):1794-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Okuyama 2016
- Okuyama Y, Oya K, Matsunaga S, Kishi T, Iwata N. Efficacy and tolerability of topiramate-augmentation therapy for schizophrenia: a systematic review and meta-analysis of randomized controlled trials. Neuropsychiatric Disease and Treatment 2016;12:3221-36. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborn 2001
- Osborn DP. The poor physical health of people with mental illness. Western Journal of Medicine 2001;175:329-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Overall 1962
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799-812. [Google Scholar]
Peeters 2003
- Peeters A, Barendregt JJ, Willekens F, Mackenbach JP, Al Mamun A, Bonneux L, et al. Obesity in adulthood and its consequences for life expectancy: a life-table analysis. Annual of Internal Medicine 2003;138:24-32. [DOI] [PubMed] [Google Scholar]
Perez‐Iglesias 2008
- Perez-Iglesias R, Crespo-Facorro B, Martinez-Garcia O, Ramirez-Bonilla ML, Alvarez-Jimenez M, Pelayo-Teran JM, et al. Weight gain induced by haloperidol, risperidone and olanzapine after 1 year: findings of a randomized clinical trial in a drug-naive population. Schizophrenia Research 2008;99(1-3):13-22. [DOI] [PubMed] [Google Scholar]
Rajkumar 2017
- Rajkumar AP, Horsdal HT, Wimberley T, Cohen D, Mors O, Borglum AD, et al. Endogenous and antipsychotic-related risks for diabetes mellitus in young people with schizophrenia: a Danish population-based cohort study. American Journal of Psychiatry 2017;174(7):686-94. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ratzoni 2002
- Ratzoni G, Gothelf D, Brand-Gothelf A, Reidman J, Kikinzon L, Gal G, et al. Weight gain associated with olanzapine and risperidone in adolescent patients: a comparative prospective study. Journal of the American Academy of Child and Adolescent Psychiatry 2002;41:337-43. [DOI] [PubMed] [Google Scholar]
Reichenberg 2007
- Reichenberg A, Harvey PD. Neuropsychological impairments in schizophrenia: integration of performance-based and brain imaging findings. Psychological Bulletin 2007;133(5):833-58. [DOI] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Reynolds 2010
- Reynolds GP, Kirk SL. Metabolic side effects of antipsychotic drug treatment--pharmacological mechanisms. Pharmacology & Therapeutics 2010;125(1):169-79. [DOI] [PubMed] [Google Scholar]
Reynolds 2017
- Reynolds GP, McGowan OO. Mechanisms underlying metabolic disturbances associated with psychosis and antipsychotic drug treatment. Journal of Psychopharmacology (Oxford, England) 2017;31(11):1430-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Richardson 2005
- Richardson CR, Faulkner G, McDevitt J, Skrinar GS, Hutchinson DS, Piette JD. Integrating physical activity into mental health services for persons with serious mental illness. Psychiatric services (Washington, D.C.) 2005;56(3):324-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ringen 2018
- Ringen PA, Faerden A, Antonsen B, Falk RS, Mamen A, Rognli EB, et al. Cardiometabolic risk factors, physical activity and psychiatric status in patients in long-term psychiatric inpatient departments. Nordic Journal of Psychiatry 2018;72(4):296-302. [PMID: ] [DOI] [PubMed] [Google Scholar]
Roberts 2021
- Roberts MT, Shokraneh F, Sun Y, Groom M, Adams CE. Classification of psychotherapy interventions for people with schizophrenia: development of the Nottingham Classification of Psychotherapies. Evidence-Based Mental Health 2021;24:62-9. [DOI: 10.1136/ebmental-2020-300151] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saari 2005
- Saari KM, Lindeman SM, Viilo KM, Isohanni MK, Jarvelin MR, Lauren LH, et al. A 4-fold risk of metabolic syndrome in patients with schizophrenia: the Northern Finland 1966 birth cohort study. Journal of Clinical Psychiatry 2005;66:559-63. [DOI] [PubMed] [Google Scholar]
Saha 2007
- Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Archives of General Psychiatry 2007;64:1123-31. [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from www.training.cochrane.org/handbook (accessed March 2021).
Seaton 2001
- Seaton BE, Goldstein G, Allen DN. Sources of heterogeneity in schizophrenia: the role of neuropsychological functioning. Neuropsychology Review 2001;11(1):45-67. [DOI] [PubMed] [Google Scholar]
Sellbom 2012
- Sellbom KS, Gunstad J. Cognitive function and decline in obesity. Journal of Alzheimer's Disease 2012;30 Suppl 2:S89-95. [DOI] [PubMed] [Google Scholar]
Shokraneh 2017
- Shokraneh F, Adams CE. Study-based registers of randomized controlled trials: starting a systematic review with data extraction or meta-analysis. BioImpacts 2017;7(4):209-17. [DOI: 10.15171/bi.2017.25] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokraneh 2019
- Shokraneh F, Adams CE. Study-based registers reduce waste in systematic reviewing: discussion and case report. Systematic Reviews 2019;8:129. [DOI: 10.1186/s13643-019-1035-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shokraneh 2020
- Shokraneh F, Adams CE. Cochrane Schizophrenia Group’s Study-Based Register of Randomized Controlled Trials: development and content analysis. Schizophrenia Bulletin Open 2020;1:sgaa061. [DOI: 10.1093/schizbullopen/sgaa061] [DOI] [Google Scholar]
Shokraneh 2021
- Shokraneh F, Adams CE. Classification of all pharmacological interventions tested in trials relevant to people with schizophrenia: a study-based analysis. Health Information and Libraries Journal 2021. [DOI: 10.1111/hir.12366] [DOI] [PubMed] [Google Scholar]
Silverman 2018
- Silverman BL, Martin W, Memisoglu A, DiPetrillo L, Correll CU, Kane JM. A randomized, double-blind, placebo-controlled proof of concept study to evaluate samidorphan in the prevention of olanzapine-induced weight gain in healthy volunteers. Schizophrenia Research 2018;195:245-51. [PMID: ] [DOI] [PubMed] [Google Scholar]
Silverstone 1988
- Silverstone T, Smith G, Goodall E. Prevalence of obesity in patients receiving depot antipsychotics. British Journal of Psychiatry 1988;153:214-7. [DOI] [PubMed] [Google Scholar]
Simpson 1970
- Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatrica Scandinavica 1970;45(S212):11-9. [DOI] [PubMed] [Google Scholar]
Siskind 2016
- Siskind DJ, Leung J, Russell AW, Wysoczanski D, Kisely S. Metformin for clozapine associated obesity: a systematic review and meta-analysis. PloS One 2016;11(6):e0156208. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Egger M, Moher D editor(s). Chapter 10: Addressing reporting biases. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Strassnig 2003
- Strassnig M, Brar JS, Ganguli R. Nutritional assessment of patients with schizophrenia: a preliminary study. Schizophrenia Bulletin 2003;29:393-7. [DOI] [PubMed] [Google Scholar]
Susce 2005
- Susce MT, Villanueva N, Diaz FJ, Leon J. Obesity and associated complications in patients with severe mental illnesses: a cross-sectional survey. Journal of Clinical Psychiatry 2005;66:167-73. [DOI] [PubMed] [Google Scholar]
Vancampfort 2017
- Vancampfort D, Firth J, Schuch FB, Rosenbaum S, Mugisha J, Hallgren M, et al. Sedentary behavior and physical activity levels in people with schizophrenia, bipolar disorder and major depressive disorder: a global systematic review and meta-analysis. World Psychiatry 2017;16(3):308-15. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Os 2009
- Van Os J, Kapur S. Schizophrenia. Lancet 2009;374(9690):635-45. [DOI] [PubMed] [Google Scholar]
Velazquez 2018
- Velazquez A, Apovian CM. Updates on obesity pharmacotherapy. Annals of the New York Academy of Sciences 2018;1411(1):106-19. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ward 2015
- Ward MC, White DT, Druss BG. A meta-review of lifestyle interventions for cardiovascular risk factors in the general medical population: lessons for individuals with serious mental illness. Journal of Clinical Psychiatry 2015;76(4):e477-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wei 1999
- Wei M, Kampert JB, Barlow CE, Nichaman MZ, Gibbons LW, Paffenbarger RS, et al. Relationship between low cardiorespiratory fitness and mortality in normal weight, overweight and obese men. JAMA 1999;282:1547-53. [DOI] [PubMed] [Google Scholar]
Weiden 2004
- Weiden PJ, Mackell JA, McDonnell DD. Obesity as a risk factor for antipsychotic noncompliance. Schizophrenia Research 2004;66:51-7. [DOI] [PubMed] [Google Scholar]
Westman 2017
- Westman J, Eriksson SV, Gissler M, Hallgren J, Prieto ML, Bobo WV, et al. Increased cardiovascular mortality in people with schizophrenia: a 24-year national register study. Epidemiology and Psychiatric Sciences October 2018;27(5):519-527. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2013
- World Health Organization. Obesity and overweight fact sheet. www.who.int/mediacentre/factsheets/fs311/en/ accessed before 22 July 2022.
WHO 2016
- World Health Organization (WHO). International Statistical Classification of Diseases and Related Health Problems 10th Revision. icd.who.int/browse10/2016/en accessed before 25 July 2022.
Wirshing 2004
- Wirshing DA. Schizophrenia and obesity: impact of antipsychotic medications. Journal of Clinical Psychiatry 2004;65 Suppl 18:13-26. [PubMed] [Google Scholar]
Xia 2009
- Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H, et al. Loss to outcomes stakeholder survey: the LOSS study. Psychiatric Bulletin 2009;33(7):254-7. [Google Scholar]
Zipursky 2005
- Zipursky RB, Gu H, Green AI, Perkins DO, Tohen MF, McEvoy JP, et al. Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. British Journal of Psychiatry 2005;187:537-43. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hahn 2014
- Hahn M, Remington G, Duncan MJ, Cohn T, Faulkner GEJ. Pharmacological interventions for reducing weight gain in schizophrenia. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD011127. [DOI: 10.1002/14651858.CD011127] [DOI] [PMC free article] [PubMed] [Google Scholar]


